Open Access Article
Open Access ArticleChanges in characteristics of silver conductive fabrics owing to perspiration and washing
Sohyun Parka,
Hyewon Kimb and
Suhyun Lee *c
*c
aDepartment of Human Ecology, Korea National Open University, Seoul, 03087, Republic of Korea
bLG Electronics Home Appliance & Air Solution Company, Seoul, 08592, Republic of Korea
cDepartment of Fashion and Textiles, Seoul National University, Seoul, 08826, Republic of Korea. E-mail: suhyun14@snu.ac.kr
First published on 27th September 2023
Abstract
The original appearance and physical properties of smart clothing as well as the electrical properties of the conductive fabric applied, despite utilization and the management environment, need to be maintained. Previous research has only investigated the washability and functional changes of smart textiles according to the environment from the perspective of sensors and fabric material; however, they have not comprehensively considered actual usage conditions. In this study, changes in the appearance, color, chemical components, wettability, and electrical properties of silver-coated conductive knitted fabrics due to exposure to perspiration and washing were investigated based on the manufacturing methods of conductive fabrics. The conductive knitted fabric exposed to perspiration exhibited the most prominent color change, and the surface became rough and hydrophilic as AgCl and AgO were formed through a chemical reaction between perspiration and silver. In contrast, the conductivity of the fabric was enhanced by the release of Ag+ ions via perspiration. After washing, the silver layer on the surface of the conductive knitted fabric peeled off due to the interference and friction between the fabrics caused by the mechanical force generated during the cleaning process. There was no obvious chemical change, but the contact angle decreased as the nano-roughness decreased owing to the removal of silver particles. The conductivity slightly increased after washing but did not show a significant difference at 10% elongation. Finally, as the frequency of exposure to perspiration and washing increased, the silver layer coating the fiber or fabric surface peeled off and was damaged, resulting in a significant color change. Additionally, the chemical composition of the silver layer was significantly altered by perspiration and water and became hydrophilic. Surface resistance also increased linearly as the frequency of exposure to perspiration and washing increased. In terms of hand value, softness and smoothness decreased, and warmness increased after several cycles. Under all conditions, the conductive fabric with silver-coated yarn maintained a more optimized appearance and electrical properties than the silver-coated fabric according to the manufacturing method. Therefore, conductive yarn is more suitable for manufacturing conductive knitted fabrics for smart clothing in terms of durability. Additionally, specific management plans based on the actual use and environment of smart clothing using conductive knitted fabric are urgently needed.
1. Introduction
The demand for smart textiles is rapidly growing. The sportswear industry is one of the first industries to utilize this growing smart textile technology. There are several smart textile products from major sportswear brands, most of which are experiencing exponential growth.1 Smart sportswear refers to wearable e-textile products or smart clothing designed to be worn on or close to the body.2,3 This could potentially drastically change the way athletes are trained and monitored physiologically, ultimately improving their performance during games.1,4,5Numerous studies on e-textile prototypes are underway, and a few products are available in laboratories worldwide; however, these products have limitations. Reliability is a major hurdle to their commercial launch.6 If e-textile products are completely integrated into a fabric structure, they must be washable and reusable to gain market acceptance in the market and attract customers.7 This means that similar to fabrics without an integrated system, smart clothing must be washable or cleanable, as they can get stained during use.2,8 Smart sportswear, which is currently the most commercially available, is exposed to mechanical and chemical environmental changes owing to the intense physical activity of the wearers, and undergoes frequent washing and drying to remove the consequent high amount of perspiration and body odor. Even so, the performance and appearance of smart clothing should not deteriorate or change because of perspiration by athletes during physical activity or due to management procedures such as washing, drying, and storage.
Silver-coated nylon yarn is the most commonly used smart textile material for wearable e-textile products. Although silver-coated nylon yarns exhibit excellent electronic properties, they are easily damaged and susceptible to environmental factors such as moisture and perspiration, and can be relatively easily corroded.7–9 During the life cycle of smart clothing with a silver-coated conductive fabric, silver may undergo various chemical changes that can limit its conductivity in the air and moisture in its environment, perspiration from the human body, or the washing process.5,10 Therefore, identifying the moisture- and perspiration-induced changes and ensuring the corrosion resistance of silver-coated fabrics are important for applications such as smart sportswear in wet environments and during contact with perspiration.7
Currently, the change in electrical resistance is measured through repeated washing to evaluate the performance and reliability of functional implementation of smart textiles. There are several standard test methods for washing smart textiles. In this study, samples were washed using the general washing methods specified in ISO 6330, and the reliability of the electrical properties was evaluated by measuring the surface resistance using the methods specified in AATCC 76 or AATCC 84. However, because basic information on the general wearing environment for smart clothing is lacking, washing evaluation is mostly based on the conclusion drawn by researchers.2 Gaubert et al.11 evaluated the effect of washing on silver-plated textile electrodes. Evaluation of the appearance and function of the smart electrode after washing showed that liquid detergent had less effect on the electrode performance than powder detergent. In addition, bleach oxidizes and destroys the silver layer, maximizing the effect of mechanical friction during washing. Previous studies suggested that the silver-plated nylon electrode loses its conductivity owing to necessitates the identification of suitable washing conditions. Chui et al.7 simulated the actual environment of silver-coated nylon fibers or fabrics and evaluated their functional stability according to environmental conditions. In particular, a washing and drying environment is created during clothing production. When the adhesion between the fibers and silver particles was attenuated, the silver layer on the fiber surface peeled off, even after washing 10 times, causing damage to the appearance and poor performance.
However, most previous studies on changes in the physical properties and functions of smart fabrics based on the actual wearing environment by monitoring physical activity, to which smart clothing is applied, are related to perspiration. Tajin et al.9 manufactured a smart antenna from silver-coated nylon yarn, and evaluated the performance of the smart antenna according to perspiration, washing, and drying conditions to apply it to clothing. They found that it was difficult to maintain the performance of silver because the resistance increased owing to changes in the chemical composition caused by perspiration and friction during washing. Therefore, based on these results, researchers have proposed further research to improve the durability of antenna performance in a dynamic environment. Yan et al.12 observed the chemical changes in fabrics coated with silver upon exposure to an artificial perspiration solution. They mixed salt, acid, and alkali by adjusting the pH of artificial perspiration and immersed fabric in this solution to evaluate the consequent changes in silver ion release, surface shape change, and chemical composition. Their results showed that the release behaviour of the silver particles varied according to the pH of the solution, and the environmental and human risk increased with the release of silver ions in an environment with high ionicity. These results can be applied to silver-based smart fabrics.
Previous studies have only investigated the washability and functional changes of smart textiles according to the environment from the perspective of sensors and fabric material. However, they have not comprehensively considered actual usage conditions. Smart clothing is in close proximity to the human body; therefore, chemical and physical changes can affect the human body. In particular, it is necessary to consider the chemical changes in smart textiles caused by sebum and perspiration from the human body. Additionally, clothing products must undergo essential management processes such as washing and drying. Therefore, it is necessary to accurately understand the changes in conductive materials by mimicking the actual wearing and management environment of smart clothing and observing the changes in physical properties and functionality when they are repeatedly exposed to perspiration and washing. Furthermore, it is necessary to identify acceptable changes in physical properties and appearance by comparing and analysing these results with changes in general textile materials, and propose practical usage and management methods.
The purpose of this study was to analyze and compare changes in the physical, chemical, and electrical properties of smart fabrics and common fabrics applied to representative smart clothing products owing to repeated exposure to perspiration and washing. In this study, changes in the appearance, color, chemical composition, electrical properties, and tactile properties of conductive knitted fabrics with silver coatings and common knitted fabrics were examined and compared in relation to changes in chemical and physical environments such as perspiration and washing. This was aimed at elucidating the behavior of smart textiles under each environmental condition and acquiring basic data to ensure product quality reliability and develop management measures for future commercialization by comprehensively exploring the scope and environment of smart textiles.
