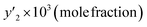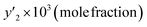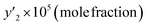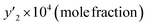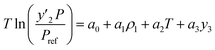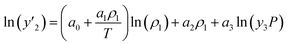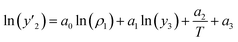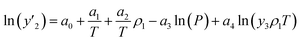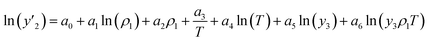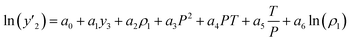Open Access Article
Open Access ArticleImproving and measuring the solubility of favipiravir and montelukast in SC-CO2 with ethanol projecting their nanonization
Adrián Rojasab,
Seyed Ali Sajadian *c,
Carol López-de-Dicastillod,
Nedasadat Saadati Ardestanie,
Gonzalo Aguila
*c,
Carol López-de-Dicastillod,
Nedasadat Saadati Ardestanie,
Gonzalo Aguila f and
Abolghasem Jouybangh
f and
Abolghasem Jouybangh
aPackaging Innovation Center (LABEN), Department of Science and Food Technology, Faculty of Technology, University of Santiago of Chile (USACH), Obispo Umaña 050, Santiago 9170201, Chile
bCenter for the Development of Nanoscience and Nanotechnology (CEDENNA), Santiago 9170124, Chile
cDepartment of Chemical Engineering, Faculty of Engineering, University of Kashan, 87317-53153, Kashan, Iran. E-mail: seyedali.sajadian@gmail.com
dPackaging Laboratory, Institute of Agrochemistry and Food Technology IATA-CSIC, Av. Agustín Escardino 7, 46980 Paterna, Spain
eNanotechnology Research Center, Research Institute of Petroleum Industry (RIPI), P.O. Box: 14857-336, Tehran, Iran
fDepartamento de Ciencias de la Ingeniería, Facultad de Ingeniería, Universidad Andres Bello, Antonio Varas 880, Santiago, Chile
gPharmaceutical Analysis Research Center and Faculty of Pharmacy, Tabriz University of Medical Sciences, Tabriz, Iran
hPharmaceutical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
First published on 22nd November 2023
Abstract
Supercritical carbon dioxide (SC-CO2)-based approaches have become more popular in recent years as alternative methods for creating micro- or nanosized medicines. Particularly, high drug solubility is required in those techniques using SC-CO2 as a solvent. During the most recent pandemic years, favipiravir and montelukast were two of the most often prescribed medications for the treatment of COVID-19. In this study, ethanol at 1 and 3 mol% was utilized as a cosolvent to increase the solubility of both medicines in SC-CO2 by a static approach using a range of temperatures (308 to 338 K) and pressure (12 to 30 MPa) values. The experimentally determined solubilities of favipiravir and montelukast in SC-CO2 + 3 mol% ethanol showed solubility values up to 33.3 and 24.5 times higher than that obtained for these drugs with only SC-CO2. The highest values were achieved in the pressure of 12 MPa and temperature of 338 K. Last but not least, six density-based semi-empirical models with various adjustable parameters were used to perform the modeling of the solubility of favipiravir and montelukast.
1. Introduction
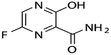
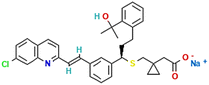
Favipiravir and montelukast are two drugs whose interest has increased principally during last years because of the fact that they can be used as potential adjuvant treatments for coronavirus (COVID-19). COVID-19 is a SARS-CoV-2 virus infection, and some infected patients can become seriously ill and require medical attention. In this way, it is essential to continue looking for alternative treatments and drugs for combating this infection. Favipiravir was one of the oral drugs approved for re-emerging pandemic influenza in Japan in 2014, and recently it has demonstrated strong in vitro antiviral efficacy against the coronavirus that causes severe acute respiratory syndrome.1 Favipiravir (prodrug) is a selective and potent inhibitor of viral RNA-dependent RNA polymerase.2 On the other hand, montelukast, an oral selective leukotriene receptor antagonist, is very successful in treating chronic asthma and some COVID-19 symptoms because it inhibits cysteinyl leukotriene.3A drug's bioavailability, which is closely related to its solubility, determines how effectively it treats a patient. Therefore, a drug's solubility and rate of dissolution are crucial factors in determining how effective it is the drug treatment. One of the most crucial factors in achieving the correct drug concentration in the systemic circulation for the pharmacological response is solubility. Nevertheless, most drugs present poor solubility, and it is recognized that in the pharmaceutical industry more than 40% of newly discovered drugs show the same handicap.4 Several approaches are employed to improve their bioavailability, and solubility studies and prediction tools have become a valuable information to optimize their reformulation processes. In this context, solutions taking into account the encapsulation and micronization of particles and drug delivery systems are being offered by the supercritical fluids technology.5–7 Specifically, SC-CO2 is considered a sustainable solvent as it has moderate critical parameters, is cheap, harmless, incombustible and can be recycled. Furthermore, carbon dioxide (CO2) is a gas under atmospheric conditions that is easily removed from the material by simple depressurization and leaves no residue. Several life cycle assessment studies have shown the use of supercritical water and CO2-based methods as sustainable processes to produce materials.8,9 Comparing to conventional processes, the use of SC-CO2 can entail da positive impact on environmental preservation, but also on economic gains, thanks to the reduction of processes time and energy expenditure, the water-free and effluent-free processes and the reduction of CO2 emission and auxiliary chemicals.10
Specifically in the use of this technology in the development of drug delivery systems, a crucial factor in the creation of micro/nano-sized drug particles in the pharmaceutical sector is the solubility of medicinal compounds in SC-CO2. Recently, data about the solubility of many pharmaceuticals in SC-CO2 have been measured experimentally and published in the literature.11,12 At pressures between 12 and 30 MPa and temperatures between 308 and 338 K, the mole fraction solubilities of favipiravir and montelukast have resulted in a range of 3.0 × 10−6 to 9.05 × 10−4 (ref. 13) and 0.4 × 10−6 to 6.12 × 10−5,14 respectively. Several studies have demonstrated that a strategy for improving these values is by incorporating a polar or non-polar cosolvent that can increase the solvating potential of SC-CO2 perhaps lowering the operating pressure.15–41 A selection of some relevant studies on improving the solubility of solutes in ternary systems (SC-CO2 + cosolvent + solute) are included in Table 1. In a recent study, Sodeifian et al. found that employing menthol as a cosolvent increased the solubility of ketoconazole in SC-CO2 at 308–338 K and 12–30 MPa from a range value between 2.00 × 10−7 and 8.02 × 10−5 to the drug solubility values of 1.20 × 10−5 to 1.96 × 10−4.42 Huang et al. have also improved the aspirin solubility in SC-CO2 by five times by incorporating acetone as a cosolvent.43 Particularly, ethanol has been one of the most commonly used polar cosolvents for the solute processing (drugs and nutraceuticals)44–46 and for the extraction of bioactive substances from plant materials47–49 due to its high solubility in SC-CO2 at moderate pressure and temperatures, low toxicity and capacity to interact with polar solutes by hydrogen bonding.
