Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Impact of residence time distributions in reacting magnesium packed beds on Grignard reagent formation – selectivity of Grignard reagent formation (part 2)†
Eva
Deitmann
ab,
Michael
Maskos
a,
Gabriele
Menges-Flanagan
 *a and
Dirk
Ziegenbalg
*a and
Dirk
Ziegenbalg
 *b
*b
aFraunhofer IMM, Carl-Zeiss-Strasse 18-20, 55129 Mainz, Germany. E-mail: gabriele.menges-flanagan@imm.fraunhofer.de
bUlm University, Helmholtz-Strasse 16, 89081 Ulm, Germany. E-mail: dirk.ziegenbalg@uni-ulm.de
First published on 28th June 2023
Abstract
Grignard reagents are used as intermediates in the production of complex molecules, since they can be used to form new carbon–carbon bonds, e.g. in the formation of active pharmaceutical ingredients. Side product formation like Wurtz coupling diminishes the selectivity in Grignard reagent formation and therefore side product formation needs to be reduced. It was found that the different pumping behaviours of a syringe pump, a valveless rotary piston pump and a micro annular gear pump and the obtained residence time distributions have an impact on the selectivity of the Grignard reagent formation. Selectivity can also be enhanced by the available magnesium surface and by choosing a tubular flow reactor instead of a batch reactor, showing the importance of choosing the right equipment and parameters for the specific reaction system.
Introduction
Organomagnesium halides, better known as Grignard reagents, are formed by the exothermic reaction of metallic magnesium (usually turnings or powder) with a halide in an anhydrous, etheric solvent. Grignard reagents can be used for the formation of new carbon–carbon bonds and are one of the most important intermediates in such reactions.1 Both on a small scale in a glass flask in chemical laboratories and on a larger scale in stirred vessels in the chemical industry, Grignard reagents are prepared in a dosing-controlled semi-batch process based on placing a certain mass of magnesium together with a small amount of the halide in the solvent in the stirred vessel and then slowly adding the remaining halide–solvent mixture.2 Because of the exothermic nature of the reaction, the reaction temperatures must be closely controlled to avoid thermal runaway and hazardous situations. A challenge of the synthesis of Grignard reagents is the Wurtz coupling product. This side product is formed by reaction of already-formed Grignard reagent with the halide reactant (Fig. 1). The formation of this side product decreases the yield of the Grignard reagent and the purity of the product obtained. Thus, the formation of the Wurtz coupling product must be avoided as much as possible. The formation of the Wurtz coupling product can be reduced by various means: low reaction temperatures, low halide concentrations and low addition rates as well as a large magnesium surface area and therewith mass transport.1 However, these approaches contradict the requirements of sustainability. A reduction of the halide concentration leads to an increase of the solvent content. Lowering of the cooling temperature causes reduced reaction rates and increasing operating costs. Some publications refer to changing the solvent from the commonly used tetrahydrofuran to the more expensive 2-methyltetrahydrofuran to minimize the amount of Wurtz coupling product.3,4 Considering the principles of green chemistry, the use of 2-methyltetrahydofuran is favourable, since the solvent can be made from renewable raw materials and since it is immiscible with water, having the potential to facilitate post-reaction processing.5 However, changing the solvent is not only more cost-intensive (at least for now) but also accompanied by a change in the whole reaction system. Since Grignard reagents are still purchasable mostly in tetrahydrofuran, the use of 2-methyltetrahydrofuran is not further considered in this publication. Alternatively, continuously operated, magnesium-filled plug flow reactor systems can be used to ideally keep contact times of the already-made Grignard reagent and the not yet reacted halide educt as short as possible. High surface-to-volume ratios, as they can be realized in micro- and millistructured reactors, ensure beneficial heat transfer to avoid hotspots within the magnesium bed which would enhance Wurtz coupling. Reaction networks involving consecutive reactions, like the Grignard reaction system, can be controlled by providing the required processing experience to the molecules.6 Thus, controlling the residence time distribution represents a meaningful strategy to achieve high yield and suppress undesired side product formation. Despite the laminar flow conditions in small-sized channels, micro- and millistructured reactors frequently possess narrow residence time distributions, potentially approaching plug flow behaviour, and the impact of this reactor technology to achieve a high degree of reaction control has been reported earlier.7–9 | ||
| Fig. 1 Formation of Grignard reagent RMgX from halide RX and magnesium Mg and subsequent formation of the Wurtz coupling product RR by reaction of Grignard reagent RMgX with halide reactant RX. | ||
The residence time distribution in packed-bed reactors is more complicated than in empty channels. In part 1 of this contribution, the influence of the pumping characteristics and the properties of magnesium turnings on the residence time distribution in a reactor cartridge filled with non-uniform, non-spherical metal turnings, has been reported. It was found that application of an oscillating flow influences the residence time distribution, and axial dispersion can be reduced by adjusting the amplitude and frequency of the oscillation. Since the magnesium reacts during the Grignard reagent synthesis, the entire magnesium bed is subject to constant changes. The impact of such changes on the residence time distribution and finally on the selectivity of the benchmark synthesis of benzylmagnesium bromide is studied in this work. Furthermore, the influence of the pump-induced flow behaviour and, in particular, the effect of oscillating flow on the selectivity of the benzylmagnesium bromide synthesis in a non-homogeneous, randomly packed bed of non-uniform, non-spherical magnesium turnings are investigated.
Experimental section
Materials and equipment
All solvents and reagents are used without further purification. The chemicals used and their suppliers were (bromomethyl)benzene (99%, Thermo Fisher Scientific Inc.), 2-phenylethylbenzene (99%, Thermo Fisher Scientific Inc.), 1,1′-biphenyl (≥99.5%, Glentham Life Sciences Ltd.), bromobenzene (≥99%, Merck KGaA), methanol (≥99.9%, Honeywell International Inc.), magnesium turnings (coarse: Merck KGaA, fine: Almamet GmbH), 1,2-xylene (99% pure, Thermo Fisher Scientific Inc.), butan-2-ol (99%, anhydrous, Thermo Fisher Scientific Inc.), tetrahydrofuran (>99.9%, p.a., Th. Geyer GmbH & Co. KG), and toluene (≥99.7%, Honeywell International Inc.).Temperatures are measured using thermocouples (type K, RS Components) and logged by a data logger (Expert Key 200L, Delphin Technology).
