Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Automated sulfur-[18F]fluoride exchange radiolabelling of a prostate specific membrane antigen (PSMA) targeted ligand using the GE FASTlab™ cassette-based platform†
Zixuan
Yang
b,
Chris
Barnes
b,
Juozas
Domarkas
a,
Joanna
Koch-Paszkowski
a,
John
Wright
a,
Ala
Amgheib
b,
Isaline
Renard
 a,
Ruisi
Fu
b,
Stephen
Archibald
a,
Ruisi
Fu
b,
Stephen
Archibald
 a,
Eric O.
Aboagye
a,
Eric O.
Aboagye
 *b and
Louis
Allott
*b and
Louis
Allott
 *a
*a
aCentre for Biomedicine and Positron Emission Tomography Research Centre, Hull York Medical School and University of Hull, Cottingham Road, Hull, HU6 7RX, UK. E-mail: louis.allott@hull.ac.uk
bComprehensive Cancer imaging Centre, Faculty of Medicine, Department of Surgery and Cancer, Imperial College London, Hammersmith Hospital, Du Cane Road, London, UK. E-mail: eric.aboagye@imperial.ac.uk
First published on 21st August 2023
Abstract
Sulfur-[18F]fluoride exchange radiochemistry is a rapid and convenient method for incorporating fluorine-18 into biologically active molecules. We report a fully automated radiolabelling procedure for the synthesis of a [18F]SO3F-bearing prostate specific membrane antigen (PSMA) targeted ligand ([18F]5) using the GE FASTLab™ cassette-based platform in a 25.0 ± 2.6% radiochemical yield (decay corrected). Uptake in vitro and in vivo correlated with PSMA expression, and the radioligand exhibited favourable biodistribution and pharmacokinetic profiles.
Fluorine-18 (18F) is an ideal positron emission tomography (PET) isotope owing to its favourable decay characteristics (t1/2 = 110 min, β+em 0.635 MeV, 97%) and therefore the development of convenient reactions to incorporate the isotope into molecules of interest is an active area of research.1,2 The facile sulfur-[18F]fluoride exchange reaction is a recent advancement in 18F radiochemistry where aryl [18F]fluorosulfates are synthesised via a 19F/18F isotopic exchange reaction in high yields, with excellent substate tolerability (Fig. 1).3 A variety of 18F-labelled molecules have been synthesised using this chemistry, but to our knowledge, an automated cassette-based procedure has not yet been described.4 Automation is a key component to the translation of PET radiopharmaceuticals into clinical studies as it allows: 1) scalability of dose while maintaining a safe working environment for production personnel through convenient shielding of the automated system; 2) good manufacturing practice (GMP) compliance to produce safe radiopharmaceutical doses for patients; 3) batch reproducibility and a reduction in batch failure rates.5,6 Cassette-based platforms like the GE HealthCare FASTLab™ and FASTLab2™ are a popular choice for radiopharmaceutical manufacturing and are installed in production facilities worldwide. Cassettes are convenient for investigational medicinal product (IMP) and commercial manufacture of radiopharmaceuticals as they can be populated with reagents and essential components in a sterile GMP environment and distributed, allowing for simple “plug and go” synthesis with no requirements for additional radiochemistry development.5,7 We have previously demonstrated the flexibility of these platforms in the synthesis of complex radiopharmaceuticals including labelled biologics, some of which have progressed into clinical studies.8–12
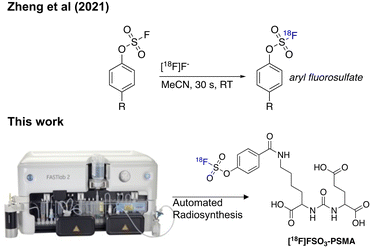 | ||
| Fig. 1 Sulfur-[18F]fluoride exchange radiochemistry developed by Zheng et al. (2021) and an overview of this study which aimed to automate the radiochemistry using a cassette-based platform. | ||
Herein we report a fully automated procedure for sulfur-[18F]fluoride radiochemistry using a commercially available cassette-based platform (GE FASTLab™), exemplified by labelling a prostate specific membrane antigen (PSMA) targeted ligand bearing an aryl fluorosulfates (SO3F) moiety.
