Open Access Article
Open Access ArticleRecent advances in metal-family flame retardants: a review
Junwei Li†
ab,
Haihan Zhao†
ab,
Huaiyin Liuab,
Jichang Sunab,
Jing Wuab,
Quanyi Liu
 *ab,
Yun Zheng
*ab,
Yun Zheng
 *c and
Penglun Zheng
*c and
Penglun Zheng
 *ab
*ab
aCollege of Civil Aviation Safety Engineering, Civil Aviation Flight University of China, Guanghan 618307, P. R. China. E-mail: zhengpenglun@cafuc.edu.cn
bCivil Aircraft Fire Science and Safety Engineering Key Laboratory of Sichuan Province, Guanghan 618307, P. R. China. E-mail: quanyiliu2005@cafuc.edu.cn
cKey Laboratory of Optoelectronic Chemical Materials and Devices, Ministry of Education, Jianghan University, Wuhan 430056, P. R. China. E-mail: zhengyun@jhun.edu.cn
First published on 26th July 2023
Abstract
The use of polymer materials is inextricably linked to our manufacturing life. However, most of them are easily combusted in the air and the combustion process generates a large amount of toxic fumes and dangerous smoke. This can result in injuries and property damage, as well as limiting their use. It is essential to enhance the flame-retardant properties and smoke suppression performance by using multiple flame retardants. Metal-based flame retardants have a unique chemical composition. They are environmentally friendly flame retardants, which can impart good smoke suppression, flame retardancy to polymers and further reduce the production of toxic gases. The differences in the compounds formed between the transition metals and the main group metals make them act differently as flame retardants for polymers. As a result, this study presents the research progress and flame-retardant mechanism of flame-retardant polymers for flame retardants from different groups of metals in the periodic table of elements in a systematic manner. In view of the differences between the main group metals and transition metals, the mechanism of their application in flame retardant polymer materials is carefully detailed, as are their distinct advantages and disadvantages. And ultimately, prospects for the development of transition metals and main group metals are outlined. It is hoped that this paper will provide valuable references and insights for scholars in the field.
1. Introduction
Synthetic and natural polymers have been widely used in many aspects of life as a result of technological advancement and rising productivity levels.1–5 Their molecular chains tend to break at high temperatures, producing various combustible gases. These polymers are highly susceptible to airborne combustion,6,7 which significantly limits their application. Furthermore, when polymers burn, a large amount of smoke and poisonous gases are released,8,9 making firefighting and rescue work difficult. Therefore, various flame retardants are typically added to the polymeric matrix to increase the fire safety of polymeric materials.10–13 Conventional flame retardants include halogen, phosphorus and nitrogen-based compounds. Halogen flame retardants produce persistent pollutants that cause irreversible and permanent pollution of the environment. And a lot of smoke and toxic gases will be released when a fire breaks out.14–16 Halogen-based flame retardants are increasingly being phased out due to the rising demand for environmental protection. There is a preference for more environmentally friendly non-halogen flame retardants,17,18 such as phosphorus and nitrogen-based. Although phosphorus19 and nitrogen-based flame retardants are effective,20,21 their combustion products are accompanied by a large volume of smoke.22 Consequently, more focus is being placed on creating environmentally acceptable flame retardants with excellent flame retardancy and they can also minimize the toxicity of smoke.In recent years, numerous studies have been conducted on different flame retardants that are utilized to enhance the flame retardancy and smoke suppression qualities of polymeric materials. Among these, metals and their compounds23 are favored by researchers for their excellent smoke-suppression properties.24–26 This can be achieved by altering the thermal degradation pattern of the polymer to provide a physical barrier and cooling. It can also promote char formation by suppressing flames in the gas phase through thermal decomposition to produce non-combustible gas dilution.27,28 This increases flame-resistance of the polymer, and they can also reduce emission of smoke and hazardous gases during polymer burning.29–31 The earliest metal-based flame retardants developed were metal hydroxides, most commonly aluminum trihydroxide (ATH)32,33 and magnesium hydroxide (MH).34–36 Jiao et al. added MH to ethylene vinyl acetate (EVA) as a flame retardant to improve its fire and smoke suppression capabilities.37 Meanwhile, the residual content of coke was also increased. In addition to the significant flame-retarding properties of metal hydroxides, metal oxides38,39 also offer outstanding performance.40 They are often used as co-effectors,41,42 with literature reporting special effects of transition metal oxides such as copper oxide,43,44 nickel oxide45 and so on. For example, Gogoi et al. synthesised a vegetable oil alkyd resin with epoxy resin (EP) as the primary raw material from Jatropha curcas oil.46 On this basis, alkyd/epoxy/NiO nanocomposites with various NiO nanoparticle mass percentages were created using a physical stirring method. The results showed that NiO nanoparticles are helpful in thermal-stability, and fire-resistant characteristics. Gradually, it was discovered that most metal oxides and hydroxides, although widely used but poorly compatible with polymers, lead to severe deterioration of mechanical properties in direct blending. So people started to focus on metal salts47 as flame retardants because they can access flame retardants by chemical bonding,48,49 thus overcoming the defects of deteriorating mechanical properties in a natural blending of hydroxides. It can also modify fabric materials to improve their thermal stability properties. For example, Zhang et al. prepared water-soluble fibres with water-soluble flame-retardant silk by using titanium sulfate, ferrous sulfate,50 and casein phosphopeptide as the primary raw materials. The results showed that the treated fabric had strong charring qualities and high thermal stability, which enhanced the flame-retardant performance of the fabric. It is due to the fact that metal ions can be used as reaction units to gain access to the flame retardant or polymer matrix, and they can also operate better as catalysts for carbon cross-linking and dehydrogenation processes. In addition, partially metastable metal ions can be used as free radical trapping agents or oxidation catalysts in the gas phase flame retardant process.51,52 In the past few years, with the continuous research and exploration of metal flame retardants, a new type of metal flame retardant material called metal organic frameworks (MOFs)53,54 has been developed. This is a new type of porous material made by connecting inorganic metal nodes with organic ligands. People take advantage of its adjustable structure, good thermal stability, excellent adsorption and catalytic properties and organic–inorganic hybridization properties to prepare polymer/MOF flame retardant composites.55,56 Sai et al. synthesized a high-porosity zirconium-based metal organic skeletal material (Zr-BDC).57 The results indicated that Zr-BDC has various effects such as catalytic oxidation, isomerization, and catalytic carbon formation of polycarbonate (PC) matrix, resulting in a dense, highly graphitized carbon layer. That means it is helpful for rescue and escapes in a real fire. As a result, metal-based flame retardants in flame retardancy and smoke suppression with low toxicity can play an excellent effect, keep developing more efficient flame retardants and smoke suppression and low toxicity of environmentally friendly flame-retardant additives are well worthy of being the focus of future research.
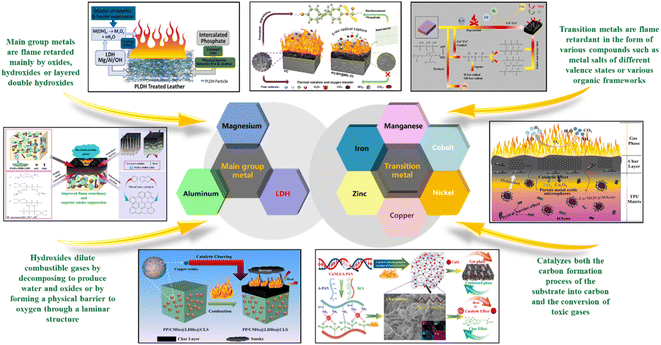
Based on the above, it is easy to find that different metal elements have different physical and chemical properties, and the compounds that can form flame retardants also differ. The electron layers of magnesium and aluminum, which are located in the main group metals of the periodic table. And they are basically in the occupied state, with fewer empty orbitals. The complexes they form are less stable and less diverse, so they generally form oxides or hydroxides for flame retardation of polymers. In contrast to the saturated state of the electronic layers of the main group elements, the electrons in the transition metals' second or penultimate third layer are unfilled and belong to an unstable structure. Since the formation of complexes requires sufficient empty orbitals to accommodate the lone pair of electrons of the ligand, the complexes formed by transition metals are much more stable than the main group metal complexes. The compounds formed are also more diverse, comprising stable oxides, hydroxides, and metal salts of different valence states. Therefore, both transition and main group metals can form corresponding stable compounds for flame and smoke suppression of polymers. In this review, based on the distribution of different metallic elements in the periodic table of elements, the differences in using different groups of metals as flame retardants are discussed and analyzed. And we hope to provide new ideas for further development of functional metal flame retardants with high efficiency, low smoke suppression and low toxicity in the future.
2. Flame-retardation mechanisms
Due to the specific chemical composition of the metal-based flame retardants themselves, this makes it possible to effectively enhance the flame retardancy and smoke suppressant properties of polymeric materials. When the polymer encounters heat and thermal decomposition, the metal oxide generated by the metal adheres to the surface of the matrix, playing a protective and physical barrier to prevent further combustion of the composite. Similarly, the thermal decomposition of metal hydroxides releases some non-combustible gases, such as H2O, which can achieve the effect of diluting the concentration of various combustible gases generated by combustion. In particular, certain metals may further catalyse the carbonation process of the polymer, thereby preventing combustion and promoting the tight formation of the carbon layer may reduce heat release. The dense carbon layer greatly reduces the area of the polymer exposed to air, consequently limiting heat release and smoke generation.58–60 The flame-retardant mechanism of metal flame retardants in polymers has been extensively studied61,62 and can be summarized in the following four main aspects.Metal-based flame retardants can catalyze a dense, intact carbon layer on the polymer matrix. It produces a highly graphitized structure that is thermally stable and prevents the release and diffusion of heat, while also providing a physical barrier against the spread of smoke. Peng et al. used solvent evaporation to prepare a series of metal oxides loaded on activated carbon (AC-MO) (Fe2O3, CuO, ZnO).63 Additionally, the vulcanization, combustion, thermal stability, and ageing characteristics of AC-MO and the flame-retardant ethylene vinylacetate copolymer rubber made from ammonium polyphosphate/dipentaerythritol synergistically expanded were examined. Fe2O3 loaded on AC improved its catalytic charring effect and enhanced the integrity and densification of the flame-retardant ethylene vinylacetate copolymer rubber char residue. Li et al. reported the reaction of phosphomolybdic acid (PMA) anions with three different metal ions (Ni, Na and Zn) to generate phosphomolybdate (PMos) for use as an enhanced flame retardant mechanism for polypropylene/intumescent flame retardants (PP/IFR).64 The results showed that PMos can facilitate the reaction between IFRs, slow down the exothermic rate during the burning process, and develop a charcoal layer with superior barrier effect. Yang et al. reported the use of tannic acid (TA)-FeIII MPNs as an efficient green flame retardant designed for the flame retardant mechanism of coatings on polyurethane (PU) foam surfaces.65 As can be seen from the Fig. 1, due to the excellent carbonization properties of FeIII, the significant TA radical scavenging ability and the cohesive phase effect of the carbonization process during catalytic combustion. The encapsulated TA-FeIII MPNs also successfully suppressed the heat release rate (HRR) and total flue gas volume, reducing the volatilization of harmful gases during combustion.
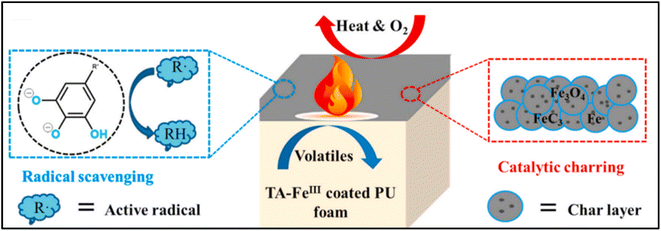 | ||
| Fig. 1 Mechanism scheme of TA-FeIII MPN flame retardant. Reproduced with permission from ref. 65. Copyright (2021) Elsevier. | ||
Secondly, the decomposition of metal hydroxides produces incombustible gases, which potently reduce the concentration of combustible gases, thereby inhibiting the release of smoke. Later, the metal hydroxides were further improved, and layered double hydroxide (LDH) was developed. Interestingly the layered structure also acts as a barrier, isolating the substrate from heat and preventing further combustion of the substrate. Ye et al. examined the cooperative effect of borosiloxane (BSil) and magnesium hydroxide in EVA/MH blends of halogen-free flame retardants.66 The mechanism of the synergistic effect of BSil and MH can be described as BSil promotes the formation of a dense burnt layer, prevents cracking of the burnt layer and actively defends the substrate polymer from further combustion. Jiang et al. successfully obtained Ni–Al LDH and used it as synergist to improve triazine-based IFR's flame retardancy and smoke suppression properties.29 The flame-retardant mechanism is shown in Fig. 2. When NiAl-LDH is added to the PP/IFR system, the alkali metal can act as a cross-linking agent, effectively improving the graphitization and thermal stability of the carbon slag and a significant increase in the residual amount of PP/IFR/Ni–Al LDH. Furthermore, the introduction of LDH and the introduction of non-combustible gases (NH3 and H2O etc.) from the decomposition of the composite effectively act as a dilution agent in the gas.
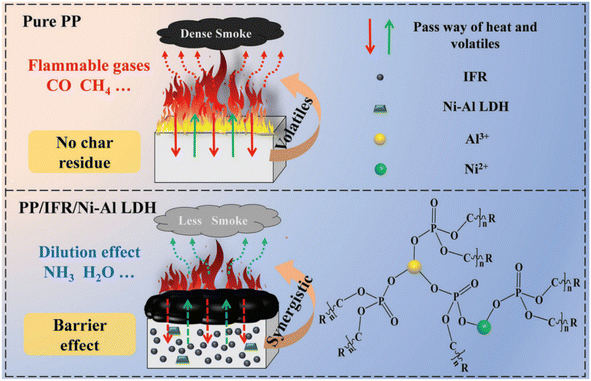 | ||
| Fig. 2 Flame-retardant and smoke-suppression mechanism of PP flame retardant composites. Reproduced with permission from ref. 29. Copyright (2021) The Authors. Macromolecular Materials and Engineering published by Wiley-VCH GmbH. | ||
Inorganic metal compounds formed by combining metals with organic acids can also exert a flame-retardant effect by decomposing to produce a corresponding metal oxide that covers the surface of the polymer matrix, protecting it from further combustion. Cheng et al. compounded melamine, melamine uric acid and polyphenylene ether with aluminum hypophosphite for flame retardant thermoplastic elastomers (blends of SEBS and polyolefin).67 The TPE-S containing 16 wt% ATH, 20 wt% cyanuric acid melamine and 10 wt% poly (epoxy phenylene) passed the UL-94 test with V-0 rating and a limiting oxygen index (LOI) value of 28.2%. The flame-retardant mechanism model for the composite is shown in Fig. 3. When the temperature is raised to 445 °C, the polymer decomposes to form AlPO4. Then the melamine cyanurate (MCA) decomposes to melt and release NH3, diluting the internal combustible gases and forming an inorganic layer. Aluminum hypophosphite, MCA and polyphenylene oxide contribute to the formation of swelling coke, which is dense and continuous. The composite decomposes to form MgO, ZnO and borophosphate in addition to concentrated aluminium phosphate, which, combined with the water generated, further dilutes the concentration of combustible gases.
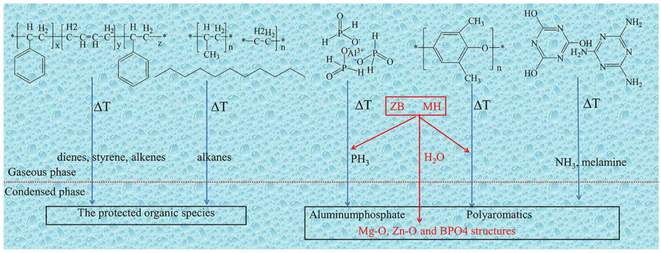 | ||
| Fig. 3 Schematic diagram of proposed flame-retardant mechanism model of TPE-S/AHP/MCA/PPO composite with replacement of ZB and MH. Reproduced with permission from ref. 67. Copyright (2019) SAGE Publications. | ||
Finally, the emerging MOFs show great potential for flame retardant applications by exploiting the catalytic carbon formation of metals and the capture of free radicals by organic flame-retardant compounds. Huang et al. designed a novel MOF through the synergistic interaction of MOF (NH2-MIL-101(Al)) and phosphorus-containing nitrogen ionic liquids ([DPP-NC(3) [PMO]) composite.68 As the Fig. 4 shows, in the gas phase, the diphenylphosphine group in the imidazolium cation of ionic liquid (IL) containing phosphorus compounds reacts with the reactive radical to inhibit flame propagation. And the catalytic oxidation of carbon monoxide (CO) by the metal cluster in NH2-MIL-101(Al) can effectively reduce the heat and CO release. In the coalescence phase, the imidazole cation and phosphomolybdate anion of IL act as catalysts to induce cross-linking, forming a stable char layer. These two effects give IL@NH2-MIL-101(Al) good refractoriness.
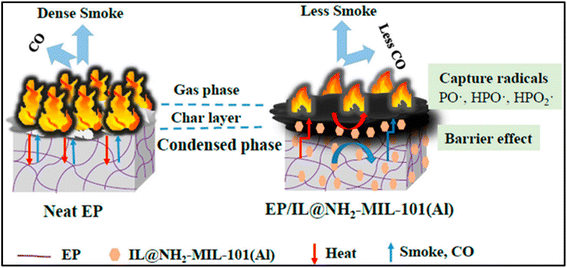 | ||
| Fig. 4 Schematic illustration of the proposed mechanism of EP/IL@NH2-MIL-101(Al). Reproduced with permission from ref. 68. Copyright (2019) Polymers. | ||
Therefore, in the burning process of polymers, all metal flame retardants can play a role in catalyzing the carbon generation of the matrix. It is worth noting that some metal hydroxides and inorganic metal compounds can also produce non-combustible gases by decomposition to dilute combustible gases and generate the corresponding mental oxide to cover the surface of the substrate and protect it from further combustion. At the same time, the metal-organic framework can also absorb some toxic gases because of its porous structure. The metal–organic framework can also adsorb some poisonous gases due to its porous structure. Overall, catalytic carbon formation of metal-based flame retardants plays a key role in enhancing flame retardancy and smoke inhibition. At the same time, it can also catalyze the conversion of CO, reducing the number of toxic gases released.
3. Classification of metal-based flame retardants
In the periodic table of elements, metal elements commonly used in flame retardant research are divided into transition and main group metals. Different metal elements can form different metal compounds, which play different roles when used as flame retardants. Therefore, the metal-based flame retardants were divided into common transition metal flame retardants, rare transition metal flame retardants, main group metal flame retardants and multi-metal compound flame retardants. This way better investigates the similarities and differences between the different groups of metal elements in the periodic table of elements to form metal compounds used as flame retardants.3.1. Common transition metal flame retardants
As transition elements in the periodic table, their electrons form chemical bonds when chemical reactions occur and can exhibit a wide range of oxidation states. Transition elements generally form ionic bonds when forming compounds in the low oxidation state and are prone to hydrate, and covalent bonds are formed when forming compounds in the high oxidation state. Transition elements have empty d orbitals that can be used for bonding and a high charge/radius ratio, readily forming stable coordination compounds with various ligands. For example, common iron, copper and zinc can be used with organic ligands to form metal complexes or MOFs for enhancing the flame retardancy of polymers. The performance of some transition metal-based flame retardants in different polymeric materials in recent years is listed in Table 1.| Sample | Polymers | Adding amount (%) | UL-94 | LOI (%) | pHRR reduction (%) | THR (MJ m−2) | Reference |
|---|---|---|---|---|---|---|---|
| Fe2O3(MMT-Fe2O3) | PVC | — | — | — | 56.6 | 27.5 | 69 |
| FeP(FeP@APP@CS) | APP | 0.4 | 0 | 28.5 | 57.2 | — | 70 |
| FeP(FeP/APP) | PEI | 45 | 0 | 31.8 | 71.8 | 74.2 | 71 |
| MIL-100(Fe)/MA | PA6 | 5 | — | — | 73.0 | 22.5 | 72 |
| Fe-OMT/MPP | 2 | 0 | 31.0 | 46.1 | 41.7 | 73 | |
| Fe-MOF | PS | 2 | — | — | 14.4 | 4.0 | 74 |
| Co-MOF | 2 | — | — | 28.0 | 17.6 | 74 | |
| ZIF-67/RGO-B | 2 | 0 | 26.4 | 65.1 | 41.4 | 75 | |
| NiO/APP/CSi-MCA | PLA | 3 | 0 | 34.1 | — | — | 76 |
| Ni(OH)2(FGO2/PP) | PP | 2 | — | — | 32.6 | 19.0 | 77 |
| ZnSO4·7H2O/IFR/PP | 1 | 0 | 32.7 | 65.0 | 26.9 | 48 | |
| HP-Mn | 12.5 | 0 | 30.7 | 74.2 | 35.8 | 78 | |
| MOF-Cu/APP | TPU | 0.125 | 0 | 27.0 | 74.5 | 69.8 | 79 |
| ZnO@MOF@PZS | PUA | 3 | 0 | 22.8 | 28.3 | 19.6 | 80 |
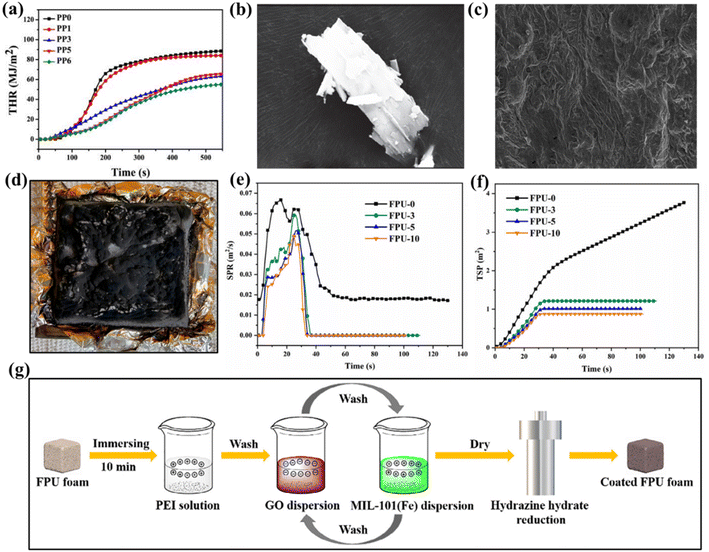 | ||
| Fig. 5 (a) THR curves of samples during the combustion in CCT; (b) SEM images of HP-Mn; (c) SEM images of the char residue obtained after CCT for PP3; (d) digital photographs of the char residue of PP3. Reproduced with permission from ref. 78. Copyright (2021) Materials; (e) SPR, (f) TSP and curves of FPU-0 and coated FPU foams; (g) preparation schematic of coated FPU foams via LBL self-assembly technology. Reproduced with permission from ref. 85. Copyright (2021) Elsevier. | ||
In future development, manganese-based flame retardants still have excellent growth potential. Using it as intumescent flame-retardant system as flame-retardant coating to cover the surface of the composite, it not only cooperates with the carbon forming agent to play flame retardant effect, but also can enhance the water resistance of cotton fabric related materials.
Due to the risk of phosphate mine depletion and increasing costs, there is a growing demand for low phosphorus or ecophosphorus based flame retardants and materials containing iron86 exhibit excellent flame retardant properties. In chemistry, the common organic ligand bound to iron is carboxylic acid.87 However, most carboxylic acid mixtures do not have flame retardant elements, and when iron and carboxylic acid compounds with no flame-retardant effect form complexes, the flame-retardant effect is not satisfactory. To address these limitations, Jiang et al. used transition metal iron and phosphorus to form an iron@ phosphorus complex (Fe-PMC) complex,88 aiming to prepare a new flame retardant with iron–phosphorus interaction and using it to replace pure phosphorus flame retardants. The synthetic route is shown in the Fig. 6(a) and the appearance of synthesized MIL-53 is shown in Fig. 6(b). The results show that Fe-PMC has a higher flame suppression capacity than the iron-only MIL-53 and that Fe-PMC reduces the laminar flame speed of cellulose and dramatically reduces the flame temperature.
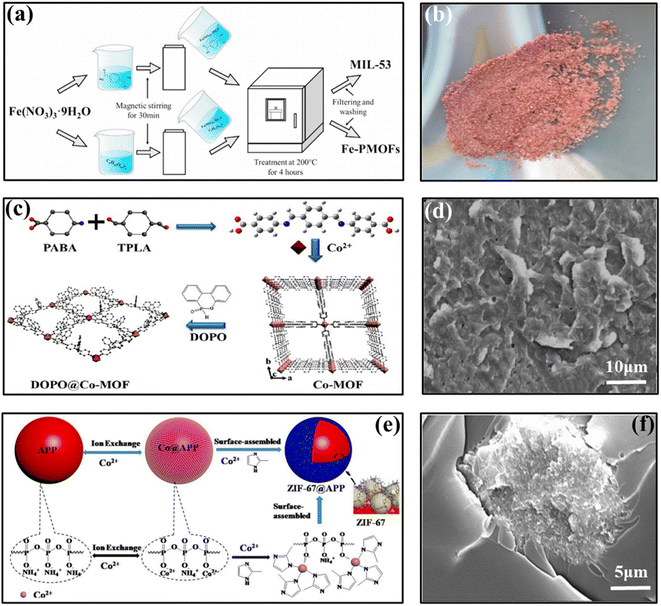 | ||
| Fig. 6 (a) Illustration of preparation of MIL-53 and Fe-PMC; (b) appearance of synthesized MIL-53 (reddish brown crystals). Reproduced with permission from ref. 88. Copyright (2022) Elsevier; (c) schematic illustration of preparation process of DOPO@Co-MOF hybrids; (d) SEM images of fractured surfaces of PLA/DOPO@Co-MOF. Reproduced with permission from ref. 90. Copyright (2018) ACS Publications; (e) the synthetic route of ZIF-67@APP; (f) the fractured morphology of 5ZIF-67@APP/EP. Reproduced with permission from Ref. 92. Copyright (2021) Elsevier. | ||
In summary, it is not difficult to find that in addition to forming complexes for flame retardancy, iron can also be used to build the recently emerging iron–metal organic frameworks, attractive new porous materials with different potential and easily customizable structures. In future developments, iron will continue to have an unshakable “place” in enhancing flame retardancy in composites.
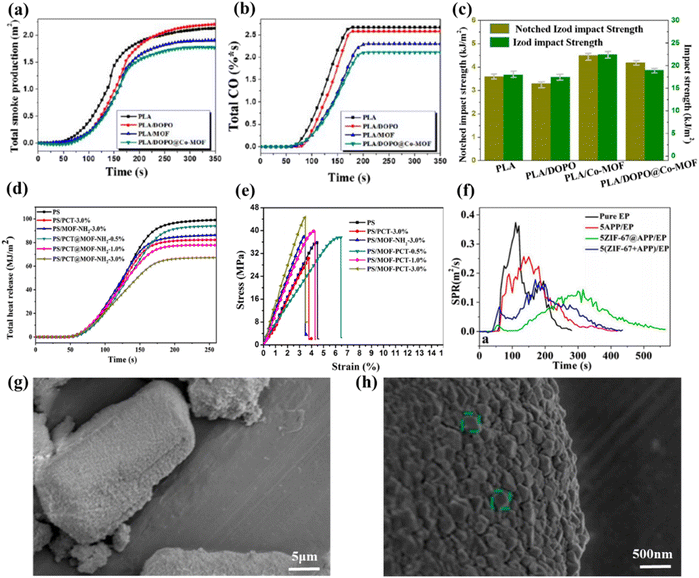 | ||
| Fig. 7 (a) TSP, and (b) total CO release curves of PLA and its assemble systems; (c) impact strength results of PLA and its composites. Reproduced with permission from ref. 90. Copyright (2018) ACS Publications; (d) THR curves of PS and its composites at a heat flux of 35 kW m2; (e) results of tensile test for PS and its composites. Reproduced with permission from Ref. 91. Copyright (2020) Elsevier; (f) the SPR curves of pure EP, 5APP/EP, 5ZIF-67@APP/EP and 5(ZIF-67 + APP)/EP; (g and h) the morphology of ZIF-67@APP. Reproduced with permission from Ref. 92. Copyright (2021) Elsevier. | ||
Of course, in addition to the common cobalt metal–organic frameworks, cobalt ions are often coordinated or modified with other phosphorus and nitrogen organic compounds to enhance the flame-retardant properties of polymers further. Shao et al. performed a coordination reaction using ZIF-67@APP to distribute it more uniformly on ammonium polyphosphate (APP).92 The synthesis route of ZIF-67@APP is shown in the Fig. 6(e) and the fractured morphology of 5ZIF-67@APP/EP is shown in Fig. 6(f). The morphology of ZIF-67@APP is shown in Fig. 7(g) and (h). The LOI value of EP was 28.5% at 5 wt% loading, and its PHRR decreased by 67.4% and SPR (Fig. 7(f)) decreased by 46.2%. In addition, ZIF-67@APP/EP exhibits better mechanical properties. Its tensile and flexural strengths are significantly improved. It can form dense and stable expanded carbon layer, which can isolate the transmission of oxygen and heat and therefore has a high fire safety. Zhou et al. synthesized cobalt oxide nanoparticles adsorbed on graphene nanosheets by a hydrothermal method.93 The mixture was introduced into the thermoplastic polyurethane (TPU) matrix as a reinforcing agent. The mixture was well scattered in the TPU and no significant aggregation occurred. Compared to pure PU, nanocomposites offer remarkable performance improvements in terms of thermal stability, flame retardancy and mechanical properties. These significant improvements in performance are mainly attributed to the “bent path” effect of graphene nanosheets.
In general, cobalt can exist in many forms to exert flame retardant effects, such as cobalt ions, cobalt metal–organic framework, etc. However, cobalt resources are less abundant and more expensive than iron. In the future, compounding with other metals can be considered to meet the cost reduction and further enhance the flame-retardant effect.
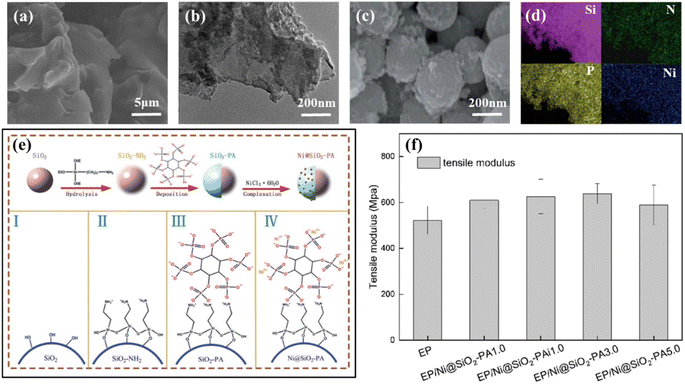 | ||
| Fig. 8 (a) SEM images from PLA3 after cone calorimeter test. Reproduced with permission from ref. 76. Copyright (2019) Elsevier; (b) TEM images of FGO1. Reproduced with permission from ref. 77. Copyright (2017) Elsevier; (c) SEM images of Ni@SiO2-PA; (d) SEM image of Ni@SiO2-PA with elemental mapping of Si, N, P, and Ni; (e) synthesis route of the nanostructure Ni@SiO2-PA; (f) tensile modulus of pure EP and its composites. Reproduced with permission from ref. 94. Copyright (2020) RSC Advances. | ||
In addition to nickel oxides and hydroxides, nickel ions can also form metal ionic compounds to enhance flame retardancy, catalyzing carbon formation from the substrate and reducing heat transfer. Sui et al. obtained a new green flame retardant (Ni@SiO2-PA) (SEM images of Ni@SiO2-PA is Fig. 8(c) and (d)) by aggregating nickel phytate as shell on amination-modified silica through electrostatic interactions.94 The synthetic route is shown in the Fig. 8(e). After adding the Ni@SiO2-PA with a core–shell structure to EP, the flame retardancy and stability of EP composites thermally were significantly improved. The flame-retardant mechanism was also speculated, and Ni@SiO2-PA initially formed a net-like structure to prevent further decomposition. The nitrogen-phosphorus synergistic flame-retardant system generated gas inhibition body and phosphorus-rich expanded carbon layer. In addition, the heat and exchange between the oxygen and the substrate are prevented by introducing Ni2+ catalyzed carbon layer. As shown in the Fig. 8(f), the mechanical properties of the composites were also improved after the addition of flame retardants. Gong et al. prepared phytic acid nickel (PA-Ni) by reacting phytic acid with nickel acetate in water and then compounded PA-Ni with pentaerythritol and APP as a flame retardant into PLA.95 The PLA composites prepared under 4 wt% PA-Ni and 11 wt% IFR could achieve UL-94 test rating of V-0. The PHRR of the PLA/11IFR/4PA-Ni composite was 62.3% lower than the original PLA, and the carbon residue was 12.5% higher.
Along with aluminium, copper, lead and zinc, nickel is the most economically valuable of the common metals. Strength, formability, and improved corrosion resistance are known properties of nickel, making nickel-containing materials necessary in harsh environments and at very high temperatures. Therefore, nickel metal flame retardants will have a broader range of applications.
Riyazuddin et al. synthesized polymeric flame retardants (ETPMP) by nucleophilic substitution reactions using ethanolamine, piperazine, and melamine, as raw materials.96 ETPMP, APP and CuO were used in various ratios to prepare intumescent flame retardants (IFR and IFR/CuO). Flame retardant and smoke suppression tests were conducted and the results showed that the addition of a small amount of CuO significantly improved the LOI results and the UL-94 to V-0 level and the strength analysis results of epoxy, EP/IFR, and EP/IFR/CuO-5% sample charred layers is shown in Fig. 9(a). Analysis of the TGA results revealed that the addition of CuO significantly increased the residual carbon content and altered the thermal degradation behaviour. SEM testing of the residual carbon after CCT (Fig. 9(b) and (c)) showed that the EP/IFR/CuO-5 wt% residual carbon was significantly smoother than the EP/IFR composite, indicating that the addition of CuO can have strong synergistic effect with IFR, forming dense carbon layer without pores and cracks, effectively blocking the flow of heat and oxygen. Jia et al. modified graphene with CuO as a co-effector for PU flame retardancy,97 and experimentally demonstrated that CuO has good smoke and toxic gas suppression effect while significantly reducing PHRR and THR. It also showed excellent synergistic effect on improving the amount and denseness of residual carbon formation. In addition to forming copper oxide for flame retardation, it is often used, as is iron, to construct MOFs for flame and smoke suppression in polymeric materials. For example, Chen et al. synthesized a copper metal–organic backbone (MOF-Cu),79 and added it to TPU matrix in synergy with APP to prepare high-performance TPU composite. The synthetic route is shown in the Fig. 9(d) and the SEM images of TPU/APP/Cu0.0625 is shown in Fig. 9(e). It was found that the PHRR and THR of TPU/Cu0.0625 wt% were reduced by 26.0% and 1.3%, respectively, compared to the pure TPU samples, while the PHRR and THR values of TPU/APP samples were reduced by 70.3% and 15.8%, respectively, compared to the pure TPU samples. And the PHRR and THR values of TPU/APP/Cu0.0625 wt% samples were further reduced by 19.2% and 63.4%, in addition, TPU/APP/Cu0.125 wt% reduced the TSR by 23.4% compared to TPU/APP. This indicates that MOF-Cu has significant flame-retardant effect and that APP shows a better synergistic flame-retardant effect with MOF-Cu.
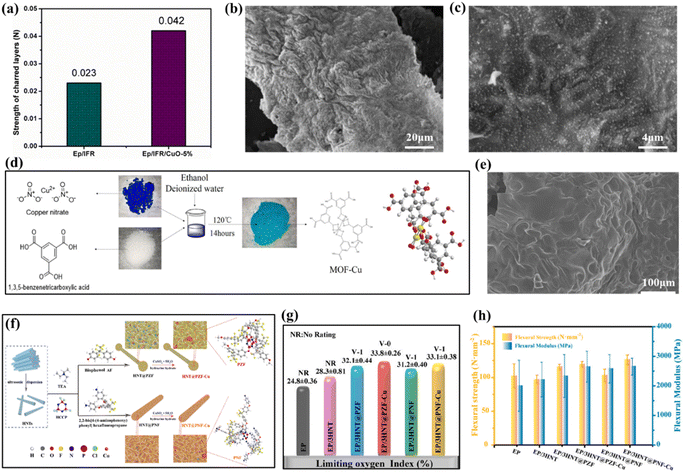 | ||
| Fig. 9 (a) Strength analysis results of epoxy, EP/IFR, and EP/IFR/CuO-5% sample charred layers; (b and c) SEM images of EP/IFR/CuO-5% charred layers with different amplifications. Reproduced with permission from ref. 96. Copyright (2020) Molecules; (d) schematic representation for the preparation of MOF-Cu; (e) SEM images of TPU/APP/Cu0.0625. Reproduced with permission from ref. 79. Copyright (2021) Polymers for Advanced Technologies; (f) schematic illustration for the synthesis process of HNT@PZF-Cu and HNT@PNF–Cu; (g) UL-94 vertical burning and LOI values; (h) flexural strength and modulus of EP nanocomposites. Reproduced with permission from ref. 99. Copyright (2021) Elsevier. | ||
Copper still achieves excellent flame retardancy when forming the complexes while also reducing the impact on the mechanical properties of the composites themselves. For example, Abd El-Wahab et al. synthesized a Schiff base sulphonamide ligand and its copper metal complexes, which were coated onto TPU surfaces at 0.5 wt% addition.98 The test results showed that the LOI increased from 20% to 45% when the TPU was blank and increased by 7% compared to the ligand alone as flame retardant, indicating that the metal complex had better flame-retardant properties than the ligand. It is worth mentioning that the complexes did not show significant damage to the mechanical properties of the substrate while improving the flame-retardant effect. Hong et al. coated the surface of kaolinite nanotubes (HNT) with amino-terminated (PNF) and hydroxyl-terminated (PZF) polyphosphonitrile, respectively.99 While copper nanoparticles (2.6 ± 0.8 nm) were immobilized on the surface of these two core–shell structures, the flame retardants were named HNT@PZF-Cu and HNT@PNF-Cu, respectively. The synthetic route is shown in the Fig. 9(f). The results showed that HNT@PZF-Cu exhibited better smoke suppression and flame retardancy against EP. This is because HNT@PZF-Cu promotes the rapid carbonization of EP nanocomposites, resulting in the formation of structurally stable carbon slag. Meanwhile, copper nanoparticles can effectively reduce the release of toxic fumes and volatile organic compounds (VOCs) during catalytic combustion. The UL-94 vertical burning and LOI values is shown in Fig. 9(g). Flexural strength and modulus of EP nanocomposites is shown in Fig. 9(h), compared to pure EP, EP materials with both flame retardants are improved.
In summary, both copper oxides and hydroxides, metal ligands and nanoparticles are equally effective in enhancing flame retardancy, but the addition of oxides or hydroxides have certain degree of impact on the mechanics of the composite, whereas metal ligands or metal ionic compounds have less impact on its mechanical properties and are a major trend for future development.
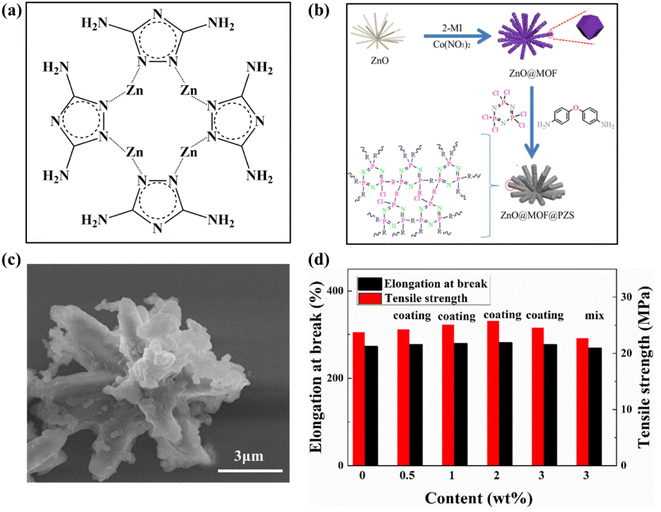 | ||
| Fig. 10 (a) Proposed molecular structure of zinc–triazole complex. Reproduced with permission from ref. 100. Copyright (2021) Elsevier; (b) schematic diagram of the synthetic route of ZnO@MOF@PZS; (c) SEM images of ZnO@MOF@PZS; (d) mechanical property curves of PUA and its composites. Reproduced with permission from ref. 80. Copyright (2021) Polymers for Advanced Technologies. | ||
It is well known, zinc is also commonly used to coat and protect steel from corrosion, so zinc metal flame retardants can be used in the future as a fire and corrosion resistant coating in production life, both to ensure the flame-retardant properties of the base material can also enhance its corrosion resistant properties.
3.2. Rare transition metal flame retardants
Rare metals are usually referred to as metals that are less abundant or sparsely distributed in nature. They are difficult to extract from raw materials and also late in industrial preparation and application. Rare metal mineral resources have a wide range of uses, especially in astronautics, atomic energy, electronics, the defense industry and other high-tech technology applications. With the continuous development of science and technology, some rare metals can also serve as efficient flame retardants in polymers.Titanium is referred to as the “space metal” due to its stable chemical characteristics, good resistance to extreme cold and heat, potent acids and bases, high strength, and low density. As a rare metal element, titanium has been studied relatively little in flame retardant research, but it can be used as co-effector in combination with certain other flame retardants to show substantial flame resistant effects. For example, Zhou et al. synthesized a diblock polymer containing DOPO.101 Through the Schiff base reaction, it continued to react with titanium-hybridized aminopropyl-polyhedral oligomeric sesquisiloxane (Ti-POSS), resulting in the creation of the novel graft copolymer PolyTi, which was then employed to modify EP materials as polymeric flame retardant. The findings indicated that, in comparison to pure EP, the Tg, LOI, coke graphitization, and mechanical properties of the EP/PolyTi composites were all improved by the addition of PolyTi. Additionally, compared to the EP/PolyTi composites, the phosphorus portion of the EP/DOPO composites migrated more readily. Apparently, the large molecule flame retardant-modified EP/PolyTi composites showed better thermal stability, flame retardancy, and migration resistance compared with the small molecule flame retardant-modified EP. Wei et al. utilized tetrabutyl titanate, tetraethyl orthosilicate, and diphenylphosphonic acid as raw ingredients by hydrothermally reaction to create a new hybrid nanorod called OPTS.102 And it contains organophosphorus–titanium–silicon (P–Ti–Si) and the synthetic route is shown in the Fig. 11(a). Based on the structural properties of OPTS, it is used to manufacture PC with high flame-retardant properties and its CCT values are shown in the Fig. 11(b–d). Just 0.1 wt% OPTS received PC 29.7% LOI value and UL-94 V-0 rating. When OPTS content was increased to 0.1 wt%, the tensile strength and elongation at break of flame-retardant PC increased to 8.0 and 8.3%, respectively.
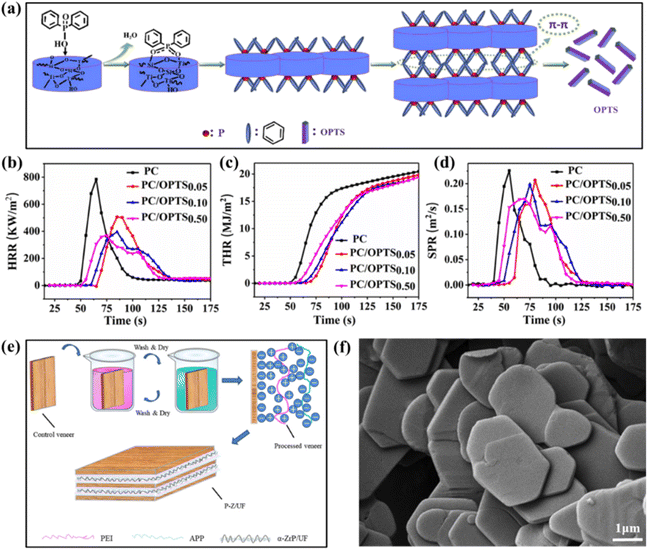 | ||
| Fig. 11 (a) Formation process of OPTS nanorods; (b) HRR, (c) THR and (d) SPR results of pure PC and PC/OPTS nanocomposites during a CCT. Reproduced with permission from ref. 102. Copyright (2019) ACS Publications; (e) preparation process of P-Z/UF flame-retardant plywood (plywood was prepared by flame-retardant processed veneers and a-ZrP/UF composite adhesive); (f) FE-SEM images of a-ZrP. Reproduced with permission from ref. 103. Copyright (2021) Elsevier. | ||
In recent years, there has been less research into the application of zirconium in flame retardancy. As a new rare metal flame retardant, zirconium has amazing corrosion resistance, high melting point, and ultra-high hardness and strength, so there is much to explore in the future for zirconium in flame retardancy.
Molybdenum also is a refractory metal, although less research has been done on the use of molybdenum as flame retardant than has been done with zirconium, molybdenum exhibits good synergy in the formation of compounds to enhance the flame-retardant properties of polymers. More research is required to determine whether molybdenum can be used widely as flame retardant in polymers.
3.3. Main group metal flame retardants
In addition to transition metals, main group elements also contain many metal elements. The main group metals, such as magnesium and aluminum, are often used in flame retardant studies. They are generally used to form mental oxides and hydroxides rather than metal–ion complexes as flame retardants. Aluminum can also form organic acid compounds to strengthen the fire resistance of polymers. The performance of some main group metal-based flame retardants in different polymeric materials in recent years is listed in Table 2.Tang et al. applied layered MH to flame-retardant thermoplastic elastomers (TPE).34 When the MH content was 35 wt%, the TPE/Mg(OH)2 composites had the best flame retardant capabilities, with delay in the ignition when compared to samples without MH addition. The TSP, HRR, THR, and mass loss rate were reduced by 56%, 31.4%, 35.6%, and 34.2%, respectively. He et al. employed organic magnesium hydroxide (OMH) (the SEM image at the same scale magnifications for OMH is shown in Fig. 12(b)) and expandable graphite (EG) as raw materials to create environmentally friendly flame-retardant unsaturated polyester resin (UPR) material.110 Instead of the conventional direct addition of MH to the UPR matrix, OMH participated in the polycondensation process of UPR as reactive monomer, which considerably increased the compatibility of the flame retardant with the substrate. Interestingly, the flame retardant UPR composites demonstrated more adequate flame retardancy when 8 wt% EG was added to the UPR/OMH matrix, with increasing in LOI from 21.7% to 28.5% and a V-0 rating in UL-94 testing. This was owing to the synergistic impact between OMH and EG. Zhao et al. created a new organic–inorganic hybrid flame retardant (DMMH) (the synthesis process is shown in the Fig. 12(a) and the SEM images of DMMH is shown in Fig. 12(d) and (e)) by neutralizing the addition reaction of maleic acid with MH and DOPO, which was then mixed with APP to create an IFR system.112 With 1.7% DMMH and 5.3% APP added, EP-7 received UL-94 V-0 rating with LOI of 26.0%. In comparison to pure EP, the samples' THR and smoke emission decreased by 54.5% and 43.6%, respectively. And the mechanical properties of the EP were unaffected by the addition of DMMH (Fig. 12(c)). Liu et al. used triethoxysilane and polymethylvinyl silicone rubber for modified MH to prepare flame-retardant composites.109 A water contact angle of 141 demonstrates the outstanding hydrophobicity of the modified MH (MMH). When the loading of MH or MMH was 70 wt%, the sample (1.6 mm) passed the UL-94 V-0 rating, indicating that its modification had no adverse effects on the flame-retardant qualities of MH. Comparing MH-O-SEBS-PP composites to MMH-O-SEBS-PP composites, the tensile strength and elongation at break of the latter improved by 20.4% and 88.9%, respectively. The mechanical properties were enhanced by the better interfacial compatibility of the flame retardant with the matrix. Additionally, after 168 hours of water treatment at 70 °C, the mechanical and flame-retardant properties of the MMH-O-SEBS-PP composites were still intact.
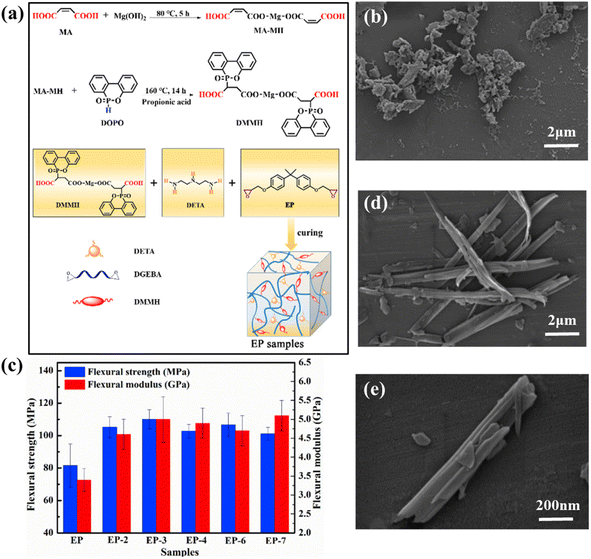 | ||
| Fig. 12 (a) Synthesis of DMMH and schematic diagram of the curing process of the EP samples. Reproduced with permission from ref. 112. Copyright (2021) Elsevier; (b) SEM image at the same scale magnifications for OMH. Reproduced with permission from ref. 110. Copyright (2019) Wiley; (c) effect of the mechanical properties of flame retardant UPR composites; (d and e) SEM images of DMMH. Reproduced with permission from ref. 112. Copyright (2021) Elsevier. | ||
In recent years, due to the traditional MH flame retardant on the mechanics of polymer materials, people are more committed to the study of LDH and found that the introduction of magnesium into LDH can better play the efficacy of flame retardants, which is the future of magnesium-based flame-retardant development trend.
Ai et al. used ATH to synthesize an organic–inorganic synergistic flame retardant.32 The LOI was 27.0% and the UL-94 rating was V-0 for PE/20% ATH/20% CP-6B. In comparison to pure PE, the PHRR of PE/20% ATH/20% CP-6B was 33.7% higher. In addition to its improved flame retardancy, ATH also has suppressive effect on the smoke and toxicity released by burning polymers. Li et al. have similarly concluded in their flame retardant study on bitumen that ATH has high flame retardant qualities and can reduce the generation of CO and aldehyde fumes.33 In addition to the excellent flame retardancy of the hydroxide formed by aluminum, compounds formed with other flame-retardant elements are also effective in enhancing the flame retardancy of polymers. For example, Wang et al. prepared flame retardant EP materials using aluminum diethyl hypophosphonate (ADP), melamine (MEL) and pentaerythritol (PER).113 The data showed that the LOI was 29.5% when ADP was only 15%, and the material passed the UL-94 V-0 rating. In the thermogravimetric test, a residual of 13.10% was achieved. When the total of 10% flame retardant was supplied, the EP-4 compound system and the EP-5 composite system both displayed outstanding flame retardancy, demonstrating that ADP, MEL, and PER have a good synergistic flame retardancy effect. A low amount of flame-retardant additive still has good flame-retardant effect when PER is present, which can accelerate the carbonization of the sample and successfully reduce smoke production. Hu et al. prepared PU elastomers based on monomethyl aluminum hypophosphonate (MeP–Al).114 The outcomes demonstrated that adding MeP–Al gave the PU the desirable flame retardancy and anti-drip qualities. With LOI value of 29.6%, samples containing 20 wt% MeP–Al passed the UL-94 V-0 rating and did not leak when burning. Additionally, there is a considerable reduction in both the heat released after burning and the organic volatilization from PU degradation. The effective action of MeP–Al in the gas and condensing phases is responsible for the good flame retardancy, anti-drip capabilities, and the significantly reduced emission of heat and organic compounds. MeP–Al breaks down into monomethylphosphonic acid in the gas phase, which dampens heat release and flames. MeP–Al transforms into tough aluminum pyrophosphonate in the condensed phase, where it interacts with PU to encourage the synthesis of stable carbonaceous coke from PU.
There is a wide range of main group metal elements, the most traditional and earliest development of MH and ATH flame retardants are the most widely used. However, as transition metals are increasingly used in flame retardant research and play outstanding smoke-suppression and flame-retardant capabilities, MH and ATH flame retardants are facing serious challenge to their status.
3.4. Multi-metal composite flame retardants
Flame retardants or synergists containing single metal element have been confirmed to be effective by abundance of research. But the different metal elements have different chemical properties and the different roles played in catalyzing the composite matrix into carbon or inhibiting smoke toxicity, and the price of different metals is also somewhat different. Therefore, some studies have shown that multi-metal collaboration can make use of the advantages between different metal elements to complement each other. This includes both transition metals and transition metals, as well as transition metals and main group metals, which can achieve better flame-retardant smoke suppression effect.Liu et al. prepared a flame retardant (MnO2@ZHS) consisting of two-dimensional manganese dioxide nanosheets and stereo-hydroxyzine stannate (ZHS) binary hybrid, and the synthesis process is shown in the Fig. 13(a).115 The microstructural analysis showed that MnO2@ZHS was successfully prepared and was morphologically and structurally intact and the TEM images of MnO2@ZHS hybrid is shown in Fig. 13(b) and (c). The MnO2@ZHS binary hybrid efficiently suppressed the PHRR of EP composites, according to CCT findings. At 2 wt% loading, the THR of EP/MnO2@ZHS may be lowered by nearly 40% compared to the control EP, which is better than that of ZHS alone. The MnO2@ZHS binary hybridization improved the density and graphitization of the residual carbon, successfully preventing oxygen and flammable gases from escaping. Shao et al. created a flame retardants, which used the Fe/Zn-LDH to modify the APP.116 The outcomes revealed that the EP containing 4 wt% Fe/Zn-LDH@APP had the greatest LOI of 29.4% and passed the UL-94 V-0 class. The fire growth rate, peak smoke production rate and PHRR were reduced by 80.7%, 48.4% and 66.4% compared to pure EP. This is due to the LDH and APP in Fe/Zn-LDH@APP may better accelerate the conversion of aliphatic molecules to aromatic or polyaromatic compounds, thereby reducing the emission of smoke and heat. Li et al. inserted ferrocene molecules into the nanocavities of the sulfonated cyclodextrins of the layered hydroxide (LDH-CD) via host-guest chemistry, the LDH-CD-Ferr formed a more homogeneous dispersion than LDH-CD in the EP matrix.117 The peak and total smoke generation rates of the EP matrix were lowered by 43 and 42% when added 6 wt% of LDH-CD-Ferr, while the LOI and PHRR were reduced by 5% and 36%, respectively. A higher quality carbonaceous layer was constructed by the catalytic charring of retained ferrocene, and oxidative degradation of fume intermediates was promoted by the delayed release of ferrocene. Chen et al. synthesized iron-based flame retardant enhancers using a hydrothermal method with iron sulfate Fe2(SO4)3 and urea as raw materials.118 The outcomes demonstrated that a modest quantity of FeOOH might significantly increase the compound's flame retardancy. The compound's LOI value increased to 30.8% when FeOOH was added at 1.5 phr, and the UL-94 vertical flame-retardant test grade reached V-0.
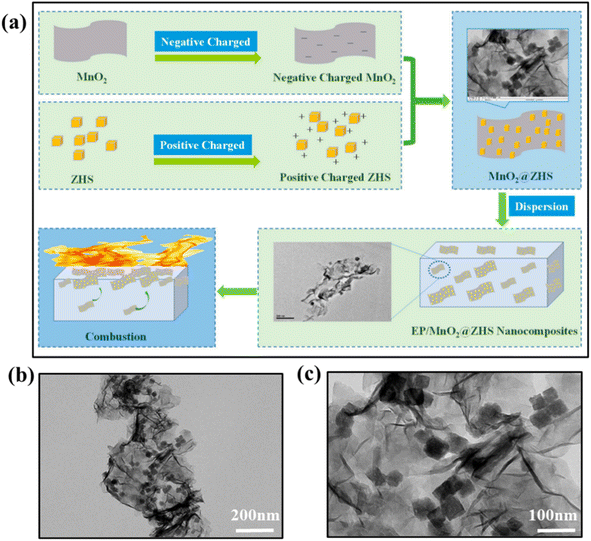 | ||
| Fig. 13 (a) Synthesis of MnO2@ZHS hybrids and flame retardancy mechanism in epoxy; (b and c) TEM images of MnO2@ZHS hybrid. Reproduced with permission from ref. 115. Copyright (2019) Polymers. | ||
Transition metals and main group metals are not completely separated from each other. On the contrary, they are inextricably linked to each other. In future research on transition metals and main group metal flame retardants, the compounding of multiple metals is also major research hotspot. However, the problems of complex operation and preparation difficulties when compounding different metals are still major challenge for the future.
In summary, combined with the research on metal-based flame retardants in recent years, as shown in Fig. 14, the main group metal elements mainly exist in the form of oxides and hydroxides for flame retardant, for example, MH can dilute the concentration of combustible gases through the water and magnesium oxide produced by thermal decomposition, thus providing a flame-retardant effect. It is worth noting that they can also form LDH to flame retardant, mainly through the formation of a layered structure to play a physical barrier role to isolate combustible gases to achieve flame retardant effect. In contrast to the more homogeneous forms of compounds formed by the main group metal elements, the transition metal elements can form many different types of compounds, for example, iron can form +2 and +3 valence compounds, while manganese has +2, +3, +4, +5, +6, +7, −1, −2 and −3 valence states. Some of the transition metal elements can form a variety of valence compounds in addition, they can also and a variety of organic flame-retardant elements to form the corresponding complexes and organic framework, etc. The common metal organic framework are IRMOF series, ZIF series, MIL series, UIO series and PCN series. Transition metals can not only use the catalytic process of the metal matrix into carbon but also catalyze the conversion of toxic gases to achieve a flame retardant and low smoke and low toxicity effect. At the same time, the synthesized organometallic compounds can also combine the flame-retardant effect of organic flame-retardant elements to further enhance the flame-retardant effect of polymers. In future metal flame retardant research, transition metal flame retardants, due to their superiority, form a wider variety of compounds and can be developed in a broader range of aspects. The limitations of the main group metal flame retardants lead to their future development needs to be combined with transition metal elements in order to play a better role. Therefore, the composite of multiple metal elements is a major research trend in the future, using multiple metal elements for synergy, and then combined with organic flame-retardant elements such as phosphorus and nitrogen, to achieve a highly efficient flame retardant and low smoke and low toxicity effect.
4. Conclusion and prospect
This paper reviews the progress of research on flame retardants of different metallic elements to provide various synergistic additives to strengthen the flame retardancy of polymers. The primary methods by which metal flame retardants work as well as the many forms of various metals used to construct flame retardant complexes to improve the flame retardancy of polymers are described. Most studies demonstrate that metals can catalyze the carbon formation process in composites to generate dense carbon layer that serves as useful physical barrier by reducing heat transmission, containing flammable gas egress, and preventing further matrix combustion. Additionally, the gases produced during burning may help dilute the oxygen and other combustible gases. According to the current literature, most metals and their compounds cannot be used alone to give polymers excellent LOI values or UL-94 vertical combustion behavior. Instead, they must act chemically modified or in synergy with other organic flame-retardant compounds and carbon formers. Moreover, some metal compounds tend to congregate within the polymer matrix and cannot be entirely disseminated, failing to achieve the maximum enhancement effect in the manufactured polymer composites, which might negatively affect the mechanical properties of the polymer itself.In general, at present, various metal flame retardants have various problems while achieving an efficient flame retardant and smoke suppression, such as how to uniformly disperse in the polymer material, certain impact on the mechanical properties of the base material and the preparation process is complicated and difficult. Therefore, in the future development stage, the research of metal flame retardants is more inclined to combine the main group metals and transition metals or a variety of transition metal elements, while introducing organic flame-retardant elements such as phosphorus, nitrogen, silicon, etc. So as to further enhance the flame retardancy of polymer engineering materials by using the catalytic carbon formation effect of metal elements and reducing the smoke and toxicity on the one hand, and combining the gas phase and condensed phase flame retardancy of organic flame-retardant elements on the other. In addition, when combining metal elements and organic flame-retardant elements, the introduction method of metal elements should be considered, and the introduction in the form of ions can effectively reduce the mechanical impact on the base material and better dispersion in the base material, which are the areas that need to be focused on when studying metal flame retardants in the future. In a word, metal flame-retardant have excellent functions of catalytic matrix carbon formation and smoke emission inhibition, making them a promising class of environmentally friendly flame retardants and smoke suppressants that are considered to have promising industrial applications in polymers.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. U2033206), the funding of Civil Aircraft Fire Science and Safety Engineering Key Laboratory of Sichuan Province (No. MZ2022JB01) and General Program of Civil Aviation Flight University of China (No. J2020-111, J2021-110).References
- S. Fatullayeva, D. Tagiyev, N. Zeynalov, S. Mammadova and E. Aliyeva, Recent advances of chitosan-based polymers in biomedical applications and environmental protection, J. Polym. Res., 2022, 29(7), 259 CrossRef CAS.
- R. Hu, A. J. Qin and B. Z. Tang, AIE polymers: Synthesis and applications, Prog. Polym. Sci., 2020, 100, 101176 CrossRef CAS.
- I. I. Kabir, C. C. Sorrell, S. S. Mofarah, W. Yang, A. C. Y. Yuen, M. T. Nazir and G. H. Yeoh, Alginate/Polymer-Based Materials for Fire Retardancy: Synthesis, Structure, Properties, and Applications, Polym. Rev., 2021, 61(2), 357–414 CrossRef CAS.
- S. Kangishwar, N. Radhika, A. A. Sheik, A. Chavali and S. Hariharan, A comprehensive review on polymer matrix composites: material selection, fabrication, and application, Polym. Bull., 2023, 80(1), 47–87 CrossRef CAS.
- G. F. Liu, X. D. Sun, X. D. Li and Z. X. Wang, The Bioanalytical and Biomedical Applications of Polymer Modified Substrates, Polymers, 2022, 14(4), 826 CrossRef CAS.
- A. B. Morgan and J. W. Gilman, An overview of flame retardancy of polymeric materials: application, technology, and future directions, Fire Mater., 2013, 37(4), 259–279 CrossRef CAS.
- L. Ahmed, B. Zhang, L. C. Hatanaka and M. S. Mannan, Application of polymer nanocomposites in the flame retardancy study, J. Loss Prev. Process Ind., 2018, 55, 381–391 CrossRef CAS.
- H. M. Chen, F. Y. Chen, H. Chen, H. S. Liu, L. Chen and L. Yu, Thermal degradation and combustion properties of most popular synthetic biodegradable polymers, Waste Manage. Res., 2023, 41(2), 431–441 CrossRef CAS PubMed.
- R. N. Walters, N. Safronava and R. E. Lyon, A microscale combustion calorimeter study of gas phase combustion of polymers, Combust. Flame, 2015, 162(3), 855–863 CrossRef CAS.
- A. Dasari, Z. Z. Yu, G. P. Cai and Y. W. Mai, Recent developments in the fire retardancy of polymeric materials, Prog. Polym. Sci., 2013, 38(9), 1357–1387 CrossRef CAS.
- E. Kicko-Walczak and G. Rymarz, Recent developments in fire-retardant thermoset resins using inorganic-organic hybrid flame retardants, J. Polym. Eng., 2018, 38(6), 563–571 CrossRef CAS.
- J. Zhang, Z. Li, X. L. Qi and D. Y. Wang, Recent Progress on Metal-Organic Framework and Its Derivatives as Novel Fire Retardants to Polymeric Materials, Nano-Micro Lett., 2020, 12(1), 1–21 CrossRef PubMed.
- E. Cakmakci and M. V. Kahraman, Boron/Phosphorus-Containing Flame-Retardant Photocurable Coatings, in Photocured Materials, ed. A. Tiwari and A. Polykarpov, 2015, pp. 150–187 Search PubMed.
- M. Liu, H. W. Yin, X. Q. Chen, J. Yang, Y. H. Liang, J. J. Zhang, F. Yang, Y. Y. Deng and S. Lu, Preliminary ecotoxicity hazard evaluation of DOPO-HQ as a potential alternative to halogenated flame retardants, Chemosphere, 2018, 193, 126–133 CrossRef CAS PubMed.
- P. Luo, L. J. Bao, F. C. Wu, S. M. Li and E. Y. Zeng, Health Risk Characterization for Resident Inhalation Exposure to Particle-Bound Halogenated Flame Retardants in a Typical E-Waste Recycling Zone, Environ. Sci. Technol., 2014, 48(15), 8815–8822 CrossRef CAS PubMed.
- B. Massoumi, M. Abbasian, R. Mohammad-Rezaei, A. Farnudiyan-Habibi and M. Jaymand, Polystyrene-modified novolac epoxy resin/clay nanocomposite: Synthesis, and characterization, Polym. Adv. Technol., 2019, 30(6), 1484–1492 CrossRef CAS.
- N. A. Isitman, H. O. Gunduz and C. Kaynak, Halogen-free Flame Retardants that Outperform Halogenated Counterparts in Glass Fiber Reinforced Polyamides, J. Fire Sci., 2010, 28(1), 87–100 CrossRef CAS.
- T. Randoux, J. C. Vanovervelt, H. Van den Bergen and G. Camino, Halogen-free flame retardant radiation curable coatings, Prog. Org. Coat., 2002, 45(2–3), 281–289 CrossRef CAS.
- V. E. Naiker, S. Mestry, T. Nirgude, A. Gadgeel and S. T. Mhaske, Recent developments in phosphorous-containing bio-based flame-retardant (FR) materials for coatings: an attentive review, J. Coat. Technol. Res., 2023, 20(1), 113–139 CrossRef CAS.
- W. C. Zhang, X. D. He, T. L. Song, Q. J. Jiao and R. J. Yang, The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins, Polym. Degrad. Stab., 2014, 109, 209–217 CrossRef CAS.
- M. Mukhtar, D. Klosterman, V. Benin and A. Morgan, Synthesis of a phosphorus-based epoxy reactive flame retardant analog to diglycidyl ether of bisphenol A (DGEBA) and its behavior as a matrix in a carbon fiber composite, Polym. Degrad. Stab., 2022, 205, 110144 CrossRef CAS.
- J. K. Liu, S. M. Shau, T. Y. Juang, C. C. Chang, S. H. A. Dai, W. C. Su, C. H. Lin and R. J. Jeng, Tailored Thermal and Mechanical Properties of Epoxy Resins Prepared Using Multiply Hydrogen-Bonding Reactive Modifiers, J. Appl. Polym. Sci., 2011, 120(4), 2411–2420 CrossRef CAS.
- Y. B. Hou, Z. M. Xu, F. K. Chu, Z. Gui, L. Song, Y. Hu and W. Z. Hu, A review on metal-organic hybrids as flame retardants for enhancing fire safety of polymer composites, Composites, Part B, 2021, 221, 109014 CrossRef CAS.
- Z. M. Xu, L. J. Duan, Y. B. Hou, F. K. Chu, S. D. Jiang, W. Z. Hu and L. Song, The influence of carbon-encapsulated transition metal oxide microparticles on reducing toxic gases release and smoke suppression of rigid polyurethane foam composites, Composites, Part A, 2020, 131, 105815 CrossRef CAS.
- F. Zhang, X. T. Li, L. Q. Yang, Y. N. Zhang and M. J. Zhang, A Mo-based metal-organic framework toward improving flame retardancy and smoke suppression of epoxy resin, Polym. Adv. Technol., 2021, 32(8), 3266–3277 CrossRef CAS.
- S. D. Zhang, X. Wang, M. Y. Ding, Y. X. Huang, L. Li and M. Z. Wang, In-situ incorporation of metal phytates for green and highly efficient flame-retardant wood with excellent smoke-suppression property, Ind. Crops Prod., 2022, 187, 115287 CrossRef CAS.
- L. L. Zhou, W. X. Li, H. B. Zhao and B. Zhao, Comparative Study of M(II)Al (M=Co, Ni) Layered Double Hydroxides for Silicone Foam: Characterization, Flame Retardancy, and Smoke Suppression, Int. J. Mol. Sci., 2022, 23(19), 11049 CrossRef CAS PubMed.
- J. L. Xu, C. S. Li, S. B. Ren, L. Niu, Q. W. Bai and X. Li, Influence of ammonium octamolybdate on flame retardancy and smoke suppression of PVC matrix flame retardant composites, Int. Polym. Process., 2022, 37(3), 316–328 CrossRef CAS.
- S. Y. Jiang, L. B. Liu, X. H. Yang, B. Li and M. J. Xu, Nickel-Aluminum Hydrotalcite for Improving Flame Retardancy and Smoke Suppression of Intumescent Flame Retardant Polypropylene: Preparation, Synergy, and Mechanism Study, Macromol. Mater. Eng., 2023, 308(4), 2200533 CrossRef CAS.
- W. H. Li, Z. Y. Lin, H. Y. Zuo, J. H. Zhong, Y. T. Xu, B. R. Zeng, W. A. Luo, G. R. Chen, C. H. Yuan and L. Z. Dai, ZIF-8@Co-doped boronate ester polymer core-shell particles: Catalytically enhancing the nonflammability and smoke suppression of epoxy resin, Polym. Degrad. Stab., 2022, 198, 109877 CrossRef CAS.
- X. Liu, J. W. Hao and S. Gaan, Recent studies on the decomposition and strategies of smoke and toxicity suppression for polyurethane based materials, RSC Adv., 2016, 6(78), 74742–74756 RSC.
- L. H. Ai, S. S. Chen, L. Yang and P. Liu, Synergistic Flame Retardant Effect of Organic Boron Flame Retardant and Aluminum Hydroxide on Polyethylene, Fibers Polym., 2021, 22(2), 354–365 CrossRef CAS.
- M. L. Li, L. Pang, M. Z. Chen, J. Xie and Q. T. Liu, Effects of Aluminum Hydroxide and Layered Double Hydroxide on Asphalt Fire Resistance, Materials, 2018, 11(10), 1939 CrossRef PubMed.
- H. Tang, K. F. Chen, X. N. Li, M. Ao, X. W. Guo and D. F. Xue, Environment-friendly, flame retardant thermoplastic elastomer-magnesium hydroxide composites, Funct. Mater. Lett., 2017, 10(4), 1750042 CrossRef.
- Y. R. Yang, M. Niu, J. M. Dai, J. Bai, B. X. Xue, Y. H. Song and Y. Peng, Flame-retarded polyethylene terephthalate with carbon microspheres/magnesium hydroxide compound flame retardant, Fire Mater., 2018, 42(7), 794–804 CrossRef CAS.
- Y. R. Yang, M. Niu, J. Bai, B. X. Xue and J. M. Dai, Preparation of microencapsulated carbon microspheres coated by magnesium hydroxide/polyethylene terephthalate flame-retardant functional fibers and its flame retardant properties, J. Text. Inst., 2018, 109(4), 445–454 CrossRef CAS.
- L. L. Jiao, P. C. Zhao, Z. Q. Liu, Q. S. Wu, D. Q. Yan, Y. L. Li, Y. N. Chen and J. S. Li, Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA, Polymers, 2022, 14(8), 1567 CrossRef CAS.
- A. M. Palve, O. V. Vani and R. K. Gupta, Metal Oxide-Based Compounds as Flame Retardants for Polyurethanes, Materials and Chemistry of Flame-Retardant Polyurethanes Volume 2: Green Flame Retardants, American Chemical Society, 2021, pp. 121–136 Search PubMed.
- N. Wu and R. J. Yang, Effects of metal oxides on intumescent flame-retardant polypropylene, Polym. Adv. Technol., 2011, 22(5), 495–501 CrossRef CAS.
- Y. Zhou, X. Liu, F. Wang, J. W. Hao and J. X. Du, Effect of Metal Oxides on Fire Resistance and Char Formation of Intumescent Flame Retardant Coating, J. Inorg. Mater., 2014, 29(9), 972–978 CrossRef CAS.
- E. Gallo, B. Schartel, D. Acierno and P. Russo, Flame retardant biocomposites: Synergism between phosphinate and nanometric metal oxides, Eur. Polym. J., 2011, 47(7), 1390–1401 CrossRef CAS.
- N. Wu, R. J. Yang, J. W. Hao and G. S. Liu, Synergistic effect of metal oxides on intumescent flame-retardant pp systems, Acta Polym. Sin., 2009,(12), 1205–1210 CrossRef CAS.
- P. Y. Shao, P. Xu, L. Zhang, Y. Xue, X. H. Zhao, Z. C. Li and Q. Li, Non-Chloride in Situ Preparation of Nano-Cuprous Oxide and Its Effect on Heat Resistance and Combustion Properties of Calcium Alginate, Polymers, 2019, 11(11), 1760 CrossRef CAS.
- P. Kobedza, A. Smejda-Krzewicka, A. Olejnik and K. Strzelec, Flame retardant and durable chloroprene rubber and styrene-butadiene rubber blends crosslinked with copper(I) oxide, Iran. Polym. J., 2021, 30(2), 149–165 CrossRef CAS.
- H. S. Ababsa, Z. Safidine, A. Mekki, Y. Grohens, A. Ouadah and H. Chabane, Fire behavior of flame-retardant polyurethane semi-rigid foam in presence of nickel (II) oxide and graphene nanoplatelets additives, J. Polym. Res., 2021, 28(3), 1–14 Search PubMed.
- P. Gogoi, B. J. Saikia and S. K. Dolui, Effects of Nickel Oxide (NiO) Nanoparticles on the Performance Characteristics of the Jatropha Oil Based Alkyd and Epoxy Blends, J. Appl. Polym. Sci., 2015, 132(8), 41490 CrossRef.
- A. A. Sertsova, S. I. Marakulin and E. V. Yurtov, Metal Compound Nanoparticles: Flame Retardants for Polymer Composites, Russ. J. Gen. Chem., 2017, 87(6), 1395–1402 CrossRef CAS.
- N. Wu, C. Ding and R. J. Yang, Effects of zinc and nickel salts in intumescent flame-retardant polypropylene, Polym. Degrad. Stab., 2010, 95(12), 2589–2595 CrossRef CAS.
- Y. Y. Zhou, R. C. Tang, T. L. Xing, J. P. Guan, Z. H. Shen and A. D. Zhai, Flavonoids-metal salts combination: A facile and efficient route for enhancing the flame retardancy of silk, Ind. Crops Prod., 2019, 130, 580–591 CrossRef CAS.
- W. Zhang, M. Wang, J. P. Guan, R. C. Tang and Y. F. Qiao, Casein phosphopeptide-metal salts combination: A novel route for imparting the durable flame retardancy to silk, J. Taiwan Inst. Chem. Eng., 2019, 101, 1–7 CrossRef CAS.
- Y. X. Wu, X. W. Cheng, J. P. Guan and G. Q. Chen, Hydrophobic and flame-retardant coating on silk via ferric salt chelation, Surf. Eng., 2021, 37(11), 1432–1439 CrossRef CAS.
- J. Phanthuwongpakdee, T. Harimoto, S. Babel, S. Dwivedi, K. Takada and T. Kaneko, Flame retardant transparent films of thermostable biopolyimide metal hybrids, Polym. Degrad. Stab., 2021, 188, 109571 CrossRef CAS.
- K. P. Song, Y. T. Pan, J. Zhang, P. A. Song, J. Y. He, D. Y. Wang and R. J. Yang, Metal-Organic Frameworks-Based Flame-Retardant System for Epoxy Resin: A Review and Prospect, Chem. Eng. J., 2023, 468, 143653 CrossRef CAS.
- H. Nabipour, X. Wang, L. Song and Y. Hu, Metal-organic frameworks for flame retardant polymers application: A critical review, Composites, Part A, 2020, 139, 106113 CrossRef CAS.
- B. Y. Hou, W. Y. Zhang, H. Y. Lu, K. P. Song, Z. S. Geng, X. M. Ye, Y. T. Pan, W. C. Zhang and R. J. Yang, Multielement Flame-Retardant System Constructed with Metal POSS-Organic Frameworks for Epoxy Resin, ACS Appl. Mater. Interfaces, 2022, 14(43), 49326–49337 CrossRef CAS.
- J. W. Jiang, S. Q. Huo, Y. Zheng, C. Y. Yang, H. Q. Yan, S. Y. Ran and Z. P. Fang, A Novel Synergistic Flame Retardant of Hexaphenoxycyclotriphosphazene for Epoxy Resin, Polymers, 2021, 13(21), 3648 CrossRef CAS.
- T. Sai, S. Y. Ran, Z. H. Guo and Z. P. Fang, A Zr-based metal organic frameworks towards improving fire safety and thermal stability of polycarbonate, Composites, Part B, 2019, 176, 107198 CrossRef CAS.
- Y. B. Hou, W. Z. Hu, Z. Gui and Y. Hu, A novel Co(II)-based metal-organic framework with phosphorus-containing structure: Build for enhancing fire safety of epoxy, Compos. Sci. Technol., 2017, 152, 231–242 CrossRef CAS.
- X. Shi, X. Dai, Y. Cao, J. Li, C. Huo and X. Wang, Degradable Poly(lactic acid)/Metal–Organic Framework Nanocomposites Exhibiting Good Mechanical, Flame Retardant, and Dielectric Properties for the Fabrication of Disposable Electronics, Ind. Eng. Chem. Res., 2017, 56(14), 3887–3894 CrossRef CAS.
- W. Z. Xu, G. S. Wang, Y. C. Liu, R. Chen and W. Li, Zeolitic imidazolate framework-8 was coated with silica and investigated as a flame retardant to improve the flame retardancy and smoke suppression of epoxy resin, RSC Adv., 2018, 8(5), 2575–2585 RSC.
- R. A. Ilyas, S. M. Sapuan, M. R. M. Asyraf, D. Dayana, J. J. N. Amelia, M. S. A. Rani, M. N. F. Norrrahim, N. M. Nurazzi, H. A. Aisyah, S. Sharma, M. R. Ishak, M. Rafidah and M. R. Razman, Polymer Composites Filled with Metal Derivatives: A Review of Flame Retardants, Polymers, 2021, 13(11), 1701 CrossRef CAS PubMed.
- P. Lyu, Y. B. Hou, J. H. Hu, Y. Y. Liu, L. L. Zhao, C. Feng, Y. Ma, Q. Wang, R. Zhang, W. B. Huang and M. L. Ma, Composites Filled with Metal Organic Frameworks and Their Derivatives: Recent Developments in Flame Retardants, Polymers, 2022, 14(23), 5279 CrossRef.
- H. Peng, Y. Zhou, J. W. Hao, Z. S. Li and H. F. Zou, Effect of Metal Oxide Supported on Active Carbon on Vulcanization, Combustion and Thermal Ageing Properties of Flame Retardant Rubber, Chem. J. Chin., 2015, 36(12), 2569–2575 CAS.
- Y. M. Li, C. L. Wang and J. Li, Improvement of Metal Caions in Polyoxometalate on Flame Retardant Efficiency of Polypropylene, J. Inorg. Mater., 2020, 35(9), 1029–1033 CrossRef.
- Z. Yang, W. C. Guo, P. Yang, J. F. Hu, G. G. Duan, X. H. Liu, Z. P. Gu and Y. W. Li, Metal-phenolic network green flame retardants, Polymer, 2021, 221, 123627 CrossRef CAS.
- L. Ye, Y. Y. Miao, H. Yan, Z. Li, Y. L. Zhou, J. X. Liu and H. Liu, The synergistic effects of boroxo siloxanes with magnesium hydroxide in halogen-free flame retardant EVA/MH blends, Polym. Degrad. Stab., 2013, 98(4), 868–874 CrossRef CAS.
- X. Cheng, J. M. Wu, C. G. Yao and G. S. Yang, Flame-retardant mechanism of zinc borate and magnesium hydroxide in aluminum hypophosphite-based combination for TPE-S composites, J. Fire Sci., 2019, 37(3), 273–300 CrossRef CAS.
- R. Huang, X. Y. Guo, S. Y. Ma, J. X. Xie, J. Z. Xu and J. Ma, Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin, Polymers, 2020, 12(1), 108 CrossRef CAS PubMed.
- Y. J. Sun, M. Gao, Z. H. Chai and H. Wang, Thermal behavior of the flexible polyvinyl chloride including montmorillonite modified with iron oxide as flame retardant, J. Therm. Anal. Calorim., 2018, 131(1), 65–70 CrossRef CAS.
- W. F. Liu, L. Nie, L. H. Luo, J. F. Yue, L. Gan, J. Lu, J. Huang and C. H. Liu, Enhanced dispersibility and uniform distribution of iron phosphonate to intensify its synergistic effect on polypropylene-based intumescent flame-retardant system, J. Appl. Polym. Sci., 2020, 137(47), 49552 CrossRef CAS.
- L. H. Luo, W. F. Liu, L. Q. Zhai, W. Xie, L. Gan, H. L. Wang, J. Huang and C. H. Liu, Synergistic flame retardancy of aqueous hybridization between iron phosphonate and ammonium polyphosphate towards polyethyleneimine-based foam, Iran. Polym. J., 2020, 29(3), 265–274 CrossRef CAS.
- Y. Y. Li, X. M. Li, Y. T. Pan, X. Y. Xu, Y. Z. Song and R. J. Yang, Mitigation the release of toxic PH3 and the fire hazard of PA6/AHP composite by MOFs, J. Hazard. Mater., 2020, 395, 122604 CrossRef CAS PubMed.
- Q. L. Tai, R. K. K. Yuen, W. Yang, Z. H. Qiao, L. Song and Y. Hu, Iron-montmorillonite and zinc borate as synergistic agents in flame-retardant glass fiber reinforced polyamide 6 composites in combination with melamine polyphosphate, Composites, Part A, 2012, 43(3), 415–422 CrossRef CAS.
- Y. B. Hou, W. Z. Hu, Z. Gui and Y. Hu, Preparation of Metal-Organic Frameworks and Their Application as Flame Retardants for Polystyrene, Ind. Eng. Chem. Res., 2017, 56(8), 2036–2045 CrossRef CAS.
- W. Z. Xu, X. L. Wang, Y. Wu, W. Li and C. Y. Chen, Functionalized graphene with Co-ZIF adsorbed borate ions as an effective flame retardant and smoke suppression agent for epoxy resin, J. Hazard. Mater., 2019, 363, 138–151 CrossRef CAS.
- D. D. Gao, X. Wen, Y. Y. Guan, W. Czerwonko, Y. H. Li, Y. Gao, E. Mijowska and T. Tang, Flame retardant effect and mechanism of nanosized NiO as synergist in PLA/APP/CSi-MCA composites, Compos. Commun., 2020, 17, 170–176 CrossRef.
- B. H. Yuan, Y. Hu, X. F. Chen, Y. Q. Shi, Y. Niu, Y. Zhang, S. He and H. M. Dai, Dual modification of graphene by polymeric flame retardant and Ni (OH)(2) nanosheets for improving flame retardancy of polypropylene, Composites, Part A, 2017, 100, 106–117 CrossRef CAS.
- F. Q. Dong, Z. L. Luo and B. B. Wang, Preparation of Mn2+ Doped Piperazine Phosphate as a Char-Forming Agent for Improving the Fire Safety of Polypropylene/Ammonium Polyphosphate Composites, Materials, 2021, 14(24), 7589 CrossRef CAS.
- X. L. Chen, X. H. Chen, S. X. Li and C. A. M. Jiao, Copper metal-organic framework toward flame-retardant enhancement of thermoplastic polyurethane elastomer composites based on ammonium polyphosphate, Polym. Adv. Technol., 2021, 32(8), 2829–2842 CrossRef CAS.
- R. Z. Wang, Y. Chen, Y. Y. Liu, M. L. Ma, Z. Y. Tong, X. L. Chen, Y. X. Bi, W. B. Huang, Z. J. Liao, S. L. Chen, X. Y. Zhang and Q. Q. Li, Metal-organic frameworks derived ZnO@MOF@PZS flame retardant for reducing fire hazards of polyurea nanocomposites, Polym. Adv. Technol., 2021, 32(12), 4700–4709 CrossRef CAS.
- Z. H. Zhang, Z. Y. Ma, D. Zhang and Y. H. Wang, Effective enhancement of intumescent flame retardant coating by coordinating manganese ions on cotton fabrics, J. Vinyl Addit. Technol., 2022, 28(3), 604–614 CrossRef CAS.
- A. Jayakumar, R. P. Antony, J. Zhao and J. M. Lee, MOF-derived nickel and cobalt metal nanoparticles in a N-doped coral shaped carbon matrix of coconut leaf sheath origin for high performance supercapacitors and OER catalysis, Electrochim. Acta, 2018, 265, 336–347 CrossRef CAS.
- I. E. Uflyand, V. N. Naumkina and V. A. Zhinzhilo, Nanocomposites of Graphene Oxide and Metal-Organic Frameworks, Russ. J. Appl. Chem., 2021, 94(11), 1453–1468 CrossRef CAS.
- Y. Xie, X. J. Liu, X. X. Ma, Y. F. Duan, Y. W. Yao and Q. Cai, Small Titanium-Based MOFs Prepared with the Introduction of Tetraethyl Orthosilicate and Their Potential for Use in Drug Delivery, ACS Appl. Mater. Interfaces, 2018, 10(16), 13325–13332 CrossRef CAS PubMed.
- Y. L. Huang and B. H. Yuan, Reduced graphene oxide/iron-based metal-organic framework nano-coating created on flexible polyurethane foam by layer-by-layer assembly: Enhanced smoke suppression and oil adsorption property, Mater. Lett., 2021, 298, 129974 CrossRef CAS.
- Y. Koshiba, Y. Takahashi and H. Ohtani, Flame suppression ability of metallocenes (nickelocene, cobaltcene, ferrocene, manganocene, and chromocene), Fire Saf. J., 2012, 51, 10–17 CrossRef CAS.
- H. Fukaya, T. Ono and T. Abe, Theoretical study of reaction of trifluoromethyl radical with hydroxyl and hydrogen radicals, J. Comput. Chem., 1998, 19(3), 277–289 CrossRef CAS.
- H. R. Jiang, Y. Hu and Y. Jiang, Synthesis and flame inhibition efficiency of a novel Fe-based metal complex with phosphorus-containing structure, J. Solid State Chem., 2022, 309, 122975 CrossRef CAS.
- X. W. Yang, L. Zhao, F. Peng, Y. Zhu and G. Y. Wang, Co-based metal-organic framework with phosphonate and triazole structures for enhancing fire retardancy of epoxy resin, Polym. Degrad. Stab., 2021, 193, 109721 CrossRef CAS.
- Y. B. Hou, L. X. Liu, S. L. Qiu, X. Zhou, Z. Gui and Y. Hu, DOPO-Modified Two-Dimensional Co-Based Metal-Organic Framework: Preparation and Application for Enhancing Fire Safety of Poly(lactic acid), ACS Appl. Mater. Interfaces, 2018, 10(9), 8274–8286 CrossRef CAS.
- Z. M. Xu, W. Y. Xing, Y. B. Hou, B. Zou, L. F. Han, W. Z. Hu and Y. Hu, The combustion and pyrolysis process of flame-retardant polystyrene/cobalt-based metal organic frameworks (MOF) nanocomposite, Combust. Flame, 2021, 226, 108–116 CrossRef CAS.
- Z. B. Shao, J. Zhang, R. K. Jian, C. C. Sun, X. L. Li and D. Y. Wang, A strategy to construct multifunctional ammonium polyphosphate for epoxy resin with simultaneously high fire safety and mechanical properties, Composites, Part A, 2021, 149, 106529 CrossRef CAS.
- K. Q. Zhou, Z. Gui, Y. Hu, S. H. Jiang and G. Tang, The influence of cobalt oxide-graphene hybrids on thermal degradation, fire hazards and mechanical properties of thermoplastic polyurethane composites, Composites, Part A, 2016, 88, 10–18 CrossRef CAS.
- Y. L. Sui, L. J. Qu, X. Y. Dai, P. H. Li, J. R. Zhang, S. Luo and C. L. Zhang, A green self-assembled organic supermolecule as an effective flame retardant for epoxy resin, RSC Adv., 2020, 10(21), 12492–12503 RSC.
- W. G. Gong, M. Fan, J. Luo, J. Liang and X. Meng, Effect of nickel phytate on flame retardancy of intumescent flame retardant polylactic acid, Polym. Adv. Technol., 2021, 32(4), 1548–1559 CrossRef CAS.
- R. Riyazuddin, S. Bano, F. M. Husain, J. A. Siddique, K. H. Alharbi, R. A. Khan and A. Alsalme, Role of Copper Oxide on Epoxy Coatings with New Intumescent Polymer-Based Fire Retardant, Molecules, 2020, 25(24), 5978 CrossRef PubMed.
- P. F. Jia, C. Ma, J. Y. Lu, W. H. Yang, X. Jiang, G. Y. Jiang, Z. T. Yin, Y. Qiu, L. J. Qian, X. L. Yu, Y. Hu, W. Z. Hu and B. B. Wang, Design of copper salt@graphene nanohybrids to accomplish excellent resilience and superior fire safety for flexible polyurethane foam, J. Colloid Interface Sci., 2022, 606, 1205–1218 CrossRef CAS.
- H. Abd El-Wahab, M. Abd El-Fattah, H. M. Z. El-alfy, M. E. Owda, L. Lin and I. Hamdy, Synthesis and characterisation of sulphonamide (Schiff base) ligand and its copper metal complex and their efficiency in polyurethane varnish as flame retardant and antimicrobial surface coating additives, Prog. Org. Coat., 2020, 142, 105577 CrossRef CAS.
- J. Hong, T. Wu, X. Wang, Z. W. Lu, J. L. Zhang, B. R. Zeng, C. H. Yuan and L. Z. Dai, Copper-catalyzed pyrolysis of halloysites@polyphosphazene for efficient carbonization and smoke suppression, Composites, Part B, 2022, 230, 109547 CrossRef CAS.
- H. Nabipour, X. Wang, L. Song and Y. Hu, Facile synthesis of a novel zinc-triazole complex for simultaneous improvement in fire safety and mechanical properties of epoxy resins, Composites, Part A, 2021, 143, 106284 CrossRef CAS.
- R. R. Zhou, L. J. Lin, B. R. Zeng, X. D. Yi, C. Y. Huang, K. P. Du, X. H. Liu, Y. T. Xu, C. H. Yuan and L. Z. Dai, Diblock Copolymers Containing Titanium-Hybridized Polyhedral Oligomeric Silsesquioxane Used as a Macromolecular Flame Retardant for Epoxy Resin, Polymers, 2022, 14(9), 1708 CrossRef CAS PubMed.
- Y. X. Wei, C. Deng, W. C. Wei, H. Chen and Y. Z. Wang, Hybrid Nanorods Composed of Titanium, Silicon, and Organophosphorus as Additives for Flame-Retardant Polycarbonate, ACS Appl. Nano Mater., 2019, 2(8), 4859–4868 CrossRef CAS.
- F. C. Xu, H. Y. Zhang and J. G. Wu, Synergistic catalytic flame retardant effect of zirconium phosphate on the poplar plywood, Constr. Build. Mater., 2021, 290, 123208 CrossRef CAS.
- Z. B. Shao, X. Song, T. C. Wang, J. Cui, W. Ye, B. Y. Xu and D. Y. Wang, Facile Fabrication of organic zirconium/inorganic phosphorus complex for super-efficiently flame-retardant epoxy resin, Compos. Commun., 2022, 36, 101360 CrossRef.
- D. Wang, J. Z. Ma, J. J. Liu, A. L. Tian and S. H. Fu, Intumescent flame-retardant and ultraviolet-blocking coating screen-printed on cotton fabric, Cellulose, 2021, 28(4), 2495–2504 CrossRef CAS.
- Z. Yang, X. L. Kang, S. K. Lu, Z. H. Wang, X. M. Fang, J. T. Li, B. Y. Liu, T. Ding and Y. Q. Xu, Synergistic effects of molybdenum disulfide on a novel intumescent flame retardant polyformaldehyde system, J. Appl. Polym. Sci., 2023, 140(4), e53385 CrossRef CAS.
- L. X. He, F. K. Chu, X. Zhou, L. Song and Y. Hu, Cactus-like structure of BP@MoS2 hybrids: An effective mechanical reinforcement and flame retardant additive for waterborne polyurethane, Polym. Degrad. Stab., 2022, 202, 110027 CrossRef CAS.
- A. J. Li, W. Z. Xu, R. Chen, Y. C. Liu and W. Li, Fabrication of zeolitic imidazolate frameworks on layered double hydroxide nanosheets to improve the fire safety of epoxy resin, Composites, Part A, 2018, 112, 558–571 CrossRef CAS.
- L. B. Liu, M. J. Xu, Y. M. Hu, Y. Xu, Z. Y. Zhang and B. Li, Surface modification of magnesium hydroxide and its application in flame-retardant oil-extended styrene-ethylene-butadiene-styrene/polypropylene composites, J. Appl. Polym. Sci., 2019, 136(9), 47129 CrossRef.
- J. N. He, W. Zeng, M. M. Shi, X. Y. Lv, H. Fan and Z. Q. Lei, Influence of expandable graphite on flame retardancy and thermal stability property of unsaturated polyester resins/organic magnesium hydroxide composites, J. Appl. Polym. Sci., 2020, 137(1), 47881 CrossRef.
- M. J. Mochane, T. H. Mokhothu and T. C. Mokhena, Synthesis, mechanical, and flammability properties of metal hydroxide reinforced polymer composites: A review, Polym. Eng. Sci., 2022, 62(1), 44–65 CrossRef CAS.
- P. F. Zhao, W. Zeng, Z. W. Yang, Y. X. Yang, J. Li, J. P. Shi, N. Wen, H. T. Li, J. Guan, Z. Q. Lei and D. L. Chen, Preparation of a novel functionalized magnesium-based curing agent as an intrinsic flame retardant for epoxy resin, Chemosphere, 2021, 273, 129658 CrossRef CAS.
- A. Q. Wang, F. Zhang, L. P. Xing, Y. L. Zhu, W. L. Xie, X. Chen, J. J. Cheng and Y. F. Cheng, Effect of aluminum diethylphosphinate and its synergist on flame-retardant effects of epoxy resin, J. Therm. Anal. Calorim., 2022, 147(13), 7277–7287 CrossRef CAS.
- Q. Hu, L. Y. Zou, Z. H. Liu, J. Chen, J. Y. Liu and X. Q. Liu, Flame-retardant polyurethane elastomer based on aluminum salt of monomethylphosphinate, J. Therm. Anal. Calorim., 2021, 143(4), 2953–2961 CrossRef CAS.
- L. Liu, W. Wang, Y. Q. Shi, L. B. Fu, L. L. Xu and B. Yu, Electrostatic-Interaction-Driven Assembly of Binary Hybrids towards Fire-Safe Epoxy Resin Nanocomposites, Polymers, 2019, 11(2), 229 CrossRef PubMed.
- Z. B. Shao, J. Cui, X. B. Lin, X. L. Li, R. K. Jian and D. Y. Wang, In-situ coprecipitation formed Fe/Zn-layered double hydroxide/ammonium polyphosphate hybrid material for flame retardant epoxy resin via synergistic catalytic charring, Composites, Part A, 2022, 155, 106841 CrossRef CAS.
- Z. Li, Z. Q. Liu, J. Zhang, C. Fu, U. Wagenknecht and D. Y. Wang, Bio-based layered double hydroxide nanocarrier toward fire-retardant epoxy resin with efficiently improved smoke suppression, Chem. Eng. J., 2019, 378, 122046 CrossRef CAS.
- X. T. Chen, Y. L. Yin, J. Lu and X. H. Chen, Preparation and properties of iron-based flame-retardant reinforcing agent, J. Fire Sci., 2014, 32(2), 179–190 CrossRef.
Footnote |
| † These authors contributed equally: Haihan Zhao, Junwei Li. |
| This journal is © The Royal Society of Chemistry 2023 |