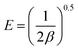Open Access Article
Open Access ArticleFluorescent molecularly imprinted polymer nanocomposite for solid-phase extraction and fluorimetric determination of hydrochlorothiazide
Ahmed S. Abo Dena *ab,
Mariam Dhaouac and
Ibrahim M. El-Sherbiny
*ab,
Mariam Dhaouac and
Ibrahim M. El-Sherbiny *a
*a
aNanomedicine Laboratories, Centre for Materials Science (CMS), Zewail City of Science and Technology, 6 October City, 12578, Giza, Egypt. E-mail: ielsherbiny@zewailcity.edu.eg; aabdelmawgoud@zewailcity.edu.eg
bPharmaceutical Chemistry Department, National Organization for Drug Control and Research (NODCAR), Giza, Egypt
cLaboratory of Materials for Energy and Environment, and Modelling, Faculty of Science, University of Sfax, 3000, Sfax, Tunisia
First published on 4th October 2023
Abstract
We report herein a fluorescent molecularly imprinted polymer (FMIP) for the solid-phase extraction (SPE) and fluorimetric determination of hydrochlorothiazide (HCTZ) in water. The FMIP is based on fluorescent polystyrene nanoparticles embedded within a molecularly imprinted polyaniline (PANI) matrix. The operational adsorption parameters such as the initial HCTZ concentration, incubation time and the solution pH were found to influence the removal efficiency. At optimum conditions, a high adsorption capacity of the FMIP was found (2.08 mg g−1). Evidence of the adsorption process was confirmed by the change in the FMIP physicochemical properties measured by FTIR absorption spectroscopy and electron microscopy. Based on the regression R2 values and the consistently low values of the adsorption statistical error functions, equilibrium data were best fitted to both Freundlich and Temkin isotherms. Moreover, the pseudo-second-order kinetic model described the adsorption kinetics, and the mechanism of the adsorption process was explained by the intraparticle diffusion model. Upon studying adsorption thermodynamics, negative ΔG values (−26.18 kJ mol−1 at room temperature) were obtained revealing that the adsorption process is spontaneous. Interestingly, the maximum adsorption capacity was obtained at 298 K, pH 7.0, and using a high HCTZ concentration, thus revealing the suitability of the proposed FMIP for easy and fast SPE of HCTZ. The FMIP showed an imprinting factor of 1.19 implying the selectivity over the corresponding FNIP. Eventually, the proposed FMIP was successfully applied to the spectrofluorimetric determination of HCTZ in aqueous samples with %recovery values close to 100%.
Introduction
Pharmaceutical compounds represent an environmental concern because of their existence in aquatic ecosystems within the effluents of pharmaceutical factories, leading to ecotoxicological effects. In addition, due to their extensive use for disease treatments in humans and animals, wastewater discharges into municipal systems present high concentrations of these compounds and their metabolites, leading to harmful effects on the environment, that could reach humans and animals via direct or indirect ways.1 Hydrochlorothiazide (HCTZ), a common pharmaceutical compound (Scheme 1), is one of the oldest members of thiazide diuretics, which comprises two sulfonamide groups. The IUPAC name of HCTZ is 6-chloro-1,1-doxo-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide. Despite its poor water solubility at room temperature (20–30 mg L−1), HCTZ remains one of the most dangerous water contaminants. It has been extensively used for the treatment of hypertension and cardiovascular morbidity,2–4 oedema (caused by using certain medications such as oestrogen and corticosteroids), liver damage, swelling (due to kidney problems), muscle spasms, erectile dysfunction, and photosensitivity. In fact, as drugs are designed to produce pharmacological effects using low doses, they are capable of producing negative eco-toxicological as well as health effects on humans and other organisms when introduced to the environment even at low concentrations.Due to their serious toxicological effects, wastewater contamination with pharmaceutical residues is a topic of great interest. Numerous water purification techniques are being used; for instance, advanced oxidative degradation, electrocoagulation, membrane filtration, biochemical degradation, flocculation, chemical precipitation, reverse osmosis, electrodialysis and ion-exchange methods. Some studies have focused on the development of methods such as solid-phase extraction (SPE), solid-phase microextraction (SPME), and ultrasound-assisted dispersive liquid–liquid microextraction. Alternatively, adsorption is considered one of the most promising techniques owing to the ease of operation, low cost, and design flexibility. It presents a profitable, robust and universal approach for the elimination of toxic pharmaceutical residues from aquatic environments.
In recent years, the adsorption of water pollutants using molecularly imprinted polymers (MIPs) has attracted the attention of researchers. MIPs are versatile and robust polymers that can be easily tailored for fast and selective separation,5 chemical sensing,6 catalysis,7,8 biological imitation,9 and other fields due to the specific active sites or cavities they have, that provide selective recognition of small or large molecules, high binding capacity, and stable physiochemical properties.10 Moreover, considering their specific adsorption power, MIPs have a great potential as carrier materials in clinical and pharmaceutical applications such as the analysis of biological samples and drug delivery.11 The MIP is firstly prepared by a simple polymerization or copolymerization method in the presence of the target template molecules (i.e. analyte molecules) and a crosslinking agent under appropriate reaction conditions. The template molecules are then removed leaving recognition cavities complementary in shape, size, and co-ordination geometry to themselves.12
The selection of the functional monomers is critical to the molecular recognition ability. Not only does an appropriate functional monomer enhance the affinity of the template molecules to the MIP, but also it reduces the nonspecific adsorption to enhance its specificity towards the template molecules.13,14 In previous studies, polyaniline (PANI)-based MIP exhibited good selective adsorption15 to template molecules such as benzophenone-4,16 ascorbic acid,17 2,4-dinitrophenol,18 antibiotic residues,19 acrylamide,20 and horseradish peroxidase.21 PANI is a synthetic semi-crystalline organic polymer that does not require high-temperature polymerization conditions nor a crosslinking agent. The recent interest in PANI applications is due to its unique properties including the ease of fabrication, low cost, high stability, chemical resistance and high electrical conductivity. Because of these unique properties, PANI is a potential candidate as a MIP adsorbent for wastewater purification.22,23 The integration of a fluorescent material with PANI-based MIPs results in excellent dual-use MIP materials. These materials can be used for the SPE as well as the fluorimetric determination of the specific analytes for which they are tailored.
In this regard, the current study aims at tailoring and synthesizing a fluorescent MIP (FMIP)-based adsorbent for the SPE and spectrofluorimetric determination of HCTZ in aqueous media. The proposed FMIP is based on a nanocomposite of polystyrene nanoparticles (PSNPs) and molecularly imprinted PANI nanofibers. The PSNPs were incorporated into the PANI matrix in order to provide the MIP with light-emitting properties that can be useful in spectrofluorimetric determination of HCTZ. The synthesized nanocomposite was characterized by thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), high-resolution transmission electron microscopy (HR-TEM), Fourier-transform infrared absorption spectroscopy (FTIR), dynamic light scattering (DLS) and zeta potential measurements. The effect of the adsorbent dose and the HCTZ initial concentration on the adsorption capacity of the FMIP was studied. Moreover, adsorption isotherms, kinetic models and the thermodynamics of the adsorption process were thoroughly investigated. To the best of our knowledge, this is the first study to apply a dual-use FMIP material for SPE and spectrofluorimetric determination of HCTZ in its aqueous solutions.
Materials and methods
Materials
HCTZ and methyclothiazide (MTZ) standards were obtained from the Egyptian Drug Authority, Egypt. For the synthesis of the molecularly imprinted PANI, aniline (Sigma-Aldrich, Germany), acetonitrile (Sigma-Aldrich, Germany) and potassium persulfate (PPS, Laborchemie Apolda GmbH, Germany) were used. Acetic acid (Honeywell International Inc., USA) and anhydrous hydrochloric acid (≥99.5%) was purchased from Sigma-Aldrich, Germany. All the used chemicals and reagents are of analytical grade, and they were used without any further purification prior to the experimental procedure. HPLC grade dichloromethane (DCM) and methanol were used during the course of the experimental work.Preparation of PSNPs
The synthesis of PSNPs was achieved through the following steps. First, accurately weighed 2.0 g of polystyrene (PS) were dissolved in 20 mL of dichloromethane (Sigma-Aldrich, Germany). Thereafter, this solution was dropped onto 100 mL of 3% (w/v) polyvinyl alcohol (PVA, Acros Organics, USA) with continuous vigorous mechanical stirring until the whole amount of dichloromethane is evaporated. Subsequently, the produced PSNPs were collected by centrifugation at 9000 rpm for 10 min. The collected amount of PSNPs was washed several times, and then left to dry overnight at 60 °C in a vacuum oven.Preparation of FMIP
Accurately weighed 100 mg of PSNPs were well dispersed in 10 mL of HCl (1 M) containing 90 μL of aniline via magnetic stirring. In addition, an acetonitrile/HCl (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) solution containing 0.297 g of dissolved HCTZ were added to the above mixture, and the resulting mixture was agitated in an ice bath at 5 °C. Thereafter, 10 mL of PPS solution (20 mg mL−1 in 1 M HCl) was dropped onto the above mixture with continuous stirring. The obtained MIP/PSNPs composite (i.e. FMIP) was collected and washed twice with distilled water, and then dried in ambient temperature. The appearance of a dark green colour indicates the formation of the emeraldine salt of PANI (Fig. 1). The FNIP was prepared by following the same procedure without the addition of HCTZ. The obtained FMIP was collected from the suspension via centrifugation at 9000 rpm for 10 min, and then washed thrice with distilled water. Subsequently, the incorporated HCTZ molecules were removed from the FMIP via repeated washing with a mixture of methanol and 1% acetic acid solution (9
10, v/v) solution containing 0.297 g of dissolved HCTZ were added to the above mixture, and the resulting mixture was agitated in an ice bath at 5 °C. Thereafter, 10 mL of PPS solution (20 mg mL−1 in 1 M HCl) was dropped onto the above mixture with continuous stirring. The obtained MIP/PSNPs composite (i.e. FMIP) was collected and washed twice with distilled water, and then dried in ambient temperature. The appearance of a dark green colour indicates the formation of the emeraldine salt of PANI (Fig. 1). The FNIP was prepared by following the same procedure without the addition of HCTZ. The obtained FMIP was collected from the suspension via centrifugation at 9000 rpm for 10 min, and then washed thrice with distilled water. Subsequently, the incorporated HCTZ molecules were removed from the FMIP via repeated washing with a mixture of methanol and 1% acetic acid solution (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) until no HCTZ could be detected spectrophotometrically at 272 nm. Thereafter, the resultant solid was washed several times with methanol to remove the excess acetic acid. The resulting FMIP was left overnight to dry at room temperature. In order to reduce any systematic errors, the prepared FNIP was handled in a similar manner.
1, v/v) until no HCTZ could be detected spectrophotometrically at 272 nm. Thereafter, the resultant solid was washed several times with methanol to remove the excess acetic acid. The resulting FMIP was left overnight to dry at room temperature. In order to reduce any systematic errors, the prepared FNIP was handled in a similar manner.
 | ||
| Fig. 1 Schematic representation of the preparation steps of FMIP and its use in the SPE and fluorimetric determination of HCTZ. | ||
Materials characterization
The FTIR spectra of the FMIP as well as the FNIP were recorded over the spectral window 4000–400 cm−1 with the aid of a Nicolet iS10 Mid infrared ATR-FTIR spectrometer (Thermoscientific, USA). Electron micrographs were recorded using a JEOL JEM-2100 HR-TEM (USA) for investigating the morphology of the prepared nanocomposites. The DLS measurements were carried out with the aid of a Malvern instrument.The effect of pH on the drug adsorption was studied by using working solutions of different pH values (pH 2–10) adjusted by a 0.1 mol L−1 solution of hydrochloric acid or sodium hydroxide. The pH of the prepared solutions was measured using a JENCO 6173 pH meter.
The specific adsorption ratio was calculated from the following formula (eqn (1)):23
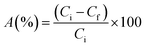 | (1) |
The adsorption capacities of the prepared FMIP and FNIP were calculated from eqn (2):23
 | (2) |
The imprinting factor (IF) was calculated as the ratio between the binding capacity of FMIP and that of the corresponding FNIP using eqn (3):
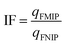 | (3) |
An adsorption isotherm is a plot that describes the adsorption equilibrium at constant pH and temperature.23,28 The Langmuir (eqn (7)), Freundlich (eqn (8)), and Temkin (eqn (9)) isotherms are the most common and traditional isotherm models used. These models were applied in the present study in order to investigate the release/retention of HCTZ from/by the FMIP in aqueous solution.
| Ka = Cad/Ce | (13) |
 | (14) |
Additionally, the change in Gibbs free energy (ΔG) was calculated from eqn (15).23,28
| ΔG = ΔH − TΔS | (15) |
Spectrofluorimetric determination of HCTZ
A standard curve was plotted by measuring the fluorescence emission intensity of solutions containing increasing concentrations of HCTZ and a fixed amount of the prepared FMIP using a Shimazdu RF-6000 Spectrofluorimeter, Japan. The concentrations of the used solutions are in the range of 2.86–22.86 μmol L−1. The emission spectra were recorded in the spectral range of 200–400 nm. For the validation of the proposed method, authentic sample solutions of HCTZ were prepared by spiking bidistilled water with different amounts of HCTZ, and then the samples were analysed using the above procedure and the recovery values as well as all the analytical parameters were calculated.Results and discussion
This section will cover the results obtained from the characterization techniques along with the optimization procedure of the adsorption conditions of the drug under investigation (HCTZ) onto the suggested FMIP. In addition, the results of the spectrofluorimetric analytical method used for the determination of HCTZ in aqueous solution using the same FMIP are introduced herein.FTTR analysis
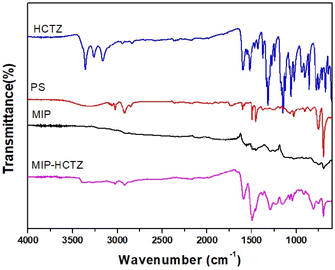
The FTIR spectrum of HCTZ (Fig. 2) shows characteristic absorption bands at 2986, 1551, 1413, 1272, 1169, 1019, 970, 828, and 674 cm−1 that can be assigned to C–H stretching, C–O stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching, pyridine stretching, ring vibration, ring breathing, P–O–C, P
N stretching, pyridine stretching, ring vibration, ring breathing, P–O–C, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretching, and C–Cl stretching, respectively.33
S stretching, and C–Cl stretching, respectively.33
In the case of pure PS, the FTIR spectrum revealed a characteristic band at about 756 and 698 cm−1 corresponding to the C–H. The sharp bands appearing at about 1600, 1492, and 1452 cm−1 indicate the presence of aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C. In addition, the presence of absorption bands at 2921 and 2848 cm−1 may correspond to the existence of a methylene group. The presence of hydroxyl groups was confirmed by the appearance of a strong broad band at 3650–3000 cm−1.34
C. In addition, the presence of absorption bands at 2921 and 2848 cm−1 may correspond to the existence of a methylene group. The presence of hydroxyl groups was confirmed by the appearance of a strong broad band at 3650–3000 cm−1.34
For the FMIP, the absence of the hydroxyl band at 3500–3300 cm−1 negates the presence of bound water molecules; indicating that the FMIP sample was well dried prior to the FTIR measurement. The broad absorption band positioned at a wavenumber higher than 2000 cm−1 is a typical band of conducting PANI. Moreover, the strong broad band positioned around 1000 cm−1 is attributed to the bond vibrations of –NH+![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) , Q
, Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH+–B, or B–NH+–B, where B is the benzenoid unit, and Q is the quinonoid unit. This band also confirms the dihedral angles distribution between B and Q rings as well as the presence of positively charged moieties in the PANI chains, thus confirming the formation of emeraldine salt.35 The large similarity between the FTIR spectrum of HCTZ and that of the unwashed FMIP (MIP-HCTZ) confirms the successful binding of HCTZ to the PANI backbone. For instance, all the characteristic HCTZ absorption bands appear at 3360, 3161, 2944, 1591,1380, 1013, 753, 552, and 540 cm−1 in the MIP-HCTZ spectrum.
NH+–B, or B–NH+–B, where B is the benzenoid unit, and Q is the quinonoid unit. This band also confirms the dihedral angles distribution between B and Q rings as well as the presence of positively charged moieties in the PANI chains, thus confirming the formation of emeraldine salt.35 The large similarity between the FTIR spectrum of HCTZ and that of the unwashed FMIP (MIP-HCTZ) confirms the successful binding of HCTZ to the PANI backbone. For instance, all the characteristic HCTZ absorption bands appear at 3360, 3161, 2944, 1591,1380, 1013, 753, 552, and 540 cm−1 in the MIP-HCTZ spectrum.
DLS measurements and electron microscopy
The average particle size of the as prepared PSNPs was investigated with dynamic light scattering measurement (Fig. 3a), and was found to be 284.4 nm. To further confirm the particle size of PSNPs obtained from DLS measurements, and to explore their morphology, the nanoparticles were imaged with transmission electron microscopy. As depicted in Fig. 3b, the prepared PSNPs exhibit a well-defined spherical shape and a homogeneous size of less than 100 nm. It is worth mentioning that the high hydrophobicity of the PSNPs prevents their agglomerations, that is why no aggregates could be seen in the TEM image. It is obvious from these two characterization techniques that there is a slight difference between the two recorded particles sizes since the size obtained from DLS measurements appears slightly larger than its TEM counterpart. In order to justify this, it is necessary to explain the principle of DLS measurements. A rapid photon counter is used in a DLS experiment to track time-dependent variations in the scattered light signal. The hydrodynamic radius may be computed using the macromolecule or particle's diffusion coefficient, which can be obtained by DLS measurements. Accordingly, the DLS instrument does not have the ability to discriminate single particles from aggregates, and hence it provides an average particle size for both, which is usually larger than the size of the particles imaged by TEM.Contact time and adsorption kinetics
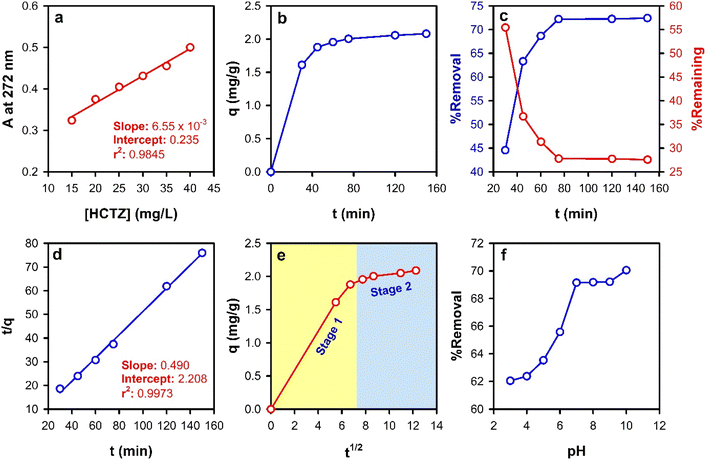
One of the important relevant parameters that control the transfer of adsorbate molecules (and the build-up of charges) from the bulk to the pores or the active sites of the adsorbent (FMIP in the present study) is the contact time. The influence of the contact time was studied over the period of 30–150 min via measuring the absorbance of the solutions at 272 nm. As illustrated in Fig. 4b, a rapid adsorption was observed in the first 30 min due to the high attractive forces between the FMIP's active sites and the HCTZ molecules. Thereafter, after 70 min, there was no significant increase in the amount of adsorbed HCTZ. After 90 min, the removal efficiency reached a plateau, meaning that the FMIP surface became saturated and the system has attained equilibrium. Therefore, the optimum contact time of the FMIP with the HCTZ solution was selected at 75 min. A fast diffusion towards the surface of the FMIP was followed as a result of the participation of the functional groups until equilibrium was attained, where about 72% removal efficiency was achieved. The change in the rate of HCTZ ion removal might be due to the fact that initially all the FMIP active sites were vacant. Afterwards, the number of the available active sites decreases continuously leading to a corresponding decrease in the rate of adsorption. The mean adsorption capacity of the proposed FMIP was found to be 2.0 mg g−1.The adsorption kinetic models are basically used to understand the mechanism of the adsorption process by fitting the obtained experimental data to the theoretical kinetic models such as PFO, PSO, Elovich, fractional power (power function), and the IPD. The application of the PSO kinetic model yielded a perfect fit to the experimental results obtained for the adsorption of HCTZ ions onto the FMIP at the tested concentration (50 mg L−1), with a coefficient of determination of R2 = 0.995. The experimental value of qe is usually higher than the value calculated from the PSO kinetic model. Curiously, qe and k2 (the adsorption rate constant) values calculated from the PSO kinetic model were found to be 2.04 mg g−1 and 5 × 10−3 g mg−1 min−1, respectively (Fig. 4d). Interestingly, it is obvious from the qe values that the experimental and PSO-calculated values are almost identical. Fitting of the experimental data to the PSO kinetic model implies that the chemisorption of HCTZ is the rate-limiting step, and the removal of HCTZ molecules from bulk solution is due to the physicochemical interactions between HCTZ and the FMIP surface.
The IPD model explains the removal mechanism of HCTZ by FMIP, as well to the PFO and the PSO models. Fig. 4e displays a two-stage adsorption process. Stage 1 resembles the intraparticle or pore diffusion, where the adsorbate molecules percolate into the interior of the adsorbent particles. This stage involves the diffusion of the adsorbate from the bulk through the liquid film that surrounds the surface of the adsorbent particles. On the other hand, stage 2 represents the equilibrium stage, where the adsorption and desorption processes of HCTZ molecules onto/from the FMIP occur at comparable rates. The calculated values of the rate constant (kIPD) are 0.28 and 0.027 mg g−1 min−1/2 for stage 1 and stage 2, respectively, demonstrating that the rate of adsorption is faster in case of the former. This can be also confirmed by comparing the values of the intercepts. Larger intercept values imply a greater effect of the boundary layer. The obtained intercept values are 0.008 mg g−1 and 1.75 mg g−1 for stage 1 and stage 2, respectively. The results of the applied adsorption kinetic models are summarized in Table 2.
| Kinetic model | Parameters | Calculated value |
|---|---|---|
| PFO | k1 (L min−1) | −0.063 |
| qe (mg g−1) | 2.17 | |
| PSO | k2 (L min−1) | 0.364 |
| qe (mg g−1) | 2.040 | |
| IPD | KIPD (stage 1) (mg g−1 min−1/2) | 0.28 |
| KIPD (stage 2) (mg g−1 min−1/2) | 0.027 |
Influence of pH
The effect of pH on the adsorption of HCTZ molecules onto the fabricated FMIP was studied and the obtained results are shown in Fig. 4f. The solution pH was varied to cover a range of 2–10. The ionic mobility, the degree of ionization, and the surface chemistry of the FMIP were influenced by the changes in solution pH. The test was conducted at the equilibrium contact time (75 min) using 20 mg of FMIP and 50 mg L−1 solution of HCTZ as an initial HCTZ concentration. At low pH values, protonation of PANI (emeraldine salt) results in charging of the FMIP surface with a large number of positive charges.23 Simultaneously, the amino groups of the HCTZ molecules get protonated forming positively charged quaternary ammonium groups. Accordingly, the repulsion between the HCTZ molecules and the surface of the adsorbent hinders the attachment of the HCTZ molecules to their specific active sites, and the rate of adsorption increases as the pH becomes more basic. The maximum adsorption capacity was attained at a solution pH of 7.0, where about 69% of the initial HCTZ amount was removed.Adsorption isotherms
The adsorption equilibrium data was evaluated with the commonly employed isotherm models such as Langmuir, Freundlich, Temkin, Dubinin–Radushkevich, Halsey and Jovanovic. Fig. 5 depicts the original linearized mathematical expressions of the applied isotherm models mentioned above.The influence of increasing the initial HCTZ concentration on the adsorption capacity of the FMIP was used to calculate the IF of the synthesized FMIP (Fig. 5a and b). This was achieved by comparing the maximum adsorption capacity recorded for the FMIP with that recorded for the corresponding FNIP, as explained in the Materials and methods section (Adsorption measurements). The calculated IF was found to be 1.19 indicating the higher selectivity of the FMIP over the FNIP towards the HCTZ molecules.
As shown in Fig. 5c, the Langmuir model has not demonstrated the best fitting to the experimental data of HCTZ adsorption onto the FMIP. The same can be noticed in the case of the Jevanovic model (Fig. 5h). This conclusion was drawn by the obtained low values of R2 which attained 0.75 and 0.95, respectively. The calculated values of qmax and bL were found to be −0.301 mg g−1 and −0.391, respectively. The negative value of qmax indicates the inadequacy of the Langmuir isotherm to describe the adsorption of HCTZ molecules onto the FMIP surface.
Unlikely, the linear correlation coefficient of Freundlich isotherm plot (R2 = 0.978) suggests that Freundlich isotherm fits the experimental data due to the multilayer adsorption to the heterogeneous surface (Fig. 5d). The characteristic parameters of the Freundlich isotherm model are the KF (adsorption coefficient) and the nF values which were found to be 3.13 and 1.22, respectively. The small value of nF indicates that the adsorption of more HCTZ molecules becomes difficult/unfavourable as the amount of the adsorbed HCTZ increases on the FMIP surface, thus interpreting the relatively long time required for attaining the equilibrium. A better fit to the experimental results, with an R2 value of 0.9851, was observed in the case of Temkin isotherm. The theoretical adsorption capacities were in good agreement with the experimental values for both the Langmuir and Freundlich isotherm models (Table 3). The values of the separation factor (RL) indicate the adsorption nature to either unfavourable or favourable, it is unfavourable if RL > 1, linear if RL = 1, favourable if 0 < RL < 1, and reversible if RL = 0. The calculated values of RL ranged from 0.0188 to 0.0761 indicating that the adsorption process is favourable.
| Isotherm model | Parameter symbol | Parameter value |
|---|---|---|
| Langmuir | qL (mg g−1) | 0.301 |
| KL (L mg−1) | 0.391 | |
| RL | 0.0188–0.0761 | |
| R2 | 0.7157 | |
| Freundlich | KF | 3.131 |
| nF | 1.220 | |
| R2 | 0.9787 | |
| Temkin | bT | 1024 |
| B (J mol−1) | 12.128 | |
| A (L g−1) | 2.447 | |
| R2 | 0.9851 | |
| D–R | QD–R (mg g−1) | 0.221 |
| KD–R | −1.146 | |
| E | −1.514 | |
| R2 | 0.9793 | |
| Halsey | nH | 1.251 |
| KH | 2.129 | |
| R2 | 0.9787 | |
| Javanovic | QJ (mg g−1) | 5.084 |
| KJ | 1.599 | |
| R2 | 0.9508 |
Temkin isotherm (Fig. 5e) assumes a linear variation of the heat of adsorption with the degree of overlap. Since the heat of adsorption is a function of temperature of all molecules in the surface layer of the adsorbent, the plot decreases linearly as a result of increasing the surface coverage. The value of the constant b determines the type of the sorption process. When bT value is less than 8 × 104, this indicates a physical adsorption. Though, the value of the term RT/bT, also termed as B, gives information about the heat of adsorption. B-Values less than 8 kJ mol−1 indicate a weak interaction between the adsorbate molecules and the adsorbent surface. The calculated bT value is 1024 and the B value was found to be 12.128 J mol−1. These values were obtained by plotting qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce, which showed an R2 value of 0.985. From regression coefficient values, it is obvious that both Temkin and Freundlich isotherms demonstrated the best fitting to the experimental results; thus confirming the involvement of physical adsorption in the governing mechanism of the removal of HCTZ by the proposed FMIP adsorbent.
Ce, which showed an R2 value of 0.985. From regression coefficient values, it is obvious that both Temkin and Freundlich isotherms demonstrated the best fitting to the experimental results; thus confirming the involvement of physical adsorption in the governing mechanism of the removal of HCTZ by the proposed FMIP adsorbent.
Adsorption thermodynamics and the effect of temperature
To build up a perspective about the mechanism of HCTZ adsorption at the surface of the FMIP, the adsorption equilibrium constant (Ka) was calculated at different test solution temperatures, and then the thermodynamic parameters were subsequently obtained. The calculated negative values of ΔG at the studied temperatures indicate that HCTZ adsorption onto the FMIP is a spontaneous process, and HCTZ molecules tend to stay in the stationary phase near to the FMIP surface. Normally, physisorption is indicated by standard free energy change values (ΔG) ranging from 0 to 20 kJ mol−1, whereas these of chemisorption range from 80 to 400 kJ mol−1. The calculated values of ΔG (Table 4) confirm that the adsorption process takes place by physisorption. Therefore, the involvement of the imprinted active sites on the FMIP surface is highly acceptable, which coincides with the calculated IF value (∼1.19).| T (K) | Ka | Slope | Intercept | ΔH (J mol−1) | ΔS (J mol−1 K−1) | ΔG (kJ mol−1) |
|---|---|---|---|---|---|---|
| 298 | 416.34 | 4.85 | −10.55 | −40.32 | −87.71 | −26.181 |
| 300 | 263.28 | −26.356 | ||||
| 305 | 165.66 | −26.795 | ||||
| 310 | 127.80 | −27.723 | ||||
| 320 | 125 | −28.111 |
Analytical applications
In order to investigate the ability of the proposed FMIP material to respond to changes in the HCTZ concentration, spiked water samples were prepared and analysed for the concentration of HCTZ using the proposed spectrofluorimetric assay method, and the analytical parameters and the recovery percentages were calculated (Table 5).| Analytical parameters | |
|---|---|
| Linear range, mol L−1 | 3.8 × 10−6-3.1 × 10−5 |
| Limit of detection (LOD), mol L−1 | 1.14 × 10−6 |
| Limit of quantification (LOQ), mol L−1 | 3.8 × 10−6 |
| Intercept (a) | 1.000 |
| Slope (b) | 0.013 |
| R2 | 0.9949 |
| Recovery study | |||
|---|---|---|---|
| Taken (μg) | Found (μg) | Recovery (%) | SD |
| Spiked distilled water samples | |||
| 1.0 | 1.00 | 100.00 | 0.45 |
| 2.0 | 2.10 | 105.0 | 0.84 |
| 4.0 | 3.95 | 98.75 | 0.46 |
| 6.0 | 6.34 | 105.7 | 0.76 |
| 8.0 | 8.23 | 102.9 | 0.24 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Spiked tap water samples | |||
| 2 | 2.05 | 102.5 | 0.32 |
| 4 | 4.13 | 103.3 | 0.27 |
| 8 | 7.94 | 99.25 | 0.51 |
The calibration curve (Fig. 6) used for the determination of the HCTZ concentration in the above-mentioned spiked samples showed linearity over the concentration range of 3.8 × 10−6–3.1 × 10−5 mol L−1 and the recovery values ranged from 98.75 to 105.7%. In addition, the R2 value of the standard plot is 0.9949. Moreover, it is worth mentioning that a low limit of detection (LOD) was obtained (1.14 × 10−6 mol L−1) indicating the sensitivity of the proposed analytical method. The low values of standard deviation (SD, 0.24–0.84) indicate the precision of the proposed procedure, while recovery values near to 100% indicate its accuracy. To investigate the selectivity of the present fluorimetric sensor, samples of HCTZ were spiked with methyclothiazide in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (HCTZ
10 (HCTZ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTZ) and then the samples were analysed using the calibration curve procedures described herein. The results indicate that despite the great structural similarity between HCTZ and MTZ, the proposed fluorimetric sensor demonstrated a high selectivity towards HCTZ as revealed by the obtained accurate recovery value (97.3%).
MTZ) and then the samples were analysed using the calibration curve procedures described herein. The results indicate that despite the great structural similarity between HCTZ and MTZ, the proposed fluorimetric sensor demonstrated a high selectivity towards HCTZ as revealed by the obtained accurate recovery value (97.3%).
 | ||
| Fig. 6 Standard plot for the determination of HCTZ using the proposed FMIP-based fluorimetric sensor. F and Fo are the fluorescence intensities of the test and blank samples, respectively. | ||
The applicability of the investigated sensor to real water sample analysis was evaluated via analysing multiple tap water samples spiked with HCTZ. The obtained high recovery and low standard deviation values (Table 5) indicate the accuracy and precision of the proposed analytical method. Table 6 compares the analytical parameters of the proposed analytical method in the present work with those of some other reported methods.
| Analytical method | LOD (μg mL−1) | Linear range (ng mL−1) | Reference |
|---|---|---|---|
| MIP-based spectrofluorometry | 339.4 | 11.31–9.2 × 103 | This work |
| Capillary electrophoresis | — | 2 × 104–6 × 105 | 29 |
| LC/TMS | 0.230 | 0.78–200 | 30 |
| Electrochemical sensor | 357.3 | 8.9 × 102–2.2 × 103 | 31 |
| Electrochemical sensor | 5.955 | 14.9–5.95 × 104 | 32 |
| Time-resolved chemiluminescence | 340.0 | 5 × 102–3 × 104 | 33 |
| 160.0 | |||
| HPLC/fluorescence | 20.00 | 20–1 × 103 | 34 |
Conclusion
Here, we studied the successful adsorption of HCTZ ions on the proposed FMIP. The synthesized FMIP material was characterized using the suitable characterization techniques such as FTIR spectroscopy, electron microscopy, and dynamic light scattering. Investigating the interaction of the HCTZ molecules with the surface functional groups of the FMIP revealed that the adsorption process takes place via a physisorption mechanism. The adsorption followed a pseudo-second-order kinetics and the highest SPE efficiency was attained at room temperature and at pH 7.0; thus allowing for facile and fast separation and analysis of HCTZ. The FMIP was successfully applied to the determination of HCTZ in aqueous samples via the spectrofluorimetric technique. The novelty of this work is that the proposed FMIP material has a dual function, since it can be used simultaneously for both the SPE extraction and the assay of HCTZ in its aqueous samples. This property allows for the fast analysis of HCTZ in complex samples which require sample pre-treatment procedure such as separation and/or masking of the undesired interfering species.Author contributions
Ahmed S. Abo Dena: conceptualization, methodology, investigation, formal analysis, data curation, visualization, writing-review and editing. Mariam Dhaou: methodology, investigation, writing-original draft. Ibrahim M. El-Sherbiny: supervision, resources, project administration, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The corresponding author would like to thank the Science, Technology and Innovation Funding Authority (STDF), Egypt (Capacity Building Fund, CB-22808 and FLUG Call 1, Project ID: 46715).Notes and references
- S. Dineva, K. Uzunova, V. Pavlova, E. Filipova, K. Kalinov and T. Vekov, J. Hum. Hypertens., 2019, 33, 766–774 CAS.
- K. M. Neff and J. J. Nawarskas, Cardiol. Rev., 2010, 18, 51–56 CrossRef PubMed.
- The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V), Arch. Intern. Med., 1993, 153, 2, 154–183 Search PubMed.
- Mortality after 10 1/2 years for hypertensive participants in the Multiple Risk Factor Intervention Trial, Circulation, 1990, 82, 5, 1616–1628 Search PubMed.
- A. Asfaram, M. Ghaedi and K. Dashtian, Ultrason. Sonochem., 2017, 34, 640–650 CrossRef CAS PubMed.
- J. W. Lowdon, H. Diliën, P. Singla, M. Peeters, T. J. Cleij, B. van Grinsven and K. Eersels, Sens. Actuators, B, 2020, 325, 128973 CrossRef CAS PubMed.
- T. Kubo and K. Otsuka, J. Pharm. Biomed. Anal., 2016, 130, 68–80 CrossRef CAS PubMed.
- X. Xin, H. Liu, N. Zhong, M. Zhao, D. Zhong, H. Chang, B. Tang, Y. He, C. Peng and X. He, Sens. Actuators, B, 2022, 357, 131468 CrossRef CAS.
- G. K. Ali and K. M. Omer, Talanta, 2022, 236, 122878 CrossRef CAS PubMed.
- W. Zhang, X. She, L. Wang, H. Fan, Q. Zhou, X. Huang and J. Z. Tang, Materials, 2017, 10(5), 475–490 CrossRef CAS PubMed.
- B. Sellergren and C. J. Allender, Adv. Drug Delivery Rev., 2005, 57, 1733–1741 CrossRef CAS PubMed.
- L. Chen, S. Xu and J. Li, Chem. Soc. Rev., 2011, 40, 2922–2942 RSC.
- H. Ran, Z. Lin, C. Hong, J. Zeng, Q.-H. Yao and Z.-Y. Huang, J. Photochem. Photobiol., A, 2019, 372, 260–269 CrossRef CAS.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- H. Freundlich, J. Phys. Chem., 1907, 57, 385–470 CAS.
- C. Ayadi, A. Anene, R. Kalfat, Y. Chevalier and S. Hbaieb, Colloids Surf., A, 2019, 567, 32–42 CrossRef CAS.
- A. K. Roy, V. S. Nisha, C. Dhand and B. D. Malhotra, J. Mol. Recognit., 2011, 24, 700–706 CrossRef CAS PubMed.
- A. Mehdinia, M. Ahmadifar, M. O. Aziz-Zanjani, A. Jabbari and M. S. Hashtroudi, Analyst, 2012, 137, 4368–4374 RSC.
- V.-P. Vu, Q.-T. Tran, D.-T. Pham, P.-D. Tran, B. Thierry, T.-X. Chu and A.-T. Mai, Vietnam J. Chem., 2019, 57, 328–333 CrossRef CAS.
- A. Drame, Š. Trafela and K. Ž. Rožman, Proceedings, 2019, 15, 37 Search PubMed.
- P. S. Pidenko, S. A. Pidenko, Y. S. Skibina, A. M. Zacharevich, D. D. Drozd, I. Y. Goryacheva and N. A. Burmistrova, Anal. Bioanal. Chem., 2020, 412, 6509–6517 CrossRef CAS PubMed.
- Á. Villabona-Ortíz, K. J. Figueroa-Lopez and R. Ortega-Toro, Sustainability, 2022, 14, 3640 CrossRef.
- H. Saad, F. A. Nour El-Dien, N. E. A. El-Gamel and A. S. Abo Dena, RSC Adv., 2021, 11, 39768–39780 RSC.
- H. Dai, Y. Huang, H. Zhang, L. Ma, H. Huang, J. Wu and Y. Zhang, Carbohydr. Polym., 2020, 230, 115599 CrossRef CAS PubMed.
- M. Ateia, D. E. Helbling and W. R. Dichtel, ACS Mater. Lett., 2020, 2, 1532–1544 CrossRef CAS.
- S. Mignardi, L. Archilletti, L. Medeghini and C. De Vito, Sci. Rep., 2020, 10, 2436 CrossRef CAS PubMed.
- K. C. Bedin, A. C. Martins, A. L. Cazetta, O. Pezoti and V. C. Almeida, Chem. Eng. J., 2016, 286, 476–484 CrossRef CAS.
- H. Saad, F. A. Nour El-Dien, N. E. A. El-Gamel and A. S. Abo Dena, RSC Adv., 2022, 12, 25487–25499 RSC.
- S. Hillaert and W. Van den Bossche, J. Pharm. Biomed. Anal., 2003, 31, 329–339 CrossRef CAS PubMed.
- F. Liu, Y. Xu, S. Gao, J. zhang and Q. Guo, J. Pharm. Biomed. Anal., 2007, 44, 1187–1191 CrossRef CAS PubMed.
- M. C. G. Santos, C. R. T. Tarley, L. H. Dall'Antonia and E. R. Sartori, Sens. Actuators, B, 2013, 188, 263–270 CrossRef CAS.
- H. Beitollahi, M. Hamzavi and M. Torkzadeh-Mahani, Mater. Sci. Eng., C, 2015, 52, 297–305 CrossRef CAS PubMed.
- J. A. Murillo Pulgarín, A. Alañón Molina and G. Pérez-Olivares Nieto, Anal. Chim. Acta, 2004, 518, 37–43 CrossRef.
- A. Hemdan, N. F. Al-Tannak and E. H. Mohamed, J. Sep. Sci., 2022, 45, 824–831 CrossRef CAS PubMed.
- V. Suendo, Y. Lau, F. Hidayat, M. Reza, A. Qadafi and A. Rochliadi, Phys. Chem. Chem. Phys., 2021, 23, 7190–7199 RSC.
| This journal is © The Royal Society of Chemistry 2023 |