| Compound | Cosolvent | Pressure range (MPa) | Temperature range (K) | Range of solubility (×105) | References |
|---|---|---|---|---|---|
| Capecitabine | Methanol 6 mol% | 10–35 | 308–348 | 3.18–120.29 | 15 |
| Ethanol 6 mol% | 0.64–71.9 | ||||
| Dimethyl sulfoxide 6 mol% | 0.85–94.8 | ||||
| Anthraquinone Violet 3RN | Methanol 6 mol% | 10–34 | 308–338 | 0.44–5.77 | 16 |
| Ammonium benzoate | Ethanol 2% molar | 11–21 | 318 | 2.33–10.63 | 17 |
| Acetone 2 mol% | 2.15–8.85 | ||||
| Ethylene glycol 2 mol% | 4.27–6.92 | ||||
| Cinnamic acid | Ethanol 2 and 4 mol% | 10–40 | 313 | 23–81 | 18 |
| Disperse yellow 119 | Ethanol 0–5 mol% | 15–30 | 353 and 393 | 0.010–3.23 | 19 |
| Disperse red 82 | 0.064–110 | ||||
| Benzamide | Ethanol 3.5 mol% | 11–21 | 318 | 5.79–74.83 | 20 |
| Acetone 3.5 mol% | 4.93–33.99 | ||||
| Ethylene glycol 3.5 mol% | 5.07–24.03 | ||||
| Ketoconazole | Menthol (mass ratio 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to ketoconazole) 2 to ketoconazole) |
12–30 | 308–338 | 2.7–1.96 | 21 |
| Phenylphosphinic acid | Methanol 1 and 4 mol% | 10–20 | 313 and 323 | 2.8–292 | 22 |
| Trioctylmethylammonium chloride | n-hexane 1.05–4.20 mol% | 10–30 | 313 and 323 | 1.8–15.3 | 23 |
| Aspirin | Stearic acid (mass ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to aspirin) 1 to aspirin) |
10–20 | 308–328 | 50.5–443.9 | 24 |
| Silymarin | Ethanol 2 mol% | 8–22 | 308–338 | 3.89–60.12 | 25 |
| Acetone 2 mol% | 5.17–101.37 | ||||
| Dichloromethane 2 mol% | 2.99–40.33 | ||||
| Nitrenpidine | Ethanol 1–7 mol% | 10–20 | 308–318 | 0.18–12.55 | 26 |
| Ferulic acid | Ethanol 5.37–10.92 mol% | 20–40 | 313–333 | 18.9–165 | 27 |
| Curcumin | Ethanol 1–5 mol% | 8–16 | 318 | 0.048–0.197 | 28 |
| Glycyrrhizin | Ethanol 2 and 4 mol% | 9–21 | 308–328 | 0.261–5.430 | 29 |
| Dexamethasone | Ethanol 3 mol% | 10–25 | 313–323 | 0.197–0.298 | 30 |
| Lutein | Ethanol 0.0211 mol mol−1 | 18.70–33.55 | 313–333 | 0.402 | 31 |
| Vitamin E acetate | Ethanol 0.5–2 mol% | 10.99–11.13 | 318 | 38.4–69.0 | 32 |
| Caffeic acid | Ethanol 2.2–10.2 mol% | 20–40 | 313–333 | 5.8–9.1 | 33 |
| 3-Aminobenzoic acid | Ethanol 2–4 mol% | 10–21 | 308–328 | Enhancement between 1.02–2.55 times | 34 |
| o-Nitrobenzoic acid | Ethanol 3.5 mol% | 10–21 | 308–328 | 0.374–3.561 | 35 |
| Ethyl acetate 3.5 mol% | 0.220–1.842 | ||||
| Capsanthin | Triolein 0.16 and 0.41 mmol mol−1 | 19–34 | 313–333 | 0.65–1.97 | 36 |
| o-tolidine | Ethanol 0.01–0.04 mol% | 11–21 | 308–328 | 1.01–5.99 | 37 |
| Ethylene glycol 0.01–0.04 mol% | 1.11–4.25 | ||||
| Benzene sulfonamide | Ethanol 3.5 mol% | 11–21 | 308–328 | 14.9–21.3 | 38 |
| Ethylene glycol 3.5 mol% | 25.1–42.4 | ||||
| Ethyl acetate 3.5 mol% | 18.9–35.3 | ||||
| Acetaminophen | Menthol | 10–25 | 313–343 | 1.44–24.91 | 39 |
| Rhodamine B | Methanol 5 mol% | 8–24 | 308–318 | 0.003372–0.076674 | 40 |
| Clozapine | Menthol 8.8 mol% | 12.3–33.7 | 313–323 | 18.8–44.8 | 41 |
| Lamotrigine | 313–323 | 0.9–3.6 |
In the current work, ethanol was used for the first time as a cosolvent approved for pharmaceutical applications with the aim of improving the solubility of favipiravir and montelukast in SC-CO2 and promoting further research dealing with their nanonization through techniques using SC-CO2 as a solvent, such as rapid expansion of supercritical fluid solutions (RESS). In this study ethanol was used at concentrations of 1 and 3 mol% when measuring the solubilities of favipiravir and montelukast in SC-CO2 at various pressures and temperatures (308–338 K) (12–30 MPa). Additionally, six density-based semi-empirical models, namely; MST,50 Sodeifian–Sajadian,51 González et al.,52 Soltani–Mazloumi,53 Garlapati–Madras,54 and Jouyban et al.,55 with four to seven adjustable parameters were used to correlate the solubilities of favipiravir and montelukast in both studied ternary systems. The results obtaining by modeling the solubility of these drugs in SC-CO2 is orientated to facilitate the development of nanodrugs formulation processes, with the consequent saving of time and resources.
2. Experimental
2.1. Materials
Montelukast (CAS No. 158966-92-8) and favipiravir (CAS No. 259793-96-9) were bought from Arasto Pharmaceutical Chemicals Co. (Tehran, Iran). Aboughadareh Co. supplied carbon dioxide (CO2) with a purity of 99.99% (CAS Number 124-38-9). (Tehran, Iran). Merck provided the methanol with a minimum purity (GC) of 99.9% and the ethanol (99.0% purity) with the CAS number 67-56-1 (Germany). The main properties of the chemicals employed in this study, which were all used without further purification, are listed in Table 2.| Material | Source | Initial mass fraction purity | Purification method | Final mass fraction purity | Analysis method |
|---|---|---|---|---|---|
| a Gas chromatography. | |||||
| Favipiravir | Arasto pharmaceutical Co. | 0.99 | None | 0.99 | HPLC |
| Montelukast | Arasto pharmaceutical Co. | 0.99 | None | 0.99 | HPLC |
| Ethanol | Merck Co. | 0.99 | None | 0.99 | GCa |
| Methanol | Merck Co. | 0.999 | None | 0.999 | GC |
| CO2 | Aboughadareh Co. | 0.9999 | None | 0.9999 | GC |
2.2. System for solubility determination
For the experiments carried out in this work, laboratory setup composed of different parts and equipment was used. This equipment is shown in Fig. 1 and includes a table describing its main components. The equilibrium cell, piping, and valves are constructed from 316 stainless steel and intended to operate under high pressure (40 MPa). At the beginning of the process, CO2 enters the refrigeration stage to liquefy the CO2 (258.15 K) and later goes through filtering (1 μm). The liquid CO2 at 6 MPa is transferred using a high-pressure pump (air-driven liquid pump, type M64, Shineeast business). A pressure transmitter and a pressure gauge are used to measure the pressure with a 0.1 MPa accuracy (WIKA, Germany). To maintain a consistent operating temperature (0.1 K), the equilibrium cell was placed within a high-precision furnace (Memmert GmbH, Germany). In the equilibrium cell, 3000 mg of the drug and a specific amount of ethanol (1 or 3 mol%) are introduced as a cosolvent and mixed through a magnetic stirrer (100 rpm). At the ends of the equilibrium cell, sintered stainless steel filters were positioned to prevent the physical transfer of undissolved drug powder. More details of this laboratory equipment for experiments can be found in previous works.56,57 Subsequently, the liquid CO2 is introduced into the equilibrium cell until operating pressure is reached, and 90 min is considered for the system to reach equilibrium. After that, a 3-valve allows SC-CO2 to be pumped from the equilibrium cell into a 300 μL sample loop. The sampling loop is depressurized into liquid ethanol using a micrometer valve that prevents ethanol from spraying out of the vessel. A syringe is used to introduce methanol into the sampling loop at the conclusion of the procedure. The volume of the final solution obtained in the collection vial corresponds to 5 mL. For each data point, this process is repeated three times.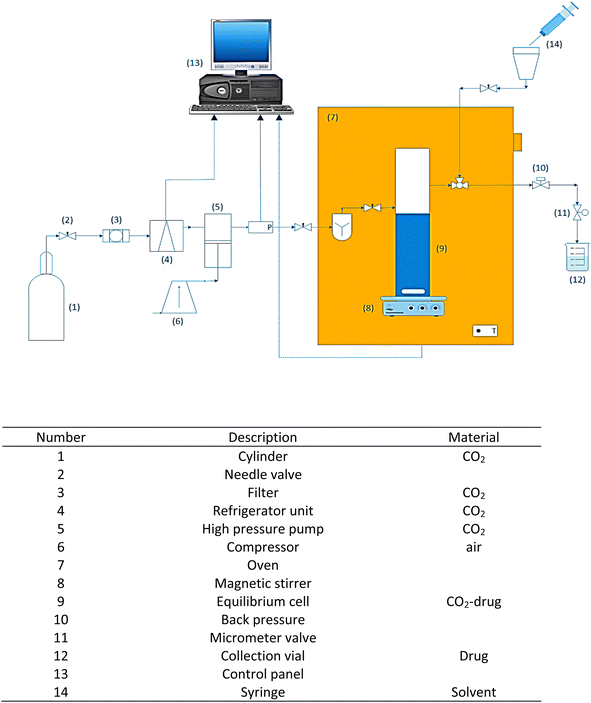 | ||
| Fig. 1 Schematic diagram of experimental apparatus for measuring favipiravir and montelukast solubility. | ||
The solubility of the drugs, obtained under different operating conditions of the previous process, is measured with a spectrophotometer (PerkinElmer), with quartz cells and a 3 cm path length. The amount of drug contained in the final solution, disposed in the collection vial, is measured using calibration curves. The concentration of drugs is analyzed with UV absorption analysis at maximum lambda.