2. Experimental section
2.1. Materials
Five fabrics were prepared as listed in Table 1. Conductive fabrics were prepared in two forms: two fabrics were dip-coated with a silver solution in the fabric state, and one fabric was dip-coated with a silver solution in the yarn state and knitted with nylon yarns. Notably, common knitted fabrics are prepared using two types of knitted fabrics based on polyester and nylon, which are primarily used in sports and fitness wear. The structures of all the fabrics were tricot-warp knitted fabrics.2.2. Test procedure
As shown in Table 2, the experimental conditions included the untreated condition, immersion in perspiration fluid only, washing only, and controlling the number of immersions in perspiration fluid and washes.
| Frequency of immersion in perspiration fluid | Frequency of washing | Cods | |
|---|---|---|---|
| Untreated | — | — | UT |
| Immersion in perspiration fluid | 1 | — | P1 |
| Washing | — | 1 | W1 |
| Immersion in perspiration fluid and washing | 1 | 1 | P1W1 |
| 5 | 5 | P5W5 | |
| 10 | 10 | P10W10 |
2.3. Characterization
Furthermore, the overall chemical composition of the sample and the chemical properties and bonding state of Ag for each treatment were examined using an X-ray photoelectron spectrometer (Versaprobe III, PHI, USA). To analyze the quantity of Ag, the binding energy was measured from 0 to 1000 eV. Additionally, CasaXPS software (Casa Software Ltd, Version 2.3.25) was used to fit the peaks for analysis of the chemical bonds of Ag.
A contact angle analyzer (Theta Lite Optical Tensiometer, KSV Instruments, Finland) was used to evaluate the hydrophobic or hydrophilic properties of the conductive knitted fabrics after exposure to perspiration fluid or washing. The samples were attached to a slide glass using a 3 M tape, and a 3.5 ± 0.3 μL drop of deionized water was deposited on the surface to be investigated. The contact angle of the water droplet was recorded 1 s after deposition. Ten different sites on each sample were measured, and the average value was calculated.
The linear electrical resistance of the conductive fabric was also measured using a DC milliohm meter. The fabric samples measured 10 × 10 cm in the wale and course directions. The sample was supported at a distance of 1 cm using two alligator clips, and the initial resistance value was measured. Subsequently, the sample was stretched to observe the change in resistance based on the elongation rate. The fabric resistance was tested at 10% elongation in the wale and course directions, respectively. Three samples were tested, and the average electrical resistance was calculated. Additionally, the percentage change in fabric resistance, tested at 10% elongations, was calculated as shown in eqn (1) below.
 | (1) |
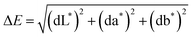 | (2) |
3. Results and discussions
3.1. Changes in characteristics of conductive fabrics due to perspiration
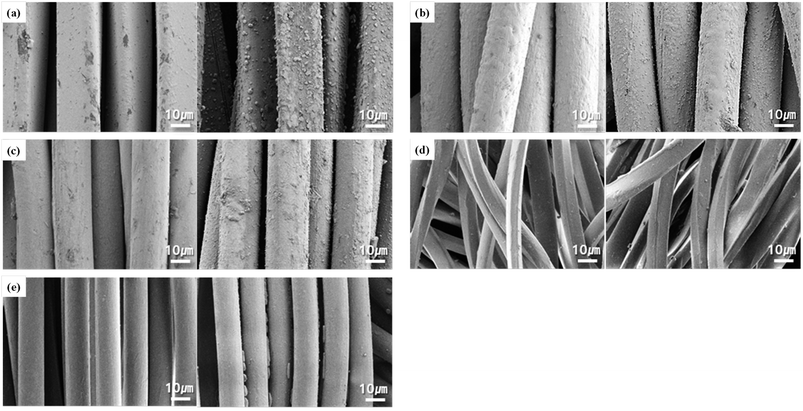
Fig. 1 shows FE-SEM images of each sample before and after immersion in the perspiration solution. In the conductive fabrics shown in Fig. 1a–c, there are numerous grains on the surface of the fibers, which may have resulted from the formation of salts upon exposure to acidic perspiration. The conductive fabric used in this study was coated with Ag in the yarn or fabric state. Acidic perspiration can result in a fast release rate and a significant release of silver.12 Silver can be corroded by a NaCl solution because a few Ag+ cations on the surface of the silver coating and Cl− in the NaCl aqueous solution form AgCl, and AgCl continues to react with Cl− anions in the NaCl aqueous solution and forms [AgCl2]− complexes, which lead to the dissolution of silver.7 Additionally, when Ag reacts with salt, it undergoes a double substitution reaction to form an insoluble precipitate.14 Therefore, the substance observed on the fiber surface in Fig. 1a–c is a precipitate. To confirm the formation of these grain components by the reaction of the conductive knitted fabric with perspiration, a quantitative analysis of the surface elements was performed using EDX, and the results are shown in Fig. 2. EDX analysis of the grains created on the surface of the conductive fibers showed the presence of C, O, Ag, Cl, and Si. C and O were detected in the fibers, and a large amount of Ag was confirmed owing to the silver coating. Si was used as a crosslinking agent to attach silver to the fiber surface. Na and Cl were not included in the fiber or silver coating agent. Therefore, they were obtained through perspiration. The Na detected in C1 and C3 (Fig. 2a and c) resulted from the recrystallization of substances that did not react or were removed after immersion in the perspiration solution into NaCl. Additionally, Cl is confirmed where silver and grains are gathered on the fiber surface. Therefore, EDX analysis confirmed that the chemical change from silver to AgCl and AgCl2 was due to perspiration. Chui et al.7 observed white grains of the same shape on the surfaces of silver-coated nylon yarns immersed in a 0.5% NaCl solution. Particularly, the conductive fabric of C1 showed a larger and clearer formation of deposits than that of C2 despite the use of the same coating method involving post-coating in a silver bath in fabric state. This is because the space between the loops in C1 is larger, resulting in a lower density than the other conductive fabrics, which is more advantageous for salt formation through the permeation of perspiration. The degree of perspiration penetration into the pores between fabrics, yarns, and fibers depends on the porosity of the weaving or knitting method. This leads to a difference in tortuosity, which affects the flow of fluid and may cause variation in water absorption between yarns and fibers.15 In the case of C3, which is a mixture of conductive yarns and nylon yarns, the silver content is low compared to that of C1 and C2; therefore, the grains formed by the reaction with perspiration are relatively small.
 | ||
| Fig. 1 FE-SEM images (×3000) of the fabric surface before and after immersion in perspiration solution: (a) C1; (b) C2; (c) C3; (d) K1; (e) K2. | ||
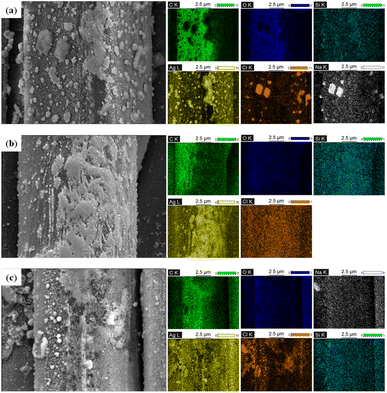 | ||
Fig. 2 FE-SEM images (×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and EDX mapping images of grains on the conductive knitted fabrics after exposure to the perspiration: (a) C1; (b) C2; (c) C3. 000) and EDX mapping images of grains on the conductive knitted fabrics after exposure to the perspiration: (a) C1; (b) C2; (c) C3. | ||
In contrast, no change was observed on the fiber surface in knitted fabrics (d) and (e). This is because the common knitted fabric does not contain substances that can chemically react with perspiration. In the case of the K2 sample, some solid materials were observed in the space between the fibers, which corresponded to the solidification of perspiration. K2 is composed of filamentous nylon fibers, allowing capillaries to be form between the fibers. Perspiration fluid is absorbed and transferred into the fabric through these capillaries. The perspiration fluid excessively adsorbed on the surface of the fiber solidified and were not removed during the drying process. When conductive fabrics and common knitted fabrics were exposed to perspiration, the color difference that may occur due to a chemical reaction with perspiration was analyzed by observing the appearance and calculating ΔE. The results are as shown in Fig. 3 and Table 3.
