Open Access Article
Open Access ArticleRecent achievements in the synthesis of benzimidazole derivatives
Nguyen Thi Chung
a,
Vo Cong Dung
b and
Dau Xuan Duc
 *a
*a
aDepartment of Chemistry, Institute of Education, Vinh University, 182 Le Duan Street, Nghe An 430000, Vietnam. E-mail: Xuanduc80@gmail.com
bCentre for Education Accreditation, Vinh University, 182 Le Duan Street, Nghe An 430000, Vietnam
First published on 7th November 2023
Abstract
Benzimidazoles are a class of heterocyclic compounds in which a benzene ring is fused to the 4 and 5 positions of an imidazole ring. Benzimidazole refers to the parent compound, while benzimidazoles are a class of heterocyclic compounds having similar ring structures, but different substituents. Benzimidazole derivatives possess a wide range of bioactivities including antimicrobial, anthelmintic, antiviral, anticancer, and antihypertensive activities. Many compounds possessing a benzimidazole skeleton have been employed as drugs in the market. The application of benzimidazoles in other fields has also been documented. The synthesis of benzimidazole derivatives has attracted much attention from chemists and numerous articles on the synthesis of this class of heterocyclic compound have been reported over the years. The condensation between 1,2-benzenediamine and aldehydes has received intensive interest, while many novel methods have been developed. In this article, we will give a comprehensive review of studies on the synthesis of benzimidazole, which date back to 2013. We have also tried to describe reaction mechanisms as much as we can. The work might be useful for chemists who work in the synthesis of heterocycles or drug chemistry.
1. Introduction
Benzimidazole, alternatively known as 1H-benzimidazole or 1,3-benzodiazole, is a bicyclic heterocyclic aromatic compound in which a benzene ring is fused to the 4 and 5 positions of an imidazole ring. Nitrogen atoms are at the 1 and 3 positions of the ring system. Benzimidazole derivatives play a crucial role in the field of medicinal chemistry because they possess a wide range of pharmacological activities such as antimicrobial, anticancer, antifungal, antileishmanial, antitubercular, antiviral and antimalarial, and some of them have been marketed as well-known drugs.1.1. Antimicrobial activity
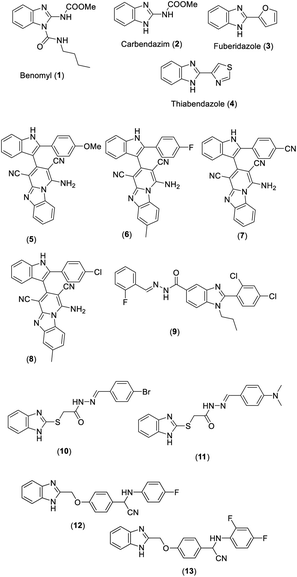
Benomyl (1), carbendazim (2), fuberidazole (3), and thiabendazole (4) are benzimidazole derivatives which have been used as fungicidal agents in the market (Fig. 1). Among indole-based pyrido[1,2-a]benzimidazoles synthesized by Kathrotiya and Patel, compounds 5, 6, and 7 displayed considerable antibacterial activity against S. typhi (MIC 50, 62.5 and 12.5 μg mL−1, respectively) compared to reference drugs ampicillin, chloramphenicol, and ciprofloxacin (MIC 100, 50 and 25 μg mL−1, respectively), while compounds 6 and 8 showed promising antifungal activity against C. albicans (MIC 250 μg mL−1) in comparison with standard griseofulvin (MIC 500 μg mL−1) (Fig. 1).1 Vasantha et al. demonstrated the synthesis of a series of N-arylidene-2-(2,4-dichlorophenyl)-1-propyl-1H-benzo[d]imidazole-5-carbohydrazide derivatives and the evaluation of these compounds for antimicrobial activity. Among them, compound 9 appeared to be a promising antibacterial and antifungal agent with an MIC value of 3.12 μg mL−1 against most bacterial and fungal strains (Fig. 1).2 A library of 2-substituted benzimidazole derivatives was synthesized and examined for antibacterial activity. Compound 10 was found to be the most potent antibacterial agent against both Gram-positive and Gram-negative bacteria compared to the reference cefadroxil, while compound 11 showed maximum activity against A. niger (MIC = 0.018 mM) (Fig. 1).3 Amongst a series of purine benzimidazole hybrids synthesized by Wang et al., compounds 12 and 13 were the most potent antibacterial agents with MIC values ranging between 3.9 and 7.8 μg mL−1 against different bacterial strains (Fig. 1).41.2. Anthelmintic activity
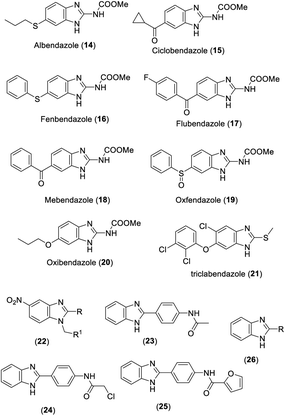
Many benzimidazoles have been developed as anthelmintic agents in the market such as albendazole (14), ciclobendazole (15), fenbendazole (16), flubendazole (17), mebendazole (18), oxfendazole (19), oxibendazole (20), triclabendazole (21), and thiabendazole (4) (Fig. 2). Faruk et al. synthesized a series of 5-nitrobenzimidazole derivatives (22) and tested them for anthelmintic activity against the adult Indian earth worm P. posthuma (Fig. 2). The results of preliminary biological tests showed that all compounds exhibited significant anthelmintic activity.5 Vilasrao et al. described the synthesis of a library of 2-substituted benzimidazoles and the evaluation of their anthelmintic activity against the adult earthworm E. fetida. Among them, compounds 23–25 exhibited excellent anthelmintic activities which are comparable to that of standard albendazole (Fig. 2).6 Sreena et al. synthesized benzimidazole derivatives (26) by the condensation reaction between o-phenylenediamine and acid and screened them for anthelmintic activity (Fig. 2). All the tested compounds exhibited significant anthelmintic activity compared with the standard piperazine citrate.71.3. Antiviral activity
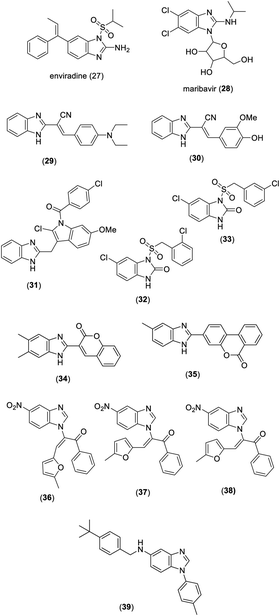
Enviradine (27) and maribavir (28) are benzimidazole-based compounds which have been used as antiviral agents (Fig. 3). Pan et al. introduced the synthesis of benzimidazole derivatives and the evaluation of these compounds for anti-HIV activity. Among them, compounds 29 and 30 showed significant activity with IC50 values of 3.45 and 58.03 nM, respectively (Fig. 3).8 Masoudi et al. prepared a series of benzimidazole derivatives and evaluated them for anti-HIV activity. Among the tested benzimidazoles, compound 31 was found to have significant activity with EC50 of 1.15 μg mL−1 against HIV-1 and HIV-2 (Fig. 3).9 Ferro et al. synthesized two series of benzimidazol-2-one derivatives and screened them for antiviral activity against HIV-1. Compounds 32 and 33 (IC50 values of 1.3 and 0.79 μM, respectively) were more potent than the standard drug nevirapine (IC50 value of 1.55 μM) (Fig. 3).10 Tsay et al. synthesized a diverse range of benzimidazole–coumarin hybrids and evaluated them for antiviral activity against the hepatitis C virus. Compounds 34 and 35 displayed potent activity with EC50 values of 3.0 and 5.5 nM, respectively (Fig. 3).11 Among benzimidazoles synthesized by Shaker et al., compounds 36–38 showed great potential to be used as potent antiviral agents due to their inhibitory effect against the rotavirus Wa strain (Fig. 3).12 Benzimidazole 39, obtained by Dai et al., was found to be a potent antiviral agent against Lassa virus envelope glycoprotein (LASV GP) pseudotypes with an EC50 value of 1.1 nM (Fig. 3).131.4. Anticancer activity
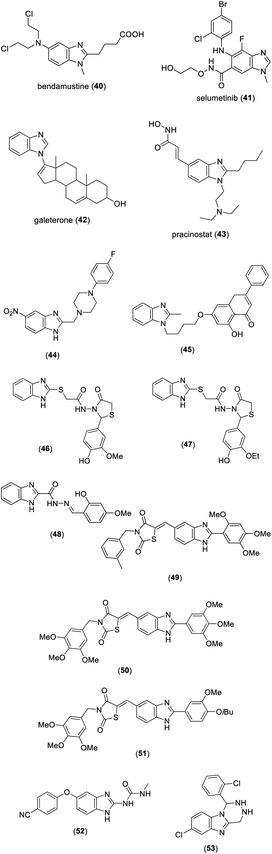
Several benzimidazole derivatives have been marketed as antitumor agents such as bendamustine (40), selumetinib (41), galeterone (42), and pracinostat (43) (Fig. 4). In a study reported by Gohary et al., compound 44 displayed significant anticancer activity with IC50 values of 0.022, 0.014, and 0.015 μM against liver cancer (HepG2), colon cancer (HCT-116), and breast cancer (MCF-7) cells,14 respectively (Fig. 4). Wang et al. demonstrated the synthesis and screening for anticancer activity of the chrysin benzimidazole derivatives. Among the synthesized benzimidazoles, compound 45 exhibited excellent activity with IC50 values of 25.72 ± 3.95 μM against MCF cells (Fig. 4).15 A series of 2-((1H-benzo[d]imidazole-2-ylthio)acetamido)-N-(substituted-4-oxothiazolidin-3-yl)acetamides was synthesized and evaluated for anticancer activity by Yadav et al. Compound 46 and 47 showed remarkable activity with IC50 values of 0.00005 and 0.00012 μM mL−1 against the HCT116 cell line, respectively (Fig. 4).16 Among benzimidazole hydrazones prepared by Onnis et al., compound 48 possessed significant anticancer activity with an IC50 value of 0.98 ± 0.02 μM against human T-lymphoblastic leukemia (CEM) cells (Fig. 4).17Sharma et al. synthesized a series of benzimidazole bearing thiazolidinedione derivatives. The bioassay study indicated that compounds 49–51 possess remarkable cytotoxicity against PC-3, HeLa, A549, and HT1080 cancer cell lines with IC50 values in the range of 0.096–0.63 μM (Fig. 4).18 In a study reported by Wang et al., compound 52 exhibited significant activity with IC50 values in the range of 0.006–1.774 μM against the K562, A431, HepG2, HeLa, and MDA-MB-435S cancer cell lines (Fig. 4).19 Among the 1,2,3,4-tetrahydro[1,2,4]triazino[4,5-a]benzimidazoles synthesized by Nassan et al., compound 53 displayed excellent activity with an IC50 value of 0.0390 μM against the human breast adenocarcinoma cell line (Fig. 4).20
1.5. Antihypertensive
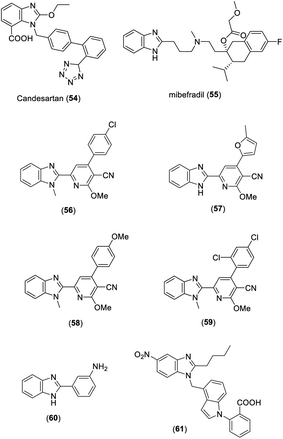
Candesartan (54) and mibefradil (55) are antihypertensive drugs that contain benzimidazole rings (Fig. 5). Abou-Seri et al. synthesized a series of 2-alkoxy-4-aryl-6-(1H-benzimidazol-2-yl)-3-pyridinecarbonitrile derivatives. The bioactivity experiment revealed that all compounds showed significant vasodilation properties. In particular, compounds 56–59 showed most prominent activity with IC50 values of 0.145, 0.202, 0.210, and 0.214 mM, respectively, compared to standard prazosin hydrochloride (IC50 of 0.487 mM) (Fig. 5).21 Khan et al. prepared a set of 2-phenyl substituted benzimidazoles and examined the antihypertensive activity of these derivatives by using the tail cuff method. Compound 60 exhibited excellent antihypertensive properties in spontaneously hypertensive rats compared to standard losartan (Fig. 5).22 Among the 5-nitro benzimidazole derivatives synthesized by Zhu et al., compound 61 was found to be the most active agent against AT1 with an IC50 value of 1.03 ± 0.26 nM (Fig. 5).231.6. Miscellaneous activities
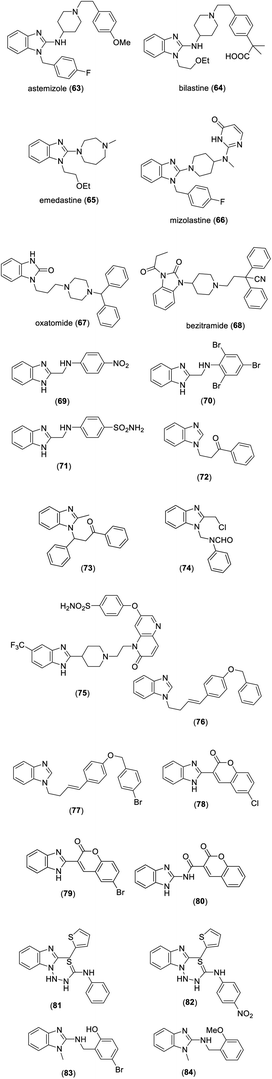
Several benzimidazole derivatives have been used as antihistamines such as astemizole (63), bilastine (64), emedastine (65), mizolastine (66), and oxatomide (67) (Fig. 6). The drug bezitramide 68 has long been used as an analgesic agent (Fig. 6). Mariappan et al. synthesized a series of 2-substituted benzimidazole derivatives and bioactive assay revealed that compounds 69–71 showed significant analgesic and anti-inflammatory activities (Fig. 6).24 In another report, compounds 72 and 73 prepared by Kumar et al. exhibited significant analgesic and anti-inflammatory properties.25 Among N-benzimidazol-1-yl methyl-benzamide derivatives synthesized by Sethi et al., compound 74 displayed significant analgesic and anti-inflammatory activity (Fig. 6).26Hameed et al. reported that compound 75 possesses significant antitubercular activity with an MIC value of 0.19 μM against fluoroquinolone-resistant strains of M. tuberculosis (Fig. 6).27 In another study, compounds 76 and 77 were found to exhibit significant antitubercular activity at an MIC value of 1.56 μg mL−1 against M. tuberculosis strains of H37Rv (Fig. 6).28 Among the benzimidazoles developed by Arora et al., compounds 78–80 showed remarkable antioxidant activity (IC50 values of 19.7, 13.9 and 1.2 μmol L−1, respectively) compared to standard butylated hydroxytoluene (BHT, IC50 of 23.4 μmol L−1) (Fig. 6).29 In a report described by Mentese et al., compounds 81 and 82 demonstrated significant antioxidant activity (Fig. 6).30 In research accomplished by Nieto-Meneses et al., a library of N-benzyl-1H-benzimidazol-2-amine derivatives was synthesized and evaluated for antileishmanial activity. Among them, compounds 83 and 84 exhibited significant antileishmanial activity against the amastigotes of L. mexicana and L. braziliensis, with IC50 values of 2.62 and 3.21 μM (Fig. 6), respectively, and their activities were 5.8 and 4.8 times better than standard miltefosine (IC50 of 15.34 μM).31 The bioactivities of benzimidazole derivatives were also demonstrated in some review articles.32–34
In addition, benzimidazoles are very important intermediates in dyes and polymer synthesis.35–37 They have also widely been used in material science such as in chemosensing,38–40 crystal engineering,41,42 fluorescence applications,43–47 and corrosion science.48,49 Furthermore, they have been employed as important intermediates in organic reactions,50–52 and as ligands for asymmetric catalysis.53–57
Owing to the vast importance of benzimidazoles in different areas, enormous efforts have been made to develop operationally simple synthetic methods for the construction of benzimidazole derivatives. The condensation reaction between 1,2-benzenediamines and aldehydes is one of the most efficient and straightforward methods for the synthesis of benzimidazoles and a great deal of studies based on this method have appeared in the literature. Furthermore, numerous novel approaches for the synthesis of benzimidazole-based compounds have been developed. In the literature, several benzimidazole synthesis review articles have been published. However, most of them are quite outdated58,59 or do not demonstrate all aspects of benzimidazole synthesis.60,61 In addition, in these review articles, reaction mechanisms were usually not described in detail. In this article, we give a brief description of the bioactivities of benzimidazole derivatives and a comprehensive overview of benzimidazole synthesis, which covers all aspects of thiazole synthesis studies dating back to 2012. We also try to describe reaction mechanisms as much as possible. The article might be useful for chemists who work in pharmaceuticals, organic synthesis, and the synthesis of heterocycles. We have previously produced reviews on the synthesis of furans, pyrroles, thiophenes, benzofurans, benzothiophenes, oxazoles, isoxazoles, and thiazoles,62–69 and this article is a continuation of our investigation into the synthesis of aromatic five-membered ring heterocycles.
