 Open Access Article
Open Access ArticlePerformance of post phthalate Ziegler Natta catalysts with activity limiting agents for propylene gas phase polymerization
Abdulrahman
Albeladi
ab,
Akhlaq
Moman
b and
Timothy F. L.
McKenna
*a
aCatalysis, Polymerization, Processes, and Materials (CP2M), UMR 5128 CNRS/UCBL/CPE-Lyon, 69616 Villeurbanne Cedex, France. E-mail: timothy.mckenna@univ-lyon1.fr
bSABIC Technology Center Riyadh, 2nd Industrial Area, Kharj Highway, Riyadh, 11551, Saudi Arabia
First published on 16th March 2023
Abstract
The performance of isopropyl myristate, methyl trimethylacetate, and dibutyl sebacate (IPM, MTMA, and DBS, respectively) as activity limiting agents (ALA) were evaluated using a post phthalate Ziegler Natta catalyst system for the gas phase polymerization of propylene at different ALA ratios to titanium (ALA/Ti) and different temperatures. ALAs are used to limit catalyst activity and prevent formation of agglomerates in the reactor in cases of thermal runaways. All the ALAs were effective at retarding the catalyst activity at temperatures higher than 90 °C when used with n-propyltrimethoxysilane (N-donor) as an external donor, even at the lowest ALA/Ti. The lowest drop in catalyst activity at the lowest reaction temperature was observed with IPM, while the highest drop was observed with MTMA. Moreover, when used without an external donor the ALAs showed a retarding effect on the catalyst at higher temperatures but to a lesser extent than when an external donor was used. Furthermore, none of the ALAs had a significant impact on the molecular weight averages or distributions, bulk density or fine content. On the other hand, the isotacticity, evaluated in terms of the isotactic pentads and the average meso sequence length (MSL), was found to increase slightly with MTMA and DBS. Finally, it was observed that the reaction between the co-catalyst and the ALA is very crucial in determining the retarding effects of the ALA.
1. Introduction
The combination of the highly exothermic nature of Ziegler Natta (ZN) catalyzed polymerizations and the need to operate at high polymerization rates makes the possibility of thermal runaway a real risk, particularly in gas phase reactors. Thermal runaway in fluidized bed reactors while the catalyst is still highly active leads to many problems ranging from increased fouling to complete loss of fluidization due to the formation of agglomerates. For propylene polymerization, the use of activity limiting agents (ALA), also called thermal runaway reducing agents (TRRA), have proven to be quite effective in deactivating the catalyst at elevated reaction temperatures higher than 80 °C which provides an effective solution to the problems mentioned. This effect is generally referred to as the self-extinguishing property of a catalyst system.1 Such modifiers are electron donating compounds similar to the external electron donors that are used in propylene polymerization to control stereoregularity but they have not been proven to be effective tacticity control agents on their own,2 hence, mostly they are injected in addition to classical tacticity control agents such as alkoxysilanes.In an overview of recent advances in fluidized bed polymerization technology, Cai et al.3 presented the advantage of using DOW's (the technology is now owned by W. R. Grace) proprietary Advance Donor Technology (ADT) with Ziegler Natta catalysts for propylene polymerization where the catalyst deactivates, or, as the authors coined it, it self-extinguishes as the temperature increases. They stated that such optimization of catalyst kinetics reduces particle agglomeration by killing the active sites before the particle temperature can reach the softening point. Another advantage they also mentioned was the increased flexibility in the reactor operating envelope, such as increasing the production rate without decreasing the polymer bed inventory or working at higher fluidized bed densities while maintaining a high production rate.
Chen and Campbell4 in their patent (US 7491670B2, entitled “A self limiting catalyst composition and propylene polymerization process”) disclosed a catalyst composition that includes an Activity Limiting Agent (ALA) to control polymerization in addition to the normal modifiers used for propylene polymerization (i.e., co-catalyst and selectivity control agents). This ALA renders the catalyst almost inactive at elevated temperatures. They discussed that the use of para-ethoxy ethyl benzoate (PEEB) as an external donor with third generation ZN catalysts provides the desired self-extinguishing property with respect to the reaction temperature but is ineffective for tacticity control with later generations of ZN catalysts. They presented many examples of combinations of different selectivity control agents, including silanes, and ALAs such as dibutylsebacate (DBS) and methyltrimethylacetate (MTMA) that seemed to lead to a significant (self-extinguishing) decrease in catalyst activity at high reaction temperatures. Looking at their data, one can see that when selectivity control agents are solely used, the catalyst still retains enough activity at temperatures close to the softening temperature of polypropylene which may lead to agglomeration and fouling, but this was not the case when ALAs were used.
