Open Access Article
Open Access ArticleSynthesis of microwave-assisted carboxamides in Triton WR-1339-induced hyperlipidemic rats: possible hypolipidemic heterocyclic compounds†
Basmah Al-Jammal‡
a,
Buthaina Hussein‡§
 *b,
Yusuf Al-Hiariab,
Tareq Al-Qirimb,
Manal Al-Najdawic,
Lama Hamadnehd,
Mohammad Alwahshb and
Balqis Ikhmaisb
*b,
Yusuf Al-Hiariab,
Tareq Al-Qirimb,
Manal Al-Najdawic,
Lama Hamadnehd,
Mohammad Alwahshb and
Balqis Ikhmaisb
aDepartment of Pharmaceutical Sciences/Faculty of Pharmacy, The University of Jordan, Amman-11942, Jordan
bFaculty of Pharmacy, Al-Zaytoonah University of Jordan, Amman-17138, Jordan. E-mail: Buthina.hussein@zuj.edu.jo
cFaculty of Pharmacy, Isra University, Amman-11622, Jordan
dDepartment of Basic Medical Sciences, Faculty of Medicine, Al-Balqa Applied University, Al-Salt, Jordan
First published on 24th July 2023
Abstract
The hypolipidemic effect of furan carboxamide derivatives was investigated using the Triton WR-1339 rat model. Nineteen compounds were synthesized, including furan-2-carboxamides of benzophenones and acetophenones (a(1–4)), anilines and amine derivatives (a(5–9)), picolinic-2-carboxamide derivatives of benzophenones and acetophenone (a(10–12)) and furan-2-carboxylate esters of benzophenones and acetophenones, substituted phenols and alcohols (b(1–7)). All the necessary steps were taken to synthesize, purify, and characterize these compounds. They were synthesized by reacting acyl chlorides of the heterocycles with their corresponding amines in the presence of pyridine and tert-butyl acetate. While the conventional heating method yielded acceptable yields for some of the reactions under reflux, the microwave synthesis reactor achieved significantly higher yields for others. Rats with hyperlipidemia were induced with Triton WR-1339 and then subjected to in vivo testing via an intraperitoneal injection of 200 mg kg−1 Triton WR-1339. The model was tested using an oral dose of bezafibrate (100 mg kg−1). After 7 hours of treatment with Triton, the new derivatives represented by compounds a(1–2), a(4–5), a7, and a(10–12) showed significant activity against the complete lipid profile, including a decrease in triglyceride, total cholesterol, and low-density lipoprotein cholesterol and an increase in high-density lipoprotein cholesterol plasma levels. At 20 mg kg−1 dose, these compounds were superior to other lipid-lowering agents in reducing triglyceride levels and slightly increased high-density lipoprotein cholesterol levels. These results indicate a mutual mechanism of action of novel compounds with fibrates, where they have a marked effect on triglyceride and high-density lipoprotein cholesterol levels; for example, a5 causes a significant reduction (p 0.0001) of triglyceride levels by 86%, and a remarkable increase (p 0.0001) in high-density lipoprotein cholesterol plasma levels by 65% as compared to hyperlipidemic rats.
Introduction
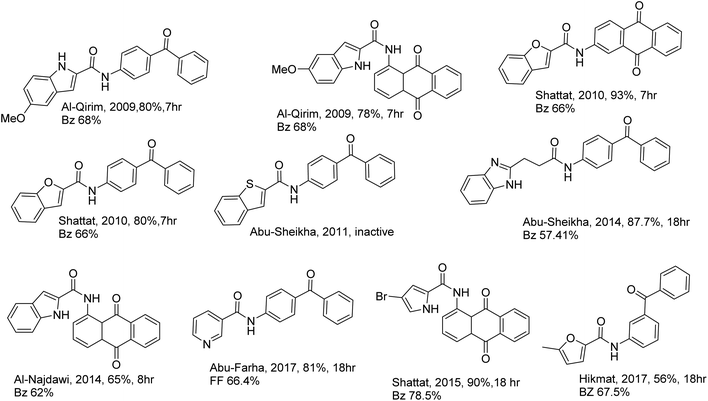
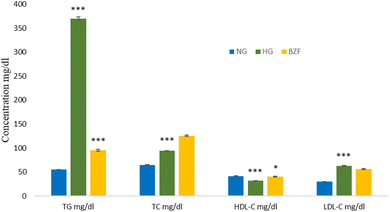
Numerous illnesses are a common source of grievance in modern life.1 Hyperlipidemia has significant adverse effects on health, causing diabetes, insulin resistance, and obesity. Also, it is considered the main risk factor for atherosclerosis leading to various cardiovascular diseases.2–4 Despite extended research to find new drugs, antihyperlipidemic therapy is still deprived of high efficacy and safety.5–7 Accordingly, there is still a need for new hypolipidemic agents. Our research team published studies that demonstrated the lipid-lowering effect of heterocyclic carboxamides, including but not limited to indole, benzofuran, furan, and pyrrole 2-carboxamide derivatives. Other compounds include nicotinic acid carboxamide derivatives (pyridine-3-carboxamide derivatives) and isonicotinic carboxamide derivatives (pyridine-4-carboxamide derivatives) that also demonstrated antihyperlipidemic activity, Fig. 1.8–15All compounds were evaluated by Triton WR-1339-induced hyperlipidemic rats, a well-known model used in screening new agents with a potential lipid-lowering activity, Fig. 2.8–15
As compared with the standard control group (NG) in Fig. 2, Triton WR-1339 leads to a remarkable increase in triglyceride (TG) (p < 0.0001) and total cholesterol (TC) (p < 0.0001) plasma concentrations when measured 7 h after Triton intraperitoneal injection (i.p.). More than six folds and TC increased the plasma TG by 1.5 folds. On the other hand, Triton WR-1339 reduce HDL-cholesterol (HDL-C) plasma levels by 0.75 fold (p < 0.0001). The Hyperlipidemic group (HG) group has also revealed that LDL cholesterol (LDL-C) increased significantly by two folds (p < 0.0001) after 7 h from Triton injection. Fig. 2 confirmed the model's validity, where the control group, the hyperlipidemic rats treated with bezafibrate (BZF), showed a significant reduction of TG and LDL-C levels compared with the HG group. It also increased the HDL-C and TC levels.
The hypolipidemic activity of carboxamide derivatives was attributed to three essential components: a heterocyclic ring, a sizeable lipophilic moiety, and a carboxamide linker.15 The previous publications8–15 have focused on carboxamide as a linker with either benzophenone or anthraquinone substitutions.
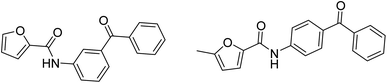
Hikmat et al., 2017, initiated a preliminary investigation on only two furan carboxamide derivatives as antihyperlipidemic agents, Fig. 3. It was found that furan benzophenone exhibited excellent antihyperlipidemic activity.14
This initial investigation of furan, which considers similar isosteric heterocycles to indole, pyrole, and benzofuran, showed excellent antihyperlipidemic activity in our previous work,8–15 in addition to its small size as a ring, its flexibility compared to the previous heterocycle ring, and the established pharmacological importance and the wide diversity of biological activities exhibited by heterocyclic carboxamide derivatives prompted this search to synthesize furan as the heterocyclic system with amide or ester linkers and investigate their effects on the activity and explore different substituents in the lipophilic part (Scheme 1). On the other hand, three picolinic-2-carboxamide derivatives have been synthesized and tested in this work as an initial investigation of a new class of different heterocycle rings.
Results and discussion
Chemistry
Procedure A, furan-2-carboxamide derivatives (a1–a9) were synthesized by coupling different amino benzophenones, acetophenones, aniline or alkyl amine to furan-2-carbonyl chloride (furoyl chloride) (2) (Scheme 2).16 Furoic acid (1) was converted to furoyl chloride (2) using thionyl chloride with toluene (solvent) under the fume hood for 30 h at reflux (90–100 °C). This reaction was time-consuming, giving low yields. Using oxalyl chloride did not improve the conditions or yields. Procedure A is based on conventional heating that consumes lots of starting materials and time, producing reasonable but not excellent yield. Therefore, a more efficient amide coupling method was necessary at this stage to produce higher yields. A microwave synthesis reactor (a more yielding and safer method) was attempted (procedure B) and compared to conventional heating as an alternative (Table 1). Using the same procedure of amide synthesis microwave conditions applied, the procedure involved adding 1 mole of the amine with pyridine and TEA, followed by adding two moles of furoyl chloride (2) in a specific glass vial (30 ml). The reactions occurred in a microwave synthesis reactor for 15 minutes at 50 or 80 °C. This purification was more straightforward, and the reaction yielded higher amounts of the desired amide compounds. The crude mixture was treated with a base in an aqueous system, followed by filtration and sometimes hot filtration to give pure products. Few reactions needed a recrystallization process but no chromatography. For Scheme 2 compounds a6, a11, b1, and b4 are novel while others are previously reported a1; a2, and a4,17 a3,18 a5; a10,19 a7,20 a8,21 a10,22 a12,23 b2,24 b3,25 b5,26 b6,27 b7.28
| Time | Temperature | Average yield | |
|---|---|---|---|
| Conventional heating (A) | 24 hours | 80–100 °C | ≈40% |
| Microwave (B) | 15 minutes | 50 or 80 °C | ≈80% |
Picolinyl chloride (4) was prepared from picolinic acid (3) using thionyl chloride (SOCl2) and toluene under reflux (80 °C). Procedure B was used for the preparation of compounds a10 and a11. At the same time, compound a12 was prepared by reacting picolinoyl chloride (4) with 4-aminoacetophenone under reflux (90 °C) for 24 hours without any catalyst. Only dioxane was added as a solvent. The reaction furnished the product (a12) but with a low yield.