 | ||
| Fig. 3 Appearance of the fabrics following exposure to perspiration: (a) C1; (b) C2; (c) C3; (d) K1; (e) K2. | ||
| L* | a* | b* | ΔE | ||
|---|---|---|---|---|---|
| C1 | Before | 54.93 ± 0.57 | 0.73 ± 0.07 | 4.90 ± 0.32 | 4.94 ± 1.94 |
| After | 51.09 ± 1.90 | 1.96 ± 0.20 | 7.64 ± 0.74 | ||
| C2 | Before | 56.56 ± 1.23 | −0.09 ± 0.22 | 5.42 ± 0.24 | 9.65 ± 0.72 |
| After | 55.40 ± 1.53 | 4.03 ± 0.10 | 13.77 ± 1.00 | ||
| C3 | Before | 60.98 ± 0.76 | 0.50 ± 0.10 | 5.28 ± 0.29 | 1.97 ± 0.54 |
| After | 59.77 ± 0.88 | 1.24 ± 0.18 | 6.53 ± 0.34 | ||
| K1 | Before | 91.30 ± 0.23 | 6.81 ± 0.08 | −17.98 ± 0.45 | 0.71 ± 0.46 |
| After | 91.22 ± 0.17 | 6.72 ± 0.08 | −17.81 ± 0.43 | ||
| K2 | Before | 92.08 ± 0.58 | 3.74 ± 0.06 | −14.63 ± 0.55 | 1.67 ± 0.58 |
| After | 91.35 ± 0.30 | 3.59 ± 0.18 | −13.41 ± 0.68 |
As a result of the observation, the color change of the conductive fabric due to exposure to perspiration could be confirmed with the naked eye, while the common knitted fabric showed a similar color, as shown in Fig. 2. According to Table 3, the L* values of all samples decreased and became somewhat darker, and the a* and b* values increased, resulting in stronger red and yellow colors. The results indicated a brownish tendency. Overall, the color change of the conductive fabrics was more pronounced after immersion in perspiration than that after immersion of common knitted fabrics in perspiration. When the fabric is immersed in water, its surface becomes negatively charged. Acidic perspiration provides a good binding force with these negatively charged fabrics, allowing them to penetrate the fibers more easily.16 Therefore, the more the perspiration penetrates the fibers, the more active the reaction with the silver deposited on the surface of the conductive fabrics, resulting in a distinct color change.
Among the conductive fabrics, especially C2 showed a significant color change after immersion in perspiration, and the ΔE value was also the highest. Compared with C1, which was coated with silver using the same method, the color change of C2 was notable. This was because the silver particles were highly reactive to acidic perspiration, and the spandex yarns blended in C2 were very vulnerable to perspiration. Spandex fibers are composed of urethane groups (–NH–CO–O–) and thus have many amino groups (–NH2). Therefore, spandex can be dyed using acid dyes.17 Based on this dyeability, spandex cause a color change through a reaction with acidic perspiration. In contrast, C3 exhibited the lowest color change and excellent durability. This is because C3 was not entirely coated with silver in the fabric state but was composed of 55% silver-coated nylon yarns and 45% normal nylon. Conductive yarns coated in the yarn state form a more uniform silver layer and stably bond with the fibers compared to conductive fabrics coated in the fabric state.18,19 Additionally, because the amount of silver exposed to the surface is small, chemical reactions or changes due to perspiration may be relatively small.
However, the color change due to exposure of common knitted fabrics to perspiration was difficult to distinguish with the naked eye, and the ΔE value was relatively low. C3, which showed the lowest color change among the conductive fabrics, exhibited an ΔE value similar to that of K2. That is, as it is difficult to distinguish this level of color difference with the naked eye, C3 may be suggested as the most suitable for use in clothing among conductive fabrics in terms of appearance changes.
| Composition | C1 | C2 | C3 | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| C12 | 55.43 | 52.20 | 53.71 | 51.98 | 68.76 | 69.26 |
| O12 | 16.27 | 25.99 | 19.70 | 29.75 | 29.28 | 28.65 |
| Cl2p | 1.39 | 2.11 | 0.88 | 1.85 | 0.00 | 0.29 |
| Ag3d | 26.91 | 19.70 | 25.71 | 16.42 | 1.96 | 1.80 |
Fig. 4 shows the XPS spectrum of Ag 3d for each conductive knitted fabric sample. The Ag 3d5/2 and Ag 3d3/2 core-level binding energies for all conductive fabrics appear at 368 and 374 eV, respectively, in good agreement with bulk Ag, indicating that Ag is bound to the fabric surface.20,21 These two bands shift to lower energies in all three samples after exposure to perspiration. This shift is caused by electron transfer from the silver layer to the perspiration solution. The binding energy of the higher ionic state of Ag is lower than that of zero-valent Ag because of shifts in the initial state potential of the ionic charge and the lattice potential.20 Additionally, new peaks appear at approximately 367 and 373 eV after perspiration, particularly for C1 and C3. The peaks at 368 and 374 eV can be assigned to the Ag 3d peaks of metallic Ag, whereas those at 367 and 373 eV can be ascribed to the Ag 3d peaks of Ag+ in AgCl.22,23
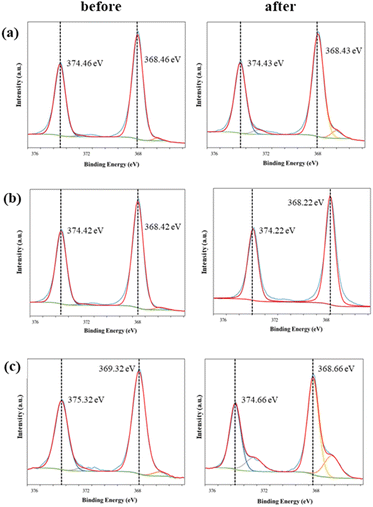 | ||
| Fig. 4 Ag 3d XPS spectra of the conductive fabrics according to exposure to perspiration: (a) C1, (b) C2, and (c) C3. | ||
In contrast, according to the contact angle measurement results in Table 5, the common knitted fabrics (K1 and K2) were immediately absorbed as soon as the water droplets fell and exhibited hydrophilic properties. PTT, nylon, and polyester are naturally hydrophobic fibers by nature.24 However, the density of the common knitted fabrics used in this study was quite low, and water easily penetrated through capillary action. Conversely, the conductive knitted fabrics (C1, C2, and C3) exhibited hydrophobicity at approximately 130° by reducing the contact angle area between water and fibers, as well as voids on the fabric surface due to the micro–nano dual roughness caused by the yarn and silver coating.24,25 After exposure to perspiration, all the samples became hydrophilic, with a contact angle of 0°. As metal oxides are capable of strong hydrogen bonding, their surfaces are typically completely wetted by water.26 Additionally, artificial perspiration absorbed into the fabric forms a heterogeneous solid surface, leading to the absorption of water droplets.25 Therefore, the oxidation and chemical changes in silver following exposure to perspiration can be confirmed through the contact angle measurements.
| C1 | C2 | C3 | K1 | K2 | |
|---|---|---|---|---|---|
| Before | 123.5 ± 4.3 | 135.1 ± 5.3 | 143.1 ± 3.8 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| After | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Wale (Ω m−2) | Course (Ω m−2) | Average (Ω m−2) | ||
|---|---|---|---|---|
| C1 | Before | 0.39 ± 0.06 | 0.38 ± 0.04 | 0.38 ± 0.05 |
| After | 0.27 ± 0.04 | 0.38 ± 0.04 | 0.32 ± 0.07 | |
| Change% | 44% | 0% | 19% | |
| C2 | Before | 0.35 ± 0.06 | 0.35 ± 0.05 | 0.35 ± 0.05 |
| After | 0.31 ± 0.05 | 0.30 ± 0.05 | 0.31 ± 0.04 | |
| Change% | 13% | 17% | 13% | |
| C3 | Before | 0.36 ± 0.06 | 0.46 ± 0.07 | 0.41 ± 0.08 |
| After | 0.27 ± 0.04 | 0.42 ± 0.04 | 0.35 ± 0.08 | |
| Change% | 33% | 10% | 17% |
Silver is highly soluble in acidic solutions. Yan et al.12 reported that when silver nanoparticle-coated nano fabric was immersed in an acidic artificial perspiration solution, a significant number of Ag+ ions was detected as silver was released. These dissolved Ag+ ions react with NaCl during perspiration, forming AgCl. However, as the conductive fabric used in this study had a compact silver coating, the silver coating became more apparent even with a small amount of silver dissolved through perspiration. This phenomenon was the main reason underlying the decrease in surface resistance.7 The effect was more pronounced in the wale direction, as the electron flow in this direction is smoother and more networked owing to the arrangement of loops. Conversely, the course direction in the warp-knitted fabric introduced contact resistance.11
Meanwhile, no difference was observed between the C1 or C2 samples, both of which were coated with silver on the fabric surface after knitting, and the C3 sample, which was knitted after applying a conductive yarn with a silver coating. The reason behind this lack of difference is that the silver layer exposed on the yarn or fabric surface chemically reacts with perspiration, regardless of the coating method.
