Leveraging cognitive resources to investigate the impact of molecular orientation on students’ activation of symmetry resources
Olivia M.
Crandell
 and
Samuel
Pazicni
and
Samuel
Pazicni
 *
*
Department of Chemistry, University of Wisconsin-Madison, Madison, Wisconsin, USA. E-mail: sam.pazicni@chem.wisc.edu
First published on 28th October 2022
Abstract
This study investigates students’ cognitive resources for identifying symmetry elements using survey data collected from 39 inorganic chemistry students from twelve undergraduate inorganic classes at universities across the United States. We propose a framework that leverages students’ knowledge of symmetry elements as a manifold of cognitive resources that can be productive or unproductive in certain contexts. This cognitive resources framework was paired with an asset-based perspective to advance work in this area beyond a deficit-framed study of “correctness”. Herein, we present findings from a qualitative analysis of the rotation and reflection symmetry elements activated for students by four select molecules. Each molecule was presented in two different static orientations to determine the impact of orientation on students’ cognitive resource activation. Our findings suggest that the orientation of static molecular images impacts the types of resources activated for students. Additionally, this study serves as an example for how to advance assessment of this topic beyond a binary approach (i.e. correct or incorrect).
Introduction
Recognizing patterns of symmetry in molecules is an essential skill in chemistry. The Anchoring Concepts Content Map (ACCM) lists symmetry as an Enduring Understanding under the Anchoring Concept of structure/function: chemical compounds have geometric structures that influence their chemical and physical behaviors (Holme and Murphy, 2012; Murphy et al., 2012). Thus, symmetry plays an important role in a chemist's ability to recognize structure–property relationships. Subdiscipline-specific applications of symmetry can be found throughout the undergraduate ACCM; for example, identifying patterns of symmetry has applications for interrogating solid-state structures introduced in General Chemistry (Holme and Murphy, 2012), determining the symmetry of orbitals and decoding spectroscopic evidence in Organic Chemistry (Raker et al., 2013) and Inorganic Chemistry (Marek et al., 2018), and predicting the symmetry and multiplicities of relative energy states in Physical Chemistry (Holme et al., 2018). These sub-disciplinary articulations are just a few examples of how students might use symmetry to approach chemistry tasks. We suggest that students might use symmetry in various tasks because not all tasks elicit evidence of students’ understanding or use of “symmetry” as it is operationalized in chemistry. For example, traditional assessment of symmetry in inorganic chemistry might ask students to identify a molecular point group using a flow chart. Students could be using a variety of processes or reasoning strategies to complete such an assessment task—indeed, studies in organic chemistry have identified multiple strategies including heuristics and algorithms for approaching visual-spatial tasks (Stieff et al., 2012).Several studies have investigated symmetry in the context of students’ spatial reasoning skills and chemistry. For example, Savec et al. (2005) examined the value of using multiple representations when solving spatial chemistry tasks that involve rotation, reflection, and three-dimensional perception, and concluded that student perception of molecular structure is best supported by using tangible models. Southam and Lewis (2013) also reported the value of targeted instruction with guided inquiry activities to moderate any differences in visuospatial ability.
More common are studies that report tools to help students visualize elements of symmetry and identify point groups. These include activities using various objects (Herman and Lievin, 1977; McKay and Boone, 2001; Flint, 2011), shapes (Gallian, 1990), artwork (Glasser, 1967), dice (Grafton, 2011), and guided inquiry activities (Luxford et al., 2012; Rattanapirun and Laosinchai, 2021) and virtual visualization tools (Cass et al., 2005; Korkmaz and Harwood, 2004; Meyer and Sargent, 2007; Tuvi-Arad and Gorsky, 2007; Sein, 2010; Tuvi-Arad and Blonder, 2010). Additionally, a virtual tool has been developed to help students understand how inter-molecular symmetry can be invoked to relate collections of molecules (Ruiz and Johnstone, 2020). While these reports provide evidence for a rich landscape related to symmetry instruction, our goal for this study was to characterize students’ recognition of specific symmetry elements from a cognitive perspective.
Students’ ideas about symmetry are not being formed primarily in our college classrooms, as students’ domain-general conceptions of symmetry have been studied at a variety of ages. Even at young ages (ages 3–7), learners are encouraged to reflect objects across horizontal, vertical, or diagonal mirror planes (Bornstein and Stiles-Davis, 1984; Schuler, 2001; Valenzeno et al., 2003; Ng and Sinclair, 2015; Nakawa, 2020). Some authors have also identified developmental trends in students’ spatial reasoning abilities across age (Bornstein and Stiles-Davis, 1984; Schuler, 2001). Students’ ability to recognize reflective symmetry has also been investigated in older children (ages 8–16) (Küchemann, 1980; Meyer et al., 1992; Hoyles and Healy, 1997; Falba and Williams, 1998; Seidel, 1998; Ramful et al., 2015; Dejarnette et al., 2016). Evidence suggests that children of all ages discern vertical reflection symmetry in objects (meaning reflecting something across a vertical line) more easily than horizontal symmetry (Fisher and Fracasso, 1987) and diagonal symmetry remains difficult even for older children (Küchemann, 1980; Bornstein and Stiles-Davis, 1984; Seidel, 1998; Ramful et al., 2015). For example, in a qualitative case study (Ramful et al., 2015), one participant (age 14) described the process of reflection as equidistance between the objects and the line of symmetry and “exactly opposite.” This conception served the student well when negotiating vertical and horizontal reflections but not diagonal ones—they often did not take angles of reflection over the diagonal line into account. That said, we feel it is important to note that there is little utility in characterizing “developmentally appropriate” visualizations for students, rather, visualization tools should be used for children as early as preschool with the goal of “producing a generation of learners well equipped to use visualizations” (Newcombe and Stieff, 2012, p. 964).
Goldmeier (1937) observed that adults perceive images with vertical symmetry to be most like a comparable object displaying both vertical and horizontal symmetry. For example, row A of Fig. 1 shows three figures: that in column A has both horizontal and vertical symmetry, while those in columns B and C have been modified to have only vertical or horizontal symmetry, respectively. Participants perceived the object with vertical symmetry (column B) to be more like the object in column A than the object in column C. In subsequent work, Fisher and Fracasso (1987) concluded that a combination of factors including relative environment (such as an inherent sense of “up and down” because of gravity) and predisposed or imposed ideas about the inherent “top and bottom” of an image or object, likely contribute to this “Goldmeier effect.” Because many molecular structures have inherent symmetry aligned with vertical and/or horizontal perspectives (depending on orientation) (row B, Fig. 1), we posited that the Goldmeier effect may be operating for undergraduate inorganic students when engaging with molecular symmetry tasks. While the aim of this work was not to investigate the Goldmeier effect specifically, this literature suggests that the orientation of the object may influence how students perceive the symmetry elements of that object.
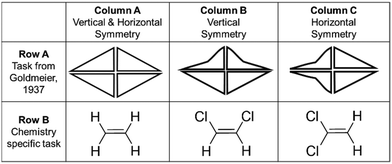 | ||
| Fig. 1 Illustrations of the Goldmeier effect, using shapes (Row A) and representations of molecules (Row B). | ||
In the work presented here, we investigated fundamental building blocks of molecular symmetry tasks: the types of rotation and reflection symmetry elements that students select for a given molecule. The tasks we present in this contribution are intended to elicit students’ foundational knowledge of symmetry elements to lay the groundwork for future research on students’ understanding and reasoning about domain-general and domain-specific applications of symmetry.
Theoretical framework
Activating cognitive resources
We have chosen to approach this work on student understanding of symmetry from an asset-based perspective. By “asset-based”, we mean forgoing a binary approach of characterizing students’ ideas as wholly correct or wholly incorrect. Instead, we choose to interpret the implications of our results from the perspective that students’ responses could be productive in a given context, even with varying levels of accuracy. While we do not deny that students may hold ideas that are scientifically inaccurate or developing in accuracy, it is more beneficial for student learning to focus on the ways students’ ideas might be productive for a particular task. Thus, defining an idea as productive or unproductive must be in reference to a specific aim. Hammer et al. (2005) refer to students’ ideas as cognitive resources and propose that these resources can be applied in productive or unproductive ways in specific contexts. These resources can be “raw” or “intuitive” as described by diSessa's phenomenological primitives (p-prims) (1988) or be learned through instruction. Asset-based perspectives have been explicitly leveraged in recent chemistry education research work to move beyond characterizing equity gaps (Tashiro and Talenquer, 2021; White et al., 2021).We argue that the productive/unproductive use continuum does not necessarily have to coincide with a continuum of scientific accuracy ranging from highly accurate to developing in accuracy. We conceive of these two continuums as orthogonal axes where ideas can range in their accuracy but also in their productivity for a particular task—we illustrate this idea in Fig. 2, using hypothetical responses for a specific task related to boron trifluoride. For example, a task could ask students to use symmetry elements to identify the point group of boron trifluoride with the support of a flow chart. A hypothetical response might be that boron trifluoride has a C3 principal rotation axis. This is scientifically accurate and would be productive for helping a student determine the point group of boron trifluoride (D3h). We place this hypothetical response in the upper right quadrant of Fig. 2 to represent how a response can be both accurate and productive for this task. However, a different student may suggest that boron trifluoride is planar and therefore contains a σh reflection plane. Identifying a σh reflection plane is scientifically accurate and productive toward determining a D3h point group; however, the student's reasoning that a planar molecule automatically contains a σh reflection plane is developing in its accuracy because this is not always true. (A σh reflection plane is defined as a plane that is perpendicular to the principal rotation axis.) This example response demonstrates how accuracy and productivity for the point group determination task might differ, thus its placement in the lower right quadrant in Fig. 2. If a student suggests that boron trifluoride does not possess an inversion center, they would have activated a resource that is scientifically accurate but not productive towards determining the point group using a flow chart as the task demands, thus its placement in the upper left quadrant of Fig. 2. Finally, there are certainly ideas that would not be productive for the task at hand nor scientifically accurate such as identifying a C4 rotation axis for BF3 (lower left quadrant). It is important to note that the characterization of these hypothetical responses is intended to illustrate how responses might be interpreted as productive or unproductive for the specified task, in this case determining the point group of boron trifluoride. Identifying that a molecule is planar and therefore contains a σh would not be productive for identifying the point group of carbon dioxide. The productivity of a resource must be considered in reference to a specific task and an instructors’ unique learning goals for that task rather than a rigid, pre-defined hierarchy.
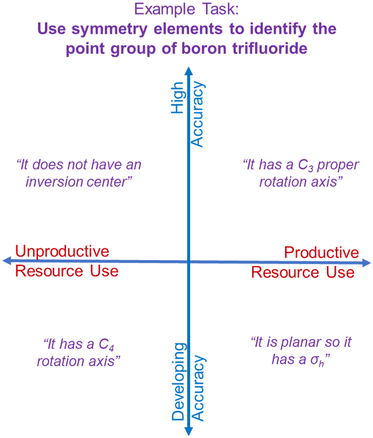 | ||
| Fig. 2 Theoretical model of how cognitive resources might be characterized as accurate or inaccurate as well as productive or unproductive for a specific prompt. | ||
We find that viewing students’ manifold of cognitive resources in an asset-based way is useful when approaching research on students’ understanding of symmetry for several reasons. First, because students’ ideas about reflection and rotation symmetry are formed in childhood in domain-general contexts (Bornstein and Stiles-Davis, 1984; Schuler, 2001; Valenzeno et al., 2003; Ng and Sinclair, 2015; Nakawa, 2020), a resources perspective considers how students’ ideas, intuitive or learned, contribute to their responses in a particular task. Second, a resources perspective provides the necessary terminology to consider students’ answers as productive or unproductive for a given context. In the context of the work presented here, we will consider each symmetry element to be a cognitive resource, and each can be characterized as accurate or inaccurate for the task at hand as presented in our Results section. We follow each discussion of accuracy versus inaccuracy with a discussion of how these activated resources with varying degrees of accuracy might be viewed as productive or unproductive in different problem-solving contexts beyond the task described in this article. We want to emphasize that this discussion of productive versus unproductive is our way of elevating this work beyond a deficit-framed discussion of “right” or “wrong” to challenge instructors and researchers to look for ways that students’ ideas could be leveraged productively in other contexts even if they are considered inaccurate in their present context.
Research questions
This study was designed to elicit evidence of activated symmetry resources for four different molecules in different orientations. Our results will discuss the different ways students’ resources could be activated accurately or inaccurately and our discussion will consider ways that ideas can be productive or unproductive for different purposes. We have chosen to apply an asset-based, resources lens to this work to illustrate a proof of concept for how researchers and instructors might reframe their evaluation of their students’ knowledge about symmetry. Our study was guided by the following research questions:1. How accurately are rotation and reflection symmetry elements activated for students by a simple prompt?
2. How does molecular orientation influence the accuracy of cognitive resources activated for students by a simple prompt?
3. What trends exist in students’ responses across molecules with similar molecular symmetry elements?
Methods
Participants
Participants were recruited from undergraduate inorganic courses across the United States. In the United States, inorganic chemistry courses are offered typically at the “foundational” and “advanced” levels; molecular symmetry is taught at both levels (Raker et al., 2015a, 2015b). Students typically take one or both courses after an introductory chemistry experience. Twelve inorganic chemistry course instructors at eleven institutions agreed to distribute information about this study to their students. We received approval from IRB offices at all institutions. Two classes were surveyed at the end of the fall 2020 academic term and the other 10 in spring 2021; data were gathered following each course's summative exam on symmetry. Across the 12 classes, a survey (described below) was offered to ∼250 students resulting in 39 complete responses. Of the 39 participants, 21 participants identified as men, 18 participants identified as women, and no participants identified as non-binary nor chose to self-describe their gender. All students who completed the survey were entered into a drawing and 20 were randomly selected to receive a $20 Amazon gift card.Survey design
This survey was designed to engage students in two types of molecular symmetry tasks: (1) identify symmetry elements and (2) perform a reflection operation. For parsimony, we will limit the discussion presented here to the results of the first task asking students to identify symmetry elements. To build these items, we chose a collection of molecules with different geometries and symmetry elements including benzene derivatives and alkenes to investigate both planar and three-dimensional geometries. We intentionally chose molecules containing no more than four different types of atoms and only five or six total atoms, save for the benzene derivative items. This choice to limit the number and types of atoms was to potentially reduce students’ cognitive load while analyzing the molecule. Additionally, in many cases, reducing the number of types of atoms increases the number of symmetry elements a molecule possesses. We selected molecules belonging to higher symmetry point groups because they contained more symmetry elements for students to analyze; however, due to the relative simplicity of these molecules, it is possible that participants have seen these molecules before. We intentionally collected data after students’ summative assessments on symmetry to, theoretically, capture students’ best understanding of this topic.To develop the survey, we conducted think-aloud cognitive interviews with two undergraduate students who had completed an inorganic chemistry course that included symmetry to understand how students were interpreting the tasks, the molecular representation choices and orientations, the length of the survey, and symmetry elements under investigation. Student feedback from these cognitive interviews informed changes to the final version of the survey. For example, we chose to limit the symmetry element options to rotation axes and reflection planes. We intentionally omitted improper rotations, identity, and inversion following our findings in the cognitive interviews. We found that students were far less familiar with those symmetry elements and investigations of those elements might be better served by detailed interviews or a more focused survey. However, our cognitive interviews found students to meaningfully discuss σv and σh reflection planes, and therefore these reflection elements were included in the final survey design.
The survey began with a statement of informed consent, asking students to identify their gender identity, list their institution, and list their completed inorganic courses. Next, students were asked to rate their confidence in their ability to (1) identify a rotation axis, (2) identify a σv reflection plane, (3) identify a σh reflection plane. Next, students were provided with a training question for each task type. Following each training question, students were provided with the scientifically accurate response (Appendix 1). Once students completed the introductory items, students were presented with 20 research tasks (12 items from Task 1 and 8 items from Task 2). The 20 research items were randomized for each participant. In this contribution, we will discuss the results from selected Task 1 items only.
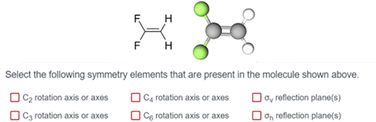
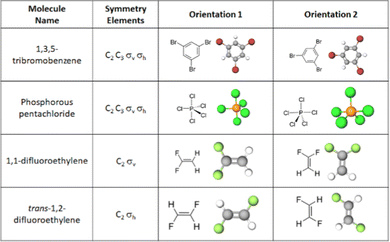
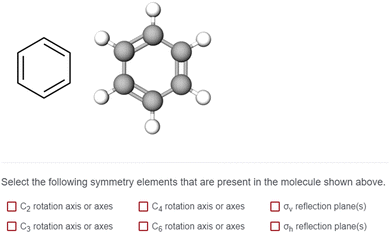
Task 1 prompted students to identify rotation and reflection elements via selection from a list. The items presented students with a molecule represented as a Lewis structure alongside a two-dimensional image of a ball-and-stick representation. The combination of Lewis structures and ball-and-stick models provided students with the identity of the atoms and the three-dimensional shape of each molecule. Our cognitive interviews provided evidence that students used both types of representations in their symmetry analyses. While we do not claim that static representations such as these are ideal for eliciting students’ visuospatial ideas, static representations are (to our knowledge) the most common way students are assessed on symmetry ideas. Thus, using these representations also increases the applicability of our study for instructors who use static representations in various teaching and assessment capacities. Students were asked to “Select the following symmetry elements that are present in the molecule shown above” from a list of six symmetry elements: C2 rotation axis, C3 rotation axis, C4 rotation axis, C6 rotation axis, σv reflection plane, and σh reflection plane (Fig. 3). Students were asked to complete this task for six different molecules. Each molecule was presented twice (in two different orientations, related by a 90° rotation) creating 12 unique items for Task 1 (Fig. 4). The 90° relationship between the two orientation options for each molecule was chosen based on findings from Fisher and Fracasso (1987) that a 90° orientation change might influence how symmetry elements are interpreted. The survey was administered using Qualtrics.
Follow-up interviews
At the end of the survey, students were asked if they would like to be contacted for a voluntary follow-up interview for which they would receive additional compensation. Nine students consented to being contacted and five of those students chose to participate in follow-up interviews. Interviews were conducted virtually using Zoom and were audio and video recorded. The first author performed the interviews. Each interview began with a discussion of informed consent and participants were encouraged to think-aloud throughout the interview. The interview protocol involved the interviewer sharing their screen to show images of molecules similar to those shown in Fig. 4 with both the Lewis structure and a ball-and-stick model. Students were encouraged to identify symmetry elements they saw in the representation by describing their thinking as well as use the Zoom annotate feature to draw axes of rotation and reflection planes to the best of their ability. The interviews were transcribed verbatim and integrated with video data of students’ annotations. Interview data were analyzed inductively in sections by molecule looking for evidence of student thinking about the difference in orientation. We will report our findings about how our five interview participants interpreted the two orientations of phosphorous pentachloride.Data trustworthiness
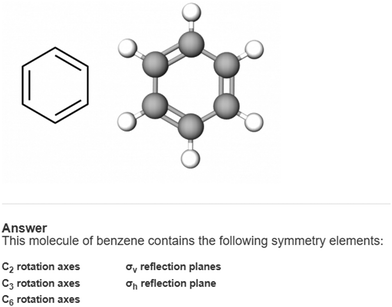
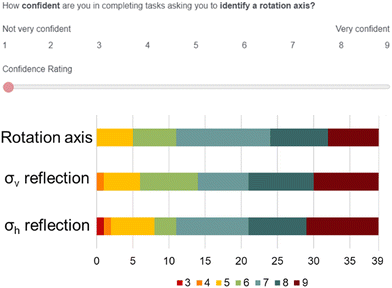
We approached analysis of the survey from a qualitative perspective, and therefore are relying less on quantitative measures of validity and reliability. Instead, we will discuss the ways that our findings are trustworthy by triangulating across multiple data sources and reducing personal researcher bias (Nobel and Smith, 2015). One way we confirmed that students were indeed answering the survey questions as intended was to gauge their personal confidence on the types of symmetry tasks. At the start of the survey, students were presented with three questions assessing their confidence with identifying rotation axes, a σv reflection plane, and a σh reflection plane. Students were asked to rank their confidence from 1 to 9 ranging from “not very confident” to “very confident” respectively. We found that students were relatively confident to very confident in their abilities on these tasks. The results of these confidence tasks are reported in Appendix 2. Next, students were presented with a practice item (Appendix 1). This practice item presented both the Lewis structure and a ball-and-stick model for benzene, with the same task structure shown in Fig. 3. Once students completed the item, we presented the scientifically accurate responses for the task and asked students if they understood what the question was asking. All 39 students confirmed that “Yes, I understand what this question is asking.”As previously discussed, the survey items were presented in random order to each participant. This was an intentional design choice to ensure that participants did not engage with orientation 1 followed by orientation 2 for every molecule. If all students had seen one orientation followed by the next, we would not be able to decouple how the orientation change influenced students’ ideas about that orientation from the fact that they were being retested on the same molecule back-to-back. We did not observe any meaningful “ordering” effects in students’ responses (Appendix 3).
Finally, we conducted follow-up interviews with five participants to elicit their reasoning when selecting symmetry elements for phosphorous pentachloride. The purpose of these interviews was to check the trustworthiness of data collected in the survey by asking students to identify the symmetry elements and explain their thinking. We found that for orientation 1, students’ responses to the survey and their responses in the interview were nearly identical. However, we did find some differences between survey responses and interview responses for orientation 2. We will discuss these differences in more detail in the Results section. These differences are interpreted not as a indication of invalid results, but rather, further evidence of the instability in the patterns of students’ resource activation for certain molecules in certain orientations which aligns with the theoretical framing of this study. However, it is important to note that the interviewer had not seen the students’ survey responses prior to conducting the interviews to avoid influencing students’ response patterns during the follow-up interviews.
Results
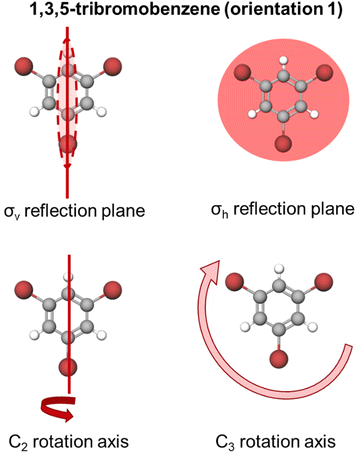
Here, we organized our findings by molecule. We will consider how orientation impacted students’ resource activation using bar charts as well as consider the collection of resources activated together for each individual participant using Sankey diagrams. We also compare across pairs of molecules for those with similar symmetry elements and those with similar architecture. Each finding is followed by a discussion of how the findings might be framed as productive or unproductive for other chemistry tasks to elevate results that might otherwise be viewed in a deficit lens to an asset-based perspective.1,3,5-Tribromobenzene
1,3,5-Tribromobenzene possesses a σh and three σv reflection planes along with one C3 and three C2 rotation axes. Visual representations of these symmetry elements are provided in Appendix 4.We recognize that this molecule possesses other elements including identity, and an S3 improper rotation axis; however, our survey was not designed to elicit students’ resources about these elements (see above). Students were asked to select the symmetry elements present in the molecule from a limited list of proper rotations and reflections (Fig. 3). In this case, σv, σh, C2, and C3 would each be scientifically accurate resources.
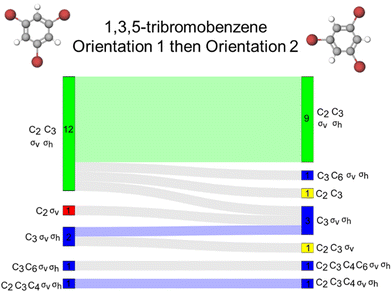
Fig. 5A reports the frequencies for each activated resource for both orientations of this molecule. We found that both orientations of the 1,3,5-tribromobenzene elicited accurate activation of σv and σh reflection planes for most students in our sample. For example, we see that for orientation 1, 37 of the 39 respondents selected a σv reflection plane and 35 respondents did so for orientation 2. We see similar trends for the σh reflection plane. Similarly, we found high counts of accurate activation of C3 rotation axis by both orientations. Interestingly, we observed that the C2 axis was not activated as often as the other resources; moreover, C2 was activated more frequently by orientation 1 than orientation 2. Fig. 5A shows that 30 of 39 participants selected a C2 axis for orientation 1 and only 27 participants for orientation 2. We call attention to this difference in C2 axis activation because, unlike the C3 axis, there are multiple C2 rotation axes present in this molecule; yet the C2 resource was activated less frequently by orientation 2.
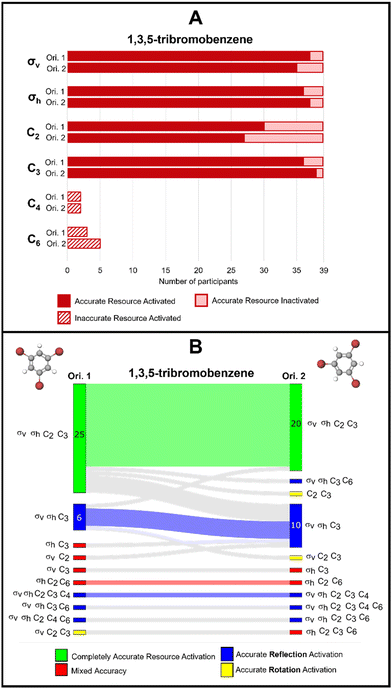 | ||
| Fig. 5 (A) Bar chart showing frequencies of students’ resource use for 1,3,5-tribromobenzene. (B) Sankey diagram showing students’ resource use across two orientations of 1,3,5-tribromobenzene. | ||
In addition to characterizing the frequencies of each activated resource, we analysed the response profiles for each participant to understand how molecular orientation influenced symmetry resource activation. To do this, we present our data using Sankey diagrams to show students’ complete response profiles across both orientations of the molecule (Fig. 5B). In these Sankey diagrams, green shading represents responses where only the accurate set of resources was activated. For example, orientation 1 of 1,3,5-tribromobenzene accurately activated σv, σh, C2, and C3 for 25 students (shown by the green bar on the left side of Fig. 5B), but orientation 2 activated this accurate set for only 20 students (green bar on the right side of Fig. 5B). Furthermore, we found that the accurate set of resources was activated consistently by both orientations for 19 students, as shown by the light green shading connecting the two sides of the Sankey diagram. Next, we found that orientations 1 and 2 activated σv, σh, and C3 together for six and 10 students, respectively. In this case, the reflection planes were activated, but not all accurate rotation resources were activated. This is indicated by blue shading in these Sankey diagrams. We also found that this set of resources was consistently activated for only four students across the two orientations; this is indicated by the blue shaded wave flowing from left to right. The rest of the Sankey diagram features blue, yellow, and red bars each representing a unique student response. Blue shading is used to represent responses where all reflection resources were accurately activated, yellow shading indicates responses where all rotation resources were accurately activated, and red shading indicates a mixture of accurate and inaccurate resource activation.
Additionally, shading is used in the waves connecting the left and right sides of the diagram to indicate how students’ responses changed across the two orientations. The use of green, blue, yellow, and red color on these waves indicates that students’ responses were consistent across both orientations. Fig. 5B shows that students who were consistent across the orientations were displaying completely accurate resource activation or accurate reflection activation most often. All other waves shaded gray show the ways students changed their responses from one orientation to the next. Using these diagrams to visualize response patterns at the participant level, we can also see that 25 students gave the same answer across both orientations of 1,3,5-tribromobenzene as indicated by the green, blue, yellow, and red waves. Thus, 14 students (36% of our sample) changed their answer in some way as indicated by the gray waves. Overall, there does not appear to be an orientation of this molecule that better supports students in activating accurate symmetry resources. Finally, we found few instances of C4 and C6 activation.
To avoid a deficit view of what students know and can do with their knowledge of symmetry applied to 1,3,5-tribromobenzene, we now leverage our proposed framework where we decouple accuracy from potential productivity. For the purposes of this task, activation of C4 and C6 rotation axes is inaccurate, and we suspect activation of these two resources would not be productive for many other tasks involving this molecule. Therefore, inaccurate resources activated by either orientation of this molecule would most likely be considered unproductive. However, most students activated some accurate resources, usually at least one accurate rotation and reflection resource suggesting that students understand what rotation and reflection elements are, which could serve as a launch point for future instruction.
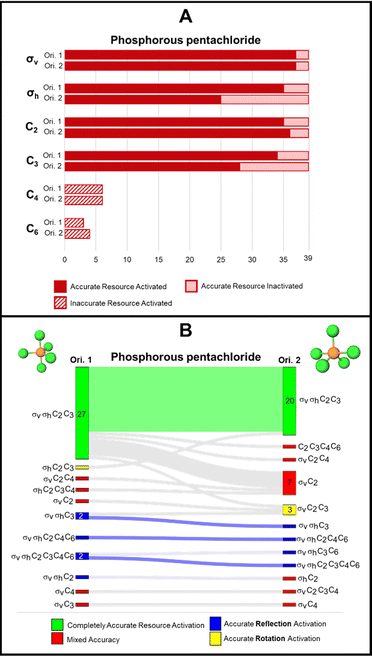
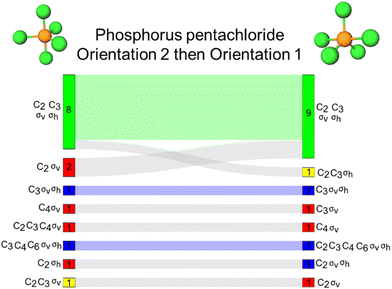
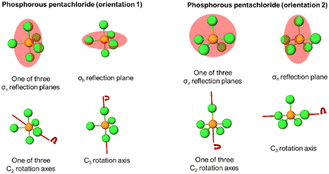
Phosphorous pentachloride
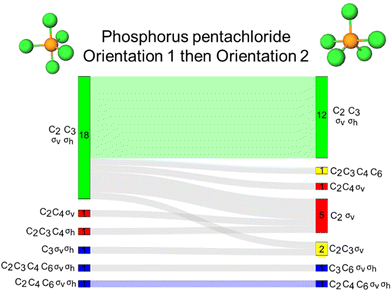
Phosphorous pentachloride, like 1,3,5-tribromobenzene, contains a σh and three σv reflection planes along with a C3 and three C2 proper rotation axes. Figures visualizing these symmetry elements in these two orientations are provided in Appendix 5. For the rotation resources, we find that the C2 rotation axis was activated for almost all students by both orientations, while the C3 was not. Specifically, the C3 axis was left inactivated for 11 students by orientation 2 of this molecule where the axis is positioned horizontally on the page. Both C4 and C6 were activated for a few students, though inaccurately, by both orientations of this molecule.The Sankey diagram in Fig. 6B reports evidence of students’ resource activation patterns across the two orientations. For example, we see that for 19 students, σv, σh, C2, C3 were activated by both orientations. Resource activation was consistent across the orientations for three other students, each with accurate reflection activation with some inaccurate rotation resources (as indicated by the blue shaded waves in Fig. 6B). Of the 39 respondents, 22 (56%) were consistent in symmetry resource activation across both orientations of phosphorus pentachloride.
As shown in Fig. 6A, we found that orientations 1 and 2 activated the σv reflection plane for 37 students, respectively. However, as shown in the accompanying Sankey diagram (Fig. 6B), this resource was not consistently activated by both orientations for the same 37 students. In contrast, the σh reflection plane was activated differently across orientations (for 35 students for orientation 1 and for 25 students for orientation 2). The σh reflection plane is coincident with the three equatorial chlorine atoms of phosphorus pentachloride. Although it is unclear why, the 90° change in orientation of phosphorus pentachloride seemed to impact how the σh reflection resource was activated for students. In our reflection interviews, we did uncover evidence that there was malleability in the activation patterns of students’ definitions of σv and σh. According to our interview data, this seems to be a minor fraction of our sample because we saw it in two of seven total interviews. We have elaborated on this evidence in Appendix 6 to be authentic to the data that we found. This finding in two of the seven interviews should not be taken as representative of our entire student sample nor should an instructor assume too much about stability in students’ definitions of reflection planes in different orientations and for different molecules. We have evidence that these definitions were stable for some but unstable for others for one specific molecule as discussed in Appendix 6 for the interested reader.
Follow-up interview data for phosphorous pentachloride
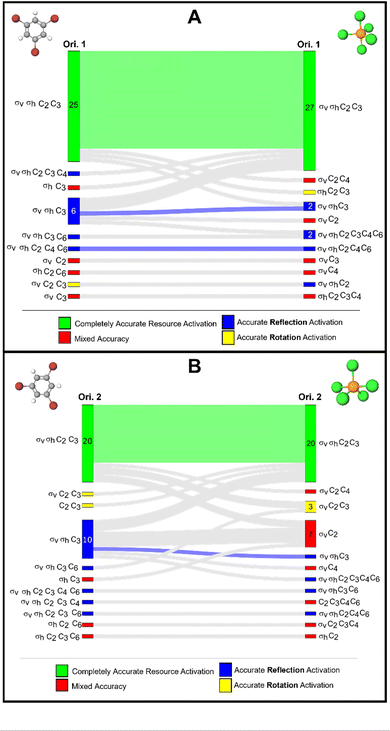
In our follow-up interviews with students, we presented students with both orientations of phosphorous pentachloride. All participants first saw orientation 1 and were encouraged to identify all the symmetry elements they could in the representation. They were not bound to only rotations and reflections, but in most cases, students did not identify other types of symmetry elements. Then, students were presented with orientation 2 of the molecule. The interviewer did not say that they were the same molecule but all five participants recognized it as the same molecule right away. Each student reported a different approach for how they would identify the symmetry elements for orientation 2. Some students reported preferences for one orientation over the other for identifying specific elements. For example, Adam stated “Yeah, I would say that it's [referring to orientation 2] not quite as clear as the first picture [orientation 1]. But overall, I still do get the same mental image from it. It just takes me a little bit to actually like, put everything together and say, oh, yeah, this is that two axial, three equatorial structure.” Another participant, Barry reported his strategy with the second orientation as being very similar to the first: “…but what I'm going to do is I'm just going to, in my mind, flip this thing [orientation 2] so it looks like the other one [orientation 1] since I've already done the work for the other one [referring orientation 1], and then do everything again. So here, check out the plane that goes through these [referring to the σhplane bisecting the equatorial atoms]. You've got the axis through each one, through these ones [referring to the σvplanes bisecting the axial atoms]… all I'm doing in my mind is I'm recreating everything that we just did 90 degrees over.” Barry went on to say that orientation 2 looked more three-dimensional to them because less atoms are depicted in the plane of the paper compared to orientation 1.Another participant, Daniel, also reported repeating his work from the first orientation when he said “Well, this is the same as the previous one just rotated 90°. So I would just, I mean, if these two were presented in succession, I would just apply whatever I did for the previous one to this one… I think I would still kind of check all the same boxes, but just in a different order” The interviewer followed up by asking “how would you rate the difficulty of it in this new orientation? The same, harder, slightly easier?” and Daniel replied “I would say probably a little easier….” The interviewer prompted Daniel to explain and Daniel responded “I think the thing that may have made it [orientation 2] easier than the other one [referring to orientation 1] is the fact that the rotational symmetry stuck out more quickly than in the other one. Maybe as the three equatorial atoms are, like, bigger in this structure than the last one. Like the triangle made by them takes up more area, I guess, relative to the diagram on the other one did, where the axial almost felt the dominant like the dominant atoms.”
When switching to orientation 2 from orientation 1, Carl commented “I definitely still see the C3pretty clearly. Just because, you know, even though this is in a different orientation, maybe the other view is probably, a bit easier to see that, especially in the notation that's on the left here [referring to the Lewis structure of for orientation 2]. Sometimes it might be a little bit confusing to see the 120° angle, everything being planar, with the chlorines right in the middle. So C3here. If you see the wedge and the dash here and see those being on the same plane, I think it's probably a little bit easier to see that there is a C2kind of going through here. That's going right down the middle of the two. So definitely this orientation kind of helps with that.” However, there was not a clear consensus among our five interview participants as to which representation they may have preferred for this task. When asked to compare which orientation was more difficult, Eileen reported that orientation 2 was a little harder.
The purpose of these interviews was not necessarily to determine which representation students preferred or even to determine which representation might be optimal. In fact, our data suggests that students had individual preferences depending on how they approached the task of identifying symmetry elements. Our primary purpose was to understand if students did indeed perceive differences between the two representations and to elicit some understanding of how those perceived differences influenced their process identifying symmetry elements. We believe these interview data illustrate the proof of concept that students did perceive differences between the orientations.
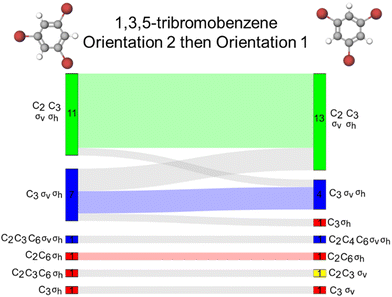
Comparing the D3h tasks
1,3,5-Tribromobenzene and phosphorous pentachloride present an interesting comparison because they contain the same symmetry elements, belong to the same point group but share very few structural motifs as one is planar without a central atom and the other adopts a trigonal bipyramidal geometry with a central atom. For these reasons, this comparison might be tenuous but even at a cursory glance, the comparison of these two molecules presents an interesting comparison. To investigate reflection and rotation resource activations in the vertical and horizontal directions, we analysed students’ responses across these two molecules comparing orientations 1 to each other and then orientations 2 to each other (Fig. 7). For orientation 1 of 1,3,5-tribromobenzene, there is a σv reflection plane in running vertically through the molecule. Similarly, orientation 1 of phosphorous pentachloride has three σv reflection planes in the vertical direction, each containing the axial chlorine atoms. We found that 24 students provided the same response across these two tasks with the set accurate resources (σv, σh, C2, and C3) being activated for 22 students (represented by the light green wave in Fig. 7A). Additionally, we find that both molecules positioned in orientation 1 activated the σv reflection plane for most students such that the σv resource was only left inactive for two students in orientation 1 of 1,3,5-tribromobenzene and two different students in orientation 1 of phosphorus pentachloride.Next, we compared orientations 2 for both 1,3,5-tribromobenzene and phosphorous pentachloride. Here, the benzene derivative features a σv reflection plane in the horizontal direction. For this orientation of phosphorus pentachloride, the σh reflection plane containing the equatorial atoms are now oriented vertically in space and the σv planes are coincident with the axial atoms that are oriented horizontally on the page. Across these two tasks, we found that the same collection of resources was activated for 16 students and, for 15 students, only the accurate set of resources (σv, σh, C2, and C3) was activated. The σv was left inactivated by the benzene derivative for four students and by phosphorus pentachloride for two students, though not necessarily the same students across the two examples (Fig. 7B). One of the most common sets of resources activated by this orientation of the benzene derivative was σv, σh, and C3. While this set was activated by phosphorus pentachloride, it was only so for a single student. The most common sets of resources activated by phosphorus pentachloride (other than the accurate set), were σv and C2 for seven students and σv, C2, and C3 for three students. Overall, the σh and C3 resources were often left inactivated by this phosphorus pentachloride orientation (Fig. 6A), a trend we did not observe for orientation 2 of the 1,3,5-tribromobenzene.
In the next sections, we discuss our findings for two structural isomers of similar molecular architecture: 1,1-difluoroethylene and trans-1,2-difluoroethylene. We will begin by discussing findings from the two orientations of 1,1-difluoroethylene.
1,1-Difluoroethylene
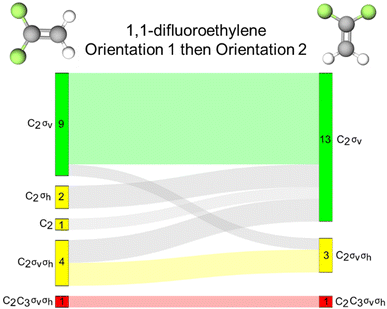
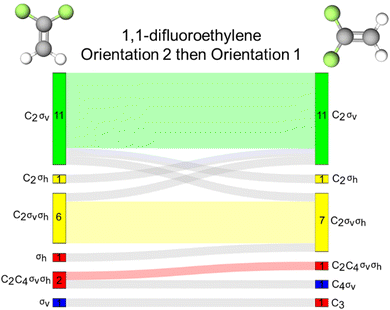
1,1-Difluoroethylene is an alkene that contains 2 σv reflection planes and a C2 rotation axis. The molecule does not contain a σh reflection plane as both reflection planes are coincident with the principal rotation axis and are therefore, defined as vertical reflection planes. We found that this distinction might be confusing for students in this context as evidenced by the 15 and 16 instances of inaccurate σh activation by orientations 1 and 2 of this molecule, respectively (Fig. 8A). We observed evidence of the same confusion in the interview quotes with phosphorus pentachloride. Looking at the response profiles provided in the Sankey diagram (Fig. 8B), we see that these instances of σh activation typically corresponded with σv activation with a few exceptions.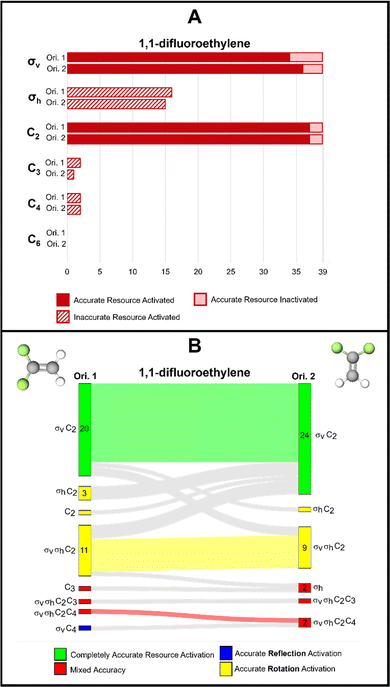 | ||
| Fig. 8 (A) Bar chart showing frequencies of students’ resource use for 1,1-difluoroethylene. (B) Sankey diagram showing students’ resource use across two orientations of 1,1-difluoroethylene. | ||
This finding could suggest two insights into students’ thinking. First, students identified the σv reflection plane that is coincident with the plane of page as a σh because that is sometimes the case with planar molecules such as the 1,3,5-tribromobenzene and the trans-1,2-difluoroethylene discussed in the next section. The second possible implication of this finding is that students do not necessarily know the conventional differences between σv and σh reflection planes. If we follow this second conjecture and assume that activation of any reflection plane is productive, we find that only 2 students did not have a reflection plane activated for orientation 1 and all students activated at least one reflection plane for orientation 2. Activation of any reflection resource (whether it is labelled accurately or not) could be productive for certain tasks, such as determining whether the molecule is chiral.
We do not have evidence to support either of these insights into student thinking as students were not asked to explicitly explain their reasoning behind each of these cognitive resources, nor was this task included in the follow up interview protocol. However, this finding maybe nonetheless of interest to instructors. If an instructor values students’ use of correct nomenclature and accurate assignment of σv and σh reflection planes, instructors might consider using a planar molecule such as this where both types of reflection are not present to elicit evidence of student reasoning. Furthermore, if instructors value students’ intuitive ideas about symmetry, they might consider asking students to draw and explain the reflection planes they see to activate relevant resources and guide students toward the more canonical use of terminology, if that is a learning goal. This task and these findings especially illuminate the utility of taking a resources perspective when considering students’ thinking about symmetry. Instead of focusing on the number of students that incorrectly identified a σh reflection plane (a deficit perspective of students’ thinking), we have chosen to highlight the numerous ways reflections were activated for students and suggest these intuitive ideas serve as building blocks to develop more desired understandings.
In addition to the reflection resources, we found that 26 students had the same collection of resources activated across these the orientations of 1,1-difluoroethylene. For these 26 students, molecular orientation did not influence how symmetry resources were activated. However, for 13 students (33% of our sample), orientation of the molecule did influence resource activation patterns, though it is not clear that one orientation better supports accurate symmetry resource activation.
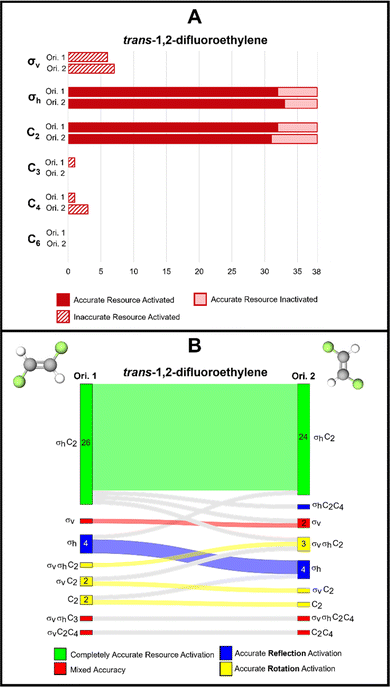
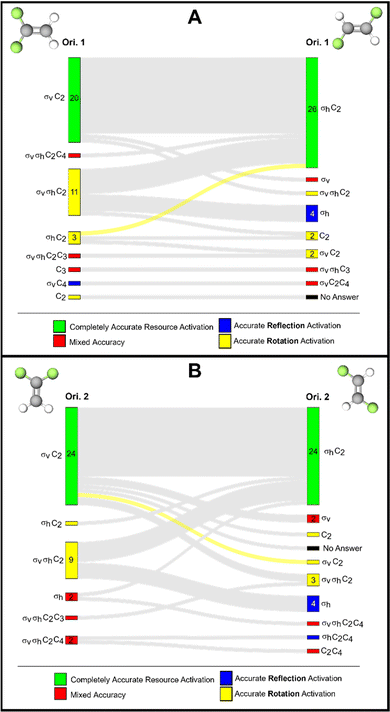
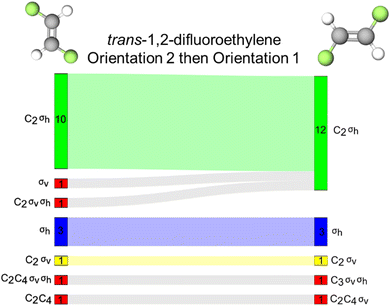
trans-1,2-Difluoroethylene
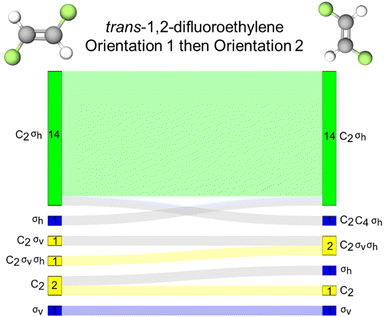
The final molecule we highlight in this report is trans-1,2,-difluoroethylene which contains a C2 and σh reflection plane. In this case, the plane of the molecule is perpendicular to the molecule's principal axis of rotation and therefore, the plane of the molecule is defined as a horizontal reflection plane. For this task pairing, we collected only 38 complete responses, compared to the 39 for the other tasks.There are relatively few instances of inaccurate resource activation, (i.e., activation of C3, C4, C6, or σv). We find that for orientations 1 and 2, the σv reflection resource was inaccurately activated for six and seven students, respectively. However, in only three instances was this resource activated without the other σh resource in both orientations. This suggests, like with 1,1,-difluoroethylene, that σv may be a productive resource for students as they are considering a reflection plane, but inaccurately label the plane.
We found that, of all the discussed tasks, changing the orientation of this molecule seemed to have the least effect on students’ resource activation, though there is not enough evidence to promote the use of orientation over another. We found that across both orientations, the same set of resources was activated for 30 of the 38 students and that for 23 of those students was the accurate set of resources (σh and C2) activated (Fig. 9B).
Comparing alkene tasks
After comparing how resource activation was impacted by different orientations of each alkene, we now will compare how similar orientations of these alkenes impacted patterns of resource activation. This comparison between 1,1-difluoroethylene and trans-1,2-difluoroethylene differs from our previous comparison of the D3h molecules, because these two alkenes do not belong to the same point group, although they are constitutional (or structural) isomers and do possess similar molecular architectures. These alkenes differ in their reflection planes: 1,1-difluoroethylene contains a σv reflection plane and trans-1,2-difluoroethylene contains a σh plane. Because of this, we would not expect the same sets of resources to be activated across these two molecules. This is indeed what we observed with the same sets of resources being activated across orientations 1 for only 1 student (Fig. 10A). For orientations 2, a similar observation was made, but with a different student than for orientations 1 (Fig. 10B). This finding suggests that students were able to distinguish between these molecules with similar molecular architecture, as we would expect. However, recall that changing the orientation of the molecule, for example, orientations 1 and 2 for 1,1-difluoroethylene, we observed different resource activation patterns when, in theory, we should not. This comparison suggests that instruction has tuned students into being sensitive to noticing differences in molecular architecture but not necessarily differences in molecular orientation.Conclusions and implications
Summary
We have leveraged the resources perspective (Hammer et al., 2005) to interpret students’ responses to symmetry tasks. Using a survey, we have investigated the frequency with which rotation and reflection symmetry elements are activated for students across two orientations of four different molecules. Even though 1,3,5-tribromobenzene and phosphorus pentachloride belong to the same point group, we found different response patterns across the two molecules as well as variation in the responses elicited from different orientations. We also report on 1,1-difluoroethylene and trans-1,2-difluoroethylene, which differ in their reflection planes. We advocate that even inaccurate activation of a reflection plane for alkenes can be leveraged productively in other types of tasks. These four molecules each presented in two orientations showed unique manifolds of activated resources and we hope that these examples provide a proof of concept of how a resources perspective can be leveraged to move beyond a binary approach of assessing symmetry.Implications for teaching
Following Rodriguez and Towns's (2019) challenge to our field, we have structured our implications for teaching by considering how these results can inform the different Professional Knowledge bases that inform pedagogical content knowledge (Chan and Hume, 2019). Specifically, we will highlight ways our results can inform instructors’ Assessment Knowledge and Curricular Knowledge (Chan and Hume, 2019).Assessment Knowledge is the knowledge of how to design formative and summative assessments for a topic. Instructors may wish to consider the potential impact of molecular orientation on students’ interpretation of reflections and rotations when designing formative assessment items. Given our findings, we suggest that instructors incorporate different orientations of static views of molecules on practice items and homework items. Doing so is a more feasible way to challenge students to consider different molecular orientations than allowing students access to virtual, manipulable 3D models, particularly on exams. These varied orientations may give instructors and students themselves deeper insights into how students identify and assign symmetry element labels. With this knowledge students’ can become aware of ways their knowledge might be applied inaccurately such as assuming the plane of a page is always a horizontal mirror plane, σh. We do not claim that the representations or orientations presented here are optimal representation choices, but rather emphasize that our study provides evidence that changes in orientations did result in different patterns of resource activation.
Next, we suggest this work has implications for instructor's Curricular Knowledge, which is the knowledge of the goals and sequence of a whole curriculum. Though a bounded task, identifying rotation and reflection symmetry elements is foundational to other applications of symmetry such as determining chirality and polarity; identifying nuclear equivalency for interpreting NMR; interpreting IR and UV-Vis absorbance spectra; crystallography; and analysing larger molecules with molecular orbital theory. We hope the results presented here encourage instructors to consider how students’ activation of symmetry elements might be leveraged productively in other types of chemistry tasks beyond the traditional symmetry unit in an upper-level inorganic chemistry course.
Implications for research
We hope this study serves as an example of how to move beyond a deficit framing when discussing student knowledge. Rather than focusing on students’ incorrect answers to these fundamental symmetry tasks, we hope we have demonstrated how researchers can adopt an asset perspective to identify ways to build more productive and robust understandings. In fact, assuming too much stability in students’ activation patterns is in many ways antithetical to our findings here which show that symmetry resource activation can be sensitive to different factors such as orientation. Our results, in total, as a collection of case studies illustrate that from a resources perspective, student knowledge should not be viewed as present or not but activated or inactivated based on factors (orientation and molecule for example). The power of leveraging a resources framework is that student knowledge can be viewed as dynamic. This article presented four case studies of fundamental symmetry tasks that we did not expect to elicit so much variability in activation.These results serve as a launch point for future research about students’ knowledge of domain-general and domain-specific applications of reflection and rotations symmetry. Additionally, this work identifies potential impacts of molecular orientation that could be leveraged in future research on problem-solving, representational competence, and spatial reasoning.
Limitations
Given the small sample size, it is difficult to draw any generalizable conclusions about students’ resource activation patterns. However, this was not the intent of this work; rather we leveraged a qualitative approach to illuminate an asset perspective for characterizing students’ cognitive resources. Even though we did not find evidence in our sample to populate each quadrant in our hypothetical model for accuracy and resource productivity, this work will serve as a launch point for future studies using more targeted interviews and more robust tasks to elicit fuller manifolds of students’ thinking.Additionally, we employed bounded tasks offering only selected rotations and reflection elements. Other studies will need to be designed to investigate more complex symmetry elements such as inversion and improper rotations. Furthermore, additional studies are needed to understand how orientation may impact students’ interpretations of symmetry when different representations are used including non-static or dynamic representations, representations in orientations differing from those studied here, and representations other than the Kekule form of benzene.
Finally, we have only limited evidence of student reasoning behind their responses. This is due to constraints presented by the COVID-19 pandemic where we were only able to collect certain types of evidence using surveys and virtual interviews. Future work will build on the lessons learned from the challenges encountered in this study.
Conflicts of interest
There are no conflicts to declare.Appendices
Appendix 1: Task 1 practice item
Appendix 2: assessment of students’ confidence in identifying symmetry elements
Appendix 3: survey results separated by order of orientation presentation
Appendix 4: visualizations of the symmetry elements present in 1,3,5-tribromobenzene
Appendix 5: Visualisations of the symmetry elements present in phosphorous pentachloride
Appendix 6: interview evidence of instability in students’ definitions of σv and σh
Interview data support the conjecture that students may conflate the definitions of σv and σh or suggest that the definitions of these elements are malleable for students based on the context. One interview participant commented “I mean, like, I have no problem saying that a plane is a σ (Sigma). That's fine. I have no problem saying that a rotation is a C. That's fine. But then I get some like, is this a σhor is this a σv? That that part like distinguishing between the two, I feel like is the more difficult part.” Another student commented that the orientation of the molecule impacts his labelling of σv and σh when he said “I think I'm mixing them around…if the C3, let's say is on the side, it all depends on how you're looking at the molecule. So if you're the C3on the side, then I would think in my mind that that would also be the σhwould be, you know, in that plane, when it could be the vertical.” This student began labelling the σv planes on orientation 1 but calling them σh's; then, on orientation 2, the student discovered their mistake and recited the accurate definition for these planes. Another participant did not use σv or σh nomenclature at all when discussing this molecule, though they did for other items in the interview. Instead, they drew the reflection planes and identified three types of planes bisecting the axial atoms (which would be the σv's) and another type of plane that would include the three equatorial atoms (which would be a σh). They did this for both orientations of the molecule. These three interview quotes are intended to show the variability in the ways that students approached identifying reflection planes in this molecule. This evidence supports the idea that not only are different reflection planes activated differently for students, but the definitions of σv and σh (and when to apply each of these labels) might be activating differently for each student and for different molecules. All five interview participants were able to articulate the two types of planes through their drawings and discussion, though use of canonical nomenclature was varied and not everyone identified all three σv planes when asked to identify and draw all reflection and rotation elements.Acknowledgements
Support for this research was provided by the University of Wisconsin-Madison, Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation. We also want to thank the instructors for their willingness to distribute the survey and the students who chose to participate.References
- Bornstein M. H. and Stiles-Davis J., (1984), Discrimination and Memory for Symmetry in Young Children, Dev. Psychol., 20(4), 637–649.
- Cass M. E., Rzepa H. S., Rzepa D. R. and Williams C. K., (2005), The use of the free, open-source program Jmol to generate an interactive web site to teach molecular symmetry, J. Chem. Educ., 82(11), 1736–1740.
- Chan K. K. H. and Hume A, (2019), Towards a Consensus Model: Literature Review of How Science Teachers’ Pedagogical Content Knowledge Is Investigated in Empirical Studies, in Hume, A., Cooper, R., Borowski, A., (ed.), Repositioning Pedagogical Content Knowledge in Teachers' Knowledge for Teaching Science, Singapore: Springer Singapore, pp. 3–76.
- Dejarnette A. F., González G., Deal J. T. and Rosado Lausell S. L., (2016), Students conceptions of reflection: opportunities for making connections with perpendicular bisector, J. Mathematics Behavior, 43, 35–52.
- diSessa A. A., (1988), Knowledge in pieces, in Forman G. and Pufall P. B. (ed.), Constructivism in the computer age, Lawrence Erlbaum Associates, Inc., pp. 49–70.
- Falba C. J. and Williams D. L., (1998), Exploring Mathematics with Interactive Computer Multimedia: An Investigation of Mirror Symmetry, Intervention School Clinic, 33(3), 187–191.
- Fisher C. B. and Fracasso M. P., (1987), The Goldmeier Effect in Adults and Children: Environmental, Retinal, and Phenomenal Influences on Judgements of Visual Symmetry. Perception, 16(1), 29–39.
- Flint E. B., (2011), Teaching point-group symmetry with three-dimensional models, J. Chem. Educ., 88(7), 907–909.
- Gallian J. A., (1990), Finite plane symmetry groups, J. Chem. Educ., 67(7), 549–550.
- Glasser L., (1967), Teaching symmetry: the use of decorations, J. Chem. Educ., 44(9), 502–511.
- Goldmeier E., (1937), Über Ähnlichkeit bei gesehenen Figuren, Psychologische Forschung, 21(1), 146–208.
- Grafton A. K., (2011), Using role-playing game dice to teach the concepts of symmetry, J. Chem. Educ., 88(9), 1281–1282.
- Hammer D., Elby A., Scherr R. and Redish E. F. (2005), Resources, framing, and transfer, in J. P. Mestre (ed.) Transfer of Learning from a Modern Multidisciplinary Perspective, Greenwich, CT: IAP, pp. 89–119.
- Herman M. and Lievin J., (1977), Group theory: from common objects to molecules, J. Chem. Educ., 54(10), 596–598.
- Holme T. and Murphy K., (2012), The ACS Exams Institute Undergraduate Chemistry Anchoring Concepts Content Map I: General Chemistry, J. Chem. Educ., 89(6), 721–723.
- Holme, T. A., Reed, J. J., Raker, J. R. and Murphy, K., (2018), The ACS Exams Institute Undergraduate Chemistry Anchoring Concepts Content Map IV: Physical Chemistry, J. Chem. Educ., 95(2), 238–241.
- Hoyles C. and Healy L., (1997), Unfolding meanings for reflective symmetry, Int. J. Comput. Math. Learn., 2, 27–59.
- Korkmaz A. and Harwood W. S., (2004), Web-supported chemistry education: design of an online tutorial for learning molecular symmetry, J. Sci. Educ. Technol., 13(2), 243–253.
- Küchemann D., (1980), Children's Difficulties with Single Reflections and Rotation, Math. School, 9(2), 12–13.
- Luxford C. J., Crowder M. W. and Bretz S. L., (2012), A symmetry POGIL activity for inorganic chemistry, J. Chem. Educ., 89(2), 211–214.
- Marek K. A., Raker J. R., Holme T. A. and Murphy K. L., (2018), The ACS exams institute undergradaute chemsitry anchoring concepts content map III: inorganic chemistry, J. Chem. Educ., 95(2), 233–237.
- McKay S. E. and Boone S. R., (2001), An early emphasis on symmetry and three-dimensional perspective in the chemistry curriculum, J. Chem. Educ., 78(11), 1487–1490.
- Meyer D. E. and Sargent A. L., (2007), An interactive computer program to help students learn molecular symmetry elements and operations, J. Chem. Educ., 84(9), 1551–1552.
- Meyer M. J., Day S. L. and Lee Y. B., (1992), Symmetry in building block design for learning disabled and nonlearning disabled boys, Perceptual Motor Skills, 74(3), 1031–1039.
- Murphy K., Holme T., Zenisky A., Caruthers H. and Knaus K., (2012), Building the ACS Exams Anchoring Concept Content for Undergraduate Chemistry, J. Chem. Educ., 89(6), 715–720.
- Newcombe S. and Stieff M., (2012), Six Myths About Spatial Thinking, Int. J. Sci. Educ., 34(6), 955–971.
- Nakawa N., (2020), Proposing and modifying guided play on shapes in mathematics teaching and learning for Zambian preschool children, S. Afr. J. Child. Educ., 10(1), a802.
- Ng O. L. and Sinclair N., (2015), Young children reasoning about symmetry in a dynamic geometry environment, Int. J. Math. Educ. Sci. Technol., 47, 421–434.
- Nobel H. and Smith J., (2015), Issues of validity and reliability in qualitative research, Evid. Based Nurs., 18(2), 34–35.
- Raker J., Holme T. and Murphy K., (2013), The ACS Exams Institute Undergraduate Chemistry Anchoring Concepts Content Map II: Organic Chemistry, J. Chem. Educ., 90(11), 1443–1445.
- Raker J. R., Reisner B. A., Smith S. R., Stewart, J. L. Crane, J. L., Pesterfield L. and Sobel S. G., (2015a), Foundation Coursework in Undergraduate Inorganic Chemistry: Results from a National Survey of Inorganic Chemistry Faculty, J. Chem. Educ., 92(6), 973–979.
- Raker J. R., Reisner B. A., Smith S. R., Stewart J. L., Crane J. L., Pesterfield L. and Sobel S. G., (2015b), In-Depth Coursework in Undergraduate Inorganic Chemistry: Results from a National Survey of Inorganic Chemistry Faculty, J. Chem. Educ., 92(6), 980–985.
- Ramful A., Ho S. Y. and Lowrie T., (2015), Visual and analytical strategies in spatial visualization: perspectives from bilateral symmetry and reflection, Math. Educ. Res. J., 27, 433–470.
- Rattanapirun N. and Laosinchai P., (2021), An Exploration-Based Activity to Facilitate Students' Construction of Molecular Symmetry Concepts, J. Chem. Educ., 98(7), 2333–2340.
- Rodriguez J. G. and Towns M. H., (2019), Alternative Use for the Refined Consensus Model of Pedagogical Content Knowledge: Suggestions for Contextualizing Chemistry Education Research, J. Chem. Educ., 96(9), 1797–1803.
- Ruiz G. N. and Johnstone T. C., (2020), Computer-Aided Identification of Symmetry Relating Groups of Molecules, J. Chem. Educ., 97(6), 1604–1612.
- Savec V. F., Vrtacnik M. and Gilbert J. K., (2005), Evaluating the Educational Value of Molecular Structure Representations, in Gilbert J. K. (ed.), Visualizations in Science Education, Dordrecht, The Netherlands: Springer, pp. 269–298.
- Schuler J., (2001), Symmetry and Young Children, Montessori Life, 12(2), 42–48.
- Seidel J., (1998), Symmetry in season, Teach. Child. Math., 4(5), 244–230.
- Sein Jr., L. T., (2010), Dynamic paper constructions for easier visualization of molecular symmetry, J. Chem. Educ., 87(8), 827–828.
- Southam D. C. and Lewis J. E., (2013), Supporting alternative strategies for learning chemical applications of group theory, J. Chem. Educ., 90(11), 1425–1432.
- Stieff M., Ryu M., Dixon B. and Hegarty M., (2012), The Role of Spatial Ability and Strategy Preference for Spatial Problem Solving in Organic Chemistry, J. Chem. Educ., 89(7), 854–859.
- Tashiro J. and Talenquer V., (2021), Exploring Inequities in a Traditional and Reformed General Chemistry Course, J. Chem. Educ., 98(12), 3680–3692.
- Tuvi-Arad I. and Blonder R., (2010), Continuous symmetry and chemistry teachers: learning advanced chemistry content through novel visualization tools, Chem. Educ. Res. Pract., 11(1), 48–58.
- Tuvi-Arad I. and Gorsky P., (2007), New visualization tools for learning molecular symmetry: a preliminary evaluation, Chem. Educ. Res. Pract., 8(1), 61–72.
- Valenzeno L., Alibali M. W. and Klatzky R., (2003), Teachers’ gestures facilitate students’ learning: a lesson in symmetry, Contemp. Educ. Psychol., 28(2), 187–204.
- White K. N., Vincent-Layton K. and Villarreal B., (2021), Equitable and Inclusive Practices Designed to Reduce Equity Gaps in Undergraduate Chemistry Courses, J. Chem. Educ., 98(2), 330–339.
| This journal is © The Royal Society of Chemistry 2023 |