Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Continuous stirred tank reactors in fine chemical synthesis for efficient mixing, solids-handling, and rapid scale-up
Nikolay
Cherkasov
 *ab,
Samuel J.
Adams
a,
Edward G. A.
Bainbridge
a and
Jonty A. M.
Thornton
a
*ab,
Samuel J.
Adams
a,
Edward G. A.
Bainbridge
a and
Jonty A. M.
Thornton
a
aStoli Chem, Prince Phillip Building, Wellesbourne, CV35 9EF, UK. E-mail: n.cherkasov@stolichem.com
bSchool of Engineering, University of Warwick, Coventry, CV4 7AL, UK
First published on 26th October 2022
Abstract
Continuous stirred tank reactors (CSTRs) facilitate chemical manufacture in continuous flow and have been used for decades. They excel at solid handling and have well understood scale-up capacity. CSTRs in series (CSTR cascades) improve residence time distribution control by exhibiting pseudo plug flow characteristics. Advanced CSTR systems incorporate multiple vessels into a single reactor to simplify design and improve reliability. This review discusses the benefits and limitations of single CSTR technology and CSTR cascades in pharmaceutical and fine chemical synthesis compared with batch, coil (tubular) and plate reactors. In addition, we cover the measurement and importance of residence time distribution in chemical and crystallisation processes. CSTRs demonstrate substantial benefits compared with batch of up to 31% yield increase, 70% cost, and 80% energy reduction. In addition, such continuous manufacturing reduces operational complexity, reliability, and plant footprint.
Introduction
Flow chemistry may be the best way for the chemicals industry to reach net zero with the proven ability to cut costs and energy consumption in half.1–3Flow chemistry brings efficiency and sustainability and has attracted global interest in the fine chemical and pharmaceutical industries over the last two decades. Indeed, flow chemistry shares many of the tenets of the 12 principles of green chemistry, including waste prevention, design for energy efficiency, real-time analysis of desired and waste products and inherently safer design.4 Flow chemistry can help address current global problems such as climate change, supply chain disruptions, and rising production costs via process intensification, reducing waste, and enhancing heat and mass transfer. Combined with real-time analysis, continuous chemical production is safer, more energy-efficient and sustainable.
Due to their simplicity and high heat & mass transfer performance, tubular, coil, chip, or plate reactors (incorrectly called plug flow reactors, discussed later) dominate continuous production at a small scale. These reactors often provide good control, but handling solids is difficult; clogging a chip reactor may lead to permanent damage. Furthermore, the flow velocity is inextricably linked to the mixing performance, heat transfer, pressure drop, and residence time (the time materials spend in the reaction vessel). Hence, the scale-up of the tubular reactors to pilot and production capacity is complex.5 Super6 compared batch and continuous hydrogenation from 30 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ton a year showing that the batch facility requires an investment of 580k$ (in US 2000 prices) while the continuous facility – 5m$.
000 ton a year showing that the batch facility requires an investment of 580k$ (in US 2000 prices) while the continuous facility – 5m$.
Continuous stirred tank reactors (CSTRs) are agitated tanks that are continuous fed and emptied; they are often used on a large scale. With decades-long performance and behaviour understanding, CSTRs are well characterised and readily available from 1 mL to above 10 m3. They can handle solids and could provide long residence time, but the resulting residence time distribution (RTD) is broad. Better residence time control can be achieved by connecting several CSTRs in a series, trading the mechanical complexity for solid handling, easy scale-up, and superior mixing.
In the current perspective on CSTRs, we review their benefits and limitations, demonstrated performance at various scales, and technological advancements in the reactor design.
Residence time distribution of CSTR cascades
Reactors vary in performance: heat transfer, mixing, ability to handle slurries, etc. When we discuss CSTRs and cascades, the RTD critically affects the reactor throughput and process selectivity.We discuss the main terms used to describe the RTD, considerations of the measurements, and examples of how residence time affects the process performance.
Key terms for the residence time discussion
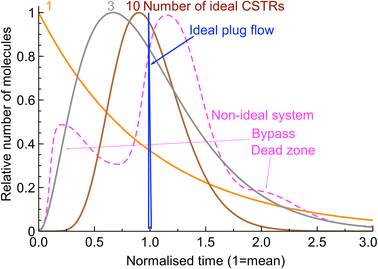
The fundamentals of residence time are discussed in detail by Fogler7 or Levenspiel.8 Briefly, the residence time of a given molecule or “fluid element” is the time it spends in a reactor. As there are many “fluid elements” in a reactor, these give rise to a distribution. The distributions of two model reactors are illustrated in Fig. 1:- ideal plug flow reactors assume no axial mixing – the elements do not mix as they move through the reactor resulting in the same residence time for all the molecules.
- ideal CSTRs assume perfect mixing inside the vessel, back-mixing the products and reactants with the inlet stream resulting in a broad distribution of residence times.
When several CSTRs are connected in series, their RTD becomes narrower described by eqn (1):9
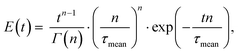 | (1) |
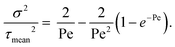 | (2) |
A Bodenstein number (Bo) is often used synonymously with Péclet, and they become indistinguishable at high values (Pe ≫ 1). Both numbers could be used to describe the breadth of the RTD and are approximately double the number of ideal CSTRs in a long series, eqn (3):
| Pe ≈ Bo = 2n − 2 | (3) |
It is worth noting that such approximations are relevant only when the distribution is sufficiently narrow and lacks notable bypass and dead zones (see Fig. 1) that must be eliminated wherever practicable and discussed by Levenspiel8 and Fogler.7
Considerations to the residence time measurements
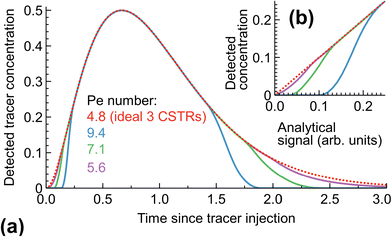
The methods for measuring RTD are extensively covered in textbooks.7 However, it is worth pointing out experimental aspects that may result in significant errors.The measurement involves the introduction of an inert tracer into the reactor. The volume of the injected tracer must be much lower than the reactor volume, and injection must not introduce substantial flow disturbances. The resulting high dilution requires a significant initial concentration of the tracer. Two issues with the injection and analytics must be considered before relying on experimental data.
Firstly, the tracer injection must indeed be instantaneous. Being obvious, this statement could be easily dismissed; however, many studies in droplet fluidics show a probability of satellite droplet formation.11,12 It is insufficient to inject the tracer rapidly – it is vital to ensure that no injection occurs after the initial pulse. In particular, the following can cause significant discrepancies:
- Satellite droplets. The rapid initial injection may create several satellite droplets seconds after the main droplet rendering the injection broad and the obtained “RTD” incorrectly broad.
- Air bubbles. If an injection is performed with a syringe (piston), the presence of air droplets may make the injection duration substantially longer. This is because air compresses during the main pulse and expands, releasing the tracer over a prolonged time.
- Liquid diffusion. If the tracer injection takes place not directly into the main fluid stream but into a side pocket such as a T-joint, the tracer enters the stream slowly. The same applies if the injection tube is wide and allows for tracer diffusion into the flow.
Secondly, the tracer outlet analytics must have a broad dynamic range (be able to resolve at least a few orders of magnitude in the concentration difference). In the RTD measurements, the initial high-concentration tracer injection diffuses to form broad distribution “tails” with low tracer concentration. If the analytical system does not detect such low-concentration areas but is calibrated for the initial solution – the “measured” distribution curve will be dramatically narrower than the real-life distribution (Fig. 2). The widely used methods to be cautious are conductivity and light absorption, often having a limited dynamic range. We use fluorescence for its simplicity, linearity, and wide range.13 Such measurement systems, however, suffer from non-linearity at high concentrations and may be prone to artefacts requiring careful system calibration.
Effects of the residence time distribution on chemistry
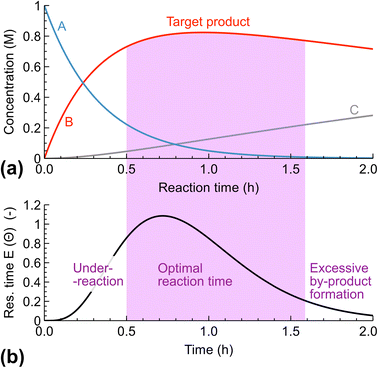
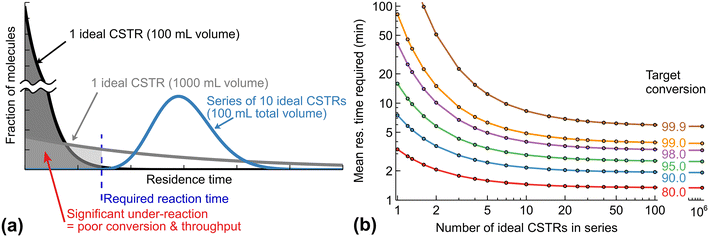
The width of RTD affects the process selectivity, quality, and throughput. When we consider a process with a possibility of significant over-reaction, the process must be stopped at a precise moment (Fig. 3). If the reactants spend too little time in the reactor, conversion decreases (due to unreacted materials). If the reactants spend too much time in the reactor, by-product formation occurs resulting in a decreased product selectivity. Even in more commonly used processes where timing is less critical, a substantial impurity formation from an altered reaction profile could be observed – these impurities may increase from ppm levels to an unacceptable level. Therefore, there is a need for a narrow RTD to maximise product purity and potentially reduce downstream processing.Fig. 4a illustrates how RTD may define the process throughput (how many kilos of product per hour are obtained). If a process requires 1 hour for completion, all of the molecules must spend at least 1 hour in the reactor for the outlet stream to observe reaction completion. In an ideal batch reactor (neglecting the start-up and cool-down time) and an ideal plug flow reactor, we expect complete conversion after 1 hour. In real reactors with a significant RTD, there is a need to increase the mean residence time to provide a 1 hour residence time for all the molecules. In the case of one CSTR, the RTD is broad and may require a mean residence time of 10 hours to ensure that 99.5% of molecules are converted. Therefore, the throughput of 1 CSTR could be substantially lower (normalised by the reactor volume) compared with an ideal plug flow if high conversion is required. Hence, the broad RTD requires slower feeding rates (to increase the mean residence time) for a given reactor volume and reduces the process throughput.
Fig. 4b provides a different view on how many ideal CSTRs in series are required to achieve a target conversion for a first-order reaction.8 A higher target conversion, obviously, requires a longer mean residence time for any series of CSTRs. The relative time increase, however, depends on the number of CSTRs. One or few CSTRs at high conversion are inefficient. For example, at 80% target conversion, 1 CSTR requires 2.2 longer mean residence time (or a correspondingly larger reactor vessel at a fixed flow rate) to achieve the same throughput as a series of 10 CSTRs. For 99% target conversion, this difference increases to a factor of 16.
In the case of polymer synthesis, the reactor's RTD is directly linked to the molecular weight distribution, copolymer composition, and branching density. The residence time determines the chain growth duration14 and thus the average molecular weight and distribution. Depending on the application, precision control of the polymer length is desired or critical to provide the required properties and performance.15
In the case of rapid polymer propagation with thousands of monomers (such as free-radical polymerisation and Ziegler–Natta polymerisation), the polymer birth conditions play an essential role in the polymer molecular weight. An ideal batch reactor with ideal back-mixing of the products and the reactants produces a broader molecular weight distribution than a single ideal CSTR.14 A continuous reactor with a narrow RTD provides consistent polymer chain birth and narrows down the molecular mass distribution.
Applications of CSTR
There are multiple reports on CSTR applications in process development and chemistry. We separate these reports by application (reaction/crystallisation) and reactor class. The reactor classes include:- conventional CSTRs and cascades are systems containing standard batch vessels and separate agitators, the most widely used due to availability. Such reactors are often connected in series to telescope processes or narrow down the RTD. The costs and complexity, however, increase proportionally to the number of individual CSTRs.
- advanced CSTRs are reactor designs with integrated series of multiple CSTRs to combine the advantages of CSTR with precise residence time control and simple operation.
Conventional CSTRs in reaction applications
Susanne et al.16 constructed a bench-scale CSTR cascade to manufacture a key benzoxazole building block (for respiratory disease treatment), Fig. 5. Benzoxazole is an expensive raw material compared to its constituent compounds. Therefore, it is an ideal candidate to synthesise in-house to dramatically reduce material expenditure. The synthesis, however, is challenging, involving a highly exothermic reaction that produces solids. The authors compared various manufacturing routes and equipment choices, such as a semi-batch system, a tubular reactor, and a CSTR cascade. The productivity of the semi-batch route was insufficient because poor heat transfer required the slow stepwise addition of reagents. Despite good heat transfer, tubular reactors were unsuitable due to major clogging problems. Hence, a series of CSTRs was the optimum design to facilitate the reaction.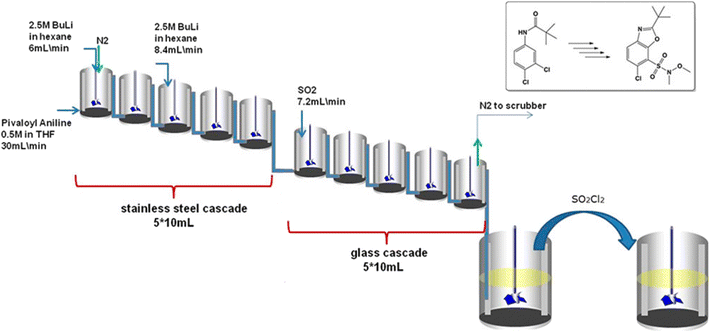 | ||
| Fig. 5 Set up for the benzoxazole synthesis containing 5 stainless steel CSTRs in series followed by 5 glass reactors followed by two batch reactors. Reprinted with permission from ref. 16. Copyright 2017 American Chemical Society. | ||
The reactor shown in Fig. 5 comprised of 10 mL CSTRs: 5 made of stainless steel followed by 5 made in glass. The system operated in 10–15 minutes compared to 12 hours in batch. The dramatic intensification was brought by rapid heat transfer and efficient mixing in small CSTRs. The reaction hear was dissipated rapidly in small CSTRs while slow heat transfer in batch required slow reagent addition. The number of CSTRs in series was a compromise between a narrow residence time (more vessels) and simplicity (fewer vessels).
The system provided an overall isolated yield of 65%, a significant increase compared to 34% observed in batch. The production was demonstrated at 17 kg of the isolated product within 72 hours at a 99.8% HPLC assay purity. The productivity, yield, and purity enhancements of the employed CSTRs translate to a 35% cost reduction leading to improved accessibility to respiratory medicine and a robust supply chain. The evidenced superior production with excellent handling of solids and heat was only made possible by the series of CSTRs.
The ability of CSTR cascades to handle rapid and hazardous chemistries safely is a significant benefit. One such example is reported by Wernik et al.17 They covered the generation of diazomethane in situ in the formation of α-chloroketones, which form the basis of several commercial human immunodeficiency virus (HIV) protease inhibitors. Fig. 6 outlines the process chemistry.
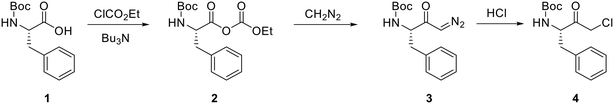 | ||
| Fig. 6 Examples of continuous synthesis of chloroketones. Reprinted from ref. 17. Copyright 2019 American Chemical Society. | ||
A tube-in-flask system was used for the preliminary studies, while diazomethane generation and dissolution were afforded by semi-permeable membrane tubing originally developed by Ley and co-workers.18 The system contained a series of 2 CSTRs, each with its membrane tube and diazomethane generator. After diazo formation, the subsequent halogenation was performed in batch. Overall, the production of α-chloroketone achieved a good yield (80–85%) and purity (98%). In addition, the cascade provided 1.54 g per hour throughput while allowing more facile scale-up opportunities than tube-in-tube laminar flow reactors.18 Other examples of CSTR cascades employed to handle hazardous chemistry have been covered by Tom et al., Yadav et al. and Duan et al.19–21
Schafer and co-workers reported two examples of CSTR cascades in the Grignard reaction of an intermediate in edivoxetine synthesis.22,23 A cascade of 3 CSTRs was used (Fig. 7). Solid magnesium was charged into tank 1 every 15 hours along with continuous addition of the solvent (4-chlorotetrahydropyran), tetrahydrofuran (THF), toluene, and a morpholine amide free base. At 35 °C, a 98% conversion in tank 1 was achieved, and tank 2 was used to increase conversion. A quench solution was fed into tank 3 to prevent side-product formation. The process was further improved by increasing scale from 250 mL per tank to 2 L per tank, while an automated magnesium charging device for tank one allowed for a viable production process. Thanks to the formation of Mg particles during start-up, no additional iodine and DIBAL were required to activate new magnesium charged into the flask. The continuous process gave product at >99% purity and >99% in situ enantiomeric excess (ee) and a yield of 89%, providing a method that could be telescoped to further products without the need for product isolation.
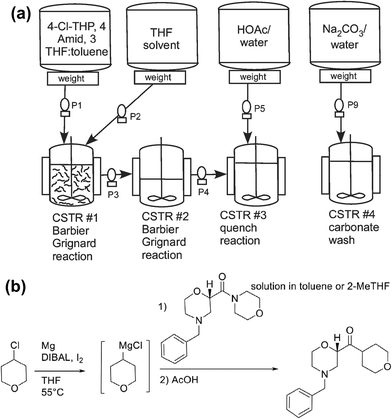 | ||
| Fig. 7 (a) Process diagram and (b) reaction scheme for the synthesis of edivoxetine intermediate. Reproduced from ref. 22 with permission from the Royal Society of Chemistry. | ||
Guan et al.24 used a commercially available lab-scale CSTR cascade (fReactor24,25) in two telescoped syntheses of ezetimibe and (R)-eslicarbazepine acetate (Fig. 8). In the first synthesis step, high-pressure hydrogenation of the ketone moiety of both substrates forms the alcohol. Next, the stream is fed into the fReactor to deprotect the second alcohol as in the case of ezetimibe or to acetylate the formed alcohol in (R)-eslicarbazepine acetate. The authors found that both APIs could be successfully telescoped via two flow systems incorporating CSTR cascade resulting in high efficiency and enantioselectivity. In the case of ezetimibe, 4.8 g per hour could be produced with 95% ee and 84% yield, while eslicarbazepine acetate could be produced at 1.6 g per hour with 98% ee and 81% isolated yield. The results showed the advantages of combining flow chemistry techniques to telescope synthetic processes avoiding intermediate purification steps.
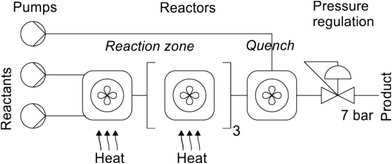 | ||
| Fig. 8 A diagram of the fReactor system used in ezetimibe and (R)-eslicarbazepine acetate synthesis. Adapted from ref. 24 with permission from Asynt and Prof. Kapur. | ||
Conventional CSTRs in crystallisation applications
Hu et al.26,27 reported examples of continuous crystallisation of small molecules in comparison with batch. CSTR cascades are useful in these scenarios because combining high viscosity and the formation of slurries means that traditional tubular reactors are impractical due to possible clogging. In addition, CSTR cascades have only short connections between tanks, making them much more suitable for slurry handling. In the first example,26 an active pharmaceutical ingredient (API) was formed by reactive crystallisation in a 5 stage multi-CSTR cascade. Despite the extra slurry handling capabilities of the system, the authors were still presented with the challenge of blockages in the tubular connectors between tanks. A burst-pumping sequence of forwards and backward flow was utilised to circumvent the problem and allow 10 wt% crystalline materials in flow. Improved control of reaction temperature in the CSTR cascade compared to batch resulted in a 1.6% increase in yield with a 0.6 mg g−1 reduction in impurity content.26Hu et al.27 developed a continuous reactive crystallisation system to produce 1 kg of an API using a tubular reactor and a 4 CSTR cascade system incorporating in-line process analytical technology. The residence time for the system was characterised by the injection of 1 mL tracer solution. The system showed a narrow RTD, allowing for an API yield of 91.3%. The high performance highlights the advantage of maintaining a narrow RTD. Process mass intensity (PMI)27 (eqn (4)) was compared for batch and continuous systems:
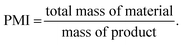 | (4) |
Continuous crystallisation has also been utilised in the semi-batch preparation of an intermediate in the synthesis of merestinib – an experimental cancer drug by Eli Lilly;28 the reaction is shown in Fig. 9.
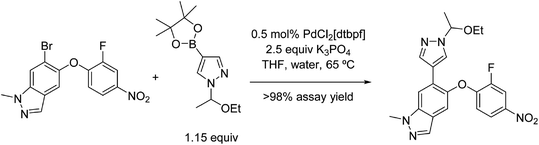 | ||
| Fig. 9 Scheme of the merestinib synthesis reprinted with permission from ref. 28. Copyright 2016 American Chemical Society. | ||
This study used intermittent flow in a single CSTR via a ‘fill-empty’ regime where reagents are pumped into the vessel and held for the reaction time before being removed to mimic plug-flow and batch characteristics. 1 kg of the starting bromide was processed in the equipment for 40 hours, resulting in a 25 g h−1 throughput and an isolated crystallisation yield of 82%. The system ran for over 100 hours, and both yield and purity were found to be robust with minimal fouling, which can often be a problem for continuous crystallisation.
Applications of advanced CSTR technologies
The major problems of conventional CSTR cascades are their mechanical complexity and costs proportional to the number of CSTRs. Over recent years, alternative reactor designs have appeared that provide a series of many CSTRs at a lower mechanical complexity. Such novel systems use multiple chambers within a single unit and have shown excellent performance in a range of applications.The Raptor is a CSTR reactor cascade containing a single cylindrical vessel, 400 mL in volume, mounted horizontally with vertical baffles separating the main channel into individual CSTR chambers.29 An internal longitudinal shaft with several impellers provides mixing at each stage – a maximum agitation rate of 1500 rpm is possible. The reactor can handle a wide range of process conditions. The Raptor is particularly suited to multiphase processes such as hydrogenation because of high gas–liquid mass transfer, good heat transfer to manage the large heat generation from reactions, and slurry handling.
Machefer et al.29 compared the performance of batch and Raptor systems in ortho methyl-cyclohexanol synthesis and found considerable improvements (Table 1). The same 300 ton per year production of ortho methyl-cyclohexanol requires a 6000 times smaller continuous reactor than batch, translating to reduced capital investment. Higher pressures and temperatures are attainable in the Raptor compared to the batch allowing for faster reactions, improved conversions (+5.1%), and acceleration of gas–liquid mass transfer by 4 orders of magnitude – this is only manageable due to superior design, smaller scale, and better heat management. The solid handling capability further enhanced the process intensification by solving clogging problems.
| Batch reactor | Raptor reactor | % difference | |
|---|---|---|---|
| Productivity | 300 t per year | 300 t per year | |
| Volume | 6 m3 | 0.001 m3 | −99.98% |
| Pressure | 15 bar | 200 bar | |
| Temperature | 100 °C | 170 °C | |
| Solvent content | 75% | 0% | −100% |
| Reaction time | 240 min | 4 min | −98% |
| Catalyst concentration | 4 wt% | 0.4 wt% | −90% |
| Conversion | 95% | 99.9% | +5% |
| Adiabatic temperature rise | 100 °C | 925 °C | |
| Total production costs | 12.4 € per kg | 3.8 € per kg | −70% |
The Raptor CSTR cascade design has successfully allowed the process intensification of the hydrogenation application, mitigating clogging problems while saving 75–85% on energy use and 70% on product costs overall, making the process scalable, greener and cost-effective.
Falß et al.30 developed a similar cascade containing 5 CSTRs to scale up the process of a continuous Buchwald–Hartwig reaction using a bulky N-heterocyclic carbene pre-catalyst (Fig. 10). This design made the reactor much less prone to blockage and offered the flexibility of CSTRs, alongside the benefits of a narrow RTD and excellent heat transfer usually seen in tube reactors. In addition, the CSTRs are all stirred by impellers attached to one central shaft, meaning there is only one moving part, simplifying the operation, reducing costs, and increasing reliability.
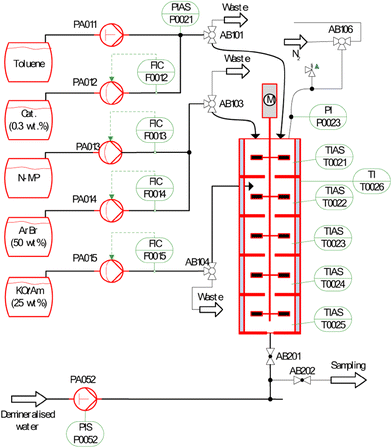 | ||
| Fig. 10 Reactor setup used by Falβ et al. to showcase continuous Buchwald Hartwig reaction. Adapted with permission from ref. 30. Copyright 2016 American Chemical Society. | ||
At a flow rate of 1 L h−1, a maximum yield of 93.2% and 92.4% product purity was obtained. The palladium content in the product was less than 1 ppm, well below the 20 ppm limit. Process intensification and vast parameter screening were the keys to the success of the synthesis since they allowed enhanced conditions to help mitigate purification steps and the need for expensive metal scavengers to meet the product specification.
The life cycle analysis on this reactor compared batch and 3 continuous processes under various conditions (Fig. 11). The CSTR cascade outperforms the original batch process in almost all areas, especially when combined with recycling (readily possible when operating in flow).
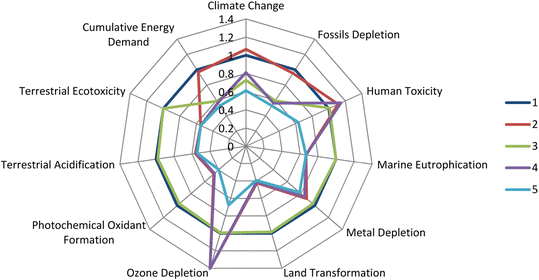 | ||
| Fig. 11 Life cycle assessment for the Buchwald–Hartwig reaction in 1 the batch process, 2 the flow process with catalyst recycling, 3 the batch process with toluene recycling, 4 the flow process with toluene recycling, 5 the flow process with all solvents recycled (including those used in catalyst manufacture). Reprinted with permission from ref. 30. Copyright 2016 American Chemicals Society. | ||
AMTech's Coflore Agitated Cell Reactor (ACR) flow reactor is an actively mixed, bench-top flow reactor where the process channel is divided into ten reactor cells similar to ten CSTRs in series. In contrast to the previous systems, the agitation is performed by moving the whole reactor rather than only the agitator parts. Fig. 12 shows one such system.31–33
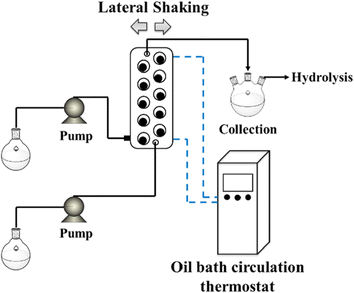 | ||
| Fig. 12 AMTech Coflore ACR system used for phosphorylation reaction. Adapted with permission from ref. 33. Copyright 2021 American Chemical Society. | ||
Yao et al.33 presented a continuous approach to the phosphorylation of 2,2′-methylene-bis(4,6-di-tert-butyl)phenol, comparing both tubular reactors and ACR. One particularly challenging aspect of this synthesis is an insoluble side product, triethylamine hydrochloride, produced during phosphorylation. Such insoluble products readily clog tubular reactors, and thus the ACR was chosen due to its ability to handle slurry. The authors found that no clogging was observed during continuous steady-state operation over 8 hours in the ACR, in contrast to the tubular system. Moreover, switching to a continuous mode improved the yield (98% in flow vs. 90% in batch) and reaction times (4 minutes in flow vs. several hours in batch). The faster reaction time was made possible by better heat transfer that allowed faster addition of POCl3. If heat transfer is slow (such as in batch) and POCl3 is added too quickly without adequate heat transfer, thermal runaway may result in a release of gaseous HCl and the formation of by-products.
Pedersen et al.34 carried out the bio-catalytic oxidation of glucose to glucono-1,5-lactone using enzymes in an ACR and a batch reactor. The ACR showed clear benefits over batch alongside some limitations. To achieve full conversion, the residence time of the ACR was 47 minutes, whereas the total time for the batch reactor was 110 minutes. The faster rate of reaction can solely be attributed to the 3.3 times higher mass transfer coefficient in the ACR (344 h−1 compared to 104 h−1 in batch).34 However, the enhanced mixing comes at a cost. The lateral shaking mechanism is likely to have caused significant backflow ultimately decreasing the number of ideal CSTRs from 10 to 2 at 0.9 ml min−1. Additionally, the mixing required at least 5000 times more energy than batch34 – “the large power consumption of the ACR will become a significant problem upon scale-up, unless a more efficient mixing method is considered”.34
Mandrelli et al. from Novartis35 designed and applied a vertically orientated cascade of CSTRs to a double alkylation of benzylamine with 1,5-dibromopentane. The reactor was constructed from Hastelloy C22 and contained a removable insert inside a jacketed vessel (Fig. 13). There were 13 CSTR stages with a height to diameter ratio of 0.6. Efficient mixing is ensured by a series of Rushton turbine agitators mounted on a central shaft connected to a magnetically coupled high-pressure drive. Three vertical supports act as baffles at each stage to further enhance mixing.
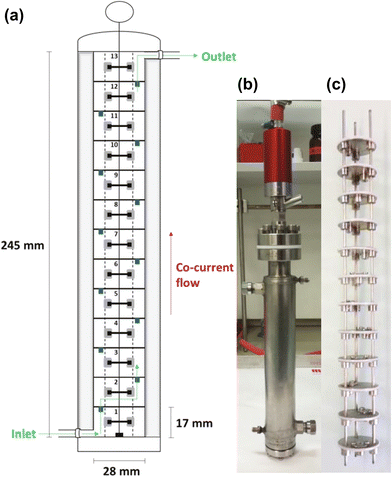 | ||
| Fig. 13 (a) Scheme and photographs of the (b) exterior and (c) interior of the 13 CSTR cascade reactor developed by Mandrelli et al. Adapted from ref. 35 with permission from The Royal Society of Chemistry. | ||
The reactor was demonstrated in biphasic (liquid–liquid) a double alkylation of benzylamine with a 1,5-dibrompentane. The reaction was chosen because Ju and Varma demonstrated that the synthesis of azacycloalkanes benefit from high-temperature operation attainable in microwave batch reactors.36 Yu et al. showed that the microwave heating increased conversion to 95% in just 20 minutes as opposed to 24 hours in a regular batch. Mandrelli et al.,35 therefore, employed rapid heating using continuous cascade reactor. The mean residence time was 25 minutes, with the process performed at 126 °C, 7 bar and a stirring rate of 1100 rpm. The high agitation rate ensured complete mixing of water and organic phases, allowing for a co-current flow. The reactor generated 17 g of the desired product at an 88% yield and <1% by-product composition in under 75 minutes of reaction time. The reactor offered further enhancement over a microwave batch reactor because of a more manageable scale-up of biphasic reactions under superheated conditions.
The advanced CSTR cascades demonstrate handling of challenging reactions at substantial process scales. Such systems overcome challenges from conventional CSTRs cascades such as connections between tanks, and complexity whilst achieving excellent solid handling, energy efficiency, and yield.
Which is the right reactor?
We have overviewed the main applications of CSTRs in reactions and crystallisations. These applications highlight the main strengths, but unfortunately CSTRs are not the ultimate system for chemical synthesis. In fact, there are no universal solutions37 and the key question is selecting the right compromise.Table 2 summarises the main characteristics of three major continuous reactor types: CSTRs, plates (or microreactors), and coils (tubular reactors). More details on all the reactors could be found in the papers by Hu27,38 and Roberge et al.39,40
| CSTRs | Plate | Coil | |
|---|---|---|---|
| Residence time | 1 min to 10 h | 1 ms to 10 min | 1 s to 1 h |
| Volume | 1 mL to 10 m3 | 1 μL to 10 L | 0.1 mL to 10 L |
| Temperature | Moderate | Widest | Moderate |
| Max pressure | Low to moderate | Extreme | Moderate |
| Heat transfer rate | Low to moderate | Moderate to extreme | Low to moderate |
| Mass transfer rate | Low to moderate | Moderate to extreme | Low to moderate |
| Handling solids | Good | None | Moderate |
| Micromixing speed | Low to moderate | Moderate to extreme | Low to moderate |
| Pressure drop | Low | High | Moderate to high |
| Residence time control | Poor to moderate | Excellent | Moderate to excellent |
CSTRs could provide large volume at the lowest cost (cost ∼ volume0.3 (ref. 39)) and correspondingly long residence time beneficial for slower processes. The large volume comes with several compromises such as limited heat and mass transfer performance which substantially depends on scale. Residence time control is also poor in a single CSTR and is limited in a CSTR cascade. Yet, a unique benefit is an ability to handle solids and slurries amply discussed in this review. In summary, CSTRs provide large volume and independent stirring control counterbalanced by the moderate cost and moderate performance.
Plate reactors are designed as an anti-thesis to batch (and CSTRs) and benefit from exceptional performance in many areas.41,42 Like the majority of physical phenomena, these characteristics decrease with scale, yet even the biggest plate reactors demonstrate good mixing, heat- and mass-transfer performance. Residence time control is most often excellent and could provide millisecond- to minute-long processes. Such characteristics, however, relate to small dimensions (limited reactor volume) and passive mixing that depends on the fluid velocity. Thus, pressure drop, heat, mass transfer and residence time are all inter-related. Hence, a plate reactor optimised for one process may show poor results in a different process or simply with different flow rates.40 A multipurpose continuous plant requires, therefore, multiple and expensive reactors. When a suitable process selected and optimised, savings and performance improvements could be exceptional in plate reactors. In summary, plates provide exceptional performance counterbalanced by the limited volume and high cost.
Characteristics of coil reactors stand in between the CSTRs and plate reactors. Coils are similar to plates relying on static mixing with inter-connected flow rate, pressure, mixing, and heat transfer. Their performance, however, falls below that for plate because coils do not have designed mixing elements. On the other hand, a simple coil structure renders them a much lower cost option compared to plates with respectable reactor volumes achievable of >300 L.38 In summary, coils' lowest cost is counterbalanced by the moderate volume and performance.
Conclusion
Continuous stirred tank reactor (CSTR) and a cascade of CSTRs is an established technology in chemical manufacture. The CSTR is one of the main tools in switching from batch to continuous and delivering its benefits.Compared with tubular reactors, CSTRs provide external agitation with impellers to:
- dramatically improve mass transfer performance in particular for multiphase systems,
- simplify scalability because mixing is not linked to the pumping rate,
- enable the handling of solids and slurries.
Compared with batch reactors, the CSTRs applications in active pharmaceutical ingredients and intermediate production could increase the product yield by up to 30% at a higher throughput and rapid scalability. The demonstrated cost reduction approaches 70% and an energy reduction of 80%.
One major problem of CSTRs, however, is broad residence time distribution and the corresponding deterioration of the volumetric throughput and reaction timing control. This problem is solved by creating a CSTR cascade with multiple reaction stages – such a cascade combines the benefits of rapid heat & mass transfer of the continuous tubular reactors with the versatility and scalability of batch.
The advanced reactor designs that incorporate multiple CSTRs in a single vessel, thus greatly simplifying and reducing the costs of the CSTR cascades, are essential. For example, the systems demonstrated by Machefer et al.,29 Falβ et al.,30 and Mandrelli et al.35 incorporate 5–13 CSTRs in a single reactor, enhancing scalability, solid handling, and mixing.
CSTR cascades are one of the vital tools in delivering greener, safer, and more efficient chemical production.
Conflicts of interest
NC is the director of Stoli Chem, the company which commercially provides one of the advanced CSTR cascade systems.Acknowledgements
Stoli Catalysts acknowledges the European Union (EU) Horizon 2020 funding (SME Instrument phase 2 project, 848926) is indispensable for this research. NC also is grateful to IChemE for the Andrew Fellowship.References
- J. Walther, R. Godawat, C. Hwang, Y. Abe, A. Sinclair and K. Konstantinov, The business impact of an integrated continuous biomanufacturing platform for recombinant protein production, J. Biotechnol., 2015, 213, 3–12, DOI:10.1016/j.jbiotec.2015.05.010.
- H. G. Jolliffe and D. I. Gerogiorgis, Plantwide design and economic evaluation of two Continuous Pharmaceutical Manufacturing (CPM) cases: Ibuprofen and artemisinin, Comput. Chem. Eng., 2016, 91, 269–288, DOI:10.1016/j.compchemeng.2016.04.005.
- S. D. Schaber, D. I. Gerogiorgis, R. Ramachandran, J. M. B. Evans, P. I. Barton and B. L. Trout, Economic analysis of integrated continuous and batch pharmaceutical manufacturing: A case study, Ind. Eng. Chem. Res., 2011, 50, 10083–10092, DOI:10.1021/ie2006752.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- Z. Dong, Z. Wen, F. Zhao, S. Kuhn and T. Noël, Scale-up of Micro- and Milli-Reactors: An Overview of Strategies, Design Principles and Applications, Chem. Eng. Sci.: X, 2021, 10, 100097, DOI:10.1016/j.cesx.2021.100097.
- J. D. Super, Selecting between batch slurry and continuous fixed bed hydrogenation, in Catal. Org. React., 2000, pp. 35–49 Search PubMed.
- H. S. Fogler, Elements of chemical reaction engineering, 1999, DOI:10.1016/0009-2509(87)80130-6.
- O. Levenspiel, Chemical Reaction Engineering, John Wiley & Sons, Inc, New York, 3rd edn, 1999 Search PubMed.
- P. Toson, P. Doshi and D. Jajcevic, Explicit residence time distribution of a generalised cascade of continuous stirred tank reactors for a description of short recirculation time (bypassing), Processes, 2019, 7(615), 1–13, DOI:10.3390/pr7090615.
- N. Cherkasov, P. Denissenko, S. Deshmukh and E. V. Rebrov, Gas-liquid hydrogenation in continuous flow – The effect of mass transfer and residence time in powder packed-bed and catalyst-coated reactors, Chem. Eng. J., 2020, 379, 122292, DOI:10.1016/j.cej.2019.122292.
- C. X. Zhao and A. P. J. Middelberg, Two-phase microfluidic flows, Chem. Eng. Sci., 2011, 66, 1394–1411, DOI:10.1016/j.ces.2010.08.038.
- P. Zhu and L. Wang, Passive and active droplet generation with microfluidics: a review, Lab Chip, 2017, 17, 34–75, 10.1039/C6LC01018K.
- L. Chatre, J. Socci, S. J. Adams, P. Denissenko and N. Cherkasov, Design of 3D-printed structures for improved mass transfer and pressure drop in packed-bed reactors, Chem. Eng. J., 2021, 420, 1–10, DOI:10.1016/j.cej.2021.129762.
- S. Zhu and A. Hamielec, Polymerization Kinetic Modeling and Macromolecular Reaction Engineering, Elsevier B.V., 2012, DOI:10.1016/B978-0-444-53349-4.00127-8.
- D. V. Rosato, Plastics Engineered Product Design, 2003 Search PubMed.
- F. Susanne, B. Martin, M. Aubry, J. Sedelmeier, F. Lima, S. Sevinc, L. Piccioni, J. Haber, B. Schenkel and F. Venturoni, Match-Making Reactors to Chemistry: A Continuous Manufacturing-Enabled Sequence to a Key Benzoxazole Pharmaceutical Intermediate, Org. Process Res. Dev., 2017, 21, 1779–1793, DOI:10.1021/acs.oprd.7b00254.
- M. Wernik, P. Poechlauer, C. Schmoelzer, D. Dallinger and C. O. Kappe, Design and Optimization of a Continuous Stirred Tank Reactor Cascade for Membrane-Based Diazomethane Production: Synthesis of α-Chloroketones, Org. Process Res. Dev., 2019, 23, 1359–1368, DOI:10.1021/acs.oprd.9b00115.
- M. O'Brien, I. Baxendale and S. Ley, Flow Ozonolysis Using a Semipermeable Teflon AF-2400 Membrane, Synfacts, 2010, 2010, 1199–1199, DOI:10.1055/s-0030-1258661.
- J. K. Tom, M. M. Achmatowicz, M. G. Beaver, J. Colyer, A. Ericson, T. L. Hwang, N. Jiao, N. F. Langille, M. Liu, M. A. Lovette, R. P. Sangodkar, S. Sharvan Kumar, S. Spada, D. Perera, J. Sheeran, K. Campbell, T. Doherty, D. D. Ford, Y. Q. Fang, E. Rossi, G. Santoni, S. Cui and S. D. Walker, Implementing Continuous Manufacturing for the Final Methylation Step in the AMG 397 Process to Deliver Key Quality Attributes, Org. Process Res. Dev., 2021, 25, 486–499, DOI:10.1021/acs.oprd.0c00440.
- M. B. Yadav, S. Kulkarni, R. A. Joshi and A. A. Kulkarni, Continuous Flow Doebner-Miller Reaction and Isolation Using Continuous Stirred Tank Reactors, Org. Process Res. Dev., 2016, 20, 1621–1625, DOI:10.1021/acs.oprd.6b00179.
- S. Duan, X. Feng, M. Gonzalez, S. Bader, C. Hayward, T. Ljubicic, J. Lu, J. Mustakis, M. Maloney, J. Rainville and X. Zhang, Developing a multistep continuous manufacturing process for (1R,2R)-2-amino-1-methylcyclopentan-1-ol, Org. Process Res. Dev., 2020, 24, 2734–2744, DOI:10.1021/acs.oprd.0c00405.
- M. E. Kopach, D. J. Roberts, M. D. Johnson, J. McClary Groh, J. J. Adler, J. P. Schafer, M. E. Kobierski and W. G. Trankle, The continuous flow Barbier reaction: An improved environmental alternative to the Grignard reaction?, Green Chem., 2012, 14, 1524–1536, 10.1039/c2gc35050e.
- T. M. Braden, M. D. Johnson, M. E. Kopach, J. McClary Groh, R. D. Spencer, J. Lewis, M. R. Heller, J. P. Schafer and J. J. Adler, Development of a Commercial Flow Barbier Process for a Pharmaceutical Intermediate, Org. Process Res. Dev., 2017, 21, 317–326, DOI:10.1021/acs.oprd.6b00373.
- F. Guan, A. J. Blacker, B. Hall, N. Kapur, J. Wen and X. Zhang, High-pressure asymmetric hydrogenation in a customized flow reactor and its application in multi-step flow synthesis of chiral drugs, J. Flow Chem., 2021, 11, 763–772, DOI:10.1007/s41981-021-00143-8.
- M. R. Chapman, M. H. T. Kwan, G. King, K. E. Jolley, M. Hussain, S. Hussain, I. E. Salama, C. González Nino, L. A. Thompson, M. E. Bayana, A. D. Clayton, B. N. Nguyen, N. J. Turner, N. Kapur and A. J. Blacker, Simple and Versatile Laboratory Scale CSTR for Multiphasic Continuous-Flow Chemistry and Long Residence Times, Org. Process Res. Dev., 2017, 21, 1294–1301, DOI:10.1021/acs.oprd.7b00173.
- C. Hu, J. E. Finkelstein, W. Wu, K. Shvedova, C. J. Testa, S. C. Born, B. Takizawa, T. F. O'Connor, X. Yang, S. Ramanujam and S. Mascia, Development of an automated multi-stage continuous reactive crystallization system with in-line PATs for high viscosity process, React. Chem. Eng., 2018, 3, 658–667, 10.1039/c8re00078f.
- C. Hu, B. T. Shores, R. A. Derech, C. J. Testa, P. Hermant, W. Wu, K. Shvedova, A. Ramnath, L. Q. Al Ismaili, Q. Su, R. Sayin, S. C. Born, B. Takizawa, T. F. O'Connor, X. Yang, S. Ramanujam and S. Mascia, Continuous reactive crystallization of an API in PFR-CSTR cascade with in-line PATs, React. Chem. Eng., 2020, 5, 1950–1962, 10.1039/d0re00216j.
- K. P. Cole, B. M. Campbell, M. B. Forst, J. McClary Groh, M. Hess, M. D. Johnson, R. D. Miller, D. Mitchell, C. S. Polster, B. J. Reizman and M. Rosemeyer, An Automated Intermittent Flow Approach to Continuous Suzuki Coupling, Org. Process Res. Dev., 2016, 20, 820–830, DOI:10.1021/acs.oprd.6b00030.
- S. Machefer, L. Falk and F. de Panthou, Intensification principle of a new three-phase catalytic slurry reactor. Part I: Performance characterisation, Chem. Eng. Process., 2013, 70, 277–288, DOI:10.1016/j.cep.2013.04.014.
- S. Falß, G. Tomaiuolo, A. Perazzo, P. Hodgson, P. Yaseneva, J. Zakrzewski, S. Guido, A. Lapkin, R. Woodward and R. E. Meadows, A Continuous Process for Buchwald-Hartwig Amination at Micro-, Lab-, and Mesoscale Using a Novel Reactor Concept, Org. Process Res. Dev., 2016, 20, 558–567, DOI:10.1021/acs.oprd.5b00350.
- D. L. Browne, B. J. Deadman, R. Ashe, I. R. Baxendale and S. V. Ley, Continuous flow processing of slurries: Evaluation of an agitated cell reactor, Org. Process Res. Dev., 2011, 15, 693–697, DOI:10.1021/op2000223.
- A. Karadeolian, D. Patel, P. Bodhuri, G. Weeratunga and B. Gorin, Continuous Flow Process for Reductive Deoxygenation of ω-Chloroketone in the Synthesis of Vilazodone, Org. Process Res. Dev., 2018, 22, 1022–1028, DOI:10.1021/acs.oprd.8b00145.
- H. Yao, L. Wan, X. Zhao, Y. Guo, J. Zhou, X. Bo, Y. Mao and Z. Xin, Effective Phosphorylation of 2,2′-Methylene-bis(4,6-di-tert-butyl) Phenol in Continuous Flow Reactors, Org. Process Res. Dev., 2021, 25, 2060–2070, DOI:10.1021/acs.oprd.1c00105.
- A. Toftgaard Pedersen, T. M. de Carvalho, E. Sutherland, G. Rehn, R. Ashe and J. M. Woodley, Characterization of a continuous agitated cell reactor for oxygen dependent biocatalysis, Biotechnol. Bioeng., 2017, 114, 1222–1230, DOI:10.1002/bit.26267.
- F. Mandrelli, A. Buco, L. Piccioni, F. Renner, B. Guelat, B. Martin, B. Schenkel and F. Venturoni, The scale-up of continuous biphasic liquid/liquid reactions under super-heating conditions: Methodology and reactor design, Green Chem., 2017, 19, 1425–1430, 10.1039/c6gc02840c.
- Y. Ju and R. S. Varma, Aqueous N-Heterocyclization of Primary Amines and Hydrazines with Dihalides: Microwave-Assisted Syntheses of N-Azacycloalkanes, Isoindole, Pyrazole, Pyrazolidine, and Phthalazine Derivatives, J. Org. Chem., 2006, 71(1), 135–141, DOI:10.1021/jo051878h.
- E. H. Stitt, Multifunctional reactors? “Up to a point lord copper”, Chem. Eng. Res. Des., 2004, 82, 129–139, DOI:10.1205/026387604772992693.
- C. Hu, Reactor design and selection for effective continuous manufacturing of pharmaceuticals, J. Flow Chem., 2021, 11, 243–263, DOI:10.1007/s41981-021-00164-3.
- D. M. Roberge, L. Ducry, N. Bieler, P. Cretton and B. Zimmermann, Microreactor Technology: A Revolution for the Fine Chemical and Pharmaceutical Industries?, Chem. Eng. Technol., 2005, 28, 318–323, DOI:10.1002/ceat.200407128.
- P. Plouffe, A. Macchi and D. M. Roberge, From batch to continuous chemical synthesis-a toolbox approach, Org. Process Res. Dev., 2014, 18, 1286–1294, DOI:10.1021/op5001918.
- V. Hessel, D. Kralisch and N. Kockmann, Novel Process Windows: Innovative Gates to Intensified and Sustainable Chemical Processes, John Wiley & Sons, 2014 Search PubMed.
- V. Hessel, D. Kralisch, N. Kockmann, T. Noël and Q. Wang, Novel process windows for enabling, accelerating, and uplifting flow chemistry, ChemSusChem, 2013, 6, 746–789, DOI:10.1002/cssc.201200766.
| This journal is © The Royal Society of Chemistry 2023 |