2. Benzimidazole synthesis by condensation of 1,2-benzenediamines with aldehydes
2.1. Using nanomaterial catalysts
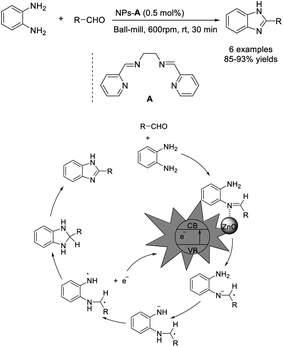
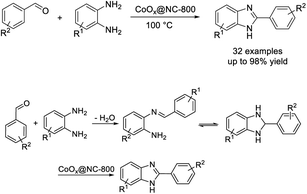
Sharma et al. achieved the synthesis of benzimidazole from 1,2-benzenediamine and various aldehydes using a ZnO-NP catalyst via a ball-milling technique. The catalyst was synthesized via a sol–gel method with in situ decoration of organic ligand A on the surface of the ZnO. The synthesis featured several advantages such as short reaction times, simple product purification, high efficiency, solvent-free conditions, recyclability of the ZnO-NP catalyst, and scalability.70 A mechanistic study suggested that the role of the catalyst is to activate the imine intermediate to accept an electron (Scheme 1).Wang et al. demonstrated a one-pot synthesis of a library of benzimidazoles via a coupling reaction between phenylenediamines and aldehydes using a cobalt nanocomposite catalyst. Broad substrate scope, high yields of products, good functional group, and scalability are the attractive features of the synthesis. Furthermore, the catalyst could be recycled by a simple procedure and reused for five runs without any significant decrease in reaction yields.71 The catalyst CoOx@NC-800 catalyzes the dehydrogenation of dihydrobenzimidazole to form the desired benzimidazole with liberation of molecular hydrogen (Scheme 2).
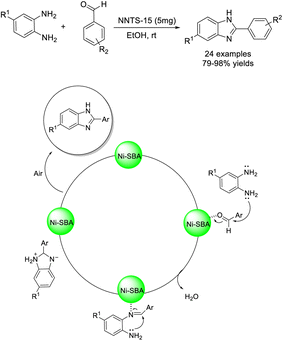
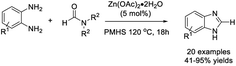
Kalhor et al. prepared a nickel-decorated SBA-15 nanocomposite (Ni/TCH@SBA-15) and employed this material as a catalyst for the construction of 2-aryl-substituted benzimidazoles. The merits of the synthesis include mild reaction conditions, easy work-up procedure, good functional group tolerance, high yields of products, and reusability of the catalyst.72 The Ni/TCH@SBA-15 was supposed to activate the aldehyde as well as the intermediate (Scheme 3).
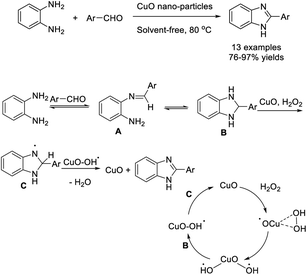
Fazlinia and Sheikh synthesized CuO nanoparticles and used this material for the assembly of 2-arylbenzimidazoles by the condensation reaction between 1,2-benzenediamine and aldehydes. Various 2-arylbenzimidazoles were obtained in high yields under solvent-free conditions in short times.73 The catalyst could be reused for eight runs without any significant loss in its catalytic activity (Scheme 4).
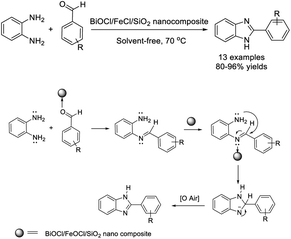
Mohammadi et al. presented the synthesis of BiOCl/FeOCl nano rods composited with spherical nano particles of SiO2 and the use of this nano catalyst for the preparation of 2-arylbenzimidazoles. The procedure showed many advantages such as short reaction times, high efficiency, mild reaction conditions, simple operation and work-up, and recyclability of the catalyst.74 The BiOCl/FeOCl/SiO2 nano material might activate the aldehyde group via the formation of a coordinate bond with the Lewis acid site of BiOCl/FeOCl over the surface of SiO2 as a mediator agent as well as the imine intermediate for ring closing reaction (Scheme 5).
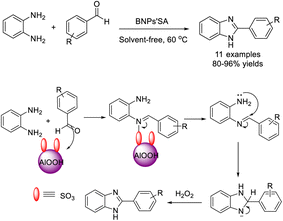
Karami et al. described the synthesis of AlOOH–SO3 nanoparticles (BNPs'SA) and applied this material as a recyclable catalyst for the construction of 2-aryl-1H-benzimidazoles. The condensation between aldehydes and o-phenylenediamines was performed under solvent-free conditions (Scheme 6). Other advantageous features of the synthesis include short reaction time, simple workup, and high yields of products.75
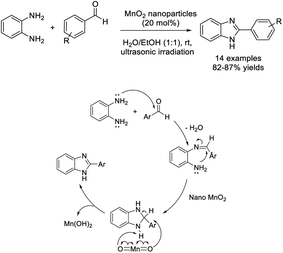
Naeimi and Babaei reported the preparation and application of MnO2 nanoparticles as an efficient oxidant agent for the synthesis of benzimidazoles.76 Various benzimidazoles were efficiently obtained in short times under ultrasound irradiation (Scheme 7).
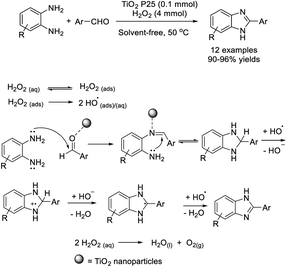
In a study introduced by Bahrami et al., the H2O2/TiO2 P25 nanoparticle system was employed as a catalyst for the assembly of a series of 2-substituted benzimidazoles. Various products were achieved in excellent yields under solvent-free conditions from 1,2-phenylenediamines and aromatic aldehydes.77 The proposed reaction mechanism is outlined below and the role of the catalyst is to activate the aldehyde as well as the imine intermediate (Scheme 8).
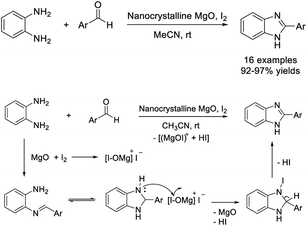
Naeimi and Alishahi established an efficient protocol for the preparation of 2-substituted benzimidazole using nanocrystalline magnesium oxide as a solid base catalyst. The protocol featured some advantages such as short reaction times, mild reaction conditions, scalability, and high yields of products.78 Moreover, the catalyst could be recovered and reused for five additional cycles without any considerable decrease in reaction yield (Scheme 9).
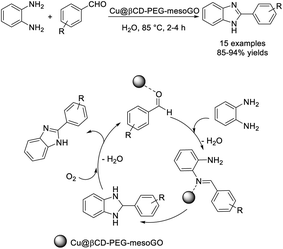
Bahadorikhalili et al. investigated the synthesis of benzimidazoles from o-phenylenediamines and benzaldehydes using a β-cyclodextrin functionalized PEGylated mesoporous silica nanoparticle-graphene oxide hybrid (Cu@βCD-PEG-mesoGO) as a catalyst. The catalyst was prepared from a mesoporous silica nanoparticle-graphene oxide hybrid by functionalization with PEG-600 ended β-cyclodextrin followed by immobilization with Cu.79 Attractive features of the benzimidazole synthesis include high yield of products, simple work-up procedure, mild reaction conditions, and recyclability of catalyst (Scheme 10). The tentative reaction mechanism suggested that the role of the catalyst is to activate the aldehyde and the imine intermediate.
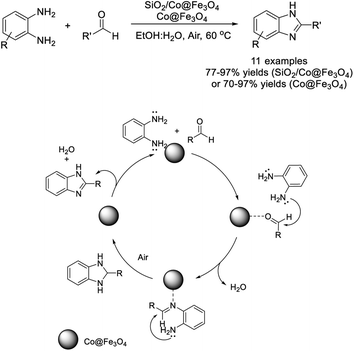
Kumara et al. introduced the employment of bare Co@Fe2O4 and SiO2/Co@Fe2O4 nanoparticles as catalysts to access 2-arylbenzimidazoles. A sequence of imine formation, cyclization, condensation, and aromatization occurs in one pot.80 The Co@Fe2O4 and SiO2/Co@Fe2O4 nanoparticles were easily recovered by using an external magnet, purified, and then used for the next several cycles without affecting the reaction yield (Scheme 11).
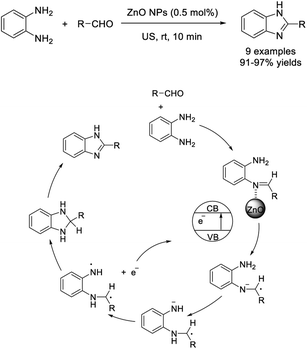
Paul et al. completed the synthesis of 2-benzimidazole derivatives using zinc oxide nanoparticles (ZnO NPs) under ultrasound (US) conditions. The catalyst was prepared using seed extract from the tender pods of Parkia roxburghii, a traditional vegetable grown abundantly in different areas of north-east India.81 Advantages of the benzimidazole synthesis include mild reaction conditions, short reaction times, recyclability of the catalyst, and excellent yields of products (Scheme 12).
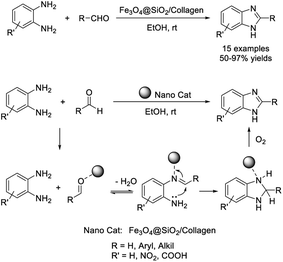
Ghafuri et al. introduced the use of Fe3O4@SiO2/collagen nanomaterial as a catalyst towards benzimidazole synthesis.82 Major advantages of the synthesis include good yields of products, short reaction times, mild reaction conditions, and environmentally benign reaction conditions (Scheme 13). The role of the catalyst is to activate aldehydes as well as the imine intermediate.
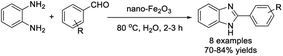
Kommula et al. developed an efficient protocol for the synthesis of benzimidazole from 1,2-diamino benzenes and substituted aromatic aldehydes using a nano-Fe2O3 catalyst (10 mol%).83 Short reaction times, high efficiency, aqueous reaction medium, and recyclability of the catalyst are the main advantages of the synthesis (Scheme 14).
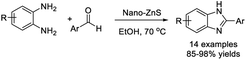
Hakimi et al. examined an efficient strategy for the construction of benzimidazole derivatives from substituted aldehydes and o-phenylenediamines using zinc sulfide nanoparticles as a catalyst (nano-ZnS).84 The strategy offered several advantageous features such as high efficiency, mild reaction conditions, short reaction times, and recovery and reuse of the catalyst (Scheme 15).
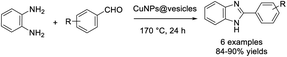
Shukla et al. prepared Cu nanoparticle loaded, surfactant free metallovesicles (CuNPs@vesicles) and used this material for the assembly of benzimidazoles via a cascade reaction.85 Products were obtained in excellent yields and the nanocatalyst could be recycled for up to five runs without affecting the reaction yields (Scheme 16).
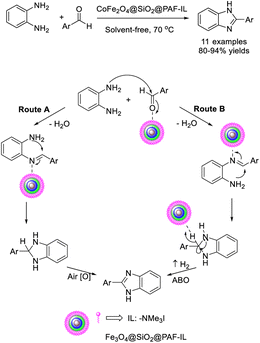
In a study reported by Bodaghifard and Shafi, a novel ionic liquid immobilized on silica-coated cobalt-ferrite magnetic nanoparticles (CoFe2O4@SiO2@PAF-IL) was synthesized, characterized, and employed as a catalyst for the synthesis of benzimidazole derivatives. The benzimidazole synthesis delivered many attractive features such as short reaction times, solvent-free and environmentally-benign conditions, high efficiency, and easy recyclability of the catalyst.86 The role of the catalyst in the proposed reaction mechanism was also described (Scheme 17).
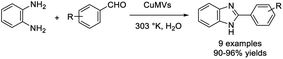
Kaur et al. demonstrated the synthesis of copper metallovesicles (CuMVs) and applied this soft nanomaterial as a catalyst for the condensation reaction between o-phenylenediamines and aromatic aldehydes in aqueous media to access 2-aryl-benzimidazoles.87 Excellent yields of products, mild reaction conditions, a harmless aqueous reaction medium, and recyclability of the catalyst make the synthesis a green and sustainable catalytic approach (Scheme 18).
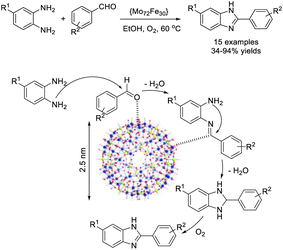
Garazhian et al. established a protocol for the synthesis of benzimidazoles using amorphous {Mo72Fe30} nanocapsules as a safe Keplerate polyoxometalate catalyst. The condensation reaction between aromatic 1,2-diamines and aldehydes was performed under aerobic and mild conditions resulting in products in high yields.88 In this procedure, the nanocatalyst presumably activates the aldehyde substrates and imine intermediates (Scheme 19).
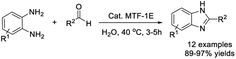
In a study presented by Roy et al., mesoporous TiO2–Fe2O3 mixed oxide material (MTF-1E) with nanoscale porosity and a high BET surface area was utilized as a catalyst for the synthesis of benzimidazole derivatives.89 The merits offered by this method include harmless solvent, mild reaction conditions, high regioselectivity, broad substrate scope, recyclability of the catalyst, and excellent yield of products (Scheme 20).
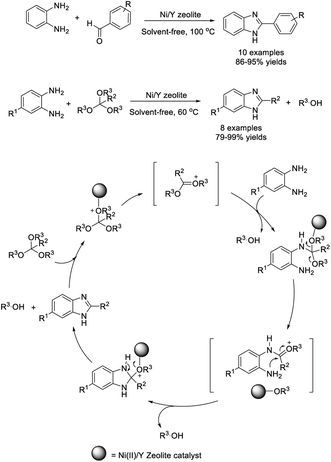
An efficient route for the construction of benzimidazole derivatives using a nano-Ni(II)/Y zeolite catalyst was investigated by Mobinikhaledi et al. The condensation reaction of o-phenylenediamines with aromatic aldehydes or orthoesters was performed under solvent-free conditions providing the desired products in good to excellent yields.90 The reaction mechanism was presented for the reaction between phenylenediamines and orthoesters (Scheme 21).
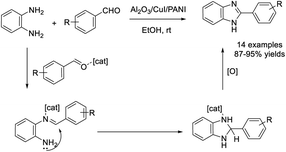
Kohli et al. described the synthesis of benzimidazole by the reaction between o-phenylenediamine and aldehydes using an Al2O3/CuI/PANI nanocomposite as a catalyst. A series of products were produced in excellent yields under mild conditions.91 Furthermore, the nanocatalyst was easily recovered and reused for five cycles without a significant loss of its catalytic activity (Scheme 22).
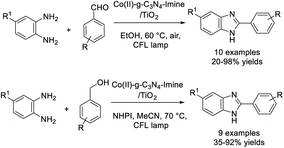
Pourmorteza et al. employed cobalt incorporated in the g-C3N4-imine/TiO2 nanohybrid as a heterojunction photocatalyst to prepare benzimidazoles from benzaldehydes and aryl diamines.92 The protocol was also successfully applied to access benzimidazoles from benzylic alcohols and aryl diamines via a one-pot reaction sequence of aerobic photooxidation of benzylic alcohols and oxidative coupling of benzaldehydes with aryl diamines (Scheme 23).
2.2. Using acid catalysts
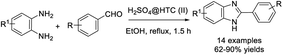
Shitole et al. accomplished the synthesis of 2-aryl-1-arylmethyl-1H-benzimidazoles from phenylenediamines and aromatic aldehydes using chlorosulfonic acid as a catalyst.93 Various products were afforded in good to excellent yields under mild conditions (Scheme 24).Mathapati et al. demonstrated an efficient strategy to access benzimidazoles through the cyclization of 1,2-phenylenediamine with aldehydes.94 Products were produced in high yields under mild reaction conditions (Scheme 25). In another report, H2SO4@HTC(II) was used as a catalyst for the synthesis of benzimidazole derivatives from o-phenylenediamines and aromatic aldehydes.95 Moderate to excellent yields of products were also obtained (Scheme 26).
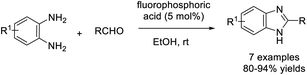
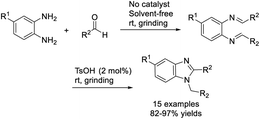
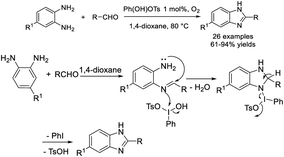
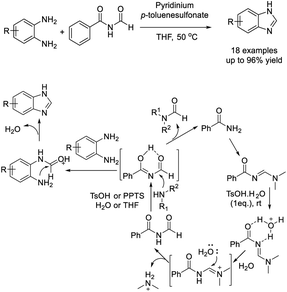
Singh et al. developed an efficient protocol for the synthesis of 1,2-disubstituted benzimidazole derivatives from o-phenylenediamines and aldehydes. The synthesis was catalyzed by p-toluenesulfonic acid and performed under grinding and solvent-free conditions.96 Short reaction time, high efficiency, simple product isolation and purification, and mild reaction conditions are the attractive features of the protocol (Scheme 27).
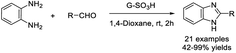
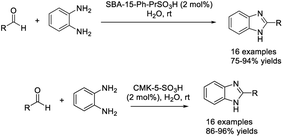
An efficient procedure for the synthesis of C-2-substituted benzimidazoles employing Brønsted acidic reduced graphene was described by Karthik and Suresh. The catalyst was prepared by a massive influx of sulphonic acid groups on the reduced graphene oxide surface.97 A library of benzimidazoles was provided in good yields under mild reaction conditions. In addition, the catalyst could be recycled for five runs without a significant decrease in reaction yields (Scheme 28). In another study introduced by Zareyee et al., organosulfonic acid-functionalized silica (SBA-15-Ph-PrSO3H) and sulfonic acid-based nanoporous carbon (CMK-5-SO3H) were utilized as catalysts to prepare 2-substituted benzimidazoles from aldehydes and benzene-1,2-diamine. High yields of products were observed for both catalysts under mild reaction conditions and in an aqueous medium.98 The catalysts CMK-5-SO3H and SBA-15-Ph-PrSO3H were readily recovered and reused in eight and six consecutive runs, respectively, without any considerable loss of activity (Scheme 29).