To conduct the experiments, the following pumps are used: Postnova syringe pump with 2.5 mL Hamilton glass syringes, programmable Landgraf syringe pump, HNP micro annular gear pump, and Ismatec valveless rotary piston pump. Pump specifications are listed in Table 1.
| Pump | Pump type | Pump name | Manufacturer | Operating conditions |
|---|---|---|---|---|
| A | Syringe pump | PN1610 syringe dosing system | Postnova Analytics GmbH | Flow rate: max. 20 mL min−1 (2.5 mL syringes) |
| B | Syringe pump | LA-120 | Landgraf Laborsysteme HLL GmbH | Stroke volume: depending on syringe size |
| No limits on number of withdrawing/dosing cycles per minute noted but pump is not designed for frequent changes | ||||
| Flow rate: max. 35 mL min−1 (depending on syringe size) | ||||
| C | Micro annular gear pump | mzr-7205 | HNP Mikrosysteme GmbH | Flow rate: 0.048–288 mL min−1 |
| Dosing precision: variation coefficient <1% | ||||
| D | Valveless rotary piston pump | Reglo-CPF digital with ISM321 drive and RH1CKC pump head | Ismatec, Cole-Parmer Instrument Company LTD | Stroke volume: 10–100 μl |
| Speed: 40–1800 min−1 | ||||
| Flow rate: 0.40–180 mL min−1 | ||||
For tempering of the reactor cartridge, a thermostatic bath (F31-C) from Julabo GmbH is used.
Analysis of benzylmagnesium bromide and phenylmagnesium bromide is done by thermometric titration (Metrohm AG) in toluene with 1 mol L−1 butan-2-ol in 1,2-xylene. Unreacted halide and formed side products are detected by quenching the Grignard reagent samples in methanol and subsequent analysis using a calibrated gas chromatograph (GC) (Varian GC 3900 system with Varian 8400 GC-autosampler).
Infrared (IR) spectroscopy is used for toluene concentration measurements during the residence time experiments (Matrix-MF with diamond micro ATR probe, Bruker Optik GmbH). The measurement data are recorded by using the software OPUS (version 6.5, Bruker Optik GmbH).
Batch syntheses are conducted in a 250 mL 4-neck flask equipped with a reflux condenser, dropping funnel, magnetic stirrer with stirring bar, thermocouple and septa.
Residence time measurements during Grignard reagent synthesis
The residence time measurements are conducted by using the displacement marking method (step function), in which a tracer-free inlet flow is exchanged for a tracer flow (or vice versa) of the same flow rate at time t = 0. The tracer-free solution contains the halide in tetrahydrofuran (THF), while 2 mol L−1 toluene is added to this solution for the tracer-containing solution. Unless otherwise specified, the measurement runs are performed by introducing the tracer toluene to the reactor system. For simultaneous Grignard reagent formation, the synthesis of phenylmagnesium bromide from bromobenzene and magnesium turnings is chosen. Coarse or fine magnesium turnings are used (refer to part 1 for the particle size distributions). Residence time measurements are started as soon as the Grignard reagent formation reaches a steady state and full conversion of the halide is measured by IR and GC analyses.Measurement runs are repeated at least three times to obtain mean values for Bodenstein number Bo and mean residence time ![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) . The procedure for performing the residence time measurements and evaluation of the gathered data is described in detail in part 1.
. The procedure for performing the residence time measurements and evaluation of the gathered data is described in detail in part 1.
Grignard reagent synthesis in a continuous flow reactor
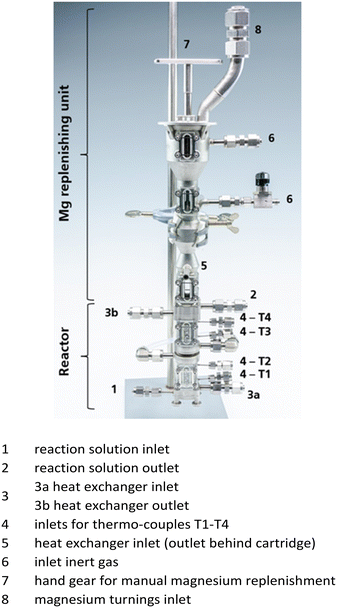
The reactor used for residence time distribution measurements is the same reactor used in part 1. It is a laboratory-scale reactor designed by Fraunhofer IMM and manufactured via 3D laser melting for the formation of organometallic halide reagents.10,11Fig. 2 displays the reactor in more detail. It consists of a reactor cartridge and a magnesium replenishing unit that can be attached to the main reactor body for replenishing magnesium turnings during Grignard reagent synthesis.The reactor is charged with magnesium (Mg) turnings (23 g for coarse magnesium turnings, 27 g for fine turnings) while the jogging motor at the bottom of the reactor is running (9 V). An inert atmosphere in the reactor is realized with argon. Four thermocouples are placed along the magnesium bed for temperature monitoring. Before starting the synthesis, the reactor is purged for 30 min by an argon flow, while the magnesium bed is compacted due to vibrations generated by the jogging motor and the reactor cartridge is already tempered. Subsequently, the reactor is filled with a solution of educt halide in tetrahydrofuran (THF) at an elevated flow rate. This leads to a temperature increase resulting from the initiation of the exothermic reaction. As soon as the reactor is completely filled with educt solution, the pump is turned off and the reactor content is allowed to rest for 10 min for initial formation of Grignard reagent and further activation of the magnesium turnings. Afterwards, the pump is turned on again, recording of measured temperatures is started and the flow rate is reduced compared to that in the initial filling step of the reactor to keep temperatures below the solvent's boiling point. To accelerate the start of the reaction, an elevated temperature of 50 °C is set. After waiting for about three residence times, samples from the outlet solution are taken and titrated by thermometric titration to determine the Grignard reagent concentration. Samples are quenched with methanol as well and analysed with GC measurements to determine side product concentration and residual halide educt concentration. Depending on the concentration of the halide used, portions of magnesium turnings are replenished at regular time periods (about 30 min) to keep the magnesium mass as constant and equal as possible for the individual residence time measurements. In the case of residence time measurements, the magnesium is replenished in between two runs to avoid disturbance of the measurement and the flow behaviour. Specific reaction conditions for the investigated Grignard reagents are summarized in Table 2.
| Phenylmagnesium bromide | Benzylmagnesium bromide | |
|---|---|---|
| Educt | Bromobenzene | (Bromomethyl)benzene |
| Concentration starting material/(mol L−1) | 1 | 0.7 |
| Solvent | THF | |
| Flow rate/(mL min−1) | 2 | |
| Hydrodynamic residence time/s | 652 (coarse) | |
| 583 (fine) | ||
| Thermostat temperature/°C | 50 (initiation) | |
| 35 (continued synthesis) | ||
Grignard reagent synthesis in a stirred tank (semi-continuous batch mode)
Intensely stirred tank reactors possess complete backmixing and thus represent one extreme case with respect to the process experience which molecules undergo. The opposite extreme case represents a continuously operated plug flow reactor with no backmixing. Thus, comparing batch and continuous mode allows a better assessment of the potential of continuous flow reactors for the use in Grignard reagent syntheses.Generally applicable parameters for each synthesis in the glass flask to allow comparability with continuous syntheses:
• All glassware used are placed in a drying oven at 115 °C for at least 12 hours, cooled in a desiccator and flushed with argon to prevent contamination with air and especially moisture to which the Grignard reagent is extremely sensitive.
• Magnesium turnings are stirred dry under argon for 30 min.
• Tempering of the flask at 35 °C.
• Waiting for a 10 min incubation period after initial addition of a first portion of the halide-in-THF solution.
• Addition of the halide/THF solution at a rate of approx. 2 mL min−1.
• 30 min post-stirring time after complete addition of the halide solution (corresponds to approx. 3 times the residence time in the continuous flow reactor).
• Synthesis of benzylmagnesium bromide from (bromomethyl)benzene and magnesium turnings.
In the continuous synthesis process, starting the synthesis at 50 °C and using an initially higher flow rate serves solely to accelerate the start of the synthesis and to reach steady state faster, and has no effect on the yield and selectivity achieved. Since no steady state is reached in a discontinuous or semi-continuous operation mode, there is no need to accelerate the start-up behaviour in a batch experiment. Therefore, and for safety reasons, the application of elevated temperatures and feed rates is not used in the batch experiments.
Batch procedure 1: literature reaction conditions
To generate a reference data set, initial reaction conditions for semi-continuous batch experiments are taken from a reference book12 and the indicated quantities are halved, resulting in a total liquid volume of 117.19 mL and a (bromomethyl)benzene concentration of 2.13 mol L−1.Tetrahydrofuran is used instead of diethyl ether, as this solvent is also used in further Grignard reagent syntheses, resulting in a different boiling point (66 °C instead of 35 °C). However, the flask is tempered at 35 °C, which corresponds to the boiling point of diethyl ether and thus allows comparability with the literature reaction conditions.
Coarse magnesium turnings (6.075 g) are filled into the flask, poured over with 25 mL THF, and 1.48 mL of (bromomethyl)benzene is added while stirring. After initiation of the reagent formation, the remaining halide 4 (28.21 mL), dissolved in 62.5 mL of THF, is added. Cooling in an ice bath is applied when boiling is too vigorous.
Batch procedure 2: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 transfer of the flow conditions to batch synthesis
1 transfer of the flow conditions to batch synthesis
For these experiments all parameters that are typically used for the continuous synthesis of benzylmagnesium bromide are applied for the semi-continuous batch experiments. 23 g magnesium turnings (coarse turnings) or 27 g magnesium turnings (fine turnings) are filled into the flask. To this 20 mL (if coarse turnings) or 15 mL (if fine turnings) of the (bromomethyl)benzene/THF solution (0.7 mol L−1) are added. This corresponds to the volume of liquid available in the reactor cartridge. After a 10-min incubation period, the remaining solution (80 mL or 85 mL, respectively) of halide 4/THF solution is added.
Batch procedure 3: literature reaction conditions adapted to flow conditions
The literature reaction conditions are adjusted to be comparable to the conditions used in the continuous reactor. Thus, the same amounts of magnesium and halide (0.07 mol each) are used. With this, high excess of magnesium is avoided while still maintaining a halide concentration of 0.7 mol L−1 in THF. 0.07 mol of magnesium turnings (1.7017 g) are introduced into the flask regardless of the magnesium turning size. In addition, 0.42 mL of (bromomethyl)benzene and 26 mL of THF are added. After an incubation period of 10 min, the remaining (bromomethyl)benzene in THF (7.89 mL in 65.49 mL) is added.Results
Choice of reaction
Within this study, the Grignard reagent formation of phenylmagnesium bromide 2 from bromobenzene 1 was used for the determination of residence time distributions under reactive conditions and the Grignard reagent formation of benzylmagnesium bromide 5 from (bromomethyl)benzene 4 was performed to study the impact on selectivity. The halides used and their corresponding Grignard reagent and Wurtz coupling product are displayed in Fig. 3. | ||
| Fig. 3 Halides (bromobenzene 1, (bromomethyl)benzene 4) and their corresponding Grignard reagent (2, 5) and Wurtz coupling product (3, 6). | ||
The synthesis of phenylmagnesium bromide from bromobenzene was chosen to determine the residence time distribution under reactive conditions due to the following reasons:
(1) It is a well-established and frequently used Grignard synthesis (the synthesis is among the 20 most cited Grignard reagents on the platform SciFinder13).
(2) It is known that this reaction does not tend to form the Wurtz coupling product 3.10 This is important for the RTD measurements since the Wurtz coupling product 3 exhibits bands in the IR spectrum at wavelengths very similar to the band positions of the IR spectrum of toluene, so the presence of 3 would thwart the calibration of the IR spectrometer for toluene.
(3) The synthesis of benzylmagnesium bromide instead is prone to the formation of the Wurtz coupling product 6.1 The Grignard reagent and the Wurtz coupling product both exhibit bands in the IR spectrum at wavelengths similar to the band positions used to determine toluene concentration during RTD measurement. Again, this would thwart the calibration of the IR spectrometer.