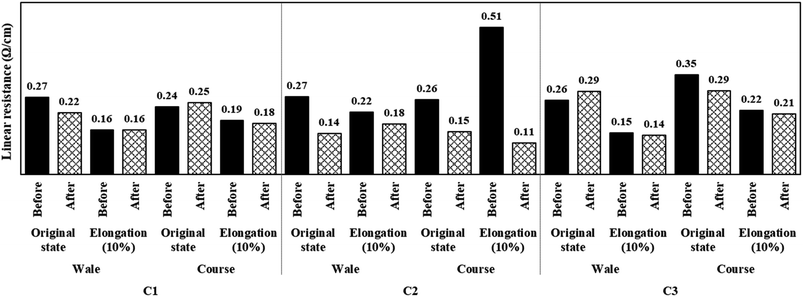
Fig. 5 shows the linear resistance values of the conductive fabrics before and after perspiration, which were measured in their original and elongated states. All samples showed lower linear resistance, regardless of the direction, when elongated by 10% compared to the original state. However, in the case of C2, a higher linear resistance was observed when stretching in the course direction because the spandex fiber was inserted. Owing to their structural characteristics, knitted fabrics have excellent stretchability.27 Spandex, contrastingly, is elastic because the soft segment is deformed by an external force owing to its polymer structure composed of soft and hard segments.28,29 Therefore, the linear resistance increased as the silver layer on the surface was partially damaged, as the fiber, and not the fabric structure, was stretched during elongation in the course direction.
Conversely, the linear resistance of each sample decreased or remained the same after exposure to perspiration, and there was no significant difference from before exposure to perspiration at 10% elongation. As observed in the surface resistance results mentioned above, Ag+ ions were eluted as perspiration and silver reacted, which made the compact silver layer clearer and improved conductivity. This trend was more clearly observed for C2. In the case of C2, the effect of the reaction between silver and perspiration was more pronounced, and the conductivity improved five times before and after exposure to perspiration in the elongation state. Meanwhile, the effect of Ag+ ions caused by perspiration appeared to be relatively small in the other samples because the electrons flowed smoothly owing to the shortened electron transport distance as the loop expanded during elongation.30
3.2. Changes in characteristics of conductive fabrics by washing
Fig. 6 displays SEM images of changes in the fiber surface after washing. In the case of conductive fabrics C1 and C2, which were coated with silver, the fiber surface was scratched, the silver-coated layer peeled off after washing, and damage to the fiber surface was evident. This is because the entire fabric was post-coated with silver, and the silver layer was removed owing to interference and friction between the fabrics caused by the mechanical force generated during the washing process. The mechanical force transmitted to the fabric during washing includes a large-scale force, which causes movement of the entire fabric; a medium-scale force, which is transmitted between yarns; and a small-scale force, which is transmitted between the fibers and causes damage to the fiber surface.32 Therefore, the fabric is damaged by the friction between the fabric and the frictional force caused by the fall that occurs when the fabric rises from the drum by centrifugal force and then falls into in the drum-washing machine.
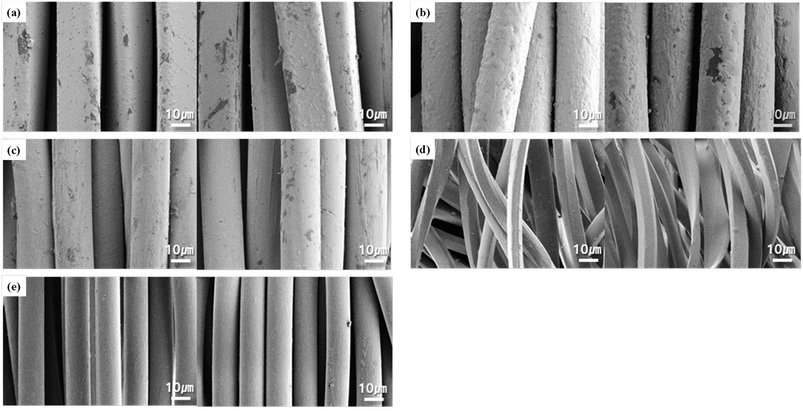 | ||
| Fig. 6 FE-SEM (×3000) of the fabric surface before and after washing: (a) C1; (b) C2; (c) C3; (d) K1; (e) K2. | ||
However, in the case of C3, the damage to the fiber surface was the least among the conductive fabrics. This indicates that the conductive fabric in C3 is made of silver-coated yarn and nylon yarn, and the area exposed to mechanical force from the washing machine is smaller compared to C1 and C2, which are coated with silver in the fabric state.33 Additionally, yarn is more flexible to mechanical forces than the fabric, resulting in less damage during washing. No damage to the fiber surface was observed in the common knitted fabrics, as shown in Fig. 6d and e. This is because the mechanical force was not sufficient to cause damage to the surface of the fabric after only one washing cycle.
Fig. 7 and Table 7 show the results of observations on the changes in appearance and the color difference between the conductive fabrics and common knitted fabrics before and after washing. In all the samples, there was no significant change in appearance after one washing cycle. The L*, a*, and b* values decreased slightly, resulting in a decrease in brightness and a stronger bluish color. However, the color change was not significant. C2, which exhibited a significant color change when exposed to perspiration among the conductive fabrics, was not significantly affected by washing alone. Thus, it was confirmed that silver had some durability against washing, as there were no significant physical and chemical changes with respect to washing. However, desorption of some silver particles was observed even after one washing. If repeated washing occurs, desorption of the coating adsorbed on the surface is induced, leading to a potential decrease in conductivity.
| L* | a* | b* | ΔE | ||
|---|---|---|---|---|---|
| C1 | Before | 54.93 ± 0.57 | 0.73 ± 0.07 | 4.90 ± 0.32 | 1.23 ± 0.46 |
| After | 54.47 ± 0.88 | 0.57 ± 0.06 | 4.10 ± 0.17 | ||
| C2 | Before | 56.56 ± 1.23 | −0.09 ± 0.22 | 5.42 ± 0.24 | 1.30 ± 0.60 |
| After | 55.60 ± 0.73 | 0.04 ± 0.23 | 4.77 ± 0.17 | ||
| C3 | Before | 60.98 ± 0.76 | 0.50 ± 0.10 | 5.28 ± 0.29 | 1.81 ± 0.44 |
| After | 61.76 ± 0.69 | 0.65 ± 0.06 | 3.86 ± 0.23 | ||
| K1 | Before | 91.30 ± 0.23 | 6.81 ± 0.08 | −17.98 ± 0.45 | 0.85 ± 0.59 |
| After | 91.19 ± 0.11 | 6.86 ± 0.12 | −18.63 ± 0.33 | ||
| K2 | Before | 92.08 ± 0.58 | 3.74 ± 0.06 | −14.63 ± 0.55 | 0.95 ± 0.44 |
| After | 91.33 ± 0.17 | 3.52 ± 0.18 | −14.11 ± 0.56 |
| Composition | C1 | C2 | C3 | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| C12 | 55.43 | 64.23 | 53.71 | 60.97 | 68.76 | 72.17 |
| O12 | 16.27 | 17.08 | 19.70 | 19.24 | 29.28 | 24.25 |
| Cl2p | 1.39 | 0.97 | 0.88 | 0.85 | 0.00 | 0.00 |
| Ag3d | 26.91 | 17.72 | 25.71 | 18.94 | 1.96 | 3.57 |
Fig. 8 shows the XPS spectrum of Ag 3d for each conductive knitted fabric after washing. The binding bands representing Ag 3d5/2 and Ag 3d3/2 shift to lower energies after washing. This shift is the result of the slight oxidation of silver due to the detergent and water used during washing. The binding energies of AgO and Ag2O are 367.4 eV and 367.8 eV, respectively.35 In contrast, since no new peak was observed after washing, there was little change in the silver components, such as AgCl.
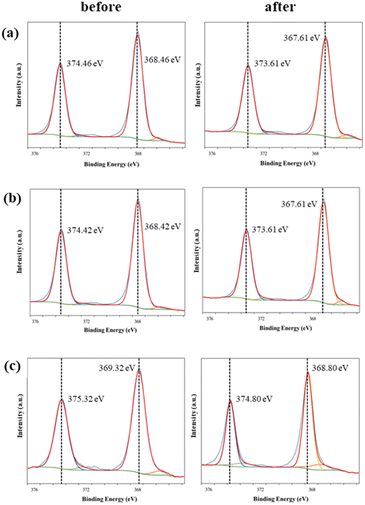 | ||
| Fig. 8 Ag 3d XPS spectra of the conductive fabrics according to exposure to washing: (a) C1, (b) C2, and (c) C3. | ||
The detachment of the silver particles from the fiber surface after washing was also confirmed by the contact angle results. As shown in Table 9, the contact angles of all silver-coated conductive knitted fabrics decreased after washing. This is the result of the oxidation of silver, increased reactivity with water, and loss of nano-roughness due to the silver particles falling off.24
| C1 | C2 | C3 | K1 | K2 | |
|---|---|---|---|---|---|
| Before | 123.5 ± 4.3 | 135.1 ± 5.3 | 143.1 ± 3.8 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| After | 119.2 ± 2.7 | 116.0 ± 7.2 | 136.3 ± 10.7 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Table 10 lists the surface resistance values of the conductive fabrics before and after washing measured in the wale and course directions, respectively. After washing, the surface resistance of all the samples increased, regardless of the wale and course direction. As confirmed by the FE-SEM image, this was because the silver layer on the surface of the conductive fabric was damaged owing to mechanical friction between the fabric and the drum wall of the washing machine during the washing process.31 However, the change in conductivity after washing showed a trend similar to the change following exposure to perspiration, regardless of the coating method.
| Wale (Ω m−2) | Course (Ω m−2) | Average (Ω m−2) | ||
|---|---|---|---|---|
| C1 | Before | 0.39 ± 0.06 | 0.38 ± 0.04 | 0.38 ± 0.05 |
| After | 0.47 ± 0.06 | 0.51 ± 0.08 | 0.49 ± 0.07 | |
| Change% | −17% | −25% | −22% | |
| C2 | Before | 0.35 ± 0.06 | 0.35 ± 0.05 | 0.35 ± 0.05 |
| After | 0.44 ± 0.2 | 0.41 ± 0.09 | 0.43 ± 0.12 | |
| Change% | −20% | −15% | −19% | |
| C3 | Before | 0.36 ± 0.06 | 0.46 ± 0.07 | 0.41 ± 0.08 |
| After | 0.52 ± 0.23 | 0.50 ± 0.11 | 0.51 ± 0.15 | |
| Change | −31% | −8% | −20% |
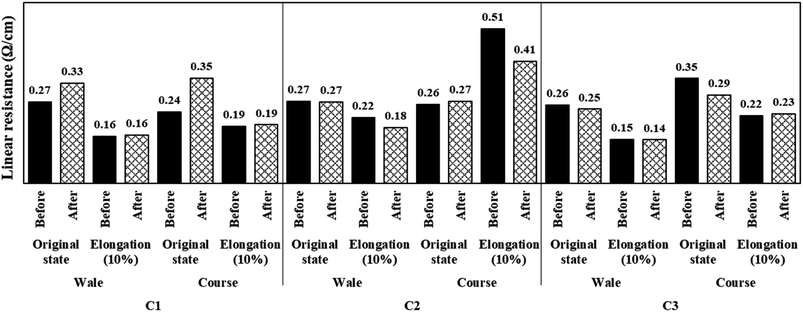
Fig. 9 shows the linear resistance values of the conductive fabrics before and after washing measured in their original and elongated states. The linear resistance of each sample increased or remained constant after washing. The largest change was observed in the C1 sample, which showed a linear resistance increase of approximately 20% or more with just one washing process in both the course and wale directions. This was because the silver coating layer on the fabric surface was partially damaged during the washing process. C2 was also coated using the same method as C1, but in the case of C2, owing to a higher gauge than C1, the pores were small and the adhesion between the silver layer and the fiber was large; therefore, the effect of washing on the mechanical behavior was relatively small. On the other hand, there was no significant difference from before washing at 10% elongation. As the loop was stretched owing to elongation, the electron movement path was shortened, and the electron flow was smoothed. In this process, damage to the silver layer due to washing did not have a significant effect on the linear resistance.
3.3. Changes in characteristics of conductive fabrics after repeated exposure to perspiration and washing
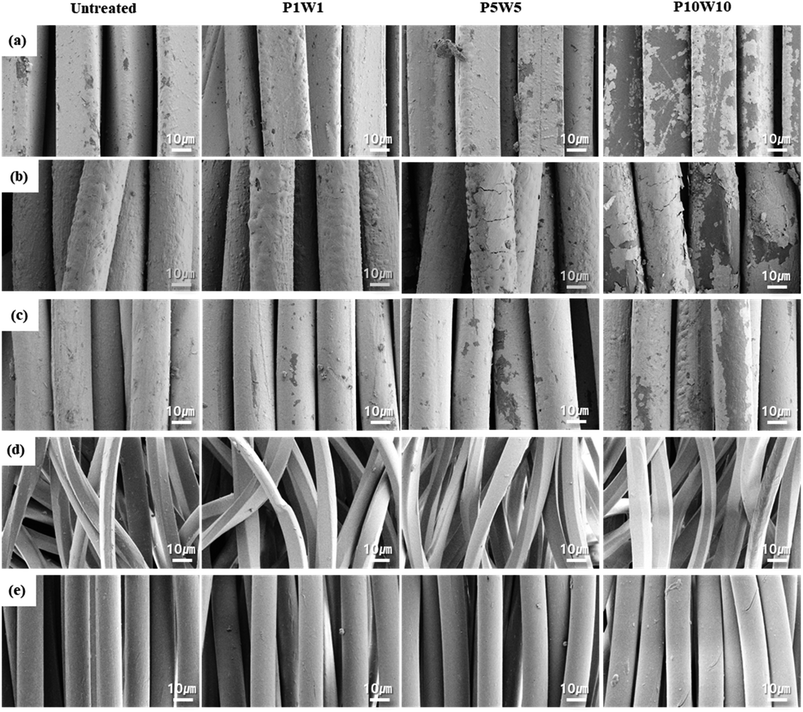 | ||
| Fig. 10 FE-SEM (×3000) of the fabric surface according to repeating exposure of perspiration and washing: (a) C1; (b) C2; (c) C3; (d) K1; (e) K2. | ||
In the case of conductive fabrics, the silver layer on the fiber surface was peeled off and damaged upon repeated exposure to perspiration and washing. A single cycle of exposure to perspiration and washing did not significantly damage the silver coating layer; however, after five cycles, the silver coating peeled off or cracked, as shown in Fig. 10a–c, and was completely peeled off after 10 cycles, thus exposing the nylon fiber surface. In particular, compared to C3, which was coated with silver in the yarn state, C1, and C2, which were post-coated with silver in the fabric state, showed relatively greater damage from repeated exposure to perspiration and washing. This is because the coating layer on the fiber surface was loosened as the silver was oxidized owing to exposure to perspiration, and the mechanical force generated during the washing process was transferred to the fabric, causing movements such as tension, bending, and elongation in the fabric.12,32 In the case of the fabric coated entirely with silver, all the yarns in the fabric were bent and stretched simultaneously, and the outermost surface-coated material fell off, causing more damage. However, in the case of C3, because the fiber was coated with silver, the fiber was flexible even when the entire fabric moved, resulting in relatively less damage and high durability.
In the common knitted fabrics K1 and K2, no change was observed on the surface of the fibers even after repeated exposure to perspiration and washing cycles because there was no separately treated material in the state of the fibers of the fabrics.
Fig. 11 and 12 show photographs and graphs of the color difference values of conductive fabrics and common knitted fabrics according to the frequency of exposure to perspiration and washing, respectively.
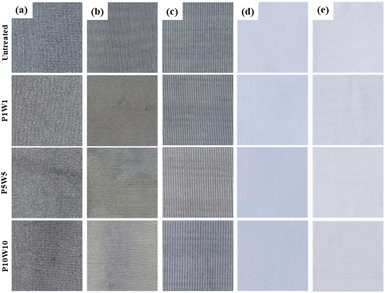 | ||
| Fig. 11 Appearance of the fabric according to repeating exposure to perspiration and washing: (a) C1; (b) C2; (c) C3; (d) K1; (e) K2. | ||
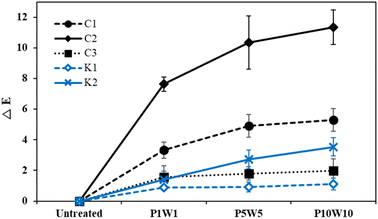 | ||
| Fig. 12 Color differences of the fabric according to repeating exposure to perspiration and washing. | ||
As shown in Fig. 11, a color change was observed in the conductive fabric as the frequency of exposure to perspiration and washing increased. C2 exhibited the largest color change and numerous stains on its surface. C1 and C3 also turned yellow and became more stained as the number of repetitions increased. Among the conductive fabrics, C3 exhibits the smallest color change. In the case of the common knitted fabrics, no color change was observed with the naked eye after repeated perspiration and washing.
