Open Access Article
Open Access ArticleThe preparation and characterization of self-healing hydrogels based on polypeptides with a dual response to light and hydrogen peroxide†
Congwei Wanga,
Dinglei Zhaob,
Yongjun Xie*b,
Qiang Zhoub and
Haiyang Yang *b
*b
aKey Laboratory of Biomimetic Sensor and Detecting Technology of Anhui Province, School of Materials and Chemical Engineering, West Anhui University, Lu'an, 237012, Anhui Province, P. R. China
bCAS Key Laboratory of Soft Matter Chemistry, School of Chemistry and Materials Science, University of Science and Technology of China, Hefei, 230026, P. R. China. E-mail: yhy@ustc.edu.cn
First published on 11th December 2023
Abstract
Injectable self-healing hydrogels are being widely used in drug delivery, tissue engineering, and other fields. Because of their excellent biocompatibility and biodegradability, polypeptides are an ideal candidate for preparing injectable self-healing hydrogels. In this study, a polypeptide-based hydrogel with dual response to hydrogen peroxide and light was obtained by copolymerizing 4-arm PEG-amine, N-(p-nitrophenoxycarbonyl)-L-methionine, and N-(p-nitrophenoxycarbonyl)-γ-o-nitrobenzyl-L-glutamate. The hydrogel exhibits injectable self-healing behavior due to the hydrophobic interactions among peptide blocks, which also act as the reservoir of hydrophobic drug molecules. In the presence of hydrogen peroxide or under light irradiation, the thioether bond in methionine was oxidized to sulfoxide, whereas the o-nitro benzyl ester bond was broken to form glutamic acid. As a result, the corresponding hydrophobic blocks of polypeptide become hydrophilic, accelerating the release of drug molecules loaded in the polypeptide hydrophobic blocks. Using this technique, the controlled release of hydrophobic drug molecules was achieved. Our efforts could provide a new strategy for the preparation of self-healing hydrogels based on polypeptides with a dual response to hydrogen peroxide and light. In this view, the practical application of polypeptides in drug delivery, tissue engineering, and other fields, could be expanded and advanced.
Introduction
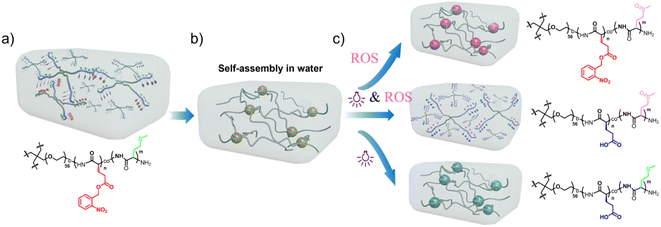
In recent years, stimulation-responsive and self-healing hydrogels have attracted extensive attention because of their great potential applications in a number of fields, such as drug delivery and tissue engineering.1–3 Among various types of hydrogels, the injectable self-healing hydrogels, which can enter the human body through a syringe injection, thus avoiding the injury caused by surgical incisions in patients, have attracted special attention in the field of drug delivery.4–7 Owing to their interactions, such as hydrogen bonding,8 π–π stacking,9 electrostatic interaction,10 and hydrophobic interaction,11–13 polypeptides can easily self-assemble into supramolecular structures. In particular, polypeptides have excellent biocompatibility and biodegradability. Therefore polypeptides are the ideal candidates for preparing injectable self-healing hydrogels for drug delivery and tissue engineering. The effective incorporation of drug molecules into polypeptides and their effective release at target locations is an area that needs further investigation.Stimulation-responsive hydrogels can change their physical and chemical properties under stimulation to achieve targeted and controlled drug release.14 So far, commonly used stimuli include temperature,15,16 light,12,17 pH,18 enzymes,19 and ROS,20 which are also one of the commonly used internal stimuli in targeted anticancer drug delivery.21 According to literature reports, high concentrations of reactive oxygen species have been found in most types of tumor tissues. Hydrogen peroxide, a reactive oxygen species having the highest concentration and best stability, is mainly produced in cell membranes, mitochondria, endoplasmic reticulum, and phagosomes and can freely diffuse among cells.22–25 The pendent group - thioether bond in L-methionine is a reactive oxygen species-responsive group. Numerous studies have reported that L-methionine in proteins can be easily oxidized by reactive oxygen species to sulfoxides or sulfones.26,27 L-Methionine exhibits a transition between hydrophobicity and hydrophilicity while responding to the reactive oxygen species.20 In our previous study,28 we found that if the reactive methionine monomer is polymerized in situ in the presence of water using 4-arm PEG-amine as an initiator, the hydrogels based on polypeptide can be conveniently obtained. In the presence of hydrogen peroxide, the thioether bond in methionine is oxidized to sulfoxide. As a result, the corresponding hydrophobic blocks of the polypeptide become hydrophilic. As a result, the hydrogels undergo the sol–gel transition owing to the action of hydrogen peroxide. Using doxorubicin as a model molecule for drug release simulation experiments, we have found that the release of doxorubicin loaded in the hydrogels was significantly accelerated, mainly because the corresponding hydrophobic blocks of polypeptide became hydrophilic in the presence of hydrogen peroxide.
However, if the polypeptide-based hydrogels were obtained by copolymerizing 4-arm PEG-amine with N-(p-nitrophenoxycarbonyl)-L-methionine alone, the encapsulated drugs should be easy to leak into humans under physicochemical conditions because the hydrophobic interactions among peptide blocks, which also act as reservoirs of hydrophobic drug molecules, are not strong enough. It is already known that light stimulus is a remote external stimulus source that can easily and conveniently modulate the mechanical properties of hydrogels in time and space.29–37 Deming et al. reported that hydrogels based on polylysine modified with photoresponsive groups were used for controlled load release. When the hydrogels were exposed to UV light, they degraded to release the dye molecules loaded therein.38 Yin et al. reported that photoresponsive polyglutamic acid can also be used for DNA delivery.39
In order to control the physicochemical properties of the hydrogels more effectively40–42 and modulate the release of the loaded drug more precisely, the new hydrogels based on a polypeptide, with dual response to hydrogen peroxide and light, by copolymerizing 4-arm PEG-amine, methionine N-carboxylic anhydride, and o-nitro benzyl glutamate N-carboxylic anhydride, is reported here. The hydrophobic interaction among the polypeptide blocks not only endows the hydrogels with self-healing properties but also provides hydrophobic micro-regions for loading hydrophobic drugs. In the presence of hydrogen peroxide or under light irradiation, the thioether bond in methionine was oxidized to sulfoxide whereas the o-nitro benzyl ester bond was broken to form glutamic acid. As a result, the corresponding hydrophobic blocks of the polypeptide became hydrophilic, accelerating the release of the drug molecules loaded among the polypeptide hydrophobic blocks. According to this technique, the controlled release of the hydrophobic drug molecules at specific sites such as tumor tissues in a physiological environment was realized.
Experimental
Materials and methods
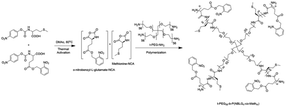
p-Nitrophenyl chloroformate was purchased from Meyer Chemical Co. Ltd. without further purity. L-Glutamate acid (Aladdin) was used as received. 1,1,3,3-Tetramethylguanidine (TMG, 98%, Aldrich) was distilled under reduced pressure. Tetra-PEG-NH2 was purchased from Sigma-Aldrich. DOX·HCl was obtained from J and K Chemicals. N,N-Dimethylacetamide (extra dry, with molecular sieves, water ≤ 50 ppm), and o-nitrobenzyl alcohol were purchased from Energy Chemicals. All other reagents were purchased from Sinopharm Chemical Reagent Co Ltd. and used as received. Water was deionized using a Milli-Q SPreagent water system (Millipore) to a specific resistivity of 18.4 mΩ cm.1H NMR (300 MHz) spectra were acquired using a 300 MHz Bruker instrument. Molecular weight and molecular weight distributions, PDI, were determined by gel permeation chromatography (GPC) equipped with a Waters 1515 pump and a Waters 2414 differential refractive index detector (set at 30 °C). It used a series of three linear Styragel columns at an oven temperature of 45 °C. The eluent was DMF at a flow rate of 1.0 mL min−1. A series of low polydispersity polystyrene standards were employed for calibration. The degrees of polymerization, DPs, were determined by 1H NMR analysis. The rheological properties of the prepared hydrogels were studied using a rheometer (TA instruments, AR-G2) with a platform of 40 mm diameter.
Synthetic procedure
p-Nitrophenyl chloroformate was added dropwise to the solution of o-nitro benzyl glutamate, and the temperature was raised to 40 °C while stirring overnight. After the reaction was completed, the obtained product was separated by column chromatography to obtain a white solid.
Results and discussion
Preparation process of the organogel/hydrogel hybrids
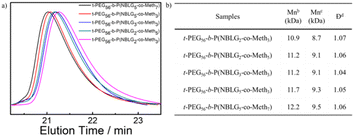
The monomer conversion and mean degree of polymerization (DP) were determined using 1H NMR from the ratio of the characteristic peak of the benzyl group on o-nitrobenzyl-L-glutamate at 5.5 ppm and the characteristic peak of the methylene group on the side chain of L-methionine at 2.55 ppm, and the characteristics of the PEG moiety at 3.5 ppm. The number-average molecular weight (Mn) was determined by 1H NMR analysis, and polydispersity index (PDI, Mw/Mn) was quantified using gel permeation chromatography (Fig. 1).
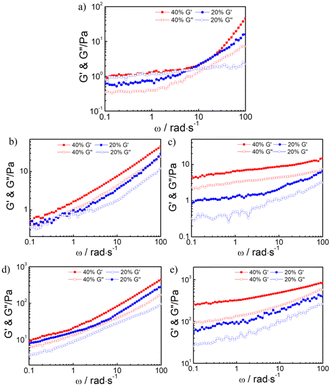
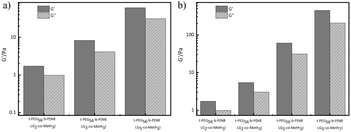
The mechanical properties of the hydrogel also appeared to be relevant to the normal development, differentiation, and motility of cell. We finally chose t-PEG56-b-P (NBLG5-co-Meth7) hydrogel with 40 wt% solid content for the next experiments, because it has the mechanical strength matching to that of the normal human soft tissue (Fig. 3).
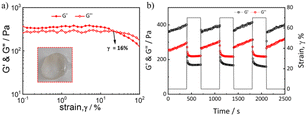
Fig. 4a shows the changes in the storage modulus and loss modulus of t-PEG56-b-P (NBLG5-co-Meth7) hydrogels with 40 wt% solid content under strain scanning. Under small strain, the storage modulus and loss modulus of the hydrogels remained unchanged and the storage modulus was greater than the loss modulus (strain γ< 10%). Under the increased strain, the storage modulus and loss modulus of the hydrogels began to decline, and the storage modulus decreased faster than the loss modulus, finally, the storage modulus and loss modulus of the hydrogel intersected under the ground strain γ = 70%, and then the loss modulus became greater than the storage modulus, which indicated that the hydrogels were converted by the sol transition, and the cross-linking network inside the hydrogels was destroyed. This excellent shear-thinning property of the prepared hydrogel makes it possible for injection with a syringe.
The self-healing properties of the hydrogels can be verified by dynamic strain scanning under a strain of 0.1% or 70% (Fig. 4b). At the initial stage, 0.1% strain was applied to the hydrogels. The storage modulus of the hydrogels was approximately 0.37 kPa and the loss modulus was 0.28 kPa. Under this strain, the storage modulus was greater than the loss modulus and substantially remained unchanged. When the strain applied to the hydrogels was changed to 70%, the storage modulus of the hydrogels immediately dropped from about 0.37 kPa to about 0.18 kPa, but the loss modulus only dropped from 0.28 kPa to 0.22 kPa. Under this strain, the storage modulus was less than the loss modulus and remained substantially unchanged. Then, the strain applied to the hydrogels was recovered from 70% to 0.1%, and the storage modulus and loss modulus can be immediately restored to their initial state under small strain. The cyclic strain test results showed that the hydrogels have excellent self-healing properties, and their mechanical properties can be restored to the initial state after several different strain scans.
 | ||
| Fig. 5 (a–d) The photographs of the reversed vial test of the dual response process of hydrogels at 37 °C. | ||
Finally, 10 μL of hydrogen peroxide was added to the vial and then the vial was irradiated with UV light for 30 minutes. In the process of radiation, the hydrogels gradually turned red, at the same time the tilted hydrogels in the vial gradually recovered to an equilibrium state (Fig. 5d). The sample was found to flow by shaking the vial, indicating that the hydrogels had undergone a complete gel–sol transition and degraded to a solution.
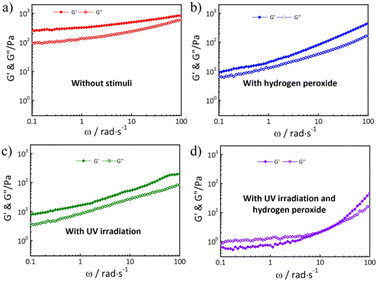
Furthermore, frequency-sweep measurements of hydrogels in different states were performed. As shown in Fig. 6a–d, the storage moduli (G′) was larger than the loss moduli (G′′), indicating the sample was in the gel state. After double stimuli, the storage moduli (G′) was smaller than the loss moduli (G′′), indicating the gel degraded to a fluidic solution (Fig. 6d). The hydrogels without any stimulation showed a significant shear thinning behavior, which indicated that the hydrogels were injectable. The gel weakened significantly after stimulation by UV radiation or hydrogen peroxide, and the viscosity of the gel decreased significantly when stimulated by both UV radiation and hydrogen peroxide (Fig. S3†).
The results are shown in Fig. 7. The cumulative release curve of the hydrogels without any external stimulus was first investigated. Under the condition of 37 °C and pH 7.4, the cumulative release of doxorubicin loaded in the hydrogels was only 26% within 24 hours. In the presence of hydrogen peroxide, the release rate of the loaded doxorubicin increased and ultimately reached 52% after 24 hours.
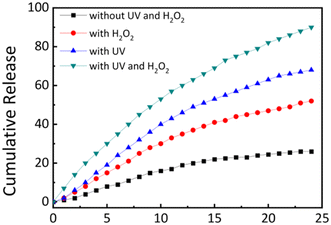 | ||
| Fig. 7 A cumulative DOX release profile from the drug-loaded hydrogel (pH 7.4 buffer, 37 °C) with different stimuli. | ||
In contrast, light irradiation stimulus has a stronger accelerating effect on the release of doxorubicin, ultimately accumulating 68% of doxorubicin after 24 hours. This may be due to the generation of the carboxyl negative ions after the bond breakage caused by light, which accelerates the release of doxorubicin by the reduction of hydrophobic interactions and repulsiveness among negative charges. Finally, under the combined action of hydrogen peroxide and ultraviolet light, the release rate of doxorubicin was significantly increased, and 90% of doxorubicin was ultimately released within 24 hours. Hydrogen peroxide combined with light accelerated the release of doxorubicin loaded in the hydrogels in the target site.
Conclusions
Herein, an injectable self-healing hydrogel, based on polypeptides and responsive to hydrogen peroxide and light, was prepared. Due to the hydrophobic interactions among peptide blocks, which also act as the reservoir of hydrophobic drug molecules, the hydrogel exhibits injectable self-healing behavior. In the presence of hydrogen peroxide or under light irradiation, the thioether bond in methionine was oxidized to sulfoxide, whereas the o-nitro benzyl ester bond was broken to form glutamic acid. As a result, the corresponding hydrophobic blocks of the polypeptide became hydrophilic, accelerating the release of the drug molecules loaded among the polypeptide hydrophobic blocks. The controlled release of the hydrophobic drug molecules at specific sites such as tumor tissues was realized accordingly.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation of China (Grant no 51273189), the National Science and Technology Major Project of the Ministry of Science and Technology of China (2016ZX05016), and the National Science and Technology of China (2016ZX05046).Notes and references
- M. R. Islam, Y. Gao and X. Li, et al., Responsive polymers for biosensing and protein delivery, J. Mater. Chem. B, 2014, 2(17), 2444–2451 RSC.
- J. L. Drury and D. J. Mooney, Hydrogels for tissue engineering: scaffold design variables and applications, Biomaterials, 2003, 24(24), 4337–4351 CrossRef CAS.
- M. E. Roth-Konforti, M. Comune and M. Halperin-Sternfeld, et al., UV Light-Responsive Peptide-Based Supramolecular Hydrogel for Controlled Drug Delivery, Macromol. Rapid Commun., 2018, 39(24), 1800588 CrossRef PubMed.
- L. Yu and J. Ding, Injectable hydrogels as unique biomedical materials, Chem. Soc. Rev., 2008, 37(8), 1473–1481 RSC.
- H. Tan and K. G. Marra, Injectable, Biodegradable Hydrogels for Tissue Engineering Applications, Materials, 2010, 3(3), 1746–1767 CrossRef CAS.
- M. K. Nguyen and D. S. Lee, Injectable Biodegradable Hydrogels, Macromol. Biosci., 2010, 10(6), 563–579 CrossRef CAS PubMed.
- C. He, S. W. Kim and D. S. Lee, In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery, J. Controlled Release, 2008, 127(3), 189–207 CrossRef CAS PubMed.
- D. J. Adams and P. D. Topham, Peptide conjugate hydrogelators, Soft Matter, 2010, 6(16), 3707–3721 RSC.
- Y. Kuang and B. Xu, Disruption of the Dynamics of Microtubules and Selective Inhibition of Glioblastoma Cells by Nanofibers of Small Hydrophobic Molecules, Angew. Chem., Int. Ed., 2013, 52(27), 6944–6948 CrossRef CAS PubMed.
- A. Aggeli, M. Bell and N. Boden, et al., Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapes, Nature, 1997, 386(6622), 259–262 CrossRef CAS PubMed.
- C. Chen, D. Wu and W. Fu, et al., Peptide Hydrogels Assembled from Nonionic Alkyl-polypeptide Amphiphiles Prepared by Ring-Opening Polymerization, Biomacromolecules, 2013, 14(8), 2494–2498 CrossRef CAS.
- D. Zhao, Q. Tang and Q. Zhou, et al., A photo-degradable injectable self-healing hydrogel based on star poly(ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier, Soft Matter, 2018, 14(36), 7420–7428 RSC.
- V. K. Kotharangannagari, A. Sánchez-Ferrer and J. Ruokolainen, et al., Thermoreversible Gel–Sol Behavior of Rod–Coil–Rod Peptide-Based Triblock Copolymers, Macromolecules, 2012, 45(4), 1982–1990 CrossRef CAS.
- L. Dong, A. K. Agarwal and D. J. Beebe, et al., Adaptive liquid microlenses activated by stimuli-responsive hydrogels, Nature, 2006, 442(7102), 551–554 CrossRef CAS.
- H. J. Oh, M. K. Joo and Y. S. Sohn, et al., Secondary Structure Effect of Polypeptide on Reverse Thermal Gelation and Degradation of l/dl-Poly(alanine)–Poloxamer–l/dl-Poly(alanine) Copolymers, Macromolecules, 2008, 41(21), 8204–8209 CrossRef CAS.
- H. J. Moon, D. Y. Ko and M. H. Park, et al., Temperature-responsive compounds as in situ gelling biomedical materials, Chem. Soc. Rev., 2012, 41(14), 4860–4883 RSC.
- M. He, J. Li and S. Tan, et al., Photodegradable Supramolecular Hydrogels with Fluorescence Turn-On Reporter for Photomodulation of Cellular Microenvironments, J. Am. Chem. Soc., 2013, 135(50), 18718–18721 CrossRef CAS PubMed.
- Q. Tang, D. Zhao and H. Yang, et al., A pH-responsive self-healing hydrogel based on multivalent coordination of Ni2+ with polyhistidine-terminated PEG and IDA-modified oligochitosan, J. Mater. Chem. B, 2019, 7(1), 30–42 RSC.
- J. Li, Y. Gao and Y. Kuang, et al., Dephosphorylation of d-Peptide Derivatives to Form Biofunctional, Supramolecular Nanofibers/Hydrogels and Their Potential Applications for Intracellular Imaging and Intratumoral Chemotherapy, J. Am. Chem. Soc., 2013, 135(26), 9907–9914 CrossRef CAS PubMed.
- Q. Xu, C. He and K. Ren, et al., Thermosensitive Polypeptide Hydrogels as a Platform for ROS-Triggered Cargo Release with Innate Cytoprotective Ability under Oxidative Stress, Adv. Healthcare Mater., 2016, 5(15), 1979–1990 CrossRef CAS PubMed.
- G. Saravanakumar, J. Kim and W. J. Kim, Reactive-Oxygen-Species-Responsive Drug Delivery Systems: Promises and Challenges, Advanced Science, 2017, 4(1), 1600124 CrossRef.
- Z. Deng, Y. Qian and Y. Yu, et al., Engineering Intracellular Delivery Nanocarriers and Nanoreactors from Oxidation-Responsive Polymersomes via Synchronized Bilayer Cross-Linking and Permeabilizing Inside Live Cells, J. Am. Chem. Soc., 2016, 138(33), 10452–10466 CrossRef CAS PubMed.
- S. Yu, C. Wang and J. Yu, et al., Injectable Bioresponsive Gel Depot for Enhanced Immune Checkpoint Blockade, Adv. Mater., 2018, 30(28), e1801527 CrossRef.
- Q. Xu, C. He and C. Xiao, et al., Reactive Oxygen Species (ROS) Responsive Polymers for Biomedical Applications, Macromol. Biosci., 2016, 16(5), 635–646 CrossRef CAS PubMed.
- C. Wang, J. Wang and X. Zhang, et al., In situ formed reactive oxygen species–responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy, Sci. Transl. Med., 2018, 10(429), eaan3682 CrossRef.
- S. Yamada, K. Koga and A. Sudo, et al., Phosgene-free synthesis of polypeptides: Useful synthesis for hydrophobic polypeptides through polycondensation of activated urethane derivatives of α-amino acids, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(17), 3726–3731 CrossRef CAS.
- J. Moskovitz, S. Bar-Noy and W. M. Williams, et al., Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals, Proc. Natl. Acad. Sci. U. S. A., 2001, 98(23), 12920–12925 CrossRef CAS.
- D. Zhao, Q. Tang and Q. Zhou, et al., A photo-degradable injectable self-healing hydrogel based on star poly (ethylene glycol)-b-polypeptide as a potential pharmaceuticals delivery carrier, Soft Matter, 2018, 14(36), 7420–7428 RSC.
- H. Yamamoto, T. Kitsuki and A. Nishida, et al., Photoresponsive Peptide and Polypeptide Systems. 13. Photoinduced Cross-Linked Gel and Biodegradation Properties of Copoly(l-lysine) Containing ε-7-Coumaryloxyacetyl-l-lysine Residues, Macromolecules, 1999, 32(4), 1055–1061 CrossRef CAS.
- S. Matsumoto, S. Yamaguchi and S. Ueno, et al., Photo gel-sol/sol-gel transition and its patterning of a supramolecular hydrogel as stimuli-responsive biomaterials, Chemistry, 2008, 14(13), 3977–3986 CrossRef CAS.
- A. M. Kloxin, A. M. Kasko and C. N. Salinas, et al., Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties, Science, 2009,(324), 59–63 CrossRef CAS.
- Y. Yang, J. Zhang and Z. Liu, et al., Tissue-Integratable and Biocompatible Photogelation by the Imine Crosslinking Reaction, Adv. Mater., 2016, 28(14), 2724–2730 CrossRef CAS PubMed.
- G. Pasparakis, T. Manouras and P. Argitis, et al., Photodegradable polymers for biotechnological applications, Macromol. Rapid Commun., 2012, 33(3), 183–198 CrossRef CAS.
- D. D. McKinnon, T. E. Brown and K. A. Kyburz, et al., Design and characterization of a synthetically accessible, photodegradable hydrogel for user-directed formation of neural networks, Biomacromolecules, 2014, 15(7), 2808–2816 CrossRef CAS.
- A. M. Kloxin, M. W. Tibbitt and K. S. Anseth, Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms, Nat. Protoc., 2010, 5(12), 1867–1887 CrossRef CAS PubMed.
- I. Tomatsu, K. Peng and A. Kros, Photoresponsive hydrogels for biomedical applications, Adv. Drug Delivery Rev., 2011, 63(14–15), 1257–1266 CrossRef CAS PubMed.
- M. A. Azagarsamy, D. D. McKinnon and D. L. Alge, et al., Coumarin-Based Photodegradable Hydrogel: Design, Synthesis, Gelation, and Degradation Kinetics, ACS Macro Lett., 2014, 3(6), 515–519 CrossRef CAS PubMed.
- G. E. Negri and T. J. Deming, Triggered Copolypeptide Hydrogel Degradation Using Photolabile Lysine Protecting Groups, ACS Macro Lett., 2016, 5(11), 1253–1256 CrossRef CAS.
- L. Yin, H. Tang and K. H. Kim, et al., Light-responsive helical polypeptides capable of reducing toxicity and unpacking DNA: toward nonviral gene delivery, Angew. Chem., Int. Ed., 2013, 52(35), 9182–9186 CrossRef CAS PubMed.
- M. K. Nguyen, C. T. Huynh and G. H. Gao, et al., Biodegradable oligo(amidoamine/β-amino ester) hydrogels for controlled insulin delivery, Soft Matter, 2011, 7(6), 2994–3001 RSC.
- J. Zhuang, M. R. Gordon and J. Ventura, et al., Multi-stimuli responsive macromolecules and their assemblies, Chem. Soc. Rev., 2013, 42(17), 7421–7435 RSC.
- A. Fraix, R. Gref and S. Sortino, A multi-photoresponsive supramolecular hydrogel with dual-color fluorescence and dual-modal photodynamic action, J. Mater. Chem. B, 2014, 2(22), 3443–3449 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05156k |
| This journal is © The Royal Society of Chemistry 2023 |