Open Access Article
Open Access ArticleElectrochemical synthesis and antimicrobial evaluation of some N-phenyl α-amino acids†
Kishanpal Singha,
Neetu Singhb,
Harvinder Singh Sohal *b,
Baljit Singh*a,
Fohad Mabood Husainc,
Mohammed Arshadd and
Mohd Adile
*b,
Baljit Singh*a,
Fohad Mabood Husainc,
Mohammed Arshadd and
Mohd Adile
aDepartment of Chemistry, Punjabi University, Patiala 147002, Punjab, India
bMedicinal and Natural Product Laboratory, Department of Chemistry, Chandigarh University, Gharuan 140413, Mohali, Punjab, India. E-mail: drharvinder.cu@gmail.com
cDepartment of Food Science and Nutrition. College of Food and Agriculture Sciences, King Saud University, Riyadh, Kingdom of Saudi Arabia
dDental Health Department, College of Applied Medical Sciences, King Saud University, Riyadh 11433, Kingdom of Saudi Arabia
eDepartment of Environmental Sciences, Dalhousie University, Truro, NS, Canada
First published on 1st November 2023
Abstract
In the present report, the authors describe a synthetic route for the generation of N-phenyl amino acid derivatives using CO2 via a C–C coupling reaction in an undivided cell containing a combination of Mg–Pt electrodes. The reactions were completed in a short time without the formation of any other side product. The final products were purified via a simple recrystallization procedure. The structures of the newly prepared compounds were established using advanced spectroscopic techniques including 1H, 13C NMR, IR, and ESI-MS. All the prepared derivatives show good-to-excellent activity when tested against bacterial and fungal strains. Interestingly, it was observed that the presence of polar groups (capable of forming H-bonds) such as –OH (4d) and –NO2 (4e) at the para position of the phenyl ring show activity equivalent to the standard drugs.
1. Introduction
Amino acids are a type of biomolecule that contains an amino group, a carboxylate group, and a side chain.1 Although hundreds of different amino acid side chains have been described and synthesized in the literature, only 20 amino acids have been identified as common building blocks of proteins.2 In addition to their role as protein building blocks, amino acids are used for a variety of other applications in organic chemistry.1 Because of their inherent chirality, they can act as ligands,3 organocatalysts4,5 and as structural linkers in agrochemicals6 and pharmaceuticals.7Furthermore, these 20 important amino acids have been connected to a number of substrates to achieve potent biological activities, such as antibacterial,8 antifungal,9 anticancer10 inhibition of Type B monoamine oxidase,11 and anti-fibrotic12 activities. It has also been observed that N-substituted amino acids are more potent for a number of activities, such as PPAR γ-agonist,13 hyperalphalipoproteinaemic,14 anti-inflammatory,9 anti-phlogistic,10 anti-hypertensive,15 anti-oxidant,16 and anti-phlogistic activity.17
To date, a number of methods have been proposed for the formulation of amino acids, including simple refluxing,18 visible light irradiation,19 microwave irradiation,20 and ultraviolet irradiation,21 but these methods all have limitations. In continuation of our work on electro-carboxylation,22–24 in the present report, we introduced a synthetic route for the production of N-substituted amino acids via an environmentally friendly electrochemical C–C coupling method using carbon dioxide.
2. Results and discussion
2.1. Optimization of reaction conditions
| Entry | Sacrificial anode | Conc. (mmol L−1) | SRP (volts) | Yield (%) |
|---|---|---|---|---|
| 1 | Ni | 0.64 | −0.19 | 64 |
| 2 | Ni | 1.12 | −0.19 | 53 |
| 3 | Ni | 1.53 | −0.19 | 41 |
| 4 | Ni | 2.15 | −0.19 | 30 |
| 5 | Al | 0.59 | −1.62 | 72 |
| 6 | Al | 1.05 | −1.62 | 63 |
| 7 | Al | 1.61 | −1.62 | 59 |
| 8 | Al | 2.15 | −1.62 | 47 |
| 9 | Mg | 0.54 | −2.36 | 92 |
| 10 | Mg | 1.05 | −2.36 | 89 |
| 11 | Mg | 1.59 | −2.36 | 77 |
| 12 | Mg | 2.15 | −2.36 | 65 |
| Entry | Current density (mA cm−2) | Temperature (°C) | Yielda (%) |
|---|---|---|---|
| a Yield refers to combined yield from all the crops. | |||
| 1 | 10 | 0 | 61 |
| 2 | 10 | 5 | 65 |
| 3 | 10 | 10 | 69 |
| 4 | 10 | 15 | 75 |
| 5 | 10 | 20 | 79 |
| 6 | 10 | 25 | 75 |
| 7 | 15 | 0 | 63 |
| 8 | 15 | 5 | 74 |
| 9 | 15 | 10 | 79 |
| 10 | 15 | 15 | 80 |
| 11 | 15 | 20 | 90 |
| 12 | 15 | 25 | 92 |
| 13 | 20 | 0 | 65 |
| 14 | 20 | 5 | 69 |
| 15 | 20 | 10 | 73 |
| 16 | 20 | 15 | 75 |
| 17 | 20 | 20 | 81 |
| 18 | 20 | 25 | 79 |
Various substitutions on the aldehyde moiety in the imine derivatives were reacted with CO2 under similar reaction conditions for the generalization of the reaction, and it was discovered that the reactions proceeded smoothly and the desired molecules were collected in high yield as well as high purity. Finally, all the collected synthesized compounds were purified by simple recrystallization in ethanol.