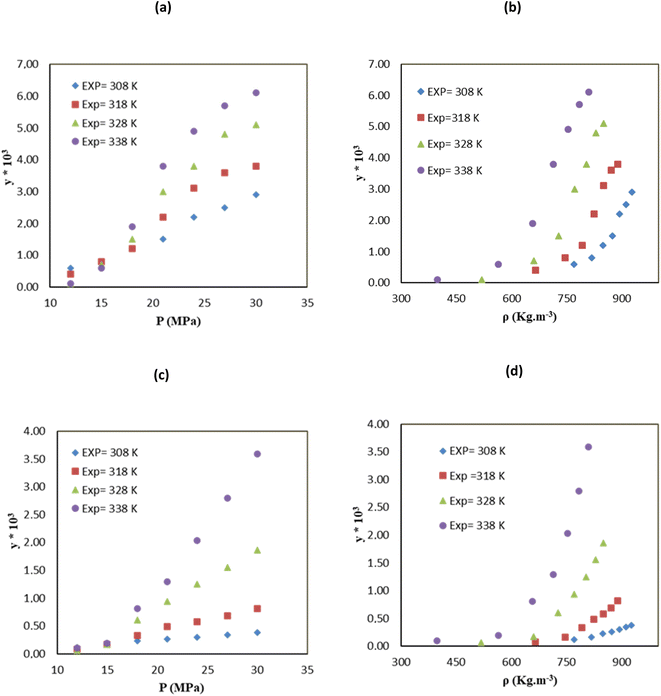
The following equations were used to calculate the equilibrium solubility of the drugs in SC-CO2 at all pressure and temperature ranges, including equilibrium molar fraction (y2) and equilibrium solubility S (g L−1):
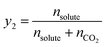 | (1) |
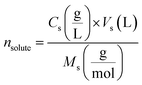 | (2) |
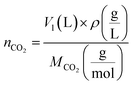 | (3) |
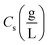 indicates the solute concentration in the collection vial as calculated by the calibration curve, Vs (L) and Vl (L) show the collection vial and sample loop volumes, and
indicates the solute concentration in the collection vial as calculated by the calibration curve, Vs (L) and Vl (L) show the collection vial and sample loop volumes, and 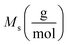 and
and 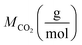 reflect the solute's and CO2's molecular weights, respectively. Eqn (4) is produced by adding eqn. (2) and (3) to eqn (1):
reflect the solute's and CO2's molecular weights, respectively. Eqn (4) is produced by adding eqn. (2) and (3) to eqn (1):
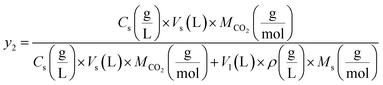 | (4) |
Eqn (5) also yielded the equilibrium solubility, S (g L−1), of the solute in SC-CO2.
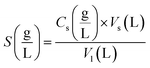 | (5) |
Information on API's physical characteristics can be found in Table 3. The National Institute of Standards and Technology (NIST) database was used to determine the density (ρ) for SC-CO2 at various temperatures and pressures.
3. Theoretical background
According to Mendez-Santiago–Teja (MST),50 Sodeifian–Sajadian,51 González et al.,52 Soltani–Mazloumi,53 Garlapati and Madras,54 and, Jouyban et al.,55 empirical models based on density were utilized in this study. These empirical models allowed to correlate the experimentally obtained solubility data. In addition, the constants of these empirical models were obtained through a regression of the experimental data. Finally, using simulated annealing (MATLAB®), it was possible to obtain the adjustable parameters.Since correlations based on density can be used to explain the solubility of solids in supercritical fluids (SCF), empirical models based on density were used. Since these empirical models depend on the pressure, temperature, and SCF density, which correspond to independent variables, in addition to constants and adjustable parameters, they have the advantage of not requiring estimation of the physicochemical features of the solid.
Through the establishment of two trustworthy statistical criteria, Average Absolute Relative Deviation (AARD%) and correlation coefficient (Radj), were used to evaluate the performance of the thermodynamics models employed in this investigation to correlate the solubility of both medicines in SC-CO2 with ethanol at 1 and 3 mol% was assessed:58
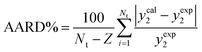 | (6) |
The experimental value of the molar solubility of favipiravir and montelukast in SC-CO2 with ethanol at 1 and 3 mol% is represented by yexp in eqn (6). The theoretical solubility values determined using the suggested thermodynamics models are represented by ycal in the meanwhile.
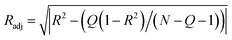 | (7) |
Radj was calculated according eqn (7). In eqn (7) N is the data points number for each set, and Q correspond to the number of independent variables.
4. Results and discussion
4.1. Experimental solubility data
To validate the equipment used to determine the solubility of favipiravir and montelukast in SC-CO2, in a previous work,59 the solubility of capecitabine and naphthalene at different temperatures and pressures was determined, using the same equipment that in this study, and compared with existing data reported by Amani et al.,60 Iwai et al.,61 Ardestani et al.,15 and Sodeifian et al.62Our research team has previously published data on the solubility of favipiravir13 and montelukast14 in SC-CO2 reported as molar fraction at various temperatures (308 to 338 K) and pressures (12 to 30 MPa) in the range of 0.03 × 10−4 to 9.00 × 10−4 and 0.04 × 10−5 to 6.12 × 10−5, respectively. In the current study, ethanol was utilized as a cosolvent to increase these medicines' solubility in SC-CO2. To improve the precision of the measurements, every experimental run was developed in triplicates. The experimental molar solubility values for favipiravir and montelukast in SC-CO2 with 1 and 3 mol% ethanol is shown in Tables 4 and 5, and 6 and 7, respectively. From these results, it can be seen that the addition of ethanol at 1 and 3 mol% increased the solubility of drugs under all the pressure and temperature parameters examined because the polarity of supercritical mixture's is increased. The solubility of other compounds in SC-CO2 has been reported to be affected by the addition of ethanol in the same way. Li et al. reported a 6.87-fold increase in benzamide solubility in SC-CO2 using ethanol at 3.5 mol% at 318 K and 18 MPa.20 Lee et al. reported 5.77 and 15.74-fold increase in the solubility of modified disperse yellow 119 and red 82 in SC-CO2 at 353.2 K, 30 MPa and using ethanol 3 mol% as cosolvent, respectively.19 Meanwhile, Ota et al. reported a 5.16-fold increase in the solubility of anthracene in SC-CO2 at 333 K, 22 MPa and using ethanol 3 mol% as a cosolvent.63 Li et al. reported a 2.96-fold increase in the solubility of p-toluenesulfonamide in SC-CO2 at 328 K, 21 MPa and using ethanol 3.5% as a cosolvent.64
| Temperatureb (K) | Pressureb (MPa) | Binary | Ternary | ||
|---|---|---|---|---|---|
| y2 × 104 (mole fraction)c | Experimental standard deviation, S (ȳ′) ×104 | e (cosolvent effect) | |||
a y2, 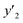 and e are molar fraction of favipiravir in SC-CO2 (binary system), in SC-CO2 with 1% of ethanol, and the cosolvent effect, respectively.b For each experimental run cosolvent effect was calculated as y2/, and e are molar fraction of favipiravir in SC-CO2 (binary system), in SC-CO2 with 1% of ethanol, and the cosolvent effect, respectively.b For each experimental run cosolvent effect was calculated as y2/, 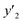 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 1 bar. Also, the relative standard deviations are obtained below 0.05 for mole fractions and solubilities.c Data taken from a previous work.13 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 1 bar. Also, the relative standard deviations are obtained below 0.05 for mole fractions and solubilities.c Data taken from a previous work.13 |
|||||
| 308 | 12 | 0.53 | 0.50 | 0.10 | 9.43 |
| 15 | 0.87 | 0.70 | 0.17 | 8.05 | |
| 18 | 1.44 | 1.00 | 0.30 | 7.14 | |
| 21 | 2.31 | 1.40 | 0.43 | 6.06 | |
| 24 | 3.42 | 1.60 | 0.77 | 4.68 | |
| 27 | 4.09 | 1.80 | 0.93 | 4.40 | |
| 30 | 5.13 | 2.00 | 1.23 | 3.90 | |
| 318 | 12 | 0.37 | 0.30 | 0.08 | 8.11 |
| 15 | 0.80 | 0.60 | 0.22 | 7.50 | |
| 18 | 1.30 | 0.70 | 0.41 | 5.38 | |
| 21 | 2.72 | 1.30 | 0.78 | 4.78 | |
| 24 | 4.29 | 2.00 | 1.30 | 4.66 | |
| 27 | 5.41 | 2.40 | 1.34 | 4.44 | |
| 30 | 6.48 | 2.80 | 1.50 | 4.32 | |
| 328 | 12 | 0.08 | 0.09 | 0.01 | 11.25 |
| 15 | 0.60 | 0.50 | 0.10 | 8.33 | |
| 18 | 1.39 | 1.00 | 0.33 | 7.19 | |
| 21 | 3.21 | 1.80 | 0.88 | 5.61 | |
| 24 | 4.75 | 2.50 | 1.39 | 5.26 | |
| 27 | 6.58 | 3.00 | 1.18 | 4.56 | |
| 30 | 7.65 | 3.40 | 1.49 | 4.44 | |
| 338 | 12 | 0.03 | 0.06 | 0.03 | 20.00 |
| 15 | 0.37 | 0.50 | 0.20 | 13.51 | |
| 18 | 1.32 | 1.10 | 0.78 | 8.33 | |
| 21 | 3.92 | 2.30 | 1.05 | 5.87 | |
| 24 | 5.6 | 3.00 | 1.59 | 5.36 | |
| 27 | 7.57 | 3.90 | 2.05 | 5.15 | |
| 30 | 9.05 | 4.40 | 2.40 | 4.86 | |
In this study, the solubility of favipiravir and montelukast in the SC-CO2 was experimentally investigated at various pressures and temperatures (308 to 338 K) (12 to 30 MPa) and reported in the range of 0.1 × 10−4 to 6.1 × 10−3 and 0.1 × 10−4 to 3.59 × 10−4, respectively for 3 mol%, which corresponded to solubility values up to 33.3 and 24.5 times higher than the obtained for these substances using pure SC-CO2 (Tables 5 and 7). Furthermore, the results in Tables 4 and 6 showed that by adding ethanol 1 mol% to SC-CO2, the solubility of favipiravir and montelukast increased 20 and, 9.5 times respectively, and mole fractions of drugs were in the range of 0.60 × 10−5 to 4.40 × 10−3 and 0.38 × 10−5 to 17.05 × 10−5.
| Temperatureb (K) | Pressureb (MPa) | Binary | Ternary | ||
|---|---|---|---|---|---|
| y2 × 104 (mole fraction)c | Experimental standard deviation, S (ȳ′) ×104 | e (cosolvent effect) | |||
a y2, 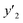 and e are molar fraction of favipiravir in SC-CO2 (binary system), in SC-CO2 with 3% of ethanol, and the cosolvent effect, respectivelyb For each experimental run cosolvent effect was calculated as y2/, and e are molar fraction of favipiravir in SC-CO2 (binary system), in SC-CO2 with 3% of ethanol, and the cosolvent effect, respectivelyb For each experimental run cosolvent effect was calculated as y2/, 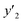 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 0.1 MPa. Also, the relative standard deviations are obtained below 0.05 for mole fractions and solubilities.c Data taken from a previous work.13 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 0.1 MPa. Also, the relative standard deviations are obtained below 0.05 for mole fractions and solubilities.c Data taken from a previous work.13 |
|||||
| 308 | 12 | 0.53 | 0.61 | 0.11 | 11.32 |
| 15 | 0.87 | 0.80 | 0.16 | 9.20 | |
| 18 | 1.44 | 1.21 | 0.29 | 8.57 | |
| 21 | 2.31 | 1.50 | 0.42 | 6.49 | |
| 24 | 3.42 | 2.20 | 0.73 | 6.43 | |
