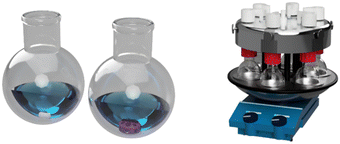Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
3D printed tetrakis(triphenylphosphine)palladium (0) impregnated stirrer devices for Suzuki–Miyaura cross-coupling reactions†
Matthew R.
Penny
,
Zenobia X.
Rao
,
Rumintha
Thavarajah
,
Ahtsham
Ishaq
,
Benjamin J.
Bowles
and
Stephen T.
Hilton
 *
*
UCL School of Pharmacy, 29-39 Brunswick Square, London WC1N 1AX, UK. E-mail: s.hilton@ucl.ac.uk
First published on 6th September 2022
Abstract
3D printed materials can be readily modified to create bespoke structures that incorporate a range of catalysts at the point of printing. In this present study we report on the design and 3D printing of tetrakis (triphenylphosphine) palladium (0) impregnated 3D printed stirrer devices that were used to catalyze a Suzuki–Miyaura reaction between biaryl compounds in a batch-based approach. It was shown that the 3D printed devices themselves are stable to solvent, reusable, easy to use, air-stable, give access to an array of biaryl compounds in excellent yields and lead to low levels of palladium loss into the reaction. Simple modification of the device's design by size reduction, meant that they could also be used to reduce the time of the Suzuki–Miyaura reaction by microwave enhanced heating. At the end of the reaction, devices can simply be removed from the flask, washed and reused, analogous to stirrer bead workflows. This makes the overall process of setting up multiple reactions simpler by obviating the need to weigh out catalysts for reactions and the device, once used, can be simply removed from the reaction media at the end of the reaction.
The palladium catalyzed Suzuki–Miyaura cross coupling reaction between an aryl halide and a boronic acid is perhaps one of the most widely used reactions in medicinal chemistry, as it provides ready access to arrays of substituted biaryl compounds.1–3 As a result of its utility, there have been a plethora of studies into both the optimization of palladium catalysts and improvements in their associated ligands.2–5 However, despite the advantages of various catalysts and their low loadings, the high cost and toxicity of palladium necessitates the extensive purification of the biaryl compounds themselves and recovery of the catalyst and associated ligands.6 To overcome these challenges, various groups have sought to embed palladium catalysts onto solid support for easy removal and recovery of the catalyst from the reaction. Solid supports investigated for palladium catalyzed Suzuki–Miyaura reactions have included carbon,7 silica8 and polymer-based systems,9 with the latter encompassing encapsulated metal catalysts.10
One novel area of solid supported catalysts which has recently gained growing attention has been the use of 3D printing to develop catalytic devices, as these can be readily used to produce objects with unique shapes and sizes with facile control over geometries.11 The use of 3D printing within chemistry has recently been reviewed and recent research shows the potential of catalytic devices that can be prepared using either fused deposition modelling (FDM), extrusion, selective laser sintering (SLS) or stereolithography (SLA) based 3D printing.11,12 As part of our research into the potential applications of 3D printing in synthetic chemistry,13–16 we first introduced the concept of 3D printed catalytically active stirrer devices in 2017 and more recently, showed that those containing pTsOH can be used in the acid-catalysis of the Mannich reaction to synthesize products in good yield and that the devices themselves, could be readily reused.12j,17,18 In this approach, normal magnetic stirrers are placed within a 3D printed outer housing containing a catalyst. The outer 3D printed structure is designed to enhance the mixing of the reaction and hence achieve a higher throughput over the surface of the device (ESI,† Fig. S5). The concept of using 3D printed stirrer devices to catalyze reactions has several advantages over traditional batch chemistry work-flows. The catalyst no longer has to be weighed before the reaction, making multiple reaction set-ups much easier, as the “catalytic stirrer” can simply be added to the reaction vessel as per most normal chemistry workflows (Fig. 1).
This avoids addition of the stirrer bead, the weighing out of catalyst and addition to the reaction. At the end of the reaction, the device (and embedded catalyst) can simply be removed from the reaction, making work-up and purification of the reaction much simpler (Fig. 1).18 Herein, we now report on the extension of this concept to incorporate a metal complex, tetrakis(triphenylphosphine)palladium (0), and an evaluation of their use as catalytic devices in the Suzuki–Miyaura reaction in both batch and microwave mediated reactions.
To demonstrate the utility of 3D printing, we wanted to design stirring devices that could be used in a parallel synthesis apparatus. Our approach involved modification of our original design so that it could be used in both round bottomed flasks and in a Radleys carousel multi-tube reactor,19 where reactions could be conducted in multiple test tubes, with stirring taking place at the edge of the stirrer hot plate. As well as changing external dimensions of the stirring device, we needed to use a rare earth magnetic flea to ensure efficient stirring due to the weaker magnetic force observed at the edge of the stirrer hotplate. The stirrer used for microwave reactions was simply scaled down to an appropriate size to fit the reaction vessel. The designs and 3D Printed devices are shown (Fig. 2).
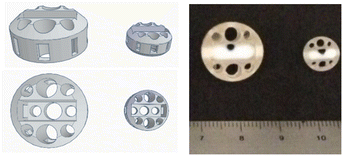 | ||
| Fig. 2 Designed carousel (left) and microwave (middle) devices and their final 3D printed congeners (right). | ||
In order to improve the utility of our 3D printed catalytically active stirring devices we believed it important to assess the solvent resistance of our SLA printed materials in order to determine the amount of catalyst that was potentially available for reaction. The poorer the solvent resistance of the device, the greater the release of catalyst throughout the course of the reaction as the printed object swells, releasing catalyst. Previous reports in the literature have shown that SLA printed devices display poor solvent resistance and it was regarded as one of the key limitations of this technology and its application to organic chemistry.12a Whilst investigating the application of SLA 3D printing in a different research area, we serendipitously discovered that 3D printed objects prepared from poly(ethylene glycol) diacrylate (PEGDA) showed excellent solvent resistance (ESI†) and therefore the devices were printed using this acrylate as the resin monomer. Tetrakis (triphenylphosphine)palladium(0) was chosen as a catalyst for this study due to its relative simplicity and widespread use in the catalysis of biaryl couplings. While it was not postulated what the exact ligand environment the palladium would be in after printing, it was decided for expediency to proceed due to its ready solubility in the PEGDA. The initial loading of Pd(PPh3)4 was 0.5% w/w (mass of catalyst to PEGDA), as higher loadings resulted in premature polymerization of the diacrylate (Fig. 3).
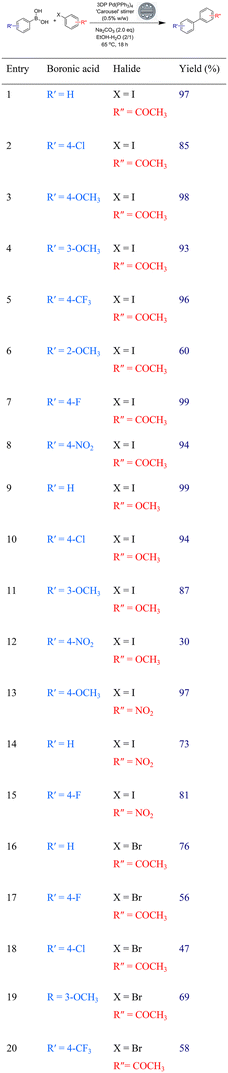
We attempted to explore the Suzuki–Miyaura reaction with a range of aryl halides and aryl boronic acids using our Pd impregnated stirring device under standard conditions, where the reactions were heated at 65 °C for 18 hours for all substrates (Table 1). The reaction was straightforward to work up as the stirrer device could be easily removed from the reaction, washed and dried. Isolated yields were excellent for the biaryl coupling of the aryl iodide substrates however the aryl bromides only gave moderate yields of the biaryl products (Table 1, Entries 16–20).