Hypolipidemic activity
(Table 2) shows the TG, TC, HDL-C, and LDL-C plasm levels after administering the compounds to Triton-treated rats. Plasma TG levels were significantly suppressed (86%, p < 0.0001), (84%, 84% p < 0.001), (82%, 80% p < 0.05) following the administration of compounds a5, a2, a7, a4, and a1, respectively concerning HG group. However, compounds a3, a6, b1, b2, b3, b4, b6, and b7 show a weak reduction in TG levels compared to the previous compounds. The TC levels were significantly decreased (21%, 5%, P < 0.0001), (14%, 33%, 27%, 10% p < 0.001), (12%, 5% p < 0.05) after administration of compounds a5, a10, a7, a11, a12, a2, a4 and a1 respectively with respect to HG group. Contrary to the BZF, which caused an increase in TC levels, our novel above compounds resulted in a significant reduction. However, no significant differences in plasma TC levels were observed compared to HG-treated rats with a3, a6, a8, a9, b1–4, b6, and b7. Furthermore, HDL-C levels, the results show that HDL-C plasma levels were significantly increased (65% p < 0.0001), (58%, 40%, 30%, 25%, p < 0.001), (24%, 19% p < 0.05) in the presence of compounds a5, a11, a2, a7, a12, a4 and a1 respectively as compared to HG-group (Table 2). In contrast, no significant difference was found in HDL-C levels with compounds a3, a6, a8, a9, a10, b1–4, b6, and b7 compared to HG-treated rats. The same pattern was seen for the LDL-C levels, where the increase in plasma LDL-C levels made by Triton WR-1339 was significantly suppressed (60%, 38%, p < 0.0001), (66%, 58%, 57%, 53% p < 0.001), (50%, 47% p < 0.05) in the presence of compounds a5, a10, a11, a12, a2, a7, a1 and a4 respectively, concerning HG-group. At the same time, no significant differences in LDL-C levels were detected with compounds with a3, a6, a8, a9, b1–4, b6, and b7 compared to HG-treated rats. In summary, a significant reduction in TG, TC, and LDL-C and an increase in HDL-C plasma levels were noticed with compounds a11, a12, a5, a2, a7, a1, and a4.| Lipid profile | TG mg dl−1 | TC mg dl−1 | HDL-C mg dl−1 | LDL-C mg dl−1 |
|---|---|---|---|---|
| a Values are expressed as mean ± SEM from five rats in each group. NG, normal control group; HG, hyperlipidemic control group; BZF; bezafibrate control group, a1–a12, b1–b4, and b6–b7 + 4% DMSO for each compound, TC; total cholesterol, TG; triglyceride, HDL-C; high-density lipoprotein-cholesterol, LDL-C; low-density lipoprotein-cholesterol. All compounds are compared with HG. HG is compared with NG.b p < 0.05.c p < 0.001.d p < 0.0001. | ||||
| NG | 55.2 ± 0.4 | 64.2 ± 0.8 | 41.2 ± 0.5 | 30 ± 0.35 |
| HG | 370 ± 3.2d | 94 ± 0.71d | 31.5 ± 0.36d | 62.2 ± 0.91d |
| BZF | 95.6 ± 1.8d | 125.3 ± 1.5 | 40.2 ± 1.3b | 56.0 ± 1.1 |
| a1 | 75.6 ± 1.2b | 89 ± 1.2b | 37.6 ± 0.41b | 31 ± 0.32b |
| a2 | 58.3 ± 0.9c | 85 ± 0.95c | 44 ± 0.65c | 27 ± 0.21c |
| a3 | 340 ± 2.8 | 96 ± 1.6 | 27 ± 0.21 | 61 ± 0.84 |
| a4 | 65 ± 0.78b | 83 ± 0.75b | 39 ± 0.12b | 33 ± 0.12b |
| a5 | 50.2 ± 1.3d | 74.5 ± 0.91d | 52 ± 0.47d | 25 ± 0.45d |
| a6 | 352 ± 4.6 | 110 ± 2.2 | 33 ± 0.66 | 67.4 ± 0.47 |
| a7 | 60.4 ± 0.85c | 81 ± 1.4c | 41 ± 0.28c | 29 ± 0.32c |
| a8 | 352.9 ± 9.5 | 92.7 ± 8.2 | 31.7 ± 3 | 63.4 ± 5 |
| a9 | 361.3 ± 7.3 | 91.6 ± 5.5 | 29.4 ± 8 | 59.1 ± 7.4 |
| a10 | 109.2 ± 4.7b | 88.7 ± 9.5b | 34.5 ± 10b | 38.6 ± 4.9b |
| a11 | 20.0 ± 2.2c | 62.2 ± 2.9c | 49.8 ± 6c | 21.2 ± 3.7c |
| a12 | 29.9 ± 4.7c | 68.8 ± 4.5c | 39.6 ± 6c | 26.12 ± 1.2c |
| b1 | 361 ± 4.1 | 93 ± 2.1 | 30 ± 0.31 | 59 ± 0.42 |
| b2 | 368 ± 4.3 | 97.3 ± 1.1 | 29 ± 0.45 | 64 ± 0.95 |
| b3 | 443 ± 2.1d | 96.2 ± 0.5d | 30.2 ± 0.22d | 71 ± 1.1d |
| b4 | 302 ± 1.1c | 81.6 ± 1.9c | 38 ± 0.51c | 56 ± 0.4c |
| b5 | NA | NA | NA | NA |
| b6 | 421 ± 1.9b | 88.4 ± 1.2b | 32 ± 0.43b | 62.2 ± 0.8b |
| b7 | 390 ± 2.2c | 91.2 ± 1.5c | 33.2 ± 0.42c | 60.1 ± 1.1c |
SAR and chemistry
This research indicates that furan carboxamides are potential antihyperlipidemic compounds. These findings go smoothly with our proposed hypothesis. As a heterocyclic ring, furan was previously established for its hypolipidemic properties with two compounds.14,29 However, this work deeply investigated carboxamide derivatives with amide and ester linkers.Table 2 demonstrates the significant reduction in TG, TC, and LDL-C and the increase in HDL-C plasma levels after the treatment with compounds a1, a2, a4, a5, a7, a10, a11, and a12, with the most significant results relating to a11 at a minimal dose (20 mg kg−1).
Surprisingly enough, the active compounds at the dose of 68 μM (20 mg kg−1) and after 7 h of Triton injection have revealed activity against the complete lipid profile, including TG, TC, HDL-C, and LDL-C, with the most significant results regarding TG plasma levels, Fig. 4. These findings introduce a new hypolipidemic compound with synergistic action at low doses against the entire lipid profile for the first time. Bearing in mind that contrary to fibrates, they reduce TC levels (for example, bezafibrate (276 μM, 100 mg kg−1 body weight)). Unexpectedly, the active compounds significantly increased HDL levels, an essential issue in hyperlipidemia with no treatment yet. One agent only addresses this problem; niacin, usually used at high and toxic doses. Furthermore, despite increasing HDL levels by both niacin and sometimes fibrates such as gemfibrozil (Lopid) or certain statins, such as simvastatin (Zocor) and rosuvastatin (Crestor), these drugs have failed to protect the human body from heart attacks.
This pattern of activity is similar to the reported fibrates reduction pattern, which points out the mutual mechanism of action of the furan compounds with fibrates in that they have marked TG and HDL-C activity. It was reported that fibrates stimulate lipoprotein lipase gene expression.30 They stimulate peroxisome proliferator-activated receptor alpha (PPARα), which controls the expression of gene products that mediate the metabolism of TGs and HDL. As a result, the synthesis of fatty acids, TGs, and VLDL is reduced, while that of lipoprotein lipase, which catabolizes TGs, is enhanced. In addition, Apo A1 and ATP binding cassette A1 production are upregulated, leading to increased reverse cholesterol transport via HDL. Consequently, fibrates reduce TGs by up to 50% and increase HDL-C by up to 20%, but LDL-C changes are variable.31
From a chemical point of view, the similarity in the structural scaffold of the furan benzophenone carboxamides and fibrates was very distinct. It was noted that fenofibrate exhibited a benzophenone group attached to a fibric acid unit. Most fibrates, including bezafibrate, have a similar scaffold to our system in that the fibric acid (A, free or ester prodrug) is attached to a lipophilic side chain (C) through an H-B acceptor linker (B) such as ether (Fig. 5).
Scaffold similarity might explain to some extent the pattern similarity on lipid profile and, of course, the close mechanism of action/targets to fibrates. However, the unusual activity of these compounds and the different patterns on TC levels might suggest an additional mechanism(s) by these compounds that need to be validated. The assumption of additional mechanism for these derivatives was verified by the publication of our team with closely related indole benzophenone derivatives,32 where it was revealed that, unlike bezafibrate,’’ most of the overexpressed genes by Triton were down-regulated by compound N-(3-Benzoylphenyl)-1H-Indole-2-carboxamide with significant decreases in Apoc3, Apob, Acaa2, Acsl1, and Slc247a5 gene expression levels. However, N-(4-benzoylphenyl)-1H-indole-2-carboxamide and bezafibrate did not significantly affect the upregulated gene expression levels in acute hyperlipidemic mice treated with Triton WR1339”.
In conclusion, it is accepted that these active compounds not only have an additional mechanism to bezafibrate but might also have additional and different mechanisms between themselves, explaining their different reduction activity on lipid profile (Fig. 6).
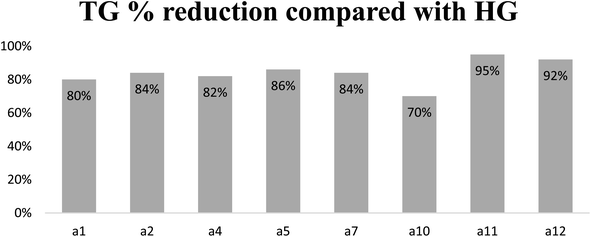 | ||
| Fig. 6 % Reduction of TG plasma levels concerning HG groups. HG: hyperlipidemic control group; TG: triglyceride. | ||
The structure–activity relationship of this work reveals that furan carboxamides have unusual activity. Ester derivatives tested (b1–4 and b6–7) of this work (Table 2) displayed no activity against plasma lipids. The possibility of fast hydrolysis of the ester compound can explain this finding.
(Table 2) shows that substitutions at 3 and 4-benzophenones (a2 and a1, respectively) were more active than position 2 (a3). The a3 derivative lost antihyperlipidemic activity ultimately. This indicates that the lipophilic side chain is essential; the extended linear form is also required in active hits. Fig. 7 shows that a3 exhibited a non-extended, non-linear, V-shaped structure. This 3D orientation makes the NH of the amide and the carbonyl group of the benzophenone in proximity, possibly permitting intra-molecular hydrogen bonding (H-B) between the molecule functionalities, concealing them from interaction with the receptor or target (Fig. 7).
Further clues to support this H-B comes from NMR spectra. It was noticed that the NH proton peak was downfield shifted in a3 to around 11 ppm, compared to a1 and a2, which appeared at a lower value of 10.4. Similarly, the keto-carbonyl in a3 was downshifted/deshielded to 197 compared to a1 and a2, which appeared at 195.
Previously, it was found that benzophenone, in particular, had a superior antihyperlipidemic effect. In this work, we revealed that lipophilic groups, including benzophenone, acetophenone, and anisidine, are all essential since they are part of the active compounds. For some compounds, 3-benzophenone showed the best percentage of reduction in lipid profile, whereas, in others, the acetophenone or anisidine effect was dominant. Such variation in the potency suggests again shared and additional mechanisms for each. Lipophilicity was the most important with extra H-B acceptor group bridge in the side chain, such as carbonyl in both benzophenone and acetophenone (a1, a2, a10, a11, and a4, a12) and methoxy oxygen in a5. While lacking, this H-B acceptor may explain the inactivity of others (a6, a8).
Picolinic acid derivatives were very comparable to furan derivatives, such as a4 and a12 which only differ in the hetercycle moiety (furan vs. pyridine), suggesting the possibility of using other heterocycle rings. Picolinic acid derivatives still need further investigation.
Referring to the previously reported derivatives [8–15] and in addition to these new derivatives, we can conclude preliminary SAR criteria for active hits, including 5 or 6-ring heterocycle (A) with H-B acceptor atom, carboxamide linker (B), lipophilic side chain (C) with H-B acceptor bridge in addition to limited size to one or two rings, and finally extended linear structure (Fig. 8).
Experimental part
Chemistry
Chemicals, reactants, and solvents were bought from commercial sources and used without further purification. Thin layer chromatography (20 cm × 20 cm, layer thickness 0.20 mm) aluminum sheets pre-coated with silica gel 60 (MACHEREY-NAGEL, Germany). Silica Gel (70–230 Mesh) from (Sigma-Aldrich, USA), was used to make preparative TLC plates that were visualized under a UV lamp (Spectroline, Model CX-20, USA). Melting points (m.p.) were measured using a Stuart scientific electro-thermal melting point apparatus at the faculty of pharmacy, University of Jordan, and are uncorrected. Nuclear Magnetic Resonance Spectra (NMR) were verified on Bruker 500 MHz-Avance III (500 MHz) at Hamdi Mango Center at The University of Jordan. Chemical shifts (δ) are described in ppm related to tetramethylsilane (TMS). H1 NMR data are reported as chemical shift (ppm), multiplicity, and coupling constant (Hz). C13-NMR data are reported as chemical shift (ppm), the corresponding carbon(s), Low resolution (LRMS), and High-resolution mass spectra (HRMS) were measured in positive or negative ion mode using electrospray ionization (ESI) technique by collision-induced dissociation on a Bruker APEX-4 (7 Tesla) instrument at the department of chemistry, The University of Jordan. Microwave 300 by Anton Paar GmbH (Microwave Synthesis Reactor) was used for the synthesis at the faculty of pharmacy, The University of Jordan.Synthesis procedures for targeted compounds
Procedure A. In a round-bottomed flask, pyridine (8.65 mmol), triethylamine (TEA, 5 mmol), and (4-amino benzophenone, acetophenone 5.07 mmol) were placed, and the mixture was stirred for 5 minutes. Furoyl chloride (20.3 mmol) was added dropwise to the mixture and left for stirring under reflux for 24 h at 100 °C. Dioxane was then added to the reaction mixture with cold water and 5 M NaOH (20 ml) solution until the pH of the media was basic. Suction filtration was carried out to yield the product. Two steps of recrystallization took place, the first one using DCM (50 ml) and charcoal with heating followed by gravity filtration, and the second one DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hexane (1
Hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); (20 ml
2); (20 ml![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 ml) that gave pale yellow powder.
40 ml) that gave pale yellow powder.
Procedure B. The starting material (e.g., p-anisidine 4.06 mmol) was weighed and placed in a 30 ml vial with pyridine (8.65 mmol, acylation catalyst) and triethylamine (5 mmol, acid scavenger). The mixture was stirred for 5 minutes, then furoyl chloride (10.14 mmol) was added dropwise, and the vial was placed in the microwave for 15 minutes at a temperature of 50 °C. The reaction was worked up by adding cold water to the solid mixture (50 ml), and the pH was adjusted to 10 using potassium carbonate (K2CO3) to remove the excess starting material, stirring for 10 minutes. Suction filtration was carried out to obtain the product.
N-(4-Benzoylphenyl)-2-furamide (a1). The product was collected as pale yellow solid (0.7 g, 47%); Rf: 0.36 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 95
MeOH, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); m.p.: 142–144 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.52 (br s, 1H, NHCO), 7.93 (br s, 3H, Ar–H-5, 2′ & 6′), 7.73 (d, J = 7.1 Hz, 2H, Ar–H-2′′ & 6′′), 7.68 (d, J = 5.4 Hz, 2H, Ar–H-3′′ & 5′′), 7.61 (br s, 1H, H-4′′), 7.51 (br s, 2H, Ar–H-3′ & 5′), 7.40 (br s, 1H, Ar–H-3), 6.69 (br s, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 195.09 (CO-ketone), 156.92 (CONH), 147.59 (C-2), 146.65 (C-5), 143.35 (C-1′), 137.97 (C-1′′), 132.75 (C-4′′), 132.22 (C-4′), 131.41 (C-2′ & 6′), 129.88 (C-2′′ & 6′′), 128.96 (C-3′′ & 5′′), 119.92 (C-3′ & 5′), 116.02 (C-3), 112.77 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + H+): found 292.09682 (C18H14NO3) requires 292.09737.
5); m.p.: 142–144 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.52 (br s, 1H, NHCO), 7.93 (br s, 3H, Ar–H-5, 2′ & 6′), 7.73 (d, J = 7.1 Hz, 2H, Ar–H-2′′ & 6′′), 7.68 (d, J = 5.4 Hz, 2H, Ar–H-3′′ & 5′′), 7.61 (br s, 1H, H-4′′), 7.51 (br s, 2H, Ar–H-3′ & 5′), 7.40 (br s, 1H, Ar–H-3), 6.69 (br s, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 195.09 (CO-ketone), 156.92 (CONH), 147.59 (C-2), 146.65 (C-5), 143.35 (C-1′), 137.97 (C-1′′), 132.75 (C-4′′), 132.22 (C-4′), 131.41 (C-2′ & 6′), 129.88 (C-2′′ & 6′′), 128.96 (C-3′′ & 5′′), 119.92 (C-3′ & 5′), 116.02 (C-3), 112.77 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + H+): found 292.09682 (C18H14NO3) requires 292.09737.