| 27 | 4.09 | 2.50 | 0.93 | 6.11 | |
| 30 | 5.13 | 2.91 | 1.20 | 5.65 | |
| 318 | 12 | 0.37 | 0.42 | 0.10 | 10.81 |
| 15 | 0.80 | 0.80 | 0.23 | 10.00 | |
| 18 | 1.30 | 1.22 | 0.39 | 9.23 | |
| 21 | 2.72 | 2.20 | 0.79 | 8.09 | |
| 24 | 4.29 | 3.10 | 1.32 | 7.23 | |
| 27 | 5.41 | 3.60 | 1.30 | 6.65 | |
| 30 | 6.48 | 3.80 | 1.52 | 5.86 | |
| 328 | 12 | 0.08 | 0.12 | 0.02 | 12.50 |
| 15 | 0.60 | 0.71 | 0.14 | 11.67 | |
| 18 | 1.39 | 1.50 | 0.36 | 10.79 | |
| 21 | 3.21 | 3.01 | 0.84 | 9.35 | |
| 24 | 4.75 | 3.83 | 1.33 | 8.00 | |
| 27 | 6.58 | 4.80 | 1.15 | 7.29 | |
| 30 | 7.65 | 5.13 | 1.48 | 6.67 | |
| 338 | 12 | 0.03 | 0.10 | 0.03 | 33.33 |
| 15 | 0.37 | 0.60 | 0.22 | 16.22 | |
| 18 | 1.32 | 1.90 | 0.76 | 14.39 | |
| 21 | 3.92 | 3.81 | 1.07 | 9.69 | |
| 24 | 5.6 | 4.90 | 1.57 | 8.75 | |
| 27 | 7.57 | 5.72 | 2.06 | 7.53 | |
| 30 | 9.05 | 6.10 | 2.44 | 6.74 | |
| Temperatureb (K) | Pressureb (MPa) | Binary | Ternary | ||
|---|---|---|---|---|---|
| y2 × 105 (molar fraction)c | Experimental standard deviation, S (ȳ′)×105 | e (cosolvent effect) | |||
a y2, 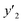 and e are molar fraction of montelukast in SC-CO2 (binary system), in SC-CO2 with 1% of ethanol, and the cosolvent effect, respectively.b For each experimental run cosolvent effect was calculated as y2/, and e are molar fraction of montelukast in SC-CO2 (binary system), in SC-CO2 with 1% of ethanol, and the cosolvent effect, respectively.b For each experimental run cosolvent effect was calculated as y2/, 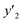 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 0.1 MPa. Also, the relative standard deviations are obtained below 0.05 for mole fractions.c Data taken from a previous work.14 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 0.1 MPa. Also, the relative standard deviations are obtained below 0.05 for mole fractions.c Data taken from a previous work.14 |
|||||
| 308 | 12 | 0.13 | 0.68 | 0.03 | 5.23 |
| 15 | 0.24 | 1.03 | 0.04 | 4.29 | |
| 18 | 0.36 | 1.47 | 0.07 | 4.08 | |
| 21 | 0.48 | 1.71 | 0.08 | 3.56 | |
| 24 | 0.61 | 2.03 | 0.09 | 3.33 | |
| 27 | 0.74 | 2.26 | 0.14 | 3.05 | |
| 30 | 0.88 | 2.52 | 0.16 | 2.86 | |
| 318 | 12 | 0.10 | 0.49 | 0.02 | 4.90 |
| 15 | 0.22 | 1.02 | 0.06 | 4.64 | |
| 18 | 0.58 | 2.20 | 0.11 | 3.79 | |
| 21 | 0.89 | 3.25 | 0.19 | 3.65 | |
| 24 | 1.22 | 3.89 | 0.23 | 3.19 | |
| 27 | 1.57 | 4.64 | 0.27 | 2.96 | |
| 30 | 1.94 | 5.50 | 0.30 | 2.84 | |
| 328 | 12 | 0.07 | 0.42 | 0.01 | 6.00 |
| 15 | 0.20 | 1.00 | 0.03 | 5.00 | |
| 18 | 0.76 | 3.52 | 0.14 | 4.63 | |
| 21 | 1.38 | 5.67 | 0.27 | 4.11 | |
| 24 | 2.12 | 7.40 | 0.40 | 3.49 | |
| 27 | 2.94 | 10.02 | 0.41 | 3.41 | |
| 30 | 3.83 | 11.72 | 0.56 | 3.06 | |
| 338 | 12 | 0.04 | 0.38 | 0.02 | 9.50 |
| 15 | 0.16 | 0.92 | 0.06 | 5.75 | |
| 18 | 0.77 | 4.18 | 0.30 | 5.43 | |
| 21 | 1.81 | 8.09 | 0.32 | 4.47 | |
| 24 | 3.20 | 10.13 | 0.63 | 3.17 | |
| 27 | 4.73 | 14.00 | 1.02 | 2.96 | |
| 30 | 6.12 | 17.05 | 1.45 | 2.79 | |
| Temperatureb (K) | Pressureb (MPa) | Binary | Ternary | ||
|---|---|---|---|---|---|
| y2 × 105 (molar fraction)c | Experimental standard deviation, S (ȳ′)×105 | e (cosolvent effect) | |||
a y2, 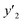 and e are molar fraction of montelukast in SC-CO2 (binary system), in SC-CO2 with 3% of ethanol, and the cosolvent effect, respectively.b For each experimental run cosolvent effect was calculated as y2/, and e are molar fraction of montelukast in SC-CO2 (binary system), in SC-CO2 with 3% of ethanol, and the cosolvent effect, respectively.b For each experimental run cosolvent effect was calculated as y2/, 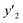 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 0.1 MPa. Also, the relative standard deviations are obtained below 0.05 for mole fractions and solubilities.c Data taken from a previous work.14 . Standard uncertainty u are u(T) = 0.1 K; u(p) = 0.1 MPa. Also, the relative standard deviations are obtained below 0.05 for mole fractions and solubilities.c Data taken from a previous work.14 |
|||||
| 308 | 12 | 0.13 | 0.11 | 0.02 | 8.4 |
| 15 | 0.24 | 0.16 | 0.03 | 6.8 | |
| 18 | 0.36 | 0.23 | 0.06 | 6.3 | |
| 21 | 0.48 | 0.26 | 0.08 | 4.8 | |
| 24 | 0.61 | 0.30 | 0.10 | 4.7 | |
| 27 | 0.74 | 0.34 | 0.13 | 4.5 | |
| 30 | 0.88 | 0.38 | 0.16 | 4.1 | |
| 318 | 12 | 0.10 | 0.08 | 0.02 | 7.9 |
| 15 | 0.22 | 0.16 | 0.05 | 7.4 | |
| 18 | 0.58 | 0.33 | 0.11 | 6.8 | |
| 21 | 0.89 | 0.49 | 0.18 | 6.0 | |
| 24 | 1.22 | 0.58 | 0.25 | 5.3 | |
| 27 | 1.57 | 0.69 | 0.25 | 4.9 | |
| 30 | 1.94 | 0.81 | 0.32 | 4.2 | |
| 328 | 12 | 0.07 | 0.06 | 0.01 | 9.1 |
| 15 | 0.20 | 0.17 | 0.03 | 8.6 | |
| 18 | 0.76 | 0.60 | 0.14 | 7.9 | |
| 21 | 1.38 | 0.94 | 0.27 | 6.8 | |
| 24 | 2.12 | 1.25 | 0.43 | 5.9 | |
| 27 | 2.94 | 1.55 | 0.39 | 5.3 | |
| 30 | 3.83 | 1.86 | 0.54 | 4.9 | |
| 338 | 12 | 0.04 | 0.10 | 0.03 | 24.5 |
| 15 | 0.16 | 0.19 | 0.07 | 11.8 | |
| 18 | 0.77 | 0.81 | 0.32 | 10.5 | |
| 21 | 1.81 | 1.29 | 0.36 | 7.1 | |
| 24 | 3.20 | 2.03 | 0.65 | 6.4 | |
| 27 | 4.73 | 2.80 | 1.01 | 5.7 | |
| 30 | 6.12 | 3.59 | 1.44 | 4.9 | |
Particularly, the improvement in the solubility of both substances can be related to the presence of hydrogen donors and acceptors moieties in their structures in which ethanol was able to interact with those molecules by hydrogen bonding.65 The largest solubility of favipiravir and montelukast in SC-CO2 with ethanol 1 and 3 mol% was obtained at the highest values of temperature (338 K) and pressure (30 MPa). At these conditions, the molar solubility of favipiravir (6.1 × 10−3) was 17 times higher than the obtained for montelukast (3.59 × 10−4) which agreed with the reported 15 times higher solubility of favipiravir than the reported for montelukast in SC-CO2 under same pressure and temperature conditions.13,14
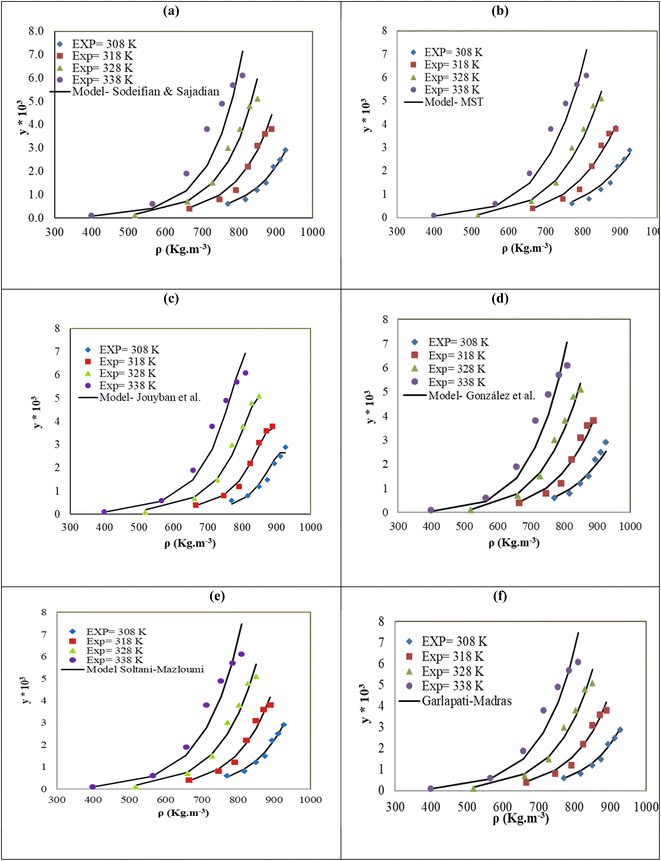
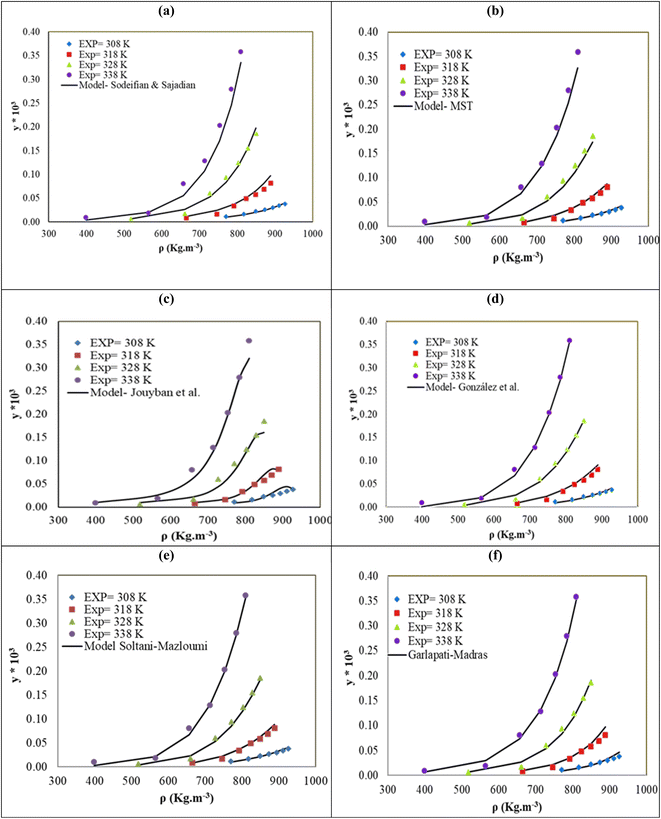
The solubility of favipiravir and montelukast in a mixture of SC-CO2 and 3 mol% ethanol is depicted in Fig. 2 as a function of operational factors (pressure, temperature, and density). As shown by the isotherms in Fig. 2, the solubility of favipiravir (Fig. 2a) and montelukast (Fig. 2c) in the supercritical mixture increased considering all the temperatures utilized in this study with rising pressure because of the well-known improvement of the solvent power of SC-CO2 as pressure rise isothermally due to the increase of density. Azim et al. reported the increase in the solubility of ibuprofen and ketoprofen as pressure raised from 8.5 MPa to 40 MPa at different constant temperature values.66 Ardestani et al. reported the increase in chloroquine's solubility in SC-CO2 as pressure raised from 12 to 40 MPa at different temperatures.67 The crossover pressure region for favipiravir in the SC-CO2 ethanol mixture was shown experimentally in Fig. 2a to be between 15 and 18 MPa, which was lower than the crossover region previously reported for favipiravir in pure SC-CO2.13 This crossover reduction by using a cosolvent has been reported for several drugs.51,68 This meant that the solubility of favipiravir increased as the temperature increased isobarically in both SC-CO2 and mixtures of 3 mol% ethanol and pure SC-CO2 using pressure values over the crossover region because the increase in favipiravir's vapor pressure was dominant over the adverse effect of decreasing CO2 density on solubility. The solubility of favipiravir, on the other hand, dropped when the temperature increased isobarically below the crossover zone since the negative influence of decreasing density over solubility was dominating. A similar crossover pressure region has been reported for haloperidol69 and ketoprofen.70 For montelukast, a little reduction in its crossover pressure from 15–16 MPa (binary system) to 15 MPa (ternary system) was obtained due to the use of ethanol 3 mol%.
 | ||
| Fig. 2 The influence of pressure and density of the SC-CO2 + 3 mol% ethanol mixture on favipiravir (a and b) and montelukast (c and d) solubility at different temperatures. | ||
4.2. Correlation of the solubility data with semi-empirical models
The solubility of favipiravir and montelukast in the two examined ternary systems (favipiravir-SC-CO2-ethanol and montelukast-SC-CO2-ethanol) was correlated in this investigation using six empirical density-based models with four to seven adjustable parameters (MST, Sodeifian–Sajadian, González et al., Soltani–Mazloumi, Garlapati–Madras, and Jouyban et al.). Table 8 shows the equations associated to each density-based model used in the present research. The average absolute relative deviation (AARD%), correlation coefficient (Radj) and the adjustable parameters obtained for each model through their correlation with the experimental solubility data of favipiravir and montelukast in SC-CO2 with ethanol 1 and 3 mol% are shown in Tables 9 and 10 respectively. Additionally, Fig. 3a–f and Fig. 4a–f, respectively, depict the experimental (points) and computed data (line) of the solubility of favipiravir and montelukast in SC-CO2 based on empirical models at various pressures and temperatures and 3 mol% cosolvent.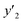 , y3 and a0–a6 are density of SC-CO2, temperature, pressure, reference pressure, mole fraction in ternary system, mole fraction of cosolvent and adjustable parameters, respectively)
, y3 and a0–a6 are density of SC-CO2, temperature, pressure, reference pressure, mole fraction in ternary system, mole fraction of cosolvent and adjustable parameters, respectively)
The correlation of the solubility of favipiravir in the ternary systems using various models yielded correlation coefficient (Radj) and AARD% higher than 0.9691, and lower than 15.91%, respectively, demonstrating that each model taken into consideration in this study has adequate accuracy to represent the solubility of favipiravir in SC-CO2 with 1 and 3 mol% of ethanol. The MST model performed the best to correlate the solubility of favipiravir, according the results shown in Table 9, in the ternary system due to presented a lower AARD% than the values obtained for the other semi-empirical models with the same number of adjustable parameters (González et al. and Sodeifian–Sajadian) and even to those obtained using the models with a higher number of adjustable parameters (Garlapati–Madras and Jouyban et al.). The more accuracy of the MST model has been previously reported for the correlation of the solubility of different solutes in SC-CO2. Esfandiari & Sajadian showed that the MST had a good degree of accuracy for simulating the solubility of glibenclamide in SC-CO2 at temperatures and pressures between 12 and 30 MPa and between 308 and 338 K, respectively.59 In order to correlate the solubility of paracetamol in SC-CO2 at pressure and temperature values ranging from 9.5 to 26.5 MPa and 311 to 358 K, respectively, Bagheri et al. found that the MST model was the most effective.71 The same predicting capacity of the MST model was reported by Zabihi et al. to estimate salsalate solubility in SC-CO2.72
| Model | a0 | a1 | a2 | a3 | a4 | a5 | a6 | AARD% | Radj |
|---|---|---|---|---|---|---|---|---|---|
| MST | −13098.6 | 4.45 | 28.37 | −5404 | — | — | — | 11.77 | 0.9894 |
| González et al. | 7.30 | 0.298 | −7068.52 | −31.91 | — | — | — | 14.92 | 0.9763 |
| Sodeifian–Sajadian | −2.82 | −1.167 | 0.0404 | 0.339 | — | — | — | 15.19 | 0.9691 |
| Soltani–Mazloumi | −15.91 | −1048.61 | 3.337 | 0.118 | 0.348 | — | — | 11.83 | 0.9886 |
| Garlapati–Madras | −52.0 | −3.68 | 0.008 | 4676.79 | 3.53 | −4.39 | 4.73 | 12.86 | 0.9849 |
| Jouyban et al. | −46.128 | 19.14 | −0.0041 | −0.0069 | 0.00131 | 0.037 | 5.451 | 12.49 | 0.9862 |
The reported Radj and AARD% values, on the other hand, resulted from the correlation of montelukast solubility in the ternary system using several semi-empirical density models, were in the range of 0.9691–0.9894, and 11.77–15.91% respectively. This result showed that various semi-empirical models correctly predicted montelukast's solubility in SC-CO2 with 1 and 3 mol% of ethanol at pressures and temperatures ranging from 12 to 30 MPa and 308 to 338 K, respectively. According to the results presented in Table 10, in this case the MST model also presented a better performance than the obtained using the semi-empirical models with more adjustable parameters to estimate montelukast's solubility in the ternary system due to presented the lowest AARD% value.
| Model | a0 | a1 | a2 | a3 | a4 | a5 | a6 | AARD% | Radj |
|---|---|---|---|---|---|---|---|---|---|
| MST | −16990 | 4.45 | 36.61 | −7281.9 | 14.76 | 0.9921 | |||
| González et al. | 7.59 | 0.44 | −11179.5 | −24.31 | 15.37 | 0.9913 | |||
| Sodeifian–Sajadian | −3.53 | −1.7924 | 0.0549 | 0.474 | — | — | — | 16.27 | 0.9892 |
| Soltani–Mazloumi | −33.82 | −17794.1 | 4.71 | 1.27 | 0.43 | 14.65 | 0.9918 | ||
| Garlapati–Madras | −41.63 | −3.10 | 0.0121 | −7961.1 | 15.01 | −4.71 | 15.95 | 0.9901 | |
| Jouyban et al. | −27.88 | 29.19 | 0.0033 | −0.0133 | 0.0025 | 0.199 | 0.031 | 17.11 | 0.9854 |
5. Conclusions
In this study, the increase in solubility of favipiravir and montelukast in SC-CO2 using ethanol 3 mol% as cosolvent was determined for the first time by spectrophotometric assays following a static method. The use of ethanol as a cosolvent increased the solubility of favipiravir and montelukast for all the studied conditions of pressure and temperature due to the increase of the polarity of the supercritical mixture. The highest solubility of favipiravir and montelukast in the ternary system (3 mol% ethanol) at different temperature (308 to 338 K) and pressure values (12 to 30 MPa) were in the range of 0.1 × 10−4 to 6.1 × 10−3 and 0.1 × 10−4 to 3.59 × 10−4, respectively, which correspond to solubility values up to 33.33 and 24.5 times higher than the obtained for these substances using pure SC-CO2. The solubility of both drugs increased with increasing pressure for all the temperatures used in this study due to the well-known improvement of the solvent power of SC-CO2 as pressure rise isothermally due to the increase of density. The highest solubility of favipiravir and montelukast in the ternary systems were obtained at the highest values of temperature (338 K) and pressure (30 MPa) and 3 mol% of ethanol as cosolvent. The experimental solubility of favipiravir and montelukast was correlated with six density-based semi-empirical models with different adjustable parameters (González et al., Mendez-Santiago–Teja, Garlapati, and Madras, Sodeifian–Sajadian, Soltani–Mazloum and Jouyban et al.). The MST model presented the best performance to correlate the solubility of favipiravir (AARD%: 11.77% and Radj: 0.9894) and montelukast (AARD%: 14.65% and Radj: 0.9918) in the ternary systems.The solubility data obtained in this study aims to support the future development of nano formulations of favipiravir and montelukast with improved solubility, bioavailability and consequently pharmacological activity by the selection of an adequate nanonization method based in supercritical carbon dioxide.
Conflicts of interest
There are no conflict to declare.Acknowledgements
The authors express their thanks to the supercritical fluids laboratory of Dr Seyed Ali Sajadian for providing the experimental facilities for this research. Furthermore, C. López de Dicastillo acknowledge the “Ramon y Cajal” Fellowship RYC2020-029874-I/AEI/10.13039/501100011033 financed by the Spanish Ministry of Science and Innovation. A. Rojas thanks the support of Agencia Nacional de Investigación y Desarrollo through the Fondecyt regular project no. 1201301, to the University of Santiago de Chile through the Postdoctoral Fellowship DICYT Codigo 082371GL_Postdoc, and to the support of the “Programa de Financiamiento Basal para Centros Científicos y Tecnológicos de Excelencia” (Project AFB220001).References
- S. Joshi, J. Parkar, A. Ansari, A. Vora, D. Talwar, M. Tiwaskar, S. Patil and H. Barkate, Int. J. Infect. Dis., 2021, 102, 501–508 CrossRef CAS PubMed.
- T. Baranovich, S.-S. Wong, J. Armstrong, H. Marjuki, R. J. Webby, R. G. Webster and E. A. Govorkova, J. Virol., 2013, 87, 3741–3751 CrossRef CAS PubMed.
- J. Barré, J. M. Sabatier and C. Annweiler, Front. Pharmacol, 2020, 11, 1344 CrossRef PubMed.
- Introduction to Biopharmaceutics and Pharmacokinetics | Applied Biopharmaceutics & Pharmacokinetics, 7e|AccessPharmacy|McGraw Hill Medical, https://accesspharmacy.mhmedical.com/content.aspx?sectionid=100668900%26bookid=1592, accessed 4 December 2022.
- R. K. Kankala, P. Y. Xu, B. Q. Chen, S. Bin Wang and A. Z. Chen, Adv. Drug Delivery Rev., 2021, 176, 113846 CrossRef CAS PubMed.
- E. Velásquez, C. Patiño Vidal, A. Rojas, A. Guarda, M. J. Galotto and C. López de Dicastillo, Compr. Rev. Food Sci. Food Saf., 2021, 20, 3388–3403 CrossRef PubMed.
- A. Rojas, A. Torres, M. J. Galotto, A. Guarda and R. Julio, Crit. Rev. Food Sci. Nutr., 2020, 60, 1290–1301 CrossRef CAS PubMed.
- S. Yu, X. Dong, P. Zhao, Z. Luo, Z. Sun, X. Yang, Q. Li, L. Wang, Y. Zhang and H. Zhou, Nat. Commun., 2022, 13, 3616 CrossRef CAS PubMed.
- S. Yu, P. Zhao, X. Yang, Q. Li, Y. Zhang and H. Zhou, Fuel, 2022, 326, 124997 CrossRef CAS.
- C. R. S. de Oliveira, P. V. de Oliveira, L. Pellenz, C. R. L. de Aguiar and A. H. da S. Júnior, J. Environ. Sci., 2023 DOI:10.1016/J.JES.2023.06.007.
- S. A. Sajadian, H. Peyrovedin, K. Zomorodian and M. Khorram, J. Supercrit. Fluids, 2023, 194, 105858 CrossRef CAS.
- G. Sodeifian, F. Razmimanesh and S. A. Sajadian, J. Supercrit. Fluids, 2019, 146, 89–99 CrossRef CAS.
- S. A. Sajadian, N. S. Ardestani, N. Esfandiari, M. Askarizadeh and A. Jouyban, J. Supercrit. Fluids, 2022, 183, 105539 CrossRef CAS PubMed.
- S. A. Sajadian, N. S. Ardestani and A. Jouyban, J. Mol. Liq., 2022, 349, 118219 CrossRef CAS.
- N. S. Ardestani, N. Y. Majd and M. Amani, J. Chem. Eng. Data, 2020, 65, 4762–4779 CrossRef CAS.
- N. Saadati Ardestani, M. Amani and L. Moharrery, Chem. Eng. Res. Des., 2020, 159, 529–542 CrossRef CAS.
- B. Li, K. Liu, J. Zhu, Y. Wang, H. Meng and J. Jin, J. Chem. Eng. Data, 2022, 67, 689–694 CrossRef CAS.
- A. Cháfer, T. Fornari, R. P. Stateva and A. Berna, J. Chem. Eng. Data, 2009, 54, 2263–2268 CrossRef.
- M. J. Lee, C. C. Ho, H. M. Lin, P. Y. Wang and J. S. Lu, J. Supercrit. Fluids, 2014, 95, 258–264 CrossRef CAS.
- J. L. Li, J. S. Jin, Z. T. Zhang and Y. B. Wang, Fluid Phase Equilib., 2011, 307, 11–15 CrossRef CAS.
- G. Sodeifian, S. A. Sajadian, F. Razmimanesh and S. M. Hazaveie, Sci. Rep., 2021, 11(1), 7546, DOI:10.1038/s41598-021-87243-6.
- D. John, C. P. Kancharlapalli and S. Nagarajan, J. Chem. Eng. Data, 2019, 64, 5775–5784 CrossRef CAS.
- K. C. Pitchaiah, N. Lamba, N. Sivaraman and G. Madras, J. Supercrit. Fluids, 2018, 138, 102–114 CrossRef CAS.
- H. Behjati Rad, J. Karimi Sabet and F. Varaminian, Chem. Eng. Technol., 2019, 42, 1259–1267 CrossRef CAS.
- G. Yang, Z. Li, Q. Shao and N. Feng, Asian J. Pharm. Sci., 2017, 12, 456–463 CrossRef PubMed.
- S. Zhan, L. Cui, Q. Zhao, L. Chen, J. Wang, S. Chen and S. Ding, J. Solution Chem., 2015, 44, 1–15 CrossRef CAS.
- R. G. Bitencourt, F. A. Cabral and A. J. A. Meirelles, J. Chem. Thermodyn., 2016, 103, 285–291 CrossRef CAS.
- S. Zhan, S. Li, Q. Zhao, W. Wang and J. Wang, J. Chem. Eng. Data, 2017, 62, 1257–1263 CrossRef CAS.
- J. F. Jia, F. Zabihi, Y. H. Gao and Y. P. Zhao, J. Chem. Eng. Data, 2015, 60, 1744–1749 CrossRef CAS.
- C. Tang, Y. X. Guan, S. J. Yao and Z. Q. Zhu, J. Chem. Eng. Data, 2014, 59, 3359–3364 CrossRef CAS.
- K. A. Araus, V. Casado, J. M. del Valle, P. S. Robert and J. C. de la Fuente, J. Supercrit. Fluids, 2019, 143, 205–210 CrossRef CAS.
- J. Cheng, S. Han, J. Song, W. Wang and Z. Jiao, J. Chem. Eng. Data, 2018, 63, 4248–4255 CrossRef CAS.
- R. G. Bitencourt, A. M. Palma, J. A. P. Coutinho, F. A. Cabral and A. J. A. Meirelles, J. Supercrit. Fluids, 2018, 138, 238–246 CrossRef CAS.
- Y. Li, Y. Ning, J. Jin and Z. Zhang, J. Chem. Eng. Data, 2013, 58, 2176–2180 CrossRef CAS.
- J. S. Jin, Z. M. Dou, H. H. Liu, H. Wu and Z. T. Zhang, J. Chem. Eng. Data, 2012, 57, 2217–2220 CrossRef CAS.
- K. A. Araus, J. M. Del Valle, P. S. Robert and J. C. De La Fuente, J. Chem. Thermodyn., 2012, 51, 190–194 CrossRef CAS.
- J. S. Jin, Z. M. Dou, G. X. Su, Z. T. Zhang and H. T. Liu, Fluid Phase Equilib., 2012, 315, 9–15 CrossRef CAS.
- J. S. Jin, Y. B. Wang, Z. T. Zhang and H. T. Liu, Thermochim. Acta, 2012, 527, 165–171 CrossRef CAS.
- J. Karimi Sabet, C. Ghotbi, F. Dorkoosh and A. Striolo, Sci. Iran., 2012, 19, 619–625 CrossRef.
- C. Zhao, J. B. Wang, I. Tabata and T. Hori, Adv. Mater. Res., 2011, 332–334, 146–151 CAS.
- M. H. Hosseini, N. Alizadeh and A. R. Khanchi, J. Supercrit. Fluids, 2010, 55, 14–22 CrossRef CAS.
- G. Sodeifian, S. A. Sajadian, F. Razmimanesh and S. M. Hazaveie, Sci. Rep., 2021, 11, 1–13 CrossRef PubMed.
- Z. Huang, Y. C. Chiew, W. D. Lu and S. Kawi, Fluid Phase Equilib., 2005, 237, 9–15 CrossRef CAS.
- A. Rojas, D. Cerro, A. Torres, M. J. Galotto, A. Guarda and J. Romero, J. Supercrit. Fluids, 2015, 104, 76–84 CrossRef CAS.
- S. H. Khudaida, W. Y. Hsieh, Y. Z. Huang, W. Y. Wu, M. J. Lee and C. S. Su, J. Supercrit. Fluids, 2023, 194, 105851 CrossRef CAS.
- C. Wang, T. Yan, T. Yan and Z. Wang, J. Mol. Struct., 2023, 1279, 134947 CrossRef CAS.
- L. López-Hortas, P. Rodríguez, B. Díaz-Reinoso, M. C. Gaspar, H. C. de Sousa, M. E. M. Braga and H. Domínguez, J. Supercrit. Fluids, 2022, 188, 105652 CrossRef.
- T. F. Gondo, M. Jönsson, E. N. Karlsson, M. Sandahl and C. Turner, J. Chromatogr. A, 2023, 1706, 464267 CrossRef CAS PubMed.
- T. Do Dat, N. D. Hai, N. T. H. Nam, N. M. Thanh, N. T. T. Huyen, L. T. T. Duong, H. M. Nam and N. H. Hieu, Biocatal. Agric. Biotechnol., 2023, 52, 102824 CrossRef CAS.
- J. Mendez-Santiago and A. S. Teja, Ind. Eng. Chem. Res., 2000, 39, 4767–4771 CrossRef CAS.
- G. Sodeifian and S. A. Sajadian, J. Supercrit. Fluids, 2019, 149, 79–87 CrossRef CAS.
- J. C. González, M. R. Vieytes, A. M. Botana, J. M. Vieites and L. M. Botana, J. Chromatogr. A, 2001, 910, 119–125 CrossRef PubMed.
- S. Soltani and S. H. Mazloumi, Chem. Eng. Res. Des., 2017, 125, 79–87 CrossRef CAS.
- C. Garlapati and G. Madras, Thermochim. Acta, 2010, 500, 123–127 CrossRef CAS.
- A. Jouyban, M. Khoubnasabjafari and H.-K. Chan, Chem. Pharm. Bull., 2005, 53, 290–295 CrossRef CAS PubMed.
- S. A. Sajadian, M. Amani, N. Saadati Ardestani and S. Shirazian, Arabian J. Chem., 2022, 15, 103821 CrossRef CAS.
- M. Amani, N. S. Ardestani, A. Jouyban and S. A. Sajadian, J. Supercrit. Fluids, 2022, 190, 105752 CrossRef CAS.
- A. Jouyban, H. K. Chan and N. R. Foster, J. Supercrit. Fluids, 2002, 24, 19–35 CrossRef CAS.
- N. Esfandiari and S. A. Sajadian, Fluid Phase Equilib., 2022, 556, 113408 CrossRef CAS.
- M. Amani, N. Saadati Ardestani and N. Y. Majd, J. CO2 Util., 2021, 46, 101465 CrossRef CAS.
- Y. Iwai, T. Fukuda, Y. Koga and Y. Arai, J. Chem. Eng. Data, 1991, 36, 430–432 CrossRef CAS.
- G. Sodeifian, F. Razmimanesh and S. A. Sajadian, J. Supercrit. Fluids, 2019, 146, 89–99 CrossRef CAS.
- M. Ota, Y. Hashimoto, M. Sato, Y. Sato, R. L. Smith and H. Inomata, Fluid Phase Equilib., 2016, 425, 65–71 CrossRef CAS.
- J. L. Li, J. S. Jin, Z. T. Zhang and X. M. Pei, J. Supercrit. Fluids, 2010, 52, 11–17 CrossRef CAS.
- F. Chang, J. Jin, N. Zhang, G. Wang and H. J. Yang, Fluid Phase Equilib., 2012, 317, 36–42 CrossRef CAS.
- M. M. Azim, I. Ushiki, A. Miyajima and S. Takishima, J. Supercrit. Fluids, 2022, 186, 105626 CrossRef CAS.
- N. S. Ardestani, M. Amani, M. Grishina and S. Shirazian, Arabian J. Chem., 2022, 15, 104371 CrossRef CAS.
- N. R. Foster, G. S. Gurdial, J. S. L. Yun, K. K. Liong, K. D. Tilly, S. S. T. Ting, H. Singh and J. H. Lee, Ind. Eng. Chem. Res., 1991, 30, 1955–1964 CrossRef CAS.
- S. H. Khudaida, Y. M. Chen, Y. F. Zheng, C. M. Hsieh and C. S. Su, J. Supercrit. Fluids, 2023, 192, 105785 CrossRef CAS.
- O. Faraz, M. Poustchi, E. Nazari Denyani, P. Movahedi, F. Rajabi Kouchi and R. Shahriari, J. Mol. Liq., 2022, 353, 118809 CrossRef CAS.
- H. Bagheri, B. Notej, S. Shahsavari and H. Hashemipour, Eur. J. Pharm. Sci., 2022, 177, 106273 CrossRef CAS PubMed.
- S. Zabihi, S. Jamshidian, F. Borousan, A. Zeinolabedini Hezave, M. Pishnamazi, A. Marjani and S. Shirazian, J. Chem. Thermodyn., 2021, 152, 106271 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |