 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Low-carbon footprint diluents in solvent extraction for lithium-ion battery recycling†
Aboudaye M. Ahameda,
Benjamin Swobodab,
Zubin Arorab,
Jean Yves Lansotb and
Alexandre Chagnes *a
*a
aUniversité de Lorraine, CNRS, GeoRessources, F-54000 Nancy, France. E-mail: alexandre.chagnes@univ-lorraine.fr
bTotalEnergy Fluids, 2 Place Jean Miller, La Défense Cedex, 92078 Paris, France
First published on 2nd August 2023
Abstract
This study investigated the influence of the diluent on the extraction properties of three extractants towards cobalt(II), nickel(II), manganese(II), copper(II), and lithium(I), i.e. Cyanex® 272 (bis-(2,4,4-trimethylpentyl)phosphinic acid), DEHPA (bis-(2-ethyl hexyl)phosphoric acid), and Acorga® M5640 (alkylsalicylaldehyde oxime). The diluents used in the formulation of the extraction solvents are (i) low-odour aliphatic kerosene produced from the petroleum industry (ELIXORE 180, ELIXORE 230, ELIXORE 205 and ISANE IP 175) and (ii) bio-sourced aliphatic diluents (DEV 2138, DEV 2139, DEV 1763, DEV 2160, DEV 2161 and DEV 2063). No influence of the diluent and no co-extraction of lithium(I), nickel(II), cobalt(II), manganese(II) and aluminum were observed during copper(II) extraction by Acorga M5640. The nature of the diluent influenced more significantly the extraction properties of manganese(II) by DEHPA as well as cobalt(II) and nickel(II) by Cyanex® 272. Life cycle assessment of the diluents shows that the carbon footprints of the investigated diluents followed the following order: (ELIXORE 180, ELIXORE 230, ELIXORE 205) from petroleum industry > kerosene from petroleum industry > diluent produced from tall oil (DEV 2063) > diluents produced from recycled plastic (DEV 2160, DEV 2161) > diluents produced from used cooking oil (DEV 2138, DEV 2139). By taking into account the physicochemical properties of these diluents (viscosity, flashpoint, aromatic content), the extraction properties of Acorga® M5640, DEHPA, Cyanex® 272 in these diluents and the CO2 footprint of the diluents, this study showed DEV2063 and DEV2139 were the best diluents. A low-carbon footprint solvent extraction flowsheet using these diluents was proposed to extract selectively cobalt, nickel, manganese, lithium and copper from NMC black mass of spent lithium-ion batteries.
1. Introduction
Population growth and rapidly evolving technologies are triggering a strong demand for metals such as lithium, nickel, cobalt, manganese and copper. These metals are most used in lithium battery manufacturing (71%).1 Lithium-ion batteries (LiBs) offer many advantages, most notably their ability to store relatively large amounts of energy compared to other systems, their high charging capacity and their long-life span.2 For this reason, lithium-ion batteries are considered the best technology for electric vehicles and stationary applications, and an exponential growth of the global demand of lithium, nickel, cobalt and manganese is expected in the coming years.3,4 The circular economy stimulates the development of sustainable and efficient recycling technologies to mitigate the environmental impact of lithium-ion battery production and to improve resource management.In practice, spent LiBs are dismantled after a deep discharge, shredded to liberate the different components of the batteries, and valuable elements (usually nickel, cobalt, manganese, lithium, copper, aluminum, and graphite) are concentrated in a blackmass by using different physical separation methods such as froth flotation, magnetic separation, eddy current, screening, gravimetric separation, etc. This blackmass undergoes hydrometallurgical operations, i.e. leaching, solvent extraction, precipitation, etc.5 Leaching, is usually carried out in sulfuric acid in the presence of hydrogen peroxide6,7 but other studies showed that hydrochloric acid could be alternatively used.8,9
Metal extraction and metal separation are key steps of recycling processes to produce high-grade salts. These steps can be performed by liquid–liquid extraction, liquid–solid extraction, precipitation–crystallization, and (electro)-membrane processes.10–14 Among these technologies, liquid–liquid extraction is widely used to extract, concentrate and separate metals from the leach solution.15,16 This technique consists of mixing the leach solution with a non-miscible extraction solvent containing an extractant and a diluent in order to extract selectively the target metal(s). The performance of the liquid–liquid operation depends on the choice of the extractant and the diluent beside the use of optimized contactors (mixer-settler or column) under optimized conditions (pH, temperature, flowrates, etc.). The diluent is usually an aliphatic diluent which exhibits high chemical stability. The use of different extractants (DEHPA, Cyanex® 272, TOPO, PC-88A, etc.) and various diluents containing more or less aromatic compounds are reported in literature for the efficient and selective recovery of lithium, cobalt, nickel, manganese and copper (Table 1). Other free-diluent systems are reported in literature to replace conventional extraction solvents such as ionic liquids or deep eutectic solvents.17,18 However, there is no study reported in literature regarding the impact of the use of these systems on the CO2 emission of the solvent extraction process. Darvishi et al.19 reported that DEHPA does not exhibit a good selectivity for cobalt–nickel separation at any concentrations and temperatures since the differences in pH of half extraction between nickel and cobalt (pH1/2,Ni–pH1/2,Co) are equal to 0.26, 0.58 and 0.59 at 25, 40 and 60 °C, respectively.
| Objectives | Aqueous phase composition (g L−1) | Diluent | Extractant | Ref. |
|---|---|---|---|---|
| Direct production of Ni–Co–Mn mixtures for cathode precursors from cobalt-rich LiBs leachates by solvent extraction | Li = 2.5 | Exxsol D80 | D2EHPA | 41 |
| Ni = 2 | ||||
| Co = 16.8 | ||||
| Mn = 2.1 | ||||
| Selective recovery of cobalt, nickel and lithium from sulfate leachate of cathode scrap of LiBs using liquid–liquid extraction | Co = 25.1 | Kerosene | PC-88A | 51 |
| Ni = 2.54 | ||||
| Li = 2.62 | ||||
| Metal separation from mixed types of batteries using selective precipitation and liquid–liquid extraction techniques | Co = 0.37 | Kerosene | Cyanex® 272 | 52 |
| Ni = 1.24 | ||||
| Cu = 0.301 | ||||
| Mn = 0.499 | ||||
| Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from LiB waste | Ni = 1.295 | Kerosene | Cyanex® 272 | 53 |
| Co = 6.976 | ||||
| Ni = 0.821 | ||||
| Mn = 0.992 | ||||
| Cu = 0.113 | ||||
| Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of LiBs | Co = 44.72 | Kerosene | Cyanex® 272 | 54 |
| Li = 5.43 | ||||
| Separation of cobalt and lithium from mixed sulphate solution using Na–Cyanex® 272 | Co = 0.01 | Kerosene | Na–Cyanex® 272 | 55 |
| Li = 0.01 | ||||
| Selective extraction and separation of metal values from leach liquor of mixed spent LiBs | Co = 32.7 | Kerosene | Na–Cyanex® 272 | 56 |
| Li = 2.26 | ||||
| Ni = 0.07 | ||||
| Mn = 18.6 | ||||
| Development of a novel solvent extraction process to recover cobalt, nickel, manganese, and lithium from cathodic materials of spent LiBs | Li = 1.441 | Kerosene | Alamine® 336 | 8 |
| Co = 3.67 | ||||
| Ni = 3.60 | ||||
| Mn = 3.61 | ||||
| Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent LiBs | Ni = 6.41 | Kerosene | DEHPA | 6 |
| Co = 6.09 | ||||
| Mn = 6.29 | ||||
| Li = 1.60 | ||||
| Recovery of metals from spent LiBs leach solutions with a mixed solvent extractant system | Co = 16.9 | Shellsol D70 | Ionquest® 801 + Acorga® M5640 | 57 |
| Li = 3.8 | ||||
| Ni = 0.15 | ||||
| Improvement of metal separation process from synthetic hydrochloric acid leaching solution of spent LiBs by solvent extraction and ion exchange | Cu = 150 | Kerosene | Cyanex® 301 | 58 |
| Co = 0.938 | ||||
| Mn = 0.15 | ||||
| Ni = 0.1 | ||||
| Li = 0.15 | ||||
| Lithium recovery from effluent of spent LiB recycling process using solvent extraction | Li = 1.85 | Kerosene | HBTA and TOPO | 59 |
| Co = 0.005 | ||||
| Ni = 0.025 | ||||
| The separation and recovery of nickel and lithium from the sulfate leach liquor of spent LiBs using PC-88A | Li = 4.82 | Kerosene | PC-88A | 7 |
| Ni = 2.54 |
Conversely, Devi et al.20 demonstrated that Cyanex® 272 is the best extractant compared to DEHPA and PC-88A to separate cobalt(II) and nickel(II). Tait et al. investigated the separation of cobalt(II) and nickel(II) by using Cyanex® 272, Cyanex® 301 and Cyanex® 302 diluted in toluene.21 They showed that Cyanex® 272 can separate cobalt(II) and nickel(II) very efficiently as the pH values of half extraction for cobalt(II) are equal to 1.3, 4.0 and 5.3 whereas the pH values of half extraction for nickel are pH1/2,Ni = 2.4, 6.6 and 7.0 when 0.25 mol per L Cyanex® 301, Cyanex® 302 or Cyanex® 272 are used as extractant, respectively. These results agree with those obtained by Sole and Hiskey.22
Cobalt(II)–manganese(II) separation was also studied by several authors. Devi et al. investigated cobalt(II)–manganese(II) separation in acidic sulfate media by using Cyanex® 272, DEHPA and PC-88A.23 They showed manganese(II) and cobalt(II) can be separated efficiently from acidic sulfate solution at pH 2.7 by using DEHPA. With respect to the efficiency of separation, these authors showed that DEHPA was better than PC88A and Cyanex® 272. Others authors24 showed that the separation was even better by controlling the pH from 2 to 4 when DEHPA was used as extractant.
The physical properties of the diluent, i.e. density, viscosity, dielectric constant and solubility parameters, influence the extraction of the metal from the leach solution into the organic phase.25 In general, aromatic diluents have higher densities than aliphatic diluents, which may impede the dispersion and coalescence whereas the polarity of the diluent has a huge effect on the extraction efficiency. Furthermore, the interactions in solution may be responsible for self-association of the extractants. The intensity of self-association depends on the polarity of the diluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) extractant molecules tend to form monomer in polar diluents and dimers, trimers or even oligomers in non-polar diluents.26,27 The average degree of polymerisation increases with a decrease in polarity of the diluent. The presence of polymeric forms decreases the loading capacity of the extractant whereas the interaction of the diluent with the extractant can decrease the extraction efficiency since the formation of extractant–diluent species results in a lower concentration of free extractants. Furthermore, diluents affect the solvation of the extractant and, hence, its extractive properties. There are only few studies regarding the effect of the nature of the diluent on the extraction performance of liquid–liquid extraction, but no study concerns the effect of diluents on cobalt(II)–nickel(II)–manganese(II) separation for LiBs recycling processes. In many cases, the distribution ratios cannot be correlated to the physical properties of the diluent, even though many attempts have been made to do so. Taube tried to correlate the extraction efficiency of uranium, plutonium and neptunium with the dipole moment and the dielectric constant of the solvent.28,29 Healy30 linked the diluent effect with the water content of the solvents. The effect of diluents on the solvent extraction of metal ions has been studied for copper(II),31–34 cobalt(II),35 nickel(II),36–38 uranium(VI).39
extractant molecules tend to form monomer in polar diluents and dimers, trimers or even oligomers in non-polar diluents.26,27 The average degree of polymerisation increases with a decrease in polarity of the diluent. The presence of polymeric forms decreases the loading capacity of the extractant whereas the interaction of the diluent with the extractant can decrease the extraction efficiency since the formation of extractant–diluent species results in a lower concentration of free extractants. Furthermore, diluents affect the solvation of the extractant and, hence, its extractive properties. There are only few studies regarding the effect of the nature of the diluent on the extraction performance of liquid–liquid extraction, but no study concerns the effect of diluents on cobalt(II)–nickel(II)–manganese(II) separation for LiBs recycling processes. In many cases, the distribution ratios cannot be correlated to the physical properties of the diluent, even though many attempts have been made to do so. Taube tried to correlate the extraction efficiency of uranium, plutonium and neptunium with the dipole moment and the dielectric constant of the solvent.28,29 Healy30 linked the diluent effect with the water content of the solvents. The effect of diluents on the solvent extraction of metal ions has been studied for copper(II),31–34 cobalt(II),35 nickel(II),36–38 uranium(VI).39
It is of great importance to take into consideration the diluent during the formulation of an extraction solvent since (i) the diluent may have a significant effect on the extraction properties of a solvent in liquid–liquid extraction, and (ii) the diluent, which is the main component of an extraction solvent, impacts directly the environmental footprint of the liquid–liquid extraction process. Furthermore, the European Regulation (EC) No. 1907/2006 of 18/12/06 (ref. 40) concerning the Registration, Evaluation, Authorization and Restriction of Chemicals encourages the development of industrial processes that use less toxic organic compounds. It is therefore necessary to use eco-friendly reagents in hydrometallurgical processes. In the case of liquid–liquid extraction, bio-sourced diluents may be an alternative to petroleum kerosene to reduce the CO2 footprint of the liquid–liquid extraction operation.
In this paper, we compared the extraction properties of different extraction solvents composed of extractants (Acorga M5640, DEHPA, Cyanex® 272) in petroleum diluents (ELIXORE180, ELIXORE230, ELIXORE 205 and ISANE IP175) and in bio-sourced aliphatic diluents (DEV 2138, DEV2139, DEV1763, DEV2160, DEV2161 and DEV2063) for the recovery of lithium(I), nickel(II), cobalt(II), manganese(II) and copper(II) from acidic sulfate media representative of a leaching solution produced by dissolving a blackmass of spent lithium-ion battery in sulfuric acid. The best extraction solvent has been selected from the extraction performance and the CO2 footprint of the diluents. Then, a flowsheet has been proposed to extract and separate the different metals contained in the leach solution by using the selected diluents.
2. Experimental
2.1. Chemical reagents
Aqueous solutions were prepared by dissolving copper sulfate (CuSO4·5H2O, Aldrich, purity ≥ 99%), lithium sulfate (Li2SO4,H2O, Aldrich, purity ≥ 99.0%), cobalt sulphate (CoSO4·7H2O, Aldrich, purity ≥ 99%), manganese sulphate (MnSO4·H2O, Aldrich, purity ≥ 99%), nickel sulfate (NiSO4·6H2O, Aldrich, ≥ 98%) and aluminum(III) sulfate (Al2(SO4)3, 14H2O, Aldrich, purity = 98%) in 0.35 mol per L sulfuric acid. The solution of 0.35 mol per L sulfuric acid was prepared by diluting an appropriate amount of concentrated sulfuric acid (Aldrich, 95%) in deionized water (resistivity > 18 MΩ cm).Table 2 shows the composition of the aqueous solution representative of a leaching solution produced by dissolving NMC cathode materials with sulfuric acid in the presence of hydrogen peroxide.41
| Element | Li | Ni | Co | Mn | Al | Cu |
| Concentration (g L−1) | 2.7 | 2.6 | 14.4 | 2.7 | 1.2 | 1.6 |
A solution of 10 mol per L sodium hydroxide was prepared by dissolving the appropriate amount of sodium hydroxide pellets (NaOH, Aldrich, purity = 99%) in deionized water (resistivity > 18 MΩ cm). This solution was used to adjust the pH of the aqueous solution during the solvent extraction experiments. The pH values of the aqueous solution before and after extraction were measured using a 1000 L pH meter (VWR) equipped with a pH electrode phenomenal 221 (VWR).
The organic phases for the liquid–liquid experiments were prepared by diluting the appropriate amounts of the extractants in the diluents. The following extractants used in the present work were kindly provided by Solvay: Cyanex® 272 (bis-(2,4,4-trimethylpentyl)phosphinic acid, purity = 90%), DEHPA (bis(2-ethylhexyl)phosphoric acid, purity = 95%) and Acorga® M5640 (5-nonyl-2-hydroxy-benzaldoxime, purity not specified). The diluents reported in Table 3 were kindly provided by TotalEnergy, except the petroleum aliphatic kerosene which was supplied by Aldrich (low-odour kerosene). It is interesting to point out that the bio-sourced diluents derived from waste oil, recycled plastic or vegetable oil exhibit higher flash points than petroleum-based diluents and they contain fewer aromatics, but they are slightly more viscous than diluents from petroleum industry.
| Category | FP (°C) | Aromatic (ppm) | υ at 40 °C (mm2 s−1) | Composition (%) | ||||
|---|---|---|---|---|---|---|---|---|
| IP | LP | A | N | |||||
| Kerosene | Fossil | 52 | — | 1.1 | 20 | 10 | 20 | 50 |
| EXILORE180 | Fossil | 66 | <300 ppm | 1.4 | 20 | 10 | 0 | 70 |
| EXILORE230 | Fossil | 104 | <300 ppm | 2.4 | 30 | 10 | 0 | 60 |
| EXILORE205 | Fossil | 75 | <300 ppm | 1.7 | 20 | 10 | 0 | 70 |
| ISANE IP175 | Fossil | 63 | <50 ppm | 1.23 | 95 | 5 | 0 | 0 |
| DEV2138 | Used cooking oil | 64 | <50 ppm | 1.17 | 80 | 20 | 0 | 0 |
| DEV2139 | Used cooking oil | 84 | <50 ppm | 1.61 | 80 | 20 | 0 | 0 |
| DEV1763 | Vegetable oil | 115 | <50 ppm | 2.37 | 95 | 5 | 0 | 0 |
| DEV2160 | Recycled plastic | 49 | <100 ppm | 1.07 | 35 | 35 | 0 | 30 |
| DEV2161 | Recycled plastic | 92 | <100 ppm | 1.88 | 30 | 40 | 0 | 30 |
| DEV2063 | Tall oil | 61 | <50 ppm | 1.23 | 45 | 10 | 0 | 45 |
2.2. Solvent extraction
Solvent extraction experiments were performed by contacting 10 mL of the aqueous phase with 10 mL of the organic phase in a centrifuge tube (50 mL). The pH of the aqueous phase was adjusted with 10 mol per L NaOH. No precipitation was observed during liquid–liquid extraction experiments (the solution was clear and no solid was formed at the bottom of the flask and at the liquid–liquid interface). The two non-miscible phases were mixed during 15 minutes at room temperature and 200 rpm with a mechanical stirring apparatus (Gerhardt Laboshake) thermostated with a Gerhardt Thermoshake, so that the equilibrium was reached. A mixing time of 15 minutes is enough since literature data showed that the steady state with the investigated extractants can be reach after contacting the aqueous and organic phases during less than 5 minutes.42,43 Samples were centrifuged at 3000 rpm for 2 min by means of a Sigma 3-16L Compact Benchtop Centrifuge in order to separate the organic and the aqueous phases. Before analysing the metal concentrations in the aqueous phases, the aqueous solutions were filtered to remove traces of organic phase with a hydrophilic filter (Minisart NML 16555K, cellulose acetate, 0.45 μm, d = 28 mm, VWR).Elemental analyses were performed using a microwave plasma-atomic emission spectrometer (MP-AES 4210, Agilent). The wavelengths used for elemental analyses of lithium, nickel, cobalt, manganese, copper and aluminum were 497.175 nm, 361.939 nm, 350.631 nm, 294.920 nm, 324.754 nm and 308.215, respectively. The samples were diluted in 2% (vol) nitric acid prepared from a concentrated solution (HNO3, 95%, Aldrich) by dilution in deionized water (resistivity > 18 MΩ cm). Standards for MP analyses were prepared by diluting commercial standards provided by VWR containing 1000 ppm Li, Co, Mn, Ni, Al and Cu in 2% (vol) HNO3. Standard compositions were adjusted by adding sulfuric acid (0.35 mol L−1) in order to reach the same sulfate concentration as in the sample to analyse, and therefore, to reduce interference phenomena during MP analyses. The metal concentrations in the aqueous solutions before and after solvent extraction were used to calculate the extraction efficiency of the metal M (M = Li, Ni, Mn, Co, Cu or Al) by using the following equation:
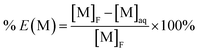 | (1) |
3. Results and discussion
3.1. Influence of diluents on the extraction curves
The influence of the diluents reported in Table 3 on the extraction efficiency by DEHPA and Cyanex® 272 of cobalt(II), nickel(II), manganese(II) and lithium(I) diluted in the acidic aqueous solution representative of leach solution (see composition in Table 2) is reported in Fig. 1. In the present study, the influence of the diluent and the extractant on the extraction efficiency of aluminum has not been studied as aluminum is usually precipitated before liquid–liquid extraction (see precipitated curves in Fig. S1, ESI†). Therefore, the composition of the aqueous solutions used in the present work contains all metals at concentrations reported in Table 2 except aluminum as it is inferred that aluminum was removed by precipitation before liquid–liquid extraction. The diluent influences the extraction properties of these metals except for copper and lithium (see below). The general rule for the extraction effect of the diluent on the extraction is that a higher dielectric constant and a higher dipole moment lead to an increase in extraction efficiency.44–46 This is explained by the fact that the interactions between the extractant molecules and the diluent molecules are stronger if the dielectric constant of the diluent is higher. These stronger interactions result in a weaker extraction of metal ions. However, in the present study, it is expected that the dielectric constant of the different diluents reported in Table 3 are similar and close to εr = 2–4. Therefore, the difference in extraction behavior observed for several systems cannot be explained by dielectric constants.Liquid–liquid extraction of cobalt(II), nickel(II) and manganese(II) from acidic aqueous media by cationic exchangers such as DEHPA, Cyanex® 272 and Acorga® M5640 involves the following general equilibrium:47–50
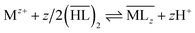 | (2) |
The following equation can be deduced from eqn (2) to fit the experimental curves of the extraction efficiency as a function of pH:
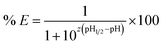 | (3) |
The extraction constant (Kex) of the extraction reaction (2) is defined as:
 | (4) |
The following equilibrium constants can be used to rewrite eqn (4):
 | (5) |
 | (6) |
 | (7) |
 | (8) |
 | (9) |
By combining eqn (4)–(9), the following expression of the extraction efficiency (in %) can be deduced:
 | (10) |
Thus, eqn (10) shows that the extraction efficiency at constant pH in the aqueous phase and at constant concentration of extractant in the organic phase can be improved provided that: (i) the metal–ligand complex is very stable in the organic phase (high value of β), (ii) the metal–ligand complex is highly soluble in the organic phase (high value of KD,ML2) and (iii) the pKa of the cationic exchanger is as low as possible. Therefore, the steric hindrance, the complexing power, and the hydrophobicity of the extractants as well as the dipolar moment and the dielectric constant of the diluent can affect the values of these constants, and therefore, the extraction properties of the extraction solvent.
The influence of the diluents on copper(II) recovery by 20% (wt) Acorga® M5640 was not reported in Fig. 1 as no significant effect was observed. At pH 0.6, the copper(II) extraction efficiency reached 80% and nearly 100% at pH 1.5 for all diluents. Likewise, the nature of the diluent did not influence lithium extraction by DEHPA or Cyanex® 272 as shown in Fig. 1a and e. Conversely, a significant effect of the diluent on the extraction efficiency of cobalt(II), nickel(II) and manganese(II) was observed when Cyanex® 272 and DEHPA were used as extractants.
The extraction efficiency curve of cobalt(II) by DEHPA is shifted towards higher pH when EXILORE 180 or kerosene is used as diluent instead of the other ones. Regarding the influence of the diluent for the extraction of nickel(II) by DEHPA, the extraction curves are located in the same region (pH1/2 = 4.2–4.4) when the diluent is kerosene EXILORE180, EXILORE230, ISANE IP175, DEV2138, DEV2139 or DEV1763. Conversely, the curves are shifted toward higher pH when these diluents are replaced by EXILORE205 or DEV2160. However, the most important effect of the diluent is observed with DEV2160 as the pH of half extraction reaches 5.5.
The behavior of manganese(II) is quite different since the extraction curves are progressively shifted toward higher pH as follows: kerosene ≈ IsaneIP75 < EXILORE180 < (EXILORE230 ≈ EXILORE205) < (DEV2138 ≈ DEV2139 ≈ DEV2063) < (DEV1763 ≈ DEV2160 ≈ DEV2161).
Likewise, the diluent influences the extraction efficiency of cobalt(II), nickel(II) and manganese(II) when Cyanex® 272 is used as extractant. The extraction efficiencies by Cyanex® 272 are shifted toward higher pH values when DEV 2261 is used instead of the other diluents for cobalt(II) extraction and when DEV 2138 is replaced by the other diluents for nickel(II) and manganese(II) extraction.
Fig. 2 displays the influence of the acidity constant (Ka), the complexation constant (β), the distribution constant of HL (KD,HL) and the distribution constant of MLz between an aqueous phase and the extraction solvent (KD,MLz) on the extraction efficiency (curves plotted by using eqn (10)). An increase of KD,HL is responsible for a shift of the extraction curve toward higher pH whereas an increase of the other constants (Ka, β, KD,MLz) is responsible for a shift toward the lower pH values. Therefore, the shift of the extraction curves towards higher pH observed previously when the diluents are changed may result from an increase of the distribution constant of HL, i.e. an increase of the solubility of the extractant into the organic phase due to an increase of the diluent–extractant interactions. The extent of the shift can be mitigated by the increase of the other constants which are responsible for shifts towards the lower pH values.
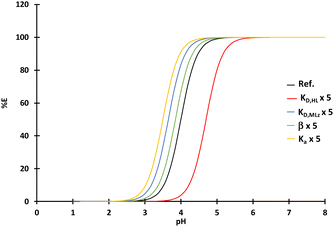 | ||
| Fig. 2 Influence of the acidity constant (Ka), the complexation constant (β), the distribution constant of HL (KD,HL) and the distribution constant of MLz (KD,MLz) on the extraction efficiency calculated by eqn (10). Thermodynamic constants for the curve quoted “Ref.”: KD,HL = 105, KD,MLz = 105, β = 105 and Ka = 10−4. For the other curves these values are the same except one constant which has been multiplied by 5 to observe their influence on the curve. | ||
3.2. Influence of diluents on the selectivity
The choice of the extraction system will rely on both the physicochemical properties (flash point, viscosity), the extraction properties (extraction efficiency, selectivity) and the environmental impact (evaluated by LCA, vide infra). The selectivity of an extraction solvent for M1 toward M2 can be assayed by calculating the difference in pH of half-extraction between M1 and M2 (pH1/2,M1–pH1/2,M2). Usually, it is inferred that a good separation can be achieved providing that pH1/2,M1–pH1/2,M2 ≳ 1.5. Fig. 3 shows the difference in pH of half-extraction between lithium(I) and cobalt(II), cobalt(II) and manganese(II) as well as nickel(II) and cobalt(II) when the extraction solvent is composed of 0.8 mol per L Cyanex® 272 and 1 mol per L DEHPA diluted in the diluents reported in Table 3.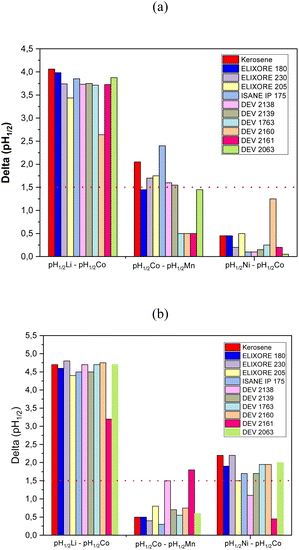 | ||
| Fig. 3 Differences in pH of half-extraction between lithium(I) and cobalt(II) (pH1/2,Li–pH1/2,Co), cobalt(II) and manganese(II) (pH1/2,Co–pH1/2,Mn), and nickel(II) and cobalt(II) (pH1/2,Ni–pH1/2,Co) when the extraction solvent is composed of (a) 0.8 mol per L DEHPA and (b) 1 mol per L Cyanex 272 solubilized in diluents reported in Table 2 (O/A = 1, T = 25 °C, extraction time = 15 min). | ||
Fig. 3 shows that pH1/2,Co–pH1/2,Mn decreased depending on the diluent as follows when DEHPA was used as extractant:
IsaneIP175 > kerosene ≫ Exilore205 ≈ Elixore230 ≈ DEV2138 ≈ DEV2161 ≈ EXILORE180 ≈ DEV2063.
The order was completely different when Cyanex® 272 was used instead of DEHPA (Fig. 3a):
DEV2161 > DEV2138 ≫ Exilore205 ≈ DEV2161 ≈ DEV2160 ≈ DEV2063 ≈ DEV1763 > kerosene ≈ EXILORE180 ≈ EXILORE230 > ISANEIP175.
Therefore, Co–Mn separation is much better with biosourced diluents (pH1/2,Co–pH1/2,Mn = 1.8 and 1.5 for DEV2161 and DEV2138, respectively) when Cyanex® 272 is used as extractant whereas fossil diluents (pH1/2,Co–pH1/2,Mn = 2.4 and 2.0 for Isane IP175 and kerosene, respectively) is better if the extraction solvent contains DEHPA. As expected, cobalt(II)–manganese(II) separation is better with DEHPA than Cyanex® 272.
Cobalt(II)–nickel(II) separation is not possible by using DEHPA as the difference of pH of half-extraction is lower than 1.5 whatever the diluent (Fig. 3b). When DEHPA was used as extractant, the values of pH1/2,Ni–pH1/2,Co decreased as follows for the investigated diluents:
Kerosene = Exilore230 > DEV2063 ≈ DEV1763 ≈ DEV2160 ≈ Exilore180 > Exilore230 ≈ IsaneIP175 ≈ Exilore205 > DEV238 > DEV2161.
Almost all diluents led to relevant values of pH1/2,Ni–pH1/2,Co event if the highest values were obtained for fossil diluents such as kerosene and Exilore230, i.e. pH1/2,Ni–pH1/2,Co = 2.2. However, biosourced diluents such as DEV1763 and DEV2160 led to interesting values of pH of half-extraction (pH1/2,Ni–pH1/2,Co = 2.0).
Therefore, the best biosourced diluent for cobalt(II)–manganese(II) separation with Cyanex® 272 by taking into account the extraction properties (pH1/2,Co–pH1/2,Mn = 1.5), the flash point (64 °C) and the viscosity (υ = 1.17 mm2 s−1) is DEV2138. Regarding the extraction properties, the best biosourced diluent for nickel(II)–cobalt(II) separation are DEV2063, DEV2160 or DEV1763 as pH1/2,Ni–pH1/2,Co = 2 in these diluents. All of these diluents exhibit a low kinematic viscosity. DEV2160 exhibits the lowest kinematic viscosity (υ = 1.07 mm2 s−1) but also the lowest flash point (FP = 49 °C). Finally, DEV1763 seems to be the best diluent as the flash point is very high (FP = 115 °C), the kinematic viscosity is high but comparable to EXILORE230 (υ = 2.4 mm2 s−1) and the difference in pH of half-extraction is high when Cyanex® 272 is used as extractant. DEV2160 is the only diluent with relevant extraction properties to separate cobalt(II) and nickel(II) when DEHPA is used as extractant even if the difference in pH of half-extraction is lower than with Cyanex® 272 (pH1/2,Co–pH1/2,Mn = 1.25). However, as explained above, this diluent exhibits very low flash point.
3.3. CO2 footprint of the diluents
The process consists in refining the feedstocks, i.e. fossil, bio-oil (tall oil, vegetable oil, used cooking oil) or recycled plastics, and purifying the products from refining by performing hydrogenation and distillation as shown in Fig. 4.For diluent coming from tall oil the process steps are: (i) conversion of wood into pulp mill, (ii) conversion of pulp mill into crude tall oil, (iii) distillation of crude tall oil to produce distilled tall oil and tall oil fatty acid, (iv) hydroprocessing of distilled tall oil and tall oil fatty acid into diesel fuels including impurities removal and hydro-isomerisation/hydrocracking, and (v) purification of biodiesel fuels into diluent by high pressurized hydrogenation. For diluent coming from spent plastics, the different process steps are: (i) waste collection of plastics, (ii) thermal cracking of the plastics which converts plastic waste into a hydrocarbons liquid, (iii) hydro-refining to remove impurities (iv) distillation, and (v) purification by high pressure hydrogenation. For diluent coming from used cooking oil, the different steps of the process are: (i) collection of used cooking oil, (ii) refining step including hydrocracking, isomerization and hydrogenation of used cooking oil to transform triglyceride into hydrocarbon liquid and (iv) purification by high pressure hydrogenation. ELIXORES are produced by high-pressure hydrogenation and distillation of diesel or kerosene feedstocks coming from refineries whereas kerosene is produced by a conventional refining process.
The LCA (cradle-to-gate) of the different routes described previously have been performed by using the EcoInvent 3.8 database. Each step contributes to the greenhouse gas emissions as shown in Table 3. It is important to note that the CO2 emissions are calculated per category of diluent (ELIXORE, kerosene, recycled plastic, etc.). This means that there is no difference in terms of CO2 emissions for the different diluents in the same category. Total emissions were calculated by summing the emissions from each process step, taking into account the inventories.
According to the ISO 14040/14044 and ISO 14067 standards, the greenhouse gas emissions of these feedstocks are 0 for recycled plastic, tall oil and used cooking oil wastes. The production of kerosene consists of two steps: oil production and refining. These two steps represent a CO2 emission of +462 kg CO2eq per t. ELIXORE diluents, based on diesel feedstock, generate more CO2 than kerosene because of the further refining and purification at the Oudalle plant.
The results presented in Table 4 show that the main stage that contribute to emits CO2 is the refining stage. In the case of bio-sourced diluents, the calculation of the global CO2 takes into account the CO2 credit. Indeed, during the growth of the plant, CO2 has been removed from the air and fixed in the biomass. After transformation, this captured CO2 is still in the product. Therefore, the percentage of carbon in the product was used to calculate the amount of CO2 which has been captured (negative value). In case of recycled plastic based diluent, the calculation of the global CO2 takes into account the avoided emission due to a usual end life of plastic, i.e. incineration.
| Process emissions | CO2 reduction | GES total | |
|---|---|---|---|
| a * means biogenetic CO2 credit and ** means avoided CO2 generated by incineration. | |||
| Tall oil (DEV2063) | +1413 | −3120* | −1707 |
| Recycled plastic (DEV2160, DEV2161) | +1495 | −3700** | −2205 |
| Used cooking oil (DEV2138, DEV2139) | +525 | −3120* | −2438 |
| ELIXORES (ELIXORE180, ELIXORE230, ELIXORE205) | +635 | 0 | +635 |
| Kerosene | +462 | 0 | +462 |
As a conclusion, the different diluents can be classified according to their carbon footprints as follow: ELIXORES (ELIXORE180, ELIXORE230, ELIXORE205) > kerosene > DEV2063 (tall oil) > DEV2160 and DEV2161 (recycled plastics) > DEV 2138 and DEV 2139 (used cooking oil).
4. Conclusion
The present study shows that the use of low-carbon footprint diluents in solvent extraction for lithium-ion battery recycling is a good alternative to fossil kerosene. In particular, the diluents DEV2139 and DEV2063 produced from used cooking oil and tall oil, respectively, are interesting as they contain very low concentration of aromatics, their flashpoints are high, their viscosity is moderate and they can be advantageously used with DEHPA and Cyanex® 272 to extract selectively manganese(II), and separate nickel(II) and cobalt(II). From the results of the present work, the flowsheet reported in Fig. 5 could be used to implement low-carbon footprint solvent extraction process for the recovery of copper, manganese, nickel, cobalt and lithium from spent lithium-ion batteries. | ||
| Fig. 5 Low-carbon footprint solvent extraction flowsheet for the recovery of metals from spent lithium-ion batteries. | ||
Fig. 5 shows a typical hydrometallurgical solvent extraction flowsheet that could be implemented to recover metals from spent lithium-ion batteries with low-carbon footprint diluents. After leaching the NMC-blackmass by sulfuric acid in the presence of hydrogen peroxide, 20% (wt) Acorga® M5640 diluted in DEV2139 or DEV2063 could be used to extract copper at pH 1.5 from the leach solution without significant co-extraction of the other metals. The pH of the resulting solution containing aluminum, cobalt, nickel, manganese and lithium could be adjusted to pH 4.6 with sodium hydroxide to precipitate aluminum. After solid/liquid separation, manganese could be extracted by using 0.8 mol per L DEHPA diluted in DEV2139 at pH 4.6. Under these conditions, losses of lithium, nickel and cobalt will be reduced. Afterwards, cobalt could be extracted selectively toward nickel by using 1 mol per L Cyanex® 272 diluted in DEV2063 at pH 4.8 and nickel could be afterwards selectively extracted towards lithium by using 1 mol per L Cyanex® 272 diluted in DEV2063 at pH 6.3 or by selective precipitation at pH 8. The resulting solution contains only lithium, which can be precipitated with sodium carbonate as lithium carbonate.
Author contributions
Conceptualization: AC; formal analysis: AMA, AC; funding acquisition: AC; investigation: AMA, AC; project administration: AC; supervision: AC; visualization: AMA, AC; writing-original draft: AMA, AC; writing-review and editing: AC, BS, ZA, JYL.Conflicts of interest
There are no conflicts to declare.References
- L. T. Peiró, G. V. Méndez and R. U. Ayres, Lithium: Sources, Production, Uses, and Recovery Outlook, JOM, 2013, 65(8), 986–996, DOI:10.1007/s11837-013-0666-4.
- M. Wakihara, Recent developments in lithium ion batteries, Mater. Sci. Eng., R, 2001, 33(4), 109–134, DOI:10.1016/S0927-796X(01)00030-4.
- A. Sonoc and J. Jeswiet, A Review of Lithium Supply and Demand and a Preliminary Investigation of a Room Temperature Method to Recycle Lithium Ion Batteries to Recover Lithium and Other Materials, Procedia CIRP, 2014, 15, 289–293, DOI:10.1016/j.procir.2014.06.006.
- K. Laadjal and A. J. M. Cardoso, Estimation of Lithium-Ion Batteries State-Condition in Electric Vehicle Applications: Issues and State of the Art, Electronics, 2021, 10(13), 1588, DOI:10.3390/electronics10131588.
- A. Masmoudi, Recyclage du lithium issu des batteries usagées par extraction liquide-liquide dans un milieu liquide ionique, Doctoral thesis, University of Strasbourg (France), 2020, Consulté le: 7 septembre 2022, Disponible sur, https://www.theses.fr/2020STRAE028 Search PubMed.
- X. Chen, Y. Chen, T. Zhou, D. Liu, H. Hu and S. Fan, Hydrometallurgical recovery of metal values from sulfuric acid leaching liquor of spent lithium-ion batteries, Waste Manag., 2015, 38, 349–356, DOI:10.1016/j.wasman.2014.12.023.
- V. T. Nguyen, J. Lee, J. Jeong, B.-S. Kim and B. D. Pandey, The Separation and Recovery of Nickel and Lithium from the Sulfate Leach Liquor of Spent Lithium Ion Batteries using PC-88A, Korean Chem. Eng. Res., 2015, 53(2), 137–144, DOI:10.9713/kcer.2015.53.2.137.
- W. Xuan, A. de Souza Braga and A. Chagnes, Development of a Novel Solvent Extraction Process to Recover Cobalt, Nickel, Manganese, and Lithium from Cathodic Materials of Spent Lithium-Ion Batteries, ACS Sustainable Chem. Eng., 2022, 10(1), 582–593, DOI:10.1021/acssuschemeng.1c07109.
- P. Zhang, T. Yokoyama, O. Itabashi, T. M. Suzuki and K. Inoue, Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries, Hydrometallurgy, 1998, 47(2), 259–271, DOI:10.1016/S0304-386X(97)00050-9.
- Lithium Process Chemistry: Resources, Extractions, Batteries and Recycling, ed. A. Chagnes and J. Swiatowska, Elsevier, 2015, p. 313, ISBN: 978-0-12-801417-2 Search PubMed.
- S. Gmar and A. Chagnes, Recent advances on electrodialysis for the recovery of lithium from primary and secondary resources, Hydrometallurgy, 2019, 189, 105124 CrossRef CAS.
- K. Shi, M. Luo, J. Ying, S. Zhen, Z. Xing and R. Chen, Extraction of Lithium from Single-Crystalline Lithium Manganese Oxide Nanotubes Using Ammonium Peroxodisulfate, iScience, 2020, 23, 101768 CrossRef CAS PubMed.
- O. Dolotko, N. Gehrke, T. Malliaridou, R. Sieweck, L. Herrmann, B. Hunzinger, M. Knapp and H. Ehrenberg, Universal and efficient extraction of lithium for lithium-ion battery recycling using mechanochemistry, Commun. Chem., 2023, 6, 49 CrossRef CAS PubMed.
- X. Li, Y. Mo, W. Qing, S. Shao, C. Y. Tang and J. Li, Membrane-based technologies for lithium recovery from water lithium resources: a review, J. Membr. Sci., 2019, 591, 117317 CrossRef CAS.
- A. Chagnes and B. Pospiech, A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries, J. Chem. Technol. Biotechnol., 2013, 88(7), 1191–1199, DOI:10.1002/jctb.4053.
- Y. Yao, M. Zhu, Z. Zhao, B. Tong, Y. Fan and Z. Hua, Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review, ACS Sustainable Chem. Eng., 2018, 6(11), 13611–13627, DOI:10.1021/acssuschemeng.8b03545.
- S. Prusty, S. Pradhan and S. Mishra, Ionic liquid as an emerging alternative for the separation and recovery of Nd, Sm and Eu using solvent extraction technique-a review, Sustainable Chem. Pharm., 2021, 21, 100434 CrossRef CAS.
- G. Zante and M. Boltoeva, Review on Hydrometallurgical Recovery of Metals with Deep Eutectic Solvents, Sustainable Chem., 2020, 1(3), 238–255, DOI:10.3390/suschem1030016.
- D. Darvishi, D. F. Haghshenas, E. K. Alamdari, S. K. Sadrnezhaad and M. Halali, Synergistic effect of Cyanex 272 and Cyanex 302 on separation of cobalt and nickel by D2EHPA, Hydrometallurgy, 2005, 77(3), 227–238, DOI:10.1016/j.hydromet.2005.02.002.
- N. B. Devi, K. C. Nathsarma and V. Chakravortty, Separation and recovery of cobalt(II) and nickel(II) from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272, Hydrometallurgy, 1998, 49(1), 47–61, DOI:10.1016/S0304-386X(97)00073-X.
- B. K. Tait, Cobalt-nickel separation: the extraction of cobalt(II) and nickel(II) by Cyanex 301, Cyanex 302 and Cyanex 272, Hydrometallurgy, 1993, 32(3), 365–372, DOI:10.1016/0304-386X(93)90047-H.
- K. C. Sole and J. B. Hiskey, Solvent extraction characteristics of thiosubstituted organophosphinic acid extractants, Hydrometallurgy, 1992, 30(1), 345–365, DOI:10.1016/0304-386X(92)90093-F.
- N. B. Devi, K. C. Nathsarma and V. Chakravortty, Separation of divalent manganese and cobalt ions from sulphate solutions using sodium salts of D2EHPA, PC 88A and Cyanex 272, Hydrometallurgy, 2000, 54(2), 117–131, DOI:10.1016/S0304-386X(99)00054-7.
- Y.-C. Hoh, W.-S. Chuang, B.-D. Lee and C.-C. Chang, The separation of manganese from cobalt by D2EHPA, Hydrometallurgy, 1984, 12(3), 375–386, DOI:10.1016/0304-386X(84)90008-2.
- C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, John Wiley & Sons, Ltd, 2003, DOI:10.1002/3527601791.indauth.
- L. Berthon, F. Testard, L. Martinet, T. Zemb and C. Madic, Influence of the extracted solute on the aggregation of malonamide extractant in organic phases: consequences for phase stability, C. R. Chim., 2010, 13(10), 1326–1334, DOI:10.1016/j.crci.2010.03.024.
- R. Chiarizia, D. R. McAlister and A. W. Herlinger, Solvent extraction by dialkyl-substituted diphosphonic acids in a depolymerizing diluent. Ii. Fe(iii) and actinide ions, Solvent Extr. Ion Exch., 2001, 19(3), 415–440, DOI:10.1081/SEI-100103278.
- M. Taube, The influence of diluent polarity on the extraction of neptunium and uranium compounds to organic media, J. Inorg. Nucl. Chem., 1960, 15(1), 171–176, DOI:10.1016/0022-1902(60)80025-5.
- M. Taube, The influence of diluent polarity on extraction of plutonium complexes to organic media, J. Inorg. Nucl. Chem., 1959, 12(1), 174–180, DOI:10.1016/0022-1902(59)80107-X.
- T. V. Healy, Synergism in the solvent extraction of di-, tri- and tetravalent metal ions—II: synergic effects in so-called inert diluents, J. Inorg. Nucl. Chem., 1961, 19(3), 328–339, DOI:10.1016/0022-1902(61)80122-X.
- R. J. Whewell, M. A. Hughes and P. D. Middlebrook, The modelling of equilibrium data for the liquid-liquid extraction of metals part IV. The effect of the diluent on the copper/LIX 64N system, Hydrometallurgy, 1979, 4(2), 125–133, DOI:10.1016/0304-386X(79)90041-0.
- B. Wionczyk and W. Apostoluk, Analysis of diluent effects and estimation of distribution constants of 8-hydroxyquinoline and its derivatives in extraction systems, Hydrometallurgy, 1997, 45(1), 73–81, DOI:10.1016/S0304-386X(96)00082-5.
- W. Tao and Y. Nagaosa, Solvent Extraction of Copper (II) with Di-2-Methylnonylphosphoric Acid in Some Organic Diluents, Solvent Extr. Ion Exch., 2003, 21(2), 273–290, DOI:10.1081/SEI-120018950.
- J. W. Mitchell and C. V. Banks, Solvent effects on solubilities and partition coefficients of some copper(II)-beta-diketone complexes, J. Inorg. Nucl. Chem., 1969, 31(7), 2105–2115, DOI:10.1016/0022-1902(69)90026-8.
- H. F. Aly, M. Raieh, S. Mohamed and A. A. Abdel-Rassoul, Influence of diluent on the synergic extraction of divalent cobalt by the thenoyltrifluoroacetone-tribenzylamine perchlorate system, J. Radioanal. Chem., 1979, 49(2), 213–224, DOI:10.1007/BF02519912.
- A. Rajab, D. Pareau, J. Moulin and A. Chesne, Effect of solvent composition and solvent-extraction of cobalt and nickel by di(2-ethylhexyl) phosphoric-acid, Bull. Soc. Chim. Fr., 1987, 29–33 CAS.
- K. Rezaei and H. Nedjate, Diluent effect on the distribution ratio and separation factor of Ni(II) in the liquid–liquid extraction from aqueous acidic solutions using dibutyldithiophosphoric acid, Hydrometallurgy, 2003, 68(1), 11–21, DOI:10.1016/S0304-386X(02)00168-8.
- S. Guo-Xin, C. Yu, S. Si-Xiu, Y. Yong-Hui and Y. Yan-Zhao, Interfacial Activity of Hdehp and Kinetics of Nickel Extraction in Various Diluents, Solvent Extr. Ion Exch., 2000, 18(3), 517–531, DOI:10.1080/07366290008934695.
- T. Sato, T. Nakamura and M. Kuwahara, Diluent Effect on the Extraction of Uranium(vi) from Hydrochloric Acid Solutions by Trioctylamine, Solvent Extr. Ion Exch., 1985, 3(3), 283–307, DOI:10.1080/07366298508918513.
- Règlement (CE) no. 1907/2006 du 18/12/06 concernant l'enregistrement, l'évaluation et l'autorisation des substances chimiques, ainsi que les restrictions applicables à ces substances (REACH), instituant une agence européenne des produits chimiques (Titre VII: art 55 à 66), AIDA, https://aida.ineris.fr/reglementation/reglement-ndeg-19072006-181206-concernant-lenregistrement-levaluation-6, consulté le 7 février, 2023 Search PubMed.
- N. Jantunen, S. Virolainen and T. Sainio, Direct Production of Ni–Co–Mn Mixtures for Cathode Precursors from Cobalt-Rich Lithium-Ion Battery Leachates by Solvent Extraction, Metals, 2022, 12, 1445, DOI:10.3390/met12091445.
- I. R. Rodrigues, C. Deferm, K. Binnemans and S. Riano, Separation of cobalt and nickel via solvent extraction with Cyanex-272: batch experiments and comparison of mixer-settlers and an agitated column as contactors for continuous counter-current extraction, Sep. Purif. Technol., 2022, 296, 121326, DOI:10.1016/j.seppur.2022.121326.
- N. Vieceli, N. Reinhardt, C. Ekberg and M. Petranikova, Optimization of Manganese Recovery from a Solution Based on Lithium-Ion Batteries by Solvent Extraction with D2EHPA, Metals, 2021, 11, 54, DOI:10.3390/met11010054.
- Y. Cui, J. Yang, G. Yang, G. Xia, Y. Nie and G. Sun, Effect of diluents on extraction behavior of rare earth elements with N,N,N′,N′-tetrabutyl-3-oxy-glutaramide from hydrochloric acid, Hydrometallurgy, 2012, 121–124, 16–21, DOI:10.1016/j.hydromet.2012.04.013.
- M. Gergoric, C. Ekberg, M. R. S. J. Foreman, B.-M. Steenari and T. Retegan, Characterization and Leaching of Neodymium Magnet Waste and Solvent Extraction of the Rare-Earth Elements Using TODGA, J. Sustain. Metall., 2017, 3(3), 638–645, DOI:10.1007/s40831-017-0122-8.
- Y. A. El-Nadi, Effect of diluents on the extraction of praseodymium and samarium by Cyanex 923 from acidic nitrate medium, J. Rare Earths, 2010, 28(2), 215–220, DOI:10.1016/S1002-0721(09)60083-1.
- I. Komasawa and T. Otake, The effects of diluent in the liquid-liquid extraction of copper and nickel using 2-hydroxy-5-nonylbenzophenone oxime, J. Chem. Eng. Jpn., 1983, 377–383 CrossRef CAS.
- P. R. Danesi, L. Reichley-Yinger, G. Mason, L. Kaplan, E. P. Horwltz and H. Diamond, Selectivity-Structure Trends In The Extraction Of Co(Ii) And Ni(Ii) By Dialkyl Phosphoric, Alkyl Alkylphosphonic,And Dialkylphosphinic Acids, Solvent Extr. Ion Exch., 1985, 3(4), 435–452, DOI:10.1080/07366298508918522.
- R. Torkaman, M. Asadollahzadeh, M. Torab-Mostaedi and M. G. Maragheh, Recovery of cobalt from spent lithium ion batteries by using acidic and basic extractants in solvent extraction process, Sep. Purif. Technol., 2017, 186, 318–325, DOI:10.1016/j.seppur.2017.06.023.
- S. Agarwal, M. T. A. Reis, M. R. C. Ismael, M. J. N. Correia and J. M. R. Carvalho, Modeling of the Extraction Equilibrium of Copper from Sulfate Solutions with Acorga M5640, Solvent Extr. Ion Exch., 2012, 30(5), 536–551, DOI:10.1080/07366299.2012.670603.
- V. Nguyen, J. Lee, J. Jeong, B.-S. Kim and B. Pandey, Selective recovery of cobalt, nickel and lithium from sulfate leachate of cathode scrap of Li-ion batteries using liquid-liquid extraction, Met. Mater. Int., 2014, 20, 357–365, DOI:10.1007/s12540-014-1016-y.
- K. Provazi, B. A. Campos, D. C. R. Espinosa and J. A. S. Tenório, Metal separation from mixed types of batteries using selective precipitation and liquid–liquid extraction techniques, Waste Manag., 2011, 31(1), 59–64, DOI:10.1016/j.wasman.2010.08.021.
- A. Porvali, et al., Mechanical and hydrometallurgical processes in HCl media for the recycling of valuable metals from Li-ion battery waste, Resour. Conserv. Recycl., 2019, 142, 257–266, DOI:10.1016/j.resconrec.2018.11.023.
- B. Swain, J. Jeong, J. Lee, G.-H. Lee and J.-S. Sohn, Direct Production of Ni–Co–Mn Mixtures for Cathode Precursors from Cobalt-Rich Lithium-Ion Battery Leachates by Solvent Extraction, J. Power Sources, 2007, 167(2), 536–544, DOI:10.1016/j.jpowsour.2007.02.046.
- B. Swain, J. Jeong, J. Lee and G.-H. Lee, Separation of cobalt and lithium from mixed sulphate solution using Na-Cyanex 272, Hydrometallurgy, 2006, 84(3), 130–138, DOI:10.1016/j.hydromet.2006.03.061.
- A. A. Nayl, M. M. Hamed and S. E. Rizk, Selective extraction and separation of metal values from leach liquor of mixed spent Li-ion batteries, J. Taiwan Inst. Chem. Eng., 2015, 55, 119–125, DOI:10.1016/j.jtice.2015.04.006.
- Y. Pranolo, W. Zhang and C. Y. Cheng, Recovery of metals from spent lithium-ion battery leach solutions with a mixed solvent extractant system, Hydrometallurgy, 2010, 102(1), 37–42, DOI:10.1016/j.hydromet.2010.01.007.
- V. N. H. Nguyen and M. S. Lee, Improvement of metal separation process from synthetic hydrochloric acid leaching solution of spent lithium ion batteries by solvent extraction and ion exchange, Physicochem. Probl. Miner. Process., 2021, 57(4), 1–17, DOI:10.37190/ppmp/136500.
- L. Zhang, et al., Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction, J. Hazard. Mater., 2020, 398, 122840, DOI:10.1016/j.jhazmat.2020.122840.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra04679f |
| This journal is © The Royal Society of Chemistry 2023 |