Cai et al.5 published a gas phase polymerization process where the fluidized bed density was higher than 128 kg m−3. Operating at a high bed density for a fluidized bed reactor means a lower superficial gas velocity which in turn means less heat is removed from the bed. On the other hand, as long as one maintains stable reactor operation, a high bed density means high space time yields that are economically desirable. However, as in the patent mentioned, the inventors employed a mixed external donor system where one of the donors was an ALA which allowed for operating the reaction beds at higher bed densities without encountering continuity issues. The use of an ALA provides the needed protection from temperature excursions as the catalyst is deactivated at elevated temperatures before reaching the softening point of the polymer. They presented two examples of using DBS in homo-polymerization and random copolymerization of propylene in vertically and horizontally stirred reactors, and compared these to two similar examples without an ALA. They showed that after a problem occurred to the agitator, the kill gas was immediately introduced and an attempt to start agitation was made after fixing the problem. Agitation could not have been started when no ALA was used due to the blocking of the reactor with agglomerates and chunks, which was not the case when an ALA was used and agitation in this case was re-started without issues.
In a patent by Basell, Morini et al.2 highlighted two negative aspects of using ALAs to control the reaction at elevated temperatures: the drop in base catalyst activity at normal operating temperatures and the drop in stereoregularity when attempting to lower the ratio of the mixed donor to the catalyst. In their patent, they revealed a catalyst composition for the polymerization of propylene containing a diether internal donor that exhibits the desired self-extinguishing property without the addition of specific donors like in the case of using ALAs. They presented several examples whereby they showed that the residual activity of their catalyst system drops to as low as 43% at 90 °C.
Several other patents disclose novel mixtures of external donors and processes where an ALA is used, including in the production of impact propylene copolymers,6 the production of high melt flow propylene polymers,7 the production of ethylene based polymers,8 the discontinuous addition of thermal runaway control agents in the commercial production of polypropylene,9 the use of monoether compounds as ALAs,10 the utilization of fluorinated fatty acids as ALAs,11 and an improved multi-zone olefin polymerization process operating in deep condensed mode employing several thermal runaway control agents.12
Although there has been a significant surge in patent applications disclosing processes and compounds where the desired self-extinguishing property can be achieved in olefin polymerization reactors since 2005 (cf.Fig. 1), there are no clear hypotheses about their mechanism of action. Vipin Raj et al.13 conducted DFT studies on two novel ALAs and concluded that interactions between the ALA and dimeric TEA lead to a decomposition reaction with the help of another TEA molecule, producing either a ketone or an aldehyde. Similarly, the interaction of the ALA with Ti on the 110 surfaces of MgCl2 produces a ketone more favorably. The decomposition reaction products lead to the poisoning of active sites as both ketones and aldehydes are known to be able to poison Ziegler Natta active sites. They also explained the efficacy of one ALA over the other in their study based on thermodynamic binding preferences supported by the computational study. Such decomposition reactions have been experimentally observed as a result of the reaction between alkyl aluminum compounds and esters by Chien et al.14 who also suggested the pathways by which such reactions occur and the role of temperature in the decomposition rates.
In this work, we evaluate the performance of a sixth generation Ziegler Natta catalyst for propylene polymerization in the gas phase developed by SABIC with three activity limiting agents. The ALAs were chosen from the cited patents and have been proven to work effectively with the corresponding catalyst systems used in the aforementioned patents. The main property we will look at is the so-called self-extinguishing property of the catalyst system. This will be conducted by varying the amounts of ALAs and combination with a selectivity control agent at different reaction temperatures. We will also take a closer look at the polymer microstructure and powder morphology (mainly the bulk density and fine content) to identify eventual effects of ALA, if any.
2. Experimental
2.1. Materials
The catalyst used in this study was a sixth generation (phthalate free) Ziegler Natta catalyst developed by SABIC. The titanium content of this catalyst was around 3 wt% and it was formulated with two different internal electron donors. The triethylaluminum purchased from Witco was diluted to around 1 M in n-heptane which was purified using an Mbraun solvent purification unit. The external electron donor (ED) n-propyltrimethoxysilane (N-donor) was purchased from ABCR and stored over molecular sieves after bubbling for a couple of hours using Argon to get rid of free oxygen. The ALAs isopropyl myristate (IPM), methyl trimethylacetate (MTMA), and dibutyl sebacate (DBS) were purchased from ABCR and were treated similarly to the electron donor. Both the ED and ALA compounds were diluted in purified heptane before being introduced to the polymerization experiments.2.2. Polymerization setup and procedure
