Open Access Article
Open Access ArticleInvestigation of simultaneous volatile organic compound removal by indoor plants using solid phase microextraction-gas chromatography-mass spectrometry
Geoffrey Peterson,
Timothy Jones,
Diana Rispoli,
Shokouh Haddadi and
Vadoud Niri *
*
Department of Chemistry, State University of New York at Oswego, Oswego, NY 13126, USA. E-mail: vadoud.niri@oswego.edu
First published on 7th September 2023
Abstract
Volatile organic compounds (VOCs) are significant indoor air pollutants, and employing plants offers a simple and cost-effective approach to reduce their concentration. It is important to determine which plant exhibits greater efficiency in removing specific VOCs. This study aimed to compare the efficacy of various common indoor plants in simultaneously removing multiple hazardous VOCs. A sealed chamber was utilized to expose five different species of houseplants to eight commonly found VOCs. The concentrations of each compound were monitored over an extended period using solid phase microextraction (SPME) coupled with gas chromatography-mass spectrometry (GC-MS). The study determined and reported the efficiency of removal per leaf area for all compounds by each plant under different conditions, including removal by the entire plant (with and without light) and removal by the plant's leaf area. The paper discusses the efficiency and rate of removal of each VOC for the tested plants, namely Chlorophytum comosum, Crassula argentea, Guzmania lingulata, Consolea falcata, and Dracaena fragrans.
Introduction
Air, being the most consumed substance by humans with an average of over 3000 gallons per person each day, is a vital necessity for life. However, air pollution poses a significant environmental threat to the health of communities worldwide. Among the various air pollutants, volatile organic compounds (VOCs) hold particular importance. Extensive research on the effects of numerous VOCs on human health has been conducted by various authoritative bodies, including the United States Center for Disease Control, the United States Occupational Safety and Health Administration, the United States Environmental Protection Agency (EPA), and numerous publicly and independently funded research institutions. Given the wide-ranging definition of VOCs, the associated health effects on humans span a vast spectrum, ranging from benign to carcinogenic and potentially lethal. For instance, benzene, which can originate from both natural processes and human activities, is a recognized carcinogenic chemical known to cause leukemia.1The EPA conducted the Total Exposure Assessment Methodology (TEAM) study from 1980 to 1985, which revealed that human exposure concentrations to volatile organic compounds (VOCs) were generally significantly higher indoors compared to outdoor environments, ranging from 2 to 5 times higher.2 This study identified several hundred VOCs, including mutagens and carcinogens.2 Exposure to VOC emissions in indoor environments is unavoidable and occurs on a daily basis. Common sources of VOC emissions include building materials, paints, cleaning supplies, solvents, preservatives, furnishings, heating fuel, cosmetics, glues, adhesives, printers, and copiers.3 The advent of more energy-efficient building designs has led to reduced external air exchange compared to older buildings. Combined with the increased use of modern synthetic building materials, this contributes to the accumulation of VOCs in indoor air.4
The growing awareness of indoor health concerns has spurred advancements in various physical and chemical techniques for remediation. Among the comprehensive strategies for volatile organic compound (VOC) removal, absorption, adsorption, condensation, chemical reaction, biofiltration, and photodegradation stand out.5–9 Activated carbon is a widely employed technique for VOC remediation. The material is processed to create a high surface area and low volume pores (1–35 nm), facilitating VOC adsorption when passed through the material.10 However, a challenge associated with this method is the proper disposal of the used material, which typically involves incineration.11 Incineration of VOCs requires high temperatures (800–1200 °C), which incur high costs and result in the formation of undesirable by-products.11
Catalytic oxidation utilizing metal catalysts has proven to be another effective remediation solution for VOCs. Transition metal oxides and noble metals have been utilized as highly active oxidation catalysts for VOC removal. However, the cost of these metals tends to be high, and they exhibit instability in the presence of organohalide reactions. Chromium, magnesium, nickel, copper, and cobalt oxides have been tested for VOC oxidation but demonstrated low activity and similar deactivation in the presence of organohalides.11
In the realm of phytoremediation, which involves using plants to address environmental pollution, biofiltration emerges as a cost-effective and efficient solution. Pioneering research conducted by the National Aeronautics and Space Administration (NASA) in the 1980s demonstrated the potential of plant systems in removing trace organic compounds.12 Building upon this study, subsequent investigations focused on the combination of activated carbon adsorption and biochemical uptake and metabolic mechanisms within plants for VOC remediation.12,13 Numerous studies evaluating the ability of plants to reduce VOCs have been published in recent decades.14–17
A study conducted by Aydogan and Montoya (2011) examined the reduction of formaldehyde within an enclosed space. The research compared different hydroponic growing media and various plant species, revealing that VOC reduction through the plant's root zone proved to be the most effective. The study monitored formaldehyde levels using a commercial device specifically designed for formaldehyde detection. Orwell et al.15,16 conducted studies comparing the removal of benzene, toluene, and m-xylene by various plant species. The findings from these studies suggest that the growth media initially respond to VOC exposure through adsorption, while the plants, along with associated rhizosphere microorganisms, absorb and metabolize the VOCs present in the suspended growth media.15,17
The mechanism of VOC uptake in plants is not fully understood and it depends on the compound and varies from plant to plant. It is understood that the microorganisms found in the rhizosphere area, generally work symbiotically with the plant.18 The VOC compound initially enters a catabolic pathway within the microorganism, producing a by-product that the plant utilizes as either an anabolic carbon source or a catabolic energy source.18 A compound may take multitude pathways to be taken up by the plant system. Transpiration, the process by which water moves through the plant, creates a natural pressure gradient resulting from the influx of soil materials (chemical compounds, nutrients, and water) and water evaporation at the stomatal complex.19,20 Lipophilic molecules are prominently sorbed at the root–soil interface and on the root surface itself, while polar solute compounds enter the plant system in the aqueous phase through apoplastic channels, guided by the same hydraulic force that drives water flow within the plant—transpiration. However, the overall hydraulic resistance depends on the age and stress levels of the plant's root material. Water and associated aqueous solute compounds (including VOCs) are then transported through the plant structure via the xylem, where sorption occurs at the cell–xylem interface, eventually reaching the leaf structure where gaseous water is released through the stomatal complex, thereby sustaining transpiration.19,20 Sorption occurs not only in the root zone but also in the stomatal complex of the aerial leaf area, as it possesses the ability to absorb gaseous chemical components.21 Phytodegradation refers to the process in which pollutants are taken up by the leaf tissue of plants and subsequently metabolized by the plant system.22 Two uptake mechanisms by the leaf contribute to the accumulation of chemical compounds from the ambient air: absorption through the cuticle and passage through the open stomata.23,24 Research focusing on the uptake of toluene and ethylbenzene found that the composition of the plant cuticle exhibited a stronger correlation with plant VOC removal than stomatal number alone.24 Regarding benzene removal, high levels of α-linoleic acid and dodecyl cyclohexane were detected in the cuticle composition of plants that effectively removed benzene.24 Metabolic pathways whereby VOCs are degraded in plant cells have been recently presented by several researchers.25–28 These studies have shown that the phytoremediation mechanisms involve phytoextraction, phytovolatilization, phytodegradation, phytostabilization, rhizodegradation, and rhizofiltration.
Solid-phase microextraction (SPME), utilized in this study, was developed in 1989 to address the need for rapid sample preparation in both laboratory and on-site settings where the investigated system is located.29 The technique involves exposing a small amount of an extracting phase dispersed on a solid support to the sample matrix. The SPME process consists of two fundamental steps: (i) partitioning of analytes between the extraction phase and the sample matrix, and (ii) desorption of concentrated extracts into an analytical instrument. SPME has become a routine method, often combined with gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS), and has been successfully applied to a wide range of compounds, particularly for the extraction of volatile and semivolatile organic compounds from various sample matrices.30
This study aimed to investigate the efficiency and simultaneous removal rate of several volatile organic compounds (VOCs) by different plant species. Solid phase microextraction (SPME) coupled with gas chromatography-mass spectrometry (GC-MS) was employed to monitor the reduction of VOCs over twelve-hour periods. The study utilized five commonly found indoor plants, namely Crassula argentea (Jade plant), Chlorophytum comosum (Spider plant), Guzmania lingulata (Bromeliad), Consolea falcata (Caribbean Tree Cactus), and Dracaena fragrans (Dracaena), which were of similar size. The investigation focused on eight prevalent VOCs: acetone, benzene, dichloromethane (methylene chloride), ethylbenzene, p-xylene, o-xylene, toluene, and trichloromethane (chloroform). Table 1 provides comprehensive information about the VOCs utilized in this experiment, including their grade, distributor, OSHA designated reference concentration, and the associated side effects of exposure.3
| VOCs | Acute effects (less than 14 days) | Chronic effects (1 year+) |
|---|---|---|
| Benzene | Drowsiness, dizziness, rapid/irregular heartbeats, tremors, confusion, unconsciousness, and death | Effects on bone marrow cause decreased RBC count, excessive bleeding, immune system effects, and leukemia |
| Toluene | Kidney, liver, cardiovascular effects, central nervous system effect ranging from tingling to unconsciousness and death | Kidney, liver, pulmonary effects, high frequency hearing loss, classified as non-carcinogen |
| Ethylbenzene | Central nervous system toxicity, liver, kidney, pulmonary effects, eye irritation | Effects on blood, liver, kidney, pulmonary systems, group D carcinogen (non-human carcinogen) |
| o-Xylene | Headache, dizziness, nausea, vomiting, central nervous system depression, death | Headaches, irritability, depression, insomnia, agitation, extreme tiredness, tremors, impaired concentration, short term memory impairment, not classified as carcinogenic |
| p-Xylene | Headache, dizziness, nausea, vomiting, central nervous system depression, and death | Headaches, irritability, depression, insomnia, agitation, extreme tiredness, tremors, impaired concentration, short term memory impairment, not classified as carcinogenic |
| Acetone | Nose, throat, lungs, eye irritation, headache, light-headedness, dizziness, confusion, unsteadiness, unconsciousness, nausea, vomiting blood | Nose, throat, lungs, eye irritation, headache, light-headedness, dizziness, confusion, unsteadiness, unconsciousness, nausea, vomiting blood |
| Dichlormethane | CNS effects (including visual, auditory, psychomotor function depression), death (at high levels) | Headache, dizziness, nausea, memory loss, effects on liver, kidneys, cardiovascular system and central nervous system, and group B2 carcinogen (probable human carcinogen) |
| Trichloromethane | Central nervous system depression, anesthesia, dizziness, headache, tiredness, death | Liver effects (jaundice, hepatitis), central nervous system depression (depression, irritability), effects on kidneys, and blood, group B2 carcinogen (probable human carcinogen) |
Materials and methods
Chemicals and supplies
Benzene, 99.5%, ethylbenzene, 99%, o-xylene, 99%, p-xylene, 99%, and trichloromethane, 99.8% were purchased form Alfa Aesar (Ward Hill, MA). Acetone, 99.9%, and dichlormethane, 99.5%, were purchased form Pharmco AAPER (Brookfield, CT). Toluene, 99.9%, was purchased from Fisher Scientific (Pittsburgh, PA). SPME fibres and Thermogreen® LB-2 Septa were obtained from Supelco (St. Louis, MO). Helium gas used as carrier gas in GC-MS was supplied by Airgas (Radnor, PA).Test plants
The plant species employed in this study included Crassula argentea (Jade plant), Chlorophytum comosum (Spider plant), Guzmania lingulata (Bromeliad plant), Consolea falcata (Caribbean Tree Cactus), and Dracaena fragrans (Dracaena plant). All plants were procured from local stores. During non-sampling cycles, the plants were maintained under carefully controlled greenhouse conditions. To ensure consistent sampling conditions, periodic trimming of new inflorescence plant growth was performed. Considering the variation in leaf area sizes among the plants, the overall leaf areas were determined for subsequent calculations. Table 2 presents the estimated leaf area in square inches.| Plant | Overall leaf area (in2) |
|---|---|
| Chlorophytum comosum | 5220 |
| Crassula argentea | 410 |
| Guzmania lingulata | 2241 |
| Consolea falcata | 1064 |
| Dracaena fragrans | 1960 |
Sampling chamber
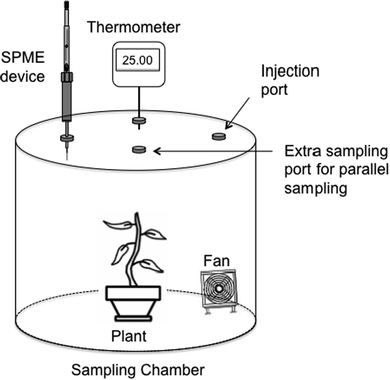
A stainless-steel sampling chamber was designed as shown in Fig. 1. The sampling chamber was constructed using an 82-quart (approximately 77.6 L) stainless steel cooking pot, chosen for its chemical inertness. To ensure an airtight seal, polyvinyl chloride (PVC) vacuum tubing was wrapped around the upper rim of the stainless-steel pot and secured with clamps. The PVC tubing was further protected by wrapping it with PTFE (Teflon) tape to maintain its chemical inertness. Four inlet ports were drilled into the stainless-steel cover of the sampling chamber. These ports served different purposes: one for standard injection, one for SPME sampling, one for mounting a thermocouple to monitor temperature during sampling, and an additional port for potential simultaneous sampling devices such as the Needle Trap Device (NTD).31 To maintain the integrity of the closed system, Thermogreen septa were installed in each inlet port. Two internal components were utilized within the sampling chamber. Firstly, an 80 mm personal computer fan was employed, rotating at a constant speed of 2500 rpm throughout the sampling process. Secondly, a portable LED light with 24 light diodes was used. The wiring for each component was carefully wrapped with PTFE tape. To close and seal the apparatus, the stainless-steel top was placed on the PVC seal of the upper rim of the sampling chamber, and pony spring clamps were applied around the perimeter of the lid. This ensured a secure and tightly sealed sampling environment.Preparation of standards
A standard solution containing benzene, toluene, ethylbenzene, o-xylene, p-xylene, dichloromethane, trichloromethane, and acetone was prepared in methanol with each compound at a concentration of 8.7%. The solution was formulated to achieve a concentration of 10 μg L−1 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg m−3 or 10 mg m−3) for each component in the sampling chamber after injecting 1 μL of the standard solution. A fresh standard solution was prepared at the start of each new plant study.
000 μg m−3 or 10 mg m−3) for each component in the sampling chamber after injecting 1 μL of the standard solution. A fresh standard solution was prepared at the start of each new plant study.
The chosen concentration is elevated compared to what is usually found in a standard indoor living environment. Nevertheless, it falls within the acceptable range, and in certain instances, it's even lower than levels that could be encountered in settings with higher pollutant concentrations, such as nail salons. It's important to highlight that in the next phase of the project, this approach will be utilized to forecast VOC removal in real-world settings, including environments such as nail salons. The concentration of VOCs in real indoor air samples varies from one location to another. Average indoor total VOC concentrations have been reported as 203 μg m−3 by Jin et al.,32 120–1620 μg m−3 by Kang et al.33 in Tianjin, China, 770–2650 μg m−3 by Akal et al.34 in Ankara, and 260–1062 μg m−3 by Mundackal and Ngole-Jeme35 in South Africa. However, the concentration range of VOCs in nail salons is typically much higher than living indoor area. For example, Zhong et al.36 reported that VOC concentrations could be as high as 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg m−3 in salons in Michigan, USA. An additional rationale for opting for higher VOC concentrations was to monitor concentration reduction over a 12 hour duration. The outcomes indicated that achieving a noticeable reduction in VOC concentrations within the lower range would necessitate an extended timeframe, requiring sampling to extend beyond 72 hours, which is not practical for our research study.
000 μg m−3 in salons in Michigan, USA. An additional rationale for opting for higher VOC concentrations was to monitor concentration reduction over a 12 hour duration. The outcomes indicated that achieving a noticeable reduction in VOC concentrations within the lower range would necessitate an extended timeframe, requiring sampling to extend beyond 72 hours, which is not practical for our research study.
Sampling procedure
The sampling procedure involved the following steps:1. The test plant was positioned at the center of the sampling apparatus on the bottom.
2. The fan, located underneath the analyte injection port in the sampling chamber, was activated.
3. During experiments involving light conditions, the 24 LED light, positioned at the bottom of the sampling chamber with focused illumination on the abaxial epidermis of the plant, was turned on.
4. A 15 cm filter paper was placed beneath the “analyte injection” port on the sampling apparatus lid. The filter paper was secured by two pieces of approximately 2 cm electrical tape, each covered with PTFE tape to minimize exposure to the analytes.
5. The stainless-steel lid of the sampling chamber was placed on the PVC seal around the rim of the sampling chamber, and 14 pony spring clamps were used to secure it.
6. The thermocouple was inserted into the “Temperature” injection port.
7. Using a GC microsyringe, exactly 1 μL of the standard solution was injected through the injection port into the sampling chamber, onto the filter paper.
8. The SPME sampling commenced 30 minutes after the standard injection by exposing the SPME fiber through the “SPME sampling” port of the chamber.
9. After a 5 minute SPME exposure, the fiber was removed and injected into the GC-MS instrument to record the chromatogram.
10. Steps 8 and 9 were repeated every 30 minutes during the first 6 hour sampling period, and then every 60 minutes during the second 6 hour sampling period.
To obtain control results and ensure that the chamber has no leak, SPME sampling was performed without introducing the plant in the chamber (Steps 3 to 11). Experiments with plants were conducted with and without covering the pot with aluminum foil. In cases where the pot was covered, the base of the selected test plant was wrapped in aluminum foil to eliminate exposure of the root area, soil, and plant pot material to the analytes, allowing for a focused investigation of VOC exposure on the leaf area. The influence of artificial light was also examined by activating the LED light.
GC-MS analysis
The analysis was conducted using a Thermo Scientific ISQ LT Single Quadrupole GC/MS instrument. For this experiment, a Thermo Scientific TG-5MS (Trace Gold) column with dimensions of 30 m × 0.25 mm and a coating of 5% diphenyl/95% dimethyl polysiloxane (PDMS) with a thickness of 0.25 μm was selected. The column had a maximum temperature of 330/350 °C. The temperature program for the column was as follows: starting at 35 °C for 1 min, ramping to 70 °C at a rate of 5 °C min−1, further increasing to 250 °C at a rate of 80 °C min−1, and holding for 1.75 min. The front inlet port of the GC instrument was operated in splitless mode at a temperature of 200 °C. The SPME liner was installed in the inlet port. The carrier gas used was hydrogen at a flow rate of 1.000 mL min−1. The SPME fiber was conditioned at the rear inlet port, which was set to 250 °C.Electron impact (EI) ionization was employed as the ionization mode for the MS. The transfer line temperature of the MS was set to 280 °C, and the ion source temperature was set to 250 °C. The method was run in scan mode (Total Ion Chromatogram, TIC) with a scanning range of 40–300 amu for compound identification. Quantification of each compound was performed by extracting selected ions from the chromatogram. The mass ranges used for identifying individual compounds are provided in Table 3.
| Chemical component | Retention time (min) | Quantitative mass (amu) |
|---|---|---|
| Acetone | 1.05 | 58.08 |
| Dichloromethane | 1.14 | 84.93 |
| Trichloromethane | 1.46 | 119.38 |
| Benzene | 1.76 | 78.11 |
| Toluene | 2.99 | 92.14 |
| Ethylbenzene | 4.90 | 106.17 |
| p-Xylene | 5.11 | 106.17 |
| o-Xylene | 5.69 | 106.17 |
Results and discussion
The following results were obtained from the experiments performed to study the removal of VOCs by different plants.Control experiment (without plant)
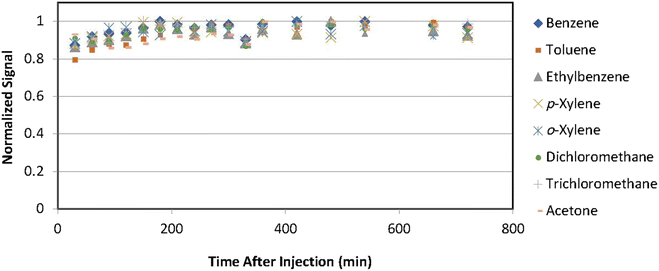
Prior to conducting experiments with plants, a control experiment was conducted using the same standard VOCs solution, but without the presence of any plants, in order to assess potential leakage and adsorption of VOCs by the sampling chamber. All experiments were performed in triplicate, and the average signals were utilized. Fig. 2 illustrates the normalized signals obtained over a 12 hour sampling period. Normalized signals were calculated by dividing the signals by the maximum signal observed for each compound, enabling better comparisons.The results of the control experiment indicate that the concentrations of all compounds remained constant during the 12 hour sampling period. The slight increase observed after injecting the solution can be attributed to the time required for evaporation and convection of compounds within the chamber. These findings suggest that there was no significant leakage or adsorption by the sampling chambers for any of the compounds.
VOC removal efficiency by different plants
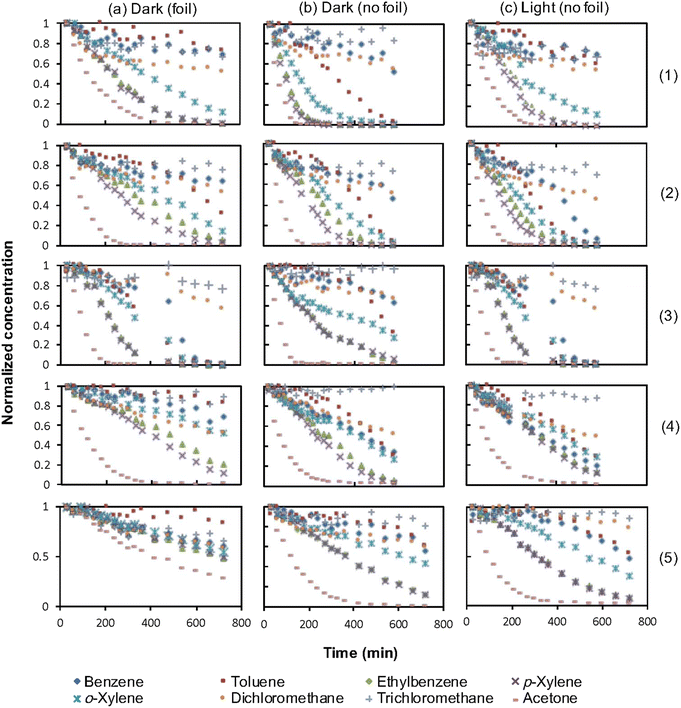
Fig. 3 presents graphs depicting the variations in VOC concentrations over a 12 hour sampling period for each plant under different conditions: (1) with the base wrapped in aluminum foil (exposing only the leaf area), (2) without foil (exposing the entire plant, including the leaf area, soil, and pot), and (3) without foil in the presence of artificial light. The results have been adjusted based on the overall leaf areas of the plants and normalized to facilitate better comparisons.The removal efficiency of each compound was calculated by comparing the concentration of each compound in the control chromatogram with that in the treatment chromatogram at the twelve-hour mark. The results are summarized in Table 4.
| Plant | Condition | VOCs removal percentage over 12 hour period | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | p-Xylene | o-Xylene | Dichloro-methane | Trichloro-methane | Acetone | ||
| Crassula argentea | Foil | 25.73 ± 1.28 | 60.22 ± 3.36 | 78.87 ± 3.78 | 85.96 ± 3.74 | 73.20 ± 2.91 | 28.30 ± 1.74 | 13.72 ± 0.68 | 72.27 ± 6.29 |
| No foil | 43.39 ± 2.16 | 88.10 ± 4.91 | 84.31 ± 4.04 | 88.37 ± 3.85 | 84.11 ± 3.35 | 30.32 ± 1.87 | 16.23 ± 0.81 | 72.02 ± 6.27 | |
| Light | 83.03 ± 4.13 | 91.23 ± 5.09 | 84.12 ± 4.03 | 88.16 ± 3.84 | 86.62 ± 3.45 | 37.36 ± 2.30 | 20.45 ± 1.03 | 71.59 ± 6.23 | |
| Chlorophytum comosum | Foil | 18.72 ± 1.01 | 15.20 ± 1.05 | 91.08 ± 2.21 | 91.21 ± 3.42 | 81.96 ± 3.01 | 35.76 ± 1.46 | 17.46 ± 1.04 | 84.20 ± 3.91 |
| No foil | 35.28 ± 1.91 | 35.51 ± 1.92 | 92.49 ± 2.25 | 92.58 ± 3.47 | 93.40 ± 3.43 | 34.93 ± 1.51 | 17.05 ± 1.14 | 83.87 ± 3.89 | |
| Light | 17.24 ± 0.93 | 27.92 ± 1.93 | 91.76 ± 2.23 | 92.06 ± 3.45 | 82.24 ± 3.02 | 33.27 ± 1.36 | 16.69 ± 0.99 | 84.32 ± 3.91 | |
| Guzmania lingulata | Foil | 31.13 ± 0.93 | 16.67 ± 1.15 | 54.20 ± 1.88 | 50.47 ± 1.75 | 38.90 ± 1.21 | 17.30 ± 0.48 | 1.09 ± 0.03 | 80.84 ± 3.72 |
| No foil | 30.59 ± 0.92 | 58.42 ± 4.02 | 84.64 ± 2.93 | 84.01 ± 2.92 | 61.24 ± 1.90 | 31.31 ± 0.87 | 1.12 ± 0.03 | 92.78 ± 4.27 | |
| Light | 92.87 ± 2.78 | 90.11 ± 6.19 | 88.02 ± 3.05 | 89.55 ± 3.11 | 88.47 ± 2.74 | 41.55 ± 1.15 | 21.21 ± 0.59 | 92.88 ± 4.27 | |
| Consolea falcata | Foil | 22.35 ± 1.02 | 3.09 ± 0.18 | 71.83 ± 2.77 | 75.04 ± 3.33 | 37.37 ± 2.28 | 37.63 ± 2.36 | 0.00 ± 0.01 | 91.48 ± 5.12 |
| No foil | 56.47 ± 2.57 | 54.61 ± 3.22 | 86.52 ± 3.33 | 83.39 ± 3.70 | 62.85 ± 3.75 | 38.05 ± 2.39 | 1.05 ± 0.56 | 89.32 ± 5.00 | |
| Light | 71.61 ± 3.26 | 57.48 ± 3.39 | 85.05 ± 3.05 | 75.77 ± 3.36 | 62.23 ± 3.71 | 41.71 ± 2.62 | 1.28 ± 0.07 | 91.29 ± 5.11 | |
| Dracaena fragrans | Foil | 36.79 ± 1.41 | 12.94 ± 0.80 | 45.08 ± 1.74 | 42.02 ± 1.67 | 39.27 ± 1.31 | 35.24 ± 1.65 | 17.87 ± 0.66 | 68.24 ± 3.40 |
| No foil | 41.05 ± 1.57 | 35.77 ± 2.21 | 80.14 ± 3.09 | 79.45 ± 3.15 | 50.54 ± 1.68 | 35.69 ± 1.39 | 17.96 ± 0.63 | 94.56 ± 4.71 | |
| Light | 48.87 ± 1.87 | 43.10 ± 2.67 | 84.60 ± 3.26 | 84.45 ± 3.34 | 63.43 ± 2.11 | 37.10 ± 1.67 | 19.61 ± 0.74 | 94.09 ± 4.69 | |
Regarding the VOC removal efficiency by different plants (Fig. 3), a general trend is observed for the “foiled” and “no foil” treatments, where the plants exhibit higher VOC removal in the “no foil” conditions compared to the “foiled” conditions. This suggests that VOC uptake occurs through both the aerial part of the plant and the soil/root system. However, exceptions to this trend were observed for the removal of dichloromethane and trichloromethane, where no significant differences are found between the two treatments, indicating that these compounds are primarily taken up by the leaves rather than the soil/root system.
Comparing the “light” and “no light” conditions reveals that, in most cases, the removal percentages either increased or remained unchanged. However, for certain compounds such as dichloromethane and trichloromethane in the case of the Dracaena plant, the removal percentages slightly decreased in the “light” treatment.
Chlorophytum comosum demonstrated over 80% effective removal of ethylbenzene, p-xylene, o-xylene, and acetone in the “foil,” “no foil,” and “light” treatments. Notably, this plant exhibited the highest reduction of ethylbenzene among all treatments. Toluene was also effectively removed by Chlorophytum comosum when considering the entire plant. This suggests a unique relationship between gaseous toluene uptake in Chlorophytum comosum under light and dark conditions. No other tested plants exhibited such a strong negative correlation between toluene uptake and exposure to light. Chlorophytum comosum displayed the highest overall reduction of p-xylene among all treatment conditions and the highest total removal of o-xylene in both the “foil” and “no foil” treatments compared to other plants tested. Even after 12 hours of exposure, benzene, dichloromethane, trichloromethane, and toluene (except for the “no foil” treatment) remained at concentrations greater than 59% of their respective initial concentrations for all treatments.
Crassula argentea showed over 80% effective removal of p-xylene in the “foil” treatment and over 80% effective removal of ethylbenzene, p-xylene, and o-xylene in the “no foil” and “light” treatments. Notably, a significant reduction of benzene was observed in the “light” treatment. Crassula argentea exhibited the highest total reduction of toluene among all tested plants under any treatment condition. However, the total concentration removed for dichloromethane and trichloromethane did not exceed 50% during the 12 hour sampling period. Trichloromethane was not effectively removed by Crassula argentea under any treatment condition, and benzene was not efficiently removed by exclusive exposure to the leaf area of the plant, as seen in the “foil” treatment.
Guzmania lingulata demonstrated over 80% effective removal of toluene, ethylbenzene, p-xylene, and acetone in the “no foil” treatment. In the “light” treatment, this plant showed 80% effective removal of benzene, toluene, ethylbenzene, p-xylene, o-xylene, and acetone. Guzmania lingulata exhibited effective removal of acetone with only aerial leaf exposure, as seen in the “foil” treatment. Overall, this plant showed the highest total removal of target VOCs among the five tested plants, effectively removing six out of the eight target VOCs in the “light” treatment. However, the total concentration removed for dichloromethane and trichloromethane did not exceed 50% during the 12 hour sampling period.
Consolea falcata demonstrated over 80% effective removal of acetone for all treatment conditions. Additionally, ethylbenzene and p-xylene were effectively removed in the “no foil” treatment. Among the five plants tested, Consolea falcata showed the highest total reduction of acetone in the “foil” and “light” conditions. However, trichloromethane was not effectively removed in any of the treatment conditions.
Dracaena fragrans showed over 80% effective removal of acetone in the “no foil” and “light” treatments, as well as p-xylene and ethylbenzene in the “light” treatment. Additionally, p-xylene was effectively removed in the “no foil” treatment. Dracaena fragrans showed the highest total removal of benzene, dichloromethane, and trichloromethane in exclusive aerial leaf exposure in the “foil” treatment among the five plants tested. However, none of these chemical agents exceeded 41% total removal after 12 hours of exposure.
Removal of different VOCs by plants
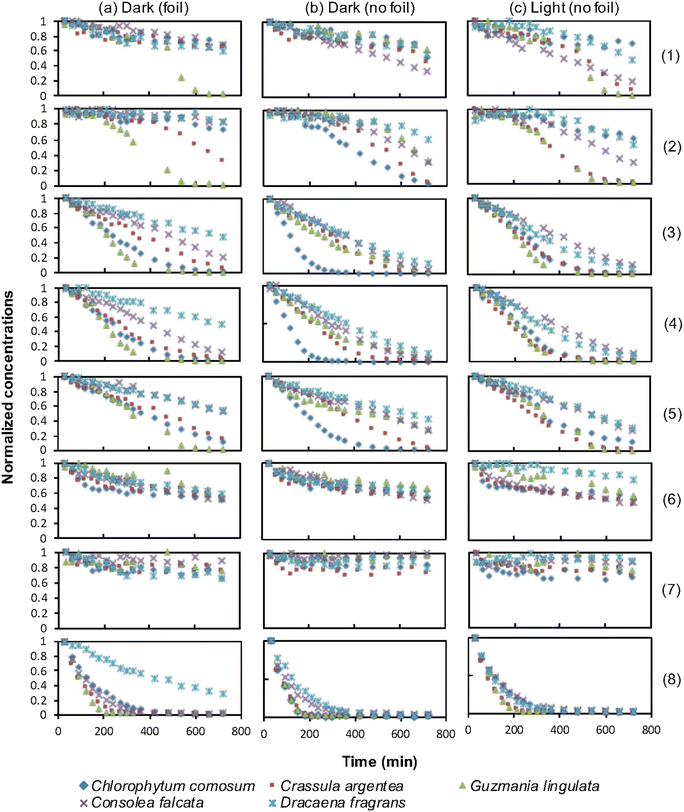
To compare the effectiveness of the plants in removing different VOCs, the change in concentration of each compound was plotted against exposure time for each plant. Fig. 4 shows the graphs for each plant under different treatments over a 12 hour period.The removal percentages of each VOC by different plants under three conditions were calculated, and the results are presented in Table 4. Standard deviations were calculated for triplicates to account for variability.
The results for the removal of each VOC by different plants can be summarized as follows:
The experimental findings supported the previous studies conducted by Sriprapat et al.23 and Treesubsuntorn et al.24 The initial hypothesis suggested that total VOC removal by CAM plants in the “light” treatment would be lower than in the dark treatment, as the stomata in the leaves of these plants remain closed during the day to reduce evapotranspiration but open at night to collect carbon dioxide. However, the results showed that VOC uptake was not decreased and, in some cases, even increased for CAM plants in the “light” treatments. The findings by Sriprapat et al.23 and Treesubsuntorn et al.24 indicated a stronger correlation between VOC uptake and cuticle composition than stomatal density. This evidence suggests that even if the stomata were closed due to the “light” treatment, it would not cause a decrease in VOC absorption by the plants.
Further investigation of the concentration of hexadecanoic acid, α-linoleic acid, and dodecyl cyclohexane in the investigated CAM plants that exhibited successful VOC removal would be particularly interesting to assess the cuticle-uptake hypothesis.
Conclusions
The results of this study indicate that certain plants have the ability to effectively remove airborne volatile organic compounds (VOCs), although the efficiency of removal varies depending on the specific compound and the uptake mechanism employed by each plant. Significant differences were observed between treatments when the percent reduction in VOC concentration exceeded the range of significant error for the two compared treatments. The findings demonstrated significant differences between multiple treatments, with a consistent positive trend observed between the “control” and “foil” treatment groups for all plants except Consolea falcata in relation to trichloromethane. A general positive trend was observed between the “foil” and “no foil” treatments for all test plants except Chlorophytum comosum. Guzmania lingulata exhibited a general positive trend from the “no foil” treatment to the “light” treatment. Benzene was generally more effectively reduced during the “light” treatments for all plants except Chlorophytum comosum.The most efficient plant-treatment combination for total VOC removal was Guzmania lingulata during the “light” treatment, achieving over 80% efficacy in removing six out of the eight targeted compounds. Additionally, all five tested plants demonstrated over 80% efficacy in the removal of acetone compared to their respective control samples. Future plans for this study involve expanding its scope by utilizing larger chambers to accommodate larger plants and increasing the number of VOCs studied. The overarching objective is to evaluate the effectiveness of selected plants in removing VOCs from real-world environments, such as nail salons. By conducting experiments in these settings, we aim to gain practical insights into the potential of indoor plants as a means of mitigating VOC concentrations and improving air quality. The comprehensive results obtained from this study can serve as a valuable resource for selecting the appropriate indoor plant based on the presence of specific VOCs in the environment. These findings can assist individuals and organizations in making informed decisions regarding indoor plant selection, thereby promoting healthier indoor environments with reduced VOC concentrations.
Author contributions
Geoffrey Peterson: investigation, writing – original draft, Timothy Jones: investigation, writing – original draft, Diana Rispoli: investigation, Shokouh Haddadi: conceptualization, methodology, writing – review & editing, Vadoud Niri: conceptualization, formal analysis, funding acquisition, methodology, project administration, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their gratitude to the Faculty Scholarly and Creative Activity Grants at SUNY Oswego for providing financial support for this research project. They also thank Daniel Stitt for his contribution in early stage of the project and Frederick Scoles for his technical support.Notes and references
- R. Snyder, Int. J. Environ. Res. Public Health, 2012, 9, 2875–2893, DOI:10.3390/ijerph9082875.
- L. A. Wallace, Int. J. Toxicol., 1989, 8, 883–895, DOI:10.3109/10915818909018049.
- United State Environmental Protection Agency. Available online: https://www.epa.gov/indoor-air-quality-iaq/volatile-organic-compounds-impact-indoor-air-quality accessed 6/8/2023.
- A. Jones, Atmos. Environ., 1999, 33, 4536–4564, DOI:10.1016/S1352-2310(99)00272-1.
- Z. Xu, N. Qin, J. Wang and H. Tong, Bioresour. Technol., 2010, 101, 6930–6934, DOI:10.1016/j.biortech.2010.03.128.
- W. B. Li, J. X. Wang and H. Gong, Catal. Today, 2009, 148, 81–87, DOI:10.1016/j.cattod.2009.03.007.
- C. Yang, G. Miao, O. Pi, O. Xia, J. Wu, Z. Li and J. Xiao, Chem. Eng. J., 2019, 370, 1128–1153, DOI:10.1016/j.cej.2019.03.232.
- M. Masi, W. G. Nissim, C. Pandolfi, E. Azzarello and S. Mancuso, J. Hazard. Mater., 2022, 422, 126875, DOI:10.1016/j.jhazmat.2021.126875.
- X. Sun, C. Li, B. Yu, J. Wang and W. Wang, J. Environ. Sci., 2023, 125, 427–442, DOI:10.1016/j.jes.2022.01.020.
- G. Tchobanoglous and F. L. Burton, Wastewater Engineering: Treatment, Disposal, and Reuse, McGraw-Hill, New York, USA, 3rd edn, 1991, p. 317 Search PubMed.
- K. Everaert and J. Baeyens, J. Hazard. Mater., 2004, 109, 113–139, DOI:10.1016/j.jhazmat.2004.03.019.
- B. Wolverton, A. Johnson and K. Bounds, Interior Landscape Plants for Indoor Air Pollution Abatement, Reported by National Aeronautics and Space Administration, Stennis Space Center, 1989 Search PubMed.
- R. Wood, R. Orwell, J. Tarran, F. Torpy and M. Burchett, J. Hortic. Sci. Biotechnol., 2002, 77, 20–129, DOI:10.1080/14620316.2002.11511467.
- A. Aydogan and L. D. Montoya, Atmos. Environ., 2011, 45, 2675–2682, DOI:10.1016/j.atmosenv.2011.02.062.
- R. L. Orwell, R. A. Wood, J. Tarran, F. Torpy and M. D. Burchett, Water, Air, Soil Pollut., 2004, 157, 193–207, DOI:10.1023/B:WATE.0000038896.55713.5b.
- R. L. Orwell, R. A. Wood, M. D. Burchett, J. Tarran and F. Torpy, Water, Air, Soil Pollut., 2006, 177, 59–80, DOI:10.1007/s11270-006-9092-3.
- S. Hong, J. Hong, J. Yu and Y. Lim, Environ. Health Toxicol., 2017, 32, e2017006, DOI:10.5620/eht.e2017006.
- M. Delhomenie and M. Heitz, Crit. Rev. Biotechnol., 2005, 25, 53–72, DOI:10.1080/07388550590935814.
- P. Schroder and C. Collins, Organic Xenobiotics and Plants: From Mode of Action to Ecophysiology, in Organic Xenobiotics and Plants, Publisher, Springer, Dordrecht, Heidelberg, London, New York, 2011, pp. 1–16. DOI: DOI:10.1007/978-90-481-9852-8.
- E. Steudle, J. Exp. Bot., 2000, 51, 1531–1542, DOI:10.1093/jexbot/51.350.1531.
- E. L. Arthur, P. J. Rice, P. J. Rice, T. A. Anderson, S. M. Baladi, K. L. D. Henderson and J. R. Coats, Crit. Rev. Plant Sci., 2005, 24, 109–122, DOI:10.1080/07352680590952496.
- L. A. Newman and C. M. Reynolds, Curr. Opin. Biotechnol., 2004, 15, 225–230, DOI:10.1016/j.copbio.2004.04.006.
- W. Sriprapat, P. Suksabye, S. Areephak, P. Klantup, A. Waraha, A. Sawattan and P. Thuravetyan, Ecotoxicol. Environ. Saf., 2014, 102, 147–151, DOI:10.1016/j.ecoenv.2014.01.032.
- C. Treesubsuntorn, P. Suksabye, S. Weangjun, F. Pawana and P. Thiravetyan, Water, Air, Soil Pollut., 2013, 224, 1736, DOI:10.1007/s11270-013-1736-5.
- K. J. Kim, C. C. Shagol, F. R. Torpy, T. Pettit and P. J. Irga, Plant physiological mechanisms of air treatment. In from biofiltration to promising options in gaseous fluxes biotreatment, Elsevier, 2020, pp. 219–244, DOI: DOI:10.1016/B978-0-12-819064-7.00011-X.
- A. Setsungnern, C. Treesubsuntorn and P. Thiravetyan, Plant Physiol. Biochem., 2017, 120, 95–102, DOI:10.1016/j.plaphy.2017.09.021.
- B. X. Y. Lee, T. Hadibarata and A. Yuniarto, Water, Air, Soil Pollut., 2020, 231, DOI:10.1007/s11270-020-04813-6.
- R. Kumar, V. Verma, M. Thakur, G. Singh and B. Bhargava, Air Qual., Atmos. Health, 2023, 16, 1501–1527, DOI:10.1007/s11869-023-01326-z.
- C. L. Arthur and J. Pawliszyn, Anal. Chem., 1990, 62, 2145, DOI:10.1021/ac00218a019.
- S. Risticevic, V. H. Niri, D. Vuckovic and J. Pawliszyn, Anal. Bioanal. Chem., 2009, 393, 781–795, DOI:10.1007/s00216-008-2375-3.
- H. L. Lord, W. Zhan and J. Pawliszyn, Anal. Chim. Acta, 2010, 677, 3–18, DOI:10.1016/j.aca.2010.06.020.
- S. Jin, L. Zhong, X. Zhang, X. Li, B. Li and X. Fang, Int. J. Environ. Res. Public Health, 2023, 20, 5829, DOI:10.3390/ijerph20105829.
- J. Kang, J. Liu and J. Pei, J. Air Waste Manage. Assoc., 2017, 67, 725–737, DOI:10.1080/10962247.2017.1280561.
- D. Akal, S. Yurdakul, M. Y. Civan, G. Tuncel and H. Y. Ersan, Environ. Forensics, 2015, 16, 173–185, DOI:10.1080/15275922.2015.1022913.
- A. Mundackal and V. M. Ngole-Jeme, Int. J. Environ. Health Res., 2022, 32, 1076–1094, DOI:10.1080/09603123.2020.1828304.
- L. Zhong, S. Batterman and C. W. Milando, Int. Arch. Occup. Environ. Health, 2019, 92, 141–153, DOI:10.1007/s00420-018-1353-0.
| This journal is © The Royal Society of Chemistry 2023 |