2.2. Chemistry
Primary analysis of the products was conducted by comparing their melting points (MP), and the spectra later assisted in the illustration of the synthesized compounds. The IR spectra of compound 3a in series 3 shows three considerable absorptions at 3149, 3029, and 1597 cm−1 corresponding to the C–H, Ar–H, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups. In the 1H NMR spectrum (500 MHz, DMSO-d6), the deshielded signal at δ 8.44 (s) is assigned to the proton of C-1 and the multiplet peak at δ 7.44–7.13 (m) is assigned to the aromatic protons. Furthermore, the presence of [M+1] and [M+2] peaks at 216 and 217 m/z, respectively, in the mass spectrum validates the formation of the expected compound.
N groups. In the 1H NMR spectrum (500 MHz, DMSO-d6), the deshielded signal at δ 8.44 (s) is assigned to the proton of C-1 and the multiplet peak at δ 7.44–7.13 (m) is assigned to the aromatic protons. Furthermore, the presence of [M+1] and [M+2] peaks at 216 and 217 m/z, respectively, in the mass spectrum validates the formation of the expected compound.
The synthesis of amino acid derivative 4a was confirmed by the downfield shift in the signal of the aromatic protons from δ 7.25 to δ 7.60, and also the singlet peak for the proton of N–H at δ 9.59 ppm. 13C-NMR exhibits signals at δ 180.5 for the carboxylic group, with other peaks δ 129.5, 129.2, 128.9, 120.8, 113.5, and 64.3 confirming the formation of the targeted compound. In the IR spectrum, an additional broad peak at 3356 cm−1 demonstrates the –OH of the carboxylic group and a peak at 2873 cm−1 validates the C–H group. Furthermore, ESI-MS fragmentation generated [M+1] and [M+2] peaks at 262 and 263 m/z respectively, confirming the formation of the desired amino acid derivative.
2.3. Plausible mechanism for the synthesis of amino acid derivatives
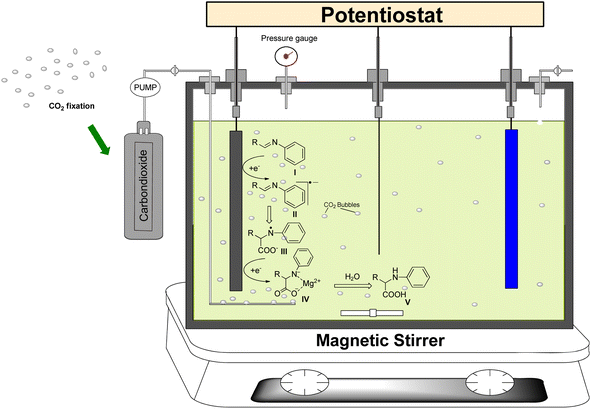
Several reports on electrocarboxylation of unsaturated organic imines are available in which an undivided cell with magnesium as a stable sacrificial anode and platinum or silver as a cathode gives a much higher yield and carboxylation selectivity.25,26 When the carboxylate ions are reduced and dissociated, a reactive radical is formed. These dissociated carboxylate ions react with the anode immediately to form Mg(OH)2. The reactive intermediate II is then reduced again to form an intermediate III anion. The nucleophile III later reacts with CO2 to produce carboxylate anion IV (Fig. 1). Finally, during the work-up, intermediate IV absorbs protons from the solution to produce the final compound.2.4. Antimicrobial activity
The anti-microbial potencies of the synthesized compounds 4a–l were investigated using the Minimum Inhibitory Concentration (MIC) method. The findings were compared to the reference drugs fluconazole and amoxicillin in their respective areas at 4 g mL−1 and 2 g mL−1, respectively. Table 4 shows that series 4a–l has good-to-great activity against the preferred strain. Only five amino acids (4a, 4d, 4e, 4f, and 4g) were found to be effective against various bacterial (B. subtilis, E. coli, S. aureus S. pyogenes, and K. pneumonia) and fungal strains (A. janus and A. niger). Further, it was observed that the presence of polar groups that are capable of forming H-bonds, such as –OH (4d) and –NO2 (4e) at the para position of the phenyl ring show maximum resistance against the microbes, which is equivalent to the standard drugs at MIC 4 μg mL−1.| Compound | Gram (+ve) bacteria | Gram (−ve) bacteria | Fungi | |||||
|---|---|---|---|---|---|---|---|---|
| B. subtilis | S. pyogenes | E. coli | K. pneumonia | S. aureus | A. janus | A. niger | A. sclerotiorum | |
| 4a | 16 | 8 | 8 | 8 | 16 | 8 | 16 | 8 |
| 4b | 16 | 16 | 8 | 16 | 16 | 16 | 8 | 16 |
| 4c | 16 | 32 | 32 | 16 | 32 | 16 | 8 | 32 |
| 4d | 8 | 4 | 8 | 4 | 8 | 16 | 8 | 16 |
| 4e | 4 | 4 | 4 | 8 | 4 | 4 | 4 | 8 |
| 4f | 8 | 8 | 16 | 8 | 16 | 16 | 16 | 8 |
| 4g | 16 | 16 | 8 | 8 | 16 | 8 | 16 | — |
