Open Access Article
Open Access ArticleEffect of water content on the homogeneous catalytic process of N-vinyl pyrrolidone synthesis from acetylene†
Jiahe
Fan
a,
Yao
Mu
a,
Shuairen
Qian
a,
Bozhao
Chu
b,
Siqing
Zhong
b and
Yi
Cheng
 *a
*a
aDepartment of Chemical Engineering, Tsinghua University, Beijing 100084, China. E-mail: yicheng@tsinghua.edu.cn
bSinopec Shanghai Research Institute of Petrochemical Technology, Shanghai 201208, China
First published on 17th January 2024
Abstract
The acetylene method is presently the most frequently employed technique for producing N-vinyl pyrrolidone (NVP). However, when using pyrrolidone potassium salt as the catalytically active substance in the conventional process, the water in the reaction system has a great impact on the catalyst activity and selectivity of the homogeneous process. This study comprehensively investigates the influence of water on the reaction within the conventional process of synthesizing NVP in an autoclave, and obtains a highly active homogeneous catalytic active substance through strict water removal. The main reaction and catalyst deactivation mechanisms are further clarified through DFT calculations. Based on the understanding of the mechanism, a one-step synthesis of NVP using KOH directly as the catalyst without removing water has been realized by introducing solvents into the stop-flow micro-tubing (SFMT) reactor with enhanced gas–liquid mass transfer to increase the amount of acetylene in the liquid phase.
Introduction
The industrial production of acetaldehyde from hydrated acetylene in 1916 marked a critical moment, pushing acetylene to the forefront of coal chemistry in the early 20th century.1,2 Acetylene displays remarkable reactivity so as to facilitate organic synthesis mostly in a short time. The vinylation reaction in which acetylene directly participates has become a practical and important route for the synthesis of vinyl monomers. N-Vinyl pyrrolidone (NVP) is one of the high value-added chemical products, which can be polymerized into polyvinylpyrrolidone (PVP), a nonionic water-soluble polymer. PVP is widely used in medicine and health, daily chemicals, pigments and coatings, office stationery, beverages and food industries.Since Walter Reppe's pioneering work3,4 on the synthesis of NVP and its polymer polyvinylpyrrolidone (PVP) from acetylene in 1939, the acetylene process has been developed into the most widely used technology in industry nowadays. The reaction path beginning with acetylene and 2-pyrrolidone is very simple and exemplified by a process catalyzed by the strong base KOH as shown in eqn (1)–(3).
 | (1) |
 | (2) |
 | (3) |
Another issue encountered with the conventional acetylene method for synthesizing NVP is that the reactant 2-pyrrolidone is prone to undergoing a ring opening reaction in the presence of strong alkali hydroxide, leading to catalyst deactivation. In addition, the deprotonation process of 2-pyrrolidone produces water, which accelerates the ring opening of 2-pyrrolidone,7–10 thereby accelerating the loss of catalyst deactivation. The ring-opening process of 2-pyrrolidone is shown in eqn (4), yielding potassium γ-aminobutyrate.
 | (4) |
Experimental and theoretical methods
Two-step synthesis of NVP in an autoclave
In industry, the conventional acetylene method utilizes high-pressure batch reactors to synthesize NVP. In order to explore the sensitivity of the catalyst to water under the process conditions, a two-step synthesis process was implemented in an autoclave.Initially, 200 g of 2-pyrrolidone (TCI, >98%) and 6 g of KOH (Aladdin, ACS) were added to the rotary evaporator flask and distilled for 2 h at 110 °C and 20 mbar. The prepared mixture, containing the catalyst and 2-pyrrolidone, was then introduced into a 500 mL autoclave. The autoclave was sealed quickly, and nitrogen (Beiwen Gas Manufacturing Plant, 99.95%) was introduced to displace the air and check the device for airtightness. With stirring set at 1000 rpm, 12.87 L of acetylene (Beiwen Gas Manufacturing Plant, 99.95%) was charged to the autoclave at a flow rate of 0.6 L min−1. Nitrogen was subsequently introduced to dilute the acetylene, maintaining a volume ratio of acetylene to nitrogen of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to ensure safe acetylene utilization. The mixture was then heated to 150 °C and allowed to react for 1 h. Upon completion of the reaction, the autoclave was cooled down by circulating cooling water. Once the autoclave temperature reached room temperature, the tail gas was purged and the liquid phase products were collected for analysis using a Shimadzu gas chromatograph (GC-2014) equipped with a flame ionization detector and a Stabilwax capillary column (30 m × 320 μm × 320 μm).