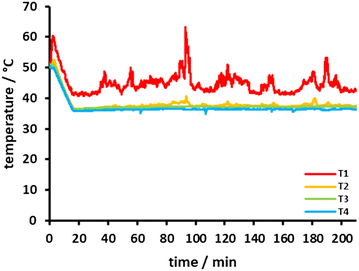
A typical temperature profile during the formation of 2 is shown in Fig. 4. Feeding of magnesium turnings into the reactor led to small temperature drops at sensor T4, indicating the contact of the thermoelement T4 with the colder magnesium turnings. If the boiling point of the chemicals involved is not exceeded, there is no formation of gas bubbles which would influence the flow behaviour within the reactor cartridge to a considerable extent.
In contrast to the use of phenylmagnesium bromide synthesis for RTD measurements, influences of pump behaviour on the selectivity of Grignard reagent formation are most readily demonstrated with syntheses that exhibit a strong affinity for the formation of the Wurtz coupling product. It is reported that the synthesis of benzylmagnesium bromide 5 can sometimes yield the Wurtz product quantitatively,1 making this synthesis a suitable benchmark reaction. Using the same process parameters (flow rate, thermostatic bath temperature, magnesium mass, solvent) as for the synthesis of phenylmagnesium bromide 2 allows comparability of residence time distributions based on the Bodenstein numbers. Only a lower halide concentration was chosen (0.7 mol L−1 instead of 1.0 mol L−1) to avoid precipitation of the Wurtz product 6 during the continuous syntheses.
Benzyl alcohol, a typical product formed by reaction of the Grignard reagent with oxygen and subsequent hydrolysis,3 was detected in the reaction solution. The fraction of benzyl alcohol was found to be up to 19% and could not be fully avoided. For the actual synthesis case, less active Grignard reagent is available for a subsequent synthesis (e.g. Grignard reaction) due to the presence of benzyl alcohol. For studying the impact of the residence time distribution on the selectivity of the Grignard formation, the consecutive reaction is irrelevant. Alcohol formation can be suppressed by consistently working under an inert atmosphere (argon) and by using dry materials and chemicals. During the experiments evidence arose that during sample collection and especially during sample processing, the alcohol is formed. Since benzyl alcohol formation is a consecutive reaction of benzylmagnesium bromide and does not occur or compete with the actual Grignard synthesis, the fraction of detected benzyl alcohol was stoichiometrically considered as benzylmagnesium bromide for the selectivity evaluation (further information on the benzyl alcohol formation can be found in the ESI†).
Residence time measurements under reactive conditions
The determination of the residence time distribution (RTD) and the Bodenstein numbers under reactive synthesis conditions provides a more accurate representation of the hydrodynamic situation in a reactor with a permanently changing magnesium bed. Mean Bodenstein numbers under reactive conditions were found to be lower than those under non-reactive conditions, but standard deviations remain higher for fine magnesium turnings than for coarse turnings (Table 3 and Fig. 5, for details of the non-reactive experiments see part 1, exemplary distribution functions in the ESI†). On average, the Bodenstein numbers decreased by about 66% and 74% for the coarse and fine magnesium turnings, respectively (mean number of RTD measurements: 3.8 for coarse turnings, 5.8 for fine turnings). The mean Bo for the use of pump D with fine magnesium turnings and displacement of the tracer toluene by pure THF even decreased by 90%.| Column | Magnesium turnings | Pump | Introduce/displace tracer | Mean Bo/1 | Standard deviation of Bo/1 | τ/s | Mean ![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) /s /s |
Δ![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) (mean- hydrodyn.)/s (mean- hydrodyn.)/s |
Δ![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) (displ.–introd.)/s (displ.–introd.)/s |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Coarse | Syringe pump A | Displace | 26 | 11 | 652 | 650 | −2 | 11 |
| 2 | Introduce | 21 | 8 | 639 | −13 | ||||
| 3 | Coarse | Micro annular gear pump C | Displace | 28 | 6 | 652 | 683 | 31 | 2 |
| 4 | Introduce | 18 | 11 | 681 | 29 | ||||
| 5 | Coarse | Valveless rotary piston pump D | Displace | 29 | 6 | 652 | 723 | 71 | 35 |
| 6 | Introduce | 20 | 4 | 688 | 36 | ||||
| 7 | Fine | Syringe pump A | Displace | 37 | 25 | 583 | 640 | 57 | 31 |
| 8 | Introduce | 42 | 23 | 609 | 26 | ||||
| 9 | Fine | Micro annular gear pump C | Displace | 46 | 12 | 583 | 651 | 68 | 8 |
| 10 | Introduce | 66 | 37 | 643 | 60 | ||||
| 11 | Fine | Valveless rotary piston pump D | Displace | 25 | 8 | 583 | 717 | 134 | 38 |
| 12 | Introduce | 68 | 34 | 679 | 96 | ||||
 | ||
| Fig. 5 Comparison of Bodenstein numbers and standard deviations for fine and coarse magnesium turnings under reactive and non-reactive conditions. Description of columns according to Table 3. | ||
The decrease in Bodenstein numbers occurs regardless of the fact that portions of the magnesium turnings are replenished during synthesis. The influence of the decreasing magnesium mass should be limited to the period of a single measurement run. This assumption does not consider that the composition of the magnesium bed changes during synthesis. The particle size distribution becomes broader since magnesium turnings react and their size decreases. Voids may form within the magnesium packing that may lead to preferential short-circuit flow paths. Since magnesium turnings are repeatedly replenished during synthesis, the reactor was operated until steady state and complete conversion of the halide reactant 1 were achieved before the first residence time experiment was started. Thus, a constant particle size distribution was assumed for every measurement run. Even though these particle size distributions do not exactly match the particle size distribution of the mixed turnings in part 1, the impact of the particle size distribution on the residence time distribution is also evident for the reactive case in Fig. 5. A broad particle size distribution (coarse Mg turnings) results in higher Bo when the tracer toluene is displaced from the reactor system.
For non-reactive measurements a significant influence of the jogging motor on the axial dispersion was observed. This effect was also noticeable under reactive conditions. When coarse magnesium turnings were used and the jogging motor was omitted, a maximum Bodenstein number of 10 was measured, regardless of the pump used (see the ESI†). This value is smaller than the Bodenstein numbers in Table 3 and Fig. 5 and illustrates the positive effect of the jogging motor on flow behaviour as already mentioned in part 1 for the non-reactive case.
For most cases, mean residence times under reactive conditions are higher than the calculated hydrodynamic residence time. For the coarse magnesium turnings, on average, the mean residence time increases by 11% compared to the unreactive case and by 4% compared to the hydrodynamic residence time. For the fine magnesium turnings, on average, the mean residence time increases by 26% compared to the unreactive case and by 13% compared to the hydrodynamic residence time. Assuming that the reaction does not influence the RTD experiments except for the changes within the magnesium bed, the magnesium bed and its impact on the flow behaviour is responsible for the increase in ![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif) .
.
Possible reasons for the increase in residence time could be:
(1) Missing compaction. The magnesium bed is subject to changes occurring due to the reacting magnesium. The decrease in magnesium mass during one measurement run is 2.4% and 2% for the coarse and the fine magnesium turnings. Although magnesium turnings are replenished after every measurement run, the compaction of the magnesium bed by means of the jogging motor might be less effective in a dry magnesium bed. There might be a small pile up of replenished magnesium turnings at the top of the bed right above the product outlet, too small to be visually noticeable by looking through the viewing window, but significant enough to form an inhomogeneous bed with voids and thus less magnesium in the reactive zone of the reactor cartridge. Under dry conditions and independent of the size of the magnesium turnings, 10% less magnesium mass fits into the reactor cartridge when the jogging motor is omitted, showing the importance of the jogging motor for the compaction of the bed.
(2) Channelling (short-circuit flow). The formation of channels within the bed due to the reacting magnesium would also cause an increase in residence time, if the velocity within such a channel is increased (less pressure drop) but the velocity within the remaining homogeneous bed is reduced. Although the recorded measurement data do not clearly exhibit a strong channelling behaviour (exemplary data in Fig. 6), the risk for channelling does remain for packed beds. Most likely there is not only one channel formed but a few channels with different characteristics, so that channelling is not quite visible within the recorded data.
 | ||
| Fig. 6 Example of a fit of a model curve (dispersion model) to the interpolated and normalized measurement data (fine magnesium turnings, pump C, introduction of the tracer). | ||
Within the present flow behaviour, most likely both reasons exist at the same time, but differentiation between both effects is out of the scope of this study.
Batch synthesis of benzylmagnesium bromide
Well-stirred batch reactors show a maximum degree of backmixing, representing one extreme case of residence time behaviour. The batch synthesis of 5 from 4 in THF is carried out using various procedures, which are described in detail in the Experimental section.Significant amounts of Wurtz product 6 were obtained using the general literature procedure for the synthesis of Grignard reagents (Table 4, batch procedure 1). The Wurtz product 6 has a melting point of 52 °C and in this synthesis it was present in such a high concentration that it precipitated in the flask, forming a thick layer of precipitate, and stirring of the reaction mixture by means of a magnetic stirrer was hardly possible (picture in the ESI†). Due to the large amount of Wurtz product, only a low concentration of Grignard reagent would be available for a subsequent synthesis. The poor stirrability of the reactor contents also means that targeted temperature control was hardly possible.
| Batch procedure | ||||||
|---|---|---|---|---|---|---|
| 1 (coarse Mg turnings) | 2 (fine Mg turnings) | 2 (coarse Mg turnings) | 3 (fine Mg turnings) | 3 (coarse Mg turnings) | ||
| Selectivity/% | Grignard reagent (5) | 11 | 81 | 82 | 74 | 68 |
| Thereof benzyl alcohol | 4 | 10 | 8 | 11 | 8 | |
| Wurtz coupling product (6) | 89 | 19 | 18 | 26 | 32 | |
| Conversion/% | Halide (4) | 100 | 98 | 100 | 95 | 95 |
If the synthesis parameters from the continuous synthesis of 5 were adapted to batch synthesis (batch procedure 2), the formation of 6 was reduced compared to the literature reaction conditions. This was partly a result of the lower halide concentration. It must be noted that intense mixing was hardly possible due to the high magnesium content in the flask (picture in the ESI†). The small volume of the halide solution initially introduced was not sufficient to completely cover the magnesium turnings. For this reason, the subsequently added halide solution initially only met the bare magnesium surface and rinsed from top to bottom through only magnesium turnings before eventually meeting the Grignard reagent in the sump of the flask. Even in a continuous synthesis process, the halide reactant is in more intense contact with the Grignard reagent. Due to the high magnesium excess, there is a large magnesium surface area available for Grignard reagent synthesis per drop of halide solution. Coarse and fine magnesium turnings have similar selectivity in batch synthesis since formation of the Wurtz product can occur only when the halide reactant comes into contact with the Grignard reagent.
Conducting the Grignard reagent synthesis in a batch under the investigated reaction conditions exhibited inadequate temperature control of the exothermic Grignard synthesis due to the poor stirrability of the magnesium turnings, exhibiting a safety hazard. Considering that Grignard reagents are rarely the desired final product and that in laboratory syntheses in batch flasks, the subsequent synthesis such as the Grignard reaction is carried out in the same flask, the high magnesium excess requires separation of the magnesium turnings from the Grignard reagent. The influence of remaining magnesium turnings can lead to problems ranging from issues with stirring to insufficient heat dissipation to undesired reactions with the magnesium turnings (e.g. in the case of a halogen atom on the electrophilic substrate for the subsequent synthesis), so that separation of the magnesium turnings may be necessary. Separation of a solid from a batch flask is not easy to achieve preparatively and, moreover, opening the flask can lead to contamination with, e.g., oxygen and moisture. The latter would lead to yield losses due to the reaction with the Grignard reagent and must be avoided urgently. To handle these challenges, laboratory syntheses in batch flasks are typically conducted with stoichiometric amounts of magnesium, so that at the end of the Grignard synthesis the magnesium is completely consumed. These points were considered during a further adjustment of the literature conditions (batch procedure 3) as a compromise between the previously investigated conditions. The adjustment of the conditions corresponds to what is expected from a laboratory synthesis in a batch flask: in the optimum case, complete conversion of the magnesium turnings and complete mixing of the reactants are achieved so that uniform tempering is possible. Independent of the size of the magnesium turnings, 5% of the halide reactant could not be converted in the flask at the end of the synthesis with condition 3, although the flask was tempered and stirred for an additional 30 min after the addition of the complete halide solution and magnesium turnings were still present (picture in the ESI†). This comparatively low conversion is related to the smaller magnesium surface, although it is not clear why the remaining 4 did not or could not react with the remaining magnesium. When using fine turnings, a higher selectivity was achieved. Assuming complete backmixing and since all other reaction conditions were constant for both types of Mg turnings, the influence of the magnesium surface is particularly evident here: a larger magnesium surface area leads to a higher yield of the Grignard reagent. The formation of side product 6 was found to be higher compared to that of the batch procedure 2, which is also due to the smaller Mg surface.
Continuous synthesis of benzylmagnesium bromide using different pumps
Lower selectivity was measured when coarse magnesium turnings were used (Fig. 7, columns 1–4) compared to the case when fine magnesium turnings were used (Fig. 7, columns 5–8). Fine magnesium turnings possess a larger magnesium surface compared to coarse turnings. Since the Grignard reagent formation is a surface reaction between halide and metallic magnesium, the reaction between magnesium and 4 on a large Mg surface proceeds fast. The data show that not only the size of the magnesium particles has an influence on the selectivity but also the type of pump used. The influence of the particle size is more pronounced than the influence of the pump type. All pumps used were calibrated before use, so that a wrong volumetric flow rate can be ruled out and the influence can be attributed to the pumping principle alone. The following order of selectivity towards 5 when using coarse magnesium turnings was found: pump A > pump D > pump C > pump C + pump B. For the fine magnesium turnings, the following order was obtained: pump C > pump C + pump D > pump A > pump D. Considering only single pumps, the lowest selectivity was measured for pump C for the coarse magnesium turnings. In contrast to that, the best selectivity was found for the fine turnings and the same pump. Similar contradictory results were obtained for pump A: the highest selectivity was registered for the coarse magnesium turnings, while for fine turnings a low selectivity was found. Pronounced formation of the Wurtz product 6 may result from limited magnesium surface accessibility, limiting the conversion of the educt 4. The available Mg surface in a bed of fine turnings is more than twice as high as in a bed of coarse turnings (see part 1). Another reason for an increasing formation of 6 might be an enhanced backmixing that widens the contact zone where 4 and 5 are simultaneously present. The effect of the available Mg surface is clearly observable when comparing the results of coarse and fine turnings. When assuming that Mg beds with similar sized turnings are comparable, the available Mg surface cannot be the reason for the differences in selectivity when using the same turnings. Therefore, differences in the residence time distribution are likely the reason for pronounced Wurtz product formation. | ||
| Fig. 7 Selectivity for the synthesis of 5 depending on the pump system used. Description of columns in Table 5. | ||
When using coarse magnesium turnings, a broader particle size distribution is present. Therefore, the magnesium bed as a whole is more inhomogeneous compared to a bed of finer turnings. According to the RTD results for non-reactive Mg beds, the small amplitudes and high frequencies of pump D have only a limited effect on the degree of backmixing. Therefore, pump C and pump D exhibit similar selectivity. Although pump A does only pulse with long periods, it does exhibit a distinct pulse generated by switching between the two alternating syringes, which is noticeable by a brief dip in volumetric flow rate (see part 1). This single but distinct pulse appears to have a positive effect on the flow behaviour in the magnesium bed, most likely because the momentum change of this pulse is large enough to induce movement of the turnings. For fine turnings, the highest selectivity was recorded with the pulsation-free pump C. Pumps or pump combinations that exhibit pulsed flow led to lower selectivity. It is concluded that for a compacted magnesium bed of fine magnesium turnings, a pulsation-free pump leads to the lowest degree of backmixing/axial dispersion. In contrast to the non-reactive RTD measurements, pulsed flow conditions seemingly do not reduce axial dispersion. For the non-reactive RTD measurements (see part 1), an increase of the Bo could be realized by tuning the oscillatory Reynolds number Re0. Experiments with pump parameters that were found to be most effective without reaction (coarse turnings: Re0 = 1.38, f = 0.0416 s−1, x0 = 0.0057 m; fine turnings: Re0 = 0.10, f = 0.6667 s−1, x0 = 0.000065 m) did not prove effective during Grignard reagent synthesis. Compared to the pulsation-free pump C, 4% (coarse turnings) or 3% (fine turnings) less 5 was obtained.
| Column | Magnesium turnings | Pump |
|---|---|---|
| 1 | Coarse | Pump A |
| 2 | Coarse | Pump C |
| 3 | Coarse | Pump D |
| 4 | Coarse | Pump C + pump B |
| 5 | Fine | Pump A |
| 6 | Fine | Pump C |
| 7 | Fine | Pump D |
| 8 | Fine | Pump C + pump D |
Correlation between Bodenstein number and selectivity
A comparison of batch with continuous experiments reveals that the yield of the desired Grignard reagent 5 was always greater in the continuous experiments. The best results obtained with the batch synthesis are similar to the worst results obtained with the continuous approach. By using a continuous synthesis process, the fraction of 6 can be reduced, the quality of the product obtained is improved and the efficiency of the synthesis is increased. The positive effect of a continuous synthesis process on the production of Grignard reagents is thus clearly demonstrated (Fig. 8). For the fine magnesium turnings, the Bodenstein numbers obtained by displacing the toluene from the reactor system were used, whereas for the coarse magnesium turnings, the Bodenstein numbers obtained by introducing the tracer toluene were used. This selection was made based on the Bodenstein numbers obtained when using a mixture of coarse and fine magnesium turnings (see part 1 for details), since the magnesium bed in the steady state of a Grignard synthesis resembles a mixture of coarser and finer magnesium turnings due to the reacting magnesium. Assigning a Bodenstein number of 0 to the batch synthesis enables evaluation of the impact of the residence time distribution on the selectivity towards the Grignard reagent. A linear correlation is found for both sizes of Mg turnings, indicating that a further improvement in Bodenstein numbers could lead to a further increase in selectivity. | ||
| Fig. 8 Selectivity as a function of Bodenstein number for the use of coarse and fine magnesium turnings. | ||
Oscillating flow operation
Due to the changing magnesium bed during Grignard reagent synthesis, the oscillation amplitude and frequency parameters identified as optimal for the non-reactive operation cannot be transferred when aiming for an increase in selectivity.Varying both parameters based on the accessible range of parameters of pump D did not increase the yield of 5 when using the fine magnesium turnings compared to synthesis using pulsation-free pump C. The selectivity decreased with increasing oscillatory Reynolds number (Fig. 9). The highest selectivity was still achieved under oscillation-free flow conditions. A similar correlation was observed for the oscillation frequencies (Fig. 10). The maximum selectivity thus shifts to smaller oscillatory Reynolds numbers in benzylmagnesium bromide synthesis. Evaluation of the effect of the stroke volume (see Fig. 11) suggests that increasing the stroke volume above a value of 100 μL (maximum stroke volume for pump D with the used pump head, highest amplitude) could lead to improved selectivity.