The polymerization setup and procedures of preparation, the conduct of the experiments, and post treatment of the polymers were the same as those described in a previous publication.152.3. Polymer characterization
The polymers in this study were characterized as follows: using GPC for molecular weight, using 13C NMR for stereoregularity, using a standard volume recipient for bulk density, and using a Malvern Mastersizer 3000 particle analyzer for particle size distribution. All the characterization methods were described in a previous publication.152.4. Diffuse reflectance infrared transmittance (DRIFT)
DRIFT analyses were performed using a Nicolet 6700 FTIR spectrometer where data are collected using OMNIC software. The treated samples were placed in airtight DRIFT cells with a CaF2 window inside the glove box. For each spectrum, 64 scans were collected with a resolution of 4 cm−1. All the treatments of the catalyst for DRIFT analysis were performed at room temperature.2.5. Experiments
The three main variables in this study were the reaction temperature, the use of an electron donor (an alkoxysilane), and the ALA/Ti ratio. All the core experiments of this work were conducted with 20 g of NaCl as a seedbed under a propylene pressure of 8.0 barg and 2.0 mol% hydrogen. The molar ratios for the co-catalyst and the ED to Ti were 51 and 3, respectively. As shown in Table 1, experiments 1 to 3 are the reference experiments where an N-donor was used and the reaction temperature was varied at three levels: 58, 78, and 94 °C. Experiments 4 to 6 are similar to the reference experiments except that no ED was added. In this experimental program, three ALAs were used: IPM, MTMA, and DBS; and their chemical structures are shown in Fig. 2. The rest of the experiments from numbers 7 to 18 were repeated for each ALA; the numbering of these experiments for later sections of this work will be consisting of two digits, the first one refers to the numbers shown in Table 1, and the second digit will refer to the ALA: 1 for IPM, 2 for MTMA, and 3 for DBS. For example, experiment number 7.3 refers to the conditions of experiment 7 with DBS as an ALA. The ALA/Ti molar ratio was varied for these experiments at three different levels: 0.3, 3.0, and 9.0 at the different reaction temperatures. For each ALA, a set of three experiments where no ED was used were also conducted. All the experiments were conducted for 60 minutes.| SN | ED/Ti | ALA/Ti | T (°C) |
|---|---|---|---|
| 1 | 3.0 | 0 | 58 |
| 2 | 3.0 | 0 | 78 |
| 3 | 3.0 | 0 | 94 |
| 4 | 0 | 0 | 58 |
| 5 | 0 | 0 | 78 |
| 6 | 0 | 0 | 94 |
| 7 | 3.0 | 0.3 | 58 |
| 8 | 3.0 | 0.3 | 78 |
| 9 | 3.0 | 0.3 | 94 |
| 10 | 3.0 | 3.0 | 58 |
| 11 | 3.0 | 3.0 | 78 |
| 12 | 3.0 | 3.0 | 94 |
| 13 | 3.0 | 9.0 | 58 |
| 14 | 3.0 | 9.0 | 78 |
| 15 | 3.0 | 9.0 | 94 |
| 16 | 0 | 3.0 | 58 |
| 17 | 0 | 3.0 | 78 |
| 18 | 0 | 3.0 | 94 |
 | ||
| Fig. 2 Chemical structures for the ALAs used in this study: A. isopropylmyristate (IPM), B. methyltrimethylacetate (MTMA), and C. dibutylsebacate (DBS). | ||
A second set of experiments were carried out, as shown in Table 2, with the objective of discriminating the possible reactions of ALA during the gas phase polymerization of propylene. This set of experiments were conducted using the same catalyst with 6 barg of propylene and 20 g of NaCl as a seedbed, where neither hydrogen nor a selectivity control agent (alkoxysilane; namely, an N-donor) was introduced in an attempt to minimize the variables. All the experiments were conducted with one ALA, dibutylsebacate (DBS), and triethylaluminum (TEA) as the co-catalyst. The four main variables considered here were the Al/Ti ratio, the ALA/Ti ratio, the reaction temperature, and the method of addition of the co-catalyst and the ALA. The methods of addition of modifiers are described below:
| SN | Al/Ti | ALA/Ti | T (°C) | Al and ALA addition method |
|---|---|---|---|---|
| 19 | 50 | 9 | 36 | Method A |
| 20 | 50 | 9 | 96 | Method A |
| 21 | 2 | 9 | 36 | Method B |
| 22 | 2 | 9 | 96 | Method B |
| 23 | 2 and 48 | 9 | 36 | Method C |
| 24 | 2 and 48 | 9 | 96 | Method C |
| 25 | 2 and 48 | 9 | 36 | Method D |
| 26 | 2 and 48 | 9 | 96 | Method D |
1. Method A: the catalyst was pre-contacted with the ALA and then vacuum dried; the catalyst was then introduced to the reactor one hour after the pre-contact step, and the co-catalyst was injected before the start of polymerization.
2. Method B: the catalyst was pre-activated with the co-catalyst which was then vacuum dried before introduction to the reactor with a time of contact of around 10 minutes; then the reaction was started without an ALA until around minute 30 when it was introduced.
3. Method C: similar to method B (the catalyst was pre-activated with an Al/Ti ratio of 2) except that the ALA added during the reaction was mixed with the co-catalyst to make a total Al/Ti ratio of 50 and injected at around minute 15 of the reaction.
4. Method D: similar to method B except that the ALA addition was performed at around minute 15 of the reaction followed by TEA injection at around minute 25.
3. Results and discussion
3.1. Reaction rates
Fig. 3-C shows the catalyst productivity as a function of temperature for the two sets of experiments: with and without an external donor; the activity in both cases follows a decreasing trend but the decrease in the presence of the ED at 94 °C compared to 58 °C is around 26% whereas it is around 89% in the absence of the ED. This behavior could mean the catalyst itself exhibits a self-extinguishing property in the absence of external donors. This catalyst has two types of internal donors, one that is not affected by the co-catalyst, and the other one which can be removed by the co-catalyst, and we believe it is possible for this second donor to poison the active sites at a higher temperature because of the possible higher rate of removal of this second internal donor at elevated temperatures by the co-catalyst.
| Experiment set | Productivity drop at 94 °C (%) | Productivity change at 58 °C (%) |
|---|---|---|
| ALA/Ti = 0.3 + N-donor | 67 | +2.00 |
| ALA/Ti = 3.0 + N-donor | 74 | −14.0 |
| ALA/Ti = 9.0 + N-donor | 93 | −19.0 |
| ALA/Ti = 3.0 | 40 | +23.0 |
Using IPM in the absence of an N-donor leads to around 20% increase in catalyst productivity, as shown in Fig. 4-E, while the reaction rate curves at 58 and 78 °C show a similar behavior to the rest of the experiments where we used an N-donor. However, at the highest temperature, we see the appearance of a second activation as evidenced by the slightly increasing reaction rate after the first 5 minutes of reaction.
One of the parameters we used in this study was the extent of the catalyst self-extinguishing property in the percentage of activity decrease at 94 °C in relation to the standard experiment at the same temperature. As we can see in Table 3 for IPM, even at the lowest ratio of ALA/Ti, a 67% drop in activity takes place compared to the standard system (STD) while the activity at the lowest temperature is not affected. Further increasing the amount of IPM increases the activity drop to 74 and 93%, however, at the highest ratio of IPM where the best self-extinguishing performance is seen, it comes at the expense of activity at the lower temperature (i.e., normal operating temperatures) with a drop of around 19% compared to the standard experiments. Finally, using IPM without an external donor does not seem to be very effective in controlling the activity at higher temperatures with the activity dropping by around 40% while the activity at the lowest temperature increased by 23%. The increase in activity at the lower temperatures is due to the lower total ED + ALA molar ratio to Ti which lowers the poisoning effect of the external bases leading to an increase in activity. However, the inefficient behavior of the ALA when no external donor is used is due to two factors: the increase of TEA concentration reacting with IPM which will be discussed later and the lower ratio of ALA to Ti.
The efficacy of MTMA in inducing self-extinguishing of the catalyst can be observed in Fig. 5-E and Table 4 where a significant drop of activity can be seen, even at the lowest ALA/Ti ratio. However, the highest activity drop is observed at the highest ALA/Ti ratio with about 96% loss of activity compared to the standard catalyst experiment at the same temperature (i.e., experiment number 3). Though the behavior of retarding activity at higher temperatures seems to be similar to IPM, the negative influence on the activity at the lowest temperature is quite significant where 50% of the activity is lost as shown in Table 4 compared to the standard system with an increase of 30% compared to the loss with IPM at the highest ALA/Ti ratio. We believe this effect is dependent on two main factors: the reactivity of MTMA with TEA and the higher diffusivity of MTMA. We will discuss in a later section of this work the possible mechanisms of actions for ALA which will shed some light on some of the differences in their self-extinguishing behaviors. Moreover, MTMA seems to be quite effective when used in the absence of an external donor in contrast to IPM where we see an 80% drop in activity at 94 °C while the negative effect on activity at 58 °C is only a 14% decrease.
| Experiment set | Productivity drop at 94 °C (%) | Productivity change at 58 °C (%) |
|---|---|---|
| ALA/Ti = 0.3 + N-donor | 50 | +18.0 |
| ALA/Ti = 3.0 + N-donor | 60 | −20.0 |
| ALA/Ti = 9.0 + N-donor | 96 | −50.0 |
| ALA/Ti = 3.0 | 80 | −14.0 |
| Experiment set | Productivity drop at 94 °C (%) | Productivity change at 58 °C (%) |
|---|---|---|
| ALA/Ti = 0.3 + N-donor | 17 | −21.0 |
| ALA/Ti = 3.0 + N-donor | 69 | −14.0 |
| ALA/Ti = 9.0 + N-donor | 97 | −40.0 |
| ALA/Ti = 3.0 | 50 | +98.0 |
3.2. Polymer microstructure and morphology
The microstructure (molecular weight and tacticity) and morphology (bulk density and fine content) analyses were performed on the most meaningful subsets of experiments, which are listed below:• The reference experiments: 1 to 3.
• The reference experiments without an external donor: 4 to 6.
• ALA experiments with the highest ALA/Ti ratio, which is 9.
• ALA experiments in the absence of an external donor.
| SN | ED | ALA | ALA/Ti | M n (kDa) | M w (kDa) | MWD |
|---|---|---|---|---|---|---|
| 1 | N | None | 0 | 40 | 217 | 5.4 |
| 2 | N | None | 0 | 33 | 166 | 5.0 |
| 3 | N | None | 0 | 24 | 124 | 5.3 |
| 4 | None | None | 0 | 32 | 163 | 5.1 |
| 5 | None | None | 0 | 27 | 138 | 5.2 |
| 6 | None | None | 0 | 13 | 83 | 6.2 |
| 13.1 | N | IPM | 9.0 | 31 | 213 | 6.9 |
| 14.1 | N | IPM | 9.0 | 29 | 190 | 6.5 |
| 15.1 | N | IPM | 9.0 | 26 | 130 | 5.0 |
| 16.1 | None | IPM | 3.0 | 35 | 191 | 5.5 |
| 17.1 | None | IPM | 3.0 | 29 | 152 | 5.2 |
| 18.1 | None | IPM | 3.0 | 20 | 115 | 5.7 |
| 13.2 | N | MTMA | 9.0 | 39 | 301 | 7.7 |
| 14.2 | N | MTMA | 9.0 | 22 | 164 | 7.6 |
| 15.2 | N | MTMA | 9.0 | 22 | 113 | 5.2 |
| 16.2 | None | MTMA | 3.0 | 30 | 187 | 6.3 |
| 17.2 | None | MTMA | 3.0 | 28 | 147 | 5.2 |
| 18.2 | None | MTMA | 3.0 | 15 | 103 | 6.9 |
| 13.3 | N | DBS | 9.0 | 43 | 233 | 5.3 |
| 14.3 | N | DBS | 9.0 | 39 | 212 | 5.4 |
| 15.3 | N | DBS | 9.0 | 26 | 151 | 5.8 |
| 16.3 | None | DBS | 3.0 | 34 | 177 | 5.3 |
| 17.3 | None | DBS | 3.0 | 34 | 142 | 4.2 |
| 18.3 | None | DBS | 3.0 | 21 | 104 | 4.9 |
The MWD results are comparable for all experiments where we do not see any major effect of ALAs or temperature except for MTMA when used with an external donor where a slight broadening is observed due to the atypical response as an effect of temperature on Mn and Mw. Additionally, when DBS is used in the absence of an external donor, the MWD shows mild narrowing at a reaction temperature of 78 °C. It is possible that ALAs participate in transfer reactions which could explain some of the subtle differences observed.
The catalyst used in this study has two internal donors to control the isotacticity of the polymer; one of them is hardly affected by TEA whereas the other one can be extracted by TEA, and because of this we can see that the mmmm pentads fluctuate within a narrow range as shown in Table 7. For all the experiments, the temperature seems to negatively affect the isotactic pentads, which is most likely due to the extraction of one of the internal donors by the co-catalyst, TEA. It is also possible that as the temperature increases, TEA decomposition reactions are more favored,24 which lowers the efficiency of the external donor in isotacticity control, as the complex between TEA and the external donor is thought to play an important role in regulating isotacticity.25
| SN | ED | ALA | ALA/Ti | mmmm | MSL |
|---|---|---|---|---|---|
| 1 | N | None | 0 | 93 | 85 |
| 2 | N | None | 0 | 92 | 72 |
| 3 | N | None | 0 | 91 | 65 |
| 4 | None | None | 0 | 92 | 70 |
| 5 | None | None | 0 | 91 | 63 |
| 6 | None | None | 0 | 89 | 47 |
| 13.1 | N | IPM | 9.0 | 94 | 99 |
| 14.1 | N | IPM | 9.0 | 94 | 101 |
| 15.1 | N | IPM | 9.0 | 93 | 88 |
| 16.1 | None | IPM | 3.0 | 92 | 73 |
| 17.1 | None | IPM | 3.0 | 92 | 70 |
| 18.1 | None | IPM | 3.0 | 90 | 52 |
| 13.2 | N | MTMA | 9.0 | 95 | 138 |
| 14.2 | N | MTMA | 9.0 | 94 | 119 |
| 15.2 | N | MTMA | 9.0 | 94 | 115 |
| 16.2 | None | MTMA | 3.0 | 92 | 69 |
| 17.2 | None | MTMA | 3.0 | 91 | 61 |
| 18.2 | None | MTMA | 3.0 | 90 | 52 |
| 13.3 | N | DBS | 9.0 | 95 | 145 |
| 14.3 | N | DBS | 9.0 | 94 | 110 |
| 15.3 | N | DBS | 9.0 | 93 | 84 |
| 16.3 | None | DBS | 3.0 | 93 | 67 |
| 17.3 | None | DBS | 3.0 | 92 | 65 |
| 18.3 | None | DBS | 3.0 | 92 | 60 |
The standard catalytic system with an N-donor as an external donor gives a maximum mmmm content of 93%, which drops to 91% at the highest polymerization temperature as shown in Table 7. However, in the absence of an external donor, the isotactic pentads did not drop significantly which as we mentioned earlier is due to the inability of TEA to extract one of the internal donors, thus, retaining a high isotacticity. A major difference when the external donor is not used is the drop in the MSL which indicates the presence of higher stereo-errors in the absence of an external donor; this correlation is shown in Fig. 7 for all the experiments where the MSL correlates very well to the mr diads which represent the stereo-irregular diads. The addition of ALAs with an external donor increased the isotactic pentads marginally but a significant increase can be observed in the MSL which is indicative of the ability of ALAs, when added with an external donor, to control the polymer stereoregularity. On the other hand, using the ALAs without an external donor leads to a similar amount of isotactic pentads to the experiments without an external donor. This shows that the ability of ALAs to regulate tacticity is nonexistent in the absence of an external donor. Nevertheless, the highest isotacticity in terms of mmmm pentads and MSL is observed when MTMA and DBS are used with an external donor.
 | ||
| Fig. 8 A. Bulk density and B. fine content as a function of temperature for ALA and ED combinations. | ||
 | ||
| Fig. 9 A. Bulk density as a function of the initial maximum activity and B. the plot indicating the temperatures for the experimental analysis of ALA and ED combinations. | ||
The fine content, as shown in Fig. 8-B, follows a similar decreasing trend with reaction temperature to bulk density. There are no major differences between all the experiments as the fine content is fluctuating within 2 vol% for most experiments. Overall, we can see that ALAs have no positive or negative influence on the bulk density of the powder or the fine content. The bulk density is showing a strong trend with the initial reaction activity regardless of the ALA or ED use, as we can see in Fig. 9-A and B, where lower reaction temperatures give a better bulk density due to the higher initial reaction rate.
3.3. Possible mechanisms of ALAs
To investigate the possible mechanisms of ALA with the catalyst that we used, we performed a series of experiments that we have described in Table 2, experiments 19 to 26. Additionally, the catalyst was analyzed using DRIFT without any treatment (R530 curve in Fig. 10), and with DBS treatment (DBS curve in Fig. 10). The treatment with DBS was performed in a glove box at room temperature where a DBS/Ti ratio of 9 was used; after the DBS comes into contact with the catalyst under mixing, the catalyst is dried and sampled for DRIFT analysis. The DRIFT spectra as shown in Fig. 10 have the following distinct peaks that are identified based on the functional groups of the internal donors and DBS:1. Peaks 1024 and 1060 belong to the first internal donor used in this catalyst.
2. Peak 1611 belongs to the second internal donor.
3. The peak appearing after DBS treatment, 1690, belongs to DBS.
4. Peaks between 3400 and 3600 belong to traces of OH on the catalyst surface.26
The addition of DBS does not influence the internal donors used in this catalyst; however, DBS seems to bind to the catalyst surface. Since no Ti vacancies are available in a non activated catalyst as is the case here, DBS is most likely bound to Mg. As we will see from the succeeding experiments, the DBS binding to the Mg does not seem to influence the self-extinguishing property of the catalyst.
In Fig. 11-A, we can see the two experiments performed at low and high temperatures where the catalyst was treated with DBS in the glove box and dried before use in polymerization. Since we used the highest DBS/Ti molar ratio of 9 and in reference to the earlier experiments described in the prior sections, at the highest temperature with DBS, we would expect complete extinction of the polymerization before 60 minutes which is not the case. This indicates that treating the catalyst with DBS does not affect the self-extinguishing property of the catalyst significantly. This is most likely the case as DBS is bound to Mg where the effect is limited to stereoregular control. In the second set of experiments shown in the same figure, B, addition of DBS during the polymerization where no TEA is present as the catalyst is preactivated and dried in the glove box before injection shows that DBS is able to retard the polymerization independent of the polymerization temperature. This means that DBS is able to adsorb on the active site directly and suppress the activity completely. To further distinguish which reaction is more critical with DBS, some TEA was mixed with DBS before injection during the reaction as seen in graph C, where at a lower temperature, the reaction proceeds as if there was no DBS added; however, at a higher temperature, we get the same effect as in graph B where the reaction rate goes to zero after the addition of DBS mixed with TEA. Though we have no direct evidence, these experiments strongly suggest that the most critical reaction happens between DBS and TEA, where either this reaction is not favored at elevated temperatures which allows the free DBS molecule to adsorb on the active site and suppress the activity, or the decomposition reaction that occurs between TEA and DBS is causing the observed deactivation; however, the decomposition reaction doesn't explain the catalyst deactivation by DBS at the lowest reaction temperature where the catalyst is pre-activated with TEA and dried unless the decomposition can occur at the active sites without the aid of the co-catalyst at low temperatures.
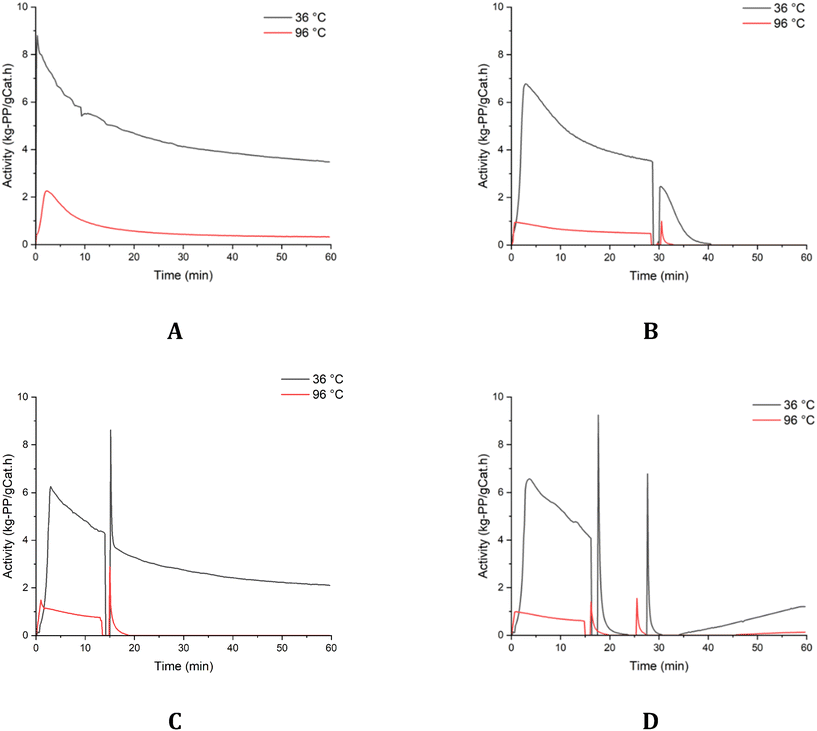 | ||
| Fig. 11 DBS experiments with different methodologies; A. experiments number 19 and 20, B. experiments number 21 and 22, C. experiments number 23 and 24, and D. experiments number 25 and 26. | ||
Finally, to understand how reversible this retarding reaction by DBS is, we performed the last set of experiments shown in graph D, where DBS is added in the middle of the reaction which leads to complete suppression of the reaction at both operating temperatures, followed by two injections of TEA at a molar ratio of 48 to Ti. We can see that in the case of the lower temperature, TEA seems to reactivate the catalyst slowly but steadily; even in the case of the higher temperature, we can see some reactivation, but it is extremely low. Though we cannot rule out the decomposition of DBS into some poisonous compounds when reacting on the active species, however, it is difficult to imagine that such a decomposition reaction is fast acting especially at a lower reaction temperature of around 36 °C. Instead, it is more likely that DBS is adsorbing directly on the active species in the absence of TEA rendering it inactive.
Based on the experiments performed in this study and in addition to the decomposition reactions that are favored at higher temperatures, we believe the following reactions might take place as well when an ALA is introduced to the reaction environment:
• A reversible ALA–TEA complex formation that is favored at low temperatures.
• Reacting with Mg in an analogous way to the alkoxysilanes which are used as external donors; this reaction could be the reason ALAs exhibit mild stereoregular control.
| DBS + TiCl4/MgCl2 → TiCl4/MgCl2·DBS |
• Adsorption on the alkylated active Ti species which leads to the complete suppression of the reaction at higher temperatures.
| DBS + MgCl2/TiCl3 → MgCl2/TiCl3·DBS |
In addition to the expected reactions that take place with ALAs, the diffusion of these molecules could play an important role as most of them are quite large molecules; we have seen a difference in the behavior of the smaller molecule that we used in this study, MTMA, compared to the aliphatic mono and diesters, IPM and DBS. Additionally, the number of functional groups could play a significant role in the reactions that took place, for example, we know from studies conducted on alkoxysilanes (i.e. external donors) that an increased number of functional groups leads to increased and unselective deactivation of active species.18
4. Conclusions
The performance of three activity limiting agents (ALA) with a sixth generation Ziegler Natta catalyst system was evaluated during the gas phase polymerization of propylene. The retarding effect of ALAs on the catalyst at elevated temperatures in order to prevent reactor instabilities in the case of thermal runaways was also studied. Additionally, we investigated the effects of ALAs on the polymer microstructure and the morphology of the powder reactor product. Finally, we looked at the possible pathways by which the retarding effect of the ALA is taking place.The activity of this catalyst system when used with an external donor decreases mildly as the reaction temperature increases from 58 to 94 °C; however, when we introduced ALAs even at the lowest ALA/Ti ratio of 0.3 along with an external donor, we observed a decrease in catalyst productivity (67, 50, and 17%) in comparison with the standard system productivity at the same temperature for IPM, MTMA, and DBS, respectively. Further increasing the ALA/Ti ratio intensified this retarding effect on activity at the highest reaction temperature; for instance at an ALA/Ti ratio of 9.0, the drops in productivity at the highest temperature in comparison with the standard system were 93, 96, and 96% for IMP, MTMA, and DBS, respectively. This significant drop in catalyst activity at a high reaction temperature is desirable in order to prevent the softening of the polymer which subsequently leads to the formation of agglomerates. However, this effect comes at the expense of a drop in catalyst productivity at the normal operating temperature of 58 °C: 19, 50, and 40% for IPM, MTMA, and DBS, respectively. An optimum balance between the retardation effect on catalyst activity at higher temperatures and the least negative effect at normal operating temperatures is achieved at an ALA/Ti ratio of around 3.0. We only tested three levels of ALA/Ti in a very wide range; the balance of activity at normal operating temperatures and the desired retardation effect can be adjusted for the operation of an industrial plant in favor of retardation when the process is facing some instabilities and towards the least negative impact on activity at normal temperatures when the process is operating smoothly. Furthermore, the retarding effect is still observed when we use the ALA without an external donor at an ALA/Ti ratio of 3.0; we do see the desirable retarding effect on activity but to a lesser extent than when an external donor is used except for MTMA; the obtained drops in productivity were 40, 80, and 50% for IPM, MTMA, and DBS, respectively. Nonetheless, when the ALAs were used without an external donor, the activity at 58 °C increased by 23 and 98% for IPM and DBS, respectively, and dropped by 14% for MTMA.
The use of ALAs with this catalyst system did not lead to any significant effects on the bulk density of the powder and the fine content. Similarly, no significant effects were observed on the molecular weight averages and distributions. However, when ALAs were used with an external donor, we observed a mild increase in isotacticity which was reflected in the isotactic pentads and more so in the MSL. The increase in isotacticity was more pronounced with MTMA and DBS than with IPM. Moreover, the use of ALAs without an external donor did not lead to any significant reduction in the isotacticity of polypropylene. Finally, it seems that besides the suspected decomposition reaction products that could poison the active sites at higher temperatures, the ALA itself is capable of reversibly deactivating the active site; this reaction is seemingly prevented when the ALA complexes with the co-catalyst which is probably more favored at lower temperatures.
Conflicts of interest
There are no conflicts of interest to declare.References
- K. P. Peil, D. R. Neithamer and D. W. Patrick, Macromol. Rapid Commun., 2004, 25, 119–126 CrossRef CAS.
- G. Morini, M. Ezzi, T. Dall'occo, D. Liguori, F. Piemontesi and V. G. Fabrizi, US Pat., 10730974, 2017 Search PubMed.
- P. Cai, L. Chen, J. W. Van Egmond and M. Tilston, Particuology, 2010, 8, 578–581 CrossRef CAS.
- L. Chen and R. E. Campbell, US Pat., 7491670, 2009 Search PubMed.
- P. Cai, J. W. Van Egmond, M. J. Fedec, J. Goad, R. C. Bradey and L. Chen, US Pat., 10647790, 2013 Search PubMed.
- W. G. Sheard and L. Chen, US Pat., 7935766, 2013 Search PubMed.
- C. Linfeng, US Pat., 7893003, 2015 Search PubMed.
- R. J. Jorgensen, S. W. Ewart, R. E. Campbell, D. Beigzadeh, R. D. Froese and P. M. Margl, US Pat., 8993692, 2015 Search PubMed.
- S. K. Gupta, M. Al-Ofi, R. R. Kumar and A. Albeledi, US Pat., 11384166, 2022 Search PubMed.
- S. Kaur, U. Sahoo, G. Signh, R. Rani and G. S. Kapur, US Pat., 10689465, 2020 Search PubMed.
- V. Gupta, R. Grubbs and S. Dhamaniya, US Pat., 20210024662, 2021 Search PubMed.
- Y. Banat, A. A. Alshaiban and O. Maidan, US Pat., 10696756, 2020 Search PubMed.
- K. V. Raj, J. Kumawat, S. Dhamaniya, M. Subramania, E. Balaraman, V. K. Gupta, K. Vanka and R. H. Grubbs, ChemCatChem, 2021, 13, 674–681 CrossRef.
- J. W. C. Chien and J. C. Wu, J. Polym. Sci., Part A-1: Polym. Chem., 1982, 20, 2445–2460 CAS.
- A. Albeladi, A. Moman and T. F. L. McKenna, Macromol. React. Eng., 2022, 2200049, DOI:10.1002/mren.202200049.
- C. C. Han-Adebekun and W. H. Ray, J. Polym. Sci., Part A-1: Polym. Chem., 1997, 35, 2063–2074 CrossRef.
- L. Wu, D. T. Lynch and S. E. Wanke, Macromolecules, 1999, 32, 7990–7998 CrossRef CAS.
- J. Seppälä, M. Härkönen and L. Luciani, Die Makromol. Chem., 1989, 190, 2535–2550 CrossRef.
- S. Kojoh, M. Kioko, N. Kashiwa, M. Itoh and A. Mizono, Polymer, 1995, 36, 5015–5018 CrossRef CAS.
- V. Busico and R. Cipullo, Prog. Polym. Sci., 2001, 26, 443–533 CrossRef CAS.
- J. C. Randall, J. Polym. Sci., Polym. Phys. Ed., 1976, 14, 2083–2094 CrossRef.
- J. C. Randall, J. Macromol. Sci., Polym. Rev., 1989, 29, 201–317 CrossRef.
- R. Paukkeri, T. Väänänen and A. Lehtinen, Polymer, 1993, 34, 2488–2494 CrossRef CAS.
- D. B. Malpass, Industrial Metal Alkyls and Their Use in Polyolefin Catalysts, in Handbook on Transition Metal Polymerization Catalysts, ed. R. Hoff, Wiley-Blackwell, Hoboken, N.J. USA, 2nd edn, 2018, ch. 1, pp. 1–30 Search PubMed.
- M. C. Sacchi, F. F. Forlini, I. Tritto, R. Mendichi, G. Zannoni and L. Noristi, Macromolecules, 1992, 25, 5914–5918 CrossRef CAS.
- A. G. Potapov, G. D. Bukatov and V. A. Zakharov, J. Mol. Catal. A: Chem., 2009, 301, 18–23 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |