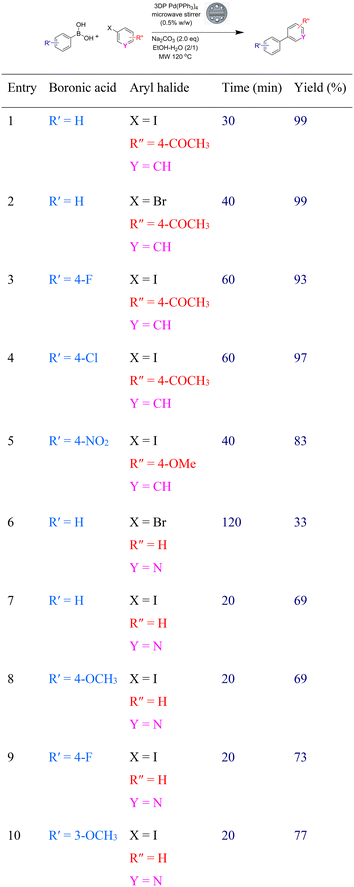
We attributed the lower yields with the bromo compounds to their reduced reactivity and to the fact that there is simply a very low loading of accessible palladium catalyst at the surface of the stirrer device. The stirrers possess a surface area of 1266 mm2 and a volume of 646 mm3, giving a surface area/volume ratio of 2.0 mm−1. However, the catalyst itself is distributed evenly throughout the device, meaning that only the catalyst near the surface is available for reaction. As such, we estimate that only 10% of the actual catalyst is available for reaction for the carousel stirrer devices (ESI†). In light of this, we envisaged that the use of microwave heating (120 °C versus 65 °C) would improve yields when using less reactive substrates. It was calculated that the microwave stirrer device contains less Pd(PPh3)4 catalyst (9 mg) per device than the related carousel congener (48 mg) but due to its greater surface area/volume ratio (4.7 mm−1), it contains a greater estimated proportion of palladium in the first 100 microns depth of its surface (20%) (ESI†). As such, we elected to explore the reaction of a range of less reactive substrates (aryl bromides and heteroaryl halides) in the Suzuki–Miyaura reaction to assess our stirrer devices (Table 2).
From the reactions, it can be seen that the use of Biotage microwave heating gave good yields of the biaryl product in reduced reaction times when compared to their carousel counterparts with their individual optimized heating times shown. Simple addition of the reactants to a microwave vial containing a Pd(PPh3)4 impregnated stirrer device and heating it at 120 °C for 20–120 minutes gave good yields of all products. Of note is the improved yield of the biaryl products from the corresponding aryl bromides when compared to that of the carousel-based reactions. In addition, the use of heteroaryl halides also gave good yields of products (33–77%) with the Pd(PPh3)4 impregnated microwave stirrer devices with both aryl iodides and bromides.
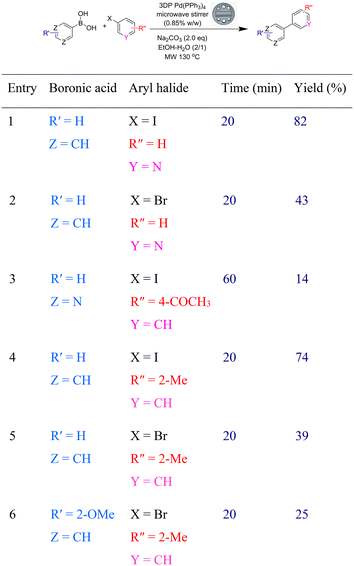
In order to improve the yields of the products from the heteroaryl halides we elected to increase the loading of Pd(PPh3)4 in the devices. When the loading was increased from 0.5% to 0.85% w/w, the reactions of both iodo- and bromopyridine afforded the biaryl products in higher yields and in reduced reaction times. The coupling of two ortho-substituted coupling partners was also successful with higher loading and at a slight higher temperature of 130 °C. Attempts to improve the yield of the reaction, by increasing the amount of Pd(PPh3)4 above 0.85% w/w led to undesired polymerization of PEGDA (Table 3).
The reusability of the stirrer devices was also investigated and it was shown that they can be used for up to 5 times with no loss of yield in the reaction between phenylboronic acid and 4-iodoacetophenone. The carousel device from the first reaction was washed, dried and used in the same Suzuki–Miyaura reaction using the same substrate and reaction molarities, giving the product in analogous yield across all reactions, clearly highlighting the potential of these devices. Noteworthy is the fact that the final printed devices containing tetrakis(triphenylphosphine)palladium(0) showed no discoloration even after 2 years at room temperature and exposure to air. Indeed, their continued reactivity was clearly demonstrated in repeat reactions where comparable yields of product were obtained when compared to freshly printed devices (Table 4).
| Use | 1st | 2nd | 3rd | 4th | 5th |
|---|---|---|---|---|---|
| Yield (%) | 97 | 99 | 99 | 99 | 97 |
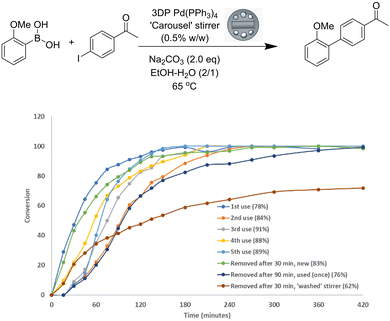
This result, despite its promise, was somewhat surprising as the devices themselves had undergone significant discoloration after coupling (ESI†), presumably as a result of decomposition and formation of elemental palladium species. In order to fully explore the reusability of the stirrer devices, we elected to follow a poorer yielding reaction to better understand the reaction progress during multiple uses of the stirrer bead. The repeated reactions were monitored by HPLC to shed some light on the mechanism of action (Fig. 4).
Unsurprisingly the 1st reaction was the fastest to go to completion, with the 2nd use of the stirrer device reaching full conversion the slowest. Interestingly the 3rd use was quicker than the 2nd (after a lag period) which suggests that either: more catalyst is becoming available due to degradation of the stirrer and release of catalyst; or a more active form of palladium is present. When the stirrer was washed with dichloromethane prior to the 4th run, an increase in rate of reaction and no lag period was observed, suggesting that more palladium is being uncovered by partial degradation of the stirrer surface. Finally, a series of experiments were conducted to investigate if the catalyst released into the reaction solution from the stirrer was responsible for the catalytic activity or if surface catalysis was dominant. When the stirrer (unused) was removed after 30 minutes, the reaction reached the same conversion in a similar time. When the experiment was carried out using a used stirrer, the difference in reaction progress was more pronounced.
To try to understand how much palladium was being lost to the reaction, we carried out an analysis of palladium leaching from the reaction. Pleasingly only 0.8% of the total amount of the palladium catalyst was lost from the carousel device. For a second use of the device, only 0.15% of palladium was lost from the device, clearly tracking the slower reaction profile in the reusability tests. This indicates that whilst some catalyst is lost from the surface of the device following the first use, the integral structure of the device is maintained, meaning that there is a lower amount of catalyst available in the second use of the device and hence results in lower leaching in the second use. Whilst the amount of catalyst lost from the microwave device was higher at 10.9%, this still represents less than 1 mg of catalyst released into the reaction as the loading of catalyst is very low in the microwave-based device. We attribute this increased loss of palladium in the microwave stirrers to the increased temperatures used in the reaction (Table 5).
In addition to the relatively low leaching of palladium as detected by ICP-OES, HPLC analysis of the crude reaction mixture also showed reduced levels of impurities in the crude reaction mixture when compared to the use of solution based “free” catalyst, further adding weight to the clear utility of these devices for the catalysis of reactions (ESI†).
Conclusions
In summary, we have demonstrated that our concept of using 3D Printed stirrer devices containing catalysts can be extended to include metal-based catalysts such as palladium and that they can be employed in the widely used Suzuki–Miyaura reaction. In addition, the reactions are simple to set up as they avoid the tedious weighing out of catalyst prior to reaction and are also easier to purify as the catalytic device can simply be removed from the reaction medium. The devices themselves are also air stable and can be used after at least two years, meaning that they are of wide utility. Further investigation as to the exact nature of the catalyst and the ranges of catalysts that can be incorporated into the stirrer devices will be reported in due course.Conflicts of interest
MRP and SH are authors of a patent related to this research.20Acknowledgements
This research was supported by the Engineering and Physical Sciences Research Council by the award of a DTP studentship (Award – 1810639) and by Scott Bader by the award of funding for a fellowship to B. J. B.Notes and references
- (a) Handbook of Combinatorial Chemistry, ed. K. C. Nicolaou, R. Hanko and W. Hartwig, Wiley-VCH, Weinham, 2002 Search PubMed; (b) T. Ruhland, S. D. Nielsen, P. Holm and C. H. Christensen, J. Comb. Chem., 2007, 9, 301–305 CrossRef CAS PubMed.
- (a) J. Boström, D. G. Brown, R. J. Young and G. M. Keserü, Nat. Rev. Drug Discovery, 2018, 17, 709–727 CrossRef PubMed; (b) S. D. Roughley and A. M. Jordan, J. Med. Chem., 2011, 54, 3451–3479 CrossRef CAS PubMed; (c) T. W. J. Cooper, I. B. Campbell and S. J. F. Macdonald, Angew. Chem., Int. Ed., 2010, 49, 8082–8091 CrossRef CAS PubMed.
- N. Miyaura, K. Yamada and A. Suzuki, Tetrahedron Lett., 1979, 20, 3437–3440 CrossRef.
- A. Biffis, P. Centomo, A. del Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249–2295 CrossRef CAS PubMed.
- L. Branzi, D. Franco, M. Baron, L. Armelao, M. Rancan, P. Sgarbossa and A. Biffis, Organometallics, 2019, 38, 2298–2306 CrossRef CAS.
- C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889–900 CrossRef CAS.
- (a) F.-X. Felpin, T. Ayad and S. Mitra, Eur. J. Org. Chem., 2006, 2679–2690 CrossRef CAS; (b) M. Seki, Synthesis, 2006, 18, 2975–2992 CrossRef.
- (a) R. Ciriminna, V. Pandarus, A. Fidalgo, L. M. Ilharco, F. Bé-land and M. Pagliaro, Org. Process Res. Dev., 2015, 19, 755–768 CrossRef CAS; (b) A. S. Díaz-Marta, S. Yañez, E. Lasorsa, P. Pacheco, C. R. Tubío, J. Rivas, Y. Piñerio, M. A. G. Gómez, M. Amorín, F. Guitián and A. Coelho, ChemCatChem, 2020, 12, 1762–1771 CrossRef.
- (a) D. Song and W.-B. Yi, J. Mol. Catal. A: Chem., 2008, 280, 20–23 CrossRef CAS; (b) Y. Wang and D. R. Sauer, Org. Lett., 2004, 6, 2793–2796 CrossRef CAS PubMed; (c) I. R. Baxendale, C. M. Griffiths-Jones, S. V. Ley and G. K. Tranmer, Chem. – Eur. J., 2006, 12, 4407–4416 CrossRef CAS PubMed; (d) C. Ramarao, S. V. Ley, S. C. Smith, I. M. Shirley and N. DeAlmeida, Chem. Commun., 2002, 1132–1133 RSC.
- (a) I. P. Beletskaya, F. Alonso and V. Tyurin, Coord. Chem. Rev., 2019, 385, 137–173 CrossRef CAS; (b) F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047–3101 CrossRef CAS.
- (a) A. J. Capel, R. P. Rimington, M. P. Lewis and S. D. R. Christie, Nat. Rev. Chem., 2018, 2, 422–436 CrossRef; (b) S. Rossi, A. Puglisi and M. Benaglia, ChemCatChem, 2018, 10, 1512–1525 CrossRef CAS; (c) C. W. Hull, US4575330, 1986 Search PubMed; (d) B. C. Gross, J. L. Erkal, S. Y. Lockwood, C. Chen and D. M. Spence, Anal. Chem., 2014, 86, 3240–3253 CrossRef CAS PubMed; (e) J. S. Manzano, Z. B. Weinstien, A. S. Sadow and I. I. Slowing, ACS Catal., 2017, 7, 7567–7577 CrossRef CAS; (f) X. Wang, Q. Guo, X. Cai, S. Zhou, B. Kobe and J. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 2583–2587 CrossRef CAS PubMed.
- (a) A. J. Capel, S. Edmondson, S. D. R. Christie, R. D. Goodridge, R. J. Bibb and M. Thurstans, Lab Chip, 2013, 13, 4583–4590 RSC; (b) V. Dragone, V. Sans, M. H. Rosnes, P. J. Kitson and L. Cronin, Beilstein J. Org. Chem., 2013, 9, 951–959 CrossRef CAS PubMed; (c) P. J. Kitson, R. J. Marshall, D. Long, R. S. Forgan and L. Cronin, Angew. Chem., Int. Ed., 2014, 53, 12723–12728 CrossRef CAS PubMed; , Angew. Chem., 2014, 126, 12937–12942 Search PubMed; (d) P. J. Kitson, M. D. Symes, V. Dragone and L. Cronin, Chem. Sci., 2013, 4, 3099–3103 RSC; (e) P. J. Kitson, M. H. Rosnes, V. Sans, V. Draone and L. Cronin, Lab Chip, 2012, 12, 3267–3271 RSC; (f) A. Ambrosi and M. Pumera, Chem. Soc. Rev., 2016, 45, 2740–2755 RSC; (g) S. Rossi, R. Porta, D. Brenna, A. Puglisi and M. Benaglia, Angew. Chem., Int. Ed., 2017, 56, 4290–4294 CrossRef CAS PubMed; , Angew. Chem., 2017, 129, 4354–4358 Search PubMed; (h) P. J. Kitson, S. Glatzel, W. Chen, C.-G. Lin, Y.-F. Song and L. Cronin, Nat. Protoc., 2016, 11, 920–936 CrossRef CAS PubMed; (i) A. S. Díaz-Marta, C. R. Tubío, C. Carbajales, C. Fernández, L. Escalante, E. Sotelo, F. Guitián, V. L. Barrio, A. Gil and A. Coelho, ACS Catal., 2018, 8, 392–404 CrossRef; (j) ed. V. Saggiomo, M. Benaglia and A. Puglisi, Wiley-VCH, 2019, pp. 369–408; (k) E. Bulatov, E. Lahtinen, L. Kivijärvi, E. Hey-Hawkins and M. Haukka, ChemCatChem, 2020, 12, 4831–4838 CrossRef CAS; (l) J. Zhu, P. Wu, Y. Chao, J. Yu, W. Zhu, Z. Liu and C. Xu, Chem. Eng. J., 2022, 433, 134341 CrossRef CAS; (m) S. Lawson, X. Li, H. Thakkar, A. A. Rownaghi and F. Rezaei, Chem. Rev., 2021, 121, 6246–6291 CrossRef CAS PubMed.
- M. Penny, Z. Cao, B. Patel, B. Sil dos Santos, C. Asquith, B. R. Szulc, Z. X. Rao, Z. Muwaffak, J. P. Malkinson and S. T. Hilton, J. Chem. Educ., 2017, 94, 1265–1271 CrossRef CAS.
- (a) A. L. Tyson, S. T. Hilton and L. C. Andreae, Int. J. Pharm., 2015, 494, 651–656 CrossRef CAS PubMed; (b) S. K. Talapatra, M. R. Penny, S. T. Hilton and F. Kozielski, J. Appl. Crystallogr., 2019, 52, 171–174 CrossRef CAS.
- (a) S. A. Mohammed, M. E. Vianna, M. R. Penny, S. T. Hilton, N. Mordan and J. C. Knowles, Dent. Mater., 2016, 32, 1289–1300 CrossRef PubMed; (b) S. A. Mohammed, M. E. Vianna, M. R. Penny, S. T. Hilton, N. Mordan and J. C. Knowles, MicrobiologyOpen, 2017, 00:e00455 CrossRef PubMed.
- (a) Z. X. Rao, B. Patel, A. Monaco, Z. Cao, M. Barniol-Xicota, E. Pichon, M. Ladlow and S. T. Hilton, Eur. J. Org. Chem., 2017, 6499–6504 CrossRef CAS; (b) C. G. W. Van Melis, M. R. Penny, A. D. Garcia, A. Petti, A. P. Dobbs, S. T. Hilton and K. Lam, ChemElectroChem, 2019, 6, 4144–4148 CrossRef CAS; (c) M. R. Penny, Z. X. Rao, B. F. Peniche and S. T. Hilton, Eur. J. Org. Chem., 2019, 23, 3783–3787 CrossRef.
- https://generic.wordpress.soton.ac.uk/dial-a-molecule/wp-content/blogs.dir/sites/50/2017/07/Hilton-Dial-a-molecule-2017-pdf-COPY.pdf, (accessed 11/05/ 2022).
- M. R. Penny and S. T. Hilton, React. Chem. Eng., 2020, 5, 853–858 RSC.
- https://www.radleys.com/range/carousel-12-plus-reaction-station/, (accessed 11/05/ 2022).
- S. T. Hilton, M. R. Penny, B. S. Dos Santos and B. Patel, Br. Pat., GB201604322D0, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2re00218c |
| This journal is © The Royal Society of Chemistry 2023 |