| 4h | 64 | 16 | 32 | 16 | 32 | 32 | 32 | 16 |
| 4i | 16 | 32 | 32 | 32 | 16 | 32 | 32 | 32 |
| 4j | 32 | 16 | 8 | — | 16 | 32 | 16 | 16 |
| 4k | 8 | 16 | 16 | 32 | — | 16 | 16 | 32 |
| 4l | 8 | 8 | 8 | 16 | 8 | 16 | 32 | 16 |
| Amoxicillin | 4 | 4 | 4 | 4 | 4 | — | — | — |
| Fluconazole | — | — | — | — | — | 2 | 2 | 2 |
3. Materials and methods
The commercial supplier Sigma Aldrich provided all of the compounds utilized in the current methods, which were all used without any further purification. 4A molecular sieves were used to preserve the commercially available solvent CH3CN (Merck) overnight. It was collected for further use in the reaction after distillation at a temperature of 80–82 °C. The distillate was placed in P2O5 for about 24 h before being diluted once to produce dry and pure CH3CN. Acetonitrile was stored in a dark-coloured and airtight bottles. All of the aqueous solutions were created using double-distilled water. We bought all of the solvents of analytical grade from Loba-Chemie. The melting points of all the developed heterocyclic compounds were measured using the open capillary method and a digital melting point instrument. Recording of IR spectra was conducted using a PerkinElmer Spectrum II with a diamond ATR. Using a Bruker Advanced NMR spectrometer, CDCl3 as the solvent and TMS as the reference, 1H and 13C NMR data were both collected at 500 MHz. The mass spectra of the compounds was recorded on the LC-MS Spectrometer Model Q-ToF-Micromass, Waters. The purity of compounds was determined using the Thin Layer Chromatography (TLC) method and UV light.3.1. Electrochemical instrumentation involved in setup
As an inert cathode and sacrificial anode electrode, platinum gauze and magnesium electrodes with dimensions of 1 cm × 1 cm × 0.1 cm and 1 cm diameter and 5 cm length, respectively, were employed. Using a DC power supply, the cathode and anode were eventually connected to the positive and negative ends of the electric circuit, respectively.
4. Experimental
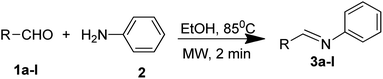
4.1. Synthesis of (E)-N,1-diphenylmethanimine (3a)
Imine derivative 3a was prepared by the reaction of 4-chlorobenzaldehyde (1.40 g, 10 mmol) with aniline (0.91 mL, 10 mmol) at 85 °C in ethanol for 2 min using a microwave reactor. The progress of the reaction was observed via thin layer chromatography (TLC) and finally worked up in chilled water. The recrystallization was conducted with ethanol to yield colorless crystalline product 3a (Scheme 1). Similarly, other imine derivatives have been synthesized using aldehydes 1b–l with aniline 2 using the same procedure to obtain 3b–l in excellent yield (Table 5).| Entry | Product | R | Rf | Yielda (%) | Melting point (°C) | Literature melting point (°C) |
|---|---|---|---|---|---|---|
| a Yield refers to total mass of collection from different crops. | ||||||
| 1 | 3a | 4-Cl C6H4 | 0.61 | 98 | 62 | 62–64 (ref. 25) |
| 2 | 3b | C6H5 | 0.66 | 98 | 55 | 54 (ref. 25) |
| 3 | 3c | 4-Me C6H4 | 0.63 | 96 | 39–41 | 38–40 (ref. 25) |
| 4 | 3d | 4-OH C6H4 | 0.69 | 95 | 96–97 | 94–96 (ref. 25) |
| 5 | 3e | 4-NO2 C6H4 | 0.71 | 97 | 90–93 | 90–92 (ref. 25) |
| 6 | 3f | 3-NO2 C6H4 | 0.68 | 98 | 64–65 | 65–66 (ref. 25) |
| 7 | 3g | 4-OMe C6H4 | 0.62 | 93 | 64–66 | 63–65 (ref. 25) |
| 8 | 3h | 4-Br C6H4 | 0.63 | 95 | 75–77 | 76–77 (ref. 25) |
| 9 | 3i | C6Hs CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
0.65 | 89 | 103–105 | 106–108 (ref. 25) |
| 10 | 3j | 2-Furyl | 0.67 | 91 | 56–58 | 55–57 (ref. 25) |
| 11 | 3k | 2-Thiophenyl | 0.63 | 92 | 61–63 | — |
| 12 | 3l | 2-Pyridyl | 0.59 | 90 | 65–66 | — |
3a: yield 98%, colourless solid, mp 62 °C. IR spectrum, ν, cm−1: 3149 (sp2 C–H), 3029 (Ar–H), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.44 (s, 1H, CH), 7.13–7.44 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 216 (M+1), 217 (M+2).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.44 (s, 1H, CH), 7.13–7.44 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 216 (M+1), 217 (M+2).