N-(3-Benzoylphenyl)-2-furamide or N-[3-(phenylcarbonyl) phenyl] furan-2-carboxamide (a2). The product was collected as pure off-white crystals. (0.25 g, 17%); Rf: 0.34 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 95
MeOH, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); m.p.: 185–187 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.39 (br s, 1H, NHCO), 8.15 (br s, 1H, Ar–H-2′), 8.06 (d, J = 7.95 Hz, 1H, Ar–H-6′), 7.90 (s, 1H, Ar–H-5), 7.72 (d, J = 7.4 Hz, 2H, Ar–H-2′′ & 6′′), 7.64 (dd, J = 7.35, 7.3 Hz, 1H, Ar–H-4′′), 7.54 (d, J = 7.55 Hz, 2H, Ar–H-3′′ & 5′′), 7.49 (m, 1H, Ar–H-5′), 7.42 (d, J = 7.55 Hz, 1H, Ar–H-4′), 7.32 (d, J = 3.25 Hz, 1H, Ar–H-3), 6.66 (d, J = 1.55 Hz, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 195.25 (CO-ketone), 156.88 (CONH), 147.70 (C-2), 146.41 (C-5), 139.24 (C-1′), 137.87 (C-1′′), 137.48 (C-3′), 133.16 (C-2′), 130.11 (C-2′′ & 6′′), 129.46 (C-6′), 129.03 (C-3′′ & 5′′), 125.32 (C-4′′), 124.65 (C-5′), 121.77 (C-4′), 115.57 (C-3), 112.68 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 314.07876 (C18H13NNaO3) requires 314.07931.
5); m.p.: 185–187 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.39 (br s, 1H, NHCO), 8.15 (br s, 1H, Ar–H-2′), 8.06 (d, J = 7.95 Hz, 1H, Ar–H-6′), 7.90 (s, 1H, Ar–H-5), 7.72 (d, J = 7.4 Hz, 2H, Ar–H-2′′ & 6′′), 7.64 (dd, J = 7.35, 7.3 Hz, 1H, Ar–H-4′′), 7.54 (d, J = 7.55 Hz, 2H, Ar–H-3′′ & 5′′), 7.49 (m, 1H, Ar–H-5′), 7.42 (d, J = 7.55 Hz, 1H, Ar–H-4′), 7.32 (d, J = 3.25 Hz, 1H, Ar–H-3), 6.66 (d, J = 1.55 Hz, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 195.25 (CO-ketone), 156.88 (CONH), 147.70 (C-2), 146.41 (C-5), 139.24 (C-1′), 137.87 (C-1′′), 137.48 (C-3′), 133.16 (C-2′), 130.11 (C-2′′ & 6′′), 129.46 (C-6′), 129.03 (C-3′′ & 5′′), 125.32 (C-4′′), 124.65 (C-5′), 121.77 (C-4′), 115.57 (C-3), 112.68 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 314.07876 (C18H13NNaO3) requires 314.07931.
N-(2-Benzoylphenyl)-2-furamide (a3). The product collected as a yellow powder (0.5 g, 17%); Rf: 0.74 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 95
MeOH, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); m.p.: 109–110 °C; 1H-NMR (500 MHz, DMSO-d6, Rotamers): δ = 11.00 (br s, 1H, NHCO), 8.04 (br s, 1H, Ar–H-5), 7.86 (d, J = 8.05 Hz, 1H, Ar–H), 7.68–7.55 (m, 4H, Ar–H), 7.54–7.41 (m, 3H, Ar–H), 7.22 (d, J = 7.1 Hz, 1H, Ar–H), 7.19 (br s, 1H, H-3), 6.60 (br s, 1H, H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 197.27 (CO-ketone), 156.41 (CONH), 147.62 (C-2), 146.42 (C-5), 137.97 (C-1′), 137.80 (C-1′′), 133.33 (Ar-CH), 133.01 (Ar-CH), 132.00 (Ar-CH), 130.13 (C-2′′ & 6′′), 128.72 (C-3′′ & 5′′), 128.44 (C-2′), 124.25 (Ar-CH), 123.38 (Ar-CH), 115.74 (C-3), 112.86 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 314.07877 (C18H13NNaO3) requires 314.07931.
5); m.p.: 109–110 °C; 1H-NMR (500 MHz, DMSO-d6, Rotamers): δ = 11.00 (br s, 1H, NHCO), 8.04 (br s, 1H, Ar–H-5), 7.86 (d, J = 8.05 Hz, 1H, Ar–H), 7.68–7.55 (m, 4H, Ar–H), 7.54–7.41 (m, 3H, Ar–H), 7.22 (d, J = 7.1 Hz, 1H, Ar–H), 7.19 (br s, 1H, H-3), 6.60 (br s, 1H, H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 197.27 (CO-ketone), 156.41 (CONH), 147.62 (C-2), 146.42 (C-5), 137.97 (C-1′), 137.80 (C-1′′), 133.33 (Ar-CH), 133.01 (Ar-CH), 132.00 (Ar-CH), 130.13 (C-2′′ & 6′′), 128.72 (C-3′′ & 5′′), 128.44 (C-2′), 124.25 (Ar-CH), 123.38 (Ar-CH), 115.74 (C-3), 112.86 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 314.07877 (C18H13NNaO3) requires 314.07931.
N-(4-Acetylphenyl)-furan-2-carboxamide (a4). The product was collected as light orange pure solid (1.52 g, 45%); Rf: 0.3 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 181–184 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.43 (s, 1H, NHCO), 7.93–7.85 (m, 5H, Ar–H-2′, 3′, 5′, 6′ & 5), 7.36 (d, J = 3.35 Hz, 1H, Ar–H-3), 6.67 (d, J = 1.7 Hz, 1H, Ar–H-4), 2.49 (s, 3H, COCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 197.04 (CO-ketone), 156.87 (CONH), 147.61 (C-2), 146.59 (C-5), 143.49 (C-1′), 132.51 (C-4′), 129.75 (C-3′ & 5′), 119.89 (C-2′ & 6′), 115.94 (C-3), 112.76 (C-4), 26.90 (CH3) ppm. HRMS (ESI, negative mode): m/z (M+ − H+): found 228.06662 (C13H10NO3) requires 228.06607.
2); m.p.: 181–184 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.43 (s, 1H, NHCO), 7.93–7.85 (m, 5H, Ar–H-2′, 3′, 5′, 6′ & 5), 7.36 (d, J = 3.35 Hz, 1H, Ar–H-3), 6.67 (d, J = 1.7 Hz, 1H, Ar–H-4), 2.49 (s, 3H, COCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 197.04 (CO-ketone), 156.87 (CONH), 147.61 (C-2), 146.59 (C-5), 143.49 (C-1′), 132.51 (C-4′), 129.75 (C-3′ & 5′), 119.89 (C-2′ & 6′), 115.94 (C-3), 112.76 (C-4), 26.90 (CH3) ppm. HRMS (ESI, negative mode): m/z (M+ − H+): found 228.06662 (C13H10NO3) requires 228.06607.
N-(4-Methoxyphenyl)-furan-2-carboxamide (a5). The product was collected as a light pink pure solid. TLC revealed that the product was pure without additional recrystallization steps. (0.54 g, 61.2%); Rf: 0.62 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 108 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.02 (s, 1H, NHCO), 7.86 (s, 1H, H-5), 7.61 (d, J = 8.8 Hz, 2H, Ar–H-2′ & 6′), 7.24 (d, J = 3.1 Hz, 1H, H-3), 6.88 (d, J = 8.8 Hz, 2H, H-3′ & 5′), 6.64 (br s, 1H, Ar–H-4), 3.69 (s, 3H, OCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 156.42 (CONH), 156.09 (C-4′), 148.20 (C-2), 145.89 (C-5), 131.98 (C-1′), 122.48 (C-3′ & 5′), 114.74 (C-3), 114.24 (C-2′ & 6′), 112.52 (C-4), 55.63(OCH3) ppm. HRMS (ESI, positive mode): m/z (M+ + Na): found 240.06311 (C12H11NNaO3) requires 240.06366.
2); m.p.: 108 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.02 (s, 1H, NHCO), 7.86 (s, 1H, H-5), 7.61 (d, J = 8.8 Hz, 2H, Ar–H-2′ & 6′), 7.24 (d, J = 3.1 Hz, 1H, H-3), 6.88 (d, J = 8.8 Hz, 2H, H-3′ & 5′), 6.64 (br s, 1H, Ar–H-4), 3.69 (s, 3H, OCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 156.42 (CONH), 156.09 (C-4′), 148.20 (C-2), 145.89 (C-5), 131.98 (C-1′), 122.48 (C-3′ & 5′), 114.74 (C-3), 114.24 (C-2′ & 6′), 112.52 (C-4), 55.63(OCH3) ppm. HRMS (ESI, positive mode): m/z (M+ + Na): found 240.06311 (C12H11NNaO3) requires 240.06366.
N-(4-Chlorophenyl)-furan-2-carboxamide (a6). Procedure B is used to get light gray pure crystals of the product (16). (1.4 g, 80.5%); Rf: 0.88 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 150–153 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.27 (br s, 1H, NHCO), 7.89 (s, 1H, H-5), 7.75 (d, J = 8.65 Hz, 2H, Ar–H-2′ & 6′), 7.35 (d, J = 8.65 Hz, 2H, Ar–H-3′ & 5′), 7.30 (d, J = 3.25 Hz, 1H, Ar–H-3), 6.66 (d, J = 1.4 Hz, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 156.69 (CONH), 147.76 (C-2), 146.32 (C-5), 138.01 (C-1′), 129.00 (C-3′ & 5′), 127.84 (C-4′), 122.31 (C-2′ & 6′), 115.49 (C-3), 112.67 (C-4) ppm.
2); m.p.: 150–153 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.27 (br s, 1H, NHCO), 7.89 (s, 1H, H-5), 7.75 (d, J = 8.65 Hz, 2H, Ar–H-2′ & 6′), 7.35 (d, J = 8.65 Hz, 2H, Ar–H-3′ & 5′), 7.30 (d, J = 3.25 Hz, 1H, Ar–H-3), 6.66 (d, J = 1.4 Hz, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 156.69 (CONH), 147.76 (C-2), 146.32 (C-5), 138.01 (C-1′), 129.00 (C-3′ & 5′), 127.84 (C-4′), 122.31 (C-2′ & 6′), 115.49 (C-3), 112.67 (C-4) ppm.
N-Butylfuran-2-carboxamide (a7). Procedure B used a light orange product with a waxy texture was obtained. (2 g, 59%); Rf: 0.38 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 39–42 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.26 (br s, 1H, NH–CO), 7.75 (br s, 1H, H-5), 7.01 (d, J = 3.1 Hz, 1H, H-3), 6.55 (d, J = 1.35 Hz, 1H, H-4), 3.16 (q, J = 6.5 Hz, 2H, CH2-1′), 1.42 (m, J = 7.25 Hz, 2H, CH2-2′), 1.25 (m, J = 7.4 Hz, 2H, CH2-3′), 0.84 (t, J = 7.3 Hz, 3H, CH3-4′); 13C-NMR (125 MHz, DMSO-d6): δ = 158.12 (CONH), 148.62 (C-2), 145.16 (C-5), 113.39 (C-3), 112.17 (C-4), 38.54 (CH2-1′), 31.77 (CH2-2′), 20.04 (CH2-3′), 14.13 (CH3-4′) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 190.08385 (C9H13NNaO2) requires 190.08440.
2); m.p.: 39–42 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.26 (br s, 1H, NH–CO), 7.75 (br s, 1H, H-5), 7.01 (d, J = 3.1 Hz, 1H, H-3), 6.55 (d, J = 1.35 Hz, 1H, H-4), 3.16 (q, J = 6.5 Hz, 2H, CH2-1′), 1.42 (m, J = 7.25 Hz, 2H, CH2-2′), 1.25 (m, J = 7.4 Hz, 2H, CH2-3′), 0.84 (t, J = 7.3 Hz, 3H, CH3-4′); 13C-NMR (125 MHz, DMSO-d6): δ = 158.12 (CONH), 148.62 (C-2), 145.16 (C-5), 113.39 (C-3), 112.17 (C-4), 38.54 (CH2-1′), 31.77 (CH2-2′), 20.04 (CH2-3′), 14.13 (CH3-4′) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 190.08385 (C9H13NNaO2) requires 190.08440.
N-Phenylfuran-2-carboxamide (a8). Procedure B was used to obtain pure gray product crystals (a8). TLC revealed that the product was pure without additional recrystallization steps. (5.4 g, 96%); Rf: 0.5 (hexane
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethylacetate, 7
ethylacetate, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); m.p.: 121–125 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.15 (s, 1H, NHCO), 7.88 (s, 1H, Ar–H-5), 7.75 (d, J = 7.9 Hz, 2H, Ar–H-2′ & 6′), 7.30 (m, 3H, Ar–H-3′,5′ & 3), 7.05 (dd, J = 7.3, 7.25, 1H, Ar–H-4′), 6.64 (d, J = 1.2 Hz, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 156.73 (CONH), 148.07 (C-2), 146.07 (C-5), 139.02 (C-1′), 129.09 (C-3′ & 5′), 124.21 (C-4′), 120.90 (C-2′ & 6′), 115.18 (C-3), 112.59 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + H+): found 188.07060 (C11H10NO2) requires 188.07115. LRMS (ESI, positive mode): m/z (M+ + H+): 188.07 (M+1, 47%), 161.04 (10%), 160.04 (100%), 82.97 (9%), 46.4 (15%), 42.0 (7%).
3); m.p.: 121–125 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.15 (s, 1H, NHCO), 7.88 (s, 1H, Ar–H-5), 7.75 (d, J = 7.9 Hz, 2H, Ar–H-2′ & 6′), 7.30 (m, 3H, Ar–H-3′,5′ & 3), 7.05 (dd, J = 7.3, 7.25, 1H, Ar–H-4′), 6.64 (d, J = 1.2 Hz, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 156.73 (CONH), 148.07 (C-2), 146.07 (C-5), 139.02 (C-1′), 129.09 (C-3′ & 5′), 124.21 (C-4′), 120.90 (C-2′ & 6′), 115.18 (C-3), 112.59 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + H+): found 188.07060 (C11H10NO2) requires 188.07115. LRMS (ESI, positive mode): m/z (M+ + H+): 188.07 (M+1, 47%), 161.04 (10%), 160.04 (100%), 82.97 (9%), 46.4 (15%), 42.0 (7%).
N-(3,5-Dimethoxyphenyl)-furan-2-carboxamide (a9). Procedure B was used to recrystallize the product from DCM/hexane (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); (15
3); (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 ml) to furnish pure golden needle-like crystals. (1.06 g, 66%); Rf: 0.6 (DCM
45 ml) to furnish pure golden needle-like crystals. (1.06 g, 66%); Rf: 0.6 (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 102–105 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.04 (s, 1H, NHCO), 7.88 (s, 1H, Ar–H-5), 7.29 (s, 1H, Ar–H-3), 7.02 (s, 2H, Ar–H-2′ & 6′), 6.65 (s, 1H, Ar–H-4), 6.22 (s, 1H, Ar–H-4′), 3.68 (s, 6H, 2OCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 160.84 (C-3′ & 5′), 156.67 (CONH), 147.92 (C-2), 146.20 (C-5), 140.69 (C-1′), 115.24 (C-3), 112.62 (C-4), 99.03 (C-2′ & 6′), 96.17 (C-4′), 55.56 (2OCH3) ppm.
2); m.p.: 102–105 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.04 (s, 1H, NHCO), 7.88 (s, 1H, Ar–H-5), 7.29 (s, 1H, Ar–H-3), 7.02 (s, 2H, Ar–H-2′ & 6′), 6.65 (s, 1H, Ar–H-4), 6.22 (s, 1H, Ar–H-4′), 3.68 (s, 6H, 2OCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 160.84 (C-3′ & 5′), 156.67 (CONH), 147.92 (C-2), 146.20 (C-5), 140.69 (C-1′), 115.24 (C-3), 112.62 (C-4), 99.03 (C-2′ & 6′), 96.17 (C-4′), 55.56 (2OCH3) ppm.
Procedure C. Picolinoyl chloride (4) (1 ml, 9.16 mmol) was added to 4-aminoacetophenone (0.5 g, 3.7 mmol) in a round bottom flask, with dioxane (20 ml) and refluxed for 24 hours at temperature 90 °C with stirring.
Work up: cold water (60 ml) was added to the mixture with stirring for 10 minutes, and the pH was adjusted to 11 using potassium carbonate (K2CO3) to remove the excess starting material.
N-(4-Benzoylphenyl)-pyridine-2-carboxamide (a10). Procedure B and the crude product were purified using preparative TLC plates to afford pure beige solid. (0.46 g, 60%); Rf: 0.64 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 161–166 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.94 (br s, 1H, NHCO), 8.72 (d, J = 4.15 Hz, 1H, H-6), 8.14 (d, J = 7.7 Hz, 1H, H-3), 8.09 (d, J = 8.3 Hz, 2H, H-2′ & 6′), 8.04 (dd, J = 7.65, 7.65 Hz, 1H, H-4), 7.74 (d, J = 8.35 Hz, 2H, H-3′ & 5′), 7.70–7.59 (m, 4H, H-2′′, 6′′, 5 & 4′′), 7.52 (dd, J = 7.4, 7.5 Hz, 2H, H-3′′ & 5′′); 13C-NMR (125 MHz, DMSO-d6): δ = 195.09 (CO-ketone), 163.49 (CONH), 150.04 (C-2), 148.96 (C-6), 143.01 (QC-Ar), 138.68 (CH–Ar), 137.95 (QC–Ar), 132.76 (CH–Ar), 132.49 (QC–Ar), 131.37 (C-3′ & 5′), 129.89 (C-2′′ & 6′′), 128.96 (C3′′ & 5′′), 127.67 (CH–Ar), 123.12 (CH–Ar), 120.09 (C-2′ & 6′) ppm.
2); m.p.: 161–166 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.94 (br s, 1H, NHCO), 8.72 (d, J = 4.15 Hz, 1H, H-6), 8.14 (d, J = 7.7 Hz, 1H, H-3), 8.09 (d, J = 8.3 Hz, 2H, H-2′ & 6′), 8.04 (dd, J = 7.65, 7.65 Hz, 1H, H-4), 7.74 (d, J = 8.35 Hz, 2H, H-3′ & 5′), 7.70–7.59 (m, 4H, H-2′′, 6′′, 5 & 4′′), 7.52 (dd, J = 7.4, 7.5 Hz, 2H, H-3′′ & 5′′); 13C-NMR (125 MHz, DMSO-d6): δ = 195.09 (CO-ketone), 163.49 (CONH), 150.04 (C-2), 148.96 (C-6), 143.01 (QC-Ar), 138.68 (CH–Ar), 137.95 (QC–Ar), 132.76 (CH–Ar), 132.49 (QC–Ar), 131.37 (C-3′ & 5′), 129.89 (C-2′′ & 6′′), 128.96 (C3′′ & 5′′), 127.67 (CH–Ar), 123.12 (CH–Ar), 120.09 (C-2′ & 6′) ppm.