According to the results in Fig. 12, the color difference increased compared to the untreated fabric as the frequency of exposure to perspiration and washing increased under all fabric conditions. The color differences according to repeated treatments were large, in the order of C2, C1, K2, C3, and K1. Among the conductive fabrics, C2, which was most vulnerable to perspiration, showed the highest color difference, whereas C3 showed minimal color change. This is because the effect of perspiration exceeded that of washing. In the results of the above examination, the fabric coated with silver in the fabric state showed the lowest durability when exposed to perspiration solution. Therefore, repeated exposure to perspiration caused a large color change as it caused continuous damage to the conductive fabric surface. In contrast, in the case of the common knitted fabrics, no color change was observed with the naked eye, but when compared through color difference values K2 showed a higher color difference value than C3, which is a conductive fabric. K2 has luster because it is thin, very flexible, and had a smooth surface, owing to the uniform arrangement of the fibers. Therefore, as the surface was worn by repeated washing cycles, the luster decreased, the L* value decreased, and the overall color difference of K2 was higher than that of K1 or C3.36
Therefore, in terms of appearance, C3 exhibited optimal durability upon subjection to repeated exposure to perspiration and washing.
| Composition | C1 | C2 | C3 | |||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| C12 | 55.43 | 63.04 | 53.71 | 59.17 | 68.76 | 67.26 |
| O12 | 16.27 | 19.33 | 19.70 | 19.00 | 29.28 | 27.05 |
| Cl2p | 1.39 | 0.91 | 0.88 | 1.28 | 0.00 | 0.75 |
| Ag3d | 26.91 | 16.72 | 25.71 | 20.55 | 1.96 | 4.94 |
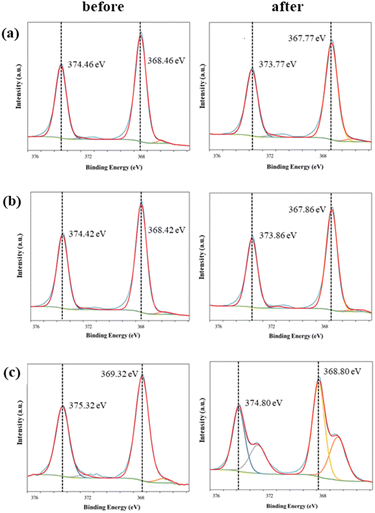 | ||
| Fig. 13 Ag 3d XPS spectra of the conductive fabrics according to exposure to perspiration and washing: (a) C1, (b) C2, and (c) C3. | ||
Fig. 14 shows the change in the contact angles of the conductive knitted fabrics according to the frequency of exposure to perspiration and washing cycles. The contact angles of the untreated sample and the 1, 5, and 10 cycles of exposure to perspiration and washings were 123.5°, 108.8°, 117.2°, and 122.7° for C1 and 135.1°, 107.4°, 114.7°, and 126.0° for C2. In C1 and C2, the contact angle decreased after a single cycle of exposure to perspiration and washing, and recovered to the level of the untreated sample upon increasing the number of cycles. This was because water penetration by capillary action on the fabric surface increased as the nano-roughness of the fabric was damaged by the loss of silver particles after a single cycle of exposure to perspiration and washing.24 However, as the silver layer peeled off from the fiber surface owing to friction between fabrics during repeated washing, the fabric surface became rough and the contact angle increased. In contrast, the contact angles of untreated and 1, 5, and 10 times of treatment for C3 were 143.1°, 123.7°, 120.2°, and 0.0°. The oxidation and desorption of silver due to exposure to perspiration and washing increased its affinity to water molecules and wicking by capillary action. However, unlike other samples, the silver-coated conductive yarn was half-inserted into the fabric, so the nano-roughness due to the peeling of the silver layer by increasing the number of washings was not induced. In particular, hydrophobicity was maintained for 5 cycles, at 10 cycles, the contact angle was 0.0°, and the water droplets were completely absorbed from the surface and became hydrophilic.
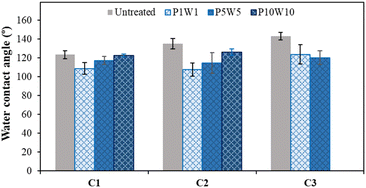 | ||
| Fig. 14 Water contact angle of the fabric according to repeating exposure to perspiration and washing. | ||
Based on these results, exposure to perspiration and washing more than 10 times significantly changed the chemical properties and wettability of the surface of the conductive knitted fabric. Therefore, to maintain the original characteristics of the conductive fabric, the cycle frequency need to be reduced to the extent possible, or a manufacturing method that improves the durability of adhesion between the silver layer and fabric need to be developed.
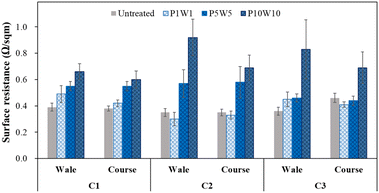 | ||
| Fig. 15 Surface resistance of the conductive fabrics according to repeating exposure to perspiration and washing. | ||
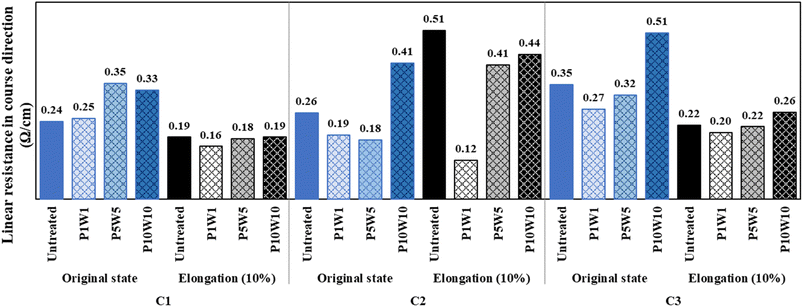
Fig. 16 and 17 show the measured linear resistance values of the conductive fabrics in their original and stretched states, respectively, after repeated exposure to perspiration and washing. The linear resistance of each sample increased as with the frequency of exposure to perspiration and washing, but the trend was inconspicuous compared to the surface resistance results. Unlike surface resistance, which calculates the resistance value within a certain area, linear resistance measures the electrical resistance of the linear distance between two probes. Therefore, the damage to the silver layer following exposure to perspiration and washing did not significantly affect the linear resistance per unit cm. These trends were more evident in the wale direction, where the yarns were continuously intertwined, and in the C1 and C2 coated in fabric states. At 10% elongation, the linear resistance decreased compared with the original state, and the electrical conductivity was improved. In addition, the differences due to repeated exposure to perspiration and washing were further reduced. Similar to the results of exposure to perspiration or washing alone, the electron movement path shortened as the loop constituting the knitted fabric was elongated. Consequently, electron movement per unit length became easier, and linear resistance decreased. However, the number of iterations reached ten, damage or chemical changes in the silver layer due to exposure to perspiration and washing affected the linear resistance. Therefore, a clear change in linear resistance is expected as the frequency of exposure to perspiration and washing is increased.
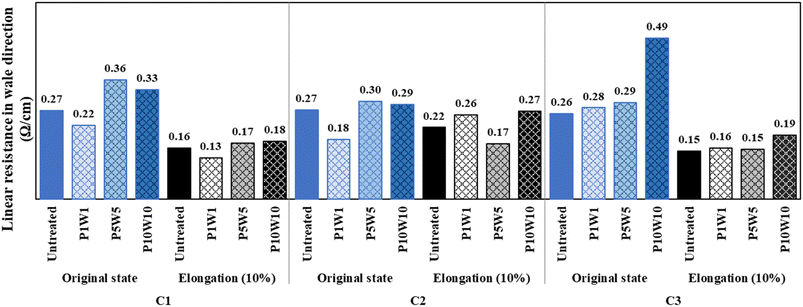 | ||
| Fig. 16 Linear resistance of the fabric in wale direction according to repeated exposure to perspiration and washing. | ||
Table 12 lists the fabric properties of the untreated samples according to the FTT test and the samples that were exposed to perspiration and washed 10 times.
| Bending | Thermal conductivity (Qmax) | Compression | Surface Friction coefficient | Surface roughness | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bending rigidity (gf mm rad−1) | Bending work (gf mm rad) | Compression work (gf mm) | Recovery rate | Amplitude (μm) | Wavelength (mm) | ||||||||||||||
| Face | Back | Face | Back | Face | Back | Face | Back | Face | Back | Face | Back | Face | Back | Face | Back | ||||
| C1 | Untreated | 49 | 52 | 181 | 183 | 0.1 | 0.1 | 168 | 129 | 0.7 | 0.8 | 0.3 | 0.3 | 22 | 23 | 1.3 | 1.1 | ||
| P10W10 | 44 | 41 | 166 | 151 | 0.1 | 0.1 | 206 | 190 | 0.7 | 0.7 | 0.2 | 0.2 | 23 | 22 | 1.5 | 1.0 | |||
| C2 | Untreated | 95 | 100 | 427 | 409 | 0.1 | 0.2 | 138 | 148 | 0.7 | 0.8 | 0.3 | 0.3 | 23 | 17 | 1.1 | 1.8 | ||
| P10W10 | 103 | 90 | 417 | 372 | 0.1 | 0.1 | 471 | 331 | 0.6 | 0.7 | 0.2 | 0.2 | 15 | 17 | 2.0 | 1.0 | |||
| C3 | Untreated | 85 | 87 | 314 | 293 | 0.1 | 0.1 | 330 | 305 | 0.6 | 0.7 | 0.4 | 0.4 | 27 | 21 | 1.5 | 1.9 | ||
| P10W10 | 79 | 82 | 342 | 311 | 0.1 | 0.1 | 294 | 222 | 0.7 | 0.7 | 0.3 | 0.3 | 27 | 31 | 1.7 | 1.8 | |||
| K1 | Untreated | 137 | 189 | 580 | 647 | 0.1 | 0.1 | 384 | 371 | 0.7 | 0.7 | 0.3 | 0.3 | 20 | 23 | 2.2 | 2.0 | ||
| P10W10 | 163 | 175 | 713 | 759 | 0.1 | 0.1 | 377 | 335 | 0.7 | 0.7 | 0.3 | 0.3 | 25 | 27 | 2.7 | 2.2 | |||
| K2 | Untreated | 108 | 122 | 346 | 364 | 0.1 | 0.1 | 249 | 250 | 0.8 | 0.7 | 0.2 | 0.3 | 17 | 14 | 2.2 | 1.9 | ||
| P10W10 | 89 | 101 | 303 | 323 | 0.1 | 0.1 | 194 | 185 | 0.7 | 0.7 | 0.5 | 0.3 | 33 | 11 | 1.5 | 1.8 | |||
In terms of changes in fabric properties owing to exposure to perspiration and washing, the bending properties tended to decrease slightly after treatment in all samples. Bending rigidity refers to the force required to bend per radian, whereas bending work refers to the work required to bend the specimen.36 Therefore, the decrease in bending rigidity after treatment is attributed to the increase in the degree of freedom of the yarn as the silver coating on the surface of the conductive fabric was peeled off, or the filament yarn of the common knitted fabric was partially untwisted by chemical and physical treatment. Ajeli et al.39 studied the bending rigidity of warp-knitted fabrics as a function of the knit structure, density, and yarn bending properties. Their results indicated that the bending rigidity increased for the fabrics with relatively high density.
There was no difference in the thermal conductivity before and after treatment or among the samples. In the case of conductive fabrics, the thermal conductivity was expected to increase as the silver layer on the surface was damaged. However, considering that there was no change in the value of Qmax, the silver particles falling off the surface or the chemical changes due to exposure to perspiration or washing were presumed to be insufficient to affect the thermal conductivities.
However, in the case of the compression properties, the compression work of C1 and C2, which were coated with silver in the fabric state, increased after treatment, unlike the other samples that were not coated. High compression implies that the force required to compress the specimen is large.36 In C1 and C2, the silver layer was oxidized and damaged owing to exposure to perspiration and washing, and the samples became bulkier than they were before treatment, thus necessitating additional force for compression. In particular, the compression work of C2 was more than twice that of the untreated samples. The resilience of the fabric sharply decreased as the chemical composition of the spandex yarn contained in the sample was changed by perspiration, and the silver layer was peeled off from the fabric surface.
In terms of surface geometry properties, the surface friction and surface roughness of conductive and common knitted fabrics differed before and after the treatment. The coefficient of friction of the common knitted fabric was similar to or slightly increased by the exposure to perspiration and washing, whereas that of the conductive fabrics decreased. Fabrics with low friction coefficients are typically relatively smooth.40 The coefficient of friction of the fabric is mainly influenced by the nature of the fabric and ambient humidity. The physical characteristics of yarns, such as the yarn count, fiber fineness, and yarn twist, together with their alignment and positioning in the fabric structure, are important for the measurement of surface friction properties.41 In the case of conductive fabrics, silver was oxidized to form AgCl particles owing to exposure to perspiration, and the silver layer was then peeled off during the washing process, exposing the inner nylon filament yarn. Unlike metals, fibers contain a certain amount of moisture because of their chemical functional groups and structural properties. As shown in the contact angle measurement results, the surface of the conductive fabric became hydrophilic following exposure to perspiration and washing. As moisture acts as a lubricant in friction, the coefficient of friction of the conductive fabric was lowered owing to the effect of increasing moisture in the sample after treatment.42 Upon treatment, the surface roughness, amplitude, and wavelength of common knitted fabrics K1 and K2 increased, but remained the same of decreased for conductive fabrics. Such a change in the surface geometry properties was due to the effect of the mechanical force from repeated washing rather than exposure to perspiration. Washing involves constant abrasion between the drum wall and fabric, which causes a change in the geometric structure of the fabric.43 The change in the surface structure due to the mechanical force of washing was more pronounced in the uncoated samples, as the fibers were more prone to swelling by water during washing.44 The common knitted fabric used in this study is a warp-knitted fabric composed of uncoated spun or filament yarns. Therefore, the amplitudes and wavelengths of K1 and K2 were higher than those of the untreated sample because the yarns swelled and dissociated owing to the mechanical and hydraulic forces during washing, resulting in a rough surface.
Table 13 lists the fabric indices of the hand value for the five fabric properties according to repeated exposure to perspiration and washing.
| Smoothness | Softness | Warmness | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Face | Back | Face | Back | Face | Back | Face | Back | ||
| C1 | Untreated | 3 | 4 | 4 | 4 | 3 | 2 | 3 | 4 |
| P10W10 | 3 | 4 | 4 | 3 | 3 | 2 | 3 | 3 | |
| C2 | Untreated | 4 | 5 | 4 | 4 | 2 | 1 | 3 | 4 |
| P10W10 | 3.5 | 4.5 | 4 | 3.5 | 3 | 1.5 | 3.5 | 3.5 | |
| C3 | Untreated | 3 | 4 | 4 | 3 | 3 | 2 | 3 | 3 |
| P10W10 | 3 | 4 | 4 | 3 | 3 | 1 | 3 | 3 | |
| K1 | Untreated | 2 | 3 | 3 | 3 | 4 | 2 | 3 | 3 |
| P10W10 | 2.5 | 3 | 3 | 2 | 4 | 2 | 3 | 3 | |
| K2 | Untreated | 3 | 4 | 4 | 3 | 3 | 2 | 3 | 3 |
| P10W10 | 3 | 4 | 4 | 3 | 3 | 2 | 3 | 3 | |
All three fabric indices changed for conductive fabrics C1, C2, and C3. In C1, the softness of the back side of the fabric and the total fabric index decreased. In C3, warmness on the back side of the fabric decreased. The greatest changes were observed in C2; smoothness and softness decreased, warmness increased, and the total fabric index decreased on both sides of the fabric. C2 was considered the most vulnerable to chemical and physical changes caused by exposure to perspiration and washing because it contained spandex yarn and was coated with silver through a post-processing method in a fabric state.
In contrast, the fabric indices of common knitted fabrics, K2 did not change, but K1 showed an increase in smoothness on the face side and a decrease in softness on the back side. This is because K1 is composed of hairy textured yarns rather than filament yarns, and the surface changes due to friction during washing exceed those of K2.45 Therefore, the general knitted fabric also showed changes in the hand value owing to repeated exposure to perspiration and washing; however, the change was insignificant compared to that of conductive fabrics.
Based on the hand value evaluation, the change in the C2 score was remarkable. Therefore, it is recommended that spandex yarns be avoided in the manufacture of conductive fabrics and that structural elasticity, such as knitted fabrics, be integrated when stretching is required. In addition, knitting or weaving after pre-coating in the yarn state rather than performing post-processing in the fabric state is advantageous in terms of fabric hand value. However, because these results are based on a limited sample size, additional studies are needed to identify more generalized changes in conductive fabrics.
4. Conclusions
In this study, considering the wearing and management of smart clothing, changes in the appearance, chemical composition, wettability, and electrical properties of conductive knitted fabrics based on exposure to perspiration and washing were investigated.Overall, the conductive knitted fabric became hydrophilic and significantly changed in appearance and color in accordance with the chemical change in the silver layer caused by perspiration and washing. Additionally, the hand values of all the conductive fabrics varied with decreasing softness and smoothness and increasing warmness after repeated exposure to perspiration and washing.
Here, different changes were observed depending on the manufacturing method used for the conductive fabric. The silver-coated fabric samples were more vulnerable to the durability of the conductivity, and considerable change was observed. Conversely, the conductivity of the sample coated with silver in the yarn state showed slight changes owing to perspiration and washing, but the changes in basic properties such as appearance, color, and hand value were similar to those of common knitted fabrics. Therefore, considering the conductivity and aesthetic characteristics of fabrics such as color and touch, the use of conductive fabric with silver-coated yarn is more optimized for smart clothing to improve the durability of the silver layer. However, because these results are limited to this study, verification using more diverse samples is required.
This study confirmed the extent of change in conductive fabrics that is acceptable by consumers by comparing the changes in the actual wearing environment of conductive fabrics used for smart clothing with those of common fabrics. The changes in the appearance and chemical composition of conductive fabrics following exposure to perspiration and washing allow for the determination of whether harmful substances are generated by the reaction of metals and chemical components in the conductive fibers and present specific manufacturing methods and directions for human-friendly smart fibers. In addition, follow-up research should determine the optimal duration of product quality and functionality through repeated use and washing of smart clothing, and exhaustively identify conditions for washing and management.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Acknowledgements
This work was supported by the New Faculty Startup Fund from Seoul National University. And this research was supported by the National Research Foundation (NRF) of Korea funded by the Korean government (MSIP) (Grant No. 2021R1F1A1060287).References
- N. A. Choudhry, L. Arnold, A. Rasheed, I. A. Khan and L. Wang, Adv. Eng. Mater., 2021, 23, 2100649 CrossRef.
- S. Rotzler, M. Krshiwoblozki and M. Schneider-Ramelow, Text. Res. J., 2021, 91, 2401–2417 CrossRef CAS.
- O. L. H. Junior, R. M. Neves, F. M. Monticeli and L. D. Agnol, Textiles, 2022, 2, 582–605 CrossRef.
- M. Ahsan, S. H. Teay, A. S. M. Sayem and A. Albarbar, Signals, 2022, 3, 113–145 CrossRef.
- M. A. Shah, B. M. Pirzada, G. Price, A. L. Shibiru and A. Qurashi, J. Adv. Res., 2022, 38, 55–75 CrossRef CAS PubMed.
- E. Ismar, S. Zaman, X. Tao, C. Cochrance and V. Koncar, Fibers Polym., 2019, 20, 2604–2610 CrossRef CAS.
- Y. T. Chui, C. X. Yang, J. H. Tong, Y. F. Zhao, C. P. Ho and L. L. Li, Text. Res. J., 2016, 86, 787–802 CrossRef CAS.
- C. Isaia, S. Mcmaster and D. Mcnally, J. Ind. Text., 2022, 51, 8528S–8548S CrossRef.
- M. A. S. Tajin, A. S. Levitt, C. L. Schauer, G. Dion and K. R. Dandekar, IEEE Antennas Wirel. Propag. Lett., 2020, 19, 542–546 Search PubMed.
- E. Spielman-Sun, T. Zaikova, T. Dankovich, J. Yun, M. Ryan, J. E. Hutchison and G. V. Lowry, NanoImpact, 2018, 11, 51–57 CrossRef.
- V. Gaubert, H. Gidik, N. Bodart and V. Koncar, Sensors, 2020, 20, 1739 CrossRef CAS PubMed.
- Y. Yan, H. Yang, J. Li, X. Lu and C. Wang, Text. Res. J., 2012, 82, 1422–1429 CrossRef CAS.
- W. Dang, L. Manjakkal, W. T. Navaraj, L. Lorenzelli, V. Vinciguerra and R. Dahiya, Biosens. Bioelectron., 2018, 107, 192–202 CrossRef CAS PubMed.
- N. Becenen and Ö. Altun, J. Text. Inst., 2018, 109, 914–919 CrossRef CAS.
- N. Asidiq and A. I. Makki, Proceeding Indonesian Textile Conference, 2019, vol. 3, pp. 103–108 Search PubMed.
- Y. Okada, T. Nagashima, H. Iizuka, M. Asano and Z. Morita, Dyes Pigm., 1997, 33, 239–250 CrossRef CAS.
- Y. M. Park, B. S. Kim and Y. A. Son, Journal of the Korea Society of Dyers and Finishers, 2006, 18, 63–68 Search PubMed.
- K. Yang, R. Torah, Y. Wei, S. Beeby and J. Tudor, Text. Res. J., 2013, 83, 2023–2031 CrossRef.
- G. B. Tseghai, B. Malengier, K. A. Fante, A. B. Nigusse and L. V. Langenhove, Sensors, 2020, 20, 6910 CrossRef CAS PubMed.
- L. He and S. C. Tjong, RSC Adv., 2017, 7, 2058 RSC.
- P. Prieto, V. Nistor, K. Nouneh, M. Otyama, M. Abd-Lefdil and R. Diaz, Appl. Surf. Sci., 2012, 258, 8807–8813 CrossRef CAS.
- Y. Zheng, J. Shu and Z. Wang, Mater. Lett., 2015, 158, 339–342 CrossRef CAS.
- Z. Wang, C. An, M. Zhang, C. Qin, X. Ming and Q. Zhang, Can. J. Chem., 2012, 90, 858–864 CrossRef.
- S. X. Jiang, W. F. Qin, R. H. Guo and L. Zhang, Surf. Coating. Technol., 2010, 204, 3662–3667 CrossRef CAS.
- N. Gao and Y. Yan, J. Bionic Eng., 2009, 6, 335–340 CrossRef.
- M. A. Osman and B. A. Keller, Appl. Surf. Sci., 1996, 99, 261–263 CrossRef CAS.
- O. Kyzymchuk and L. Melnyk, J. Eng. Fibers Fabr., 2018, 1–10 Search PubMed.
- A. A. Almetwally and M. M. Mourad, J. Text. Inst., 2014, 105, 235–245 CrossRef CAS.
- E. M. Hicks, A. J. Ultee and J. Drougas, Science, 1965, 147, 373–379 CrossRef PubMed.
- S. H. Rho, S. Lee, W. Jeong and D. Y. Lim, Text. Res. J., 2022, 92, 1550–1564 CrossRef CAS.
- S. U. Zaman, X. Tao, C. Cochrane and V. Koncar, Mater. Sci. Eng., 2019, 012071 Search PubMed.
- C. Yun and C. H. Park, Text. Res. J., 2016, 86, 563–572 CrossRef CAS.
- C. J. Hurren, A. Kaynak and X. Wang, Res. J. Text. Apparel, 2007, 11, 11–17 CrossRef.
- T. M. Benn and P. Westerhoff, Environ. Sci. Technol., 2008, 42, 4133–4139 CrossRef CAS PubMed.
- M. Montazer, A. Shamei and F. Alimohammadi, Mater. Sci. Eng., C, 2014, 38, 170–176 CrossRef CAS PubMed.
- A. B. H. Musa, B. Malengier, S. Vasile, L. V. Langenhove and A. D. Raeve, Autex Res. J., 2018, 18, 51–60 CrossRef.
- I. Kim, H. Shahariar, W. F. Ingram, Y. Zhou and J. S. Jur, Adv. Funct. Mater., 2019, 29, 1807573 CrossRef.
- S. Vasile, B. Malengier, A. D. Raeve and F. Deruyck, Text. Res. J., 2019, 89, 98–112 CrossRef CAS.
- S. Ajeli, A. Jeddi, A. Rastgo and R. E. Gorga, J. Text. Inst., 2009, 100, 496–506 CrossRef.
- S. A. Mooneghi, S. Saharkhiz and M. H. Varkiani, J. Eng. Fibers Fabr., 2014, 9, 1–18 Search PubMed.
- K. A. Beyene and S. Gebeyaw, RJTA, 2022, 26, 359–370 CrossRef CAS.
- R. R. Moorthy and P. Kandhavadivu, J. Text. Appar. Technol. Manag., 2015, 9, 1–14 Search PubMed.
- W. Huang, Y. Xing, Y. Yu, S. Shang and J. Dai, Appl. Surf. Sci., 2011, 257, 4443–4448 CrossRef CAS.
- C. Yun, S. Park and C. H. Park, Text. Res. J., 2013, 8, 1786–1795 CrossRef.
- S. Vasile, B. Malengier, A. D. Raeve and A. B. H. Musa, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 254, 1–7 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |