Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Characterizing students’ peer–peer questions: frequency, nature, responses and learning
Grace
Tiffany
a,
Krystal
Grieger
 b,
Kassidy
Johnson
c and
James
Nyachwaya
b,
Kassidy
Johnson
c and
James
Nyachwaya
 *d
*d
aDepartment of Chemistry and Biochemistry, North Dakota State University, USA. E-mail: grace.tiffany@ndsu.edu
bDepartment of Chemistry and Biochemistry, North Dakota State University, USA. E-mail: Krystal.grieger@ndsu.edud
cSchool of Education, North Dakota State University, USA. E-mail: kassidy.l.johnson@ndsu.edu
dDepartments of Chemistry and Biochemistry and School of Education, North Dakota State University, USA. E-mail: James.nyachwaya@ndsu.edu
First published on 22nd February 2023
Abstract
In group activities, students work collaboratively to accomplish specific objectives. Students have to engage and interact with each other in order to complete collaborative assignments. One way that students stay engaged is through asking questions. In the research reported here, we looked at peer-to-peer questions in the context of a collaborative activity. Specifically, we examined the frequency of questions, types of questions, types of responses elicited by student questions, whether peer question-and-answer interactions led to verbalized learning, and the disciplinary content of the questions asked by students in their groups. Our results show that there was a wide range in the frequency of questions asked across groups. The types of questions asked were broadly classified as confirmation seeking, clarification seeking, information seeking, and questions seeking understanding. Types of responses elicited included explanations (conceptual), informational, unsure, and no response. Most of the question-and-answer exchanges did not lead to verbalized learning. Some types of question-and-answer combinations were more likely to lead to verbalized learning than others. The most commonly asked disciplinary content questions sought facts and descriptions of procedures. Questions seeking conceptual understanding, which are more likely to lead to learning, were least common. Implications for instruction and research are discussed.
Introduction
Questioning is central to meaningful learning. Questions in an educational setting include questions from students to teachers, from teachers to students, questions from a textbook to students, or questions from students to other students. Questions can serve a number of functions, including confirmation of expectations, solving problems, and filling gaps in knowledge and understanding (Biddulph and Osborne, 1982). Students’ questions can also be a source of feedback for teachers, because they can reveal students conceptual understanding, as well as the nature and quality of their ideas (Woodward, 1992; Watts et al., 1997). Student questions show that they are thinking about the ideas at hand and are making an effort to link the ideas to what they already know (Chin and Osborne, 2008).Questioning is also central to inquiry in science and is one of the Science and Engineering Practices (SEPs) identified in the Next Generation Science Standards (NGSS) (Lead States, 2013). Benefits of questioning include increasing student motivation to learn, promoting and supporting productive discussions in the classroom, supporting knowledge construction, and improving reading comprehension (Chin and Osborne, 2008; Yu, 2009). Despite the known benefits, research has shown that students seldom ask questions, and when they do, the questions are low-level, require minimal inferences, focus on surface features, and require little-to-no cognitive processing (Chin and Brown, 2002).
In recent years, there has been widespread recognition of the importance of structuring learning environments and activities that actively engage students in their own learning (National Research Council, NRC, 2012; Freeman et al., 2014; Theobald et al., 2020). In such environments, students work collaboratively in groups to accomplish assigned tasks. As part of their intellectual involvement in the tasks (Kuh, 2009), students suggest and contribute ideas towards solving assigned problems. An important aspect of peer-peer interaction in groups is the questions students ask each other. Peer-to-peer questioning is a behavioral form of student engagement (Fredricks et al., 2004). This study sought to characterize student questions across different student groups, uncover how often students asked each other questions, the nature of questions asked, what types of responses were given, and determine whether there was evidence that through asking questions in groups, learning occurred.
Much of the existing research on student questions was conducted in K-12 settings and involved looking at the nature of questions students asked during inquiry laboratories, and questions asked during argumentation. At the college level, in addition to the fact that there is limited research around student questions, the main approach used in the studies involved explicitly prompting students to ask questions or giving students some structure for asking questions, such as reading text and asking questions, asking questions after lecture, and looking at data and asking questions (Marbach-Ad and Sokolove, 2000; Harper et al., 2003; Kastens et al., 2020). The research we report here is different in the sense that we explored unprompted student peer-to-peer questions spontaneously generated during a group activity. Specifically, we sought to answer the following research question:
What is the nature and outcomes of students’ peer-to-peer questions during a collaborative activity?
This study builds on and contributes to existing research on the processes and outcomes of student collaborative work in active learning classrooms, recognizing that such environments enable students to interact with each other as they work towards a shared goal. Examples of these research studies include the ways of student reasoning that constitute chemistry classroom practice (Cole et al., 2012), development of sociochemical norms (Becker et al., 2013), nature of chemical reasoning manifested during argumentation (Moon et al., 2017), influence of group dialogue on student understanding of science concepts (Warfa et al., 2018), social metacognition in small-group problem solving (Halmo et al., 2022), patterns of student engagement during collaborative activities (Reid et al., 2022), and student perceptions of behavioral, cognitive and emotional engagement (Naibert et al., 2022). Interestingly, asking questions, which is the subject of our study, was identified as a form of behavioral engagement in the Naibert et al. (2022) study. Our study adds to existing knowledge by analyzing students’ peer-to-peer questions during a collaborative group activity.
Literature review
One of the core roles of science education is to help students develop the ability to ask questions (Bybee, 2000; Hakkarainen, 2003; Chin and Osborne, 2008). However, the number of questions posed by students during instruction is very small (Nystrand et al., 2003; Kaya and Kablan, 2013). Teachers’ actions are central to encouraging student questions (van Zee et al., 2001). Classrooms where teachers elicit student questions and encourage discussion are more likely to encourage question generation among students (Penuel et al., 2004). Since questions during discourse can benefit students, teachers should provide opportunities for and encourage discourse in their classrooms.As an important cognitive strategy, generating questions helps focus students’ attention on the content, in addition to helping check for understanding of content (Rosenshine et al., 1996). Questions initiate communication (Costa et al., 2000; Dogan and Yucel-Toy, 2021) and trigger critical thinking processes. Questions play an important role during discourse, either in the way of challenging someone's views or sustaining a conversation (Chin and Osborne, 2010).
Students’ questions can be lower level, requiring mere recall of memorized information and simple observation (Chin and Brown, 2000b; Lai and Law, 2013; Zhang et al., 2007). Student questions can also be higher order, which require finding more information, relating students’ current knowledge to new information, and integrating information from multiple sources (Chin and Brown, 2000b). When compared to lower level questions, higher order questions have the potential to encourage critical thinking and allow students to evaluate and synthesize information (Chin and Osborne, 2008), ultimately leading to conceptual understanding and construction of new knowledge (Hakkarainen, 2003; Hofstein et al., 2005; Zhang et al., 2007). Despite the potential benefits, asking higher order questions by students is a learned skill (Chin and Brown, 2002). Thus, students need to be explicitly trained to ask questions. Graeser and Person (1994), in their research on questioning, developed a taxonomy of questions generated by tutors and students during tutoring sessions. They found that deep reasoning questions elicited explanatory reasoning and were associated with higher learning outcomes in the tutoring environments that they investigated.
The size of the groups and the achievement gap between group members can affect questioning behavior. In a study involving middle school students, Myoung-Sook et al. (2004) studied the types and frequencies of student–student questions. They found that student–student questions fell into two broad categories: information-type questions and thought-type questions. Most of the information type questions were procedural, while the thought-type questions were comprehension questions. Information-type questions were asked more frequently than thought-type questions. The researchers found that in smaller groups, there were more question–answer interactions. They also found that when the achievement gap between members was large, the frequency of the student-to-student questions was low.
The nature and types of questions that students ask are influenced by prior knowledge. In a study where students were prompted to write or ask questions after reading a text, Scardamalia and Bereiter (1992) found that if the text was closely related to their prior knowledge, students asked questions seeking explanations, inferences, and applications and integration of information. When the text topic was unfamiliar to students, they asked basic questions that were meant to help familiarize themselves with the topic. The types of questions asked after reading a text is also dependent on the students’ comprehension level with high reading comprehension levels associated with higher-level questions (Taboada and Guthrie, 2006).
Despite the importance of prior knowledge in one's ability to ask good questions, it is possible for students to ask good questions in some contexts. In a study involving college students, Kastens et al. (2020) presented students from different majors and multiple institutions with data on sea level and climate on paper and interactively on a screen, and asked them to come up with questions based on the data, as well as questions that they would like to ask a scientist. Their results showed wide individual differences in the quantity and quality of questions asked. They also found that student questions differed by experimental condition (paper or interactively). Further, they found that over 70% of their participants generated at least one question at the highest level of Bloom's taxonomy. They concluded that it is possible for students to ask good questions about data even before the data is explained to them.
Peer-to-peer questioning is a feature of effective group dynamics because the questions asked support knowledge construction. Indeed, students ask each other questions in dialogic conversations in a classroom to either clarify ideas or solicit information (Kaya, 2015). Students ask questions because there may be gaps between their prior knowledge and the information they are given. For example, they may not understand what a particular statement means. Questions asked between members of a group can help coordinate their interactions, focus the group on the task at hand, activate prior knowledge, seek explanations, clarify doubts, seek justification for one's reasoning, and even stimulate a different kind of thinking (Chin and Osborne, 2008).
In traditional science classrooms, where discourse is usually monologic, it is teachers who usually ask questions, with minimal student input (Nystrand et al., 2003). In what Mehan (1979) called the initiation–response–evaluation (I–R–E) structure, a teacher asks a question, a student responds, followed by the teacher's evaluation of the student's response. In the rare occasions then, when students ask questions, those questions are few at best. The I–R–E pattern of classroom discourse does not allow students to ask their own questions or express their own ideas (Erdogan, 2017).
Given the role that questions can play in knowledge construction, attention should be given to promoting students’ ability to ask higher order questions. The quality of questions affects the nature of knowledge generated and therefore the level of understanding (Harper et al., 2003). However, asking higher order questions is a learned skill – students don’t just acquire it. In fact, as Chin and Brown (2002) note, students do not spontaneously ask such questions. It is therefore important that students are given opportunities where they can ask questions of each other. More importantly, they should receive feedback on the nature of their questions, and in the process be ‘explicitly trained’ how to ask questions (Chin and Osborne, 2008), and encouraged to ask ‘how and why’ questions. Teachers can encourage such questions by engaging students in problem solving activities (Chin and Kayalvizhi, 2002), as well as in student-led investigations (Hofstein et al., 2005). In the study reported here, students worked in groups to complete an activity on precipitation. Such an activity provided opportunities for students to ask each other questions within their groups.
The kind or types of questions asked elicit different types of responses. Chin and Brown (2002) found that basic information questions were either ignored or led to little meaningful discussion. However, good questions elicit interesting and productive answers. One way of helping students learn how to ask good questions is to have them pose questions regularly to enable them to develop questioning as a habit of mind (Chin, 2004).
The types of tasks that students engage in influence the kinds of questions that they ask. For example, a task that asks students to follow laid down procedures will most likely elicit factual questions meant to ensure that they are following expectations, as opposed to eliciting curiosity about scientific phenomena (Chin, 2002). On the contrary, an open-ended activity that requires creative thinking from students is likely to elicit more wonderment type questions.
Different types of questions can have varying outcomes. Questions seeking explanations are more likely to contribute to knowledge construction than fact-seeking questions because they can lead to a deeper level of understanding (Hakkarainen, 2003; Zhang et al., 2007). Factual questions seek descriptions of phenomena and definitions of terms, while explanation questions seek for mechanisms and relationships (Zhang et al., 2007). Research has indeed shown that when student groups ask questions targeting facts or information, the knowledge they construct consists of mainly simple facts or information. Groups asking explanation seeking questions are more likely to construct explanations and draw relationships and interconnections (Van Aalst, 2009).
Theoretical framework
This study is grounded in the theories of social constructivism and knowledge building pedagogy. Social constructivism draws on the notion that knowledge construction is a socially shared activity, and that knowledge construction and learning occur in a social context from which they cannot be separated (Vygotsky, 1978). Under social constructivism, knowledge and understandings of the world are developed jointly by individuals, and meanings are developed in coordination with others (Kundi and Nawaz, 2010). Social constructivists believe that meaningful learning occurs when individuals are engaged in social activities such as interaction and collaboration. Learning is therefore a social process, and meaningful learning occurs when individuals are engaged in a social activity that happens within a community (Adams, 2006). Development of ideas in such a context is a collaborative process where students work together and evaluate each other's ideas. Indeed, constructivist classrooms entail group work and dialogue as well as shared norms (Smith et al., 2000). It can be argued that an environment where students collaborate with one another, such as during problem solving, is necessary for them to build knowledge (Powell and Kalina, 2009). In the current study, students worked collaboratively to complete the assigned activity. As they worked together, students proposed and shared ideas, and asked and answered each other's questions.It is worth noting that in addition to knowledge construction, collaborative learning environments can be designed to support the development of important skills among students. To ensure that these skills develop among students, it is imperative that they are explicitly taught and practiced through the careful design of learning activities. In science education, one approach which draws on social constructivism and explicitly aims to promote the development of these skills is Process Oriented Guided Inquiry (POGIL) (Farrell et al., 1999). In a POGIL environment, learners are actively engaged in mastering content as they construct their own understanding of concepts, while also developing important cognitive and affective skills (process skills) in self-managed teams. These skills include teamwork, communication, critical thinking, problem solving, metacognition, information processing and assessment (Spencer and Moog, 2008). Since its inception in 2003, the POGIL project has developed and disseminated guided inquiry materials that focus on mastering course content and developing these process skills (Spencer and Moog, 2008).
As a pedagogical approach, knowledge building emphasizes that students have a collective responsibility in building and advancing their knowledge especially in a collaborative learning setting (Bereiter, 2002; Scardamalia, 2002), where knowledge building is a collective achievement. Asking questions and receiving answers play an important role in knowledge building. Group learning provides a context for students to ask questions. In such contexts, a student's question can encourage others in a group to think about both the content and solutions to a problem, as well as lead to more questions from other students, thereby helping in knowledge construction (Chin and Brown, 2002). Asking questions leads to construction of knowledge by stimulating the generation of explanations and proposing solutions to problems (Chin, 2004). In some cases, such questions can lead to hypothesizing, predicting, thought experimenting, and explaining, which in turn can help students fill in missing pieces of information or resolve conflicts in their understanding (Chin and Brown, 2000). During group collaborative activities, one student's questions can influence group members’ thinking.
Methods
Research setting
Before this research study was conducted, Institutional Review Board (IRB) approval was sought and obtained. Consent was also obtained from all participants. The data reported in this study came from a non-majors General Chemistry (I) course in the spring semester at a research university in the midwestern United States. The class met for 50 minutes three times a week for 16 weeks. To help facilitate in-class activities, the instructor worked with a Learning Assistant (LA). Instruction in the course consisted of lecture, clicker questions followed by small and whole class discussions, as well as in-class collaborative activities. The required text for the course was Tro's (2019) General Chemistry textbook.In this course, students usually complete 3–5 graded collaborative problem solving activities over the course of the semester. The class had just completed the chapter “Reactions in Aqueous Medium,” which covered the concept of precipitation. The topic covered writing complete molecular equations of reactions, writing complete ionic and net ionic equations, and linking the process to the identity of the precipitate. To supplement instruction, YouTube videos were used to specifically illustrate the process of precipitation.
At our institution, laboratories are treated as separate courses. Effort is made to align laboratory experiments with what students are covering during lecture throughout the semester. During the week prior to the activity, students completed a laboratory activity involving reactions, in which they wrote out equations to help determine whether a reaction occurred.
Data collection
In this basic qualitative study (Merriam and Tisdell, 2015), we sought to understand peer–peer questioning among students during an in-class collaborative group activity. At the end of the unit on “Reactions in Aqueous Medium,” students were assigned an in-class collaborative activity that addressed precipitation. Prior to the activity, we created a video where we mixed lead(II) nitrate and potassium iodide to illustrate the precipitation of lead(II) iodide. The two beakers containing lead(II) nitrate and potassium iodide were labeled with the respective ions in each solution. Students were asked to work in self-selected groups of 2–4 to answer the questions shown in Fig. 1. They were also asked to record their conversations and share the audio files with the instructor. It is worth noting that in the laboratory course, students go through a similar exercise in which they must determine whether a reaction will occur when two solutions are mixed. Part of that process involves writing complete and net ionic equations, which requires understanding and applying the solubility rules. Students had 20 minutes to complete the activity. Analysis of the recordings revealed that groups took between seven and eighteen minutes to complete the activity and that some groups had five students, contrary to the instructions to limit the group to 4 students. There were 167 students who consented to take part in the study, comprising 49 groups. Transcripts from eight groups could not be transcribed because the audio files could not be opened, leaving us with data from 41 groups.In this study, oral and written data were collected from one activity. As part of data processing, all audio files of student conversations were transcribed verbatim, and the transcribed data was subsequently coded and analyzed. Transcription was followed by fact checking (Tracy, 2013), which involved listening to the recordings while simultaneously reading transcripts to ensure accuracy and make corrections as necessary. During transcribing, we listened to each student speaking in turn, making sure that we separated each speaker's vocalizations accurately. Each speaker was assigned a number based on the order in which they spoke (speaker 1 as the first to speak in the conversation, speaker 2 as the next to speak, etc.). Generic references were used to identify students as they took turns speaking (student 1, 2, 3). The transcripts were also anonymized by replacing group names with pseudonyms. The transcripts were then organized for coding and analysis by ensuring that each group's transcript was separate.
Coding and analysis
Data coding and analysis involved quantifying qualitative verbal data, for which the research team co-developed codes through multiple iterations and extensive discussions. The codes were informed by emerging trends from data as well as prior research. As part of the coding process, we tallied up occurrences of each coded category and looked for emerging patterns. Four researchers coded and analyzed the transcript data. The analysis focused on the frequency and types of student questions, the nature of interactions based on the number of peer-to-peer questions, the types of responses the questions elicited, the disciplinary content of the student questions, and whether the question-and-answer interactions led to verbalized learning. We defined verbalized learning as instances in which students gained knowledge that they did not have through a question-and-answer exchange.First, we identified the inquiries in the transcripts that could be classified as questions. For our analysis, we defined a question as an information seeking inquiry (Van der Meij, 1994). We identified and counted instances when questions were asked within each group's transcript with 100% inter-rater agreement. To further analyze the data, we adopted Carlsen's (1991) suggestion that among other features, the context, content, responses, and reactions of speakers can provide a basis for analyzing student questions, with the latter two being especially relevant to our study.
Next, all four researchers read through the transcripts from five groups and analyzed the student questions for the type of question, the level of interactions based on the number of peer-to-peer questions asked, the type of response, whether verbalized learning occurred, and the disciplinary content of the questions. We determined the type of questions asked by examining the objective of the question or what type of information the question sought. For the level of interactions based on the number of questions asked, we looked at the average number of distinct individual utterances during the activity, the average number of words per minute, and the number of peer-to-peer questions per minute. For the type of response given or received, we characterized the content of student responses to peer questions, for example, an explanation given after a question was asked. We also analyzed and quantified the types of questions asked and the responses they elicited, for example, a question seeking clarification of a procedure and receiving an informational response. We analyzed question-and-answer combinations to see if verbalized learning occurred, more specifically that at the end of the exchange, one of the group members knew something they did not know before. Finally, we analyzed each question to determine the disciplinary content, specifically for questions about content underlying the activity. The average inter-rater agreement for the five transcripts was 96%. Differences were resolved through discussion. The remaining transcripts were then equally divided, with two researchers coding one half of the transcripts and two researchers coding the other half. The coded transcripts were then exchanged and reviewed to ensure consistency in coding. All four researchers then met to discuss and resolve any differences. The excerpt below illustrates our coding and analyses.
Student 2: combine the two that would be soluble right? (1)
Student 1: Yes so we would do the Pb and the…
Student 2: Is the NO3Pb um soluble? (2)
Student 1: I actually don’t know.
Student 3: Uh… Where's the solubility rules? (3)
Student 1: Umm…let me go back.
Student 2: Yeah
Student 1: Solubility… rules! Ok, so Pb
Student 3: So look for NO3, and then see what it doesn’t…
Student 1: NO3has no exceptions, so it's soluble… right? (4)
Student 2: Okay so would we do PbNO3plus… K plus I? (5)
Student 1: Okay so potassium… Ok. Iodine is insoluble… [said simultaneously with 2]
Student 2: So that's insoluble you said? (6)
Student 1: Um…Iodine is only insoluble with Pb two plus
Using this excerpt, we noted the following:
(a) There were six (6) questions asked (bolded, italicized, and labelled 1–6).
(b) Based on the information being sought, questions 1, 4, 5 and 6 were coded as confirmation questions, while questions 2 and 3 were coded as information seeking questions.
(c) When student 2 asked the question about NO3Pb (referring to lead(II) nitrate), an information seeking question, the student got an informational response (which ends with question 4), that the salt would be soluble.
(d) When question 2 was asked, student 1 responded with “I actually don’t know.” At the end of the excerpt, the student had gained new knowledge and exhibited verbalized learning.
(e) Question 2 asked whether NO3Pb (referring to lead(II) nitrate) is soluble, question 6 asked about and sought to confirm that the salt in question is insoluble. The disciplinary content of these questions was solubility.
In the next section, the themes identified here will be defined and explained, then data will be presented and described.
Results
This basic qualitative study (Merriam and Tisdell, 2015) sought to characterize student peer-to-peer questions. We first sought to establish question frequency and extent of interactions, question type (confirmation seeking, clarification seeking, information seeking, or questions seeking understanding) and response type (e.g., informational, conceptual/explanation, ‘unsure’ or ‘no response’). Then we looked at the specific disciplinary content of each question. Finally, we evaluated question–answer exchanges for verbalized learning.In the following sections, we present results of the study as organized by the goals of the study.
Question frequency
We counted the total number of questions asked as recorded in the transcripts; a total of 652 questions were asked. The average number of questions asked per group (n = 41; 2–5 students per group) was 16.3 questions with a standard deviation of 9.3 questions. Overall, the number of questions asked within each group ranged from 1 to 42.Extent of interactions
We examined the number of questions asked to gauge the level of interaction in groups. Looking at the range of question frequency (1–42), we picked the groups with 42 questions, 20 questions and 7 questions respectively to help get a sense of interactions within the groups. In the group with the highest number of questions asked, among the three group members, there were on average 70 distinct individual utterances during the activity. These utterances were questions, responses to the questions, and discussions. On average, in this group, students asked four questions per minute, and exchanged 137 words per minute over the twelve 12 minutes it took to complete the activity. In a group where 20 questions were asked, there were on average, 45 distinct individual utterances, an average of 1.5 questions asked per minute, and exchanged 102 words per minute over the 13 minutes the group took to complete the activity. Additionally, in a group where 7 questions were asked, there were 21 distinct individual utterances, asked about one question per minute, and exchanged an average of 58 words per minute over the 8 minutes the group took to complete the activity.Question type
The questions asked by students fell into four categories: confirmation seeking; clarification seeking; information seeking, and questions seeking understanding. The figure below (Fig. 2) is a visual summary of the observed question types and their relative proportions.In the following sections, each of these categories is described, followed by sample student transcripts to illustrate each category.
Student 2: Based on the ions in two solutions what are the formulas of the compounds (reading question)
Student 3: So, you’re going to get PbI2 and NO3K or KNO3.
Student 2: Oh, does it want after? Did it just want a molecular formula? Oh, never mind. Ok. I see what you’re saying. PbI2, that one was the precipitate right?
Student 1: And then, yeah, K, it's going to be K first, so KNO3.
Student 2: That one's aqueous one, right?
Student 1: Predict the products and write a molecular equation. Each solution.
Student 2: Sorry. Predict the products
In the conversation above, the student asked for confirmation twice but did not receive a response. As we discuss later, this was a trend we noted in student interactions. In total, there were 292 confirmation seeking questions, the most common type of questions asked.
Student 2: Do we need to write the sol solid?
Student 1: uh. Do we need to measure the conductivity of one of the solutions and then slowly add the second solution to it? How would the conductivity of the mixture.
Student 2: mmhmm.
Student 1: Suppose
Student 2: What about the conductivity?
Student 1: Conductivity mmm Conductor not this page (student appears to be checking notes).
Student 1: Maybe just put conduct
Student 2: Or are we supposed to draw the graph for
Student 1: Yeah
Student 2: What are we supposed to do though?
Student 1: I think what we should draw, draw the numbers that were
Student 2: Yeah, like what are we going to draw?
Student 1: Hmm. Should I ask them.
Student 2: Yeah.
Student 1: Him or
Student 2: Do you know what I’m supposed to draw on the number seven?
In the excerpt above, the two students asked each other what the prompt was asking, and student 2 asked what they were supposed to draw in the italicized questions. In total, 73 of the questions asked within the groups fit into this category.
Student 1: Which is a molecular equation? I don’t…
Student 3: Ionic equation, the… I think an ionic equation would be the balanced form, right?
Student 2: Do you want to write it? Is that what you were saying?
Student 3: Um… I don’t know.
Student 1: It includes like… the precipitate as the product, I think. Something like that.
Student 2: Oh
Student 1: But it just kind of leaves out…
Student 3: So what was the precipitate in this reaction, do we know that?
Student 2: What was that one chart that showed… or expressed as…
Student 3: oh, so it’d be Pb plus NO3 plus K plus I.
Student 2: Oh, the ionic you’d just split them up kinda more?
Student 3: Yeah, There expresses disassociated ions… We’re looking at the same page.
Student 2: Okay, so it’d be… and then do you write the charges with it then?
Student 3: Yes.
In the excerpt above, the student wants to know what the precipitate was in the equation. The group then proceeds to look for a chart on solubility to ultimately determine the precipitate. In total, information seeking questions were asked 241 times and were the second most common type of question asked.
Student 1: Yeah so remember, think back to electrolytes. So they conduct electricity because of ions, right?
Student 2: Yeah.
Student 1 So, you have ions, here you don’t.
Student 2 So how does that affect conductivity?
Student 1: So it’ll be less… is that the word?
Student 2: Yeah.
Student 1: So it decreases. So we’d expect it to decrease cause the final products don’t have ions. Because the reaction causes there to be less ions
Student 2: In the… what do you call it? The products. The products have less than what you started with, so it decreases.
Student 2: In the… or is that enough?
Student 1: Yeah.
In the excerpt above, the student wants to understand how having ions or not, as proposed by student 1, affects conductivity. In total, questions seeking understanding were asked 46 times, and were the least common type of question asked. In addition, although we had anticipated that the last prompt in the task, which asked for an explanation, would be where most questions seeking understanding would be asked, this was not the case. For instance, there were questions asking why the predicted products would be correct; why certain ions were considered to be spectator ions; or why conductivity would change.
Table 1 below shows per group the average frequency and standard deviation for each type of question.
| Confirmation seeking | Information seeking | Seeking understanding | Clarification seeking | Total questions | |
|---|---|---|---|---|---|
| Average | 7.30 | 6.02 | 1.15 | 1.83 | 16.3 |
| St. dev. | 5.4 | 3.9 | 1.5 | 1.7 | 9.3 |
Patterns in student answers to their peer questions
a. Types of responses elicited
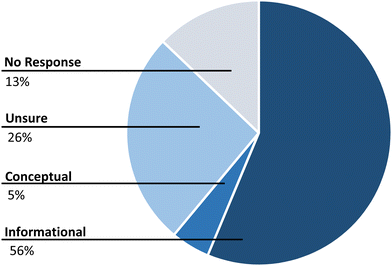
We also characterized the responses given to peer questions. Ideally, a specific type of question would elicit a response appropriate to the question. For example, a question that requires an explanation would get an explanation, not just information or even no response at all. Generally, in this study, peer responses were categorized as being informational, explanation/conceptual, unsure, or no response. As can be seen in the pie chart (Fig. 3), the most common type of response was informational (56%). At 5.0%, conceptual responses, or responses that involved an explanation had the lowest number of occurrences. In about 13% of the time, a question was posed but no response was given by peers. In 26% of the questions asked, the response was ‘I am not sure’.Each of the categories of student responses is described below, and a sample excerpt is provided as an illustration.
Student 1: Do we know anything about? [flipping pages] What do we know about electrolytes?
Student 2: Weak electrolytes, strong. Materials are completely dissolved as ions. Dissolved in mostly. umm. Would this [inaudible]
Student 1: Maybe, yeah.
Student 2: So are we talking about these two separate things?
Student 1: yes.
Student 2: Ok. So, it looks like with K, its soluble
Student 1: It will always be soluble and
Student 2: And then.
Student 1: NO3 will always be.
Student 2: Oh, ok. So if they are both going to dissolve, what does that mean?
Student 1: So that means they, um, completely dissolve. They will, they will be good conductors of electricity.
Student 2: Wouldn’t it go down? (silence) yeah.
Student 3: So, It’d go like that, and then we have to explain it.
Student 2: So it goes down because PbI2 is insoluble.
Student 1: And the strong…
Student 2: Strong acid. Which doesn’t… So the solution doesn’t dissolve completely, making… resulting in not all the ions being able to conduct electricity.
Student 1: Yeah.
Student 3: What?
Student 2: Okay. The conductivity goes down because PbI2is insoluble, which… um… causes the solution to not dissolve completely, not allowing for all the ions to be available to conduct electricity.
Student 1: Yeah, I think that makes sense.
Student 2: Cause the ions, the solution not to dissolve completely leaving ions not available
Student 3: to conduct electricity.\
Student 1: Complete ionic equation… is the net ionic equation, is that…
Student 2: hmm
Student 1: with all the, um, like the numbers before?
Student 2: Oh, I’m not sure.
Student 1: Like the moles of it. I don’t know the difference between net ionic and complete ionic…
Student 2: How do I do this… Let me see. Kay…. Okay, so, does that look right for number 3? Because I don’t know the difference between a net ionic equation and a complete ionic equation.
Student 1. Maybe? But I am not sure, so…
Student 2: Hold on, it might be in Chapter 4 notes. I remember like there was a table that showed what… um… what…
Student 1: Precipitation.
Student 2: I can’t find it now. There was a table that…
Student 3: I think this is what we’re looking for. This is the same stuff.
Student 2: Oh.
Student 1: Does it say which is the?
Student 2: Go down to the next side. Oh, that's something else.
Student 3: That's something else. Different example.
Student 2: Well… Man, I wish I could find that table.
Student 3: This is aqueous though.
Student 1: I’m pretty sure it's, the uh, the yellow stuff, the Pb…
Student 3: Pb is lead isn’t it?
Student 2: He really did not give us enough time to work on this.
Student 3: Yeah
b. Question type and type of response elicited
We also looked at the different types of questions and the responses they elicited as shown in Fig. 4 below. For confirmation seeking, clarification seeking and information seeking questions, the most common response elicited was informational, followed by ‘unsure’ and ‘no’ response, with the least common response elicited being conceptual. Questions seeking understanding received more conceptual responses than the other categories of questions, higher proportions of ‘unsure’ and ‘no’ responses, and a smaller proportion of informational responses.Student 1: Are we supposed to name what they are?
Student 2: Uh. [laughs.] I don’t know. What is Pb?
Student 3: That's lead. NO3 is nitrate
Student 2: Lead nitrate and potassium iodide.
Student 1: Yep.
Indeed, this is an interesting group exchange because Student 1 asked an information question about the expectations of the assignment. The ensuing question-and-answer exchange helped student 1 learn the name of the compound.
In the excerpt below, one of the students did not know if a salt was soluble:
Student 2: combine the two that would be soluble right?
Student 1: Yes so we would do the Pb and the…
Student 2: Is the NO3Pb um soluble?
Student 1: I actually don’t know.
Student 3: Uh… Where's the solubility rules?
Student 1: Umm… let me go back.
Student 2: Yeah
Student 1: Solubility… rules! Ok, so Pb
Student 3: So look for NO3, and then see what it doesn’t…
Student 1: NO3 has no exceptions, so it's soluble… right?
Student 2: Okay so would we do PbNO3 plus… K plus I?
Student 1: Okay so potassium… Ok. Iodine is insoluble… [said simultaneously with 2]
Student 2: So that's insoluble you said?
Student 1: Um… Iodine is only insoluble with Pb two plus
In this excerpt, every other utterance is a question. Through a combination of confirmation and information seeking questions, student 2 knows that lead(II) nitrate is a soluble salt. It is interesting that student 2 refers to Pb(NO3)2 as NO3Pb. Overall, most of the questions asked during the collaborative activity did not lead to verbalized learning. Our analysis showed that only about 15% of the questions asked led to verbalized learning.
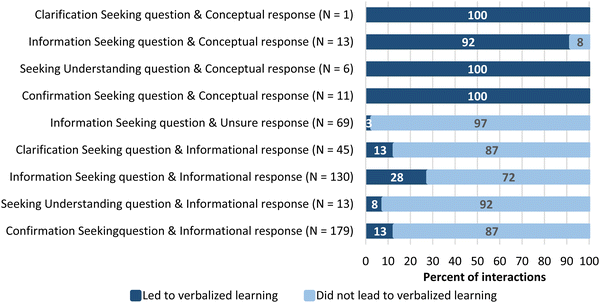
There were three question-and-answer combinations that led to learning every single time they occurred. The combination of a question seeking understanding with a conceptual answer only occurred 6 times and each occurrence led to verbalized learning. A confirmation seeking question and conceptual response occurred 11 times and seeking clarification and conceptual response occurred once, and again, every instance led to verbalized learning. Conceptual responses overall were much less frequent within the transcripts, but frequently led to verbalized learning when they were given. The figure below (Fig. 5) shows the different question-and-answer/response combinations and whether they led to verbalized learning.
Note that Fig. 6 shows only the top five most asked questions, not every question asked. It is interesting how prevalent the questions about molecular, ionic, and net ionic equations were, likely because these were among the first questions students had to answer. It is also worth noting that over half of the top five most asked questions were lower-level questions.
Discussion and conclusions
In this study, we sought to understand the nature and outcomes of students’ peer-to-peer questions during a collaborative activity. Peer-to-peer questions during groupwork is a form of student interaction and engagement (Fredricks et al., 2004). Our study adds to recent research in chemistry education on student engagement during collaborative activities which identified behavioral, cognitive, and emotional dimensions of engagement (Naibert et al., 2022) and the association between the dimensions of student engagement and the cognitive level of assigned tasks (Reid et al., 2022).Questions can foster discussion and debate, which in turn may enhance discourse in student groups (Chin and Osborne, 2008). Indeed, this study's group transcripts showed that groups that asked more questions had more interactions through questions, answers, and discussion, supporting the argument that questions can indeed enhance discussions during group work. For example, in the group in which 42 questions were asked, students exchanged on average 137 words per minute. However, in a group where only eight questions were asked, on average, students exchanged 58 words per minute. The results of this study showed that as students worked together to complete the assigned tasks, they asked each other questions and that the number and frequency of questions mirrored the level of interaction of students across the groups. Research has shown that student interaction during collaborative learning can influence the outcome of an activity. Students with higher levels of interaction during collaborative learning tend to show higher quality cognitive interactions (Barron, 2003; Sinha et al., 2015).
Asking and answering questions as a generative activity allowed students in our study to find and put together missing pieces of information or knowledge, which in turn led to verbalized learning in collaborative groups. Indeed, student questions directed learning and drove knowledge construction (Chin and Brown, 2000a). For example, when a student asked about the solubility of lead(II) iodide, the group was able to determine that the salt was the precipitate and used this information to predict and explain how conductivity would change as a result of precipitation. Cooperative learning enhances learning by offering opportunities for learners to develop joint understanding of concepts (Eilks and Byers, 2009; Becker et al., 2013; Warfa et al., 2018). In collaborative learning, learners engage in cognitive and social processes which influences the group performance (Miyake and Kirschner, 2014). Some of the questioning behaviors observed in our study align with research on social processing in groups. Two modes of social processing pertinent to our work are collaborative and tutoring (Reid et al., 2022). Collaboration occurred as students worked together to complete the assigned task. This collaboration was also mediated by peer-to-peer questions. Tutoring was evident when students responded to their peers’ questions, especially those questions that led to verbalized learning.
Different types of questions were asked across groups. In addition, the question type shaped its function during the group activity. The most commonly asked question types were confirmation and information seeking questions. Clarification (procedural) questions helped groups make sure they were completing the activity as required – that student responses were complying with and relevant to the instructions. Although these types of questions no doubt play a role during group discourse and allowed for groups to complete the assigned activity, they played a limited role in promoting learning because they elicited clarification responses. In asking confirmation questions, students wanted to check if their ideas were right or if their group members agreed with them. Of the top five types of content questions asked, questions seeking understanding were the least common. These questions would provide an opportunity for conceptual understanding and meaningful learning from each other to occur. Such questions can lead to a deeper level of understanding (Hakkarainen, 2003; Zhang et al., 2007). In this study, questions that sought an explanation about why conductivity would change as predicted are an example of such questions. In particular, a correct explanation would help students connect conductivity to the ideas of aqueous solutions and electrolytes, the particulate nature of matter, and precipitation.
One of our findings showed that student questions elicited various types of responses. For the different types of questions asked, the most common response was informational. This shows that students did not always receive appropriate responses when they asked questions. It would be ideal if a student got a conceptual response or explanation if they asked a question seeking conceptual understanding. There were instances in which a confirmation question was asked, and it elicited an explanation, and there were cases in which an explanation was sought through asking a question, but the response was either ‘unsure’ or informational. Of course, as instructors we would prefer that in cases in which an explanation is sought, that an explanation is provided.
One of the touted benefits of collaborative learning is that it allows students to learn from each other (Eilks and Byers, 2009; Becker et al., 2013; Warfa et al., 2018). Learning improves as students take an active role through taking turns in dialogue with each other, asking questions, and clarifying and explaining ideas (Chi and Wylie, 2014). Our results showed that asking questions and getting answers from peers led to verbalized learning even though it did not happen every time. Again, because different questions elicited different types of responses, some responses led to learning while others did not. Our results showed that there were fewer instances overall in which asking and answering questions led to verbalized learning, which we can attribute to the nature of most of the questions asked. Our results showed that most of the questions asked were clarification questions, in which students sought to make sure they were clear about what they were being asked to do. Such questions would, at best, elicit an assurance or explanation of the instructions. There were also a number of cases where responses of “unsure” were given, which would not lead to learning. There was an instance where a student started out with the right conception but ended up with the wrong conception as a result of the question-and-answer interaction in the group. This situation, of course, is counterproductive, and one in which students could benefit from immediate feedback. Unfortunately, this kind of corrective help is not always possible given the context of many student group activities.
Nevertheless, student questions can be a valuable tool to an instructor, especially in uncovering misconceptions or lack of understanding. Questions can provide insight into what students know and understand (Chin and Osborne, 2008). Unfortunately, students seldom ask questions in class. However, this study proved to be a useful tool to get a window into what questions students may and do have. From our results, the most common question was about the meaning and difference between equation types. Other questions asked involved the solubility of salts, the meaning of conductivity, and why conductivity would change as predicted in individual groups. We noticed from the transcripts that even though the most common questions were about the meaning of molecular and ionic equations, most groups were ultimately successful at the task of writing and balancing molecular, ionic, and complete ionic equations. However, most groups did not explicitly link lead(II) iodide to the precipitate, and most did not correctly predict how conductivity would change due to precipitation. The content embedded in students’ questions, such as why conductivity would decrease due to precipitation can reveal their level of thinking (Chin and Brown, 2000), which is important feedback to the instructor.
Asking questions and receiving answers play an important role in knowledge building. The most common disciplinary content questions from our results focus on facts about definitions and procedures, which are a foundation upon which conceptual knowledge can be built – it is necessary to build a factual foundation in order for one to build deep conceptual knowledge. Factual questions seek descriptions of phenomena and definitions of terms, while explanation questions seek for mechanisms and relationships (Zhang et al., 2007). Research has indeed shown that when student groups ask questions targeting facts or information, the knowledge they construct consists of mainly simple facts or information. Groups asking explanation seeking questions are more likely to construct explanations and draw relationships and interconnections (Van Aalst, 2009). Questions seeking explanations, which we saw in this study, are more likely to contribute to knowledge construction than fact-seeking questions as they can lead to a deeper level of understanding (Hakkarainen, 2003; Lee et al., 2006; Zhang et al., 2007).
There are a number of factors that affect the process and outcome of student engagement in collaborative classroom activities. These factors include course expectations, the nature of the task that students complete, students’ prior experiences, the cognitive level of assigned tasks, and existing norms in the classroom (Becker et al., 2013; Zagallo et al., 2016; Warfa et al., 2018; Reid et al., 2022). Students in this study completed a structured activity, where they were asked to answer questions listed in a particular order. The questions asked during the activity were specific to the context. The results we reported in this study came from the first activity of the semester, meaning that students did not have prior experience completing such tasks in the course. In the course, activities like the one reported here count towards the course grade, which we believe incentivizes students to put their best effort in completing it. In the lecture hall where the class met, students sat where there was an open seat. Students did not choose their groupmates, and were asked to work with those seated next to them. This may have impacted the level of interaction especially in the beginning when students may not have been familiar with each other and may also account for the low number of questions in some groups. We should also note that students were not explicitly instructed to ask each other questions, specifically because we did not want to interfere with the spontaneous, needs-based nature of these questions. Interestingly, students indeed asked each other questions, and these questions played a role in completion of the activity.
Limitations
One of the limitations of this study was that the data were collected from one institution and one activity from a single course. Although a lot of data in the form of audio transcripts was collected, other activities, courses, and contexts would provide insights that might be more generalizable on peer-peer questions during collaborative activities. A second limitation is that questioning was not purposefully integrated into the activity. For example, prior to the activity, students did not have any training or feedback on asking and responding to their peers’ questions during collaborative activities. Furthermore, there was not a requirement to ask questions even though we strongly believe it is important to ask each other questions as students work collaboratively and co-construct knowledge. The value of this work is that it gives us a window into this aspect of student interaction during collaborative activities. A third limitation is that students formed groups with those who usually sit next to them. Therefore, it is possible that group dynamics played a role in the group questioning behaviors. The fourth limitation is that this study only focuses on the questions raised by students in their groups. We did not look at relationships between questioning behavior in groups and conceptual understanding and student performance in the course. This is a potential area of future research.Implications for instruction and research
Our results add to what is already known about the importance of collaborative learning (e.g.Eilks and Byers, 2009; Becker et al., 2013; Warfa et al., 2018). Collaborative learning or problem solving in our case provided opportunities for students to ask each other questions, probably more than they would to the instructor, especially in large enrollment courses and should therefore be encouraged. The data that this research drew on was collected right before the COVID-19 related disruption to in-person learning. Therefore, this was the first and only activity for that semester. It is possible that with more opportunities, we might have seen different questioning behaviors. Like any other skill, it takes practice and multiple opportunities to get better at asking questions. Future research will focus on the evolution of students’ questioning behaviors over the course of a semester.We recognize that asking good higher-level questions is a learned skill. Students can be taught to ask such questions, and then encouraged to ask those questions when working in groups. Instructors can devote class time to talk about what makes a question good or what Harpa et al. (2003) call ‘desirable question behavior’ (p. 788). To explicitly teach this question behavior, one can provide students with good question stems (King, 1994), or show and discuss a question taxonomy such as Bloom's with students (Marbach-Ad and Sokolove, 2000). Thus, modelling and encouraging students to ask higher-level questions may be beneficial. Existing studies at the college level involved explicitly prompting students to ask questions (Marbach-Ad and Sokolove, 2000; Harper et al., 2003; Kastens et al., 2020). A potential area of future research could be to examine whether explicitly prompting students to ask questions impacts the nature of unprompted peer-to-peer questions in collaborative settings.
Student questions raised in settings such as the one used in this study could reveal gaps in student knowledge that would otherwise remain unknown to the instructor until there is an assessment like an exam. In addition, because of the size of the large enrollment classes, students are less likely to ask questions in front of the whole class (Good et al., 1987). However, silence does not mean that students do not have questions; therefore, finding a way to access those questions is important. Collecting and analyzing audio transcripts from student groups is time consuming but offers an option, especially for spontaneous peer-to-peer questions. Technology, especially for transcribing audio transcripts can help cut down on transcribing time, and thereby help instructors access the transcripts in a shorter time to be able to use the information for instruction. Other approaches, such as those used by Marbach-Ad and Sokolove (2000) and Harpa et al. (2003) have proved useful in promoting students’ questioning in college science classrooms. Technology can also be used in classrooms to allow students to anonymously submit questions. As students have opportunities to ask questions and see those questions addressed in class, this could model the process of appropriately responding to questions.
As noted above, factors such as course expectations, the nature of the task that students complete, students’ prior experiences, the cognitive levels of assigned tasks, and existing norms in the classroom can all affect the process and outcome of in-class collaborative activities (Becker et al., 2013; Zagallo et al., 2016; Warfa et al., 2018; Reid et al., 2022). In the context of asking questions, instructors can design activities that encourage students to pose certain types of questions, such as those of a certain cognitive complexity (Chin and Osborne, 2008; Kastens et al., 2020). A potential area of future research is how the content, context, and type of collaborative activity that students engage in influences the types of questions that they ask within their groups and the nature of interactions they engender. In the current study, students watched a brief recorded video of a precipitation reaction and were asked a series of questions (see Fig. 1 above). The questions students asked each other are influenced by the nature of the activity. For example, in many of the groups, clarification questions were asked first since students needed to make sure that they understood instructions. The patterns in the order of questions also follow the order of prompts in the assigned task. The content that questions addressed, such as the meaning of conductivity and electrolytes, were context dependent. Therefore, would a different activity, such as data interpretation lead to different types of questions?
In the study reported here, we focused on students’ peer-to-peer questions. Our study did not look at the relationship between the questioning behavior in groups and success in the assigned task. Additionally, future research could look at the following questions: Do groups where more questions were asked answer more questions correctly than groups with fewer questions? Did the types of questions asked in each group affect performance in the task? How do group characteristics (such as gender, major, or number of students) affect questioning behavior?
Finally, our results showed some instances in which students asked questions in their groups but did not get a response. Even though this could imply that group members did not know the answer, students should be explicitly taught the importance of responding to each other, even if it means saying “I do not know.” This is an important norm that could be learned if explicitly taught. Students could also be encouraged to ask for help, even if they only get a hint that helps them answer their own question, or to look for additional information that could be useful, from sources such as textbooks. For collaborative learning to be productive, active and reciprocal participation is necessary since not responding to a peer's question could potentially hinder productive collaborative learning.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank ND EPSCOR for funding to support undergraduate research. We would also like to thank Brent Hill, Aubrey Renfrew, Drew Jordahl, Kristina Caton and Amos Tarfa for their contributions to research and editing the manuscript.References
- Adams P., (2006), Exploring social constructivism: Theories and practicalities, Education, 34(3), 243–257.
- Barron B., (2003), When smart groups fail, J. Learn. Sci., 12(3), 307–359.
- Becker N., Rasmussen C., Sweeney G., Wawro M., Towns M. and Cole R., (2013), Reasoning using with particulate nature of matter: An example of a sociochemical norm in a university- level physical chemistry class, Chem. Educ. Res. Pract., 14, 81–94.
- Bereiter C., (2002), Education and Mind in the Knowledge Age, Lawrence Erlbaum Associates, Inc.
- Biddulph F. and Osborne R., (1982), Some issues relating to children's questions and explanations. LISP(P) Working paper no. 106. Waikato, New Zealand: University of Waikato.
- Bybee R. W., (2000), Teaching science as inquiry, in Minstrell J. and van Zee E. H. (ed.), Inquiring into inquiry learning and teaching in science, Washington, DC: American Association for the Advancement of Science, pp. 20–46.
- Carlsen W. S., (1991), Questioning in classrooms: a sociolinguistic perspective, Rev. Educ. Res., 61, 157–178.
- Chi M. T. H. and Wylie R., (2014), The ICAP framework: Linking cognitive engagement to active learning outcomes, Educ. Psych., 49, 219–243.
- Chin C., (2002), Student-generated questions: Encouraging inquisitive minds in learning science, Teach. Learn., 23(1), 59–67.
- Chin C., (2004), Students’ questions: Fostering a culture of inquisitiveness in science classrooms, Sch. Sci. Rev., 86(314), 107–112.
- Chin C. and Brown D. E., (2000a), Learning deeply in science: An analysis and reintegration of deep approaches in two case studies of Grade 8 students, Res. Sci. Educ., 30(2), 173–197.
- Chin C. and Brown D. E., (2000b), Learning in science: A comparison of deep and surface approaches, J. Res. Sci. Teach., 37(2), 109–138.
- Chin C. and Brown D. E., (2002), Student-generated questions: A meaningful aspect of learning in science, Int. J. Sci. Educ., 24(5), 521–549.
- Chin C. and Kayalvizhi G., (2002), Posing problems for open investigations: What questions do pupils ask? Res. Sci. Tech. Educ., 20(2), 269–287.
- Chin C. and Osborne J., (2008), Students’ questions: a potential resource for teaching and learning science, Stud. Sci. Educ., 44(1), 1–39.
- Chin C. and Osborne J., (2010), Students' questions and discursive interaction: Their impact on argumentation during collaborative group discussions in science, J. Res. Sci. Teach., 47, 883–908.
- Cole R., Becker N., Towns M. H., Sweeny G., Wawro M. and Rasmussen C., (2012), Adapting a methodology from mathematics education research to chemistry education research: documenting collective activity, Int. J. Sci. Math. Educ., 10, 193–211.
- Costa J., Caleira H., Gallastegui R. J. and Jose O., (2000), An analysis of question asking on scientific texts explaining natural phenomena, J. Res. Sci. Teach., 37(6), 602–614.
- Dogan F. and Yucel-Toy B., (2021), Students’ question asking process: a model based on the perceptions of elementary school students and teachers, As. Pac. J. Educ., 42(2), 1–16.
- Eilks I. and Byers B., (2009), Innovative methods of teaching and learning chemistry in higher education, London: RCS Publishing.
- Erdogan I., (2017), Turkish Elementary Students’ Classroom Discourse: Effects of Structured and Guided Inquiry Experiences That Stimulate Student Questions and Curiosity. Int. J. Environ. Sci. Educ., 12(5), 1111–1137.
- Farrell J. J., Moog R. S. and Spencer J. N., (1999), A Guided-Inquiry General Chemistry Course, J. Chem. Educ., 76(2–4), 570–574.
- Fredricks J. A., Blumenfeld P. and Paris A., (2004), School engagement: Potential of the concept, state of the evidence, Rev. Educ. Res., 74(1), 59–109.
- Freeman S. et al., (2014), Active Learning Increases Student Performance in Science, Engineering, and Mathematics, Proc. Natl. Acad. Sci. U. S. A., 111, 8410–8415.
- Good T. L., Slavings R. L., Harel K. H. and Emerson H. (1987). Student passivity: A study of question asking in K-12 classrooms, Soc. Educ., 60, 181–199.
- Graeser A. and Person N., (1994), Question asking during tutoring, Am. Educ. Res. J., 31(1), 104–137.
- Hakkarainen K., (2003), Progressive inquiry in a computer-supported biology class, J. Res. Sci. Teach., 40(10), 1072–1088.
- Halmo S. M., Bremers E. K., Fuller S. and Stanton J. D., (2022), “Oh that makes sense”: Social Metacognition in small-group problem solving, CBE–Life Sci. Educ., 21(58), 1–20.
- Harper K. A., Etkina E. and Lin Y., (2003), Encouraging and analyzing student questions in a large physics course: Meaningful patterns for instructors, J. Res. Sci. Teach., 40(8), 776–791.
- Hofstein A., Navon O., Kipnis M. and Mamlok-Naaman R., (2005), Developing students’ ability to ask more and better questions resulting from inquiry-type chemistry laboratories, J. Res. Sci. Teach., 42(7), 791–806.
- Kastens K. A., Zrada M. and Turrin M., (2020), What kinds of questions do students ask while exploring data visualizations? J. Geo. Educ., 68(3), 199–219.
- Kaya S., (2015), The effect of the type of achievement grouping on students’ question generation in science, Aust. Educ. Res., 42, 429–441.
- Kaya S. and Kablan Z. (2013). Assessing the relationship between learning strategies and science achievement at the primary school level, J. Baltic Sci. Educ., 12(4), 525–534.
- King A. (1994). Guiding knowledge construction in the classroom: Effects of teaching children how to question and how to explain, Am. Educ. Res. J., 31(2), 338–368.
- Kuh G. D., (2009), The national survey of student engagement: conceptual and empirical foundations, New Dir. Institutional Res., 141, 5–20.
- Kundi G. M. and Nawaz A., (2010), From objectivism to social constructivism: The impacts of information and communication technologies (ICTs) on higher education, J. Sci. Tech. Educ. Res., 1(2), 30–36.
- Lai M. and Law N., (2013), Questioning and the quality of knowledge constructed in a CSCL context: a study on two grade-levels of students, Inst. Sci., 41(3), 597–620.
- Lee E. Y. C., Chan C. K. K. and van Aalst J. (2006), Student assessment of collaborative learning in a CSCL environment, Int. J. Comput. Support. Collab. Learn., 1(1), 57–87.
- Marbach-Ad G. and Sokolove P. G., (2000), Can Undergraduate Students Learn to ask Higher Level Questions? J. Res. Sci. Teach., 37(8), 854–870.
- Mehan H., (1979), Learning lessons: Social organization in the classroom, Cambridge, MA: Harvard University Press.
- Merriam S. B. and Tisdell E. J., (2015), Qualitative research: A guide to design and implementation, 4th edn, San Francisco, CA: Jossey-Bass.
- Miyake N. and Kirschner P. A., (2014), The social and interactive dimensions of collaborative learning, in Sawyer K. R. (ed.), The Cambridge Handbook of the learning Sciences, New York: Cambridge University Press, pp. 418–438.
- Moon A., Stanford C., Cole R. and Towns M., (2017), Analysis of inquiry materials to explain complexity of chemical reasoning in physical chemistry students’ argumentation, J. Res. Sci. Teach., 54(10), 1322–1346.
- Myoung-Sook L., Kwang-Hee J. and Jin-Woong S., (2004), Types and frequencies of questions - answers by middle school students in a small group activity during school experiments, J. Kor. Ass. Sci. Educ., 24(2), 277–286.
- Naibert N., Vaughan E. B., Brevick K. and Barbera J., (2022), Investigating student perceptions of behavioral, cognitive and emotional engagement at the activity level in general chemistry, J. Chem. Educ., 99, 1358–1367.
- National Research Council, (2012), A Framework for K-12 Science Education: Practices, Crosscutting Concepts, and Core Ideas. Washington, DC: The National Academies Press.
- NGSS Lead States, (2013), Next Generation Science Standards: For States, By States. Washington, DC: The National Academies Press.
- Nystrand M., Wu L. L., Gamoran A., Zeiser S. and Long D. A., (2003), Questions in time: Investigating the structure and dynamics of unfolding classroom discourse, Disc. Proc., 35(2), 135–198.
- Penuel W. R., Yarnall L., Koch M. and Roschelle J., (2004), Meeting teachers in the middle: Designing handheld computer-supported activities to improve student questioning, in Kafai Y. B., Sandoval W. A., Enyedy N., Nixon A. S. and Herrera F. (ed.), Proceedings of the International Conference of the Learning Sciences, Mahwah, NJ: Lawrence Erlbaum, pp. 404–411.
- Powell K. C. and Kalina C. J., (2009), Cognitive and social constructivism: Developing tools for an effective classroom, Education, 130(2), 241–250.
- Reid J., Gunes Z. D. K., Fateh S., Fatima A., Macrie-Shuck M., Nennig H., T. Quintilla, F. States, N. E. Syed, A. Cole, R. Rushton, Shah G. L. and Talanquer V., (2022), Investigating patterns of student engagement during collaborative activities in undergraduate chemistry courses, Chem. Educ. Res. Pract., 23, 173–188.
- Rosenshine B., Meister C. and Chapman S., (1996), Teaching students to generate questions: A review of the intervention studies, Rev. Educ. Res., 66 (2), 181–221.
- Scardamalia M., (2002), Collective Cognitive Responsibility for the Advancement of Knowledge, in Liberal Education in a Knowledge Society, Carus Publishing Company, pp. 67–98.
- Scardamalia M. and Bereiter C., (1992), Text-based and knowledge-based questioning by children, Cog. Inst., 9(3), 177–199.
- Sinha S., Rogat T. K., Adams-Wiggins K. R. and Hmelo-Silver C. E. (2015). Collaborative group engagement in a computer-supported inquiry learning environment, Int. J. Comp. Supp. Coll. Learn., 10(3), 273–307.
- Smith C. L., Maclin D., Houghton C. and Hennessey M. G., (2000), Sixth-grade students’ epistemologies of science: The impact of school science experiences on epistemological development. Cog. Inst., 18(3), 349–422.
- Spencer J. N. and Moog R. S., (2008), The process oriented guided inquiry learning approach to teaching physical chemistry, in Ellison M. D. and Schoolcraft T. A. (ed.), Advances in teaching physical chemistry, Washington, DC: American Chemical Society, pp. 268–279.
- Taboada A. and Guthrie J. T., (2006), Contributions of student questioning and prior knowledge to construction of knowledge from reading information text, J. Lit. Res., 38(1), 1–35.
- Theobald E. J., Hill M. J., Tran E., Agrawal S., Nicole Arroyo E., Behling S., Chambwe N., Cintrón D. L., Cooper J. D., Dunster G., Grummer J. A., Hennessey K., Hsiao J., Iranon N., Jones L., Jordt H., Keller M., Lacey M. E., Littlefield C. E. and Freeman S., (2020), Active learning narrows achievement gaps for underrepresented students in undergraduate science, technology, engineering, and math, Proc. Natl. Acad. Sci. U. S. A., 117(12), 6476–6483.
- Tracy S. J., (2013), Qualitative Research Methods: Collecting Evidence, Crafting Analysis, Communicating Impact, Wiley-Blackwell.
- Tro N. J., (2019), Chemistry: A Molecular Approach, 5th edn, Pearson Education.
- Van Aalst J., (2009), Distinguishing knowledge-sharing, knowledge construction, and knowledge-creation discourses, Comp.-Supp. Coll. Learn., 4, 259–287.
- Van der Meij H., (1994), Student questions: a componential analysis. Learn. Indiv. Diff., 6(2), 137–161.
- van Zee E. H., Iwasyk M., Kurose A., Simpson D. and Wild J., (2001), Student and teacher questioning during conversations about science, J. Res. Sci. Teach., 38(2), 159–190.
- Vygotsky L. S., (1978), Mind in society: The development of higher psychological processes, Cambridge, MA: Harvard University Press.
- Warfa A. R. M., Nyachwaya J. and Roehrig G., (2018), The influences of group dialog on individual student understanding of science concepts, Int. J. STEM Educ., 5, 46.
- Watts M., Gould G. and Alsop S., (1997), Questions of understanding: Categorizing pupils’ questions in science, Sch. Sci. Rev., 79(286), 57–63.
- Woodward C., (1992), Raising and answering questions in primary science: Some considerations, Eval. Res. Educ., 6(2–3), 145–153.
- Yu F. Y., (2009), Scaffolding student-generated questions: Design and development of a customizable online learning system, Comp. Hum. Beh., 25(5), 1129–1138.
- Zagallo P., Meddleton S. and Bolger M. S., (2016), Teaching real data interpretation with models (TRIM): Analysis of student dialogue in a large-enrollment cell and developmental biology course, CBE–Life Sci. Educ., 15(2), 1–18.
- Zhang J., Scardamalia M., Lamon M., Messina R. and Reeve R., (2007), Socio-cognitive dynamics of knowledge building in the work of 9- and 10-year-olds, Educ. Tech. Res. Dev., 55(2), 117–145.
| This journal is © The Royal Society of Chemistry 2023 |