Open Access Article
Open Access ArticleApplication of biodegradable cholinium ionic liquids for the extraction of 5-hydroxymethylfurfural (HMF) from honey†
Aleksandar Marić *a,
Pavle Jovanova,
Slobodan Gadžurić
*a,
Pavle Jovanova,
Slobodan Gadžurić b,
Tatjana Trtić-Petrovićc,
Marijana Sakača,
Aleksandar Totd,
Marko Bertić
b,
Tatjana Trtić-Petrovićc,
Marijana Sakača,
Aleksandar Totd,
Marko Bertić e and
Milan Vraneš
e and
Milan Vraneš b
b
aInstitute of Food Technology in Novi Sad, University of Novi Sad, Bulevar Cara Lazara 1, 21000 Novi Sad, Republic of Serbia. E-mail: aleksandar.maric@fins.uns.ac.rs
bFaculty of Sciences, University of Novi Sad, Department of Chemistry, Biochemistry and Environmental Protection, Trg Dositeja Obradovića 3, 21000 Novi Sad, Republic of Serbia
cLaboratory of Physics, Vinča Institute of Nuclear Sciences, University of Belgrade, National Institute of the Republic of Serbia, 11001 Belgrade, Republic of Serbia
dApplied Physical Chemistry, Department of Chemistry, KTH Royal Institute of Technology, Stockholm, SE-10044, Sweden
eUniversity of Freiburg, Fahnenbergplatz, 79085 Freiburg im Breisgau, Germany
First published on 7th November 2023
Abstract
5-Hydroxymethylfurfural (HMF), a Maillard reaction product, can be formed when honey is subjected to heat treatment or a long storage time, becoming volatile and toxic depending on its concentration. The fact that, until today, there is no literature data on the extraction of 5-hydroxymethylfurfural (HMF) from honey using ionic liquids directed the investigation of the influence of biodegradable cholinium ionic liquids on the formation of aqueous biphasic systems and the application of these systems for the extraction of HMF from honey. The influence of anions of synthesised ionic liquids on the construction of biphasic systems in which an inorganic salt was used as a salting agent was investigated. Then, the extraction of HMF in these systems was examined, and the mechanisms of HMF extraction using ionic liquids were explained using computer simulations. Examining the effect of cholinium ionic liquids (choline chloride ([Ch][Cl]), cholinium nicotinate ([Ch][Nic]), cholinium propionate ([Ch][Prop]), and cholinium butyrate ([Ch][But])) on the formation of aqueous biphasic systems by comparing the phase diagrams, it was concluded that the ability of ionic liquids to form an aqueous biphasic system with tripotassium phosphate (K3PO4) decreases in the following order: [Ch][But] ≈ [Ch][Prop] > [Ch][Nic] > [Ch][Cl]. By applying all tested aqueous biphasic systems for the extraction of HMF from honey, an extraction efficiency of more than 89% was achieved. Complete extraction was achieved using the extraction system with [Ch][But], while the weakest ability to extract HMF was exhibited by the system with [Ch][Cl]. The mechanisms of HMF extraction using ionic liquids are explained on the basis of the optimised structures of the ionic liquid systems with HMF, together with the visualisation of non-covalent interactions, and on the basis of the calculated binding energies ΔGbin, which can be used as a good predictor of the extraction potential of newly synthesised ionic liquids.
Introduction
Honey is a valuable food product in terms of nutritional value and therapeutic potential consumed without prior technological processing.1 However, certain ingredients in honey, such as heavy metals, alkaloids, and 5-hydroxymethylfurfural can contribute to its toxicity. For that reason, continuous monitoring and selective and precise methods for their determination are of exceptional importance.5-Hydroxymethylfurfural (5-(hydroxymethyl)furan-2-carbaldehyde, HMF) is a furan compound formed as an intermediate in the Maillard reaction following dehydration of sugar in an acidic medium during thermal treatments applied on foods. In acidic conditions, HMF can be formed even at low temperatures, although its content increases with an increase in temperature. In addition to temperature dependence, the amount of HMF formed in food also depends on pH value, water activity, and the concentration of divalent cations in the medium.2,3
HMF in honey derives from hexoses, i.e., glucose and fructose, as the main sugar compounds, even at low temperatures during a long storage period because honey is an acidic medium.2 HMF in honey represents a parameter that indicates its toxicity and freshness.4 The permissible concentration of HMF, according to Codex Alimentarius, is up to 40 mg kg−1. Exceptions are types of honey originating from the tropics (80 mg kg−1).5 The amount of up to 10 mg per kg HMF is considered as naturally presents in honey.6
In vitro toxicological studies have shown that HMF is toxic in high concentrations. Acceptable daily intake from food for human beings was suggested to be 2 mg of HMF per kg body weight.7 Also, the range of 2–30 mg was cited for HMF per person per day as acceptable.8 Ulbricht et al. (1984)9 reported that the toxic effects of HMF were registered with a dose greater than 75 mg HMF per kg body weight. HMF can cause irritation of the eyes, upper respiratory tract, skin, and mucous membranes. Also, in vitro animal experiments have shown that exposure to higher concentrations of HMF can cause skin cancer, kidney tumors and significantly increase the incidence of hepatocellular adenomas.10,11 According to the conventional Ames test, the attention in toxicological studies has been paid to the conversion of HMF to sulfoxymethylfurfural (SMF), a mutagenic compound. SMF is thought to be a major initiator of skin tumors as well as liver and kidney damage.12 However, many literature data indicate the possibility of applying HMF and its derivatives in various industry fields, such as the pharmaceutical and petrochemical industries, for the production of resins, green polymers, fungicides, and solvents.13–15
For all the reasons mentioned above, it is of great interest to choose a suitable extraction technique for the extraction of HMF from honey to ensure its safety for consumption.
Until now, the most commonly used methods for the extraction of HMF from foods, including honey, were mainly based on the application of solid–liquid (SP) extraction. Driffield et al. (2005)16 used SP extraction to isolate HMF from honey using toxic solvents, acetonitrile and methanol, while the procedure required considerable time. Gürkan and Altunay (2015)17 developed a method of preconcentration and separation of HMF using ultrasonic extraction with micelles. Both liquid–liquid extraction with the help of salting out18 and dispersive liquid–liquid microextraction19 were applied for HMF extraction and they also used toxic and volatile aromatic or chlorinated organic solvents.
Ionic liquids (ILs) are solvents of the 21st century and they are considered alternatives to volatile and toxic organic solvents in many technological and industrial processes.20 ILs have found significant analytical chemistry applications: in liquid–liquid extraction and solid phase extraction (as sample preparation methods),21 gas and liquid chromatography, electrophoresis, and other analytical techniques.22,23 By properly selecting and combining cations and anions, it is possible to adjust their selectivity and successfully extract non-polar and polar compounds. By selecting an adequate ionic liquid, a high extraction efficiency of the chosen compound can be achieved, and the amount of solvent can be significantly reduced. ILs can often be successfully recycled, leading to a significant reduction in the cost of the process itself. It should be added that using these compounds can reduce environmental harm due to their biodegradability. At the same time, their non-flammability ensures the safety of the process.23,24
Emphasis should be placed on the development and implementation of energy-efficient processes using non-toxic chemicals to identify and determine compounds (such as HMF) that, if present in large quantities, reduce honey quality and safety. Based on the above facts, it is crucial to examine the possibility of honey compound extraction (HMF) following the modern concept of sustainable development and environmental protection, using less toxic, chemically and thermally stable and non-flammable solvents – ionic liquids. Previous research based on the use of green solvents, primarily ionic liquids, has exclusively focused on the conversion of biomaterials (fructose, cellulose, sucrose) into HMF.25–29 Until today there are no literature data on the extraction of HMF from honey using ionic liquids. Previous studies on the toxicity of cholinium cation-based ionic liquids in combination with different linear alkanoate anions demonstrated that these ionic liquids are environmentally friendly and biodegradable.30 Furthermore, Vraneš et al. (2017)31 examined the toxicity of vitamin-based cholinium ionic liquids and established that ionic liquids, in which both cation and anion are biologically active components, can be classified as non-toxic. Therefore, the idea was to investigate (1) the influence of biodegradable choline-based ionic liquids on the formation of aqueous biphasic systems and (2) the application of these systems for the extraction of HMF from honey. The influence of the anions of the synthesized choline-based ionic liquids on the construction of biphasic systems in which tripotassium phosphate (K3PO4) was used as a salting agent was examined. The choice of this salting-out agent relies on its strong salting-out effect, following the position of the inorganic salt in the Hoffmeister series, as well as considerations of biocompatibility and environmental impact.
Afterwards, the extraction of HMF in these systems was investigated and the mechanisms of HMF extraction using ionic liquids were explained using computer simulations.
Materials and methods
Materials
The honey solution was prepared by dissolving 10 g of honey in 50 mL of distilled water using a magnetic stirrer (Witeg, Germany) (500 rpm for 10 minutes), and after that, the mixture was filtered through a 0.45 μm nylon micro filter (Amtast, Lakeland, FL, USA).Analytical standard of 5-hydroxymethylfurfural (mass fraction purity w ≥ 98.0%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Tripotassium phosphate was supplied from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade methanol and formic acid (HCOOH) were purchased from Merck (Darmstadt, Germany). All chemicals used for the synthesis of ionic liquids were of analytical grade. Choline hydroxide solution (46 wt%) (Sigma-Aldrich, St. Louis, MO, USA) and propanoic, nicotinic, butyric, and oxalic acid dihydrate (Merck, Darmstadt, Germany) were used without further purification.
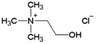
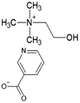
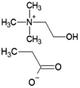
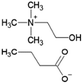
Ionic liquid, choline chloride, [Ch][Cl], was purchased from Sigma-Aldrich (St. Louis, MO, USA). The synthesis of the other three ionic liquids, choline propionate ([Ch][Prop]), choline butyrate ([Ch][But]), and choline nicotinate ([Ch][Nic]) were carried out by potentiometric titration. The synthesis of [Ch][Prop] and [Ch][But] was done according to Muhammed et al. (2012),32 while synthesis of [Ch][Nic] was carried out as described by Vraneš et al. (2017).31 An aqueous solution of choline hydroxide (ChOH) was used as a titration agent, whose concentration (c = 3.51 mol L−1) was determined by titration with a standard solution of oxalic acid dihydrate. The predetermined amount of nicotinic, butyric, or propanoic acid was dissolved in water at the room temperature. The solution was titrated with choline hydroxide while monitoring the change in pH value. The solution was overtitrated with the addition of ChOH to determine the endpoint of the titration. Based on the zero of the second derivative of the titration curve, the pH value at the endpoint of the titration was read and adjusted by the addition of nicotinic, butyric, or propanoic acid to correspond to the pH value at the endpoint of the titration (pH [Ch][Nic] = 7.95; pH [Ch][But] = 10.40; pH [Ch][Prop] = 10.30).
Excess water was removed by evaporation on a rotary vacuum evaporator (Reacti-Vap, Thermo Fisher Scientific, USA) at a water bath temperature lower than the decomposition temperature of the synthesized ionic liquids of T = 343.15 K for 120 min. The procedure was continued using a stronger (industrial) vacuum pump with heating until the water was removed. After evaporation to a constant mass (±1.0 mg), the ionic liquids were stored in a desiccator over phosphorus pentoxide.
The structures of the synthesized ionic liquids were confirmed by IR spectra (Fig. S1a–c†).
The structures of the applied ILs are given in Table 1.
Determination of phase diagrams
The binodal curves' construction was based on the cloud point titration method at temperature T = 298 ± 1 K and standard atmospheric pressure. A turbid mixture was achieved by adding a solution of K3PO4 dissolved in a 20% solution of honey (ωsalt = 55%) dropwise to a solution of ionic liquid prepared in a 20% honey solution (ωIL = 60%).The amount of added salt was measured by an analytical balance (AND HA-180M, Tokyo, Japan) with an uncertainty of ±10−4 g. Furthermore, an aqueous honey solution was added to the mixture until it became apparent, and the amount of added honey solution was measured using the analytical balance. After each addition, the mixture was stirred using a vortex mixer (Boeco V1 plus, Hamburg, Germany) at 2500 rpm. This procedure was repeated until sufficient points were obtained to construct binodal curves. Ternary phase diagrams were fitted using the Merchuk equation:33
Y = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(BX0.5 − CX3) exp(BX0.5 − CX3)
| (1) |
Extraction of hydroxymethylfurfural using aqueous biphasic systems (ABS)
Characterized aqueous biphasic systems based on ionic liquids [Ch][Cl], [Ch][Nic], [Ch][Prop] and [Ch][But] and K3PO4 were applied to extract HMF from honey samples. In order to evaluate the extraction capabilities of ionic liquids (ILs), the identical composition of the ABS within the two-phase region as the extraction point for all the ABS systems was chosen (Fig. S2†). A series of biphasic three-component systems with the following composition was made in triplicate: 25% ionic liquid, 35% K3PO4 and 40% aqueous honey solution (20% v/v), in which the concentration of HMF was 5, 10, 20, 40 and 60 mg kg−1, respectively. The prepared mixtures were shaken for 2 min, centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and left for 3 h to balance the phases. After reaching thermodynamic equilibrium, the phases were separated, and the concentration of HMF was determined using high-performance liquid chromatography with DAD.
000 rpm for 10 min and left for 3 h to balance the phases. After reaching thermodynamic equilibrium, the phases were separated, and the concentration of HMF was determined using high-performance liquid chromatography with DAD.
The chromatographic separation and quantification of HMF were performed using the HPLC method described by Ariffin et al. (2014)34 and Tomasini et al. (2012)35 with some modifications. A liquid chromatograph (Agilent 1200 series, Agilent Technologies Santa Clara, CA, USA), equipped with a DAD detector and an Eclipse XDB-C18, 1.8 μm, 4.6 × 50 mm column (Agilent) was used for quantification of HMF in the obtained extracts. The analyte was separated using column temperature of 30 °C and a sample injection volume of 10 μL. The mobile phase consisted of two eluents, H2O (0.1% HCOOH) (A) and methanol (B), delivered at a flow rate of 0.75 mL min−1. The isocratic elution was employed with the ratio A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B (90
B (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v). The DAD wavelength was set at 284 nm. The total run time of the analysis was 10 min. The method recovery (R) was ≥93% for the investigated HMF concentrations. The limit of detection (LOD) and limit of quantification (LOQ) were 0.1 mg kg−1 and 0.3 mg kg−1, respectively.
10, v/v). The DAD wavelength was set at 284 nm. The total run time of the analysis was 10 min. The method recovery (R) was ≥93% for the investigated HMF concentrations. The limit of detection (LOD) and limit of quantification (LOQ) were 0.1 mg kg−1 and 0.3 mg kg−1, respectively.
All methodology steps are present in a flowchart (Fig. S3†).
Computational setup
All the calculations presented in this paper were performed in Jaguar software, implemented as a part of the Schrödinger Suite 2015-4 package. The initial phase considered the geometrical optimization of each molecule. The optimization was undergone in the implicit water solvation phase, applying Poisson–Boltzmann finite elements (PBF) solvent model. After successful optimization, the optimized molecules of ionic liquids and HMF were combined, and molecular pair was used for further simulations. Upon initial geometrical optimization, the density functional theory (DFT) calculations were applied. The used functional was B3LYP exchange–correlation functional (B3LYP-D3) followed by 6-41+G(d,p) basis set. The validity of the results was checked by Hessian analysis, which showed no presence of imaginary frequencies proving that all optimized structures are true minima. The non-covalent interactions (NCI) in the optimized structures were analyzed by the method of Johnson et al. (2010).36 Based on DFT calculations, ion-pair binding energies (ΔGbin) between all constituents were calculated. To avoid a basis-set superposition error (BSSE) binding energies (ΔGbin) were counter-poise corrected using a standard approach by Boys and Bernardi (1970).37 For the estimation of binding energies, eqn (2) was used:| ΔGbin = Ecp(I–II) − Emin(I) − Emin(II) + ΔZPVE | (2) |
Results and discussion
Phase diagrams of aqueous biphasic systems
The new phase diagrams of the ABSs based on the targeted ILs combined with inorganic salt (K3PO4) at 296.15 ± 1 K and atmospheric pressure p = 0.1 MPa were determined in this work. The experimental binodal data for the ternary mixtures of the studied ILs are reported in Table S1.† The phase diagrams (Fig. 1) are shown in molality units, moles of solute (IL or salt) per kilogram of solvent, which allow comparison of diagrams obtained for different ILs. The experimental data were fitted by the empirical relation of Merchuk (eqn (1)) and the regression parameters for this equation were estimated by least-squares regression. The calculated coefficients of the Merchuk equation (A, B, and C) for the investigated ABS, corresponding standard deviations (σ), and correlation coefficients (R2) are given in Table S2.† The high values of the regression parameters and low values of the standard deviations prove that the Merchuk equation is suitable to fit the experimental data. In all phase diagrams, the biphasic region is localized on the right side of binodal curves.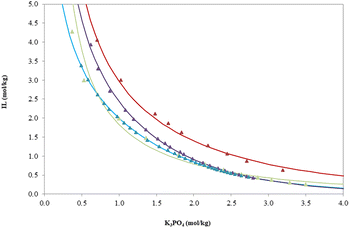 | ||
Fig. 1 Ternary phase diagrams of the studied systems {IL + K3PO4 + H2O} at T = 296.15 K and atmospheric pressure (p = 0.1 MPa). Legend:  [Ch][Prop], [Ch][Prop],  [Ch][But], [Ch][But],  [Ch][Nic] and [Ch][Nic] and  [Ch][Cl]. [Ch][Cl]. | ||
Based on the phase diagrams, an insight into the miscibility/immiscibility of phases is obtained depending on the composition of the mixture (mass fraction of ionic liquid and inorganic salt). If the solubility curve is closer to the coordinate axes, i.e. the wider the two-phase region, a smaller amount of salt is needed to salt out a certain amount of ionic liquid.38 Phase diagrams based on choline-based ionic liquids and salts (Fig. 1) provide the insight into the influence of ionic liquids on the ability to form an aqueous biphasic system.
When a salt with a high charge density such as K3PO4 is dissolved in water, the isolated ions are surrounded by a layer of water molecules, a phenomenon known as ionic hydration. Thus, when K3PO4 is added to an aqueous medium containing an ionic liquid, the two solutes compete for solvent molecules. Inorganic ions are more capable of forming hydration complexes and, therefore, there is a “migration” of solvent molecules from the ions of the ionic liquid to the ions of the inorganic salt, which consequently leads to liquid–liquid demixing. As a result, the ionic liquid-rich phase separates from the rest of the inorganic salt solution.39
The condition for the formation of an aqueous biphasic system is that the ions of the ionic liquid have a delocalized charge and, consequently, are poorly hydrated in the solution. Accordingly, choline-based ionic liquids with aliphatic anions or fully hydrogenated C-atoms of the anion(s) can achieve phase separation by adding the phosphate salt solution.
By comparing the phase diagrams of aqueous biphasic systems based on choline-based ionic liquids containing the same inorganic salt, it is possible to analyze the influence of the anions of the ionic liquid on the ability to form the system (Fig. 1). The ability of the studied ionic liquids to form an aqueous biphasic system with K3PO4 decreases in the following order: [Ch][But] ≈ [Ch][Prop] > [Ch][Nic] > [Ch][Cl].
The ionic liquid that separated most easily from the aqueous solution was [Ch][But]. An increase in the number of carbon atoms in an ionic liquid leads to a decrease in its hydrophilic nature, which implies a lower affinity for water. However, it can be seen from Fig. 1 that [Ch][Nic] also builds a biphasic system, and this ability can be attributed to the presence of an aromatic ring with a large number of carbon atoms, which contributes to the efficiency of the formation of aqueous biphasic systems. Taking into account the values of the n-octanol–water distribution coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow) for the anions of ionic liquids as a measure of the differential solubility of a given substance between octanol and water, i.e. a measure of the hydrophobicity of four choline-based ionic liquids, the following sequence was obtained: log
Kow) for the anions of ionic liquids as a measure of the differential solubility of a given substance between octanol and water, i.e. a measure of the hydrophobicity of four choline-based ionic liquids, the following sequence was obtained: log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow ([But]) = 0.78 > log
Kow ([But]) = 0.78 > log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow ([Prop]) = 0.25 > log
Kow ([Prop]) = 0.25 > log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow ([Nic]) = 0.15 > log
Kow ([Nic]) = 0.15 > log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow ([Ch][Cl]) = −3.70.40 The series of log
Kow ([Ch][Cl]) = −3.70.40 The series of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values for the investigated ionic liquids agrees with the obtained capacities for the formation of biphasic systems from Fig. 1, that is, the higher the hydrophobicity of the ionic liquid, the easier it is salted out by K3PO4.
Kow values for the investigated ionic liquids agrees with the obtained capacities for the formation of biphasic systems from Fig. 1, that is, the higher the hydrophobicity of the ionic liquid, the easier it is salted out by K3PO4.
Nevertheless, it should be emphasized that the ability of choline-based ionic liquids to separate phases is lower than that of ionic liquids containing other types of cations. Thus, for example, ionic liquids with chloride anion and different cations, 1-butyl-3-methylimidazolium, 1-butyl-1-methylpyrrolidinium, tetrabutylphosphonium or tetrabutylammonium, are more easily isolated than [Ch][Cl]. This is an inherent consequence of the presence of three methyl groups and a hydroxyl group on the cholinium cation, which enhances its affinity for water.41 The presence of –OH groups which enable the formation of strong hydrogen bonds with water molecules and obtaining dipole–dipole interactions, as well as the absence of hydrophobic chains in the choline molecule leads to a weakening of the hydrophobic solvation of the molecule.42
Extraction of HMF
In all cases, the concentration of HMF in the lower phase (salt) was below the detection limit (0.1 mg kg−1), indicating high partition coefficients in the upper phase rich in ionic liquid. Extraction yields (R) of HMF extracted by different aqueous biphasic systems were calculated by measuring the concentration of HMF in the ionic liquid-rich phase (the extraction phase) to evaluate the extraction efficiency of certain aqueous biphasic systems. The corresponding chromatograms of HMF detected in the ionic liquid phase are shown in Fig. 2.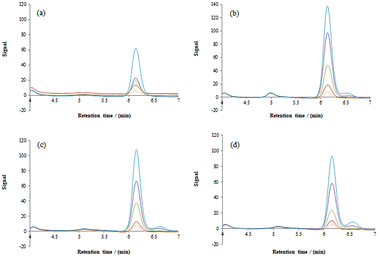 | ||
Fig. 2 Chromatograms of HMF detected in the ionic liquid phase (a) [Ch][Cl] (b) [Ch][Nic] (c) [Ch][But] and (d) [Ch][Prop]. Legend:  5 mg kg−1, 5 mg kg−1,  10 mg kg−1, 10 mg kg−1,  20 mg kg−1, 20 mg kg−1,  40 mg kg−1 and 40 mg kg−1 and  60 mg per kg HMF. 60 mg per kg HMF. | ||
The extraction yield (R) of HMF was used to evaluate the efficiency of analyte extraction using aqueous biphasic systems and it was determined using the eqn (3):
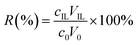 | (3) |
Fig. 3 illustrates the extraction efficiencies of 5-hydroxymethylfurfural (HMF) determined using eqn (3). The data presented in Fig. 3 represents the average extraction efficiencies obtained from three independent repetitions, accompanied by their corresponding standard deviations.
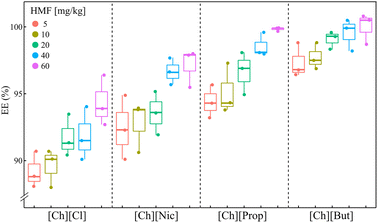 | ||
| Fig. 3 Extraction efficiency (EE, %) using the aqueous biphasic systems composed of choline-based ILs. | ||
This statistical approach provides valuable insights into the variability within the extraction process and enables a robust assessment of the reliability and precision of the calculated HMF extraction efficiencies.
From the graph shown in Fig. 3, it can be seen that more than 89% extraction efficiency was achieved by applying all the systems. Complete extraction (EEHMF = 100%) was achieved using the extraction system with [Ch][But], while the system with [Ch][Cl] showed the weakest ability to extract HMF.
The explanation of the extraction mechanism, that is, the types of interactions that occur using aqueous biphasic systems based on cholinium ionic liquids, which ensure appropriate extraction efficiencies, can be obtained using computer simulation.
Computer simulations of interactions of ionic liquids with HMF
The optimized structures of HMF ionic liquid systems and visualization of non-covalent interactions (NCI) are presented in Fig. 4 and 5. Fig. 4 shows ionic liquids with halide anions to distinguish the influence and potential of different cations for interactions with HMF.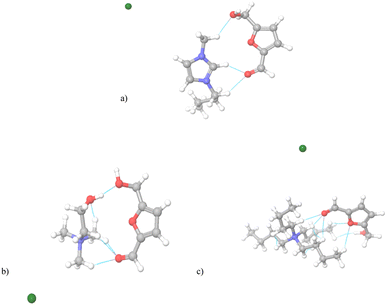 | ||
| Fig. 4 The geometrical optimized system of ionic liquids with HMF. (a) [Bmim][Cl], (b) [Ch][Cl], (c) [N4444][Cl]. | ||
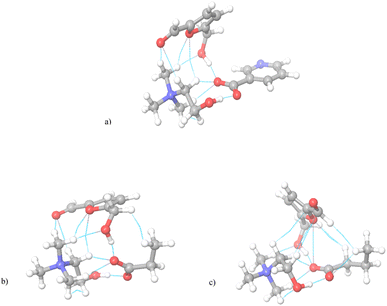 | ||
| Fig. 5 The geometrical optimized system of ionic liquids with HMF. (a) [Ch][Nic], (b) [Ch][Pro], (c) [Ch][But]. | ||
In each system, the optimized structures indicate that the halide anions do not affect the HMF–ionic liquid interactions, as all anions are “repelled” from the HMF–IL pair. Therefore, the structure of the cation is decisive for the type of interactions with HMF. As an initial step, an NCI study was carried out and it was concluded that the imidazolium-based cations have 3 NCIs with HMF, one via H-bond between the H2 atom of the imidazolium ring and the oxygen of the keto group from HMF. The additional stability of the structure is accomplished via two van der Waals interactions between alkyl groups of imidazolium side chain and oxygens from HMF. Indeed, the most determining interaction is H-bond (between –OH group of cholinium and HMF), but easily approachable methyl groups of cholinium provide three more van der Waals-type of interactions with HMF, contributing to the overall number of 4 NCI. The largest number of interactions with HMF (6 NCI) was obtained using tetrabutylammonium cation due to the pronounced possibility of forming dispersive-type interactions with alkyl side chains. After NCI analysis, ΔGbin was calculated (in eqn (2) I is given for the cation, while II is HMF) and the results are shown in Table 2. The obtained data show that the affinity of the imidazolium cation to HMF is the weakest. Contrary, the binding energy values for systems with cholinium and tetrabutylammonium cations are similar, suggesting that the contribution of H-bonding to the total binding is significantly greater than that of dispersive interactions.
| Ionic liquid | ΔGbin/kJ mol−1 |
|---|---|
| [Bmim][Cl] | −47.93 |
| [Ch][Cl] | −65.27 |
| [N4444][Cl] | −68.61 |
| [Ch][Nic] | −84.83 |
| [Ch][Pro] | −98.16 |
| [Ch][But] | −101.23 |
Due to the high binding energy and the best experimental results, further research was directed only toward the cholinium cation. In the next step, the influence of different anions on the binding energy of cholinium ionic liquids was examined, i.e. the chloride anion was replaced by nicotinate, propionate, or butyrate, and the obtained results are shown in Fig. 5. With the inclusion of more complex organic anions, the interaction pattern between ionic liquid and HMF is drastically changed. The geometrical position of cation and anion in each IL–HMF complex was mostly the same. The presence of the carboxylic group in anions mainly contributed to the weakening of cation–HMF interactions. The previous main contributor to cation–HMF stability, the –OH group of cholinium cation was excluded from interaction by the –COO− group of anion. In that way, cation and anion of investigated ionic liquid forms a strong H-bond, leaving only methyl groups and van der Waals interactions of cholinium cation to interact with HMF. In these systems, the anion contribution to the overall stability of IL–HMF complex becomes significant. The presence of –COO− group not only stabilizes cation–anion interaction, but also provides the ability to form H-bond with the –OH group of HMF as well. By comparing the overall number of NCI, the same cation–HMF interactions were obtained between HMF and [Ch][Nic] and [Ch][Pro]. In both situations, methyl groups of cations established van der Waals interactions with oxygen of HMF, followed by similar orientation of HMF. The orientation of HMF induced the additional interaction of propionate aliphatic groups with HMF leading to one more NCI in [Ch][Pro] compared to [Ch][Nic]. The higher flexibility of the aliphatic anion compared to the aromatic-type allowed the closer proximity of propinate to HMF, leading to overall better binding. The influence of aliphatic side chain flexibility thoroughly changed the HMF orientation in the system [Ch][But]–HMF. Compared to systems with the other two cholinium ILs, the HMF is rotated for 90°, followed by a completely different interaction pattern. Foremost, the number of cation–HMF interactions decreased to 4. However, anion contribution was even more important represented in 4 NCI. Despite the usual H-bond formation, three additional van der Waals interactions of the butyrate alkyl chain with HMF were established increasing the stability of IL–HMF complex. The increase in NCI was followed by the rise of ΔGbin (Table 2).
The obtained theoretical results (ΔGbin) were correlated with the experimentally determined extraction efficiency (Fig. 6). From the correlation shown, an increase in extraction efficiency can be observed with an increase in the absolute value of the binding energy between the ionic liquid and HMF.
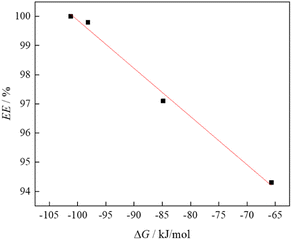 | ||
| Fig. 6 The dependance of extraction efficiency binding energies obtained using density functional theory (DFT) calculations. | ||
This dependence follows the linear trend, yielding the correlation coefficient R2 of 0.9903, indicating that the estimate of ΔGbin can be a good predictor of the extraction potential of newly synthesized ionic liquids.
Based on computer simulations of HMF interactions with ionic liquids and certain binding energies, it can be concluded that the influence of ionic liquid anions is crucial for HMF extraction. Research by Berton et al. (2021),43 who applied aqueous biphasic systems based on ([Ch][OAc], [Ch][Pro], [Ch][But] and [Ch][Hex]) and inorganic salts and extracted herbicides with extraction efficiencies greater than 90% confirms this statement. Shahriari et al. (2013)44 also extracted antibiotics with high yields using systems based on choline ionic liquids. Furthermore, Nie et al. (2018)45 utilized magnetic cholinium ionic liquids in combination with inorganic salts for the extraction of berberine hydrochloride from Rhizoma coptidis with extraction efficiencies greater than 97%.
Conclusions
Extraction of HMF from honey using aqueous biphasic systems based on cholinium ionic liquids involved testing: (1) the influence of cholinium ionic liquids on the formation of aqueous biphasic systems and (2) the efficiency of these systems for the extraction of HMF from honey. The influence of anions of synthesized cholinium ionic liquids on the construction of aqueous biphasic systems in which K3PO4 salt was used as a salting agent was examined. To examine the influence of the anionic part of ionic liquids on the construction of aqueous biphasic systems, the following ionic liquids were used – cholinium chloride ([Ch][Cl]), cholinium nicotinate ([Ch][Nic]), cholinium propionate ([Ch][Prop]) and cholinium butyrate ([Ch][But]). By comparing the phase diagrams of aqueous biphasic systems based on cholinium ionic liquids, it was concluded that the ability of ionic liquids to form an aqueous biphasic system with K3PO4 decreases in the following order: [Ch][But] ≈ [Ch][Prop] > [Ch][Nic] > [Ch][Cl]. Characterized aqueous two-phase systems based on investigated ionic liquids and K3PO4 were applied to extract HMF from honey. A series of biphasic three-component systems was created with the following composition: 25% ionic liquid, 35% K3PO4 and 40% aqueous honey solution (20% v/v), in which the concentration of HMF was 5, 10, 20, 40 and 60 mg kg−1, respectively. By applying all the described systems, an extraction efficiency of more than 89% was achieved. Complete extraction (EEHMF = 100%) was achieved using the extraction system with [Ch][But], while the system with [Ch][Cl] showed the weakest ability to extract HMF. Structures of ionic liquid systems with HMF were optimized, along with visualization of non-covalent interactions and calculated binding energy ΔGbin. Binding energies had almost the same values for ionic liquids with [Pro]− and [But]−, while the affinity of HMF to ionic liquid with nicotinamide anion was significantly lower.The obtained theoretical results (ΔGbin) were correlated with the experimentally determined extraction efficiencies. From the correlation, an increase in extraction efficiency was observed with an increase in the absolute value of the binding energy between the ionic liquid and HMF. This dependence follows the linear trend, where the correlation coefficient R2 of 0.9903 was obtained, indicating that the estimation of ΔGbin can be a good predictor of the extraction potential of newly synthesized ionic liquids.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Ministry of Science, Technological Development and Innovations, Republic of Serbia (contract number: 451-03-47/2023-01/200222) and by The Science Fund of the Republic of Serbia, grant no. 6673, PROTein from Rapeseed Oil Processing Waste: Application in Food and Wastewater Treatment – PROTOPOWER.Notes and references
- A. Ajibola, J. P. Chamunorwa and K. H. Erlwanger, Nutr. Metab., 2012, 9, 61 CrossRef PubMed.
- M. B. Sakač, P. T. Jovanov, A. Z. Marić, L. L. Pezo, Ž. S. Kevrešan, A. R. Novaković and N. M. Nedeljković, Food Chem., 2019, 276, 15–21 CrossRef PubMed.
- H. Zhao, N. Cheng, Y. Zhang, Z. Sun, W. Zhou, Y. Wang and W. Cao, LWT, 2018, 96, 133–139 CrossRef CAS.
- U. M. Shapla, M. Solayman, N. Alam, M. I. Khalil and S. H. Gan, Chem. Cent. J., 2018, 12, 35 CrossRef CAS PubMed.
- Codex Alimentarius Commission, Revised Codex Standards for Honey, Codex Standard 12-1981, Rev. 2, 2001 Search PubMed.
- A. S. Alqarni, A. A. Owayss and A. A. Mahmoud, Arab. J. Chem., 2016, 9, 114–120 CrossRef CAS.
- A. N. Zaitzev, T. A. Simonian and A. L. Pozdniakov, Vopr. Pitan., 1975, 1, 52–55 Search PubMed.
- K. E. Appel, R. Gürtler, K. Berg, G. Heinemeyer, A. Lampen and K. E. Appel, Mol. Nutr. Food Res., 2011, 55, 667–678 CrossRef PubMed.
- R. J. Ulbricht, S. J. Northup and J. A. Thomas, Toxicol. Sci., 1984, 4, 843–853 CrossRef CAS.
- M. R. Farag, M. Alagawany, M. Bin-Jumah, S. I. Othman, A. F. Khafaga, H. M. Shaheen, D. Samak, A. M. Shehata, A. A. Allam and M. E. Abd El-Hack, Molecules, 2020, 25, 1941 CrossRef CAS PubMed.
- M. M. Marcondes, F. Della Betta, S. K. T. Seraglio, M. Schulz, P. Nehring, L. V. Gonzaga, R. Fett and A. C. O. Costa, J. Food Compos. Anal., 2021, 100, 103927 CrossRef CAS.
- E. Capuano and V. Fogliano, LWT--Food Sci. Technol., 2011, 44, 793–810 CrossRef CAS.
- R. J. van Putten, J. C. van der Waal, E. de Jong, C. B. Rasrendra, H. J. Heeres and J. G. de Vries, Chem. Rev., 2013, 113, 1499–1597 CrossRef CAS PubMed.
- Y. B. Yi, J. W. Lee and C. H. Chung, Curr. Org. Chem., 2014, 18, 1149–1158 CrossRef CAS.
- I. K. M. Yu and D. C. W. Tsang, Bioresour. Technol., 2017, 238, 716–732 CrossRef CAS PubMed.
- M. Driffield, D. Chan, R. Macarthur, S. MacDonald, P. Brereton and R. Wood, J. AOAC Int., 2005, 88, 121–127 CrossRef CAS PubMed.
- R. Gürkan and N. Altunay, J. Food Compos. Anal., 2015, 42, 141–151 CrossRef.
- W. Chen, S. Wu, J. Zhang, F. Yu, X. Miao and X. Tu, Anal. Methods, 2019, 11, 4835–4841 RSC.
- M. Madani-Tonekaboni, M. Kamankesh and A. Mohammadi, J. Food Compos. Anal., 2015, 40, 1–7 CrossRef CAS.
- M. G. Freire, A. F. M. Cláudio, J. M. M. Araújo, J. A. P. Coutinho, I. M. Marrucho, J. N. C. Lopes and L. P. N. Rebelo, Chem. Soc. Rev., 2012, 41, 4966 RSC.
- I. Kiszkiel-Taudul, B. Starczewska and M. Jarosz, J. Food Compos. Anal., 2023, 115, 104944 CrossRef CAS.
- J. L. Anderson and D. W. Armstrong, Anal. Chem., 2003, 75, 4851–4858 CrossRef CAS PubMed.
- S. Pandey, Anal. Chim. Acta, 2006, 556, 38–45 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- L. Chen, Y. Xiong, H. Qin and Z. Qi, ChemSusChem, 2022, 15, e202102635 CrossRef CAS PubMed.
- A. Chinnappan, C. Baskar and H. Kim, RSC Adv., 2016, 6, 63991–64002 RSC.
- A. H. Jadhav, H. Kim and I. T. Hwang, Catal. Commun., 2012, 21, 96–103 CrossRef CAS.
- N. A. S. Ramli and N. A. S. Amin, BioEnergy Res., 2020, 13, 693–736 CrossRef CAS.
- P. V. Rathod, R. B. Mujmule, W.-J. Chung, A. R. Jadhav and H. Kim, Catal. Lett., 2019, 149, 672–687 CrossRef CAS.
- M. Petkovic, J. L. Ferguson, H. Q. N. Gunaratne, R. Ferreira, M. C. Leitão, K. R. Seddon, L. P. N. Rebeloa and C. S. Pereira, Green Chem., 2010, 12, 643–649 RSC.
- M. Vraneš, A. Tot, S. Papović, D. Četojević-Simin, S. Markov, A. Velićanski, M. Popsavin and S. Gadžurić, J. Mol. Liq., 2017, 247, 411–424 CrossRef.
- N. Muhammad, M. I. Hossain, Z. Man, M. El-Harbawi, M. A. Bustam, Y. A. Noaman and C. Y. Yin, J. Chem. Eng. Data, 2012, 57, 2191–2196 CrossRef CAS.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 711, 285–293 CrossRef CAS PubMed.
- A. A. Ariffin, H. M. Ghazali and P. Kavousi, Food Chem., 2014, 154, 102–107 CrossRef CAS PubMed.
- D. Tomasini, M. R. F. Sampaio, S. S. Caldas, J. G. Buffon, F. A. Duarte and E. G. Primel, Talanta, 2012, 99, 380–386 CrossRef CAS PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- A. Dimitrijević, A. Jocić, N. Zec, A. Tot, S. Papović, S. Gadžurić, M. Vraneš and T. Trtić-Petrović, J. Ind. Eng. Chem., 2019, 76, 500–507 CrossRef.
- T. Mourão, A. F. M. Cláudio, I. Boal-Palheiros, M. G. Freire and J. A. P. Coutinho, J. Chem. Thermodyn., 2012, 54, 398–405 CrossRef.
- ChemSpider-Search and Share Chemists, https://www.chemspider.com, 2022, accessed 13 September 2022.
- H. Passos, A. R. Ferreira, A. F. M. Cláudio, J. A. P. Coutinho and M. G. Freire, Biochem. Eng. J., 2012, 67, 68–76 CrossRef CAS.
- M. Vraneš, A. Tot, S. Papović, J. Panić and S. Gadžurić, J. Chem. Thermodyn., 2018, 124, 65–73 CrossRef.
- P. Berton, H. Tian and R. D. Rogers, Molecules, 2021, 26, 1702 CrossRef CAS PubMed.
- S. Shahriari, L. C. Tomé, J. M. M. Araújo, L. P. N. Rebelo, J. A. P. Coutinho, I. M. Marrucho and M. G. Freire, RSC Adv., 2013, 3, 1835–1843 RSC.
- L. Nie, H. Song, A. Yohannes, S. Liang and S. Yao, RSC Adv., 2018, 8, 25201–25209 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06077b |
| This journal is © The Royal Society of Chemistry 2023 |