3b: yield 98%, colourless solid, mp 55 °C. IR spectrum, ν, cm−1: 3060 (sp2, C–H), 3028 (Ar–H), 1590 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.13 (s, 1H, CH), 7.10–7.35 (m, 10H, Ar–H). Mass spectrum, m/z (Irel, %): 182 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.13 (s, 1H, CH), 7.10–7.35 (m, 10H, Ar–H). Mass spectrum, m/z (Irel, %): 182 (M+1).
3c: yield 96%, colourless solid, mp 39–41 °C. IR spectrum, ν, cm−1: 3140 (sp2 C–H), 3030 (Ar–H), 1586 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.28 (s, 1H, CH), 7.06–7.26 (m, 9H, Ar–H), 2.14 (s, 3H, CH3). Mass spectrum, m/z (Irel, %): 196 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.28 (s, 1H, CH), 7.06–7.26 (m, 9H, Ar–H), 2.14 (s, 3H, CH3). Mass spectrum, m/z (Irel, %): 196 (M+1).
3d: yield 95%, colourless solid, mp 96–97 °C. IR spectrum, ν, cm−1: 3315 (O–H), 3142 (sp2 C–H), 3010 (Ar–H), 1584 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 9.1 (s, 1H, OH), 8.28 (s, 1H, CH), 7.09–7.27 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 198 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 9.1 (s, 1H, OH), 8.28 (s, 1H, CH), 7.09–7.27 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 198 (M+1).
3e: yield 97%, pale yellow solid, mp 90–93 °C. IR spectrum, ν, cm−1: 3170 (sp2 C–H), 3039 (Ar–H), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.59 (s, 1H, CH), 7.23–7.84 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 227 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.59 (s, 1H, CH), 7.23–7.84 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 227 (M+1).
3f: yield 98%, pale yellow solid, mp 64–65 °C. IR spectrum, ν, cm−1: 3152 (sp2 C–H), 3033 (Ar–H), 1608 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.52 (s, 1H, CH), 7.22–7.79 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 227 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.52 (s, 1H, CH), 7.22–7.79 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 227 (M+1).
3g: yield 93%, colourless solid, mp 64–66 °C. IR spectrum, ν, cm−1: 3122 (sp2 C–H), 3019 (Ar–H), 1582 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.24 (s, 1H, CH), 7.09–7.23 (m, 9H, Ar–H), 3.92 (s, 3H, OCH3). Mass spectrum, m/z (Irel, %): 212 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.24 (s, 1H, CH), 7.09–7.23 (m, 9H, Ar–H), 3.92 (s, 3H, OCH3). Mass spectrum, m/z (Irel, %): 212 (M+1).
3h: yield 95%, colourless solid, mp 75–77 °C. IR spectrum, ν, cm−1: 3148 (sp2 C–H), 3024 (Ar–H), 1599 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.27 (s, 1H, CH), 7.15–7.42 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 261 (M+1), 262 (M+2).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.27 (s, 1H, CH), 7.15–7.42 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 261 (M+1), 262 (M+2).
3i: yield 89%, yellow solid, mp 103–105 °C. IR spectrum, ν, cm−1: 3149 (sp2 C–H), 3015 (Ar–H), 1581 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.27 (s, 1H, CH), 6.89 (s, 1H,
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.27 (s, 1H, CH), 6.89 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.27 (s, 1H,
CH), 7.27 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.11–7.34 (m, 10H, Ar–H). Mass spectrum, m/z (Irel, %): 208 (M+1).
CH), 7.11–7.34 (m, 10H, Ar–H). Mass spectrum, m/z (Irel, %): 208 (M+1).
3j: yield 91%, yellow solid, mp 56–58 °C. IR spectrum, ν, cm−1: 3144 (sp2 C–H), 3033 (Ar–H), 1601 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.43 (s, 1H, CH), 7.17–7.45 (m, 8H, Ar–H). Mass spectrum, m/z (Irel, %): 172 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.43 (s, 1H, CH), 7.17–7.45 (m, 8H, Ar–H). Mass spectrum, m/z (Irel, %): 172 (M+1).
3k: yield 92%, yellow-brown solid, mp 61–63 °C. IR spectrum, ν, cm−1: 3140 (sp2 C–H), 3025 (Ar–H), 1599 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.47 (s, 1H, CH), 7.16–7.48 (m, 8H, Ar–H). Mass spectrum, m/z (Irel, %): 188 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.47 (s, 1H, CH), 7.16–7.48 (m, 8H, Ar–H). Mass spectrum, m/z (Irel, %): 188 (M+1).
3l: yield 90%, brown solid, mp 65–66 °C. IR spectrum, ν, cm−1: 3132 (sp2 C–H), 3029 (Ar–H), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). 1H NMR spectrum, δ, ppm (J, Hz): 8.46 (s, 1H, CH), 7.14–7.51 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 183 (M+1).
N). 1H NMR spectrum, δ, ppm (J, Hz): 8.46 (s, 1H, CH), 7.14–7.51 (m, 9H, Ar–H). Mass spectrum, m/z (Irel, %): 183 (M+1).
4.2. Synthesis of anilino(phenyl)acetic acid (4a)
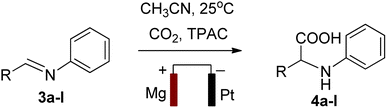
To obtain amino acid derivative 4a, an undivided electrochemical cell consisting of Mg as a sacrificial anode and Pt as an inert cathode was used, which was cleaned with diluted HNO3, rinsed with distilled water and dried in an oven. Then, 0.54 mmol of imine derivative 3a was added to a 100 mL quantity of CH3CN containing 5 mmol of TPAC as a supporting electrolyte. In the next step, the designed electrolytic solution mixture was electrolyzed at 25 °C by maintaining a constant current density of 15 mA cm−2. A continuous flow of CO2 gas was also passed into the solution to maintain the required pressure (1 atm) over a 10 hours period to obtain compound 4a.Following this, the excess solvent was reduced under low pressure, while the solid residue was retained. Furthermore, to eliminate ionic residues from the solid, the extraction was carried out in a separating funnel with diethyl ether, and the product was left to dry using anhydrous MgSO4. Finally, compound 4a was obtained by recrystallizing the isolated crude product with ethanol (Scheme 2). Similarly, the other imine derivatives 3b–l were converted into amino acid derivatives 4b–l using the same procedure (Table 6).
| Entry | Product | R1 | Rf | Yielda (%) | Melting point (°C) |
|---|---|---|---|---|---|
| a Yield refers to total mass of collection from different crops. | |||||
| 1 | 4a | 4-Cl C6H4 | 0.66 | 92 | 196–197 |
| 2 | 4b | C6H5 | 0.69 | 91 | 183–185 |
| 3 | 4c | 4-Me C6H4 | 0.61 | 86 | 174–175 |
| 4 | 4d | 4-OH C6H4 | 0.72 | 84 | 216–217 |
| 5 | 4e | 4-NO2 C6H4 | 0.70 | 91 | 210–212 |
| 6 | 4f | 3-NO2 C6H4 | 0.64 | 89 | 191–192 |
| 7 | 4g | 4-OMe C6H4 | 0.66 | 86 | 206–208 |
| 8 | 4h | 4-Br C6H4 | 0.69 | 85 | 178–180 |
| 9 | 4i | C6Hs CH = CH | 0.73 | 82 | 225–226 |
| 10 | 4j | 2-Furyl | 0.66 | 87 | 199–201 |
| 11 | 4k | 2-Thiopehyl | 0.67 | 86 | 188–190 |
| 12 | 4l | 2-Pyridyl | 0.63 | 83 | 178–181 |
4a: yield 92%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3356 (OH), 2970 (Ar–H), 2873 (C–H), 1619 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.85 (s, 1H, OH), 9.59 (s, 1H, NH), 8.54 (s, 1H, CH), 7.14–7.80 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 145.9, 135.0, 133.1, 129.5, 129.2, 128.9, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 262 (M+1), 263 (M+2).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.85 (s, 1H, OH), 9.59 (s, 1H, NH), 8.54 (s, 1H, CH), 7.14–7.80 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 145.9, 135.0, 133.1, 129.5, 129.2, 128.9, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 262 (M+1), 263 (M+2).
4b: yield 91%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3455 (OH), 3052 (Ar–H), 2825 (C–H), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.79 (s, 1H, OH), 9.62 (s, 1H, NH), 8.23 (s, 1H, CH), 7.22–7.53 (m, 10H, ArH). 13C NMR spectrum, δ, ppm: 188.3, 145.9, 136.9, 129.7, 129.5, 129.1, 127.5, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 228 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.79 (s, 1H, OH), 9.62 (s, 1H, NH), 8.23 (s, 1H, CH), 7.22–7.53 (m, 10H, ArH). 13C NMR spectrum, δ, ppm: 188.3, 145.9, 136.9, 129.7, 129.5, 129.1, 127.5, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 228 (M+1).
4c: yield 86%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3440 (OH), 3031 (Ar–H), 2978 (C–H), 1610 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.83 (s, 1H, OH), 9.51 (s, 1H, NH), 8.37 (s, 1H, CH), 7.14–7.739 (m, 9H, ArH), 2.23 (s, 3H, CH3). 13C NMR spectrum, δ, ppm: 180.5, 155.9, 147.2, 143.9, 139.6, 139.5, 139.4, 130.8, 123.5, 74.3, 31.3. Mass spectrum, m/z (Irel, %): 242 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.83 (s, 1H, OH), 9.51 (s, 1H, NH), 8.37 (s, 1H, CH), 7.14–7.739 (m, 9H, ArH), 2.23 (s, 3H, CH3). 13C NMR spectrum, δ, ppm: 180.5, 155.9, 147.2, 143.9, 139.6, 139.5, 139.4, 130.8, 123.5, 74.3, 31.3. Mass spectrum, m/z (Irel, %): 242 (M+1).
4d: yield 84%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3428 (OH), 3031 (Ar–H), 2978 (C–H), 1625 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.87 (s, 1H, OH), 9.55 (s, 1H, NH), 8.42 (s, 1H, CH), 7.21–7.43 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 157.3, 145.9, 129.5, 128.9, 120.8, 116.3, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 244 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.87 (s, 1H, OH), 9.55 (s, 1H, NH), 8.42 (s, 1H, CH), 7.21–7.43 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 157.3, 145.9, 129.5, 128.9, 120.8, 116.3, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 244 (M+1).
4e: yield 91%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3478 (OH), 3057 (Ar–H), 3020 (C–H), 1612 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 13.15 (s, 1H, OH), 9.87 (s, 1H, NH), 8.69 (s, 1H, CH), 7.38–8.03 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 146.7, 145.9, 143.0, 129.5, 128.9, 127.9, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 273 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 13.15 (s, 1H, OH), 9.87 (s, 1H, NH), 8.69 (s, 1H, CH), 7.38–8.03 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 146.7, 145.9, 143.0, 129.5, 128.9, 127.9, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 273 (M+1).
4f: yield 89%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3470 (OH), 3053 (Ar–H), 3017 (C–H), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 13.03 (s, 1H, OH), 9.83 (s, 1H, NH), 8.63 (s, 1H, CH), 7.37–7.99 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 148.3, 145.9, 136.8, 135.8, 130.0, 129.5, 123.5, 122.7, 120.8, 113.5, 63.3. Mass spectrum, m/z (Irel, %): 273 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 13.03 (s, 1H, OH), 9.83 (s, 1H, NH), 8.63 (s, 1H, CH), 7.37–7.99 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 180.5, 148.3, 145.9, 136.8, 135.8, 130.0, 129.5, 123.5, 122.7, 120.8, 113.5, 63.3. Mass spectrum, m/z (Irel, %): 273 (M+1).
4g: yield 86%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3421 (OH), 3039 (Ar–H), 2983 (C–H), 1605 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.80 (s, 1H, OH), 9.51 (s, 1H, NH), 8.37 (s, 1H, CH), 7.22–7.53 (m, 9H, ArH), 3.98 (s, 3H, OMe). 13C NMR spectrum, δ, ppm: 180.5, 159.4, 145.9, 129.5, 129.2, 128.5, 120.8, 114.7, 113.5, 64.3, 55.8. Mass spectrum, m/z (Irel, %): 258 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.80 (s, 1H, OH), 9.51 (s, 1H, NH), 8.37 (s, 1H, CH), 7.22–7.53 (m, 9H, ArH), 3.98 (s, 3H, OMe). 13C NMR spectrum, δ, ppm: 180.5, 159.4, 145.9, 129.5, 129.2, 128.5, 120.8, 114.7, 113.5, 64.3, 55.8. Mass spectrum, m/z (Irel, %): 258 (M+1).
4h: yield 85%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3446 (OH), 3037 (Ar–H), 2989 (C–H), 1622 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.89 (s, 1H, OH), 9.62 (s, 1H, NH), 8.42 (s, 1H, CH), 7.22–7.57 (m, 9H, ArH), 5.34 (d, 2H). 13C NMR spectrum, δ, ppm: 180.5, 145.9, 135.9, 132.0, 131.9, 129.5, 121.9, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 307 (M+1), 308 (M+2).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.89 (s, 1H, OH), 9.62 (s, 1H, NH), 8.42 (s, 1H, CH), 7.22–7.57 (m, 9H, ArH), 5.34 (d, 2H). 13C NMR spectrum, δ, ppm: 180.5, 145.9, 135.9, 132.0, 131.9, 129.5, 121.9, 120.8, 113.5, 64.3. Mass spectrum, m/z (Irel, %): 307 (M+1), 308 (M+2).
4i: yield 82%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3442 (OH), 3050 (Ar–H), 2987 (C–H), 1616 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.92 (s, 1H, OH), 9.63 (s, 1H, NH), 8.61 (s, 1H, =CH), 7.48 (s, 1H, CH), 7.23–7.58 (m, 10H, ArH), 6.91 (s, 1H,
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.92 (s, 1H, OH), 9.63 (s, 1H, NH), 8.61 (s, 1H, =CH), 7.48 (s, 1H, CH), 7.23–7.58 (m, 10H, ArH), 6.91 (s, 1H, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH). 13C NMR spectrum, δ, ppm: 184.1, 147.6, 136.4, 129.5, 128.6, 128.5, 127.9, 123.3, 120.8, 113.5, 72.3. Mass spectrum, m/z (Irel, %): 254 (M+1).
CH). 13C NMR spectrum, δ, ppm: 184.1, 147.6, 136.4, 129.5, 128.6, 128.5, 127.9, 123.3, 120.8, 113.5, 72.3. Mass spectrum, m/z (Irel, %): 254 (M+1).
4j: yield 87%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3434 (OH), 3049 (Ar–H), 2997 (C–H), 1633 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.95 (s, 1H, OH), 9.57 (s, 1H, NH), 8.62 (s, 1H, CH), 7.28–7.68 (m, 8H, ArH). 13C NMR spectrum, δ, ppm: 178.5, 145.9, 142.8, 139.3, 129.5, 120.8, 118.6, 113.5, 110.7, 60.0. Mass spectrum, m/z (Irel, %): 218 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.95 (s, 1H, OH), 9.57 (s, 1H, NH), 8.62 (s, 1H, CH), 7.28–7.68 (m, 8H, ArH). 13C NMR spectrum, δ, ppm: 178.5, 145.9, 142.8, 139.3, 129.5, 120.8, 118.6, 113.5, 110.7, 60.0. Mass spectrum, m/z (Irel, %): 218 (M+1).
4k: yield 86%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3439 (OH), 3047 (Ar–H), 2998 (C–H), 1628 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.87 (s, 1H, OH), 9.58 (s, 1H, NH), 8.61 (s, 1H, CH), 7.23–7.62 (m, 8H, ArH). 13C NMR spectrum, δ, ppm: 178.5, 145.9, 137.5, 129.5, 128.1, 126.1, 121.3, 120.8, 113.5, 65.5. Mass spectrum, m/z (Irel, %): 234 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.87 (s, 1H, OH), 9.58 (s, 1H, NH), 8.61 (s, 1H, CH), 7.23–7.62 (m, 8H, ArH). 13C NMR spectrum, δ, ppm: 178.5, 145.9, 137.5, 129.5, 128.1, 126.1, 121.3, 120.8, 113.5, 65.5. Mass spectrum, m/z (Irel, %): 234 (M+1).
4l: yield 83%, colourless solid, mp 183–185 °C. IR spectrum, ν, cm−1: 3445 (OH), 3055 (Ar–H), 3002 (C–H), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR spectrum, δ, ppm (J, Hz): 12.93 (s, 1H, OH), 9.65 (s, 1H, NH), 8.63 (s, 1H, CH), 7.34–7.79 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 178.5, 155.4, 148.6, 145.9, 136.2, 129.5, 121.9, 120.9, 120.8, 113.5, 72.9. Mass spectrum, m/z (Irel, %): 229 (M+1).
O). 1H NMR spectrum, δ, ppm (J, Hz): 12.93 (s, 1H, OH), 9.65 (s, 1H, NH), 8.63 (s, 1H, CH), 7.34–7.79 (m, 9H, ArH). 13C NMR spectrum, δ, ppm: 178.5, 155.4, 148.6, 145.9, 136.2, 129.5, 121.9, 120.9, 120.8, 113.5, 72.9. Mass spectrum, m/z (Irel, %): 229 (M+1).
4.3. Anti-microbial evaluation
The newly synthesized compounds 4a–l were tested against three Gram −ve (Escherichia coli MTCC 443, Klebsiella pneumonia MTCC 3384, and Staphylococcus aureus MTCC 96), two Gram +ve (Bacillus subtilis MTCC 441, and Streptococcus pyogenes MTCC 442) and Fungus (Aspergillus janus MTCC 2751, Aspergillus niger MTCC 281, and Aspergillus sclerotiorum MTCC 1008) samples. Nutrient broth was used to store the bacteria samples after they had been cultured at 37 °C for 24 hours. The fungal strains, on the other hand, were grown in malt extract for 72 hours at 28 °C before inoculation. A serial dilution procedure was used to test each produced chemical in triplicate after it was dissolved in DMSO at doses of 128, 64, 32, 16, 8, 4, and 2 g mL−1.5. Conclusion
In this work, the authors synthesized 12 potent N-phenyl amino acid derivatives from imines and CO2 via direct C–C coupling reaction in an undivided cell containing a combination of Mg–Pt electrodes. The products are obtained in a single step with adaptability and diversity in excellent yield and superior purity. This procedure is efficient in terms of labor, cost, and waste production, as well as the absence of harsh reaction conditions. All the synthesized amino acid derivatives show good-to-excellent resistance against the microbes. However, those with polar groups like –OH and –NO2 at the para position on the phenyl ring show equivalent resistance compared to the standard drugs amoxicillin and fluconazole.Abbreviations
| TPAC | Tetrapropylammonium chloride |
| TPAB | Tetrapropylammonium bromide |
| TBABF4 | Tetrapropylammonium tetrafluoroborate |
| MIC | Minimum inhibitory concentration |
| SRP | Standard reduction potential |
| ATR | Attenuated total reflectance |
| TLC | Thin layer chromatography |
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors would like to thank the Researchers Supporting Project Number (RSPD2023R729), King Saud University, Riyadh, Saudi Arabia. The authors are also grateful to UGC-BSR (New Delhi) Research Fellowship Scheme 2014–2015 (No. 15510), Punjabi University, Patiala, and Chandigarh University, Gharuan for providing financial assistance for research.References
- M. Akram, H. Asif, M. Uzair, N. Akhtar, A. Madni, S. M. Ali Shah, Z. ul Hassan and A. Ullah, J. Med. Plants Res., 2011, 5, 3997–4000 CAS.
- R. Uy and F. Wold, Science, 1977, 198, 890–896 CrossRef CAS PubMed.
- F. L. Zhang, K. Hong, T. J. Li, H. Park and J. Q. Yu, Science, 2016, 351, 252–256 CrossRef CAS PubMed.
- X. Zhao, B. Zhu and Z. Jiang, Synlett, 2015, 26, 2216–2230 CrossRef CAS.
- A. Dominguez-Huerta, I. Perepichka and C. J. Li, Commun. Chem., 2018, 1, 1–7 CrossRef CAS.
- C. Lamberth, Tetrahedron, 2010, 66, 7239–7256 CrossRef CAS.
- P. Méndez-Samperio, Infect. Drug Resist., 2014, 7, 229–237 CrossRef PubMed.
- H. M. Kaminski and J. B. Feix, Polymers, 2011, 3, 2088–2106 CrossRef CAS PubMed.
- I. Sakıyan, E. Loǧoǧlu, S. Arslan, N. Sari and N. Şakiyan, BioMetals, 2004, 17, 115–120 CrossRef PubMed.
- A. I. Abd-Elhamid, H. El-Gendi, A. E. Abdallah and E. M. El-Fakharany, Pharmaceutics, 2021, 13, 1595 CrossRef CAS PubMed.
- F. Leonetti, C. Capaldi, L. Pisani, O. Nicolotti, G. Muncipinto, A. Stefanachi, S. Cellamare, C. Caccia and A. Carotti, J. Med. Chem., 2007, 50, 4909–4916 CrossRef CAS PubMed.
- G. Li, C. Lee, A. Thomas Read, K. Wang, J. Ha, M. Kuhn, I. Navarro, J. Cui, K. Young, R. Gorijavolu, T. Sulchek, C. Kopczynski, S. Farsiu, J. Samples, P. Challa, C. Ross Ethier and W. Daniel Stamer, Elife, 2021 DOI:10.7554/eLife.60831.
- F. Peiretti, R. Montanari, D. Capelli, B. Bonardo, C. Colson, E. Z. Amri, M. Grimaldi, P. Balaguer, K. Ito, R. G. Roeder, G. Pochetti and J. M. Brunel, J. Med. Chem., 2020, 63, 13124–13139 CrossRef CAS PubMed.
- K. H. Baggaley, R. Fears, H. Ferres, G. R. Geen, I. K. Hatton, L. J. A. Jennings and A. W. R. Tyrrell, Eur. J. Med. Chem., 1988, 23, 523–531 CrossRef CAS.
- J. L. Stanton, N. Gruenfeld, J. E. Babiarz, M. H. Ackerman, R. C. Friedmann, A. M. Yuan and W. Macchia, J. Med. Chem., 1983, 26, 1267–1277 CrossRef CAS PubMed.
- I. Tumosienė, I. Jonuškienė, K. Kantminienė, V. Mickevičius and V. Petrikaitė, Int. J. Mol. Sci., 2021, 22, 7799 CrossRef PubMed.
- O. Bruno, S. Schenone, A. Ranise, F. Bondavalli, W. Filippelli, G. Falcone, G. Motola and F. Mazzeo, Farmaco, 1999, 54, 95–100 CrossRef CAS PubMed.
- M. Hamdi, R. Sakellariou and V. Spéziale, J. Heterocycl. Chem., 1992, 29, 1817–1819 CrossRef CAS.
- M. Á. Vázquez, M. Landa, L. Reyes, R. Miranda, J. Tamariz and F. Delgado, Synth. Commun., 2004, 34, 2705–2718 CrossRef.
- M. Gopalakrishnan, P. Sureshkumar, V. Kanagarajan and J. Thanusu, Res. Chem. Intermed., 2007, 33, 541–548 CrossRef CAS.
- Y. J. Chen, M. Nuevo, T. S. Yih, W. H. Ip, H. S. Fung, C. Y. Cheng, H. R. Tsai and C. Y. R. Wu, Mon. Not. R. Astron. Soc., 2008, 384, 605–610 CrossRef CAS.
- D. S. Malhi, H. S. Sohal, K. Singh, Z. M. Almarhoon, A. Ben Bacha and M. I. Al-Zaben, ACS Omega, 2022, 7, 16055–16062 CrossRef CAS PubMed.
- S. Garg, H. S. Sohal, D. S. Malhi, M. Kaur, K. Singh, A. Sharma, V. Mutreja, D. Thakur and L. Kaur, Curr. Org. Chem., 2022, 26, 899–919 CrossRef CAS.
- K. Singh, H. S. Sohal and B. Singh, Asian J. Chem., 2021, 33, 839–845 CAS.
- C. H. Li, X. Z. Song, L. M. Tao, Q. G. Li, J. Q. Xie, M. N. Peng, L. Pan, C. Jiang, Z. Y. Peng and M. F. Xu, Tetrahedron, 2014, 70, 1855–1860 CrossRef CAS.
- V. G. Koshechko, V. E. Titov, V. N. Bondarenko and V. D. Pokhodenko, J. Fluorine Chem., 2008, 129, 701–706 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03592a |
| This journal is © The Royal Society of Chemistry 2023 |