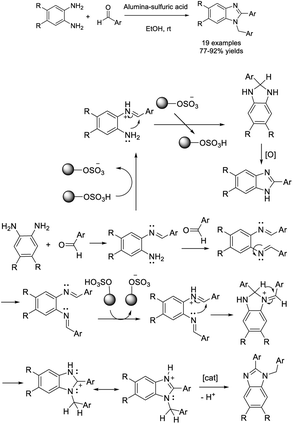
Pramanik et al. discovered an environmentally friendly strategy for the assembly of 2-aryl-1-arylmethyl-1H-benzimidazoles using alumina-sulfuric acid as a solid acid catalyst. A wide range of products were achieved in good to excellent yields under mild reaction conditions. Furthermore, the heterogeneous solid acid catalyst could be efficiently recycled seven times.99 A plausible reaction mechanism was also provided based on isotope labelling experiments, in which the catalytic behavior of alumina-sulfuric acid was explained (Scheme 30).
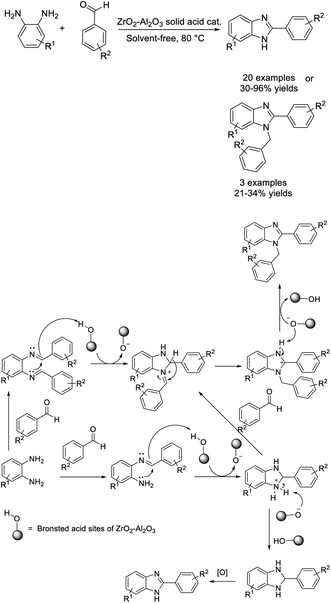
In a study reported by Thimmaraju et al., a ZrO2–Al2O3 solid acid synthesized by a solution combustion method was employed as a catalyst for the synthesis of substituted benzimidazoles. In general, benzimidazoles were isolated in good yields under thermal conditions. The catalyst could be recycled and reused for up to five consecutive reaction cycles in the synthesis of benzimidazoles.100 A proposed reaction mechanism was also described (Scheme 31).
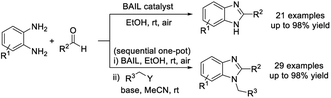
Senapak et al. established an efficient protocol for the synthesis of 2-substituted benzimidazoles by the condensation reaction of o-phenylenediamines with aldehydes using Brønsted acidic ionic liquid [DodecIm][HSO4] as a reusable catalyst. A library of benzimidazoles were afforded in high yield under mild reaction conditions. Noticeably, the protocol was also successfully employed for the construction of 1,2-disubstituted benzimidazoles via sequential one-pot reactions, when alkyl halides were added.101 Moreover, the catalyst could be recycled at least four times without significant loss in activity (Scheme 32).
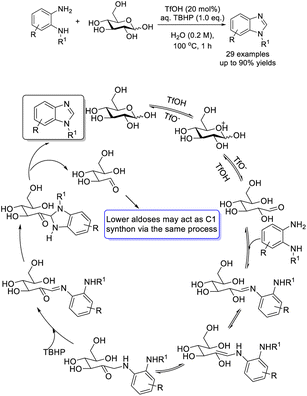
Raja et al. utilized glucose as a methine source for the synthesis of benzimidazoles from o-phenylenediamines via an oxidative cyclization reaction. A wide range of N-substituted benzimidazoles was provided in good yields using TsOH as a catalyst.102 A plausible reaction pathway was also suggested (Scheme 33).
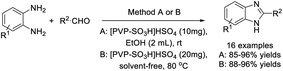
Roudsari et al. designed an efficient approach for the synthesis of 2-substituted benzimidazoles using polymeric-based solid acid [PVP-SO3H]HSO4 as a catalyst. The synthesis could be performed in EtOH at room temperature or in solvent-free conditions at 80 °C.103 Products were obtained in excellent yields in both cases. The solid acid catalyst could be recovered and reused for several runs without significant decrease in reaction yields (Scheme 34).
2.3. Using metal catalysts
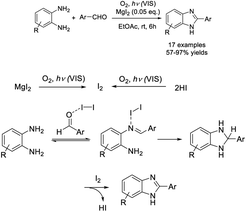
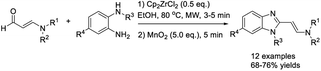
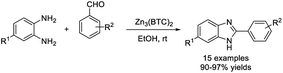
Ghosh and Subba performed the condensation reaction of o-phenylenediamines with aldehydes to prepare 2-substituted benzimidazoles using MgCl2·6H2O as a catalyst.104 A series of products were obtained in high yields in short times (Scheme 35). In another report, Nagasawa et al. employed a small amount of MgI2 as a catalyst to access benzimidazoles from aromatic aldehydes and diamines. The photooxidative synthesis used molecular oxygen as the terminal oxidant and showed broad substrate scope.105 A diverse series of products was obtained in high yields (Scheme 36).Sun et al. demonstrated a method for the preparation of 2-aminovinyl benzimidazoles using bis(cyclopentadienyl)-zirconium(IV) dichloride (Cp2ZrCl2) as a catalyst.106 The condensation reaction between 1,2-phenylenediamines and N-arylated/N,N-dialkylated 3-aminoacroleins under microwave irradiation gave the desired products in short times (Scheme 37). Sajjadifar et al. prepared and applied a Zn3(BTC)2 metal–organic framework as a catalyst for the construction of 2-aryl-1H-benzimidazole from aldehydes and o-phenylenediamines.107 A wide range of products was produced in excellent yields under mild reaction conditions and the catalyst could be recycled for four additional runs without a significant loss of its catalytic activity (Scheme 38). Nale et al. employed N-substituted formamides as C1 sources for the synthesis of a series of NH-benzimidazoles via the condensation reaction with 1,2-phenylenediamines.108 A diverse range of products were achieved in moderate to excellent yields under solvent-free conditions (Scheme 39).
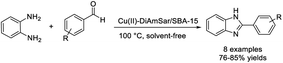
Bardajee et al. demonstrated the use of periodic mesoporous silica (SBA-15) containing a Cu(II) organometallic complex as a catalyst for the synthesis of benzimidazoles. The catalyst was obtained by coordination of Cu(II) ions with the diaminosarcophagine ligand and then grafting onto the surface of SBA-15.109 Eight benzimidazoles were formed in good yields under solvent-free conditions (Scheme 40).
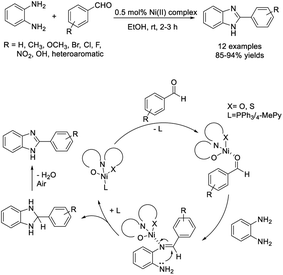
Agrahari et al. synthesized new pincer type Ni(II)–Schiff base complexes including [NiL1(PPh3)], [NiL2(PPh3)], [NiL3(PPh3)], [NiL4(PPh3)], and [NiL4(4-MePy)] and utilized these compounds as catalysts for the condensation reaction between aldehydes and o-phenylenediamine. The reactions proceeded smoothly at room temperature with low catalyst loading affording products in good to excellent yields.110 A proposed reaction mechanism was also presented (Scheme 41).
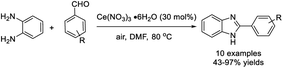
Martins et al. developed a strategy for the assembly of 2-substituted benzimidazoles starting from 1,2-diaminobenzene and aldehydes in good yields.111 The synthesis used air as an efficient oxidant and Ce(NO3)3·6H2O as a promoter (Scheme 42).
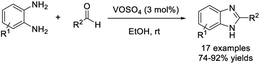
Digwal et al. described an efficient method for the construction of benzimidazoles using VOSO4 as a catalyst.112 The method featured many merits such as high yields of products, mild reaction conditions, broad substrate scope, scalability, and recyclability of catalyst (Scheme 43).
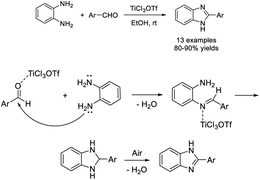
Azizian et al. established an efficient approach for the synthesis of benzimidazoles by the condensation reaction of o-phenylenediamine with aldehydes using TiCl3OTf as a catalyst.113 Thirteen products were afforded in good to excellent yields under mild reaction conditions. The proposed reaction mechanism is also included (Scheme 44).
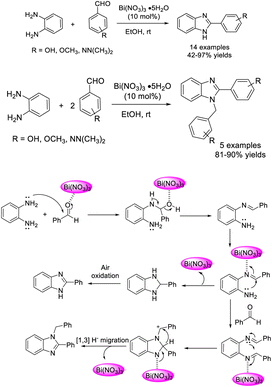
Mahire et al. accomplished the synthesis of 2-substituted benzimidazole and 1,2-disubstituted benzimidazole derivatives using bismuth nitrate as a catalyst.114 A wide range of products were produced in good yields at room temperature. A tentative reaction mechanism describing the role of the bismuth nitrate catalyst was also suggested (Scheme 45).
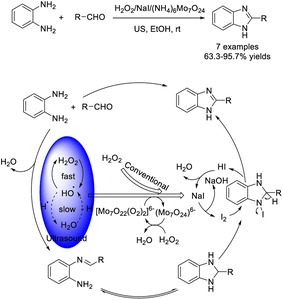
Bai et al. investigated an efficient strategy to access 2-substituted benzimidazoles by the reaction of aldehydes and o-phenylenediamine using sodium iodide and ammonium molybdate as co-catalysts and hydrogen peroxide as an oxidant.115 The products were obtained in high yields under ultrasound irradiation. A plausible reaction mechanism was also outlined (Scheme 46).
A simple, convenient, and highly efficient procedure for the assembly of 2-arylbenzimidazoles using a chromium(III)–salen complex as a catalyst and air as a green oxidant was completed by Sharghi et al. Mild reaction conditions, short reaction times, scalability, simple isolation of products, and excellent yields are the main attractive features of the synthesis.116 Moreover, the catalyst could be simply recovered and used for at least eight consecutive runs without considerable loss of its catalytic activity (Scheme 47).
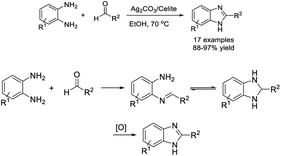
Soleimani and Khodaei designed a facile protocol for the construction of 2-substituted benzimidazoles by reactions of 1,2-phenylenediamines with aryl aldehydes using Ag2CO3/celite as a solid catalyst.117 A library of products was delivered in excellent yields in short times in ethanol (Scheme 48).
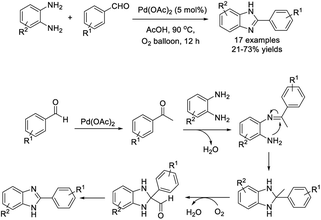
A palladium catalyzed, one-pot approach for the synthesis of benzimidazoles using molecular oxygen as a sole oxidant and Pd(OAc)2 as a catalyst was disclosed by Shaikh et al. A variety of products was isolated in moderate yields.118 A reaction pathway was also proposed (Scheme 49).
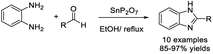
Merroun et al. discovered a green methodology to prepare benzimidazoles through a condensation reaction of 1,2-diamino benzene and aromatic aldehydes using SnP2O7 as a catalyst.119 The catalyst was obtained by adding MAP to a solution of SnCl2. Ten products were produced with excellent yields in short reaction times, and the catalyst was reused five times without a significant decrease in the reaction yield (Scheme 50).
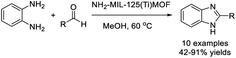
Sankar et al. designed a facile procedure for the synthesis of various benzimidazoles using NH2-MIL-125(Ti) MOF as a catalyst.120 The products were delivered in good yields and the catalyst could be recycled for five consecutive cycles without considerable loss of activity (Scheme 51).
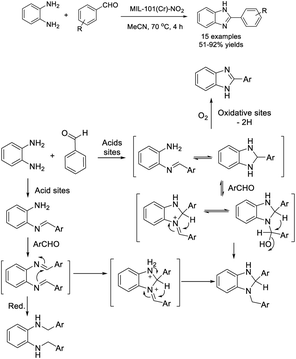
Vallés-García et al. described the preparation of a nitro functionalized chromium terephthalate [MIL-101(Cr)-NO2] metal organic framework and the application of this material for the construction of benzimidazoles.121 Fifteen compounds were produced in high yields with good catalyst recyclability (Scheme 52).
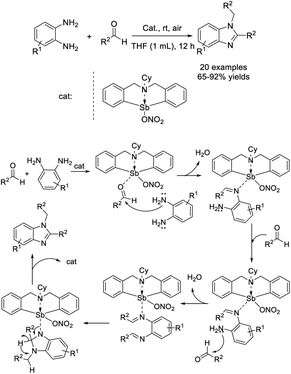
Zhou et al. synthesized a novel organoantimony complex of 6-cyclohexyl-6,7-dihydrodibenzo[c,f][1,5]azastibocin-12(5H)-yl nitrate and employed this material as a catalyst for the preparation of benzimidazole derivatives starting from aldehydes and arylenediamines.122 A wide range of products were furnished in good to excellent yields. Furthermore, the complex could be easily recycled five times in scale enlarged synthesis without any significant loss of catalytic activity (Scheme 53).
2.4. Without using a catalyst
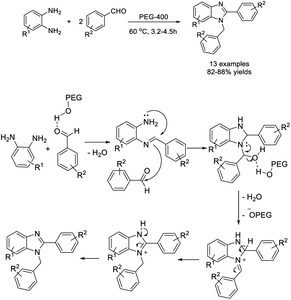
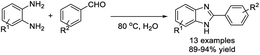
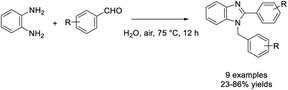
Mekala et al. used polyethylene glycol (PEG-400) as an effective medium for the one-pot synthesis of 1,2-disubstituted benzimidazoles from 1,2-phenylenediamines and aryl aldehydes.123 A set of products was synthesized in high yields and PEG-400 could be reused three times without loss of activity. A suggested reaction mechanism was also presented (Scheme 54).Bala et al. obtained 2-substituted benzimidazoles from 1,2-phenylenediamines and aryl aldehydes by heating this mixture in water at 80 °C.124 Various products were prepared in excellent yields without using a catalyst (Scheme 55). In another report, Mahato et al. demonstrated a metal-free approach for the synthesis of 1,2-disubstituted benzimidazoles. The synthesis was performed in water in the presence of air.125 Products were provided in high yields and the method could be applied on the gram scale (Scheme 56).
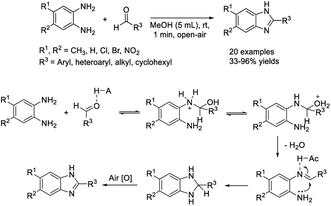
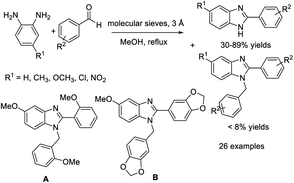
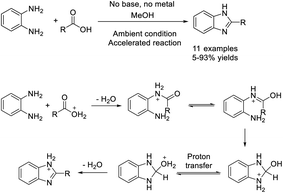
In research described by Elumalai et al., MeOH was chosen as the medium for the assembly of 2-substituted benzimidazoles by the condensation of 1,2-diaminoarenes and aldehydes in the presence of air.126 A library of products was achieved in good yields in a short time under mild, catalyst and additive-free reaction conditions. The synthesis could also be expanded to the multi-gram scale (Scheme 57). Chaturvedia et al. introduced the construction of 2-arylbenzimidazoles with antimycobacterial activity in a molecular sieve-MeOH system from o-phenylenediamines and aromatic aldehydes. In general,127 products were afforded in good yields in a relatively short time (Scheme 58). All synthesized compounds were screened for antitubercular activity against M. tuberculosis using the BACTEC radiometric method. Compounds A and B showed potential antitubercular activity against M. tuberculosis H37RV with MIC values of 16 μM and 24 μM, respectively (Scheme 58).
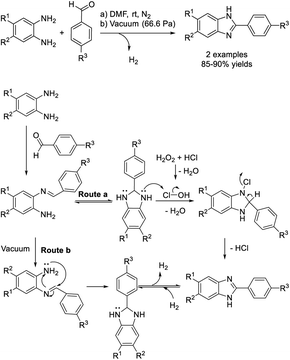
Sutapin et al. carried out the condensation of phenylenediamines with aromatic aldehydes to give benzimidazoles under harsh conditions without using any catalyst.128 Two products were formed in excellent yields under reduced pressure at 66.6 Pa and at room temperature (Scheme 59).