 | ||
| Fig. 9 Selectivity as a function of oscillatory Reynolds number for the use of fine magnesium turnings (pump C + pump D). | ||
 | ||
| Fig. 10 Selectivity as a function of oscillation frequency for the use of fine magnesium turnings (pump C + pump D). | ||
 | ||
| Fig. 11 Selectivity as a function of oscillation amplitude for the use of fine magnesium turnings (pump C + pump D). | ||
While the use of the programmable syringe pump allowed for an increase in amplitude, only lower oscillation frequencies could be realized. The oscillating flow conditions did not lead to an improvement in selectivity (Fig. 12). Regardless of Re0, the selectivity remained at about 80%. Even though the same Re0 range was accessed as with pump D, the selectivity obtained with the syringe pump was lower. Since only the relation between the amplitude and the frequency of the pulsation was changed, it can be assumed that in the reactive case the influence of the amplitude on the selectivity is more pronounced than the effect of the frequency. Thus, Re0 does not seem to be the best suited indicator for the realized flow conditions in a reacting magnesium bed. The interaction of these two parameters is hidden.
 | ||
| Fig. 12 Selectivity as a function of the oscillatory Reynolds number for the use of fine magnesium turnings (pump C + pump B). | ||
Varying the oscillation parameters when using coarse magnesium turnings leads to a similar result to that when using the finer magnesium turnings. A slight negative dependency of the selectivity on the oscillatory Reynolds number was observed (Fig. 13). The results scatter more compared to that when using fine turnings. Interestingly, for some data points selectivity values above the value of an oscillation-free flow were recorded (Re0 = 0). The data suggest that some positive effect of the oscillating flow might occur when using the coarser magnesium turnings. A separate analysis of the influence of the oscillating frequency and the amplitude does not yield a clear picture. The selectivity dependency on the frequency scatters strongly around a selectivity of about 75% (Fig. 14). It is concluded that the stroke frequency has no influence on the selectivity for a bed of coarse magnesium turnings, at least in the applied frequency range. The influence of the amplitude is not much clearer. At the highest amplitude, a negative impact on the selectivity can be observed. For the remaining data points scattering is too strong to draw a final conclusion (Fig. 15). The significant scattering of the data points leads to the conclusion that the partial positive effect on selectivity is not a result of the oscillating flow, but more likely is related to less uniform and less reproducible magnesium beds due to the broader particle size distribution and larger magnesium turnings. The characteristics of the magnesium bed seem to dominate when coarse magnesium turnings are used.
 | ||
| Fig. 13 Selectivity as a function of oscillatory Reynolds number for the use of coarse magnesium turnings (pump C + pump B). | ||
 | ||
| Fig. 14 Selectivity as a function of oscillation frequency for the use of coarse magnesium turnings (pump C + pump B). | ||
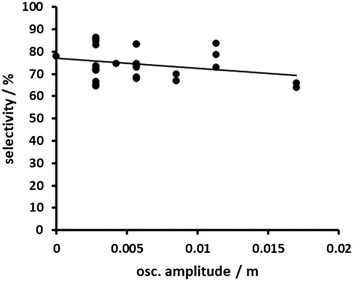 | ||
| Fig. 15 Selectivity as a function of oscillation amplitude for the use of coarse magnesium turnings (pump C + pump B). | ||
In contradiction to the hypothesized improvement of the selectivity by reducing the degree of backmixing, as indicated by non-reactive RTD measurements, a significant improvement of the selectivity for both fine and coarse magnesium turnings could not be proven. This is explained by the fact that for each positive deflection of amplitude, the flow velocity across the magnesium bed is briefly increased, although the total volume of fluid conveyed remains unchanged. The higher the amplitude at a constant frequency, the higher the Re0 and also the resulting flow velocity. The flow rate in a continuous synthesis corresponds to the feed rate in a batch synthesis and there, too, the faster addition of the halide reactant leads to increased formation of the Wurtz coupling product.1 The formation of channels and insufficient compaction of the magnesium bed during synthesis lead to an inhomogeneous bed which enhances irregular flow conditions and consequently thwart the efforts to minimize axial dispersion by means of a superimposed oscillatory flow as is also reflected in the significantly lower Bodenstein numbers of the reactive measurements.
Conclusions
The investigations demonstrate that minimizing the degree of backmixing represents a valid strategy to deal with Grignard reagents that are prone to the formation of Wurtz products. The elaborated correlations between the Bodenstein number and the selectivity towards the desired Grignard reagent product, that also include batch syntheses, underline the general applicability of the concept. This finding is of special relevance for Wurtz-affine reactions that require advanced reaction control, which, as has been demonstrated, can be realized with a suitable reactor or flow conditions. Consequently, a change of the solvent and an associated in-depth change of the entire reaction system and potentially the product can be avoided. Furthermore, it is evident that Bo of 50 or above is required to lead to a significant improvement of the selectivity. A particular challenge is the realization of a narrow residence time distribution under reactive conditions. Since the magnesium bed participates in the reaction and the Mg turnings change their size during the reaction progress, knowledge gained for the non-reactive case cannot be transferred directly. The residence time distribution is much broader under reactive conditions and especially a manipulation of the hydrodynamics in the magnesium bed by means of oscillating flows strongly deviates. The inhomogeneity of the bed seems to be the most important parameter in this case, which governs the residence time distributions that can be realized.Although the applicability of the oscillating flow conditions to generate narrow residence time distributions could not be proven under reactive conditions, the results achieved for the non-reactive case in part 1 of this contribution clearly show the potential of this approach. These results also give evidence that a meaningful tuning of the oscillatory flow conditions to the characteristics of fixed beds that participate in the reaction as reactant can open the avenue to a broader application of oscillating flow conditions as a tool for reaction control.
In general, a continuously operated flow reactor yields higher selectivity and consequently product quality for Grignard reagent syntheses than a synthesis in a semi-continuous batch process due to the reduced degree of backmixing. The comparatively low reaction volume of flow reactors provides a more precise temperature control and comes along with a reduced hazard potential that would stem from the release of toxic and hazardous chemicals in the event of leakage. The resulting smaller reactor dimensions, even on a large industrial scale, and the possibility of modularization of the production plant allow flexibility of the production process in terms of adaptation to different environments and available space. To sum up, the continuous synthesis of a Grignard reagent has the potential to improve product quality, increase process safety and enable process flexibility.
Notation
| Bo | Bodenstein number | 1 |
| f | Frequency | s−1 |
| Re0 | Oscillatory Reynolds number | 1 |
| t | Time | s |
![[t with combining macron]](https://www.rsc.org/images/entities/i_char_0074_0304.gif)
|
Mean residence time | s |
| x 0 | Amplitude | m |
Greek letters
| τ | Hydrodynamic residence time | s |
Author contributions
Conceptualization: E. D. (supporting), D. Z. (lead); data curation: E. D. (lead); formal analysis: E. D. (lead); funding acquisition: E. D. (supporting), M. M. (lead); investigation: E. D. (lead); methodology: E. D. (equal), D. Z. (equal); project administration: E. D. (lead); resources: E. D. (supporting), D. Z. (supporting), G. M.-F. (supporting), M. M. (supporting); supervision: D. Z. (lead), G. M.-F. (supporting), M. M. (supporting); validation: E. D. (lead), D. Z . (supporting); visualization: E. D. (lead); writing – original draft: E. D. (lead); writing – review & editing: E. D. (equal), D. Z. (equal).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the Fraunhofer IMM and the Fraunhofer organization for internal funding including the funding via the Fraunhofer development and career program TALENTA. The authors thank Lisa Pokropp (Fraunhofer IMM) for the creation of the table of contents graphic.References
- P. E. Rakita and G. S. Silverman, Handbook of Grignard reagents, ed. M. Dekker, New York, Basel, Hong Kong, 1996 Search PubMed.
- H. Kryk, G. Hessel, W. Schmitt and N. Tefera, Safety aspects of the process control of Grignard reactions, Chem. Eng. Sci., 2007, 62, 5198–5200 CrossRef CAS.
- A. Kadam, M. Nguyen, M. Kopach, P. Richardson, F. Gallou, Z.-K. Wan and W. Zhang, Comparative performance evaluation and systematic screening of solvents in a range of Grignard reactions, Green Chem., 2013, 15, 1880 RSC.
- D. F. Aycock, Solvent Applications of 2-Methyltetrahydrofuran in Organometallic and Biphasic Reactions, Org. Process Res. Dev., 2007, 11, 156–159 CrossRef CAS.
- A.-G. Sicaire, M. A. Vian, A. Filly, Y. Li, A. Bily and F. Chemat, in Alternative Solvents for Natural Products Extraction, ed. F. Chemat and M. A. Vian, Springer Berlin Heidelberg, Berlin, Heidelberg, 2014, pp. 253–268 Search PubMed.
- T. van Gerven and A. Stankiewicz, Structure, Energy, Synergy, Time—The Fundamentals of Process Intensification, Ind. Eng. Chem. Res., 2009, 48, 2465–2474 CrossRef CAS.
- M. Saber, J. M. Commenge and L. Falk, Microreactor numbering-up in multi-scale networks for industrial-scale applications: Impact of flow maldistribution on the reactor performances, Chem. Eng. Sci., 2010, 65, 372–379 CrossRef CAS.
- S. Hafeez, G. Manos, S. M. Al-Salem, E. Aristodemou and A. Constantinou, Liquid fuel synthesis in microreactors, React. Chem. Eng., 2018, 3, 414–432 RSC.
- S. R. L. Gobert, S. Kuhn, L. Braeken and L. C. J. Thomassen, Characterization of Milli- and Microflow Reactors: Mixing Efficiency and Residence Time Distribution, Org. Process Res. Dev., 2017, 21, 531–542 CrossRef CAS.
- G. Menges-Flanagan, E. Deitmann, L. Gössl, C. Hofmann and P. Löb, Scalable Continuous Synthesis of Grignard Reagents from in Situ-Activated Magnesium Metal, Org. Process Res. Dev., 2020, 24, 315–321 CrossRef CAS.
- G. Menges-Flanagan, E. Deitmann, L. Gössl, C. Hofmann and P. Löb, Scalable Continuous Synthesis of Organozinc Reagents and Their Immediate Subsequent Coupling Reactions, Org. Process Res. Dev., 2021, 25, 427–433 CrossRef CAS.
- Organikum, Organisch-chemisches Grundpraktikum, Wiley VCH, Weinheim, 23rd edn, 2009 Search PubMed.
- M. Berton, K. Sheehan, A. Adamo and D. T. McQuade, Disposable Cartridge Concept for On-Demand Synthesis of Turbo Grignards, Knochel-Hauser Amides and Magnesium Alkoxides, Beilstein J. Org. Chem., 2020, 16, 1343–1356 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Distribution functions for residence time measurements, pictures of Grignard reagent synthesis in a stirred tank (semi-continuous batch mode), explanation of benzyl alcohol formation, conversions and selectivity. See DOI: https://doi.org/10.1039/d3re00191a |
| This journal is © The Royal Society of Chemistry 2023 |