1 to ensure safe acetylene utilization. The mixture was then heated to 150 °C and allowed to react for 1 h. Upon completion of the reaction, the autoclave was cooled down by circulating cooling water. Once the autoclave temperature reached room temperature, the tail gas was purged and the liquid phase products were collected for analysis using a Shimadzu gas chromatograph (GC-2014) equipped with a flame ionization detector and a Stabilwax capillary column (30 m × 320 μm × 320 μm).
One-step synthesis of NVP in the stop-flow micro-tubing reactor
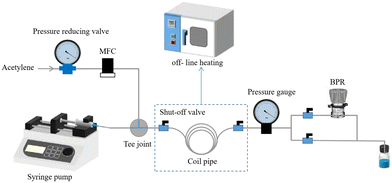
Based on the understanding of the reaction mechanism, we make efforts to promote the main reaction by increasing the amount of acetylene dissolved in the liquid phase, thereby reducing the occurrence of ring-opening side reactions caused by the presence of water. Accordingly, this would enable the direct synthesis of NVP in one step, simplifying the complex conventional two-step process. It is worth noting that autoclaves often have insufficient gas–liquid contact efficiency and have difficulty meeting the demand for increasing the amount of acetylene in liquid. In order to enhance the mass transfer process, we adopted stop-flow micro-tubing (SFMT) reactors16 to achieve the direct synthesis of NVP. Initially, 10 g of 2-pyrrolidone and 0.3 g of KOH were blended in a specific amount of solution by stirring at room temperature to form a reaction solution. The liquid flow rate was controlled at 0.5 mL min−1 with a syringe pump, and the acetylene gas rate was controlled at 20 sccm with a mass flow meter. Gas–liquid dispersion was achieved by means of tee fittings, and the system pressure was controlled at 0.2 MPa by means of a backpressure valve. When the dispersion was complete, the shut-off valves at both ends of the coils (PTFE tubing, O.D. 1/16′′, I.D. 0.03′′, 1 m, volume = 7.5 mL) were closed and the coils were removed and placed in the oven at the specified reaction temperature. After a given reaction time, heating was stopped and the liquid phase products were collected for GC analysis after cooling. The experimental setup of the SFMT reactor is shown in Fig. 1.Theoretical understanding of the reaction mechanism
Currently, the theories and computations17 of quantum chemistry have penetrated into various branches of chemistry, which can be used to calculate reaction barriers and investigate reaction mechanisms. One of the most popular methods for studying the reactivity of small molecules in organic and inorganic chemistry is density functional theory (DFT). By means of averaging electronic kinetic and potential energies, coupled with the application of variational or numerical techniques, DFT provides an approximate solution to the Schrödinger equation. This significantly simplifies electronic structure calculations. Among various density functionals, the B3LYP18 functional excels in accurately optimizing the geometry of small molecule organic systems lacking extensive conjugation features and weak interactions. The M06-2X19 functional, when combined with higher-level basis sets, offers superior precision in computing thermodynamic parameters such as reaction energies, isomerization energies, and barriers in organic systems. Therefore, this research employed B3LYP/6-311G**20,21 for both geometry optimization and vibration analysis. Transition states were located, and corresponding IRC paths were confirmed at the same computational level. Subsequently, single-point energy calculations were conducted using the M06-2x/def2-tzvp22 level of theory. The reactions were conducted in the liquid phase, and solvation effects were accounted for using the implicit solvent model SMD.23 Custom parameters were established for the solvent model of 2-pyrrolidone, with detailed methodology and optimized structures outlined in the ESI.† The computations were carried out using the GAUSSIAN 1624 software package, and the wave function analysis was performed using the Multiwfn program.25 Thermodynamic energies of individual substances were calculated at a standard temperature of 298.15 K, utilizing the Shermo program.26Substance geometries were displayed using CYLview.27 The IRI contour plot was rendered using VMD.28
Results and discussion
Investigation of catalyst sensitivity to water in a conventional two-step process
The conventional process in an autoclave uses a two-step process for the synthesis of N-vinylpyrrolidone. Studies12 have reported that the removal of water is very critical in the first step of the reaction between KOH and 2-pyrrolidone to prepare the catalyst potassium pyrrolidone. To investigate the influence of water removal on catalyst activity during the catalyst preparation process, this study implemented varying intervals of nitrogen purging during the decompression distillation process for catalyst synthesis, thereby regulating the extent of water removal in the synthesized catalyst. The experimental results are shown in Fig. 2. When the number of nitrogen purges is increased to 4 times (i.e., a nitrogen purge was conducted every 30 minutes), the yield of NVP exceeds 60%. However, with an increase in the number of nitrogen purges from 4 to 6 times, the impact on both the yield and conversion rate of the reaction is marginal. Upon further elevating the number of purges to 12 times, the yield of the target product experiences a decline. This phenomenon could be attributed to the inherent moisture content present in the nitrogen cylinder itself. While nitrogen purging effectively expels generated water vapor, some residual moisture is inevitably introduced, leading to a reduction in catalyst activity.In addition to the influence of water removal degree during catalyst synthesis on catalyst activity, we have also observed that the catalytic performance of the high-activity catalyst can be adversely affected by water. While the catalyst exhibits high activity immediately after synthesis with the introduction of six intermittent nitrogen purges, it gradually undergoes a loss in clarity and a subsequent decrease in catalytic activity upon exposure to air during storage. To address the issue, we have implemented a continuous nitrogen purging approach to protect the freshly prepared catalyst. Nevertheless, it is important to note that this method only provides a limited delay in catalyst deactivation. As shown in Fig. 3, when the freshly prepared catalyst is exposed to air for a mere 15 minutes prior to use in the reaction, there is a significant reduction in catalyst activity, resulting in a drop in the conversion rate of 2-pyrrolidone from 75.5% to less than 60%. In the absence of nitrogen sweep protection, the decline in activity is even more pronounced.
 | ||
| Fig. 3 Effect of catalyst storage time on catalytic activity: (a) conversion of 2-pyrrolidone; (b) selectivity to NVP. | ||
The deactivation of the catalyst is accompanied by the turbidity of the solution. In order to ascertain the composition of the turbid substance, we fully exposed the prepared catalyst solution to air for a week and then filtered and collected the white precipitate at the bottom of the bottle. After drying, the infrared ray (IR) spectrum of the precipitate was measured, as shown in Fig. 4a. The peaks at 3430 cm−1, 1100 cm−1, and 862 cm−1 correspond to N–H stretching vibration, C–N stretching vibration, and N–H bending vibration bonds, respectively, and can basically be considered as characteristic peaks attributed to amino groups. The peaks at 1640 cm−1 and 1390 cm−1 correspond to the symmetric and antisymmetric stretching vibration peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylates, and the stronger absorption peak at 1450 cm−1 corresponds to the C
O in carboxylates, and the stronger absorption peak at 1450 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration absorption peak of carbonates. Further, we used X-ray diffraction (XRD) to analyze the precipitate, as shown in Fig. 4b. Compared with the standard card, most of the diffraction peaks belong to potassium bicarbonate. Therefore, it is reasonable to speculate that the turbid substance is a mixture of potassium 4-aminobutyrate and potassium bicarbonate.
O vibration absorption peak of carbonates. Further, we used X-ray diffraction (XRD) to analyze the precipitate, as shown in Fig. 4b. Compared with the standard card, most of the diffraction peaks belong to potassium bicarbonate. Therefore, it is reasonable to speculate that the turbid substance is a mixture of potassium 4-aminobutyrate and potassium bicarbonate.
 | ||
| Fig. 4 (a) IR plot; (b) XRD plot of the white solid obtained during the preservation of the catalysts. | ||
To further elucidate the sensitivity of the catalytic activity of the potassium pyrrolidone catalyst to water content, we introduced a minute quantity of distilled water into the freshly prepared catalyst prior to the reaction, aiming to directly assess the impact of the additional water content on catalyst activity. As can be seen in Fig. 5, at an added water content of 0.08 wt%, equivalent to 800 ppm, there is an approximate 20% reduction in the conversion of 2-pyrrolidone. As the water content is incrementally increased, the conversion rate continues to decrease. When the amount of water added increases to 0.96 wt%, the conversion of 2-pyrrolidone is only 27.4%. Regarding its effect on selectivity, with a gradual increase in added water content from 0 to 0.47 wt%, the impact on selectivity is minimal. However, with a further escalation in water content, the selectivity to NVP drops from the initial 90% to approximately 70%. From the aforementioned analysis, it is evident that the activity of the prepared potassium pyrrolidone catalyst is exceedingly sensitive to water. Even the introduction of minute quantities of water leads to catalyst deactivation, resulting in a substantial reduction in the conversion of 2-pyrrolidone.
Mechanistic study of the NVP synthesis and catalyst deactivation process
Through experimental investigations and characterization of precipitates in catalyst solution, we have preliminarily understood the catalyst deactivation process of potassium pyrrolidone during the synthesis of NVP. Further studies require theoretical calculations to gain a more profound understanding of the main reaction mechanism and the process of catalyst deactivation, as well as to fully elucidate the mechanism of the role of water in this process.The catalytic molecule for this reaction is the potassium salt of pyrrolidone, made up of a potassium cation and a pyrrolidone anion. During the reaction, the potassium salt of pyrrolidone is initially acquired through decompression distillation aided by intermittent nitrogen purging. The subsequent vinylation reaction proceeds in two stages. Firstly, an acetylene molecule is incorporated between the cation and anion of the catalytically active molecule, which then promptly attaches the pyrrolidone anion to establish the intermediate transition state molecule. The transition state molecule combines with the reactant 2-pyrrolidone to generate the product NVP and another potassium salt of pyrrolidone, as the catalytically active molecule to catalyse the reaction continually. Calculations show that there is no energy barrier for the synthesis of potassium pyrrolidone and the protonation of the final product potassium salt, and that the energy barrier of the reaction exists mainly in the nucleophilic attack of the acetylene by potassium pyrrolidone, a process which has also been identified as an energetically critical step of the reaction. The critical step proceeds in two transition states: the first transition state (TS1) being the binding of the anion to the cis-acetylene and the second transition state (TS2) being the binding of the anion to the trans-acetylene, as shown in Fig. 6. The distance between the N atom of pyrrolidone and the C atom of acetylene in the TS1 transition is 2.00 Å. In the TS2, the two atoms are much closer, with a distance of 1.96 Å, and the corresponding free energy barriers for the activation of the process are ΔG≠TS1 = 126.3 kJ mol−1 and ΔG≠TS2 = 116.5 kJ mol−1, respectively. Although the product energy of the key step corresponding to TS1 is lower, the latter step of proton capture does not have an activation free energy barrier and can be considered to occur rapidly to achieve the generation of the final product. Hence, from an energy point of view, the key reaction step tends to occur through TS2. This energy law is consistent with the methanol vinylation reaction29,30 and similarly explains the well-known Z-stereoselectivity of monosubstituted nucleophilic adducts of acetylene.31
It can be seen from the aforementioned energy relations of the main reaction mechanism that the catalyst synthesis process does not occur via the transition state, as there is no transition state energy barrier. The free energy difference of the process is only ΔG = −11 kJ mol−1, so it can be regarded as a reversible reaction. During the synthesis of the catalyst, the positive shift of the equilibrium is promoted by the continuous rotary evaporation of water out of the system, generating a large amount of potassium pyrrolidone. When the synthesized catalyst is exposed to water, the equilibrium shifts in the reverse direction, leading to the consumption of potassium pyrrolidone and regeneration of KOH. The analysis above is supported by experimental evidence and the characterization of catalyst deactivating substances. When the catalyst comes into contact with water, KOH precipitation causes turbidity in the system. With ample exposure to air, CO2 absorption by KOH results in the formation of potassium bicarbonate.