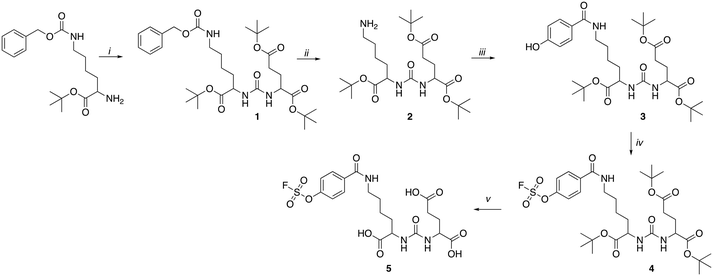
PET imaging of PSMA expression is a crucial biomarker in metastatic castrate resistant prostate cancer and a variety of radiopharmaceuticals for imaging and molecular radiotherapy have been developed.13 The Lys-urea-Glu motif is a simple ligand, which effectively targets PSMA and has been utilised in a variety of applications from radiopharmaceutical development to drug delivery.14,15 A SO3F-bearing Lys-urea-Glu dipeptide was synthesised following Scheme 1. In brief, H-Lys(Z)-OtBu hydrochloride was reacted with 4-nitrophenyl chloroformate in an inert atmosphere followed by the addition of H-Glu(OtBu)-OtBu hydrochloride to form 1 in a 55% yield after preparative HPLC purification.
Hydrogenation of 1 over palladium on carbon (10%) quantitatively liberated the free base 2 which was reacted with commercially available 4-hydroxybenzoic acid to produce 3. The SO3F moiety was incorporated into 4 using commercially available 4-[(acetylamino)phenyl]imidodisulfuryl difluoride (AISF) before the hydrolysis of the t-butyl protecting groups in acidic conditions to give the final radiochemistry reference material 5 (SO3F-PSMA). All compounds were characterised by MS, 1H-NMR, 13C-NMR and 19F-NMR where appropriate (ESI† Fig. S1–S17). A fluorosulfonyl (SO2F) derivative was also synthesised for comparison (ESI,† section 2).
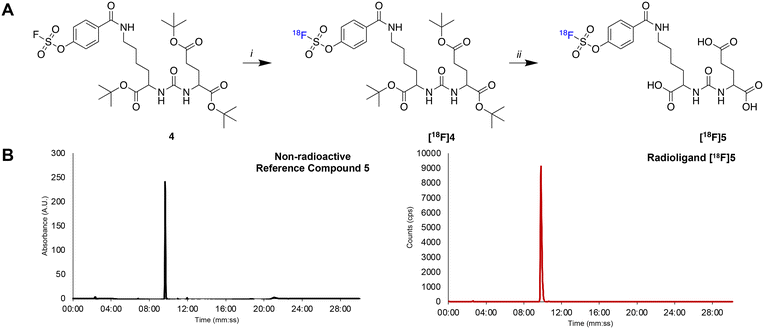
An automated radiosynthesis method to produce [18F]SO3F-PSMA ([18F]5) was developed using the GE FASTLab™ cassette-based platform (Fig. 2). The radiochemistry was performed in two steps within 70 min (Scheme 2). In brief, aqueous [18F]fluoride was dried in the presence of K2CO3 and Kryptofix222™ to which precursor 3 (5 μmol) in acetonitrile was added. The reaction proceeded at room temperature for 5 min, before hydrolysis of the t-butyl protecting groups in acidic conditions. The crude mixture was purified by semi-preparative HPLC and the desired radioligand was isolated, concentrated and reformulated in ethanol using a tC18 plus SPE cartridge. The radiochemical yield (RCY) non-decay corrected was 25.0 ± 2.6% (n = 3), and the molar activity was 0.13 ± 0.08 GBq μmol−1 (n = 3) from low starting activities of 1.75 ± 0.5 GBq (n = 3). Generally, the molar activities of radioconjugates labelled via19F/18F isotopic exchange are notoriously low as unlabelled precursor cannot be isolated from labelled product; however, the molar activities reported here were sufficient for biological evaluation, even when starting from low levels of [18F]fluoride. The use of larger activities of [18F]fluoride would undoubtedly lead to higher molar activity products, and could be investigated in the future, but was not necessary for this study. The identity and radiochemical purity of [18F]5 was determined by HPLC (Scheme 2B). The stability of [18F]5 in formulation was >99% over the duration of testing (4 hours). Given the simplicity of the radiochemistry and the previously exemplified tolerability of sulfur-[18F]fluoride exchange reactions towards an extensive scope of substrates, it is anticipated that this automated procedure could be adapted to label other ligands, though substrate specific optimisation such as precursor quantity and purification strategy will be required. An SO2F bearing PSMA conjugate was also successfully radiolabelled using this method (ESI†). To our knowledge, this is the first time that an SO2F moiety has been labelled via isotopic exchange as literature examples displace a chloro-leaving group (SO2Cl).16
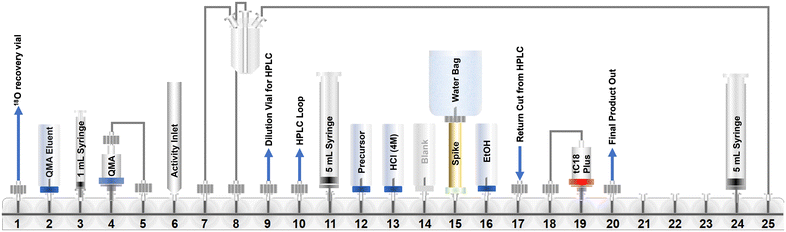 | ||
| Fig. 2 A schematic representation of the GE FASTLab™ cassette developed for the automated radiosynthesis of [18F]5. Full details of the cassette setup and radiochemistry methodology are presented in the ESI† (section 3). | ||
Evaluation of [18F]5 was performed in vitro and in vivo in PSMA expressing models to confirm the targeting properties of the radioligand. The uptake of [18F]5 was evaluated in a panel of cell lines with differential expression of PSMA (ESI† Fig. S20); PSMA expression was confirmed in the cell lines by flow cytometry (ESI† Fig. S19). While cellular uptake of [18F]5 was generally low (0.05–0.5% ID g−1), a clear and statistically significant trend was observed where uptake of [18F]5 was highest in PSMA positive cells. Uptake in LNCaP and C4-2B cells was 7.7 and 8.7-fold higher, respectively, compared to PSMA negative prostate cancer cells PC3, and normal cells PNT1A. These data confirm that [18F]5 retained PSMA-target recognition which encouraged in vivo studies.
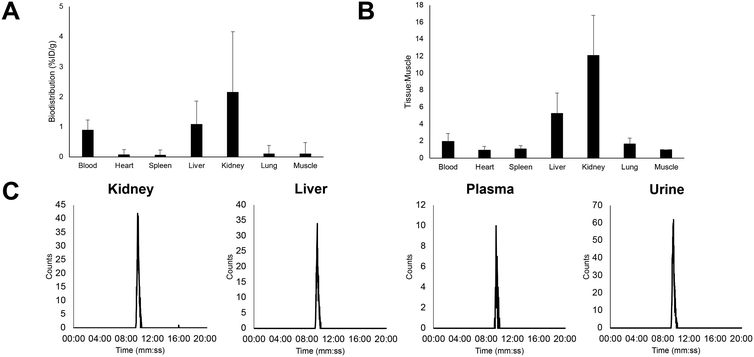
The biodistribution of [18F]5 was first evaluated by ex vivo gamma counting of tissue (Fig. 3A) in naïve mice. Renal elimination was the primary route clearance (2.2 ± 2.0% ID g−1), which is characteristic for hydrophilic peptides. Uptake in muscle was low (0.1 ± 0.4% ID g−1), ideal for high contrast PET images in tumour bearing animals. PET imaging in naïve mice showed low skeletal uptake, which was indicative of in vivo stability towards defluorination (ESI† Fig. S21). Radioactive metabolite analysis highlighted that [18F]5 exhibited excellent stability in key tissues and organs (Fig. 3C). An [18F]FSO2-PSMA ligand was also evaluated in vivo and extensive defluorination was observed, which was in agreement with in vitro metabolite studies reported in the literature (ESI† Fig. S26).16
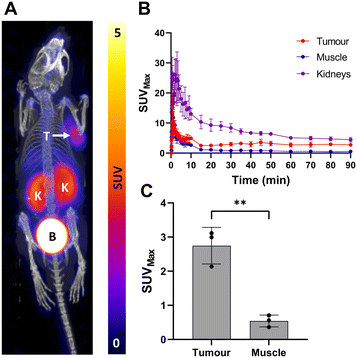
Compound [18F]5 was then evaluated in vivo using LNCaP tumour bearing mice (n = 3). Dynamic PET imaging over 90 min showed significant tumour uptake and tumour![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) muscle contrast (Fig. 4). Time activity curves showed consistent retention of radioactivity in the tumour after ca. 30 min including significant and sustained tumour
muscle contrast (Fig. 4). Time activity curves showed consistent retention of radioactivity in the tumour after ca. 30 min including significant and sustained tumour![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) muscle contrast (5.6 ± 2.7) even at 90 min post-injection (Fig. 4C). All PET images and TACs are presented in the ESI† (Fig. S22–S24).
muscle contrast (5.6 ± 2.7) even at 90 min post-injection (Fig. 4C). All PET images and TACs are presented in the ESI† (Fig. S22–S24).
Sulfur-[18F]fluoride exchange radiochemistry is a facile technique for labelling biologically relevant molecules, and is a welcome addition to the radiochemistry toolbox of 18F-fluorination chemistry. This cassette-based automated radiolabelling method will help progress sulfur-[18F]fluoride exchange radiochemistry towards clinical evaluation as GMP grade radiopharmaceuticals. This work has built a platform for others to develop and optimise for their substrates of interest; work to further improve labelling efficiency, evaluate the use of clinically relevent activities of fluorine-18 (>30 GBq), and optimise for clinically relevant molar activities would be required to translate radioligands produced using this protocol into clinical applications. We exemplified the automated method through the synthesis of radioligand [18F]5, which not only effectively demonstrated the utility of the platform, but has highlighted a new radioligand with favourable in vitro selectivity towards PSMA expressing cell lines, and favourable in vivo uptake, metabolic stability, biodistribution and pharmacokinetics profile. Further biological investigation of this radioligand is warranted.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by PhD studentship (ZY). LA acknowledges funding from the Royal Society (RGS\R2\212001) and Royal Society of Chemistry (E21-3266433901), and SJA & LA acknowledge the Medical Research Council (MR/X013774/1) for small animal PET and CT scanner provision. SJA acknowledges funding from the Engineering and Physical Science research Council (EP/V055836/1). EOA acknowledges funding from Imperial Confidence in Concept, Medical Research Council (MR/N020782/1), Cancer Research UK National Cancer Imaging Accelerator (C2536/A28680) and Experimental Cancer Medicines Centre (C1312/A25149).Notes and references
- A. F. Brooks, J. J. Topczewski, N. Ichiishi, M. S. Sanford and P. J. H. Scott, Chem. Sci., 2014, 5, 4545–4553 RSC.
- S. J. Archibald and L. Allott, JNMMI Radiopharm. Chem., 2021, 6, 30 CrossRef PubMed.
- Q. Zheng, H. Xu, H. Wang, W.-G. H. Du, N. Wang, H. Xiong, Y. Gu, L. Noodleman, K. B. Sharpless, G. Yang and P. Wu, J. Am. Chem. Soc., 2021, 143, 3753–3763 CrossRef CAS PubMed.
- N. Walter, J. Bertram, B. Drewes, V. Bahutski, M. Timmer, M. B. Schütz, F. Krämer, F. Neumaier, H. Endepols, B. Neumaier and B. D. Zlatopolskiy, Eur. J. Med. Chem., 2022, 237, 114383 CrossRef CAS PubMed.
- C. Barnes, M. Nair, E. O. Aboagye, S. J. Archibald and L. Allott, React. Chem. Eng., 2022, 7, 2265–2279 RSC.
- L. Allott and E. O. Aboagye, Mol. Pharmaceutics, 2020, 17, 2245–2259 CrossRef CAS PubMed.
- L. Bruton and P. J. H. Scott, in Handbook of Radiopharmaceuticals, 2020, pp. 437–456 Search PubMed.
- L. Allott, A. Amgheib, C. Barnes, M. Braga, D. Brickute, N. Wang, R. Fu, S. Ghaem-Maghami and E. O. Aboagye, React. Chem. Eng., 2021, 6, 1070–1078 RSC.
- L. Allott, C. Barnes, D. Brickute and E. O. Aboagye, React. Chem. Eng., 2019, 4, 569–574 RSC.
- L. Allott, S. Dubash and E. O. Aboagye, Cancers, 2020, 12, 865 CrossRef CAS PubMed.
- S. R. Dubash, N. Keat, K. Kozlowski, C. Barnes, L. Allott, D. Brickute, S. Hill, M. Huiban, T. D. Barwick, L. Kenny and E. O. Aboagye, Eur. J. Nucl. Med. Mol. Imaging, 2020, 47, 2549–2561 CrossRef CAS PubMed.
- S. R. Dubash, N. Keat, P. Mapelli, F. Twyman, L. Carroll, K. Kozlowski, A. Al-Nahhas, A. Saleem, M. Huiban, R. Janisch, A. Frilling, R. Sharma and E. O. Aboagye, J. Nucl. Med., 2016, 57, 1207–1213 CrossRef CAS PubMed.
- E. O. Aboagye, T. D. Barwick and U. Haberkorn, Ca-Cancer J. Clin., 2023, 73, 255–274 CrossRef PubMed.
- K. Kopka, M. Benešová, C. Bařinka, U. Haberkorn and J. Babich, J. Nucl. Med., 2017, 58, 17s–26s CrossRef CAS PubMed.
- J. B. Lee, K. Zhang, Y. Y. Tam, J. Quick, Y. K. Tam, P. J. Lin, S. Chen, Y. Liu, J. K. Nair, I. Zlatev, K. G. Rajeev, M. Manoharan, P. S. Rennie and P. R. Cullis, Mol. Ther.--Nucleic Acids, 2016, 5, e348 CrossRef CAS PubMed.
- L. Matesic, N. A. Wyatt, B. H. Fraser, M. P. Roberts, T. Q. Pham and I. Greguric, J. Org. Chem., 2013, 78, 11262–11270 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical synthesis and characterisation, biological evaluation in vitro and in vivo. See DOI: https://doi.org/10.1039/d3re00307h |
| This journal is © The Royal Society of Chemistry 2023 |