N-(3-Benzoylphenyl)-pyridine-2-carboxamide (a11). Procedure B and the crude product were purified using preparative TLC plates afford pure beige solid. (0.3 g, 39%); Rf: 0.67 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 122–123 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.94 (br s, 1H, NHCO), 8.69 (d, J = 4.4 Hz, 1H, H-6), 8.37 (br s, 1H, H-2′), 8.15 (d, J = 8.05 Hz, 1H, H-6′), 8.12 (d, J = 7.8 Hz 1H, H-3), 8.02 (dd, J = 7.65,7.65 Hz, 1H, H-4), 7.74 (d, J = 7.3 Hz, 2H, H-2′′ & 6′′), 7.67–7.60 (m, 2H, H-4′ & 5′), 7.56–7.48 (m, 3H, H-5,3′′ & 5′′), 7.45 (d, J = 7.65 Hz, 1H, H-4′′); 13C-NMR (125 MHz, DMSO-d6): δ = 196.05 (CO-ketone), 163.39 (CONH), 150.19 (C-2), 148.91 (C-6), 139.04 (QC–Ar), 138.59 (CH–Ar), 137.94 (QC–Ar), 137.48 (QC–Ar), 133.17 (CH–Ar), 130.15 (C-2′′ & 6′′), 129.44 (CH–Ar), 129.04 (C-3′′ & 5′′), 127.51 (CH–Ar), 125.49 (CH–Ar), 124.85 (CH–Ar), 122.99 (CH–Ar), 121.92 (CH–Ar) ppm.
2); m.p.: 122–123 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.94 (br s, 1H, NHCO), 8.69 (d, J = 4.4 Hz, 1H, H-6), 8.37 (br s, 1H, H-2′), 8.15 (d, J = 8.05 Hz, 1H, H-6′), 8.12 (d, J = 7.8 Hz 1H, H-3), 8.02 (dd, J = 7.65,7.65 Hz, 1H, H-4), 7.74 (d, J = 7.3 Hz, 2H, H-2′′ & 6′′), 7.67–7.60 (m, 2H, H-4′ & 5′), 7.56–7.48 (m, 3H, H-5,3′′ & 5′′), 7.45 (d, J = 7.65 Hz, 1H, H-4′′); 13C-NMR (125 MHz, DMSO-d6): δ = 196.05 (CO-ketone), 163.39 (CONH), 150.19 (C-2), 148.91 (C-6), 139.04 (QC–Ar), 138.59 (CH–Ar), 137.94 (QC–Ar), 137.48 (QC–Ar), 133.17 (CH–Ar), 130.15 (C-2′′ & 6′′), 129.44 (CH–Ar), 129.04 (C-3′′ & 5′′), 127.51 (CH–Ar), 125.49 (CH–Ar), 124.85 (CH–Ar), 122.99 (CH–Ar), 121.92 (CH–Ar) ppm.
N-(4-Acetylphenyl)-pyridine-2-carboxamide (a12). The crude product obtained from procedure C recrystallized from methanol, including the charcoal step, has furnished pure yellow solid. (P4AP, 39, 0.14 g, 15.7%); Rf: 0.55 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 166–170 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.94 (s, 1H, NHCO), 8.71 (d, J = 3.95 Hz, 1H, H-6), 8.13 (d, J = 7.65 Hz, 1H, H-3), 8.04 (d, J = 8.4 Hz, 3H, H-3′,5′ & 4), 7.93 (d, J = 8.45 Hz, 2H, H-2′ & 6′), 7.65 (dd, J = 5.3, 6.4 Hz, 1H, H-5), 2.51 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ = 197.09 (CO-ketone), 163.44 (CONH), 150.15 (C-2), 148.96 (C-6), 143.17 (QC-Ar), 138.68 (CH–Ar), 132.74 (QC–Ar), 129.74 (C-3′ & 5′), 127.66 (CH–Ar), 123.09 (CH–Ar), 120.06 (C-2′ & 6′), 26.96 (CH3) ppm.
2); m.p.: 166–170 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 10.94 (s, 1H, NHCO), 8.71 (d, J = 3.95 Hz, 1H, H-6), 8.13 (d, J = 7.65 Hz, 1H, H-3), 8.04 (d, J = 8.4 Hz, 3H, H-3′,5′ & 4), 7.93 (d, J = 8.45 Hz, 2H, H-2′ & 6′), 7.65 (dd, J = 5.3, 6.4 Hz, 1H, H-5), 2.51 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ = 197.09 (CO-ketone), 163.44 (CONH), 150.15 (C-2), 148.96 (C-6), 143.17 (QC-Ar), 138.68 (CH–Ar), 132.74 (QC–Ar), 129.74 (C-3′ & 5′), 127.66 (CH–Ar), 123.09 (CH–Ar), 120.06 (C-2′ & 6′), 26.96 (CH3) ppm.
Procedure B. The starting material (hydroxy benzophenone, alcohols, and phenols) (21.2 mmol) was placed in a 30 ml vial with pyridine (8.65 mmol, acylation catalyst) and triethylamine (TEA, 5 mmol, acid scavenger). The mixture was stirred for 5 minutes, then furoyl chloride (FC, 2, 3 ml, 30.43 mmol) was added dropwise, and the vial was placed in the microwave for 15 minutes at a temperature of 800 C. The crude mixture was treated with potassium carbonate in cold water (70 ml, 2×) and stirred for 10 minutes. Suction filtration was then carried out to obtain the crude product. Two steps of recrystallization were carried out, the first one using DCM (60 ml) and charcoal (spoonful) with heating followed by gravity filtration, and the second one was carried out to the solid produced in the first step using methanol (15 ml) that furnished the product.
O-(4-Benzoylphenyl)-2-furester or O-[4-(phenylcarbonyl) phenyl] furan-2-carboxylate (b1). This derivative was synthesized following procedure A
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) The starting material 4-hydroxybenzophenone (5.05 mmol) was placed in a round bottom flask under the fume hood. Pyridine (8.65 mmol, acylation catalyst) and triethylamine (5 mmol, acid scavenger) were added and followed by the addition of furoyl chloride (10.15 mmol) gradually to the reaction mixture at room temperature. The reaction mixture was stirred under reflux for 24 h at 75 °C. After 24 h, the TLC showed that the ester was formed as a major product, with a different spot on the baseline for the hydrolyzed furoic acid. Cold water was added to the solid mixture (50 ml), and the pH was adjusted to 10 using potassium carbonate (K2CO3) to remove the excess starting material. A white suspension was filtered by suction filtration using a Buchner funnel under a vacuum to obtain the product of interest. Recrystallization from methanol has furnished the ester as off-white crystals. (0.96 g, 66%); Rf: 0.95 (DCM
The starting material 4-hydroxybenzophenone (5.05 mmol) was placed in a round bottom flask under the fume hood. Pyridine (8.65 mmol, acylation catalyst) and triethylamine (5 mmol, acid scavenger) were added and followed by the addition of furoyl chloride (10.15 mmol) gradually to the reaction mixture at room temperature. The reaction mixture was stirred under reflux for 24 h at 75 °C. After 24 h, the TLC showed that the ester was formed as a major product, with a different spot on the baseline for the hydrolyzed furoic acid. Cold water was added to the solid mixture (50 ml), and the pH was adjusted to 10 using potassium carbonate (K2CO3) to remove the excess starting material. A white suspension was filtered by suction filtration using a Buchner funnel under a vacuum to obtain the product of interest. Recrystallization from methanol has furnished the ester as off-white crystals. (0.96 g, 66%); Rf: 0.95 (DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 124–125 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.09 (br s, 1H, H-5), 7.81 (d, J = 7.4 Hz, 2H, Ar–H-3′ & 5′), 7.72 (d, J = 6.50 Hz, 2H, Ar–H-2′ & 6′), 7.64 (m, 1H, H-4′′), 7.58 (br s, 1H, Ar–H-3), 7.54 (d, J = 6.55 Hz, 2H, Ar–H-2′′ & 6′′), 7.44 (d, J = 7.4 Hz, 2H, Ar–H-3′′ & 5′′), 6.78 (br s, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 195.19 (CO-ketone), 156.33 (COO-ester), 153.58 (C-2), 149.41 (C-5), 143.16 (C-1′), 137.38 (C-1′′), 135.26 (C-4′), 133.20 (C-4′′), 131.88, 130.05, 129.08, 122.58 (C-2′,3′,5′,6′,2′′,3′′,5′′ and 6′′), 121.15 (C-3), 113.34 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 315.06278 (C18H12NaO4) requires 315.06333.
2); m.p.: 124–125 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.09 (br s, 1H, H-5), 7.81 (d, J = 7.4 Hz, 2H, Ar–H-3′ & 5′), 7.72 (d, J = 6.50 Hz, 2H, Ar–H-2′ & 6′), 7.64 (m, 1H, H-4′′), 7.58 (br s, 1H, Ar–H-3), 7.54 (d, J = 6.55 Hz, 2H, Ar–H-2′′ & 6′′), 7.44 (d, J = 7.4 Hz, 2H, Ar–H-3′′ & 5′′), 6.78 (br s, 1H, Ar–H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 195.19 (CO-ketone), 156.33 (COO-ester), 153.58 (C-2), 149.41 (C-5), 143.16 (C-1′), 137.38 (C-1′′), 135.26 (C-4′), 133.20 (C-4′′), 131.88, 130.05, 129.08, 122.58 (C-2′,3′,5′,6′,2′′,3′′,5′′ and 6′′), 121.15 (C-3), 113.34 (C-4) ppm. HRMS (ESI, positive mode): m/z (M+ + Na+): found 315.06278 (C18H12NaO4) requires 315.06333.
2-Acetylphenyl furan-2-carboxylate (b2). White pure product collected. (0.4 g, 21%); Rf: 0.71 (DCM
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 98
MeOH, 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); m.p.: 91–94 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.07 (br s, 1H, H-5), 7.91 (d, J = 7.7 Hz, 1H, H-3′), 7.64 (t, J = 7.7, 7.7 Hz, 1H, H-5′), 7.53 (d, J = 3.2, 1H, H-3), 7.42 (t, J = 7.55, 7.55 Hz, 1H, H-4′), 7.33 (d, J = 8.05, 1H, H-6′), 6.76 (d, J = 1.5 Hz, 1H, H-4), 2.48 (br s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ = 197.83 (CO-keton), 156.61 (CO-ester), 149.15 (C-5), 148.16 (C-2), 143.56 (C-1′), 134.15 (C-3′), 131.25 (C-2′), 130.90 (C-5′), 127.05 (C-4′), 124.47 (C-6′), 120.83 (C-3), 113.23 (C-4), 29.98 (CH3) ppm.
2); m.p.: 91–94 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.07 (br s, 1H, H-5), 7.91 (d, J = 7.7 Hz, 1H, H-3′), 7.64 (t, J = 7.7, 7.7 Hz, 1H, H-5′), 7.53 (d, J = 3.2, 1H, H-3), 7.42 (t, J = 7.55, 7.55 Hz, 1H, H-4′), 7.33 (d, J = 8.05, 1H, H-6′), 6.76 (d, J = 1.5 Hz, 1H, H-4), 2.48 (br s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ = 197.83 (CO-keton), 156.61 (CO-ester), 149.15 (C-5), 148.16 (C-2), 143.56 (C-1′), 134.15 (C-3′), 131.25 (C-2′), 130.90 (C-5′), 127.05 (C-4′), 124.47 (C-6′), 120.83 (C-3), 113.23 (C-4), 29.98 (CH3) ppm.
Phenylfuran-2-carboxylate (b3). White pure solid. (1.7 g, 43%); Rf: 0.88 (DCM only); m.p.: 44–47 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.06 (br s, 1H, H-5), 7.52 (d, J = 3.2 Hz, 1H, H-3), 7.42 (dd, J = 7.9, 7.55 Hz, 2H, H-3′ & H5′), 7.27 (dd, J = 7.15, 7.5 Hz, 1H, H-4′), 7.22 (d, J = 7.85 Hz, 2H, H-2′ & 6′), 6.75 (d, J = 1.6 Hz, 1H, H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 156.84 (COO-ester), 150.35 (C-2), 149.08 (C-5), 143.49 (C-1′), 130.09 (C-3′ & 5′), 126.62 (C-4′), 122.32 (C-2′ & 6′), 120.62 (C-3), 113.20 (C-4) ppm.
3,5-Dimethoxyphenyl furan-2-carboxylate (b4). Light brown pure crystals formed. (1.08 g, 67.14%); Rf: 0.47 (DCM only); m.p.: 78–80 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.05 (s, 1H, H-5), 7.50 (d, J = 3.2 Hz, 1H, H-3), 6.74 (d, J = 1.45 Hz, 1H, H-4), 6.43 (br s, 2H, H-2′ & 6′), 6.40 (br s, 1H, H-4′), 3.70 (br s, 6H, 2OCH3); 13C-NMR (125 MHz, DMSO-d6): δ = 161.31 (C-3′ & 5′), 156.58 (CO-ester), 151.94 (C-2), 149.05 (C-5), 143.48 (C-1′), 120.59 (C-3), 113.19 (C-4), 100.92 (C-2′ & 6′), 98.69 (C-4′), 55.97 (2OCH3) ppm.
Butylfuran-2-carboxylate (b5). A dark brown oily pure product was obtained. (0.84 g, 30.5%); Rf: 0.81 (DCM only); 1H-NMR (500 MHz, DMSO-d6): δ = 7.91 (br s, 1H, H-5), 7.23 (d, J = 3.3 Hz, 1H, H-3), 6.63 (t, J = 1.65 Hz, 1H, H-4), 4.18 (t, J = 6.45 Hz, 2H, CH2-1′), 1.59 (m, J = 7.2 Hz, 2H, CH2-2′), 1.33 (m, J = 7.4 Hz, 2H, CH2-3′), 0.86 (t, J = 7.4 Hz, 3H, CH3-4′); 13C-NMR (125 MHz, DMSO-d6): δ = 158.43 (CO-ester), 147.97 (C-5), 144.41 (C-2), 118.65 (C-3), 112.69 (C-4), 64.57 (CH2-1′), 30.66 (CH2-2′), 19.07 (CH2-3′), 13.96 (CH3-4′) ppm.
4-Chlorophenyl furan-2-carboxylate (b6). The fluffy white pure product formed. (0.85 g, 50%); Rf: 0.95 (DCM only); m.p.: 86–90 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.07 (br s, 1H, H-5), 7.53 (d, J = 3.4 Hz, 1H, H-3), 7.47 (d, J = 8.65 Hz, 2H, H-3′ & 5′), 7.29 (d, J = 8.65 Hz, 2H, H-2′ & 6′), 6.76 (d, J = 1.55 Hz, 1H, H-4); 13C-NMR (125 MHz, DMSO-d6): δ = 156.59 (CO-ester), 149.24 (C-2), 149.11 (C-5), 143.24 (C-1′), 130.81 (C-4′), 129.99 (C-3′ & 5′), 124.31 (C-2′ & 6′), 120.91 (C-3), 113.26 (C-4) ppm.
4-Methylphenyl furan-2-carboxylate or p-tolylfuran-2-carboxylate (b7). White pure solid formed. (1.04 g, 56%); Rf: 0.88 (DCM only); m.p.: 53–56 °C; 1H-NMR (500 MHz, DMSO-d6): δ = 8.04 (br s, 1H, H-5), 7.50 (d, J = 3.4 Hz, 1H, H-3), 7.20 (d, J = 8.2 Hz, 2H, H-3′ & H5′), 7.09 (d, J = 8.3 Hz, 2H, H-2′ & 6′), 6.74 (d, J = 1.7 Hz, 1H, H-4), 2.28 (s, 3H, CH3); 13C-NMR (125 MHz, DMSO-d6): δ = 156.98 (COO-ester), 148.99 (C-5), 148.13 (C-2), 143.57 (C-1′), 135.82 (C-4′), 130.41 (C-3′ & 5′), 121.97 (C-2′ & 6′), 120.47 (C-3), 113.16 (C-4), 20.86 (CH3) ppm.
An experimental model of hyperlipidemia
Animals and treatments
Adult male Wistar rats, weighing about 200 g, were bred in the animal house (faculty of pharmacy, Al-Zaytoonah University, Amman, Jordan). They were provided ad-libitum (without restriction) access only to tap water throughout the experimental duration. Rats were kept in a 12 h light–dark cycle under settings of constant humidity and temperature (22 ± 2 °C). All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of “Al-Zaytoonah University, Amman, Jordan” and experiments were approved by the Research Ethics Committee (IRB 22/5/2020-2021).12,15 Triton was dissolved in distilled water and administered intraperitoneal to the rats (200 mg kg−1 body weight) to induce hyperlipidemia. Dose selection was based on previous work by our group and literature recommendations.8,12–14,33Experimental design
Overnight fasted rats were randomly divided into five major groups of five animals. The first group, a standard control group (NG), received an intraperitoneal administration of normal saline. The second DMSO control group (DCG) received an intraperitoneal administration of 4% DMSO, while the third hyperlipidemic group (HG) received an intraperitoneal injection of Triton (dissolved in distilled water). The fourth group (BZF) was intraperitoneally injected with Triton and intragastrically treated with bezafibrate (100 mg kg−1 body weight) dissolved in 4% DMSO.10,34 The fifth group was carried out for each compound with its given code. Rats were intraperitoneally injected with Triton and intragastrically treated with 1 ml of the target compounds (20 mg kg−1 body weight) dissolved in 4% DMSO/distilled water or corn oil.10,12 This procedure was utilized to prepare compounds 1 to 9 using distilled water, while compounds 10–19 were dissolved in corn oil. After 7 h of treatments, rats were anesthetized with diethyl ether, and blood was collected from the renal artery.8,9 The blood samples were immediately centrifuged (3000 rpm for 10 min), and the serum was used for lipid analysis by an enzymatic method with an automatic analyzer (Model Erba XL-300, Germany, Mannheim, Germany).Conflicts of interest
There are no conflicts to declare.References
- W. A. H. M. Dayyih and Z. Zakareia, Influence of Castor Oil on Glycated Hemoglobin (HbA1c) on Induced Type 2 Diabetes Mellitus in Rats, Jordan J. Pharm. Sci., 2021, 341–349 Search PubMed.
- M. Farnier and J. Davignon, Current and future treatment of hyperlipidemia, Am. J. Cardiol., 1998, 82(4), 3J–10J CrossRef CAS PubMed.
- J. C. Fruchart, F. Sacks, M. P. Hermans, G. Assmann, W. V. Brown, R. Ceska and T. Kadowaki, The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia, Am. J. Cardiol., 2008, 102(10), 1K–34K CrossRef PubMed.
- P. Nimkuntod and P. Tongdee, Association between subclinical atherosclerosis among hyperlipidemia and healthy subjects, J. Med. Assoc. Thailand, 2015, 98(Suppl 4), S51–S57 Search PubMed.
- D. Bhatnagar, Lipid-lowering drugs in the management of hyperlipidaemia, Pharmacol. Ther., 1998, 79(3), 205–230 CrossRef CAS PubMed.
- K. S. Jain, M. K. Kathiravan, R. S. Somani and C. J. Shishoo, The biology and chemistry of hyperlipidemia, Bioorg. Med. Chem., 2007, 15(14), 4674–4699 CrossRef CAS.
- G. F. Shattat, A review article on hyperlipidemia: types, treatments and new drug targets, Biomed. Pharmacol. J., 2015, 7(1), 399–409 Search PubMed.
- T. Al-Qirim, M. Shahwan, G. Shattat, Y. Al-Hiari, G. A. Sheikha and S. Zaidi, Pharmacological evaluation of novel indole-2-carboxamides as potent lipid-lowering agents in Triton-WR-1339-induced hyperlipidemic rats, Zeitschrift für Naturforschung C, 2009, 64(9–10), 619–625 CrossRef CAS PubMed.
- G. Shattat, T. Al-Qirim, K. Sweidan, M. Shahwan, W. El-Huneidi and Y. Al-Hiari, The hypolipidemic activity of novel benzofuran-2-carboxamide derivatives in Triton WR-1339-induced hyperlipidemic rats: a comparison with bezafibrate, J. Enzyme Inhib. Med. Chem., 2010, 25(6), 751–755 CrossRef CAS PubMed.
- M. Al-Najdawi, Y. Hiari, T. Qirim, G. Shattat, M. Al-Zweri and G. A. Sheikha, Synthesis and pharmacological evaluation of novel unsubstituted indole-anthraquinone carboxamide derivatives as potent antihyperlipidemic agents, Zeitschrift für Naturforschung C, 2014, 69(1–2), 21–28 CrossRef CAS PubMed.
- F. S. Ghassan, M. A. Ghassan, M. Tariq, R. Huwaitat, E. H. Waseem, R. A. KHALAF and L. Hamadaneh, Novel Pyrrole Derivatives as Potent lipid-Lowering Agents in Triton-WR-1339-Induced Hyperlipidemic Rats, Lat. Am. J. Pharm., 2015, 34(6), 1258–1264 Search PubMed.
- R. Abu Farha, Y. Bustanji, Y. Al-Hiari, T. Al-Qirim, G. Abu Shiekha and R. Albashiti, Lipid lowering activity of novel N-(benzoylphenyl) pyridine-3-carboxamide derivatives in Triton WR-1339-induced hyperlipidemic rats, J. Enzyme Inhib. Med. Chem., 2016, 31, 138–144 CrossRef CAS PubMed.
- R. Abu Farha, Y. Bustanji, Y. Al-Hiari, S. Bardaweel, T. Al-Qirim, G. Abu Sheikha and R. Albashiti, Pharmacological Evaluation of Novel Isonicotinic Carboxamide Derivatives as Potential Anti-Hyperlipidemic and Antioxidant Agents, Arch. Pharm., 2017, 350(10), e1700024 CrossRef.
- S. Hikmat, T. Al-qirim, D. Alkabbani, G. Shattat, G. A. Sheikha, D. Sabbah and Y. Al-hiari, Synthesis and in vivo anti-hyperlipidemic activity of novel n-benzoylphenyl-2-furamide derivatives in Wistar rats, Trop. J. Pharm. Res., 2017, 16(1), 193–201 CrossRef CAS.
- G. A. Sheikha, M. M. Bkhaitan, H. Kalloush, L. Hamadneh, R. A. Khalaf, T. Al-Qirim and Y. Al-Hiari, Synthesis of Novel Benzimidazole-2-carboxamide Derivatives and in Vivo Antihyperlipidemic Activity Evaluation, Chem. Pharm. Bull., 2018, 66(4), 423–426 CrossRef CAS PubMed.
- C. A. Montalbetti and V. Falque, Amide bond formation and peptide coupling, Tetrahedron, 2005, 61(46), 10827–10852 CrossRef CAS.
- k Sweidan, G. Idrees, L. Abu-Qatouseh, M. Nawaz, M. Khanfar, R. Joshi, E. Mallah and M. S. Mubarak, Synthesis, Characterization, and Antimicrobial Evaluation of New Furan-2-Carboxamide Derivatives, Lett. Org. Chem., 2022, 19(4), 314–325 CrossRef CAS.
- A. Al-Sammarra, M. Al-Najdawi, M. M. Saleh, Y. M. Al-Hiar and R. Al-Bashiti, Synthesis and Biological Evaluation of Furyl-Carboxamide Derivatives as Potential Anticancer Agents, J. Turk. Chem. Soc., Sect. A, 2022, 9(3), 909–918 CrossRef.
- S. Hwang, S. Y. Choi, J. H. Lee, S. Kim, J. In and S. K. Ha, et al., Identification of a potent and noncytotoxic inhibitor of melanin production, Bioorg. Med. Chem., 2010, 18(15), 5602–5609 CrossRef CAS PubMed.
- B. L. Miller, B. R. McNaughton, P. Gareiss, and G. Scott, Small-molecule modulators of melanin expression, Google Patents US 9162984 B2 2015-10-20, 2015.
- S. Sun, D. Guo, F. Li and J. Wang, From imines to amides via NHC-mediated oxidation, Org. Chem. Front., 2022, 9(2), 356–363 RSC.
- A. An, W. Song, Z. Peng, Y. Zhang and W. Dong, Transition-Metal-Free Hypervalent Iodine(III) Reagent-Promoted Site-Selective Solid-Phase Synthesis of Mononitroarylamines, ChemistrySelect, 2018, 3(45), 12946–12950 CrossRef.
- M. Martínez, N. Rodríguez, R. Gómez Arrayás and J. C. Carretero, Copper-catalyzed ortho-C–H amination of protected anilines with secondary amines, Chem. Commun., 2014, 50(21), 2801–2803 RSC.
- V. F. de Souza AAX, G. S. Coelho, J. P. A. Sales, A. J. Romanha, S. M. F. Murta, C. M. Carneiro and J. G. Taylor, Synthesis of 3, 5-diarylisoxazole derivatives and evaluation of in vitro trypanocidal activity, J. Braz. Chem. Soc., 2018, 29, 269–277 Search PubMed.
- N. Rajendran, K. Kamaraj, S. Janakiraman, M. Saral A, P. H. Dixneuf and C. B. Bheeter, A sustainable approach to amide bond formation via CO bond cleavage of simple esters in water, ChemRxiv, 2022, 24(21), 3855–3860 Search PubMed.
- X. Yang, Y. Guo, T. Hong'en, R. Liu and R. Zhou, Metal-free acceptorless dehydrogenative cross-coupling of aldehydes/alcohols with alcohols, Green Chem., 2023, 25(4), 1672–1678 RSC.
- S. B. S. Alwar, Catalytic Activity in the Hydrolysis of Phenyl Esters of [alpha]-Furoic Acid, Asian J. Chem., 2008, 20(1), 495 CAS.
- K. Thakar and A. Padhye, Synthesis and antifungal activity of 3-furyl-and 3-thienyl-substituted-1, 2-benzisoxazoles, J. Indian Chem. Soc., 1985, 16(26), 715–720 Search PubMed.
- M. T. Alnajdawi, The synthesis of novel heterocyclic carboxamide derivatives and evaluation of their antihyperlipidemic and antioxidant activity, PhD dissertation, Amman, The University of Jordan, 2011.
- N. Katsiki, D. Nikolic, G. Montalto, M. Banach, D. P. Mikhailidis and M. Rizzo, The role of fibrate treatment in dyslipidemia: an overview, Curr. Pharm. Des., 2013, 19(17), 3124–3131 CrossRef CAS PubMed.
- G. Steiner, Atherosclerosis in type 2 diabetes: a role for fibrate therapy?, Diabetes Vasc. Dis. Res., 2007, 4(4), 368–374 CrossRef.
- L. Hamadneh, L. Al-Essa, S. Hikmat, T. Al-Qirim, G. A. Sheikha, Y. Al-Hiari and G. Shattat, N-(3-Benzoylphenyl)-1H-Indole-2-Carboxamide decreases triglyceride levels by downregulation of Apoc3 gene expression in acute hyperlipidemic rat model, Mol. Cell. Biochem., 2017, 431(1–2), 133–138 CrossRef CAS PubMed.
- J. B. Majithiya, A. N. Parmar and R. Balaraman, Effect of Curcumin on Triton WR 1339 induced hypercholesterolemia in mice, Indian J. Pharmacol., 2004, 36(6), 381–384 Search PubMed.
- T. Nakajima, N. Tanaka, H. Kanbe, A. Hara, Y. Kamijo, X. Zhang and T. Aoyama, Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: a novel peroxisome proliferator-activated receptor α-independent mechanism, Mol. Pharmacol., 2009, 75(4), 782–792 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03581f |
| ‡ Both authors contributed equally. |
| § Author for correspondence and reprint requests. |
| This journal is © The Royal Society of Chemistry 2023 |