On the other hand, considering the effect of ring opening as a side reaction process, the calculations show that pyrrolidone and KOH are used as reactants and undergo two transition states to ultimately produce the ring-opening product potassium 4-aminobutyrate (see Fig. 7). The visual analysis of IRI (interaction region indicator) values32 allows for a more accurate determination of the structure of unstable intermediates (see Fig. 8). This allows for the assessment of the plausibility of experiencing the TS1 transition state. It can be seen from the results that the O of OH− in KOH is indeed bonded to the C of pyrrolidone and the intermediate exists unstably in this structure. The total activation free energy barrier of this ring opening process is ΔG≠ = 145.1 kJ mol−1. From the perspective of the energy, this process presents a higher energy barrier than the dominant transition state in the main reaction (ΔG≠TS2 = 116.5 kJ mol−1). The ring opening process is not considered to be a fast reaction process, and the specific reaction rate is further influenced by kinetic factors.
However, it should be noted that the process of TS2 faces a high-energy barrier under alkaline conditions due to the restricted transfer of protons from C to O. An excess of water in the system can facilitate the proton transfer, leading to a significant reduction in the energy barrier  . At this point, the energy barrier of the open-loop process is lower than that of the main reaction, eliminating the energy advantage of the main reaction. Therefore, the control of water volume plays a crucial role in the occurrence of the reaction. The process of preparing potassium pyrrolidone must achieve rapid water removal, reduce the contact time between potassium pyrrolidone and water, and avoid the occurrence of ring-opening reactions.
. At this point, the energy barrier of the open-loop process is lower than that of the main reaction, eliminating the energy advantage of the main reaction. Therefore, the control of water volume plays a crucial role in the occurrence of the reaction. The process of preparing potassium pyrrolidone must achieve rapid water removal, reduce the contact time between potassium pyrrolidone and water, and avoid the occurrence of ring-opening reactions.
Through theoretical calculations, we have obtained the mechanism of NVP synthesis under the catalysis of potassium pyrrolidone and the deactivation mechanism of the catalyst process. The complete process of the catalytic cycle and catalyst deactivation is shown in Scheme 1. It can be seen that the role of water in the catalyst deactivation process is very important. Therefore, it is necessary to use a two-step method in the conventional synthesis process and strictly control the water content during the preparation and storage of the catalyst to ensure the catalytic activity.
 | ||
| Scheme 1 Schematic of the reaction mechanism of NVP synthesis and the catalyst deactivation process. | ||
An improved technique for synthesizing NVP via the one-step acetylene method without water removal
Based on the experimental study and in-depth understanding of the mechanism, the use of conventional batch reactors to synthesize NVP has high demand for water removal. During the catalyst synthesis process, it is necessary to use rotary evaporation to quickly remove water to avoid ring-opening side reactions caused by heating in the presence of water for a long time. Catalyst storage also requires avoiding contact with moisture in the air. These requirements greatly increase the complexity and uncertainty of the process control. Therefore, how to avoid the step of removing water during the reaction process has become our concern.Notably, theoretical calculations have shown that the main reaction has a clear energy advantage when there is no additional water as a proton transfer agent to facilitate the ring-opening step. Therefore, we believe that the main reaction can be promoted by increasing the concentration of acetylene in the liquid phase, and potassium pyrrolidone can be quickly consumed to inhibit the occurrence of ring-opening side reactions. However, it is difficult to achieve efficient gas–liquid contact in conventional autoclave reactors, as well as to increase the main reaction rate. In addition, continuous contact of the liquid phase will lead to the aggregation of water. With the promotion of water, side reactions can occur to a greater extent. The presence of a small amount of water will promote the ring opening of side reactions, resulting in catalyst deactivation. Therefore, we propose to use SFMT reactors for direct synthesis of NVP. Unfortunately, experimental results show that the process cannot be realized directly using the SFMT reactors, where almost no target product NVP is produced. Based on the previous work33 on the solubility of acetylene within the group, the solubility of acetylene in 2-pyrrolidone is low, so the amount of acetylene in the liquid phase cannot be greatly increased only by improving the gas–liquid contact. As an alternative, by introducing a solvent that can increase the solubility of acetylene (see the ESI† Table S2 for the acetylene solubility data), the target reaction can be promoted. The results show that with the introduction of solvents DMSO and NMP, the selectivity and conversion rate can be significantly improved as shown in Fig. 9. It is worth noting that the addition of DMSO results in a more significant increase in conversion rate compared to NMP. Although theoretical predictions indicate that NMP dissolves slightly more acetylene, its promoting effect is not as pronounced as that of DMSO. This means that solvent molecules also have an impact on the reaction. The specific mechanism of its action deserves further investigation and is beyond the scope of this study.
 | ||
| Fig. 9 The influence of solvent introduction on the reactions in the SFMT reactors (solvent to 2-pyrrolidone molar ratio: 3). | ||
Further study on the effect of DMSO addition on conversion and selectivity shows a positive correlation, as shown in Fig. 10. As the amount of DMSO added increases, both the conversion of 2-pyrrolidone and selectivity to NVP increase significantly. When the solvent dosage reaches 50 mL, the selectivity to NVP exceeds 95%. In addition, as the amount of solvent is further increased to 100 mL, the yield of NVP reaches 68%. The increase in the amount of solvent reduces the local aggregation of water and the contact with the ring-opening reaction substrates, and increases the amount of acetylene in the liquid phase. It is beneficial to improve the competitiveness of the main reaction and reduce the impact of the presence of water on the ring-opening deactivation of the catalyst to a certain extent, which also proves the rationality of the mechanism analysis.
 | ||
| Fig. 10 Effect of solvent DMSO addition on the reactions in the SFMT reactors (10 g 2-pyrrolidone, 0.3 g KOH). | ||
Conclusion
The effect of water content on the catalyst activity during the two-step synthesis of NVP in a conventional autoclave has been investigated experimentally. The high activity catalyst can be obtained by controlling the water content. Moreover, the reaction mechanism of the main reaction and the process of deactivating the catalyst by ring opening are further elucidated with theoretical calculations. The specific mechanism by which water promotes catalyst deactivation is clarified. On the one hand, the excess water affects the reaction equilibrium of the catalyst preparation step and thus promotes the formation of KOH and 2-pyrrolidinone. On the other hand, the ring opening process is also promoted in the presence of water, which accelerates the catalyst deactivation. Based on the understanding of the reaction mechanism, the content of acetylene in the liquid phase is increased by using SFMT reactors and introducing solvents to facilitate the main reaction. On this basis, we can successfully achieve a one-step synthesis of NVP that does not require water removal to prepare catalysts. These findings provide a reference for improving subsequent reactor and process design.Data availability
The information related to the theoretical calculations is available in the ESI† for this article.Author contributions
JF, YM and SQ did all the experimental work. JF and BC completed the theoretical calculations for this study. JF wrote the original draft of the manuscript, and all authors contributed to the revision and editing of the manuscript. YC and SZ supervised the project and acquired funding for the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful for the financial support from the National Natural Science Foundation (No. 22278235 and No. 21991104) and Sinopec Shanghai Research Institute of Petrochemical Technology.Notes and references
- T. Trotus, T. Zimmermann and F. Schuth, Chem. Rev., 2014, 114, 1761–1782 CrossRef PubMed.
- B. A. Trofimov and N. K. Gusarova, Russ. Chem. Rev., 2007, 76, 507 CrossRef CAS.
- W. Reppe, H. Krzikalla and O. Dornheim, DE Pat., 877757, 1939 Search PubMed.
- R. E. Oesper, J. Chem. Educ., 1950, 27, 648 CrossRef CAS.
- M. Vicari, BASF and acetylene. 70 years of reppe chemistry - long-standing reliability and promising future - and now, the only natural gas based clean technology for acetylene production, Germany, 2013 Search PubMed.
- W. Hanford and D. Fuller, Ind. Eng. Chem., 2002, 40, 1840 Search PubMed.
- M. Vinnik, Y. V. Moiseev and L. Palagina, Dokl. Akad. Nauk, 1961, 138, 149–152 CAS.
- R. J. Washkuhn and J. R. Robinson, J. Pharm. Sci., 1971, 60, 1168–1175 CrossRef CAS PubMed.
- K. Bowden and K. Bromley, J. Chem. Soc., Perkin Trans. 2, 1990, 12, 2111–2116 RSC.
- H. K. Chenault, Handbook of Pyrrolidone and Caprolactam Based Materials: Synthesis, Characterization and Industrial Applications, 2021, vol. 6, pp. 1–69 Search PubMed.
- M. Schmidt-Radde, M. Heider and A. Dams, US Pat., 5670639, 1997 Search PubMed.
- T. Mutaffis, F. Carluccio and M. Chiddix, US Pat., 3185706, 1965 Search PubMed.
- R. Vogelsang, S. Kashamore and W. Staffel, CN Pat., 101896458, 2010 Search PubMed.
- H. Li, P. Zhang and Q. Han, CN Pat., 101701009A, 2010 Search PubMed.
- L. Yan, B. Chu, S. Zhong, Z. Fu and Y. Cheng, Chem. Eng. J. Adv., 2020, 2, 100018 CrossRef CAS.
- F. Xue, H. Deng, C. Xue, D. K. B. Mohamed, K. Y. Tang and J. Wu, Chem. Sci., 2017, 8, 3623–3627 RSC.
- I. N. Levine, Quantum chemistry, Pearson, Boston, 7th edn, 2014 Search PubMed.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- J.-P. Blaudeau, M. P. McGrath, L. A. Curtiss and L. Radom, J. Chem. Phys., 1997, 107, 5016–5021 CrossRef CAS.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 2008, 72, 650–654 CrossRef.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- V. Barone, G. Petersson and H. Nakatsuji, GAUSSIAN 16 (Revision C.01), Gaussian Inc., Wallingford, CT, 2016 Search PubMed.
- T. Lu and F. W. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- T. Lu and Q. X. Chen, Comput. Theor. Chem., 2021, 1, 231–239 CAS.
- C. Legault, CYLview, 1.0 b, Université de Sherbrooke, 2009 Search PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics Modell., 1996, 14, 33–38 CrossRef CAS PubMed.
- N. M. Vitkovskaya, E. Y. Larionova, V. B. Kobychev, N. V. Kaempf and B. A. Trofimov, Int. J. Quantum Chem., 2008, 108, 2630–2635 CrossRef CAS.
- N. M. Vitkovskaya, E. Y. Larionova, V. B. Kobychev, N. V. Kaempf and B. A. Trofimov, Int. J. Quantum Chem., 2011, 111, 2519–2524 CrossRef CAS.
- S. I. Miller and G. Shkapenko, J. Am. Chem. Soc., 1955, 77, 5038–5041 CrossRef CAS.
- T. Lu and Q. Chen, Chem.: Methods, 2021, 1, 231–239 CAS.
- Y. Mu, L. Yan, B. Chu, S. Zhong, J. Fan and Y. Cheng, Chem. Eng. Sci., 2023, 276, 118824 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3re00652b |
| This journal is © The Royal Society of Chemistry 2024 |