2.5. Using natural sources as catalysts
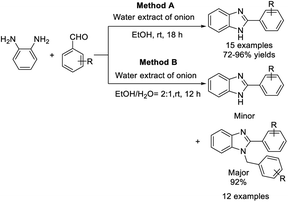
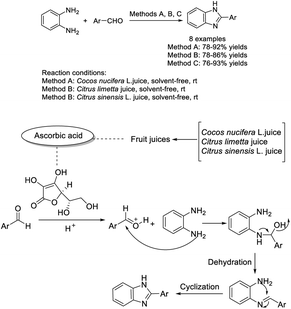
Prabakaran et al. developed an efficient methodology for the synthesis of 2-substituted benzimidazole derivatives using water extract of onion as a catalyst. The methodology provided 2-substituted benzimidazoles from aromatic aldehydes possessing electron-withdrawing groups, whereas aromatic aldehydes having electron donating groups selectively produced 1,2-disubstituted benzimidazoles.129 The products were obtained in high yields under mild reaction conditions (Scheme 60).Gulati et al. introduced a facile protocol for the synthesis of benzimidazoles mediated by fruit juices including Cocos nucifera L. juice, Citrus limetta juice and Citrus sinensis L. juice, by the condensation of substituted aldehydes and o-phenylenediamine. Eight compounds were furnished under solvent-free conditions at room temperature.130 All synthesized compounds were evaluated for herbicidal activity against Raphanus sativus L. (radish) seeds, antifungal activity against R. Solani and C. gloeosporioides, and antibacterial activity against Erwinia cartovora and Xanthomonas citri. The bioassay results indicated that strong electron-withdrawing groups at the phenyl ring showed a better activity profile than electron-donating groups. A reaction pathway involving ascorbic acid catalysis was also suggested (Scheme 61).
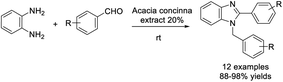
Kadu et al. utilized the aqueous extract of Acacia concinna pods as a catalyst for the synthesis of 1,2-disubstituted benzimidazole derivatives.131 A series of products were achieved in excellent yields under mild reaction conditions in short reaction times (Scheme 62).
2.6. Using other catalysts
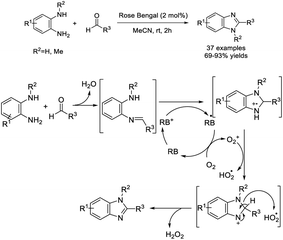
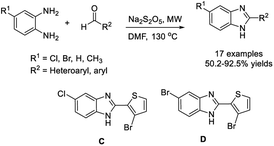
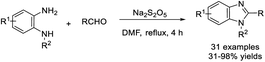
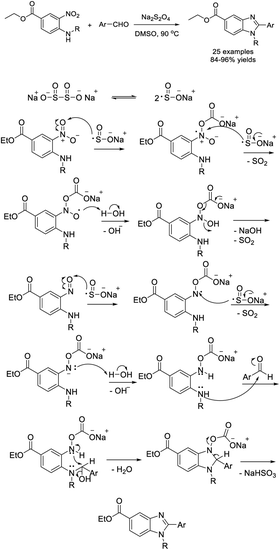
Kovvur et al. demonstrated a facile method for the synthesis of functionalized benzimidazoles from o-phenylenediamines and aldehydes using rose bengal as a photocatalyst. A huge library of benzimidazoles were afforded under mild reaction conditions.132 A plausible reaction mechanism was also proposed (Scheme 63).Shi et al. developed a practical method for the synthesis of 2,5-disubstituted benzimidazoles using Na2S2O5 as a catalyst and an oxidant. A wide range of products was obtained in moderate to excellent yields under microwave irradiation.133 Some synthesized compounds showed strong antibacterial and antifungal activity (Scheme 64). Bioassay study revealed that several synthesized compounds exhibited good antifungal activity and broad-spectrum antibacterial activity. The MIC of compounds C and D against the tested bacteria were both less than 4 μg mL−1, and the lowest one was 2 μg mL−1. In addition, compound D showed good antifungal activity against C. albicans with an MIC of 4 μg mL−1.
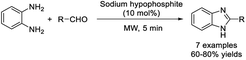
Tayade and Pawar illustrated the synthesis of 2-substituted benzimidazoles from aldehydes and o-phenylenediamine using sodium hypophosphite as a catalyst.134 The method featured some advantages such as high yield, clean and easy work-up, readily available catalyst, and short reaction time (Scheme 65).
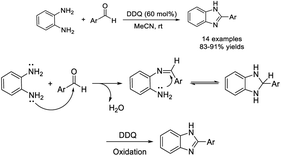
Hossein Naeimi and Zahra Babaei completed a mild and facile method for the synthesis of a series of 2-substituted benzimidazoles in the presence of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in acetonitrile.135 Fourteen products were formed under mild reaction conditions in excellent yields (Scheme 66).
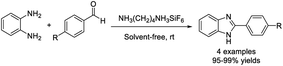
Benzekri et al. introduced a protocol for the preparation of benzimidazole derivatives using a hybrid crystal NH3(CH2)4NH3SiF6 as a mild and efficient heterogeneous catalyst.136 The protocol offered some merits such as short reaction times, absence of solvent, high efficiency, and recyclability of the catalyst (Scheme 67).
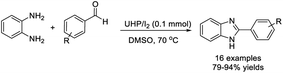
Alapati et al. reported an efficient strategy for the synthesis of substituted benzimidazoles from o-phenylenediamines and aryl aldehydes using UHP and I2 in DMSO.137 A wide range of products were isolated in good to excellent yields (Scheme 68).
Ramya et al. demonstrated a highly efficient approach for the synthesis of a variety of 2-arylbenzimidazoles using Koser's reagent [PhI(OH)OTs] as a catalyst. The advantageous features of the synthesis include short reaction time, simple operation, high efficiency, low catalyst loading, and scalability of the catalyst.138 Some synthesized benzimidazoles displayed significant antiproliferative activity (Scheme 69).
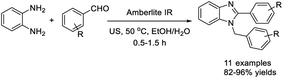
Nile et al. established a simple method to access 2-aryl-1-arylmethyl-1H-benzimidazoles using Amberlite IR-120 catalyst.139 The method exhibited several salient features such as green solvent, simple work-up procedure, short reaction time, and good to excellent yields of products (Scheme 70).
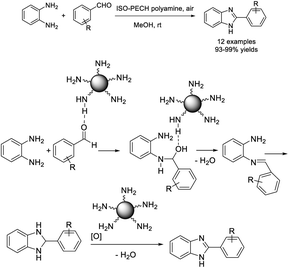
Kottayil et al. prepared a polyamine by the ring opening polymerization of epichlorohydrin and employed it for the synthesis of 2-arylbenzimidazoles from o-phenylenediamine and different aldehydes.140 Low catalyst loading, short reaction time, mild reaction conditions, high efficiency, simple work up and easy product purification, and recyclability of the catalyst are the salient features of the synthesis. A tentative reaction pathway was also illustrated (Scheme 71).
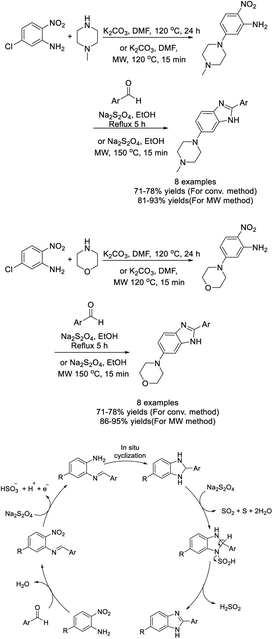
Akande et al. performed the synthesis of a diverse library of arylated benzimidazoles using sodium metabisulfite (Na2S2O5) as a catalyst.141 Products were obtained in modest to excellent yields under thermal conditions. Some synthesized compounds showed remarkable antidiabetic and antioxidant activities (Scheme 72).
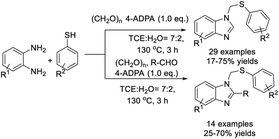
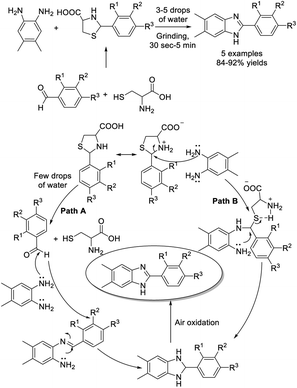
Tian et al. achieved a methodology for the synthesis of N-thiomethyl benzimidazoles from o-phenylenediamines, thiophenols, and aldehydes. Three C–N bonds and one C–S bond were formed in one pot with high chemoselectivity.142 A vast variety of products were delivered in acceptable yields (Scheme 73).
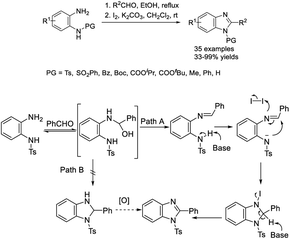
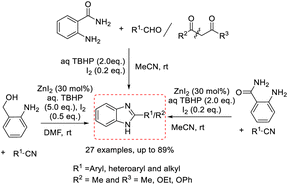
Hu et al. investigated a practical method for the construction of N-protected benzimidazoles based on intramolecular C–H amidation using molecular iodine as an oxidant.143 A diverse series of products were achieved in modest to excellent yields at room temperature with a broad substrate scope (Scheme 74). A reaction mechanism was also illustrated.
3. Benzimidazole synthesis by condensation of 1,2-benzenediamine or its analogues with other substrates
3.1. By condensation of 1,2-benzenediamines with primary alcohol
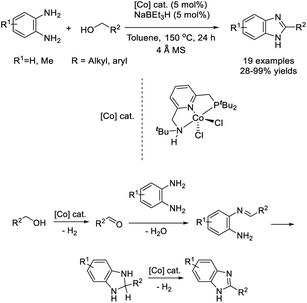
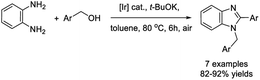
Daw et al. developed a base-metal-catalyzed dehydrogenative strategy for the synthesis of 2-substituted benzimidazoles from primary alcohols and aromatic diamines.144 A diverse series of products were provided in good yields (in most cases) under cobalt pincer complex catalysis (Scheme 75).Sharma et al. described the synthesis of four complexes [(η5-Cp*)Ir(L)Cl][PF6] by treatment of different lignans with [(η5-Cp*)IrCl(μ-Cl)]2, at 25 °C, followed by NH4PF6. These complexes then were examined as catalysts for the preparation of 1,2-disubstituted benzimidazoles from benzylic alcohols and o-phenylenediamines.145 Products were obtained in good to excellent yields under thermal and aerobic conditions (Scheme 76).
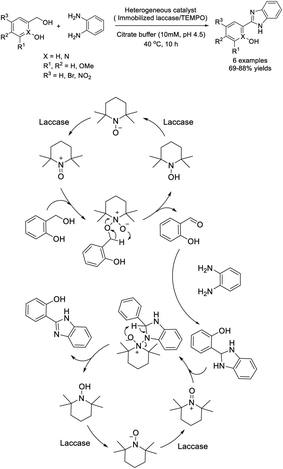
Mogharabi-Manzari et al. designed the synthesis of a high-performance heterogeneous catalyst by separately immobilizing laccase and TEMPO on magnetic nanoparticles. This catalyst was applied for the synthesis of benzimidazole from benzylic alcohols and o-phenylenediamines. Various products were afforded in good yields and the catalyst could be recycled for several runs without significant loss of catalytic activity.146 A reaction pathway was also described (Scheme 77).
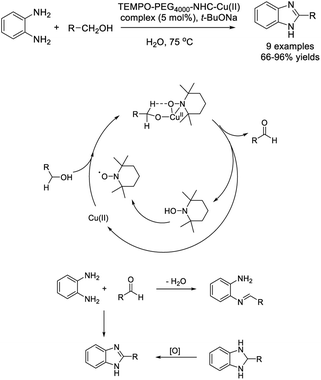
Wang et al. employed an amphiphilic catalyst TEMPO–PEG4000–NHC–Cu(II) complex for the one-pot aerobic oxidative synthesis of benzimidazoles from benzyl alcohols and o-phenylenediamines.147 Moderate to excellent yields of products were observed and the catalyst could be recycled and reused without considerable decrease in reaction yields after six runs (Scheme 78). A reaction mechanism was also suggested.
Marri et al. designed an efficient procedure for the one-pot synthesis of benzimidazoles by the reaction between benzyl alcohols and 1,2-diaminoarenes.148 A diverse library of products was produced in moderate to excellent yield under solvent- and catalyst-free conditions (Scheme 79).
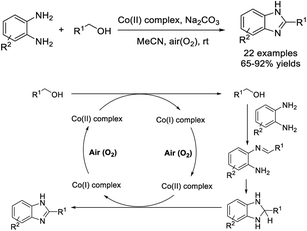
Zuo et al. demonstrated the synthesis of a Co(II) complex from quinalic acid and Co(OAc)2·4H2O and the use of this complex as a catalyst for a dehydrogenative coupling of aromatic diamines and primary alcohols to prepare 2-substituted benzimidazoles. A wide range of products were furnished in good to excellent yields under aerobic mild reaction conditions.149 A plausible reaction mechanism was proposed based on functional theory study (Scheme 80). In particular, a compound with anti-Parkinson activity was obtained on the gram-scale.
3.2. By condensation of 1,2-benzenediamines with primary amines
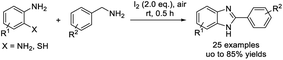
A novel strategy for the synthesis of benzimidazoles from 1,2-diaminoarenes and various benzylamines was completed by Naresh et al.150 The iodine-mediated synthesis was performed under aerobic and mild conditions to give products in moderate to good yields (Scheme 81).Dong et al. disclosed an oxidative coupling of benzylamines with 1,2-diaminoarenes to prepare benzimidazole derivatives.151 Fourteen products were delivered in moderate to excellent yields under an atmosphere of oxygen by using a 4,6-dimethoxysalicylic acid catalyst (Scheme 82).
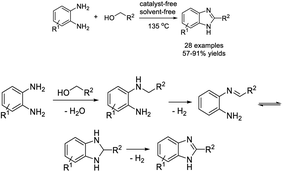
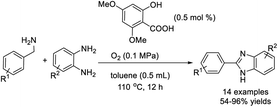
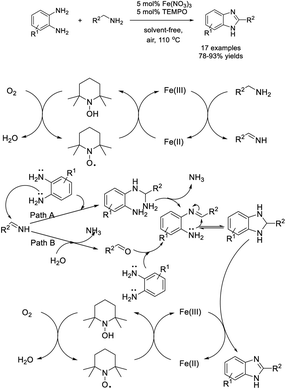
Yu et al. established an efficient approach to access benzimidazoles by the reaction between aromatic primary amines and o-phenylenediamines using Fe(NO3)3 and TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxyl) as a catalytic system and air as an oxidant.152 Various products were formed in good to excellent yields under aerobic and solvent-free conditions (Scheme 83). A tentative reaction pathway was presented.
Vasu et al. described a straightforward approach for the assembly of benzimidazoles using an Al-MCM-41 heterogeneous catalyst. Numerous products were afforded in good yields.153 Furthermore, the synthesis could be expanded to a gram scale reaction and the catalyst could be recycled for up to five cycles without considerable loss of its catalytic activity (Scheme 84).
Bermejo-López et al. discovered a new approach for the synthesis of benzimidazole, aerobic photo-oxidative cross-condensation of o-phenylenediamines with benzylic amines using a PCN-222 Pd complex as a catalyst. Products were obtained in moderate to excellent yields under mild conditions and blue light irradiation.154 The catalyst PCN-222(Pd) could be recycled maintaining yields over 90% after five runs. The approach was efficiently applied for the synthesis of an antitumor agent PMX-610 (Scheme 85).
3.3. By condensation of 1,2-benzenediamines with β-keto ester
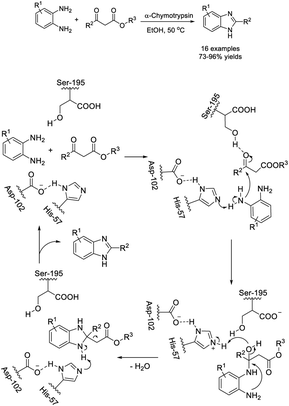
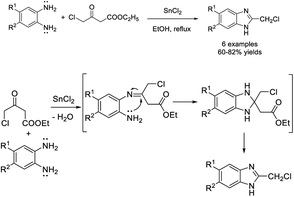
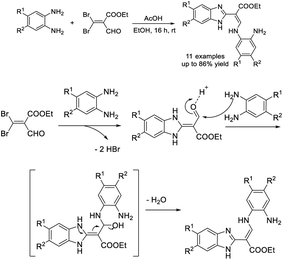
Liu et al. introduced the synthesis of 2-substituted benzimidazoles from o-phenylenediamines and diverse β-ketoesters using α-chymotrypsin as a catalyst. A wide range of benzimidazole derivatives were produced in good to excellent yields.155 A reaction mechanism was also suggested for the synthesis (Scheme 86).Dayakar et al. developed a protocol for the construction of 1H-benzimidazoles through the condensation of o-phenylenediamines with ethyl 4-chloro-3-oxobutanoate using an SnCl2 catalyst.156 A reaction pathway was also described for the reaction (Scheme 87).
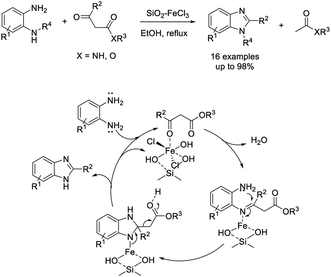
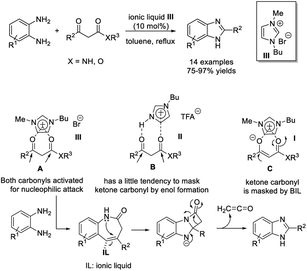
The Maiti group introduced an efficient methodology to access substituted benzimidazoles via the reaction between β-ketoesters or amides and o-phenylenediamines using silica supported ferric chloride (3 mol%) as a catalyst.157 Products were isolated in high yields and the catalyst could be recycled for up to five cycles without a considerable decrease in the reaction yields (Scheme 88). A reaction mechanism involving the role of the catalyst was proposed. The group also employed 1-butyl-3-methyl imidazolium bromide as a catalyst for the assembly of benzimidazoles. The condensation reaction between 1,2-diaminobenzenes and β-ketoesters or amides occurred at elevated temperature and formed products in good to excellent yields.158 A reaction pathway involving the formation of a 1,5-benzodiazepinone intermediate was presented (Scheme 89).
