 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Guided inquiry-based learning in secondary-school chemistry classes: a case study
Gábor
Orosz
 ab,
Veronika
Németh
ab,
Veronika
Németh
 bc,
Lajos
Kovács
bc,
Lajos
Kovács
 bd,
Zoltán
Somogyi
bd,
Zoltán
Somogyi
 be and
Erzsébet
Korom
be and
Erzsébet
Korom
 *bf
*bf
aUniversity of Szeged, Doctoral School of Education, Petőfi sgt. 32-34, H-6722 Szeged, Hungary
bMTA–SZTE Science Education Research Group, University of Szeged, Petőfi sgt. 32-34, H-6722 Szeged, Hungary. E-mail: korom@edpsy.u-szeged.hu
cUniversity of Szeged, Department of Physical Chemistry and Materials Science, Rerrich Béla tér 1, H-6720 Szeged, Hungary
dUniversity of Szeged, Department of Medicinal Chemistry, Dóm tér 8, H-6720 Szeged, Hungary
eImre Somogyi Primary School, Szelei út 1, H-2740, Abony, Hungary
fUniversity of Szeged, Department of Learning and Instruction, Petőfi sgt. 32-34, H-6722 Szeged, Hungary
First published on 4th August 2022
Abstract
Guided inquiry-based learning has been shown to be a promising method for science education; however, despite its advantages it is rarely used in chemistry teaching in Hungary. One of the reasons for this is the lack of tried-and-tested inquiry-based teaching materials with detailed guides that teachers can readily use in their classrooms. As part of a four-year research project, new teaching materials were designed to foster scientific reasoning and scientific process skills in chemistry education in Hungary. From these materials, in this study, a guided inquiry-based chemistry task was tested with 9th-grade students (N = 88) who had no previous experience with the method. Before the activity, the students’ mid-term grades were collected, and the Lawson Classroom Test of Scientific Reasoning (LCTSR) was administered to describe the sample. During the activity, students worked in groups (n = 21). Data were collected through content analysis of the student worksheets, classroom observations using a rubric, and student questionnaires to explore the learning paths and identify possible obstacles. Our findings support that guided inquiry learning is suitable for students who are new to the method if appropriate scaffolding is given. The data showed the phases of the inquiry cycle in which more guidance is necessary. Formulating hypotheses, recording observations, and evaluating the hypotheses based on the evidence were found to be the most critical steps in the learning process. More than half of the groups disregarded the collected evidence and accepted their original hypotheses, despite their unproven validity, suggesting that they did not understand the true nature of the scientific inquiry. Chemistry grades and the LCTSR scores could not predict reliably the students’ success in solving the inquiry task. The results of the student questionnaire showed that the students enjoyed the inquiry session. They mostly found their work successful, but they overestimated the level of their inquiry skills in some cases.
Introduction
Significance of the study
In Hungarian public education, chemistry is taught in grades 7 to 10. Students wishing to continue their studies in chemistry can do so in the form of facultative courses in grades 11 and 12. Although the new Chemistry Framework Curricula of Hungary (Educational Authority of Hungary, 2020a, 2020b) include the development of inquiry skills, the emphasis remains on the teaching of disciplinary knowledge. Relatively little time is available for the study of an extensive body of knowledge with the result that teacher-centred learning dominates in the classroom. Students have little opportunity to do experiments or solve problems. It is, therefore, crucial to make the most of the few classes during which students can have an active role and use those classes to realise a variety of educational goals.For teachers to feel confident in conducting an inquiry-based classroom activity, they must have easy access to tried-and-tested lesson plans that can be implemented in the classroom. Although the current teaching materials may include a few problems or experiments, inquiry-based activities are under-represented and no guidance is typically given on how to implement them.
Our study discusses a guided inquiry-based chemistry activity that we tested with 9th-grade students who had had no previous experience with guided inquiry-based learning and were following the compulsory Chemistry Framework Curricula for high schools. However, they had already learned about the methods of scientific inquiry during their regular chemistry classes where key concepts such as research questioning, hypothesis, observation, experiments, etc., had been introduced, and they had previously carried out some structured experiments. The main purpose of the study was to find out how they got along in an unfamiliar guided inquiry learning situation. We observed the process of inquiry to explore the learning paths, and to gather information about how a decision influences the next steps. Based on this we identified when more support was needed during the inquiry cycle to provide recommendations for teachers. Our second aim was to explore how the students felt during the activity and how they rated their performance.
Within the Hungarian context, our research builds upon the results of Szalay and Tóth (2016), who transformed traditional chemistry experiments into inquiry activities. In a further study, they carried out a four-year programme during which high-performing students participated in six inquiry activities each school year, focusing on designing experiments. These students were attending six-year secondary schools specialising in gifted education and had been admitted following a selection process (Szalay et al., 2020; Szalay et al., 2021). By contrast, our programme targets students attending ordinary four-year secondary schools, for most of whom chemistry education ends in the 10th grade. Under these circumstances, the goal of chemistry education is to establish scientific literacy and teach the reasoning and inquiry skills needed for success in everyday life, such as data collection and analysis, evidence-based decision-making, and cooperation.
Integrating guided inquiry into the traditional curricular framework is a complex task (e.g., Dobber et al., 2017). Smithenry (2010) argues that three decisions need to be made to achieve this goal: (1) we should identify the learning units or topics that are suitable for inquiry learning; (2) we should select the activities to prepare students for inquiry-based learning; and (3) we need to decide how to link the series of inquiry activities, and how each activity is going to build upon the previous ones during the school year. Our case study is the first step in the process of integrating guided inquiry-based learning in the chemistry curriculum while remaining within the bounds of traditional classroom instruction.
Types of inquiry-based learning
Inquiry-based learning (IBL) is a student-centred method (Kuhn et al., 2000; Reid and Ali, 2020) where “learning is driven by a process of enquiry” (Khan and O’Rourke, 2004, p. 1). To answer a question or solve a problem, students conduct experiments following the steps of scientific discovery, they collect and analyse data, and they draw conclusions, thus acquiring new knowledge and skills (Bell et al., 2010; Pedaste et al., 2015). The method is based on a constructivist approach to learning, which contends that learners create their understanding by taking an active part in the learning process (Driver and Oldham, 1986; Bednar et al., 1992; von Glasersfeld, 1995; Bächtold, 2013). Constructivism has often been seen as an alternative to transmissive teaching (Ültanir, 2012; Bada and Olusegun, 2015), but evidence has shown that while constructivism is a very good paradigm for understanding learning, it does not in itself provide a complete solution as a teaching strategy (Phillips, 1995; Terhart, 2003; Hyslop-Margison and Strobel, 2008; Hattie, 2009; Hattie and Yates, 2013; Reid and Ali, 2020). Learner-centred approaches (including IBL) are less useful for knowledge acquisition and understanding, but can be effective for developing skills and attitudes, and when learners need to apply their existing understanding in new contexts (Reid and Ali, 2020). IBL is therefore an approach where the emphasis is not entirely on transferring knowledge but on fostering inquiry skills and understanding the nature of science (Schwartz et al., 2004).Depending on the amount of information provided to students and on the involvement of the teacher in the activity, three levels of inquiry-based learning can be distinguished (e.g., Hegarty, 1978; Tafoya et al., 1980; Spronken-Smith and Walker, 2010; Zion and Mendelovici, 2012). The first level is structured inquiry, where the problem and the experimental procedures are provided by the teacher but the solution is suggested by the students based on the results of their observations and measurements. This type of activity offers limited opportunity for independent thinking and the students’ attention is channelled into a predetermined path leading to the solution. This method may be useful for practising relatively simple inquiry skills (e.g., observation, measurement, data interpretation, discussion of results and documentation) or for introducing students to inquiry-based learning. The next level is represented by guided inquiry, where the problem is still given by the teacher but all further steps of the process (e.g., the formulation of hypotheses, and designing of the experiment) are executed by the students. These activities provide an opportunity for cooperative learning and leave more room for students’ creativity and the development of their skills and abilities. The highest level of inquiry-based learning is open inquiry, where the teacher's role is limited to specifying the topic and learning goals, and students choose the problem and research questions they wish to study. This type of activity gives the greatest level of freedom and demands the most advanced inquiry skills, reasoning ability and creativity, but it also means the highest level of responsibility.
The above classification is not universal in the literature. Some researchers add a still lower level called confirmatory inquiry, where students know the outcome of the experiment in advance and their only task is to reproduce the results (Tafoya et al., 1980; Staver and Bay, 1987; Bell et al., 2005; Xu and Talanquer, 2013). Wenning (2007) gives a spectrum of inquiry levels, ranging from discovery learning to free-inquiry labs, where, as the intellectual level of the learners increases, control is increasingly transferred from the teacher to the learners.
Bruck et al. (2008) point out that the same level of IBL tends to be defined in different ways in secondary and in higher education (e.g., during the guided inquiry in higher education, students receive the experimental design, but they have to make their own decisions as regards data analysis). To avoid terminological difficulties, some researchers simply assign numerical labels to individual levels, where a higher value indicates greater student freedom (e.g., Fay et al., 2007). Since our study tests IBL with secondary school students, we shall follow the system of Zion and Mendelovici (2012).
Although IBL is widely accepted as a fundamental approach to science instruction (e.g., National Research Council 1996; Dillon, 2009), opinions differ as to its efficiency (e.g., Khishfe and Abd-El-Khalick, 2002; Berg et al., 2003; Mayer, 2004; Minner et al., 2010; Furtak et al., 2012). Inquiry-based learning as a teaching strategy – similarly to discovery, problem-based and experiential learning – does not necessarily lead to more successful learning in terms of subject-content acquisition (Reid and Ali, 2020). IBL is more effective for older and more able learners, but the stress of working-memory overload can affect them too. Younger learners need more support because of their less developed cognitive structures and more limited prior knowledge (Kirschner et al., 2006; Hattie and Yates, 2013; Reid and Ali, 2020). Therefore, it is important to adjust the implementation methods to the learning environment. Reduced teacher involvement may increase the cognitive load. If an inquiry activity overloads the students’ working memory, it may result in a decreased level of understanding and fragmented knowledge or even the emergence of misconceptions. The solution lies in appropriately guided inquiry learning with the correct balance of learner freedom and teacher support (Kirschner et al., 2006); at the same time, scaffolding should build on the students’ thinking (Hmelo-Silver et al., 2007). Research on the views and experiences of students and teachers regarding inquiry-based learning can help to identify forms of support.
Benefits and challenges of inquiry-based learning: students’ experiences
Several studies have investigated students' perceptions and opinions about inquiry-based learning. Through interviews, Baldock and Murphrey (2020) explored the perceptions of inquiry-based learning among secondary-school students studying agriculture. Students felt that their critical thinking improved because they had to think harder, solve problems and work more autonomously than in traditional lessons. Although students liked the opportunity to work independently, half of them required the teacher to provide background information before inquiry-based learning. The authors point out that this is in line with the research of Edelson et al. (1999), who emphasised that a lack of background knowledge about scientific research is one of the major challenges in implementing inquiry-based learning. Therefore, learners need sufficient information to know how to start an investigation and then be supported to find the answers themselves. Students should be given information about the nature of the inquiry-based learning process and an explanation of why it is used.Eltanahy and Forawi (2019) surveyed 8th-grade students with a questionnaire in a study on how a new science textbook facilitated the implementation of inquiry-based learning. The majority of students mentioned that they would prefer to learn science using the inquiry-based method, as traditional teaching prefers that they memorise information. Students had a positive attitude towards inquiry activities in science lessons and therefore showed more interest in learning about science. They felt that more authentic learning improved their cognitive and scientific reasoning skills, made it easier for them to understand the material and helped them to organise their knowledge. However, they felt that they needed consistent practice to become more responsible. They experienced it as a difficulty. They usually did not have enough time to discuss their results with their peers or retry procedures to gain a better understanding or confirmation.
Vekli's (2021) study shows that secondary school students' perceptions of inquiry-based learning skills in science differ by gender, grade level, grade point average, family income level, the mother's and father's educational attainment, and the frequency of information communication technology use. Girls and lower-school students perceive their inquiry-based learning skills as better. A higher parental education and a better income status have a positive effect on the perception of science-learning skills of secondary-school students, while the frequency of information communication technology use has a negative effect.
The type of inquiry-based learning that learners find more appropriate varies. For example, a survey of university students by Chatterjee et al. (2009) indicated that the majority of students preferred instruction-based experiments. However, in a comparative analysis of university students' perceptions of learning processes and intended learning outcomes, Spronken-Smith et al. (2012) found that students rated open and discovery-oriented research higher than guided or structured and information-oriented research.
These findings confirm the need to consider several factors when designing and implementing inquiry-based learning, such as learners' cognitive abilities, reasoning skills, prior knowledge and experience of inquiry.
Benefits and challenges of guided inquiry-based learning: teachers’ experiences
Guided inquiry-based learning supports the development of science-process skills, and it may also be a promising tool in the application of knowledge and shaping attitudes. Several studies have demonstrated the effects of the method in connection with science instruction in different age groups (e.g., Minner et al., 2010; Furtak et al., 2012; Firman et al., 2019). The following discussion focuses on some of those studies that are specifically concerned with chemistry education.Barthlow and Watson (2014) observed that 10th- and 11th-grade students who had studied chemistry using the Process Oriented Guided Inquiry Learning (POGIL) method had developed fewer misconceptions in connection with the particle nature of matter than did their peers who had learned via traditional methods. Ural's (2016) research revealed that guided inquiry learning had a positive effect on university students’ attitudes towards chemistry laboratory classes, improved their performance, and decreased their anxiety regarding laboratory activities. Similar results are reported by Vishnumolakala et al. (2017), who studied the effects of POGIL on attitudes towards chemistry and self-efficacy among first-year university students with minimal prior knowledge of chemistry. Students’ intellectual accessibility and emotional satisfaction increased, indicating a positive change in their attitudes towards chemistry. Also, at the end of the semester, the students rated their self-efficacy in chemistry learning higher than at the beginning. Kadioglu-Akbulut and Uzuntiryaki-Kondakci (2021) studied the effects of guided inquiry learning on self-regulated learning in dynamic-equilibrium and acid–base reactions among 11th-grade students. Although there was no significant change in the students’ learning strategies, the method had a positive effect on achievement, the participants’ use of metacognitive strategies increased, and they gave more accurate explanations for the observed phenomena. Tornee et al. (2019) found that Grade 11 students’ learning achievement in chemistry and problem-solving competency was significantly higher when they learned using guided inquiry.
Despite the numerous advantages of guided inquiry learning, the method is rarely employed in the classroom (Wallace and Kang, 2004; Capps and Crawford, 2013; Engeln et al., 2013; Hofer et al., 2018). In a review of the literature, Cheung (2007) identified a number of factors that may influence, and sometimes potentially hinder, the implementation of guided inquiry learning in teaching practice. The following paragraphs briefly discuss these factors based mainly on Cheung's (2007) work supplemented by additional research relevant to chemistry lessons.
The role of support in guided inquiry learning
Teachers play a crucial role in inquiry-based learning since students will not understand the purposes and processes behind the inquiry activity simply by engaging in hands-on activities (Trumbull et al., 2005), and nor will they gain a deeper understanding of the nature of science (Schwartz et al., 2004). It is the teacher's task to ensure that students develop an understanding of why they are doing what they are doing. It is the teacher who helps the students make sense of the inquiry process, bring the activity to a successful conclusion, and reflect on the outcome (Quintana et al., 2004). de Jong and Lazonder (2014) organised the different forms of teacher guidance into a hierarchical system based on the strength of support: process constraints, status overviews, prompts, heuristics, scaffolds, and explanations. The first three concern the organisation of the inquiry process: the identification, structuring and timing of the steps of the procedures. The last three offer specific guidance for the implementation of the steps with increasing levels of detail. Lazonder and Harmsen's (2016) results reveal that instructors’ contributions influence the learning outcomes, learning activities and performance success, but there is no clear association between the type of teacher guidance and its effectiveness. The best kind of support is therefore not necessarily the most extensive type. The authors further note that the choice of support should be based on the students’ experience and skills rather than on their age.When introducing inquiry-based learning, teachers can also transform traditional cookbook-style laboratory activities into inquiry-based activities (e.g., Szalay and Tóth, 2016). Volkmann and Abell (2003) recommend two types of support for this: (1) using an inquiry analysis tool, teachers can check that the task engages students in investigating scientific questions, supports the students in collecting data themselves and formulating evidence-based explanations, provides opportunities for students to evaluate their thinking and modify their explanations in light of the evidence, and allows them to communicate with each other to discuss their ideas; and (2) by considering a total of ten principles concerning questions, evidence, explanations and communication, teachers can ensure that the students are engaged in scientific inquiry.
Inquiry-based learning creates a new and complex classroom situation compared with traditional learning, so both students and teachers need time to adapt to a more open learning situation. Changes in teaching should therefore be introduced slowly, and new activities should be introduced gradually, for example, instead of having the teacher prepare tables in advance, the students should figure out what data to record and how (Colburn, 2000; Puddu, 2017). More autonomy can be motivating for learners; however, care should be taken not to overload them with too much information (Reid, 2021).
Aims of the study
The purpose of our study was to explore how students solve an unfamiliar guided inquiry task. We compared data from different sources to identify learning paths, correct and erroneous solutions, typical approaches and difficulties, and to find out how students evaluated the activity and their work.We formulated the following research questions (RQs) regarding (1) the learning and teaching process, and (2) student attitudes and self-evaluation:
(1) RQ1: What are the common solution pathways? RQ2: How do students perform on the steps of the inquiry process? RQ3: Can students transfer what they learned from the structured labs to the guided inquiry activity? RQ4: To what extent do student grades in chemistry and the level of scientific-reasoning skills predict the success of completing the inquiry task?
(2) RQ5: What are students’ views on the inquiry activity? RQ6: Is there a difference between the self-assessments of students who successfully completed the activity and those who did not?
Development and implementation of the guided inquiry activity
Description of the problem
The title of the activity is “What happened to the horn salt?”. It is a guided inquiry activity taking up two 45 minute class periods. The problem forming the basis of the activity is the following:“As we were tidying up the chemistry storage room, we found an interesting thing: an old bag of horn salt, which we often use for experimenting, was empty even though the packaging was unharmed. This finding seems to be in contradiction with the law of conservation of mass. We need your help to solve this chemical puzzle.”
Household horn salt (NH4HCO3), commonly used in Hungary and in many other countries as an effective leavening agent for the baking of flat, low-moisture goods such as cookies and crackers, spontaneously decomposes with time into volatile compounds (carbon dioxide, ammonia, and water), which may pass through the packaging via diffusion according to this equation: NH4HCO3 → NH3↑ + H2O↑ + CO2↑. To solve the problem, students first need to identify the compound. The scientific name found on the packaging (ammonium bicarbonate) may be of help here, although the more explicative alternative name (ammonium hydrogen carbonate) could have provided more clues. The confusing archaic names (staghorn salt, baker's ammonia, salt volatile, hartshorn or salt of hartshorn) relate to compound mixtures containing varying amounts of ammonium bicarbonate, ammonium carbonate and ammonium carbamate, but the Hungarian equivalents of these names are practically unknown to the students. Although even contemporary products contain small amounts of ammonium carbonate and ammonium carbamate in addition to ammonium bicarbonate (Wen and Brooker, 1995), the presence of these materials does not interfere with the process observed during the activity; therefore, they were not included in the worksheet to keep things simple. Once the material has been identified, the hypotheses can be formulated based on qualitative observations and prior knowledge about the substance. The solid material may disappear from the sachet if it first turns into a gas in some way. This may happen through physical changes or chemical reactions. The name indicates that the material is an ionic compound, whose particles are held together by primary bonds (ionic bonds). Ionic compounds melt at a very high temperature because the bonds are difficult to break. A change of aggregation state is therefore unlikely to take place at room temperature. Another argument in favour of a chemical process is that horn salt smells strongly of ammonia, which can only be the result of chemical change. The reaction rate increases with increasing temperature, so the decomposition can be accelerated. It is therefore sensible to test the hypothesis by heating the material and then identifying the resulting gases. This is not absolutely necessary for the solution, however; it is sufficient to cool and trap the gases. We can conclude from the result that we are indeed witnessing a chemical transformation rather than a physical change since the original crystal structure cannot be regained by cooling. Therefore, knowledge about the nature of materials and their changes is required to solve the problem, which can be expected from a student in the 9th grade. The design and execution of the experiments further require practical skills and procedural and epistemological knowledge.
When selecting the problem, our main considerations were that it should relate to a real situation, and that it should be simple, but the solution should not be obvious. It also had to relate to the subject matter of 9th-grade chemistry, provide an opportunity to apply prior knowledge of general and inorganic chemistry and be easy to embed in the yearlong curriculum. It can be used to solidify knowledge of material structure, for instance, or to deepen the students’ understanding of the difference between physical and chemical changes. The student worksheet (Appendix 1) describes the problem and lists the steps of the experiment. The instructions are supplemented by some information about the inquiry process (e.g., what a hypothesis is and the role of an experiment in testing the hypothesis).
Preparation in chemistry class for the inquiry session
A week before the inquiry lesson, students performed the following structured experiments in a chemistry class:1. they heated hydrated copper sulfate and observed the water being expelled and the water vapour condensing;
2. they heated ammonium chloride in a 90-degree elbow pipe and observed the difference between the resulting gases (ammonia and hydrogen chloride) concerning the rate of diffusion using universal pH indicator paper;
3. they blew air into lime water through a drinking straw and observed the precipitation caused by the reaction between the carbon dioxide in the exhaled air and the calcium hydroxide in the lime water; and
4. they sublimated iodine in a test tube and observed iodine crystals forming on the cold sections of the tube.
The experiments were aligned with the curriculum and provided the students with knowledge and experience to solve the following inquiry task. The students practised basic laboratory skills (e.g., heating a substance in a test tube, testing for the presence of carbon dioxide using limewater), making observations and drawing conclusions. They reviewed the differences between physical and chemical changes, they experienced the pungent smell of ammonia, and they saw water vapour condensing onto the wall of the test tube. All of these could have been useful for interpreting their observations during the inquiry lab. There was, however, no explicit allusion to this during either the preparatory class or the inquiry activity. We were interested in finding out whether the students were able to transfer their previous experiences to the new context.
The guided inquiry lesson
The inquiry activity took place in two consecutive chemistry-class periods. The first lesson was spent performing the experiment and completing the worksheet, while the second lesson was dedicated to the completion of the student questionnaire, the discussion of the results and reflection.Students worked in groups of three to five people (seven groups per class, giving a total of 21 groups). The students were free to choose the partners they wished to work with. After introducing the problem, every student received a copy of the worksheet, which they filled in during the activity in their group. At the start of the activity, every group was given some horn salt in a paper sachet. Students could ask for further equipment or materials they needed for their experiment from the teacher. Each group was observed by a pre-service teacher who did not assist the students or interfere in their work in any way but evaluated their progress using the rubric method. The in-service teacher also monitored the groups and helped the students by asking questions if they could not move on their own, but did not provide explicit answers or solutions. At the end of the first period, every group submitted a completed worksheet. During the second period, which took place the following week, the solutions of the groups were discussed and evaluated, reflecting on the nature of the inquiry.
Training of teachers and observers
Before starting the programme, the participating in-service teachers were introduced to the aims of the study, reviewed their tasks and responsibilities, and discussed the learning goals, the steps of the inquiry activity, the lesson plan, and the timing to ensure that every class would receive the same instructions and support. Teachers were asked to guide students with questions, but they were not allowed to give the correct solutions at any stage of the inquiry until the whole process had been completed. Training sessions were held for the pre-service chemistry teachers who would observe the students to ensure the consistent use of the rubric and the uniform interpretation of the criteria. Participants went over the aims and the possible solutions of the inquiry activity and received detailed guidance on the method of monitoring the work and using the rubric. Levels of inquiry-skills development were discussed and interpreted under the guidance of the research supervisors. For each skill, examples – situations described by the research leaders – were used to practise evaluating student activities according to the levels of the rubric. Where there was a difference of opinion, a common interpretation was agreed upon.Methods
To explore and analyse the learning process, we used the case study method (Hamilton and Corbett-Whittier, 2013; Yin, 2018) to evaluate the implementation of the guided inquiry from multiple perspectives (experts, pre-service teachers as observers, and learners). Our study followed a mixed-methods research design (Tashakkori and Teddlie, 2003) using numerous methods (content analysis, rubrics, and student questionnaires). The research involved a sample of 9th-grade students (N = 88, Mage = 15.43, SD = 0.71; 33.0% male, 67.0% female) from three classes in a secondary school in Hungary. At the time of the experiment, the students from every class had had the same number of chemistry-class periods and had covered the same content knowledge. They were all following the usual chemistry curriculum with two class periods per week, primarily conducted using the traditional, teacher-centred method of instruction.Data collection proceeded by observing the current ethical requirements. Parents’ or caregivers’ written permission was obtained for the observations. The questionnaire was filled in voluntarily and anonymously, but each responder's group was indicated to allow us to link the answers with the worksheet and the group rubric data.
Data collection and analysis
Both qualitative and quantitative data were used to answer the research questions. Before the inquiry-based session, students' mid-term chemistry grades and their scores on the Lawson Classroom Test of Scientific Reasoning (LCTSR; Lawson, 2000) were collected to characterise the sample. During the inquiry activity, qualitative observations were made to monitor students’ inquiry skills and thinking through analysis of the content of the student worksheets and the assessment rubric. Student attitudes towards the activity and self-evaluations were measured using a paper-and-pencil questionnaire.| Criterion | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|
| RU1: Interpretation of the problem | The problem is not understood. Teacher's help is needed to identify the material | The problem is mostly understood. Teacher's help is needed to identify the material and write the formula | The problem is understood. The material is identified but the teacher's help is needed to write the formula | The problem is understood. The material is successfully identified, and the formula is written correctly |
| RU2: Observation of properties | No observations are made | Observations are incomplete, only one property of the material is noted | Observations are incomplete, only some properties of the material are noted | Observations are complete, only material properties observable through the senses are noted |
| RU3: Formulating hypotheses | No specific hypothesis is formulated | A hypothesis is formulated but no justification is given | A hypothesis is formulated and justified using personal experiences | A hypothesis is formulated, and a scientific justification is given |
| RU4: Experimental design | No experiment is designed without the teacher's help | An experiment is suggested but it is not suitable for the testing of the hypothesis | The experiment is suitable for the testing of the hypothesis, but the description of the procedures is incomplete | The experiment is suitable for the testing of the hypothesis and every step of the procedure is described |
| RU5: Use of equipment | Choice of equipment is arbitrary; teacher's assistance is needed | Equipment is chosen via trial and error, there is uncertainty as to the functions of the tools, the teacher's help is needed | Most of the equipment is chosen correctly and the functions of most tools are known but some omissions occur | All equipment is known and chosen correctly |
| RU6: Execution of experiment | The equipment is not used appropriately, execution is unprofessional, and safety measures are only observed when prompted | There is some uncertainty about the use of equipment and procedures, the teacher's help is needed for their execution | The equipment is used essentially correctly, procedures are implemented, and only minor teacher assistance is needed | Equipment is used correctly, procedures are implemented, and safety measures are observed without fail |
| RU7: Recording of observations | Records are incomplete and the language is inadequate. Teacher's help is needed | The records are partially correct. Only minor assistance is needed | Experiences are recorded without help but some omissions remain | Every relevant experience is appropriately recorded |
| RU8: Analysis of experiences | The chemical processes behind the observed phenomenon are unfamiliar, there is no sign of understanding why things happened | There is a partial understanding of the chemical processes behind the observed phenomenon. Knowledge is uncertain | There is an advanced understanding of the chemical processes behind the observed phenomenon, but the teacher's help is needed to discover cause-and-effect relationships | There is a full understanding of the chemical processes, and cause-and-effect relationships are identified without help |
| RU9: Conclusions | No conclusions are drawn without the teacher's help | The conclusions are incomplete; the teacher's help is needed for interpretation of the evidence | The conclusions are essentially correct. Only minor assistance is needed in linking the evidence to the hypothesis | Scientifically correct conclusions are drawn. The evidence is used to evaluate the hypothesis |
| RU10: Cooperation | Not all members participate in the group work. Cooperation is intermittent | The members participate in the work with varying intensity but continuously. Ideas are discussed sporadically | Participation in the work is constant but the tasks are distributed unevenly | The members contribute to the work continuously and efficiently. The work is distributed evenly |
| RU11: Communication | Communication is difficult, there is no discussion. Scientific terminology is not used or is used incorrectly | Communication is a little difficult, there is little discussion. Scientific terminology is used uncertainly | Communication is quite good, there is a fair amount of discussion. Scientific terminology is used properly | Communication is smooth, and all ideas are discussed. Scientific terminology is used with precision |
The observers did not assist the work of the observed group, they only intervened in the case of a risk of accident. The data collected using the rubric were scored by assigning numerical values to the levels (Level 1 = 1, Level 2 = 2, Level 3 = 3, Level 4 = 4) and internal consistency was assessed, which proved to be acceptable (Cronbach's α = 0.834). The qualitative analysis of the student worksheets examined the activities and performance of the groups, and the level of inquiry skills was rated based on the rubric.
The data collected by the observers using the rubric method add valuable information to the data obtained via analysis of the worksheets since the former characterises the events observed during the activity. The data reveal to what extent the groups needed to rely on the help of the teacher at the different stages of the activity, how efficient the members were in distributing the tasks, and to what extent they were successful in cooperating and communicating.
Results
Overall assessment of student worksheets
Students first had to characterise horn salt: they were asked to give its scientific name, write the formula, and describe the observable physical properties of the material. Students found the first of the three tasks to be the easiest since we had chosen a brand of horn salt that had the scientific name written on the sachet. Nineteen groups (90%) found this information and gave the correct answer, while the remaining two groups did not answer the question. Constructing the chemical formula was more challenging for the students, but still 11 groups (52%) were able to provide the correct solution (NH4HCO3), and three other groups (14%) got at least one of the polyatomic ions right, which indicates that most students were able to link the name of the compound and its symbolic representation. Looking at the scientific name and formula of the compound, the students were able to deduce the crystal type of the material, which could help them to predict the chemical properties of the substance. The observable physical properties of the material were described in varying levels of detail. Ten groups (48%) noted the characteristic smell and two of those (10%) recognised it as ammonia. The most frequently recorded property was the material's solid state (14 groups, 67%). Six groups (29%) gave no answer. The next task was the formulation of a hypothesis. The hypotheses were evaluated with reference to their scientific plausibility and their testability. A hypothesis was judged to be correct if it referred to the transformation of the material and explained why the sachet appeared to be empty. Most hypotheses (19 hypotheses, 90%) were testable in a school laboratory regardless of the scientific plausibility. Only two groups (10%) formulated hypotheses that were untestable, and these were also scientifically implausible (for example “because the ammonium consumes the carbonate”, Group 1; and “because the carbonate ion combined with oxygen to form carbon dioxide”, Group 5). Thirteen groups (62%) explained the transformation of the matter or the causes of the transformation but did not mention why the sachet appeared to be empty (“Because the substance sublimated due to the heat”, Group 17; and “Because it was kept in a warm and damp environment”, Group 11.) These hypotheses were categorised as incomplete. Two groups (10%) formulated a hypothesis that included both considerations (e.g., “Because it is a leavening agent. After a while the bag expanded and it diffused out as a gas”, Group 21; and “Due to heat it has undergone a chemical change that caused the substance to turn into a liquid or gas, which then leaked out of the bag”, Group 7). Four groups (19%) referred to the storage conditions in their hypothesis (e.g., “It was not stored in a dry and cool place”, Group 9). The most frequent explanations were the following: decomposition, sublimation, reaction with humidity in the air and reaction with oxygen in the air.Students were also asked to explain how they arrived at their hypotheses. The most common explanations (9 groups, 43%) were based on the instructions written on the packaging of the horn salt: “Store in a cool dry place.” This is of course an acceptable explanation both for decomposition and sublimation since these processes occur at a faster rate at higher temperatures. It is not a particularly valuable answer, however, since it requires no additional knowledge. Three groups (14%) noted that horn salt was used as a leavening agent in cakes, which could support the hypothesis that it decomposed or sublimated. Only two groups (10%) argued that the material gave out a pungent smell, which could have been the result of decomposition, even though ten of the groups had noted the characteristic smell in their answer to the previous question. One of the groups gave the creative answer that the material obeyed the principle of minimum energy.
The next task was to design an experiment to test the hypotheses. The groups designed relatively simple test-tube experiments. Most of the groups (17 groups, 81%) simply heated the horn salt in a test tube. The remaining groups added some water to the horn salt before heating it. Four groups also planned to identify one of the products: two groups tested for ammonia using universal indicator paper and the other two groups tested for carbon dioxide with a lit wooden stick. Nine of the groups (43%), planned an experiment that did not provide suitable data to test their hypotheses (e.g., Group 1 formulated the hypothesis that “the ammonium consumes the carbonate” and planned an experiment during which they heated the horn salt in a test tube).
Most students successfully listed the materials and equipment for their experiment. Every group requested a spirit burner and test tubes, but some groups forgot to ask for test-tube holders and racks, cloths, or trays. Since not having these at hand could have increased the risk of accidents (e.g., injuries when holding the test tube over the burner), the teacher drew the students’ attention to these tools when distributing the equipment on the lists. The students were not allowed to modify their lists at that stage. We also identified some uncertainties with naming the equipment (candle for spirit burner, dish for beaker, tongs for test-tube holder).
Before performing the experiment, the groups had to write down what outcome they predicted if their hypothesis were true. The expected outcome matched the hypothesis for ten of the groups (48%). Fifteen groups (71%) gave a version of one of the following responses: “the horn salt will disappear/sublimate/decompose/evaporate”. Evaporation and sublimation are factually incorrect. Although students may experience that the horn salt disappears, this observation alone will not allow them to decide whether the phenomenon is sublimation or decomposition, since both processes result in the solid horn salt being dispelled from the test tube. If a group wants to test a hypothesis of sublimation, the correct prediction is that the horn salt in the test tube first turns into a gas and is then deposited again on the cold part of the tube. None of the groups derived this prediction. The groups that hypothesised decomposition had to observe compounds different from horn salt emerging from the reaction even if they could not identify those compounds. Five groups (24%) expected to detect one of the products of the decomposition.
When heating the horn salt in the test tube, the students could observe countless phenomena. The most common of these was that the quantity of solid material was gradually reduced until, with persistent heating, it eventually disappeared from the test tube. The pungent smell of the released ammonia and the drops of water deposited on the wall of the test tube could also be observed. Ten of the groups (48%) recorded every important piece of information. The appearance of water was most likely to be noted (11 groups, 52%), while ammonia was recorded by 48% of the groups. Some of them noted the characteristic pungent smell of the substance, while others saw the blue colouration of the universal indicator paper if they held it to the mouth of the test tube. The carbon dioxide was identified by four (19%) of the groups with the help of a lit wooden stick.
Several students found it quite difficult to draw their conclusions. Five groups (24%) simply repeated their experiences (e.g., “H2O and CO2were produced and a gas that stinks. An alkaline gas of pH 8 was produced, which we think is ammonia”, Group 5) but did not deduce the type of change. Ten groups (48%) arrived at incomplete or inaccurate conclusions (e.g., “The resulting substance is alkaline. Heat really transforms this substance”, Group 2). Three of these continued to argue that what they had witnessed was sublimation even though the evidence contradicted this explanation (e.g., “Sublimation has taken place. Horn salt contains water (because it has condensed)”, Group 20). Only six groups (29%) arrived at correct conclusions (e.g., “Carbon dioxide was produced because the gas did not fuel the combustion. Horn salt did decompose because more than one substance was produced”, Group 3).
Finally, in light of the results of the experiment, the students had to evaluate their initial hypotheses. Seven groups (33%) correctly accepted or rejected their hypotheses and supported their decision with evidence (e.g., Group 10 hypothesised that “Water got into the bag somehow”. Then they concluded that “The hypothesis does not match the experimental results because nothing happened to the horn salt if we held the bag above boiling water”.). Three groups (14%) believed that their hypotheses had been corroborated even though their observations did not support that claim (e.g., Group 19 hypothesised that “The horn salt is not in the bag, because it has evaporated. It was not kept in a dry and cool place, so the leavening agent evaporated, and the powder disappeared”. They heated the salt in a test tube. After their experiment, they concluded that “The hypothesis is consistent with the experimental results because water, boiler scale and NH3gas were produced. The horn salt disappeared, as it did from the bag when it was stored in a too warm place”.). These students failed to realise that the formation of new products indicates a chemical reaction, not a physical change.
Interestingly, nine groups accepted their hypotheses because they were able to reproduce the phenomenon (e.g., Group 11 hypothesised that “The horn salt is not in the bag because it was held in damp and warm conditions”. They added water to the salt and heated the mixture. They concluded that “The horn salt disappears due to heating, so we got what we had expected, therefore our hypothesis was correct”). These students might have misunderstood the question and wanted to find out why the salt disappeared, not what happened to it. Two groups (10%) gave no answer to this question. We should note that out of the 21 groups, only three rejected their hypotheses even though the teacher had repeatedly assured the students that refuting an incorrect hypothesis could be a perfect solution. The students, however, might have wished to prove that they had been correct from the beginning, even if the evidence contradicted this conclusion.
The distribution of the total scores received for the worksheet indicates that the performance of the groups varies greatly (min. 9.52%, max. 85.71%, M = 53.05%, SD = 19.08%). The performance of the seven groups successfully completing the inquiry (M = 65.33%, SD = 12.86%) was significantly better (t = 4.881, p < 0.001) than the performance of the groups that encountered obstacles or made mistakes (M = 47.17%, SD = 19.02%).
Solution pathways evident in the worksheets
The reasoning processes of the groups are summarised in Tables 2–5. The students’ hypotheses were grouped into four major categories based on the change they hypothesised: (1) change in aggregation state, (2) decomposition, (3) reaction with water and (4) other reactions. Since subsequent stages of the activity are determined by the hypothesis, the groups were categorised (I–IV) essentially through the type of their hypothesis. To facilitate the tracking of their progress, Tables 2–5 show the groups of students as a function of the hypothesis category. Since we wished to identify and characterise the different paths in the students’ reasoning, we also examined the solutions of the groups in terms of the four categories.| Inquiry stage | Solution (Category I) |
|---|---|
| IS1: Hypothesis | Change of aggregation state |
| Groups 2, 4, 7, 8, 13, 17, 19, 20, 21 |
| There is no justification | Based on erroneous information | Relies on the given information | Relies on everyday information | Relies on scientific knowledge | |
|---|---|---|---|---|---|
| IS2: Justification of hypothesis | 7, 13, 20, 21 | — | 4, 8, 19 | 2 | 17 |
| Heating | Heating and cooling | Heating and identifying compounds | Heating and adding water | |
|---|---|---|---|---|
| IS3: Experimental design | 13, 17, 19, 20, 21 | 2 | — | 4, 7, 8 |
| Inadequate or uninterpretable | The horn salt disappears | Change of aggregation state | The emergence of new material | |
|---|---|---|---|---|
| IS4: Predictions | 8 | 2, 4, 13, 17, 19 | 7, 20, 21 | — |
| Ambiguous | Reference to one product | Reference to two products | Reference to three products | |
|---|---|---|---|---|
| IS5: Record of experiences | 7 | 8, 13, 20 | 2, 4, 17, 19, 21 | — |
| Inadequate or uninterpretable | Repetition of experiences | Conceptual mistake | Correct but incomplete | Correct (decomposition) | |
|---|---|---|---|---|---|
| IS6: Conclusions | 7 | 2, 4 | 17, 19, 20, 21 | — | 8, 13 |
| No answer | The hypothesis is accepted because the material disappeared, but the evidence is not interpreted | The hypothesis is accepted despite contradictory evidence | The hypothesis is rejected based on evidence refuting it | The hypothesis is accepted based on supporting evidence | |
|---|---|---|---|---|---|
| IS7: Evaluation of hypothesis | — | 2, 4, 7, 20, 21 | 17, 19 | 8, 13 | — |
| Inquiry stage | Solution (Category II) |
|---|---|
| IS1: Hypothesis | Decomposition |
| Groups 3, 12, 14, 15, 16, 18 |
| There is no justification | Based on erroneous information | Relies on the given information | Relies on everyday information | Relies on scientific knowledge | |
|---|---|---|---|---|---|
| IS2: Justification of hypothesis | 14, 18 | — | 15 | 12 | 3, 16 |
| Heating | Heating and cooling | Heating and identifying compounds | Heating and adding water | |
|---|---|---|---|---|
| IS3: Experimental design | 3, 12, 15 | 18 | 14, 16 | — |
| Inadequate or uninterpretable | The horn salt disappears | Change of aggregation state | The emergence of new material | |
|---|---|---|---|---|
| IS4: Predictions | 15 | 12 | 18 | 3, 14, 16 |
| Ambiguous | Reference to one product | Reference to two products | Reference to three products | |
|---|---|---|---|---|
| IS5: Record of experiences | 18 | — | 12 | 3, 14, 15, 16 |
| Inadequate or uninterpretable | Repetition of experiences | Conceptual mistake | Correct but incomplete | Correct (decomposition) | |
|---|---|---|---|---|---|
| IS6: Conclusions | — | — | — | 14, 16 | 3, 12, 15, 18 |
| No answer | The hypothesis is accepted because the material disappeared, but the evidence is not interpreted | The hypothesis is accepted despite contradictory evidence | The hypothesis is rejected based on evidence refuting it | The hypothesis is accepted based on supporting evidence | |
|---|---|---|---|---|---|
| IS7: Evaluation of hypothesis | — | 12, 18 | — | — | 3, 14, 15, 16 |
| Inquiry stage | Solution (Category III) |
|---|---|
| IS1: Hypothesis | Reaction with water |
| Groups 6, 9, 10, 11 |
| There is no justification | Based on erroneous information | Relies on the given information | Relies on everyday information | Relies on scientific knowledge | |
|---|---|---|---|---|---|
| IS2: Justification of hypothesis | — | 6 | 9, 10, 11 | — | — |
| Heating | Heating and cooling | Heating and identifying compounds | Heating and adding water | |
|---|---|---|---|---|
| IS3: Experimental design | — | — | — | 6, 9, 10, 11 |
| Inadequate or uninterpretable | The horn salt disappears | Change of aggregation state | The emergence of new material | |
|---|---|---|---|---|
| IS4: Predictions | 10, 11 | 6, 9 | — | — |
| Ambiguous | Reference to one product | Reference to two products | Reference to three products | |
|---|---|---|---|---|
| IS5: Record of experiences | 11 | 6, 9 | 10 | — |
| Inadequate or uninterpretable | Repetition of experiences | Conceptual mistake | Correct but incomplete | Correct (decomposition) | |
|---|---|---|---|---|---|
| IS6: Conclusions | 11 | 6 | — | 9 | 10 |
| No answer | The hypothesis is accepted because the material disappeared, but the evidence is not interpreted | The hypothesis is accepted despite contradictory evidence | The hypothesis is rejected based on evidence refuting it | The hypothesis is accepted based on supporting evidence | |
|---|---|---|---|---|---|
| IS7: Evaluation of hypothesis | 6 | 9, 11 | — | 10 | — |
| Inquiry stage | Solution (Category IV) |
|---|---|
| IS1: Hypothesis | Other reaction |
| Groups 1, 5 |
| There is no justification | Based on erroneous information | Relies on the given information | Relies on everyday information | Relies on scientific knowledge | |
|---|---|---|---|---|---|
| IS2: Justification of hypothesis | 5 | 1 | — | — | — |
| Heating | Heating and cooling | Heating and identifying compounds | Heating and adding water | |
|---|---|---|---|---|
| IS3: Experimental design | 1 | — | 5 | — |
| Inadequate or uninterpretable | The horn salt disappears | Change of aggregation state | The emergence of new material | |
|---|---|---|---|---|
| IS4: Predictions | 1 | — | — | 5 |
| Ambiguous | Reference to one product | Reference to two products | Reference to three products | |
|---|---|---|---|---|
| IS5: Record of experiences | — | 1 | — | 5 |
| Inadequate or uninterpretable | Repetition of experiences | Conceptual mistake | Correct but incomplete | Correct (decomposition) | |
|---|---|---|---|---|---|
| IS6: Conclusions | 1 | 5 | — | — | — |
| No answer | The hypothesis is accepted because the material disappeared, but the evidence is not interpreted | The hypothesis is accepted despite contradictory evidence | The hypothesis is rejected based on evidence refuting it | The hypothesis is accepted based on supporting evidence | |
|---|---|---|---|---|---|
| IS7: Evaluation of hypothesis | 1 | — | 5 | — | — |
Category I: The change of aggregation state was the most frequent hypothesis (Table 2, IS1), nine groups began their journey on this path (Groups 2, 4, 7, 8, 13, 17, 19, 20, 21). One of these groups (Group 17) relied on their prior knowledge of chemistry (“Sublimation: solid → gas, the initial material is solid, one half of the definition is fulfilled, based on what the packing says the substance should be stored in a cold place, [since it can sublimate]”, Group 17); another one (Group 2) relied on everyday knowledge (“Cakes and other materials expand when they are hot and shrink when they are cold”); and three (Groups 4, 8, 19) on the warning found on the packaging that the product should be stored in a cool dry place. The remaining groups in this category (Groups 7, 13, 20, 21) did not justify their hypotheses (IS2). The groups designed simple experiments (IS3). Most of them heated the horn salt in a test tube (Groups 13, 17, 19, 20, 21); one group (Group 2) followed the heating stage with a cooling stage; and three groups (Groups 4, 7, 8) added water. This latter idea was presumably prompted by the instruction on the packaging (“Store in a cool dry place”) but it led them in the wrong direction because once the horn salt has been dissolved in water, changes are difficult to observe. To the question of what the students expected from the experiment, five groups (Groups 2, 4, 13, 17, 19) wrote only that the horn salt would disappear. However, it is unclear if they meant that the substance disappeared from view or completely decomposed. Three groups (Groups 7, 20, 21) predicted a change in aggregation state (IS4). One group (Group 8) gave an uninterpretable answer. When recording their experiences (IS5), all but one group identified some material distinct from horn salt. Three groups (Groups 8, 13, 20) mentioned one new material and five groups (Groups 2, 4, 17, 19, 21) mentioned two. Water and ammonia were the easiest to recognise: the former from the vapour deposited on the wall of the test tube and the latter from its pungent smell. Carbon dioxide is a colourless and odourless gas and could only be identified by the groups that, using the name of the material as their clue, predicted its formation and made plans to reveal its presence. Only two groups (Groups 8, 13) managed to draw appropriate conclusions from their observations (IS6). They realised that what happened was not a change of aggregation state but decomposition. Four groups made some factual errors. Group 17 identified ammonia based on its pungent smell but misidentified the liquid deposited on the wall of the test tube as lime water. Group 19 reasoned that “the bonds between the horn salt molecules broke up at a high temperature”, which suggests that they understand what happens at the molecular level but are not aware that the substance is made of ions. Group 20 noted the water on the wall of the test tube but believed that the water of crystallisation had been expelled and the remaining matter sublimated. They did not note the pungent smell of ammonia during their observations and forgot that ionic crystals were unlikely to sublimate under those circumstances and the anhydrous salt should have stayed in the test tube. Group 21 concluded that the horn salt had turned into a gas because the pungent smell (that they detected when they opened the sachet before executing the experiment) spread. They identified the water during their experiment and observed a gas with a pungent odour that produced an alkaline pH value when dissolved in water (ammonia). They concluded that the smell must be given out by the horn salt, forgetting that ionic compounds are not volatile and are therefore odourless. Two groups (Groups 2 and 4) repeated their experiences instead of drawing a conclusion, and one group (Group 7) gave an answer that was uninterpretable. The step of hypothesis evaluation was successfully completed by the groups who had drawn the correct conclusion (Groups 8 and 13, IS7). They rejected their initial hypotheses of a change of aggregation state and thus completed the inquiry with success. The remaining groups accepted their hypothesis of a change of aggregation state even though the results of their experiments refuted it.
Category II: The second most frequent hypothesis was a factually correct one, namely that the horn salt decomposed (Table 3, IS1). Six groups (Groups 3, 12, 14, 15, 16, 18) formulated this hypothesis but none of them explained where the products disappeared (IS2). Three groups (Groups 3, 14, 16) relied on their knowledge of chemistry in formulating their hypothesis; one group (Group 12) relied on the everyday experience that there is an unpleasant smell of ammonia when baking cakes; one group (Group 15) relied on the information provided on the packaging; and one group (Group 18) gave no explanation. All of these groups decided to heat the horn salt (IS3). Group 18 included a step of cooling after the procedure of heating as well. When making their predictions (IS4), three groups (Groups 3, 14, 16) alluded to the formation of new materials. Group 12 wrote that the material would disappear but did not explain it further. Group 18 expected sublimation, which was in contradiction with their own hypothesis. The experiment they designed, and their predictions suggest that some students confuse a change of aggregation state with decomposition. Group 15 recorded their observations instead of their predictions (“There is water in the test tube, then we bubble CO2through it. Water condenses on the wall of the tube with a sidearm and the formed gas is alkaline”). Looking at the records of observations (IS5), four groups (Groups 3, 14, 15, 16) mentioned all three products. Group 12 identified water and carbon dioxide. Group 18 only wrote that material was deposited on the wall of the test tube but gave no further description of it. In their conclusions (IS6), four groups (Groups 3, 12, 15, 18) mentioned decomposition and two groups (Groups 14 and 16) identified the products based on their observed properties but did not refer to the type of change. All groups were satisfied that their evidence supported their hypothesis (IS7). Groups 12 and 18, however, wrote that the material disappeared from the test tube. We can be certain that Group 18 rejected the idea of the change of aggregation state since they clearly indicated decomposition in their conclusions.
Category III: The hypothesis in this category was that the substance reacts with water (Table 4, IS1). Four groups chose this hypothesis (Groups 6, 9, 10, 11). Three of them (Groups 9, 10, 11) relied on the information given on the packaging, and one group (Group 6) made an erroneous claim (“aluminium (sic!) retains heat”, IS2). All four groups designed an experiment where the material had to be heated (IS3). Given their hypothesis, this procedure is not logical, however. It was presumably prompted by the instruction regarding storage. Three of the groups (Groups 6, 9, 11) dissolved the material in water and then heated it, which is not ideal, since water makes it more difficult to observe the change. Group 10 first held the sachet above water vapour to find out whether it would become damp, and when no such event occurred, they performed another experiment where the pure material was heated. Two of the groups (Groups 6 and 9) expected the horn salt to disappear (IS4). The other two groups (Groups 10 and 11) gave no interpretable answer (“horn salt in the bag” and “true” respectively). As regards their observations (IS5), three groups mentioned the formation of new materials. Groups 6 and 9 observed one new material, while Group 10 noted two. Group 11 simply wrote that the horn salt disappeared. It was difficult for these groups to draw conclusions (IS6). Only Group 10 concluded that the material decomposed because of heating and their original hypothesis turned out to be incorrect. Although Group 9 delineated the identity of the products from the name of the material and observed the appearance of water vapour, they did not mention decomposition in their conclusions. Group 6 reported their observations again and Group 11 wrote “It disappears due to heating”. Finally, when testing the hypothesis (IS7), only Group 10 realised that their original hypothesis was incorrect and thus completed the inquiry successfully. Two groups (Groups 9 and 11) kept their original hypothesis, arguing that the material did disappear, even though the evidence suggested otherwise. Group 6 gave no response.
Category IV: Two groups (Groups 1 and 5) fell into this category, where the hypothesis was some sort of chemical reaction but different from that in other categories (Table 5, IS1). Group 1 thought that “ammonia consumes the hydrogen carbonate,” which they explained by the mildly corrosive effect of ammonia. Group 5 believed that “carbonate ion combined with oxygen and carbon dioxide is produced” but gave no explanation for this hypothesis (IS2). These ideas are factually incorrect and cannot be tested under classroom conditions. The reasoning of Group 1 may be rooted in misconceptions regarding the conservation of mass. Group 5 made no mention of what would happen to other components of the compound. The experiment designed (IS3) by Group 5 involved the heating of the material and the identification of the carbon dioxide thus formed. Group 1 also planned to heat the horn salt, which did not match their hypothesis. They presumably did not have any ideas and copied the design from their peers. Group 5 predicted the disappearance of the horn salt and the formation of carbon dioxide. Group 1 made no predictions (IS4). Group 5 mentioned all three products when recording their observations (IS5). Group 1 only identified the water. Neither group succeeded in drawing appropriate conclusions from their experiences (IS6). Group 5 repeated their observations and accepted their original hypothesis even though materials other than carbon dioxide were also formed. Group 1 arrived at the mistaken conclusion that the material sublimated, and they did not evaluate their hypothesis (IS7).
In summary, we can conclude that seven groups (Groups 3, 14, 15, 16, 8, 10, 13) successfully completed the task. Four of them (Groups 3, 14, 15, 16) formulated a factually correct initial hypothesis and then accepted it based on the results of their experiments. Three groups (Groups 8, 10, 13) started with a factually incorrect but testable hypothesis and designed an appropriate experiment, the results of which led them to the correct conclusion that their hypothesis was mistaken.
Analysis of inquiry skills based on the assessment rubric
The mean scores of the groups for each criterion of the assessment rubric are shown in Fig. 1. The results complement the data collected with the student worksheet by showing when the students needed more support, how they handled the laboratory equipment, how well they worked together as a group and how successful they were at communicating ideas using the subject-specific terminology. There is a moderate positive correlation between the total score of the worksheets and the rubric data (Spearman's r = 0.59, p = 0.005).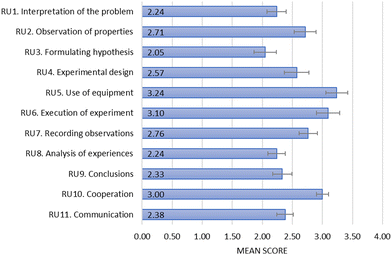 | ||
| Fig. 1 Mean performance of the groups on the inquiry skills, cooperation and communication based on the data collected using the rubric. | ||
Students performed the best on the use of equipment (RU5) and the execution of experiment (RU6) criteria, indicating that most of them handled the laboratory equipment appropriately and needed only minor teacher assistance. The lowest scores were recorded for the formulating hypothesis criterion (RU3) supporting what we found with the student worksheets that this was the most challenging step for the students during the inquiry. The rubric allowed us to obtain information about the cooperation (RU10) and communication (RU11) within the groups as well. The mean level of cooperation (RU10) was 3, meaning that most students participated in the group work, but they did not take an equal share of the task. The mean scores on the communication (RU11) criterion are closer to the level of 2, indicating that students had difficulty with communicating their ideas using the subject-specific terminology correctly in most of the groups.
Analysis of students’ attitudes
The anonymous questionnaire was completed by 78 students (response rate: 89%). The questionnaire comprised items about the students’ opinions on the inquiry activity, the steps of the inquiry, and their experience of working in groups. The data were analysed by comparing the ratings of two subsamples that were defined based on their performance indicated by the worksheets (those who completed the task successfully and those who did not, see Tables 2–5).The difference between the two subsamples was analysed using Independent Samples t-tests, and effect sizes are given in Hedges’ g data (see Table 6). Successful problem solvers were more likely to report that they had sufficient knowledge for the successful completion of the inquiry task than were unsuccessful participants (S3). This difference is statistically significant with a large effect size (Hedges’ g = 0.75). The laboratory equipment was familiar to both groups but neither group found the preparatory student experiments useful for solving the inquiry problem. Regarding the steps of the inquiry, the students in both groups found the formulation of the hypothesis the most challenging, and the use of the laboratory equipment the most straightforward. Although successful problem solvers reported higher scores on items regarding group work (S11–S14) the differences are not statistically significant. Overall, the students believed that the members in their group listened to what they had to say, and most students participated in the activity with pleasure and found group work efficient.
| Statement | Successful problem solvers | Unsuccessful participants | t | df | p | g | ||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | |||||
| a T-test t(df), probability (p) value and Hedges’ effect size (g) of the difference between the mean Likert-scale ratings of students successfully solving the inquiry problem (N = 23) and students who failed to solve the inquiry problem (N = 55). Statements for which there is a significant difference between the two groups are in italic type. | ||||||||
| S1: The activity was novel to me | 4.00 | 1.24 | 4.05 | 1.18 | −0.184 | 76 | 0.855 | −0.05 |
| S2: I was pleased to participate in the activity | 4.74 | 0.45 | 4.60 | 0.71 | 0.868 | 76 | 0.388 | 0.21 |
| S3: My prior knowledge was sufficient for solving the problem | 3.87 | 0.76 | 3.22 | 0.94 | 3.179 | 51 | 0.002 | 0.75 |
| S4: I understood the task | 4.61 | 0.58 | 4.24 | 0.84 | 1.900 | 75 | 0.061 | 0.47 |
| S5: I found the task easy | 3.39 | 0.94 | 3.13 | 0.88 | 1.157 | 74 | 0.251 | 0.29 |
| S6: Formulating a hypothesis was easy | 3.30 | 0.93 | 3.20 | 1.06 | 0.410 | 76 | 0.683 | 0.10 |
| S7: Designing the experiment was easy | 3.96 | 0.88 | 3.89 | 0.63 | 0.325 | 31 | 0.747 | 0.08 |
| S8: I was familiar with the experimental equipment | 4.65 | 0.71 | 4.71 | 0.71 | −0.322 | 76 | 0.749 | −0.08 |
| S9: I knew how to use the experimental equipment appropriately | 4.65 | 0.65 | 4.61 | 0.79 | 0.220 | 75 | 0.826 | 0.05 |
| S10: It was easy to draw conclusions based on the experiment | 3.96 | 1.11 | 3.66 | 1.04 | 1.147 | 76 | 0.255 | 0.28 |
| S11: I enjoyed working in my group | 4.87 | 0.34 | 4.56 | 0.92 | 2.138 | 75 | 0.036 | 0.44 |
| S12: The group listened to what I had to say | 4.65 | 0.57 | 4.55 | 0.88 | 0.536 | 76 | 0.593 | 0.13 |
| S13: My group worked efficiently | 4.30 | 1.02 | 3.98 | 1.19 | 1.133 | 76 | 0.261 | 0.28 |
| S14: Everyone had their equal share of the work in my group | 4.04 | 1.07 | 3.71 | 1.26 | 1.118 | 76 | 0.267 | 0.28 |
| S15: There was enough time to solve the problem | 3.56 | 1.41 | 3.48 | 1.11 | 0.253 | 34 | 0.801 | 0.07 |
| S16: I participated in activities with a similar structure before | 3.61 | 1.12 | 3.25 | 1.14 | 1.257 | 76 | 0.213 | 0.31 |
| S17: The student experiments we had conducted before (heating of hydrated copper sulfate, decomposition of ammonium chloride, blowing air into lime water, sublimation of iodine) helped me solve the problem | 2.27 | 0.93 | 2.18 | 0.91 | 0.395 | 75 | 0.694 | 0.10 |
The student questionnaire also helped us to identify the possible causes of any lack of success. The two groups with the poorest performance as indicated by the worksheets (Groups 1 and 6) appear to have different difficulties. The students in Group 1 all agreed that their group did not work efficiently, they could not distribute the tasks, and there was an unpleasant atmosphere and too little time. The members of Group 6 thought that there was not enough time, and the task was relatively difficult, but they enjoyed the activity and the atmosphere. The difference between these two groups is also reflected by the cooperation section of the assessment rubric.
Relationships between success in solving the problem, chemistry grades and LCTSR scores
The students in the sample mostly had chemistry grades of 4 and 3 (M = 3.66, SD = 0.88) on the five-point scale of the Hungarian grading system, where 1 is a failure and 5 indicates an excellent performance. As there was no significant correlation between the chemistry grades and the scores received for the worksheets (r(87) = 0.21, p = 0.054), the grades did not predict successful problem-solving. There is only one significant correlation with the statements of the student questionnaire: students with a higher grade in chemistry enjoyed participating in the activity more, r(72) = 0.36, p = 0.001.Our 9th-grade students achieved similar LCTSR scores (M = 48.05%, SD = 18.40%) to those reported in other studies (Bao et al., 2009). The results of the scientific-reasoning test did not show a significant relationship with the success of guided inquiry based on the results of the student worksheets, r(78) = −0.15, p = 0.19.
When examining the relationship with the student questionnaire, we found that in the subsample of successful problem solvers, the LCTSR test scores correlate with only one of the variables in the student questionnaire: there is a significant negative correlation with the agreement with the statement S14 (“Everyone had their equal share of the work in my group”), r(17) = −0.54, p = 0.018. Among unsuccessful participants, there was no significant correlation between the LCTSR results and the variables of the student questionnaire.
Discussion
In the present study, we examined how 9th-grade students solve a chemistry problem using guided inquiry. The solution required the application of both their prior knowledge of chemistry (e.g., properties of ionic compounds, chemical reactions, law of conservation of matter) and everyday experiences (baking powder in cakes raises the pastry). Our primary goal was to reveal the learning paths and identify the challenges. Secondly, we wanted to find out how students evaluated the activity and their own performance. Following a mixed-methods design, we collected qualitative data using student worksheets and quantitative data via rubrics and questionnaires. The results are summarised in the order of the research questions.RQ1. Based on students’ hypotheses we identified four solution pathways. Pathway II (decomposition because of heating and the production of ammonia, water vapour and carbon dioxide) is accurate both from a factual and a research methodological point of view. Pathways I (change of aggregation state) and III (interaction with water) start with factually incorrect, but testable, hypotheses. Pathway IV (other, scientifically implausible interaction) contained untestable hypotheses that could not lead to successful problem-solving. There were also differences within each solution pathway, so five types of outcomes were identified: 1. The hypothesis was accepted based on supporting evidence (4 groups); 2. The hypothesis was rejected based on evidence refuting it (3 groups); 3. The hypothesis was accepted despite contradictory evidence (3 groups); 4. The hypothesis was accepted because the material disappeared, but the evidence was not interpreted (9 groups); 5. No solution (2 groups). We considered inquiry-based learning successful for both outcomes 1. and 2.
RQ2. Most groups used the available resources to find the scientific name of the horn salt, and more than half of them could construct its chemical formula. The rest of the students were not able to demonstrate representational skills, since they might have had problems with accessing the required conceptual information, visual information, and the connection between them (Wu et al., 2001). Knowing the scientific name and chemical formula of horn salt could help to identify the components of the substance and to recall the knowledge about ionic compounds, but it was not an essential requirement for the solution. We found the formulation of a hypothesis to be one of the most critical steps in the inquiry process. Those who formulated an untestable hypothesis had very little opportunity to learn from the activity. Shute and Glaser (1990) reached similar conclusions using Smithtown, an intelligent computer-based, guided-discovery tutoring system. Njoo and De Jong (1993) pointed out that students were more active and gained better scores on domain correctness if they were provided with the hypotheses. It is therefore important that instructors guide students towards testable hypotheses before they move to the next step in the inquiry cycle.
The formulation of a hypothesis is influenced by several factors, including how the learners interpret the task. In our study, several groups believed that the purpose of the experiment was to reproduce the phenomenon, i.e., to make the horn salt disappear. The task was to find out what caused the disappearance of the material, and what transformation must have taken place. Schauble et al. (1991) called this the engineering model of experimentation, in which students try to reach the desired outcome instead of finding out the causal relations (science model of experimentation). It is thus very important to clarify the goals at the start of an inquiry activity, as was also emphasised by Millar et al. (1994): “[…] the purpose of science investigating needs to be made clear to children, through explicit discussion and examples, rather than taken for granted.” (p. 245).
Students typically justified their hypotheses using the information available on the paper bag or via everyday experiences. However, two groups used their scientific observations: the pungent smell of the material, which may have been the result of decomposition.
Most groups designed a test-tube experiment in which the substance was heated. However, the experimental design was not always aligned with their hypothesis, and in several cases, they had to modify their list of materials and equipment as the work progressed.
The recording of observations was another critical step. Some groups overlooked important details (e.g., the formation of new substances such as ammonia and water, which is evidence of a chemical change). Several groups required help in analysing their observations and drawing conclusions because of insufficient prior knowledge or undeveloped reasoning skills. In some cases, the groups simply re-reported their observations and did not attempt to draw conclusions.
When evaluating the hypothesis, more than half of the groups disregarded the evidence or accepted their original hypotheses despite the observations that refuted them. This is in line with Dunbar's (1993) finding that students’ goals may limit their cognitive processes: if they aim to support their hypothesis, they tend to overlook confronting evidence, which leads to confirmation bias. Another explanation might be that students wanted to appear successful, wished to find a good solution and did not understand what counted as a successful inquiry. Students are often focused on achieving desired outcomes rather than on understanding causes and effects and making connections (Hmelo-Silver, 2006). It is therefore important to explain at the start of the activity that even if a plausible hypothesis turns out to be mistaken, valuable information has been gained. These were pointed out and clarified during the second lesson of the investigation when students were reflecting on the whole learning process.
RQ3. Students could not transfer what they had learned from the previous four structured experiments into the inquiry context for planning and conducting the experiments. However, some students recognized similarities between the horn salt experiment and the previous copper(II) sulfate pentahydrate experiment. They inferred that horn salt contained water of crystallisation since when heated it released water vapour. Although this is not the case, the idea itself is productive. Students applied their previous knowledge in a new context to explain an unfamiliar phenomenon, which is the third level of transfer (context transfer) in Haskell's taxonomy (Haskell, 2001). Knowledge transfer is not easy, but it can be learned with teacher support. Students need some explicit guidance to make connections between previous and new events and to identify the similarities and differences between previous situations and new ones (Anderson and Beavis, 2020).
RQ4. Students' chemistry grades did not significantly correlate with their performance on the inquiry task. However, students with better chemistry grades enjoyed the activity more, which could be a result of their higher interest in chemistry, their conscientiousness and more confident prior knowledge. Compared with previous research (e.g., Wu et al., 2016), the level of scientific reasoning in our study did not predict the effectiveness of inquiry-based learning. This can be explained by the fact that the pupils worked in groups and individual differences may have been balanced out.
RQ5. Most of the students found the inquiry activity novel and challenging. Students with better grades in chemistry derived more pleasure from the work, which suggests that everything that plays a role in a school grade in chemistry (e.g., the quality of knowledge about chemistry, the amount of effort invested in the study of chemistry, and interest in chemistry) may have an effect on their attitude towards inquiry learning. By contrast, Bolte et al. (2013) found that inquiry learning was less popular with conscientious and hard-working students. Our results, of course, come from just a single inquiry activity. Further research is needed to establish to what extent students achieving good results in traditional chemistry education would prefer inquiry learning if it were used with greater frequency during the school year.
RQ6. Successful problem solvers were more likely to believe that their chemistry knowledge was sufficient to solve the problem. Both successful and unsuccessful participants found formulating hypotheses and drawing conclusions challenging but they believed that they had performed well in other parts of the inquiry. This was especially striking in the case of unsuccessful problem solvers, who made factual and methodological mistakes, which suggests that they have inaccurate notions of their chemistry knowledge and inquiry skills.
Implications of the results
On the basis of the results we identified the critical parts of the inquiry cycle where teachers should give more support to their students, especially if they are new to this type of learning. At the beginning of inquiry learning, teachers should discuss the purpose of the inquiry with the class to ensure that every student understands the problem and the research question. It is especially important to check whether everyone understands the research question.Later, teachers may interrupt the activity occasionally and help students recall the necessary chemical concepts (in our case, the formula for horn salt, and the properties of ionic compounds). They could also facilitate the transfer of everyday knowledge (e.g., baking powder raises the pastry) and of the observations of previous chemistry experiments (e.g., the heating of copper(II) sulfate pentahydrate and the identification of the products of decomposition) into the given situation. They should check if the hypothesis is reasonable and give additional support to those who formulated untestable hypotheses.
Understanding the difference between observation, explanation, and inference as well as what evidence-based hypothesis testing means is also crucial for inquiry learning. Teachers can add short explanations to the worksheet or have a class discussion about these concepts prior to the activity.
Conclusions
In this study, we observed how students solve a chemistry problem using guided inquiry learning. We used a worksheet to guide the students through the steps, but on purpose we did not lead them to the correct solution. The groups worked independently, and the results were discussed and evaluated after the activity. By permitting different hypotheses and experimental designs, we allowed the students to make decisions, test their ideas and learn from the consequences. During the discussion in the second lesson, they realised that a problem may have several different approaches and that an inquiry can be successful even if the results do not support the hypothesis. If they reflect on the learning process, they can enrich their understanding. Therefore, as much time should be dedicated to reflection as to the inquiry activity itself.Our study demonstrates that guided inquiry-based learning is suitable for students who follow the regular chemistry curriculum and are new to this type of learning if they receive appropriate teacher guidance. It is therefore important to test and refine inquiry lesson plans, monitor, and interpret the learning processes to provide instructors with as much information as possible about the expected solutions and likely mistakes and difficulties. This information will help instructors to prepare for the inquiry session and forestall failures that may demotivate students.
The data-collection methods used in our research can also be applied in teaching practice. The teacher can analyse and evaluate the worksheets completed by the groups. The rubric method can be used by the teacher to diagnose the development of students’ skills at the group level during the inquiry-based activity. If the teacher leads the lesson alone and has no assistants, he/she can monitor 2–3 aspects at a time. The rubric can also be introduced to the students in the discussion following the inquiry activity, to support reflection and self-assessment.
Our results demonstrated that even a simple problem can offer multiple learning opportunities. Based on the student's answers, teachers can identify knowledge gaps and misconceptions. They can clarify the content and point out the connections with previously learned materials and everyday experiences. Inquiry-based learning allows teachers to reflect on the scientific inquiry and the nature of science with the class. Such discussions could be more efficient in the context of the first-hand experiences of students, to which they have just been exposed, compared with a regular class of information transfer.
Limitations of the study
During the guided inquiry activity, only one observer assessed each group's inquiry skills because there were few pre-service chemistry teachers available (seven people). Therefore, we could not assign two observers to each group and test inter-rater reliability. For a deeper understanding of the students’ thinking and a more detailed analysis of the communication in the groups, further research is needed, where the research methods should be complemented by student interviews and video and audio recordings of the groups’ work.Author contributions
Author contributions have been assigned according to the Contributor Roles Taxonomy (CRediT) system (Consortia Advancing Standards in Research Administration Information, 2021) as follows: conceptualization (G. O., Z. S., V. N., L. K., E. K.); data curation (G. O., Z. S., E. K.); formal analysis (G. O., E. K.); funding acquisition (E. K.); investigation (G. O., Z. S., V. N.); methodology (G. O., V. N., Z. S., L. K., E. K.); project administration (V. N., E. K.); resources (E. K.); supervision (V. N., E. K.); validation (V. N., E. K.); visualization (G. O., E. K.); writing – original draft (G. O., E. K.); writing – review and editing (G. O.; L. K., E. K.).Conflicts of interest
There are no conflicts to declare.Appendix 1: student worksheet
What happened to the horn salt?
As we were tidying up the chemistry storage room, we found an interesting thing: an old bag of horn salt, which we often use for experimenting, was empty even though the packaging was unharmed. This finding seems to be in contradiction with the law of conservation of mass. We need your help to solve this chemical puzzle.Preparation
Give the scientific name of horn salt and write down its chemical formula. Observe its physical properties and record your findings.Formulating hypotheses
During the scientific inquiry, scientists formulate statements attempting to explain an observed phenomenon. We call these statements hypotheses. Now it is your turn. Formulate a hypothesis about what happened to the horn salt.Horn salt cannot be found in the bag, because …
Explain why you think so.
Designing experiments
Many different hypotheses can be formulated but we must select the one that fits best to our observations. One of the ways a hypothesis can be corroborated or rejected is by conducting an experiment. Design an experiment through which you can test your hypothesis. Write down the steps of your experiment in order.Now make a list of the tools and materials you need to carry out your experiment. When you are ready, show your list to the teacher who will provide you with the requested things. Don’t forget to indicate how much of everything you need.
| Tools | Materials | Safety issues |
|---|---|---|
| ……………………………. | ……………………………. | ……………………………. |
| ……………………………. | ……………………………. | ……………………………. |
| ……………………………. | ……………………………. | ……………………………. |
| ……………………………. | ……………………………. | ……………………………. |
| ……………………………. | ……………………………. | ……………………………. |
| ……………………………. | ……………………………. | ……………………………. |
What do you think will happen during the experiment if your hypothesis is correct?
Experimenting
Conduct your experiment. Record every observation you make below.Hypothesis evaluation
Now that you have experimental evidence, it is time to look at your hypothesis again. Decide whether it is supported or should be rejected based on your findings.Based on the evidence we found, our hypothesis is supported/rejected because…
Thank you for your effort!
Acknowledgements
This study was funded by the Content Pedagogy Research Program of the Hungarian Academy of Sciences (Project No.: 472021). Many thanks for all the in-service teachers, pre-service teachers’, and students’ work. The publication was funded by the University of Szeged Open Access Fund (Grant No. 5850).References
- Anderson M. and Beavis A., (2020), Teaching for learning transfer. A literature review for the Victorian Curriculum and Assessment Authority, 2018, Melbourne, Australia: Victorian Curriculum and Assessment Authority.
- Bächtold M., (2013), What do students “construct” according to constructivism in science education? Res. Sci. Educ., 43(6), 2477–2496.
- Bada D. and Olusegun S., (2015), Constructivism learning theory: A paradigm for teaching and learning, Int. J. Res. Method Educ., 5(6), 66–70.
- Baldock K. and Murphrey T. P., (2020), Secondary students’ perceptions of inquiry-based learning in the agriculture classroom, J. Agric. Educ., 61(1), 235–246.
- Bao L., Cai T., Koenig K., Fang K., Han J., Wang J., Liu Q., Ding L., Cui L., Luo Y., Wang Y., Li L. and Wu N., (2009), Learning and scientific reasoning, Science, 323(5914), 586–587.
- Bao L., Xiao Y., Koenig K. and Han J., (2018), Validity evaluation of the Lawson classroom test of scientific reasoning, Phys. Rev. Phys. Educ. Res., 14(2), 1–19.
- Barthlow M. and Watson S., (2014), The effectiveness of process-oriented guided inquiry learning to reduce alternative conceptions in secondary chemistry, Sch. Sci. Math., 114(5), 246–255.
- Bednar A. K., Cunningham D. J., Duffy T. M. and Perry J. D., (1992), Theory into practice: How do we link? in Duffy T. M. and Jonassen D. H. (ed.), Constructivism and the technology of instruction, Hillsdale, NJ: Erlbaum, pp. 17–34.
- Bell R. L., Smetana L. and Binns I., (2005), Simplifying inquiry instruction: Assessing the inquiry level of classroom activities, Sci. Teach., 72(7), 30–33.
- Bell T., Urhahne D., Schanze S. and Ploetzner R., (2010), Collaborative inquiry learning: Models, tools, and challenges, Int. J. Sci. Educ., 32(3), 349–377.
- Berg C. A. R., Bergendahl V. C. B., Lundberg B. K. S. and Tibell L., (2003), Benefiting from an open-ended experiment? A comparison of attitudes to, and outcomes of, an expository versus an open-inquiry version of the same experiment, Int. J. Sci. Educ., 25(3), 351–372.
- Boesdorfer S. B. and Livermore R. A., (2018), Secondary school chemistry teacher's current use of laboratory activities and the impact of expense on their laboratory choices, Chem. Educ. Res. Pract., 19(1), 135–148.
- Bolte C., Streller S. and Hofstein A., (2013), How to motivate students and raise their interest in chemistry education, in Eilks I. and Hofstein A. (ed.), Teaching Chemistry – A Studybook. A Practical Guide and Textbook for Student Teachers, Teacher Trainees and Teachers, Rotterdam: Sense Publishers, pp. 67–95.
- Bruck L., Bretz S. and Towns M., (2008), Characterizing the level of inquiry in the undergraduate laboratory, J. Coll. Sci. Teach., 38(1), 52–58.
- Capps D. K. and Crawford B. A., (2013), Inquiry-based instruction and teaching about nature of science: Are they happening? J. Sci. Teacher Educ., 24(3), 497–526.
- Chatterjee S., Williamson V., McCann K. and Peck M., (2009), Surveying students' attitudes and perceptions toward guided-inquiry and open-inquiry laboratories, J. Chem. Educ., 86(12), 1427–1432.
- Cheung D., (2007), Facilitating chemistry teachers to implement inquiry-based laboratory work, Int. J. Sci. Math. Educ., 6(1), 107–130.
- Cheung D., (2011), Teacher beliefs about implementing guided-inquiry laboratory experiments for secondary school chemistry, J. Chem. Educ., 88(11), 1462–1468.
- Colburn A., (2000), An inquiry primer, Sci. Scope, 23(6), 42–44.
- Consortia Advancing Standards in Research Administration Information (CASRAI), (2021), CRediT – Contributor Roles Taxonomy, https://credit.niso.org/ (accessed 24 August, 2022).
- Csapó B. and Molnár G., (2019), Online diagnostic assessment in support of personalized teaching and learning: The eDia system, Front. Psychol., 10, 1522.
- Dai D. Y., Gerbin K. A. and Daley M. J., (2011), Inquiry-based learning in China: Do teachers practice what they preach, and why? Front. Educ. China, 6(1), 139–157.
- de Jong T. and Lazonder A. W., (2014), The guided discovery learning principle in multimedia learning, in Mayer R. E. (ed.), The Cambridge Handbook of Multimedia Learning. Cambridge Handbooks in Psychology, Cambridge: Cambridge University Press, 2nd edn, pp. 371–390.
- Dillon J., (2009), On scientific literacy and curriculum reform, Int. J. Environ. Sci. Educ., 4(3), 201–213.
- Dixon N., (2011), For teachers, there is a rhetoric and a reality to scientific inquiry, in Yeomans E. (ed.), Perspectives on education: Inquiry-based learning, London, UK: Wellcome Trust, pp. 16–19.
- Driver R. and Oldham V., (1986), A constructivist approach to curriculum development in science, Stud. Sci. Educ., 13(1), 105–122.
- Dobber M., Zwart R., Tanis M. and van Oers B., (2017), Literature review: The role of the teacher in inquiry-based education, Educ. Res. Rev., 22, 194–214.
- Dunbar K., (1993), Concept discovery in a scientific domain, Cogn. Sci., 17(3), 397–434.
- Edelson D. C., Gordin D. N. and Pea R. D., (1999), Addressing the challenges of inquiry-based learning through technology and curriculum design, J. Learn. Sci., 8(3–4), 391–450.
- Educational Authority of Hungary, (2020a), Kerettanterv az általános iskola 5–8. évfolyama számára. Kémia 7–8. évfolyam [Chemistry Framework Curricula of Hungary, grade 7 – grade 8], https://www.oktatas.hu/kozneveles/kerettantervek/2020_nat/kerettanterv_alt_isk_5_8 (accessed 18 January, 2022).
- Educational Authority of Hungary, (2020b), Kerettanterv a gimnáziumok 9–12. évfolyama számára. Kémia 9–10. évfolyam [Chemistry Framework Curricula of Hungary, grade 9 – grade 10], https://www.oktatas.hu/kozneveles/kerettantervek/2020_nat/kerettanterv_gimn_9_12_evf (accessed 18 January, 2022).
- Eltanahy M. and Forawi S., (2019), Science teachers’ and students’ perceptions of the implementation of inquiry-based learning instruction in a middle school in Dubai, J. Educ., 199(1) 13–23.
- Engeln K., Euler M. and Maass K., (2013), Inquiry-based learning in mathematics and science: A comparative baseline study of teachers’ beliefs and practices across 12 European countries, ZDM, 45(6), 823–836.
- Fay M., Grove N., Towns M. and Bretz S., (2007), A rubric to characterize inquiry in the undergraduate chemistry laboratory, Chem. Educ. Res. Pract., 8(2), 212–219.
- Firman M., Ertikanto C. and Abdurrahman A., (2019), Description of meta-analysis of inquiry-based learning of science in improving students’ inquiry skills, J. Phys. Conf. Ser., 1157(2), 022018 DOI:10.1088/1742-6596/1157/2/022018 (accessed 18 January, 2022).
- Furtak E. M., (2006), The problem with answers: An exploration of guided scientific inquiry teaching, Sci. Educ., 90(3), 453–467.
- Furtak E., Seidel T., Iverson H. and Briggs D., (2012), Experimental and quasi-experimental studies of inquiry-based science teaching, Rev. Educ. Res., 82(3), 300–329.
- Gado I., (2005), Determinants of K-2 school teachers' orientation towards inquiry-based science activities: A mixed method study, Int. J. Sci. Math. Educ.3(4), 511–539.
- Hamilton L. and Corbett-Whittier C., (2013), Using Case Study in Education Research, London: Sage Publications.
- Harlen W., (2013), Inquiry-based learning in science and mathematics, Rev. Sci. Math. ICT Educ., 7(1), 9–33.
- Harwood C. J., Hewett S. and Towns M. H., (2020), Rubrics for assessing hands-on laboratory skills, J. Chem. Educ., 97(7), 2033–2035.
- Haskell R. E., (2001), Teaching for Transfer: Cognition, Instruction, and Reasoning, San Diego, CA: Academic Press.
- Hattie J., (2009), Visible learning. A synthesis of over 800 meta-analyses relating to achievement, Abingdon, UK: Routledge.
- Hattie J. and Yates G. C., (2013), Visible learning and the science of how we learn, New York, NY: Routledge.
- Hegarty E. H., (1978), Levels of scientific enquiry in university science laboratory classes: Implications for curriculum deliberations, Res. Sci. Educ., 8(1), 45–57.
- Hmelo-Silver C. E., (2006), Design principles for scaffolding technology-based inquiry, in O’Donnell A. M., Hmelo-Silver C. E. and Erkens G. (ed.), Collaborative reasoning, learning and technology, Mahwah, NJ: Erlbaum, pp. 147–170.
- Hmelo-Silver C., Duncan R. G. and Chinn C., (2007), Scaffolding and achievement in problem-based and inquiry learning: A response to Kirschner, Sweller and Clark (2006), Educ. Psychol., 42(2), 99–107.
- Hofer E., Abels S. and Lembens A., (2018), Inquiry-based learning and secondary chemistry education – a contradiction? RISTAL, 1(1), 51–65.
- Hyslop-Margison E. J. and Strobel J., (2008), Constructivism and education: Misunderstandings and pedagogical implications, Teach. Educ., 43(1), 72–86.
- Kadioglu-Akbulut C. and Uzuntiryaki-Kondakci E., (2021), Implementation of self-regulatory instruction to promote students’ achievement and learning strategies in the high school chemistry classroom, Chem. Educ. Res. Pract., 22(1), 12–29.
- Kaiser G., (2006), The mathematical beliefs of teachers about application and modelling—results of an empirical study, in Novotaná J., Moraová H., Krátká M. and Stehlíková N. (ed.), Proceedings of the 30th Conference of the International Group for the Psychology of Mathematics Education. Vol. 3, Prague: PME, pp. 393–400.
- Kang J. and Keinonen T., (2016), Examining factors affecting implementation of inquiry-based learning in Finland and South Korea, Probl. Educ. 21st Cent., 74(1), 31–48.
- Khan P. and O’Rourke K., (2004), Guide to curriculum design: enquiry-based learning, Imaginative Curriculum Network, University of Manchester, Higher Education Academy, http://www.ceebl.manchester.ac.uk/resources/guides/kahn_2004.pdf (accessed 18 January, 2022).
- Khishfe R. and Abd-El-Khalick F., (2002), Influence of explicit and reflective versus implicit inquiry-oriented instruction on sixth graders' views of nature of science, J. Res. Sci. Teach., 39(7), 551–578, DOI: 10.1002/tea.10036.
- Kirschner P., Sweller J. and Clark R., (2006), Why minimal guidance during instruction does not work: An analysis of the failure of constructivist, discovery, problem-based, experiential, and inquiry-based teaching, Educ. Psychol., 41(2), 75–86.
- Kuhn D., Black J., Keselman A. and Kaplan D., (2000), The development of cognitive skills to support inquiry learning, Cogn. Instr., 18(4), 495–523.
- Lawson A. E., (2000), Classroom test of scientific reasoning. Multiple choice version, Arizona State University, https://www.public.asu.edu/~anton1/AssessArticles/Assessments/Mathematics%20Assessments/Scientific%20Reasoning%20Test.pdf (accessed 24 August, 2022).
- Lazonder A. and Harmsen R., (2016), Meta-analysis of inquiry-based learning: effects of guidance, Rev. Educ. Res., 86(3), 681–718.
- Mayer R., (2004), Should there be a three-strikes rule against pure discovery learning? The case for guided methods of instruction, Am. Psychol., 59(1), 14–19.
- McKeown T. R., Abrams L. M., Slattum P. W. and Kirk S. V., (2016), Enhancing teacher beliefs through an inquiry-based professional development program, J. Educ. Environ. Sci. Health, 2(1), 85–97.
- Millar R., Lubben F., Got R. and Duggan S., (1994), Investigating in the school science laboratory: Conceptual and procedural knowledge and their influence on performance, Res. Papers Educ., 9(2), 207–248.
- Minner D. D., Levy A. J. and Century J., (2010), Inquiry-based science instruction—What is it and does it matter? Results from a research synthesis years 1984 to 2002, J. Res. Sci. Teach., 47(4), 474–496.
- National Research Council, (1996), National science education standards, Washington, DC: National Academies Press.
- Njoo M. and De Jong T., (1993), Exploratory learning with a computer simulation for control theory: Learning processes and instructional support, J. Res. Sci. Teach., 30(8), 821–844.
- Oliver M., Romero-Ariza M., Quesada A., Abril A. and Sorensen P., (2019), Highly recommended and poorly used: English and Spanish Science Teachers’ views of inquiry-based learning (IBL) and its enactment, Eurasia J. Math. Sci. Technol., 16(1), em1793.
- Orosz G. and Korom E., (2019), A természettudományos gondolkodás mérése: A Lawson-teszt hazai kipróbálásának tapasztalatai [Measuring scientific thinking: adapting the Lawson test to Hungarian], in Molnár E. K. and Dancs K. (ed.), 17th Conference on Educational Assessment. Programme and abstracts, Szeged, Hungary: University of Szeged, p. 71.
- Panadero E. and Jonsson A., (2013), The use of scoring rubrics for formative assessment purposes revisited: A review, Educ. Res. Rev., 9, 129–144.
- Pedaste M., Mäeots M., Siiman L. A., de Jong T., van Riesen S. A. N., Kamp E. T., Manoli C. C., Zacharia Z. C. and Tsourlidaki E., (2015), Phases of inquiry-based learning: Definitions and the inquiry cycle, Educ. Res. Rev., 14(1), 47–61.
- Phillips D. C., (1995), The good, the bad, and the ugly: The many faces of constructivism, Educ. Res., 24(7), 5–12.
- Puddu S., (2017), Implementing Inquiry-based Learning in a Diverse Classroom: Investigating Strategies of Scaffolding and Students' Views of Scientific Inquiry, Berlin: Logos Verlag.
- Quintana C., Reiser B., Davis E., Krajcik J., Fretz E., Duncan R., Kyza E., Edelson D. and Soloway E., (2004), A scaffolding design framework for software to support science inquiry, J. Learn. Sci., 13(3), 337–386.
- Reid N., (2021), The Johnstone triangle. The key to understanding chemistry, Cambridge: Royal Society of Chemistry.
- Reid N. and Ali A. A., (2020), Making sense of learning. A research-based approach. Evidence to guide policy and practice, with an emphasis on secondary stages, Cham: Springer.
- Roehrig G. H. and Luft J., (2004), Inquiry teaching in high school chemistry classrooms: the role of knowledge and beliefs, J. Chem. Educ., 81(10), 1510–1516.
- Schauble L., Klopfer L. and Raghavan K., (1991), Students' transitions from an engineering to a science model of experimentation, J. Res. Sci. Teach., 28(9), 859–882.
- Schwartz R., Lederman N. and Crawford B., (2004), Developing views of nature of science in an authentic context: An explicit approach to bridging the gap between nature of science and scientific inquiry, Sci. Educ., 88(4), 610–645.
- Shute V. J. and Glaser R., (1990), A large-scale evaluation of an intelligent discovery world: Smithtown, Interact. Learn. Environ., 1(1), 51–77.
- Silm G., Tiitsaar K., Pedaste M., Zacharia Z. C. and Papaevripidou M., (2017), Teachers’ readiness to use inquiry-based learning: An investigation of teachers’ sense of efficacy and attitudes toward inquiry-based learning, Sci. Educ. Int., 28(4), 315–325.
- Smithenry D., (2010), Integrating guided inquiry into a traditional chemistry curricular framework, Int. J. Sci. Educ., 32(13), 1689–1714.
- Spronken-Smith R. and Walker R., (2010), Can inquiry-based learning strengthen the links between teaching and disciplinary research? Stud. High. Educ., 35(6), 723–740.
- Spronken-Smith R., Walker R., Batchelor J., O’Steen B. and Angelo T., (2012), Evaluating student perceptions of learning processes and intended learning outcomes under inquiry approaches, Assess. Eval. High. Educ., 37(1), 57–72.
- Staver J. R. and Bay M., (1987), Analysis of the project synthesis goal cluster orientation and inquiry emphasis of elementary science textbooks, J. Res. Sci. Teach., 24(7), 629–643.
- Szalay L. and Tóth Z., (2016), An inquiry-based approach of traditional ‘step-by-step’ experiments, Chem. Educ. Res. Pract., 17(4), 923–961.
- Szalay L., Tóth Z. and Kiss E., (2020), Introducing students to experimental design skills, Chem. Educ. Res. Pract., 21(1), 331–356.
- Szalay L., Tóth Z. and Borbás R., (2021), Teaching of experimental design skills: Results from a longitudinal study, Chem. Educ. Res. Pract., 22(4), 1054–1073.
- Tafoya E., Sunal D. and Knecht P., (1980), Assessing inquiry potential: A tool for curriculum decision makers, Sch. Sci. Math., 80(1), 43–48.
- Tashakkori A. and Teddlie C. (ed.), (2003), Handbook on mixed methods in the behavioral and social sciences, Thousand Oaks, CA: Sage Publications.
- Terhart E., (2003), Constructivism and teaching: A new paradigm in general didactics? J. Curric. Stud., 35(1), 25–44.
- Tornee N., Bunterm T., Lee K. and Muchimapura S., (2019), Examining the effectiveness of guided inquiry with problem-solving process and cognitive function training in a high school chemistry course, Pedagogies, 14(2), 126–149.
- Trumbull D., Bonney R. and Grudens-Schuck N., (2005), Developing materials to promote inquiry: Lessons learned, Sci. Educ., 89(6), 879–900.
- Ültanir E., (2012), An epistemological glance at the constructivist approach: constructivist learning in Dewey, Piaget, and Montessori, Int. J. Instr., 5(2), 1308–1470.
- Ural E., (2016), The effect of guided-inquiry laboratory experiments on science education students' chemistry laboratory attitudes, anxiety and achievement, J. Educ. Train. Stud., 4(4), 217–227.
- Vekli G. S., (2021), What factors affect middle school students’ perceptions of inquiry learning towards science? Pedagog. Res., 6(4), em0108.
- Vishnumolakala V., Southam D., Treagust D., Mocerino M. and Qureshi S., (2017), Students’ attitudes, self-efficacy and experiences in a modified process-oriented guided inquiry learning undergraduate chemistry classroom, Chem. Educ. Res. Pract., 18(2), 340–352.
- Volkmann M. J. and Abell S. K., (2003), Rethinking laboratories: Tools for converting cookbook labs into inquiry, Sci. Teach., 70(6), 38–41.
- von Glasersfeld E., (1995), A constructivist approach to teaching, in Steffe L. P. and Gale J. (ed.), Constructivism in education, Hillsdale, NJ: Erlbaum, pp. 3–15.
- Wallace C. S. and Kang N. H., (2004), An investigation of experienced secondary science teachers' beliefs about inquiry: An examination of competing belief sets, J. Res. Sci. Teach., 41(9), 936–960.
- Wen N. and Brooker M., (1995), Ammonium carbonate, ammonium bicarbonate, and ammonium carbamate equilibria: A Raman study, J. Phys. Chem., 99(1), 359–368.
- Wenning C. J., (2007), Assessing inquiry skills as a component of scientific literacy, J. Phys. Teach. Educ. Online, 4(2), 21–24.
- Wu H., Krajcik J. and Soloway, E., (2001). Promoting understanding of chemical representations: Students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Wu H. L., Weng H. L. and She H. C., (2016), Effects of scaffolds and scientific reasoning ability on web-based scientific inquiry, Int. J. Contemp. Educ. Res., 3(1), 12–24.
- Xu H. and Talanquer V., (2013), Effect of the level of inquiry of lab experiments on general chemistry students’ written reflections, J. Chem. Educ., 90(1), 21–28.
- Yin R. K., (2018), Case study research and applications (6th edn), Thousand Oaks, CA, Sage Publications.
- Zhou S., Han J., Koenig K., Raplinger A., Pi Y., Li D., Xiao H., Fu Z. and Bao L., (2016), Assessment of scientific reasoning: The effects of task context, data, and design on student reasoning in control of variables, Think Skills Creat., 19, 175–187.
- Zion M. and Mendelovici R., (2012), Moving from structured to open inquiry: Challenges and limits, Sci. Educ. Int., 23(4), 383–399.
| This journal is © The Royal Society of Chemistry 2023 |