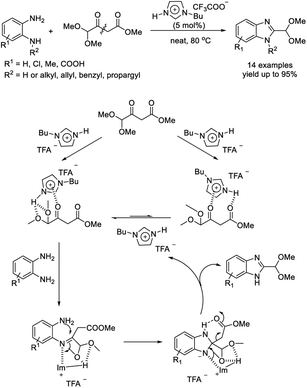
Deb et al. established a straightforward approach for the synthesis of dimethyl acetal protected benzimidazole-2-carboxaldehydes from 1,2-diaminobenzenes and methyl 4,4-dimethoxy-3-oxobutanoate using imidazolium ionic liquid HBIm·TFA as a catalyst.159 Advantages of the approach include short reaction times, mild reaction conditions, high efficiency, easy purification of products, recyclability of the catalyst, and scalability (Scheme 90).
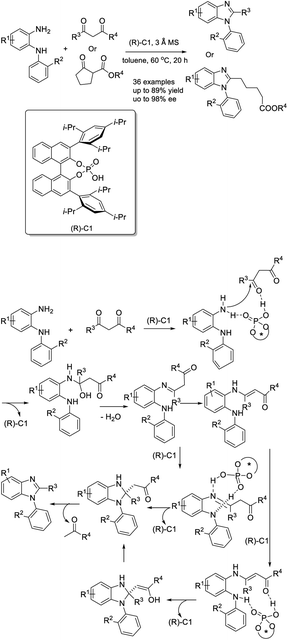
Man et al. demonstrated a novel procedure for the preparation of N-aryl benzimidazoles through the condensation of N1-(aryl)benzene-1,2-diamines with multicarbonyl compounds. A huge library of products was achieved in high yields with excellent enantioselectivity. In addition, the synthesis could be expanded to the gram scale.160 A reaction mechanism describing the catalyst's role was outlined (Scheme 91).
3.4. By condensation of 1,2-benzenediamines with carboxylic acid and its derivatives
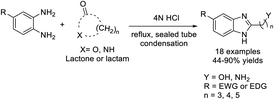
Basuri et al. designed a novel route for the synthesis of benzimidazoles from 1,2-aromatic diamines and carboxylic acids. The condensation reaction occurs in electrostatically charged microdroplets generated using a nano-electrospray (nESI) ion source.161 Ten of eleven products were obtained in high yields (Scheme 92).Castillo-Aguilera et al. accomplished the synthesis of a range of benzimidazol-2-yl alkylalkanols and benzimidazol-2-yl alkylamines through the reaction of o-phenylenediamine with lactones or lactams.162 The synthesis was suitable for a wide range of substrates (Scheme 93).
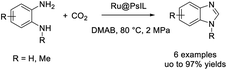
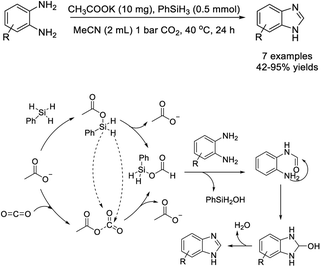
Saptal et al. prepared ruthenium nanoparticles (Ru NPs) supported on polymeric ionic liquids (PILs) and employed this material as a catalyst for the synthesis of benzimidazoles from 1,2 diamines and carbon dioxide.163 Products were afforded in high yields and the catalyst could be recycled for five consecutive runs without significant decrease in reaction yield (Scheme 94). Interestingly, this protocol also could be also applied for the tandem reduction of 2-nitroamines and CO2 to synthesize benzimidazoles, although lower yields were observed.
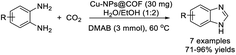
Khatun et al. synthesized copper nanoparticle embedded COF (Cu-NPs@COF) and utilized this material as a catalyst to access benzimidazoles via the incorporation of carbon dioxide in o-phenylenediamines.164 Products were achieved in moderate to excellent yields. Furthermore, the catalyst could be reused for up to six cycles without considerable loss of its catalytic activity (Scheme 95).
Li et al. utilized CO2 as a low-cost C1 resource to obtain benzimidazole based on a base-promoted reductive functionalization with 1,2-phenylenediamines.165 The synthesis used inexpensive acetate salt CH3COOK as a catalyst and delivered products in moderate to excellent yields under mild conditions (40 °C, 1 bar CO2) (Scheme 96). A reaction pathway was also suggested.
Huang et al. reported the synthesis of benzimidazoles starting from N-formyl imide and 1,2-phenylenediamines.166 Moderate to excellent reaction yields were observed for a wide variety of products (Scheme 97).
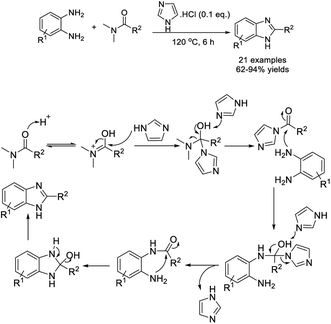
Gan et al. discovered a facile strategy for the construction of benzimidazoles and 2-substituted benzimidazoles by the reaction between o-phenylenediamines and DMF derivatives.167 The imidazolium chloride-catalyzed cyclization reaction formed products in moderate to excellent yields with broad substrate scope (Scheme 98). A tentative reaction pathway was also suggested.
3.5. By reactions of 1,2-benzenediamines with other functional groups
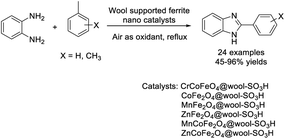
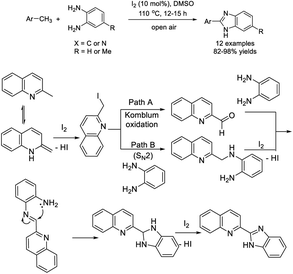
Shaabani and Hezarkhani synthesized biopolymer-supported ferrite nanocatalysts and applied them as catalysts for the assembly of 2-aryl-1H-benzimidazole using air as an oxidant.168 A diverse range of products were achieved in modest to excellent yields and the catalysts could be recycled several times without loss of their activity (Scheme 99).Yaragorla and Babu established an oxidative Csp3–H functionalization of 2-methylazaarenes for the construction of 2-azaarenyl benzimidazoles.169 The I2–DMSO-promoted reaction generated products in good to excellent yields (Scheme 100). A proposed reaction mechanism was illustrated for the 2-methyl quinoline substrate.
Garrido et al. demonstrated a straightforward approach for the preparation of functionalized benzimidazoles by the reaction between 3,3-dihalogenoacrolein and diaminobenzenes.170 The one-pot reaction afforded products in good yields under metal-free and mild conditions (Scheme 101). A plausible reaction mechanism was also described.
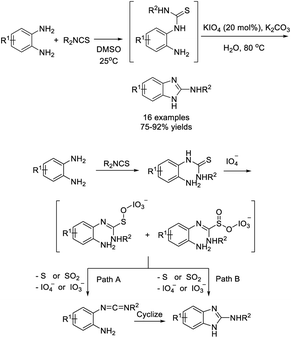
Duangkamol et al. discovered an efficient oxidative cyclodesulfurization method to access substituted 2-aminobenzazoles starting from isothiocyanates and o-phenylenediamines using periodate as a terminal oxidant.171 A wide range of products were furnished in moderate to excellent yields (Scheme 102).
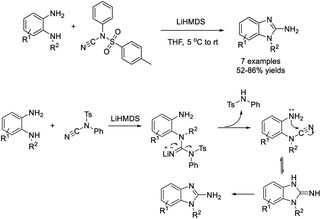
Kasthuri et al. illustrated a facile synthesis of 2-aminobenzimidazole derivatives from N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) with various benzene-1,2-diamines.172 Products were isolated in moderate to good yields in a relatively short reaction time (Scheme 103).
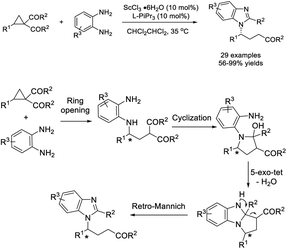
Xia et al. disclosed an efficient methodology for the construction of benzimidazoles containing chiral side chains starting from cyclopropyl ketones with aryl 1,2-diamines under Sc(III) complex catalysis. A sequence of ring-opening/cyclization/retro-Mannich reactions was proposed to occur.173 A huge library of products was achieved in moderate to excellent yields with excellent enantioselectivities (Scheme 104).
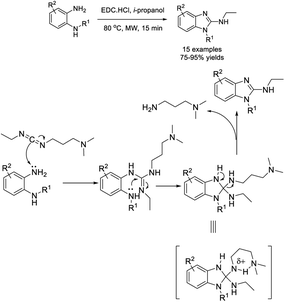
Rapolu et al. described an efficient one-pot procedure for the preparation of 2-ethylamino benzimidazole from o-phenylenediamines with EDC·HCl.174 The microwave-assisted synthesis gave products in high yields in a short reaction time. A reaction mechanism was also outlined (Scheme 105).
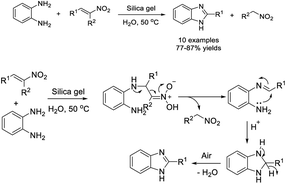
Li et al. obtained benzimidazoles by treatment of nitroolefins and o-phenylenediamine with silica gel catalyst in water.175 Products were generated in high yields with operational simplicity. A plausible reaction mechanism was described (Scheme 106).
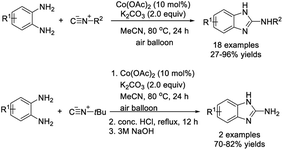
Liu et al. designed a protocol to access 2-aminobenzimidazoles from 2-aminoanilines and isonitriles.176 The cobalt-catalyzed synthesis delivered 2-aminobenzimidazoles in modest to excellent yields (Scheme 107).
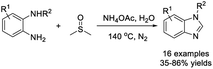
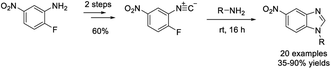
Zhu et al. illustrated a practical protocol to access 2-unsubstituted benzimidazoles by treatment of 2-aminoanilines with DMSO at elevated temperature.177 The NH4OAc-promoted reaction provided products in acceptable yields with broad substrate scope (Scheme 108).
Almansour et al. disclosed a versatile green method for the highly selective construction of 2-aryl benzimidazoles from aromatic 1,2-diamines with a series of substituted arylthioprolines.178 Five products were afforded in very good yields in short reaction times under mild reaction conditions (Scheme 109).
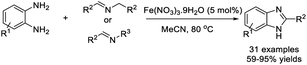
Yu et al. established an efficient procedure to access benzimidazoles from imine derivatives with o-phenylenediamines by a Fe(NO3)3·9H2O catalyzed aerobic oxidation reaction.179 A huge library of products was furnished in moderate to excellent yields (Scheme 110).
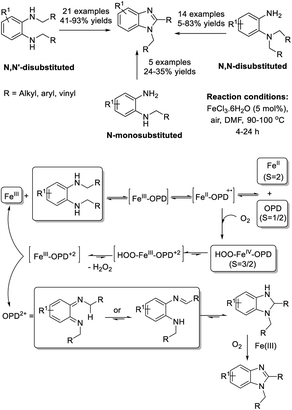
Thapa et al. developed an iron-catalyzed oxidative coupling approach to access 1,2-disubstituted benzimidazoles from mono- and di-substituted ortho-phenylenediamines.180 The synthesis used O2 as an oxidant and was suitable for a wide range of substrates (Scheme 111).
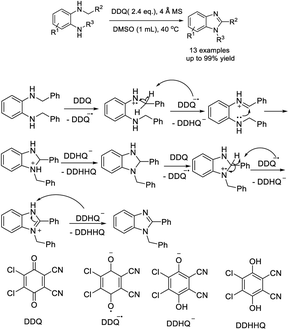
Ma et al. prepared 1,2-disubstituted benzimidazoles from N,N′-dialkyl o-phenylenediamines via intramolecular dehydrogenative coupling under the oxidation of DDQ.181 Products were furnished in high yields under mild conditions. A plausible reaction mechanism was suggested (Scheme 112).
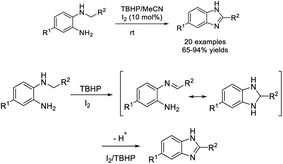
A facile methodology for the synthesis of 2-substituted benzimidazole was introduced by Patil et al. via a tandem reaction following sp3 C–H functionalization using tert-butyl hydroperoxide (TBHP) as an oxidant.182 A diverse range of products were generated in moderate to excellent yields under mild conditions (Scheme 113).
3.6. By reactions of o-nitroaniline
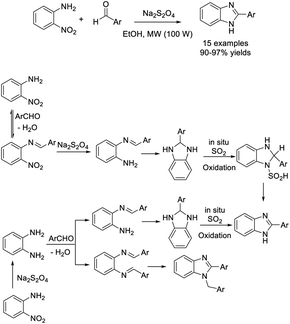
Naeimi et al. examined an efficient protocol to access 2-substituted benzimidazoles from o-nitroaniline and aryl aldehydes using Na2S2O4 as a catalyst.183 A series of products was provided in excellent yields in a short time under microwave irradiation (Scheme 114).Arya et al. prepared silver nanoparticles using green algae (Botryococcus braunii) and employed this material as a catalyst for the synthesis of benzimidazoles from o-nitroaniline and aldehydes.184 Products were generated in moderate to good yields under mild reaction conditions (Scheme 115).
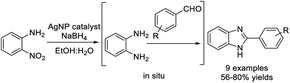
Özil et al. designed the synthesis of a series of 2-(aryl)-6-morpholin-4-yl(or 4-methylpiperazin-1-yl)-1H-benzimidazole derivatives from 5-morpholin-4-yl(or 4-methylpiperazin-1-yl)-2-nitroaniline and various aldehydes. The microwave synthesis gave better yields than the conventional heating method. All the synthesized species exhibited very high radical scavenging activities using the ABTS˙+ and DPPH˙ methods. Some compounds showed much better α-glucosidase inhibitory activity than the standard acarbose.185 The α-glucosidase inhibitory study indicated that the hydroxyl group at the para position is essential to the exhibition of biological activity, and that the introduction of a piperazine or morpholine group would potentially increase the binding affinity and yield higher inhibitory activity. A reaction mechanism was proposed for the formation of benzimidazoles (Scheme 116).
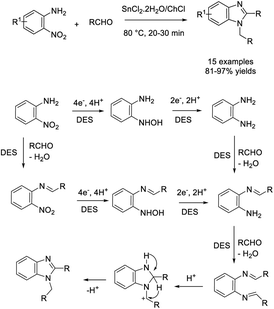
Rodríguez-Huerto et al. developed an efficient method to access N-arylmethyl-2-substituted benzimidazoles from nitroarenes and aldehydes.186 The synthesis was performed in a tin(II) chloride dihydrate/choline chloride eutectic mixture to provide products in good to excellent yields. A reaction pathway was also described (Scheme 117).
Kumar et al. introduced an efficient approach to construct 1,2-disubstituted benzimidazole-5-carboxylates from 4-(alkyl/arylamino)-3-nitrobenzoates and aldehydes via a one-pot nitroreductive cyclization process using sodium dithionite.187 A diverse library of products was produced in good to excellent yields in a very short reaction time (Scheme 118). A reaction mechanism was also presented.
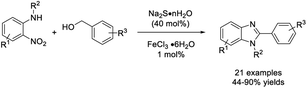
Nguyen et al. completed the synthesis of benzimidazoles starting from o-nitroanilines and benzyl alcohols through an unbalanced redox condensation reaction using sodium sulfide (40 mol%) in combination with iron(III) chloride hexahydrate (1 mol%) as a catalyst system.188 A wide range of products was furnished in modest to excellent yields (Scheme 119).
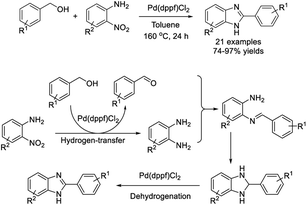
Li et al. used [1,10-bis(diphenylphosphino)ferrocene]dichloropalladium(II) (Pd(dppf)Cl2) as a catalyst for the synthesis of 2-substituted benzimidazoles via a hydrogen-transfer strategy.189 A set of 2-substituted benzimidazoles were obtained in good to excellent yields (Scheme 120).
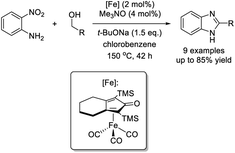
Putta et al. demonstrated an iron-catalyzed hydrogen transfer protocol to access benzimidazoles via the redox condensation of o-nitroanilines with primary alcohol.190 Various products were generated in good yields under thermal conditions (Scheme 121).
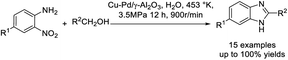
Feng et al. utilized a Cu–Pd/γ-Al2O3 catalyst for the assembly of benzimidazole derivatives via a sequence of hydrogenation transfer and cyclization coupling. The use of Cu and Pd was responsible for the dehydrogenation of the primary alcohol and hydrogenation of o-nitroaniline.191 Various products were furnished in high yields and the catalyst could be recycled for several runs without a significant decrease in reaction yields (Scheme 122).
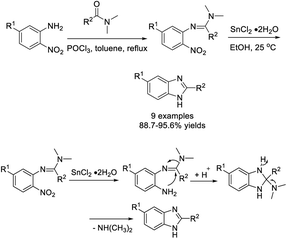
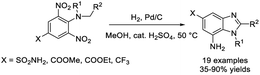
Duan et al. demonstrated a methodology to access 2,5-disubstituted benzimidazoles from 2-nitro-5-substituted anilines by a reductive cyclization reaction using stannous chloride dihydrate as a catalyst.192 All products were furnished in excellent yield under mild reaction conditions (Scheme 123).
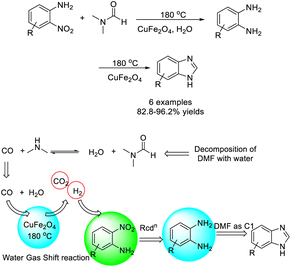
Rasal et al. developed a method for the one-pot synthesis of benzimidazole from o-nitroanilines and DMF using CuFe2O4 as a catalyst.193 The catalyst was easily recovered by an external magnetic field and reused at least three times without considerable loss of its activity (Scheme 124).
4. Benzimidazole synthesis without using 1,2-benzenediamine or its analogues
The Mamedov group explored a facile approach for the assembly of 7-(benzimidazol-2- yl)lumazines (7-(benzimidazol-2-yl)pteridine-2,4(1H,3H)-diones) from quinoxalin-2-one derivatives and 2,4,5-triamino-6-oxypyrimidines. A sequence of addition of nucleophile, ring opening, and ring closure was proposed to occur in one pot.194 A vast number of products was achieved in high yields (Scheme 125). The group also achieved an efficient strategy for the construction of substituted 2,2′-bibenzimidazoles starting from 3-cyanoquinoxalin-2(1H)-ones and 1,2-diaminobenzenes.195 The reaction proceeded via a sequence of nucleophilic addition, electrophilic substitution, and Mamedov rearrangement. A wide range of products was obtained in high yields (Scheme 126).Mahesh et al. established a novel methodology toward substituted benzimidazoles from anilines, aldehydes, and TMSN3.196 The one-pot, multicomponent reaction proceeds smoothly to afford products in modest to good yields with broad substrate scope (Scheme 127). A reaction pathway was also described.
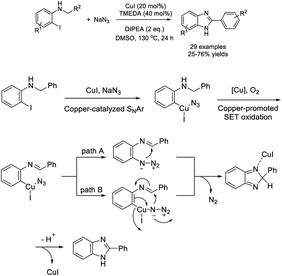
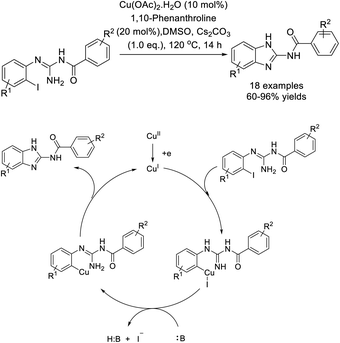
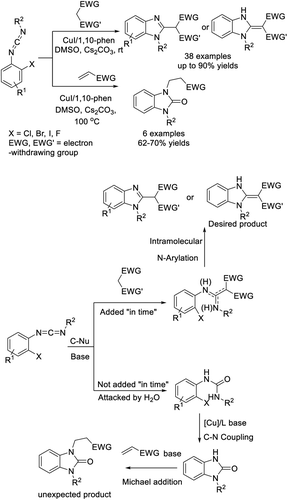
Chen et al. demonstrated an efficient protocol to access benzimidazole derivatives starting from N-alkyl-2-iodoaniline and sodium azide via copper-catalyzed double C–N bond formation.197 A sequence of SNAr reaction, aerobic oxidation of C(sp3)–H bonds and intramolecular C–N bond formation under CuI catalysis was proposed to occur. A library of products was obtained in acceptable yields (Scheme 128).
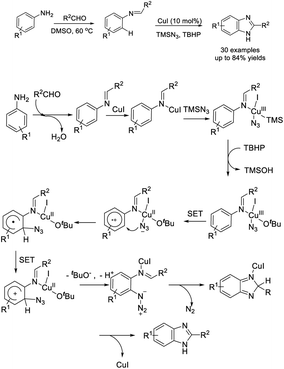
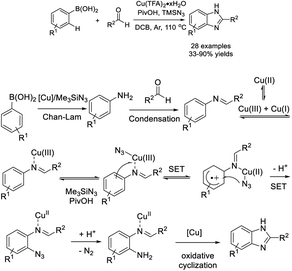
Xie et al. developed an efficient strategy for the preparation of benzimidazole from arylboronic acid and aldehydes, and TMSN3.198 A mechanistic study revealed that a sequence of copper-promoted Chan–Evans–Lam coupling, C–H amination, and oxidative cycloaddition occurred (Scheme 129).
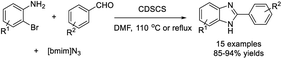
Somayeh Behrouz disclosed a highly efficient procedure for the assembly of 1-H-2-substituted benzimidazole derivatives from various 2-bromoanilines, aldehydes, and [Bmim]N3. The one-pot three-component reaction was catalyzed by copper-doped silica cuprous sulfate (CDSCS) and gave products in good to excellent yields.199 Furthermore, the catalyst could be recovered and reused for several runs without considerable loss of its activity (Scheme 130).
Boratyński et al. introduced the synthesis of new enantiomeric benzimidazoles from enantiomeric trans-1,2-diaminocyclohexane in several steps200 (Scheme 131).
Li et al. discovered an efficient protocol to access 2-substituted benzimidazoles based on an NNP-type pincer ligand promoted Cu-catalyzed Ullmann condensation reaction of o-halobenzanilides with ammonia.201 A diverse library of products was afforded in high yields. A tentative reaction mechanism was also presented (Scheme 132).
Zhang et al. described a methodology for the preparation of N-Ts benzimidazoles via a Beckmann-type rearrangement of o-aminoaryl N–H ketimines, which were generated by condensation of ammonia with ketones. (Diacetoxyiodo)benzene might act as an efficient oxidant to trigger the [1,2]-aryl migration.202 The method was successfully applied to the preparation chlormidazole and clemizole, two marketed drugs (Scheme 133).
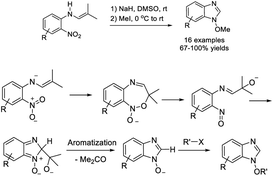
Ansari et al. obtained N-alkoxy-2H-benzimidazoles from 2-nitro-N-(2-methyl-1-propen-1-yl)benzenamines by treatment with potassium tert-butoxide followed by the addition of MeI.203 Various products were produced in moderate to excellent yields under mild reaction conditions. A reaction pathway was also proposed (Scheme 134).
Joardar et al. achieved a one-pot intramolecular nitro reduction/cyclization reaction for the synthesis of substituted benzimidazoles.204 A wide range of products was isolated in moderate to excellent yields under mild conditions (Scheme 135).
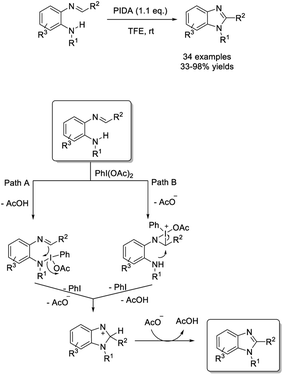
Maitia and Mala introduced an efficient approach to access substituted benzimidazoles via phenyliodine diacetate (PIDA)-mediated C(sp2)–H amidation.205 A huge number of benzimidazole products were furnished in good to excellent yields under mild reaction conditions in the open air (Scheme 136).
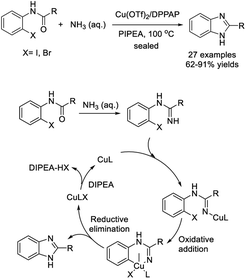
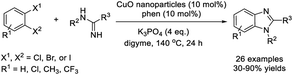
Yu et al. developed a procedure for the assembly of 1,2-disubstituted benzimidazoles from 1,2-dihaloarenes and N-arylamidines using copper oxide nanoparticles as a catalyst. In general, the products were formed in good to excellent yields.206 In addition, the catalyst could be recycled and reused without any considerable loss of the catalytic activity (Scheme 137).
Shaik et al. investigated the synthesis of 2-(N-aryolamino)benzimidazoles involving a C–N cross-coupling reaction.207 A diverse range of products was achieved in moderate to excellent yields (Scheme 138).
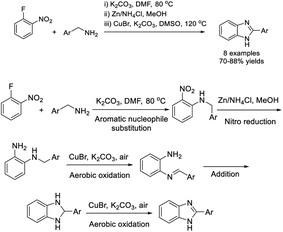
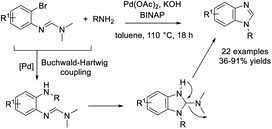
Mirza and Zeeb established a novel method to access benzimidazole derivatives from 1-fluoro-2-nitrobenzene and various arylamines.208 The CuBr-catalyzed oxidation and cyclization reaction provided products in good yields (Scheme 139).
Liu et al. demonstrated a metal-free synthesis of N-1-alkyl-2-unsubstituted benzimidazoles from o-fluoro aryl formamidines and primary amines.209 The one-pot displacement of –F by the primary amine and cyclization was performed in a microwave reactor (Scheme 140).
Kurhade et al. disclosed an efficient one-pot protocol for the construction of N-substituted benzimidazoles starting from 2-fluoro-5-nitrophenylisocyanide and primary amines.210 Various products were generated in modest to excellent yields (Scheme 141).
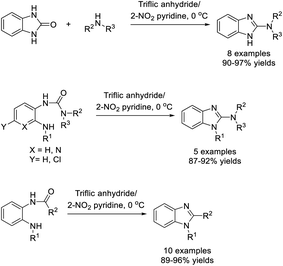
Quadri et al. completed the preparation of 2-substituted benzimidazoles from 2(3H)-benzimidazolones and amines mediated by 2-nitropyridine and triflic anhydride.211 Eight products were achieved in excellent yields (Scheme 142).
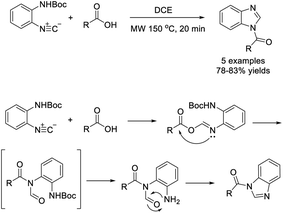
Chen et al. prepared benzimidazole derivatives by treatment of 2-(N-Boc-amino)phenylisocyanide with carboxylic acids In DCE.212 Five products were obtained in good yields under microwave irradiation (Scheme 143).
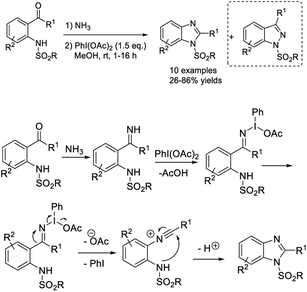
In a study reported by Saha et al., 2-substituted benzimidazoles were synthesized from 2-aminobenzyl alcohol/2-aminobenzamide and different coupling partners (nitriles, aldehydes and 1,3-diketones). A sequence of C–N bond formation, cyclization, subsequent ring contraction and dehydrogenation was proposed to occur.213 The iodine and TBHP promoted syntheses furnished products in modest to good yields under mild reaction conditions (Scheme 144).
Liu et al. introduced a facile strategy to access 2-substituted benzimidazoles from o-haloarylcarbodiimides with active methylene compounds.214 The copper-catalyzed synthesis was performed under mild reaction conditions and resulted in products in good yields (in most cases) for a wide range of substrates (Scheme 145).
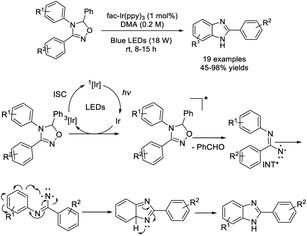
Park et al. developed a methodology for the assembly of benzimidazoles starting from oxadiazolines through nitrene intermediates. A sequence of homolytic N–O bond cleavage and concomitant C–N bond cleavage was proposed to generate nitrene, which the underwent intramolecular aromatic substitution to furnish functionalized benzimidazoles.215 A wide range of products were afforded in acceptable to excellent yields under mild photocatalytic reaction conditions (Scheme 146).
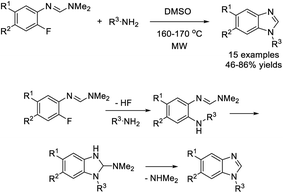
Bie et al. investigated a novel approach to access N-substituted benzimidazoles using aromatic formamidines and primary amines as starting materials.216 The Pd-catalyzed reaction furnished products in moderate to good yields with broad substrate scope (Scheme 147).
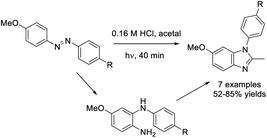
Chen et al. examined a procedure to prepare 1-aryl-1H-benzimidazoles from 4-methoxyazobenzenes.217 The products were generated in good yields when the reactants were irradiated in acetal containing 0.16 M hydrochloric acid for 40 minutes (Scheme 148).
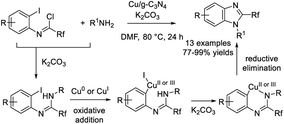
Xu et al. achieved an efficient protocol for the construction of 2-trifluoromethyl-benzimidazoles starting from trifluoroacetimidoyl chlorides and amines using a copper doped g-C3N4 catalyst.218 Various products were furnished in good to excellent yields (Scheme 149).
5. Conclusion
In conclusion, this article has summarized studies on the synthesis of benzimidazole derivatives over a decade and given brief descriptions of the biological applications of benzimidazole derivatives. The condensation of 1,2-benzenediamine or its analogues with aldehydes or primary alcohols has received much attention from chemists, and numerous studies based on this method have been published recently. Furthermore, many novel methods using different substrates with high efficiency or employing environmentally benign procedures have been investigated. Undoubtedly, more facile, environmentally friendly, and efficient strategies to prepare benzimidazole-based compounds will be developed in the near future. The application of benzimidazole synthesis to natural product synthesis and drug synthesis is probably the next challenge in the field. This review article summarizes up-to-date articles, deals with a great number of studies, covers all aspects of benzimidazole synthesis, and describes the reaction pathways in most studies.Author contributions
Vo Cong Dung: data curation; Nguyen Thi Chung: formal analysis; Dau Xuan Duc: writing original draft, review, and editing.Conflicts of interest
There are no conflicts to declare.References
- H. G. Kathrotiya and M. P. Patel, J. Chem. Sci., 2013, 125, 993–1001 CrossRef CAS.
- K. Vasantha, G. Basavarajaswamy, M. Vaishali Rai, P. Boja, V. R. Pai, N. Shruthi and M. Bhat, Bioorg. Med. Chem. Lett., 2015, 25, 1420–1426 CrossRef CAS PubMed.
- S. Yadav, B. Narasimhan, S. M. Lim, K. Ramasamy, M. Vasudevan, S. A. A. Shah and A. Mathur, J. Basic Appl. Sci., 2018, 5, 100–109 Search PubMed.
- I. N. Shaikh, K. M. Hosamani and M. M. Kurjogi, Arch. Pharm., 2018, 351, 1700205 CrossRef PubMed.
- A. Faruk, K. D. Biplab, S. Kamal, C. Arpita and K. Pallab, Int. J. Drug Res. Technol., 2014, 4, 31–38 Search PubMed.
- J. K. Vilasrao, B. S. Manish, M. J. Deepali, S. G. Priyanka and L. G. Priyanka, Int. J. Res. Pharm. Chem., 2012, 2, 105–112 Search PubMed.
- K. Sreena, R. Ratheesh, M. Rachana, M. Poornima and C. Shyni, Hygeia, 2009, 1, 21–22 Search PubMed.
- T. Pan, X. He, B. Chen, H. Chen, G. Geng, H. Luo, H. Zhang and C. Bai, Eur. J. Med. Chem., 2015, 95, 500–513 CrossRef CAS PubMed.
- N. A. Al-Masoudi, N. N. A. Jafar, L. J. Abbas, S. J. Baqir and C. Z. Pannecouque, Für Naturforschung, 2011, 66, 953–960 CrossRef CAS.
- S. Ferro, M. R. Buemi, L. De Luca, F. E. Agharbaoui, C. Pannecouque and A. M. Monforte, Bioorg. Med. Chem., 2017, 25, 3861–3870 CrossRef CAS PubMed.
- S. C. Tsay, J. R. Hwu, R. Singha, W. C. Huang, Y. H. Chang, M. H. Hsu, F. K. Shieh, C. C. Lin, K. C. Hwang, J. C. Horng, E. D. Clercq, E. Vliegen and J. Neyts, Eur. J. Med. Chem., 2013, 63, 290–298 CrossRef CAS PubMed.
- Y. M. Shaker, M. A. Omar, K. Mahmoud, S. M. Elhallouty, W. M. El-Senousy, M. M. Ali, A. E. Mahmoud, A. H. Abdel-Halim, S. M. Soliman and H. I. El Diwani, J. Enzyme Inhib. Med. Chem., 2015, 30, 826–845 CrossRef CAS PubMed.
- D. Dai, J. R. Burgeson, D. N. Gharaibeh, A. L. Moore, R. A. Larson, N. R. Cerruti, S. M. Amberg, D. E. Hruby and D. Dai, Bioorg. Med. Chem. Lett., 2013, 23, 744–749 CrossRef CAS PubMed.
- N. S. El-Gohary and M. I. Shaaban, Eur. J. Med. Chem., 2017, 131, 255–262 CrossRef CAS PubMed.
- Z. Wang, X. Deng, S. Xion, R. Xiong, J. Liu, L. Zou, Z. Xie, Y. Chen, Y. Liu, X. Zheng and G. Tang, Nat. Prod. Res., 2017, 32, 2900–2909 CrossRef PubMed.
- S. Yadav, B. Narasimhan, S. M. Lim, K. Ramasamy, M. Vasudevan, S. A. A. Shah and M. Sevaraj, Chem. Cent. J., 2017, 11, 137 CrossRef PubMed.
- V. Onnis, M. Demurtas, A. Deplano, G. Balboni, A. Baldisserotto, S. Manfredini, S. Pacifico, S. Liekens and Z. Balzarini, Molecules, 2016, 21, 579 CrossRef PubMed.
- P. Sharma, T. S. Reddy, N. P. Kumar, K. R. Senwar, S. K. Bhargava and N. Shankaraiah, Eur. J. Med. Chem., 2017, 138, 234–245 CrossRef CAS PubMed.
- W. Wang, D. Kong, H. Cheng, L. Tan, Z. Zhang, X. Zhuang, H. Long, Y. Zhou, Y. Xu, X. Yang and K. Ding, Bioorg. Med. Chem. Lett., 2014, 24, 4250–4253 CrossRef CAS PubMed.
- H. B. El-Nassan, Eur. J. Med. Chem., 2012, 53, 22–27 CrossRef CAS PubMed.
- S. M. Abou-Seri, K. Abouzid and D. A. Abou El Ella, Eur. J. Med. Chem., 2011, 46, 647–658 CrossRef CAS PubMed.
- M. T. Khan, M. T. Razi, S. U. Jan, M. Mukhtiar, R. Gul, S. IzharUllah, A. Hussain, A. M. Hasmi, M. T. Ahmad, N. R. Shahwani and I. Rabbani, Pak. J. Pharm. Sci., 2018, 31, 1067–1074 CAS.
- W. Zhu, Y. Da, D. Wu, H. Zheng, L. Zhu, L. Wang, Y. Yan and Z. Chen, Bioorg. Med. Chem., 2014, 22, 2294–2302 CrossRef CAS PubMed.
- Q. Li, Q. Hu, X. Wang, Y. Zong, L. Zhao, J. Xing, J. Zhou and H. Zhang, Chem. Biol. Drug Des., 2015, 86, 509–516 CrossRef CAS PubMed.
- N. Kumar, C. S. Sharma, M. S. Ranawat, H. P. Singh, L. S. Chauhan and N. Dashora, J. Pharm. Invest., 2015, 45, 65–71 CrossRef CAS.
- R. Sethi, S. Arora, D. Saini, T. G. Singh and S. Jain, Acta Pol. Pharm., 2017, 74, 1413–1425 CAS.
- P. S. Hameed, A. Raichurkar, P. Madhavapeddi, S. Menasinakai, S. Sharma, P. Kaur, R. Nandishaiah, P. Panduga, V. K. Sambandamurthy and D. Sriram, ACS Med. Chem. Lett., 2014, 5, 820–825 CrossRef PubMed.
- N. Anand, K. K. G. Ramakrishna, M. P. Gupt, V. Chaturvedi, S. Singh, K. K. Srivastava, P. Sharma, N. Rai, R. Ramachandran, A. K. Dwivedi, V. Gupta, B. Kumar, S. Pandey, P. K. Shukla, S. K. Pandey, J. Lal and R. P. Tripathi, ACS Med. Chem. Lett., 2013, 4, 958–963 CrossRef CAS PubMed.
- R. K. Arora, N. Kaur, Y. Bansal and G. Bansal, Acta Pharm. Sin. B, 2014, 4, 368–375 CrossRef PubMed.
- E. Menteşe, F. Yılmaz, N. Baltaş, O. Bekircan and B. Kahveci, J. Enzyme Inhib. Med. Chem., 2015, 30, 435–441 CrossRef PubMed.
- R. Nieto-Meneses, R. Castillo, A. Hernández-Campos, A. Maldonado-Rangel, J. B. Matius-Ruiz, P. J. Trejo-Soto, B. Nogueda-Torres, M. A. Dea-Ayuaela, F. Bolás-Fernández, C. Méndez-Cuesta and L. Yépez-Mulia, Exp. Parasitol., 2018, 184, 82–89 CrossRef CAS PubMed.
- S. R. Brishty, M. J. Hossain, M. U. Khandaker, M. R. I. Faruque, H. Osman and S. M. A. Rahman, Front. Pharmacol, 2021, 12, 762807 CrossRef CAS PubMed.
- Salahuddin, M. Shaharyar and A. Mazumder, Arabian J. Chem., 2017, 19, S157–S173 CrossRef.
- G. Gajanan, S. Shital, T. Vipul, V. Babar, J. Dnyaneshwar, D. Vaibhav and C. Vaibhav, Journal of Pharmaceutical Chemistry and Drug Formulation, 2021, 3, 23–32 Search PubMed.
- M. Berrada, F. Carriere, Y. Abboud, A. Abourriche, A. Benamara, N. Lajrhed, M. Kabbaj and M. Berrada, J. Mater. Chem., 2002, 12, 3551–3559 RSC.
- S. K. Kim, S. W. Choi, W. S. Jeon, J. J. O. Park, T. Ko, H. Chang and J. C. Lee, Macromolecules, 2012, 45, 1438–1446 CrossRef CAS.
- N. Esmaeili, E. M. Gray and C. J. Webb, ChemPhysChem, 2019, 20, 2016–2053 CrossRef CAS PubMed.
- W. W. H. Wong, M. S. Vickers, A. R. Cowley, R. L. Paul and P. D. Beer, Org. Biomol. Chem., 2005, 3, 4201–4208 RSC.
- N. Singh and D. O. Jang, Org. Lett., 2007, 9, 1991–1994 CrossRef CAS PubMed.
- K. S. Moon, N. Singh, G. W. Lee and D. O. Jang, Tetrahedron, 2007, 63, 9106–9111 CrossRef CAS.
- C. J. Matthews, V. Broughton, G. Bernardinelli, X. Melich, G. Brand, A. C. Willis and A. F. Williams, New J. Chem., 2003, 27, 354–358 RSC.
- L. Li, T.-L. Hu, J.-R. Li, D.-Z. Wang, Y.-F. Zeng and X.-H. Bu, CrystEngComm, 2007, 9, 412–420 RSC.
- P. C. Tway and L. J. C. Love, J. Phys. Chem., 1982, 86, 5223–5226 CrossRef CAS.
- P. Choudhury, S. Panja, A. Chatterjee, P. Bhattacharya and S. Chakravorti, J. Photochem. Photobiol., A, 2005, 173, 106–113 CrossRef.
- Y. Wu, P. V. Lawson, M. M Henary, K. Schmidt, J.-L. Brédas and C. J. Fahrni, J. Phys. Chem. A, 2007, 111, 4584–4595 CrossRef CAS PubMed.
- P. Chaudhuri, B. Ganguly and S. Bhattacharya, J. Org. Chem., 2007, 72, 1912–1923 CrossRef CAS PubMed.
- A. Sannigrahi, D. Arunbabu, R. M. Sankar and T. Jana, Macromolecules, 2007, 40, 2844–2851 CrossRef CAS.
- K. F. Khaled, Electrochim. Acta, 2003, 48, 2493–2503 CrossRef CAS.
- J. M. Roque, T. Pandiyan, J. Cruz and E. García-Ochoa, Corros. Sci., 2008, 50, 614–624 CrossRef CAS.
- G. A. Molander and K. Ajayi, Org. Lett., 2012, 14, 4242–4245 CrossRef CAS PubMed.
- Y. Bai, J. Lu, Z. Shi and B. Yang, Synlett, 2001, 544–546 CrossRef CAS.
- E. Hasegawa, A. Yoneoka, K. Suzuki, T. Kato, T. Kitazume and K. Yanagi, Tetrahedron, 1999, 55, 12957–12968 CrossRef CAS.
- A. Figge, H. J. Altenbach, D. J. Brauer and P. Tielmann, Tetrahedron: Asymmetry, 2002, 13, 137–144 CrossRef CAS.
- M. A. Pujar, T. D. Bharamgoudar and T. D. Transition, Met. Chem., 1988, 13, 423–425 CrossRef CAS.
- V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. G. Fin, J. Am. Chem. Soc., 2007, 129, 12696–12704 CrossRef CAS PubMed.
- M. Kose and V. Mckee, Polyhedron, 2014, 75, 30–39 CrossRef CAS.
- V. O. Rodionov, S. I. Presolski, S. Gardinier, Y.-H. Lim and M. G. Fin, J. Am. Chem. Soc., 2013, 135, 1626 CrossRef CAS.
- A. Chawla, G. Kaur and A. K. Sharma, Int. J. Pharm. Phytopharm. Res., 2012, 2, 148–159 CAS.
- S. I. Alaqeel, J. Saudi Chem. Soc., 2017, 21, 219–237 CrossRef.
- V. A. S. Pardeshi, N. S. Chundawat, S. I. Pathan, P. Sukhwal, T. P. S. Chundawat and G. P. Singh, Synth. Commun., 2021, 51, 485–513 CrossRef CAS.
- M. Faheem, A. Rathaur, A. Pandey, V. K. Singh and A. K. Tiwari, ChemistrySelect, 2020, 5, 3981–3994 CrossRef CAS.
- D. X. Duc, Mini-Rev. Org. Chem., 2019, 16, 422–452 CrossRef CAS.
- D. X. Duc, Curr. Org. Synth., 2020, 24, 622–657 Search PubMed.
- D. X. Duc, Mini-Rev. Org. Chem., 2021, 18, 110–134 CrossRef.
- D. X. Duc, Curr. Org. Synth., 2020, 24, 498–517 CrossRef PubMed.
- D. X. Duc, Curr. Org. Chem., 2020, 24, 2256–2271 CrossRef CAS.
- D. X. Duc and N. T. Chung, Curr. Org. Chem., 2021, 25, 1755–1782 CrossRef CAS.
- D. X. Duc and V. C. Dung, Curr. Org. Chem., 2021, 25, 2938–2989 CrossRef CAS.
- D. X. Duc and N. T. Chung, Curr. Org. Chem., 2021, 25, 702–730 Search PubMed.
- H. Sharma, N. Singh and D. O. Jang, Green Chem., 2014, 16, 4922–4930 RSC.
- Z. Wang, T. Song and Y. Yang, Synlett, 2019, 30, 319–324 CrossRef CAS.
- M. Kalhor, F. Rezaee-Baroonaghi, A. Dadras and Z. Zarnegar, Appl. Organomet. Chem., 2019, e4784 CrossRef.
- A. Fazlinia and S. Sheikh, Nano-Met. Chem., 2017, 48, 126–130 Search PubMed.
- H. Mohammadi, C. Karami, M. Bahri, M. M. Gilany and M. A. Taher, Nano-Met. Chem., 2017, 47, 1334–1341 CAS.
- C. Karami, M. Abdollahifar, F. Jahani, A. Farrokhi and M. A. Taher, Inorg. Nano-Met. Chem., 2016, 47, 626–631 CrossRef.
- H. Naeimi and Z. Babaei, Polycyclic Aromat. Compd., 2015, 36, 490–505 CrossRef.
- K. Bahrami, M. M. Khodaei and F. Naali, J. Exp. Nanosci., 2015, 11, 148–160 CrossRef.
- H. Naeimi and N. Alishahi, J. Exp. Nanosci., 2015, 10, 222–234 CrossRef CAS.
- S. Bahadorikhalili, L. Ma'mani, H. Mahdavi and A. Shafiee, Microporous Mesoporous Mater., 2018, 262, 207–216 CrossRef CAS.
- K. S. J. Kumara, G. Krishnamurthy, N. S. Kumar, N. Naik and T. M. Praveen, J. Magn. Magn. Mater., 2018, 451, 808–821 CrossRef.
- B. Paul, S. Vadivel, S. S. Dhar, S. Debbarma and M. Kumaravel, J. Phys. Chem. Solids, 2017, 104, 152–159 CrossRef CAS.
- H. Ghafuri, E. Esmaili and M. Talebi, C. R. Chim., 2016, 19, 942–950 CrossRef CAS.
- D. Kommula and S. R. M. Madugula, J. Iran. Chem. Soc., 2017, 14, 1665–1671 CrossRef CAS.
- F. Hakimi, D. M. Niri, S. H. Banitaba and E. Golrasan, Asian J. Green Chem., 2020, 4, 239–248 CAS.
- F. Shukla, M. Das and S. Thakore, J. Mol. Liq., 2021, 336, 116217 CrossRef CAS.
- M. A. Bodaghifard and S. Shafi, J. Iran. Chem. Soc., 2021, 18, 677–687 CrossRef CAS.
- N. Kaur, S. Kaur, G. Kaur, A. Bhalla, S. Srinivasan and G. R. Chaudhary, J. Mater. Chem. A, 2019, 7, 17306–17314 RSC.
- Z. Garazhian, A. Rezaeifard and M. Jafarpour, RSC Adv., 2019, 9, 34854–34861 RSC.
- S. Roy, B. Banerjee, N. Salam, A. Bhaumik and A. M. Islam, ChemCatChem, 2015, 7, 2689–2697 CrossRef CAS.
- A. Mobinikhaledi, M. Zendehdel, F. Goudarzi and G. R. Bardajee, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2016, 46, 1526–1531 CrossRef CAS.
- S. Kohli, G. Rathee, S. Hooda and S. Chandra, Dalton Trans., 2021, 50, 7750–7758 RSC.
- N. Pourmorteza, M. Jafarpour, F. Feizpour and A. Rezaeifard, RSC Adv., 2022, 12, 22526–22541 RSC.
- N. V. Shitole, K. S. Niralwad, B. B. Shingate and M. S. Shingare, Arabian J. Chem., 2016, 9, 5858–5860 CrossRef.
- S. R. Mathapati, K. N. Patil, S. S. Mathakari, A. W. Suryawanshi and A. H. Jadhav, Phosphorus, Sulfur Silicon Relat. Elem., 2021, 196, 538–547 CrossRef CAS.
- S. Belkharchach, H. Ighachane, A. Rochdi, M. A. Ali and H. B. Lazrek, Org. Prep. Proced. Int., 2021, 53, 268–277 CrossRef CAS.
- C. Kathing, N. G. Singh, R. Nongrum and R. Nongkhlaw, Russ. J. Org. Chem., 2020, 56, 1628–1634 CrossRef CAS.
- M. Karthik and P. Suresh, New J. Chem., 2018, 42, 17931–17938 RSC.
- D. Zareyee, S. R. Tuyehdarvary, L. Allahgholipour, Z. Hossaini and M. A. Khalilzadeh, Synlett, 2016, 27, 1251–1254 CrossRef CAS.
- A. Pramanik, R. Roy, S. Khan, A. Ghatak and S. Bhar, Tetrahedron Lett., 2014, 55, 1771–1777 CrossRef CAS.
- N. Thimmaraju, S. Z. M. Shamshuddin, S. R. Pratap, K. Raja, M. Shyamsundar and T. F Mohankumar, Synth. Commun., 2016, 46, 1537–1559 CrossRef CAS.
- W. Senapak, R. Saeeng, J. Jaratjaroonphong, V. Promarak and U. Sirion, Tetrahedron, 2019, 75, 3543–3552 CrossRef CAS.
- D. Raja, A. Philips, P. Palani, W.-Y. Lin, S. Devikala and G. C. Senadi, J. Org. Chem., 2020, 85, 11531–11540 CrossRef CAS PubMed.
- F. P. Roudsari, M. Seddighi, F. Shirini and H. Tajik, Org. Prep. Proced. Int., 2020, 52, 340–353 CrossRef CAS.
- P. Ghosh and R. Subba, Tetrahedron Lett., 2015, 56, 2691–2694 CrossRef CAS.
- Y. Nagasawa, Y. Matsusaki, T. Hotta, T. Nobuta, N. Tada, T. Miura and A. Itoh, Tetrahedron Lett., 2014, 55, 6543–6546 CrossRef CAS.
- Q. Sun, C.-J. Wang, S.-S. Gong, Y.-J. Ai and H.-B. Sun, Chin. Chem. Lett., 2015, 26, 297–300 CrossRef CAS.
- S. Sajjadifar, Z. Arzehgar and A. Ghayuri, J. Chin. Chem. Soc., 2018, 65, 205–211 CrossRef CAS.
- D. B. Nale and B. M. Bhanage, Synlett, 2015, 26, 2835–2842 CrossRef CAS.
- G. R. Bardajee, M. Mohammadi and N. Kakavand, Appl. Organomet. Chem., 2016, 30, 51–58 CrossRef CAS.
- B. Agrahari, S. Layek, R. Ganguly, N. Dege and D. D. Pathak, J. Organomet. Chem., 2019, 89, 13–20 CrossRef.
- G. M. Martins, T. Puccinelli, R. A. Gariani, F. R. Xavier, C. C. Silveira and S. R. Mendes, Tetrahedron Lett., 2017, 58, 1969–1972 CrossRef CAS.
- C. S. Digwal, U. Yadav, A. P. Sakla, P. V. Sri Ramya, S. Aaghaz and A. Kamal, Tetrahedron Lett., 2016, 57, 4012–4016 CrossRef CAS.
- J. Azizian, P. Torabi and J. Noei, Tetrahedron Lett., 2016, 57, 185–188 CrossRef CAS.
- V. N. Mahire and P. P. Mahulikar, Chin. Chem. Lett., 2015, 26, 983–987 CrossRef CAS.
- G.-Y. Bai, X.-W. Lan, G.-F. Chen, X.-F. Liu, T.-Y. Li and L.-J. Shi, Ultrason. Sonochem., 2014, 21, 520–526 CrossRef CAS PubMed.
- H. Sharghi, M. Aberi, M. M. Doroodmand and P. Shiri, J. Iran. Chem. Soc., 2017, 14, 1557–1573 CrossRef CAS.
- E. Soleimani and M. M. Khodaei, J. Iran. Chem. Soc., 2015, 12, 1281–1285 CrossRef CAS.
- A. Shaikh, O. Ravi, P. S. Ragini, N. Sadhana and S. R. Bathula, Tetrahedron Lett., 2020, 151356 CrossRef CAS.
- Y. Merroun, S. Chehab, T. Ghailane, M. Akhazzane, A. Souizi and R. Ghailane, React. Kinet., Mech. Catal., 2019, 126, 249–264 CrossRef CAS.
- V. Sankar, P. Karthik, B. Neppolian and B. Sivakumar, New J. Chem., 2020, 44, 1021–1027 RSC.
- C. Vallés-García, M. Cabrero-Antonino, S. Navalón, M. Álvaro, A. Dhakshinamoorthy and H. García, J. Colloid Interface Sci., 2020, 560, 885–893 CrossRef PubMed.
- C. Zhou, J. Lei, Y. Liu, C.-T. Au, Y. Chen and S.-F. Yin, Appl. Organomet. Chem., 2020, 34, e5881 CrossRef CAS.
- R. S. Mekala, S. K. Balam, J. P. S Harinath, R. R. Gajjal and S. R. Cirandur, Cogent Chem., 2015, 1, 1049932 CrossRef.
- M. Bala, P. K. Verma, D. Sharma, N. Kumar and B. Singh, Mol. Diversity, 2015, 19, 263–272 CrossRef CAS PubMed.
- R. K. Mahato, P. K. Mudi, M. Deb, B. Biswas and B. Biswas, Asian J. Org. Chem., 2021, 10, 2954–2963 CrossRef.
- V. Elumalai and J. H. A. Hansen, Synlett, 2020, 31, 547–552 CrossRef CAS.
- A. K. Chaturvedi, A. K. Verma, J. P. Thakur, S. Roy, S. B. Tripathi, B. S. Kumar, S. Khwaja, N. K. Sachan, A. Sharma, D. Chanda, K. Shanker, S. Saikia and A. S. A. Negi, Bioorg. Med. Chem., 2018, 26, 4551–4559 CrossRef CAS PubMed.
- C. Sutapin and S. Chirachanchai, Synth. Commun., 2018, 48, 650–655 CrossRef CAS.
- K. Prabakaran, S. Loganathan and M. S. Perumal, J. Heterocycl. Chem., 2021, 58, 340–349 CrossRef.
- S. Gulati, R. Singh, S. Sangwan and S. Rana, J. Iran. Chem. Soc., 2021, 18, 167–179 CrossRef CAS.
- V. R. Kadu, H. V. Chavan and S. S. Gholap, Polycyclic Aromat. Compd., 2021, 41, 1263–1273 CrossRef CAS.
- J. Kovvuri, B. Nagaraju, A. Kamal and A. K. Srivastava, ACS Comb. Sci., 2016, 18, 644–650 CrossRef CAS PubMed.
- Y. Shi, K. Jiang, R. Zheng, J. Fu, L. Yan, Q. Gu, Y. Zhang and F. Lin, Chem. Biodiversity, 2019, 16, e1800510 CrossRef PubMed.
- A. P. Tayade and R. P. Pawar, Polycyclic Aromat. Compd., 2022, 42, 1474–1478 CrossRef CAS.
- H. Naeimi and Z. Babaei, J. Chin. Chem. Soc., 2015, 62, 41–46 CrossRef CAS.
- Z. Benzekri, H. Serrar, S. Sibous, S. Boukhris, A. Ouasri, A. Rhandour and A. Souizi, Green Chem. Lett. Rev., 2016, 9, 223–228 CrossRef CAS.
- M. L. P. R. Alapati, S. R. Abburi, S. B. Mukkamala and M. K. Rao, Synth. Commun., 2015, 45, 2436–2443 CrossRef CAS.
- P. V. S. Ramya, S. Angapelly, R. S Rani, C. S. Digwal, C. G. Kumar, B. N. Babu, L. Guntuku and A. Kamal, Arabian J. Chem., 2020, 13, 120–133 CrossRef.
- S. H. Nile, B. Kumar and S. W. Park, Arabian J. Chem., 2015, 8, 685–691 CrossRef CAS.
- H. Kottayil, S. Machingal, S. M. B. Parackal, S. M. Alungal, L. V. Theresa, A. Govindan and S. Krishnapillai, J. Heterocycl. Chem., 2020, 57, 3310–3317 CrossRef CAS.
- A. A. Akande, U. Salar, K. M. Khan, S. Syed, S. A. Aboaba, S. Chigurupati, A. Wadood, M. Riaz, M. Taha, S. Bhatia, Kanwal, S. Shamim and S. Perveen, ACS Omega, 2021, 6, 22726–22739 CrossRef CAS PubMed.
- J. Tian, S. Yuan, F. Xiao, H. Huang and G.-J. Deng, RSC Adv., 2019, 9, 30570–30574 RSC.
- Z. Hu, T. Zhao, M. Wang, J. Wu, W. Yu and J. Chang, J. Org. Chem., 2017, 82, 3152–3158 CrossRef CAS PubMed.
- P. Daw, Y. Ben-David and D. Milstein, ACS Catal., 2017, 7, 7456–7460 CrossRef CAS.
- A. K. Sharma, H. Joshi, R. Bhaskar and A. K Singh, Dalton Trans., 2017, 46, 2228–2237 RSC.
- M. Mogharabi-Manzari, M. Kiani, S. Aryanejad, S. Imanparast, M. Amini and M. A. A. Faramarzi, Adv. Synth. Catal., 2018, 360, 3563–3571 CrossRef CAS.
- Z.-G. Wang, X.-H. Cao, Y. Yang and M. Lu, Synth. Commun., 2015, 45, 1476–1483 CrossRef CAS.
- M. R. Marri, S. Peraka, A. K. Macharla, N. Mameda, S. Kodumuri and N. Nama, Tetrahedron Lett., 2014, 55, 6520–6525 CrossRef CAS.
- M. Zuo, W. Guo, Y. Pang, R. Guo, C. Hou, S. Sun, H. Wu, Z. Sun and W. Chu, New J. Chem., 2020, 44, 14490–14495 RSC.
- G. Naresh, R. Kant and T. Narender, J. Org. Chem., 2014, 79, 3821–3829 CrossRef CAS PubMed.
- C.-P. Dong, Y. Higashiura, K. Marui, S. Kumazawa, A. Nomoto, M. Ueshimaand and A. Ogawa, ACS Omega, 2016, 1, 799–807 CrossRef CAS PubMed.
- J. Yu, Y. Xia and M. Lu, Synth. Commun., 2014, 44, 3019–3026 CrossRef CAS.
- A. Vasu, R. Naresh, G. K. Rai, Y. D. Rohini, B. Murali, M. Ramulamma, A. Ramunaidu and N. A. Narender, Green Chem., 2021, 23, 9439–9446 RSC.
- A. Bermejo-López, S. Carrasco, P. J. Tortajada, K. P. M. Kopf, A. Sanz-Marco, M. S. Hvid, N. Lock and B. Martín-Matute, ACS Sustainable Chem. Eng., 2021, 9, 14405–14415 CrossRef.
- L.-S. Liu, Z.-B. Xie, C. Zhang, L.-H. Fu, H.-B. Zhu and Z.-G. Le, Green Chem. Lett. Rev., 2018, 11, 503–507 CrossRef CAS.
- C. Dayakar, D. Jyothi, P. Suman and B. C. Raju, Synth. Commun., 2015, 45, 1642–1651 CrossRef CAS.
- S. Majumdar, A. Chakraborty, S. Bhattacharjee, S. Debnath and D. K. Maiti, Tetrahedron Lett., 2016, 57, 4595–4598 CrossRef CAS.
- A. Chakraborty, S. Majumdar and D. K. Maiti, Tetrahedron Lett., 2016, 57, 3298–3302 CrossRef CAS.
- B. Deb, A. Chakraborty, J. Hossain and S. A. Majumdar, SynOpen, 2020, 04, 89–95 CrossRef CAS.
- N. Man, Z. Lou, Y. Li, Y. Yang, Y. Zhao and H. Fu, Org. Lett., 2020, 22, 6382–6387 CrossRef CAS PubMed.
- P. Basuri, L. E. Gonzalez, N. M. Morato, T. Pradeep and R. G. Cooks, Chem. Sci., 2020, 11, 12686–12694 RSC.
- O. Castillo-Aguilera, P. Depreux, A. Ballée, F. Beaurain, P. B. Arimondo and L. Goossens, Synlett, 2020, 31, 1216–1220 CrossRef CAS.
- V. B. Saptal, T. Sasaki and B. M. Bhanage, ChemCatChem, 2018, 10, 2593–2600 CrossRef CAS.
- R. Khatun, S. Biswas, I. H. Biswas, S. Riyajuddin, N. Haque, K. Ghosh and S. M. Islam, J. CO2 Util., 2020, 40, 101180 CrossRef CAS.
- W. Zhao, H. Li, Y. Li, J. Long, Y. Xu and S. Yang, Sustainable Chem. Pharm., 2020, 17, 100276 CrossRef.
- H. Huang, X. Lin, S. Yen and C. Liang, Org. Biomol. Chem., 2020, 18, 5726–5733 RSC.
- Z. Gan, Q. Tian, S. Shang, W. Luo, Z. Dai, H. Wang, D. Li, X. Wang and J. Yuan, Tetrahedron, 2018, 74, 7450–7456 CrossRef CAS.
- A. Shaabani and Z. Hezarkhani, Appl. Organomet. Chem., 2017, 31, e3542 CrossRef.
- S. Yaragorla and P. V. Babu, Tetrahedron Lett., 2017, 58, 1879–1882 CrossRef CAS.
- A. Garrido, P. Delaye, F. Quintin, M. Abarbri, P. Lameiras, A. Gueiffier, J. Thibonnet and J. Petrignet, Synthesis, 2019, 51, 4006–4013 CrossRef CAS.
- C. Duangkamol, W. Phakhodee and M. Pattarawarapan, Synthesis, 2020, 52, 1981–1990 CrossRef CAS.
- M. Kasthuri, H. Sharath Babu, K. S. Kumar, C. Sudhakar and P. V. N. Kumar, Synlett, 2015, 26, 897–900 CrossRef CAS.
- Y. Xia, L. Lin, F. Chang, Y. Liao, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 12228–12232 CrossRef CAS PubMed.
- T. Rapolu, K. V. P. Kumar, K. R. Babu, S. K. Dende, R. R. Nimmareddy and L. K. Reddy, Synth. Commun., 2019, 49, 1308–1315 CrossRef CAS.
- C. Li, F. Zhang, Z. Yang and C. Qi, Tetrahedron Lett., 2014, 55, 5430–5433 CrossRef CAS.
- J. Liu, S. Morgan and J. Hoover, ChemCatChem, 2019, 12, 1297–1301 CrossRef.
- X. Zhu, F. Zhang, D. Kuang, G. Deng, Y. Yang, J. Yu and Y. Liang, Org. Lett., 2020, 22, 3789–3793 CrossRef CAS PubMed.
- A. I. Almansour, R. S. Kumar and N. A. Arumugam, J. King Saud Univ., Sci., 2020, 32, 3153–3158 CrossRef.
- J. Yu and M. Lu, Synth. Commun., 2015, 45, 2148–2157 CrossRef CAS.
- P. Thapa, P. M. Palacios, T. Tran, B. S. Pierce and F. W. Foss, J. Org. Chem., 2020, 85, 1991–2009 CrossRef CAS PubMed.
- Y. Ma, R. Xiong, Y. Feng, X. Zhang and Y. Xiong, Tetrahedron, 2020, 76, 131474 CrossRef CAS.
- S. V. Patil, S. S. Patil and V. D. Bobade, Arabian J. Chem., 2016, 9, 5515–5521 Search PubMed.
- H. Naeimi and N. Alishahi, J. Ind. Eng. Chem., 2014, 20, 2543–2547 CrossRef CAS.
- A. Arya, V. Mishra and T. S. Chundawat, Chem. Data Collect., 2019, 20, 100190 CrossRef CAS.
- M. Özil, C. Parlak and N. Baltaş, Bioorg. Chem., 2018, 76, 468–477 CrossRef PubMed.
- P. A. Rodríguez-Huerto, D. Peña-Solórzano and C. Ochoa-Puentes, Chem. Pap., 2021, 75, 6275–6283 CrossRef.
- V. Kumar, B. Poojary, A. Prathibha and N. Shruthi, Synth. Commun., 2014, 44, 3414–3425 CrossRef CAS.
- T. B. Nguyen and L. E. A. Al-Mourabit, Synthesis, 2015, 47, 1741–1748 CrossRef CAS.
- X. Li, R. Hu, Y. Tong, Q. Pan, D. Miao and S. Han, Tetrahedron Lett., 2026, 57, 4645–4649 CrossRef.
- R. R. Putta, S. Chun, S. H. Choi, S. B. Lee, D.-C. Oh and S. Hong, J. Org. Chem., 2020, 85, 15396–15405 CrossRef CAS PubMed.
- F. Feng, J. Ye, Z. Cheng, X. Xu, Q. Zhang, L. Ma, C. Lu and X. Li, RSC Adv., 2016, 6, 72750–72755 RSC.
- L.-P. Duan, Q. Li, N. B. Wu, D.-F. Xu and H.-B. Zhang, Chin. Chem. Lett., 2014, 25, 155–158 CrossRef CAS.
- K. B. Rasal and Y. D. Yadav, Catal. Today, 2018, 309, 51–60 CrossRef CAS.
- V. A. Mamedov, N. A. Zhukova, A. T. Gubaidullin, V. V. Syakaev, M. S. Kadyrova, T. N. Beschastnova, O. B. Bazanova, I. K. Rizvanov and S. K. Latypov, J. Org. Chem., 2018, 83, 14942–14953 CrossRef CAS PubMed.
- T. N. Beschastnova, D. N. Buzyurova, I. K. Rizvanov, S. K. Latypov and O. G. Sinyashin, J. Org. Chem., 2019, 84, 13572–13581 CrossRef PubMed.
- D. Mahesh, P. Sadhu and T. Punniyamurthy, J. Org. Chem., 2015, 80, 1644–1650 CrossRef CAS PubMed.
- Z. Chen, H. Li, G. Cao, J. Xu, M. Miao and H. Ren, Synlett, 2017, 28, 504–508 CrossRef CAS.
- Z. Xie, F. Zhou and K. Ding, Adv. Synth. Catal., 2020, 362, 3442–3446 CrossRef CAS.
- S. Behrouz, J. Saudi Chem. Soc., 2018, 22, 261–268 CrossRef CAS.
- P. J. Boratyński, A. E. Nowak and J. Skarżewski, Synthesis, 2015, 47, 3797–3804 CrossRef.
- M. Li, Y. Tang, H. Gao, G. Rao and Z. Mao, Asian J. Org. Chem., 2020, 9, 1027–1031 CrossRef CAS.
- X. Zhang, R. Huang, J. Marrot, V. Coeffard and Y. Xiong, Tetrahedron, 2015, 71, 700–708 CrossRef CAS.
- N. H. Ansari and B. C. G. Söderberg, Tetrahedron Lett., 2017, 58, 4717–4720 CrossRef CAS PubMed.
- S. Joardar, A. Bhattacharyya and S. Das, Synthesis, 2014, 46, 3121–3132 CrossRef CAS.
- S. Maitia and P. Mala, Adv. Synth. Catal., 2015, 357, 1416–1424 CrossRef.
- D. Yu, Q. You, X. Zhang, G. Tao and W. Zhang, Appl. Organomet. Chem., 2016, 30, 695–698 CrossRef CAS.
- B. V. Shaik, M. Seelam, R. Tamminana and P. R. Kammela, Tetrahedron, 2019, 75, 3865–3874 CrossRef CAS.
- B. Mirza and M. Zeeb, Synth. Commun., 2014, 45, 524–530 CrossRef.
- X. Liu, H. Cao, F. Bie, P. Yan and Y. Han, Tetrahedron Lett., 2019, 60, 1057–1059 CrossRef CAS.
- S. Kurhade, A. Rossetti and A. Dömling, Synthesis, 2016, 48, 3713–3718 CrossRef CAS.
- S. A. I. Quadri, T. C. Das and M. Farooqui, ChemistrySelect, 2017, 2, 1802–1807 CrossRef CAS.
- Z.-Z. Chen, Y. Tang, L. Zuo, D.-Y. Tang, J. Zhang and Z.-G. Xu, Synlett, 2014, 25, 2518–2520 CrossRef CAS.
- M. Saha and A. R. Das, Tetrahedron Lett., 2018, 59, 2520–2525 CrossRef CAS.
- H. Liu, J. Tang, L. Jiang, T. Zheng, X. Wang and X. Lv, Tetrahedron Lett., 2015, 56, 1624–1630 CrossRef CAS.
- D. D. Park, K. H. Min, J. Kang, H. S. Hwang, V. K. Soni, C.-G. Cho and e. j. Cho, Org. Lett., 2020, 22, 1130–1134 CrossRef CAS PubMed.
- F. Bie, Y. Yao, H. Cao, Y. Shi, P. Yan, J. Ma, Y. Han and X. Liu, Synth. Commun., 2021, 51, 2387–2396 CrossRef CAS.
- P.-Y. Chen, C.-H. Hsu, T.-Y. Ho and J.-H. Ho, RSC Adv., 2021, 11, 6662–6666 RSC.
- T. Xu, Y. Zhang, L. Yi, W. Lu, Z. Chen and X.-F. Wu, Mol. Catal., 2011, 513, 111767 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |