Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of Balanites aegyptiaca parts: metabolome providing insights into plant health benefits and valorization purposes as analyzed using multiplex GC-MS, LC-MS, NMR-based metabolomics, and molecular networking†
Mohamed A. Farag‡
 *a,
Mostafa H. Baky‡
*a,
Mostafa H. Baky‡ b,
Ibrahim Morgan
b,
Ibrahim Morgan c,
Mohamed R. Khalifad,
Robert Rennertc,
Osama G. Mohamedae,
Magdy M. El-Sayedf,
Andrea Porzelc,
Ludger A. Wessjohann
c,
Mohamed R. Khalifad,
Robert Rennertc,
Osama G. Mohamedae,
Magdy M. El-Sayedf,
Andrea Porzelc,
Ludger A. Wessjohann c and
Nehal S. Ramadang
c and
Nehal S. Ramadang
aPharmacognosy Department, College of Pharmacy, Cairo University, Cairo 11562, Egypt. E-mail: mohamed.farag@pharma.cu.edu.eg; Tel: +011-202-2362245
bPharmacognosy Department, Faculty of Pharmacy, Egyptian Russian University, Badr City, Cairo 11829, Egypt
cDepartment of Bioorganic Chemistry, Leibniz Institute of Plant Biochemistry (IPB), Weinberg 3, Halle (Saale) 06120, Germany
dGlobal Public Health Institute, American University in Cairo, New Cairo, Egypt
eNatural Products Discovery Core, Life Sciences Institute, University of Michigan, Ann Arbor, MI 48109, USA
fDairy Science Department, National Research Centre, Giza 12622, Egypt
gChemistry of Tanning Materials and Leather Technology Department, National Research Centre, Dokki, Giza 12622, Egypt
First published on 20th July 2023
Abstract
Balanites aegyptiaca (L.) Delile (Zygophyllaceae), also known as the desert date, is an edible fruit-producing tree popular for its nutritional and several health benefits. In this study, multi-targeted comparative metabolic profiling and fingerprinting approaches were conducted for the assessment of the nutrient primary and secondary metabolite heterogeneity in different parts, such as leaves, stems, seeds, unripe, and ripe fruits of B. aegyptiaca using nuclear magnetic resonance (NMR), ultra-performance liquid chromatography (UPLC-MS), and gas chromatography mass-spectrometry (GC-MS) based metabolomics coupled to multivariate analyses and in relation to its cytotoxic activities. NMR-based metabolomic study identified and quantified 15 major primary and secondary metabolites belonging to alkaloids, saponins, flavonoids, sugars, and amino and fatty acids. Principal component analysis (PCA) of the NMR dataset revealed α-glucose, sucrose, and isorhamnetin as markers for fruit and stem and unsaturated fatty acids for predominated seeds. Orthogonal projections to latent structure discriminant analysis (OPLS-DA) revealed trigonelline as a major distinctive metabolite in the immature fruit and isorhamnetin as a major distinct marker in the mature fruit. UPLC-MS/MS analysis using feature-based molecular networks revealed diverse chemical classes viz. steroidal saponins, N-containing metabolites, phenolics, fatty acids, and lipids as the constitutive metabolome in Balanites. Gas chromatography-mass spectroscopy (GC-MS) profiling of primary metabolites led to the detection of 135 peaks belonging to sugars, fatty acids/esters, amino acids, nitrogenous, and organic acids. Monosaccharides were detected at much higher levels in ripe fruit and disaccharides in predominate unripe fruits, whereas B. aegyptiaca vegetative parts (leaves and stem) were rich in amino acids and fatty acids. The antidiabetic compounds, viz, nicotinic acid, and trigonelline, were detected in all parts especially unripe fruit in addition to the sugar alcohol D-pinitol for the first time providing novel evidence for B. aegyptiaca use in diabetes. In vitro cytotoxic activity revealed the potential efficacy of immature fruit and seeds as cytotoxic agents against human prostate cancer (PC3) and human colorectal cancer (HCT-116) cell lines. Collectively, such detailed profiling of parts provides novel evidence for B. aegyptiaca medicinal uses.
1. Introduction
Recently, the growth of consumer demand for herbal supplements and functional foods owing to their medicinal value warranted the development of analytical tools to ensure their quality characteristics and active ingredient identification as novel quality verification techniques.1 Metabolite fingerprinting is one of the growing approaches found to be suitable to verify the quality of food-based matrices by profiling their components.2 Balanites aegyptiaca L. belongs to the family Zygophyllaceae, a fruit-producing tree widely distributed in Africa and in particular the tropical and subtropical regions of Africa.3 B. aegyptiaca is also indigenous in Egypt.4 B. aegyptiaca unripe and fully ripe fruits are nutritionally important as sweet and edible and consumed either fresh or processed into commercial beverages.3 Moreover, seeds yield fixed oil rich with significant nutrients and could be used for both culinary and biodiesel purposes.5B. aegyptiaca is commercially important as its leaves and fruits are used as livestock fodder; timber is used in furniture manufacturer; wood can be used for the production of charcoal for both fuel and industrial purposes; lastly, seed oil is used as biodiesel.3 Besides its industrial and food value, B. aegyptiaca exerts several health benefits being traditionally used for the treatment of several ailments.1 B. aegyptiaca is reported for its biological importance and benefits human health as being antidiabetic, antioxidant, anti-inflammatory, hepatoprotective, anticancer, and antimicrobial.3 Roots and bark are used as emetic, purgative, anthelmintic, and anti-malarial 1materials. The fruits are used for jaundice treatment and seed oil as a laxative, antihemorrhoids, stomach aches, jaundice, yellow fever, syphilis, and epilepsy.3
With regards to the chemical composition of B. aegyptiaca, ripe fruits, leaves, and seeds are rich in carbohydrates, protein, amino acids, vitamins, and fatty acids such as oleic, linoleic, and palmitic.6 Several phytochemicals are reported from B. aegyptiaca including polyphenols, flavonoids, saponins, coumarins, alkaloids, and steroids.7 Such rich chemical makeup in different parts of Balanites warrants the need for analytical tools, which can dissect metabolic pathways in each part and prioritize its potential uses based on detailed metabolites profiling.
In recent years, metabolomics is a growing analytical approach increasingly used for the quality assessment of food products to assure their quality in both targeted and untargeted manners.8 Metabolomics can be typically employed using either fingerprinting in which extracts are directly analyzed using spectroscopic techniques such as NMR versus profiling by performing a chromatographic step pre-detection aiding to improve sensitivity level and metabolites identification score.9 Although less sensitive than MS, NMR can provide the standardization of plant extracts using quantitative NMR with no need for standards.2 Recently, nuclear magnetic resonance (NMR) as a potent tool has been employed in conjunction with the liquid chromatography linked to MS (LC/MS) approach for the identification of major metabolites in diverse herbal crude extracts as indicated by various 1D and 2D techniques.10 Furthermore, 1H-NMR is employed for absolute quantitation of major identified metabolites in the specified sample extracts for further standardization objectives where the signal integration of the compound is directly proportional to its molar concentration.11 In order to categorize the complexity of obtained NMR datasets, multivariate analyses are frequently employed including unsupervised tools such as principal component analysis (PCA), hierarchical cluster analysis (HCA), and supervised orthogonal projections to latent structures discriminant analysis (OPLS-DA).9 The integration of multivariate analysis towards the analysis of spectral datasets provides a more powerful approach for addressing the chemical profile heterogeneity amongst different parts of Balanites, besides its quality assessments. For the assessment of metabolites heterogeneity among different parts of Balanites and to aid in identifying markers for each, a multiplex approach comparing MS and NMR-based metabolomics was employed for the analysis of stem, seed, leaf and fruits at different maturity stages for the first time. Compared to GC-MS employed for primary metabolites profiling, UHPLC-HRMS/MS was adopted for secondary metabolites profiling considering their polar non-volatile nature, whereas NMR provided standardization of its extracts. This work aimsed to investigate metabolome in the different B. aegyptiaca parts viz. immature fruit (BIF), mature fruit (BMF), leaf (BL), seed (BS), and stem (BST) in the context of its primary and secondary metabolites accounting for the plant health value. Further, cytotoxicity assessment of Balanites was performed in relation to their chemical profiles.
2. Materials and methods
2.1. Plant materials, chemicals and cell lines
Balanites aegyptiaca (L.) Delile samples including immature fruit (BIF), mature fruit (BMF), leaf (BL), seed (BS), and stem (BST) were collected from Wadi El Gemal National Park, Marsa Alam, Red Sea Governorate in Egypt in January 2022, and the plant was botanically identified by Eng. Ahmed Abdel-Razek, Senior Environmental Researcher Wadi El-Gamal-Hamata National Park (WGNP).Hexamethyldisiloxane (HMDS) and methanol-d4 (99.80% D) were supplied by Deutero GmbH (Kastellaun, Germany). Formic acid and acetonitrile of LC-MS grade were obtained from J. T. Baker (The Netherlands), and Milli-Q water was used for LC analysis. All other standards as well as chemicals were obtained from Sigma Aldrich (St. Louis, MO, USA). Two human cell lines were used to determine the anti-proliferative and cytotoxic activity of the extracts, namely PC-3 prostate cancer and HCT-116 colorectal cancer cells. Both cell lines were obtained from DSMZ (Braunschweig, Germany) by the Leibniz Institute of Plant Biochemistry (IPB), where all cell-based assays were conducted. Cell culture materials including the basal media RPMI 1640 and McCoy's 5A, phosphate-buffered saline (PBS), 0.05% trypsin–EDTA, and fetal calf serum (FCS) were purchased from Capricorn Scientific (Ebsdorfergrund, Germany). For the cell viability and cytotoxicity assays, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), crystal violet (CV) and paraformaldehyde (PFA) were used from Sigma Aldrich (St. Louis, MO, USA). The acetic acid solution was purchased from Carl Roth (Karlsruhe, Germany).
2.2. Sample preparation for NMR and MS analyses
The sample preparation was following the protocol described in Zayed et al.12 In brief, 120 mg of each dried organ powder was homogenized with 5 mL 100% methanol with 10 μg mL−1 of umbelliferone as an internal standard using a Turrax mixer (9469×g) for five 20 s periods, with 1 min interval to avoid warming. Afterwards, vigorous centrifugation (3000×g for 30 min) was performed to remove plant debris.2.3. NMR data acquisition
All spectra were recorded on an Agilent VNMRS 600 NMR spectrometer operating at a proton NMR frequency of 599.83 MHz using a 5 mm inverse detection cryoprobe, digital resolution 0.367 Hz per point (32k complex data points), pulse width (pw) = 2.1 μs (30°), relaxation delay = 18 s, acquisition time = 2.0 s, number of transients = 160, and temperature = 297 K. Zero filling up to 128 K and an exponential window function with lb = 0.4 was used prior to Fourier transformation. The 2D-NMR spectra were recorded at a frequency of 599.83 MHz using standard CHEMPACK 6.2 pulse sequences (TOCSY and HSQC) implemented in standard VNMRJ 4.0 A spectrometer software. The HSQC experiment was optimized for 1JCH = 146 Hz with DEPT-like editing and 13C-decoupling during the acquisition time.2.4. NMR data processing and multivariate data analyses
Spectra were imported to Mnova Mestrelab Research version 14 software. The spectra were referenced to internal HMDS at 0.062 ppm for 1H-NMR. Spectral intensities were reduced to integrated regions, referred to as buckets, of equal width (0.04 ppm) for all spectral (δ 0.4–10.0 ppm) and aromatic (δ 5.5–10.0 ppm) regions. The spectral regions corresponding to the residual solvent signals, δ 5.45–5.87 ppm (water), δ 3.33–3.28 ppm (methanol) and δ 7.62–7.42, δ 8.85–8.69 ppm (impurities), were removed before multivariate analyses. This binning allowed the evaluation of the absolute quantification of the identified metabolites.SIMCA-P version 13.0 (Umetrics, Umea, Sweden) was used to import the bucket table. Hierarchical cluster analysis (HCA), principal component analysis (PCA), and orthogonal projection to latent structure-discriminant analysis (OPLS-DA) were performed with all variables mean-centered and scaled to Pareto variance. The seven-fold cross-validation approach was used to determine the optimal number of main components requisite for data modelling, and the distance to the model (DModX) test was employed to confirm the presence of outliers. Permutation tests, receiver operating characteristic (ROC) curves, and CV-ANOVA were used to verify the constructed OPLS-DA models (ANOVA of cross-validated residuals). The S-plot, which was reported with covariance (p) and correlation (pcor), besides, the variable influence in the projection (VIP) was then analyzed to identify metabolite markers (VIP).
2.5. Quantification of major metabolites via 1H-NMR
The peak area of the selected proton signals pertaining to the target compounds and the peak area of the internal standard (HMDS) were manually integrated for all samples for the quantification of metabolites indicated in Tables 1 and 2 using the NMR technique.| Metabolite | Assignment | δ 1H (ppm) | δ 13C in HSQC (ppm) | TOCSY correlations δ 1H (ppm) | Parts |
|---|---|---|---|---|---|
| a Overlapped.b nd, not detected; BIF, Balanites immature fruit; BMF, Balanites mature fruit; BL, Balanites leaf; BS, Balanites seed; BST, Balanites stem. | |||||
| Alkaloids | |||||
| Trigonelline (N1) | C-2 | 9.3 (s) | 149.2 | — | BIF, BMF, BL, BS, BST |
| C-4 | 8.95 (d, J = 8.0 Hz) | 145.6 | 9.02 (H-6), 8.14 (H-5) | ||
| C-5 | 8.14 (dd, J = 8.0, 6.0 Hz) | 128.6 | 8.95 (H-4), 9.02 (H-6) | ||
| C-6 | 9.02 (d, J = 6.1 Hz) | 144.9 | 8.14 (H-5) | ||
| N–CH3 | 4.4 (s) | nd | — | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Saponins | |||||
| Diosgenin (N2) | C-3 | 5.02 (m) | 82.9 | 4.58 (H-6) | BIF, BMF, BL, BS, BST |
| C-6 | 4.58 (dd, J = 5.5, 1.98 Hz) | 121.2 | 5.02 (H-3) | ||
| C-18 | 0.8 (s) | 18.03 | — | ||
| C-19 | 1.02 (s) | 21.2 | — | ||
| C-27 | 0.78 (d, J = 6.3 Hz) | 17.5 | — | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Flavonoids | |||||
| Isorhamnetin (N3) | C-6 | 6.25 (d, J = 1.92 Hz) | 101.5 | 6.51 (H-8) | BMF, BL, BS, BST |
| C-8 | 6.51 (d, J = 1.92 Hz) | nd | 6.25 (H-6) | ||
| C-3′ (OCH3) | 3.70a (s) | 56.5 | Overlapped | ||
| C-5′ | 6.74 (d, J = 8.52 Hz) | nd | 7.13 (H-6′) | ||
| C-6′ | 7.13 (br.d, J = 8.52 Hz) | 121.6 | 6.74 (H-5′) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Sugars | |||||
| α-Glucose (N4) | C-1 | 5.19 (d, J = 3.72 Hz) | 95.2 | 3.46 (H-2), 3.70 (H-3) | BIF, BMF, BL, BS, BST |
| C-2 | 3.46a (m) | 75 | 3.70 (H-3) | ||
| C-3 | 3.70a | 76.2 | Overlapped | ||
| β-Glucose (N5) | C-1 | 4.41 (d, J = 5.88 Hz) | 100.2 | nd | BIF, BMF, BL, BS, BST |
| C-6 | 3.64 | 65.5 | Overlapped | ||
| Galactose (N6) | C-1 | 4.31 (d, J = 7.34 Hz) | 105.9 | 3.46 (H-2) | BLF, BLMF, BL, BS, BST |
| C-2 | 3.46a | 75 | 4.31 (H-1) | ||
| Sucrose (N7) | C-1 | 4.66 (br s) | 91.6 | 3.50 (H-2), 3.70 (H-3) | BIF, BMF, BL, BS, BST |
| C-2 | 3.50 (dd, J = 3, 9.9 Hz) | 79.2 | Overlapped | ||
| C-3 | 3.70 | 74.7 | Overlapped | ||
| C-6 | 3.87 (d, J = 1.93 Hz) | 63.7 | nd | ||
| C-2′ | 4.13a (br s) | 79.1 | 3.70 (H-4′ and H-5′) | ||
| C-3′ | 4.13a | 78.0 | 3.70 (H-4′ and H-5′) | ||
| C-4′ | 3.70a (s) | 83.7 | Overlapped | ||
| C-5′ | 3.70a (s) | 62.0 | Overlapped | ||
| C-6′ | 3.65 s | 65.4 | nd | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Amino acids/nitrogenous compounds | |||||
| Alanine (N8) | C-2 | 3.59a | 52.1 | 1.54 (H-3) | BIF, BIMF, BL, BS, BST |
| C-3 | 1.54 (d, J = 7.3 Hz) | 18.4 | 3.59 (H-2) | ||
| Valine (N9) | C-4 | 1.04 (d, J = 6.96 Hz) | 19.4 | Overlapped | BIF, BIMF, BL, BS, BST |
| C-5 | 1.08 (d, J = 6.96 Hz) | 20.1 | Overlapped | ||
| Phenylalanine (N10) | C-2 | 3.80 (m) | 58.5 | Overlapped | BIF, BIMF, BL, BS, BST |
| C-4′ | 7.34a | 128.3 | 7.30 (H-3′ and H-5′), 7.27 (H-2′ and H-6′) | ||
| C-3′/C-5′ | 7.30a | 128.3 | Overlapped | ||
| C-2′/C-6′ | 7.27a | 128.3 | Overlapped | ||
| Threonine (N11) | C-2 | 3.74a | 61.6 | 1.24 (H-4), 4.16 (H-3) | BIF, BMF, BL, BS, BST |
| C-3 | 4.16a | 67.7 | 3.74 (H-2), 1.24 (H-4) | ||
| C-4 | 1.24 (d, J = 6.33 Hz) | 19.3 | 3.74 (H-2), 4.16 (H-3) | ||
| Choline (N12) | N–(CH3)3 | 3.25 (s) | 55.9 | — | BLF, BLMF, BL, BS, BST |
| N–CH2 | 3.40 | 72.9 | 4.22 (O–CH2) | ||
| O–CH2 | 4.22 m | 63.3 | 3.40 (N–CH2) | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Fatty acids | |||||
| ω-9, ω-6, ω-3 fatty acids | Olefinic carbons | 5.30–5.35 (m) | nd | 2.81 (bis-allylic CH2), 2.01 (allylic CH2) | BIF, BMF, BL, BS, BST |
| Allylic CH2 | 2.01 (m) | nd | 1.28 (CH2)n, 5.30 (olefinic CH) | ||
| ω-9 oleic acid (N13) | t-CH3 | 0.89 (t, 6.96 Hz) | 15.65 | 1.28 (CH2)n | BLF, BLMF, BL, BS, BST |
| (CH2)n | 1.28 (br s) | 31.7 | 0.89 (t-CH3), 1.60 (H-3), 2.29 (H-2), 2.01 (allylic CH2) | ||
| C-2 | 2.29 (t, 7.44 Hz) | nd | 1.28 (CH2)n, 1.60 (H-3) | ||
| C-3 | 1.60 (m) | 27.3 | 1.28 (CH2)n, 2.29 (H-2) | ||
| ω-6 fatty acid (N14) | Bis-allylic CH2 | 2.77 (br s) | nd | 5.33 (olefinic CH) | BIF, BMF, BL, BS, BST |
| ω-3 fatty acid (N15) | t-CH3 | 0.94 (t, J = 7.4 Hz) | 15.65 | nd | BIF, BMF, BL, BS, BST |
| Bis-allylic CH2 | 2.81 (t, J = 4.5 Hz) | 27.7 | 5.33 (olefinic CH) | ||
| Metabolite | Protons used in quantification | BIF | BMF | BL | BS | BST |
|---|---|---|---|---|---|---|
| a nd: not detected. | ||||||
| Trigonelline | H-2 | 1.08 ± 0.05 | 0.2 ± 0.01 | 0.7 ± 0.1 | 0.3 ± 0.06 | 0.4 ± 0.2 |
| Diosgenin | H-3 | Overlap | Overlap | 2.5 ± 1.5 | 4.7 ± 0.9 | 0.4 ± 0.2 |
| Isorhamnetin | H-6 | nda | 0.2 ± 0.03 | 1.3 ± 0.7 | 0.5 ± 0.2 | 0.09 ± 0.05 |
| α-Glucose | H-1 | 0.02 ± 0.002 | 0.2 ± 0.008 | 0.003 ± 0.004 | 0.02 ± 0.0007 | 0.01 ± 0.003 |
| β-Glucose | H-1 | 0.08 ± 0.002 | 0.03 ± 0.005 | 0.007 ± 0.001 | 0.04 ± 0.01 | 0.005 ± 0.003 |
| Galactose | H-1 | 0.02 ± 0.001 | Overlap | 0.003 ± 0.0004 | 0.02 ± 0.0007 | 0.001 ± 0.0006 |
| Sucrose | H-1 | 0.8 ± 0.4 | 2.9 ± 0.9 | 0.6 ± 0.3 | 0.2 ± 0.4 | 0.3 ± 0.4 |
| Alanine | H-3 | Overlap | Overlap | 2.08 ± 0.5 | 2.8 ± 0.3 | 0.8 ± 0.07 |
| Valine | H-5 | 6.1 ± 0.3 | Overlap | 2.08 ± 0.2 | 2.7 ± 0.1 | 0.7 ± 0.04 |
| Phenylalanine | H-3′, H-4′, H-5′ | Overlap | Overlap | 0.4 ± 0.04 | Overlap | 0.2 ± 0.1 |
| Threonine | H-4 | 10.6 ± 0.9 | 14.2 ± 0.4 | 3.9 ± 0.5 | 6.2 ± 0.4 | 1.2 ± 0.2 |
| Choline | N–(CH3)3 | 0.4 ± 0.03 | 1.3 ± 0.1 | 0.3 ± 0.05 | 0.7 ± 0.05 | 0.2 ± 0.01 |
| ω-9 fatty acid | t-CH3 | 12.7 ± 0.08 | 25.6 ± 2.1 | 17.9 ± 1.3 | 89.7 ± 1.6 | 10.7 ± 0.6 |
| ω-3 fatty acid | Bis-allylic CH2 | Overlap | 9.3 ± 0.3 | 3.2 ± 1.1 | Overlap | 1.5 ± 0.4 |
| ω-6 fatty acid | Bis-allylic CH2 | 9.5 ± 1.8 | 13.7 ± 0.4 | Overlap | 44.7 ± 1.6 | 0.3 ± 0.2 |
The following eqn (1) was applied for the calculations and the protocol was adopted from:12
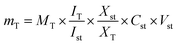 | (1) |
mT: mass of the target compound in the solution used for 1H-NMR measurement in μg. MT: molecular weight of the target compound (g mol−1). IT: relative integral value of the 1H-NMR signal of the target compound. ISt: relative integral value of the 1H-NMR signal of the standard compound. XSt: number of protons belonging to the 1H-NMR signal of the standard compound. XT: number of protons belonging to the 1H-NMR signal of the target compound. CSt: concentration of the standard compound in the solution used for 1H-NMR measurement (mmol L−1). VSt: volume of the solution used for 1H-NMR measurement in mL.
2.6. Analysis, modelling and quantification of UPLC/PDA/MS dataset
2.7. GC-MS profiling and modelling of silylated primary metabolites
The protocol to validate silylation was adopted as previously reported.19 Soluble sugars, amino acids, organic acids, and fatty acids were quantified using standard curves of glucose, glycine, citric and stearic acids and results were expressed as mg g−1. Four serial dilutions were prepared from 10 to 600 μg mL−1 for establishing the standard curves. Calibration curves for glucose, glycine, citric acid, and stearic acids displayed a 0.9948 correlation coefficient.20Identification of components was performed by comparing their retention indices (RI) in relation to n-alkanes (C6–C20), mass matching to NIST, WILEY library database, and standards if available. Peaks were first deconvoluted using AMDIS software (https://www.amdis.net) accessed on 28 November 2019 (ref. 8) before mass spectral matching. Data were then subjected to principal component analysis (PCA), hierarchical clustering analysis (HCA), and partial least squares discriminant analysis (OPLS-DA) using SIMCA-P version 13.0 software package (Umetrics, Umeå, Sweden). Markers were subsequently identified by analyzing the S-plot, which was declared with covariance (p) and correlation (pcor). All variables were mean-centered and scaled to Pareto variance. Model validation was assessed by computing the diagnostic indices, viz. Q2 and R2 values, and permutation testing.
2.8. Cytotoxicity assay
After 48 h incubation, in the case of MTT assay, the treatment solution was discarded, and the cells were washed with 50 μL per well of PBS. Afterwards, the cells were stained with 50 μL of MTT working solution (0.5 mg MTT per mL in RPMI 1640) for 30 min. Subsequently, the staining solution was discarded, and the formazan dye – converted by the viable cell fraction – was dissolved using DMSO. Finally, the formazan absorbance was measured at 570 nm (and background at 670 nm for data normalization) by using a SpectraMax iD5 multi-well plate reader (Molecular Devices, San Jose, CA, USA).23 For CV assay, cells were washed with 50 μL per well of PBS, followed by cell fixation using 4% (w/v) PFA in PBS for 10 min. After fixation, the PFA solution was discarded, and the plates were dried for another 10 min. For CV staining, cells were incubated with 50 μL per well of CV solution (0.1% (w/v) in PBS) for 15 min. Afterwards, the cells were washed with ddH2O and dried overnight. Subsequently, the CV stain was dissolved using 33% (v/v) acetic acid in ddH2O, and absorbance was measured with the SpectraMax iD5 using the aforementioned parameters as well.24 Data were determined in three independent biological replicates, each with technical quadruplicates. IC50 curves and values of the extracts were calculated by using GraphPad Prism® version 8.0.2 using sigmoidal dose–response curve fitting (variable slope, four parameters).
2.9. Statistical analysis
Duplicate samples of each Balanites part for each treatment/period were taken. Each sample was analyzed individually in triplicate. The data are presented as mean (standard deviation of three determinations. A probability value of P < 0.05 was considered to denote the statistically significant differences.3. Results and discussion
To provide a comparative assessment of datasets derived from various metabolomic platforms including NMR, UPLC-MS, and GC-MS metabolomics, a one-pot extraction method was developed (see Section 2.2 Sample preparation for NMR and MS analyses) was established. Chemometric tools were employed to categorize samples, assure analytical robustness, establish similarities as well as differences amongst samples, and lastly compare the different analytical platforms based on the model's power for the classification potential for the first time in Balanites.3.1. NMR fingerprinting of Balanites parts
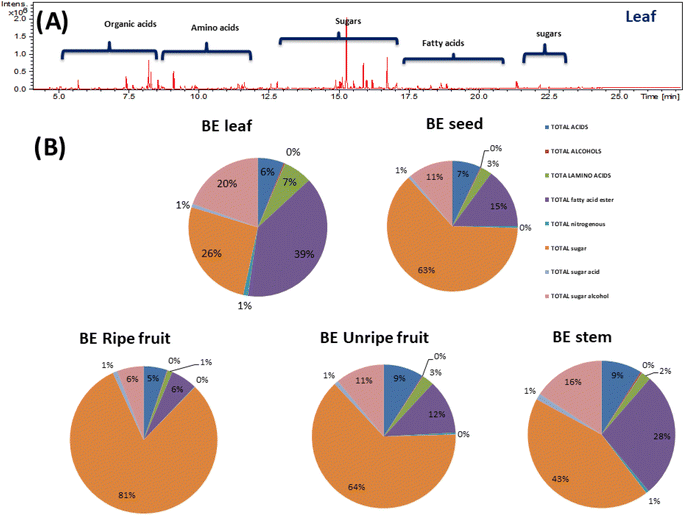
NMR-based metabolomics fingerprinting is a well-established technique for the identification and quantification of metabolites in crude extracts providing a readout of metabolite classes abundance in extracts owing to its universal detection.25 A total of 15 metabolites were identified based on 1D- and 2D-NMR analyses (Fig. S1†) and listed alongside their chemical shifts as well as their distribution amongst the different Balanites parts in Table 1. The peak assignments were identified according to the previously reported literature,4 besides the further confirmation of signal assignments using 2D-NMR experiments including 1H–13C HSQC (heteronuclear single quantum coherence spectroscopy) and 1H–1H TOCSY (total correlation spectroscopy). Two regions mainly appeared for all parts in the 1H-NMR spectra (Fig. S1†): an up-field region (δ 0.5 to 5.5 ppm) mostly relevant to primary metabolites viz. saponins (N2), sugars (N4–N7), amino acids (N8–N11), nitrogenous compounds (N12) and fatty acids (N13–N15) and a down-field region (δ 5.5–9.5 ppm) assigned to secondary metabolites, i.e., alkaloids (N1), flavonoids (N3), and phenylalanine (N10).3.2. Quantification of major Balanites metabolites using NMR
The absolute quantification of identified metabolites in Balanites parts was also estimated using 1H-NMR by integrating their well-determined respective peaks in the NMR spectra expressed as μg per mg dry powder (Table 2). Fatty acids amounted to a major metabolite class in all Balanites parts at the highest level of 134.4 μg mg−1 in Balanites seeds (BS) versus lowest level at ca. 12.5 μg mg−1 in the Balanites stem (BST), consistent with the fact of the storage of plant fatty acids in seeds.35 The abundance of unsaturated fatty acids in all Balanites parts especially seeds is suggestive of their importance as functional foods to decrease serum LDL cholesterol and the risk of atherosclerosis.36,37 Regarding amino acids, threonine represented the major amino acid in all parts ranging from 1.2 to 14.2 μg mg−1. L-Threonine is an essential amino acid known for its role in immune function regulation and improving antidiabetic activity.38,39 Valine and alanine were detected at lower levels ranging from 0.7 to 6.1 and 0.8 to 2.8 μg mg−1, respectively. Further, choline was quantified in all organ extracts, however, at low concentrations at ca. (0.2–1.3 μg mg−1). With regard to sugars, sucrose was the predominant form at 0.2 to 2.9 μg mg−1, whereas glucose (α- and β-forms) and galactose were detected at trace levels in all parts and suggestive that di-sugars account for Balanites taste.The hypo-glycemic alkaloid, trigonelline1 was detected in all parts ranging from 0.2 to 1.08 μg mg−1, with immature fruit being the most rich. Whereas, diosgenin to represent saponins was detected at the highest level in seeds at 4.7 μg mg−1 versus 0.4 μg mg−1 in the stem. The flavonoid isorhamnetin was detected in all parts at 0.1–1.3 μg mg−1, except for immature fruits.
3.3. Multivariate data analysis of NMR dataset
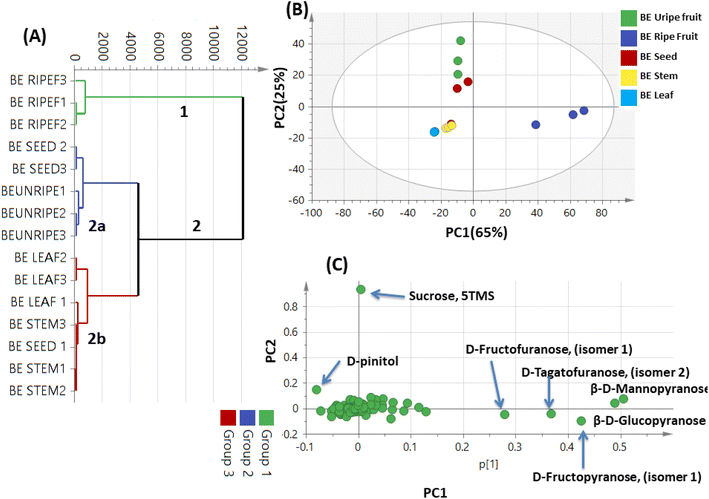
A total of 15 samples were subjected to modelling in this study (5 different parts from the same plant; each represented by 3 biological replicates) for identification of markers among the different parts. Both PCA and HCA were initially constructed to discriminate between parts' metabolic profiles in two attempts, from all spectral width (δ 0.4–10.0 ppm) and from the aromatic region only (δ 5.5–10.0 ppm) to more focus on secondary metabolites in Balanites parts, i.e., alkaloids, and flavonoids (Fig. 1).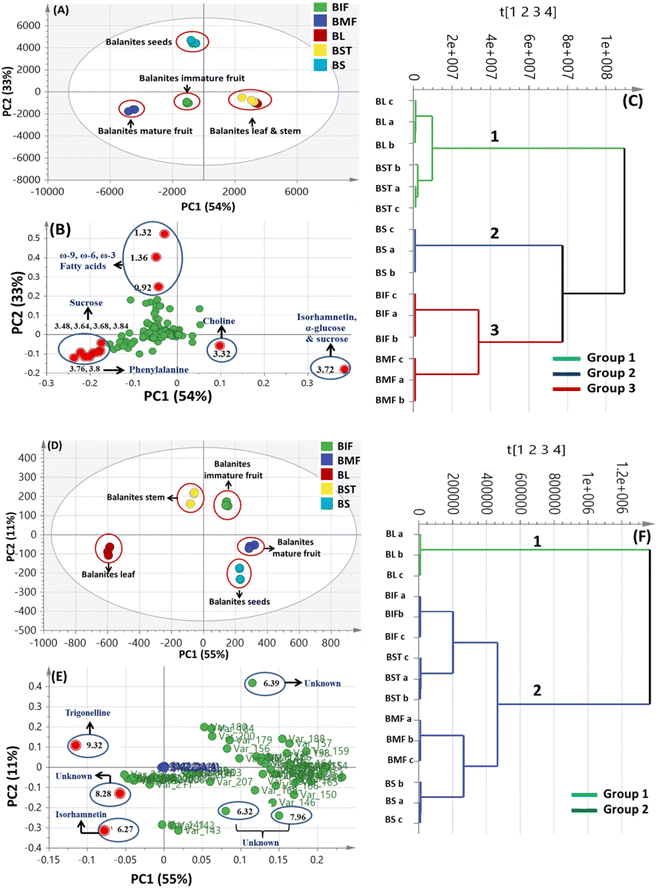 | ||
| Fig. 1 Unsupervised multivariate data analyses of Balanites parts dataset in the full 1H-NMR scale (δH 0–10 ppm) (A–C) and the aromatic region (δH 5.5–10 ppm) in (D–F). (A and D) Principal component analysis (PCA) score plot of PC1 vs. PC2. (B and E) Loading plot for PC1 and PC2 showing the major signals contributing to sample discrimination. (C and F) Hierarchical cluster analysis (HCA). The metabolites assignment is summarized in Table S1.† The model is colored according to marked groups. | ||
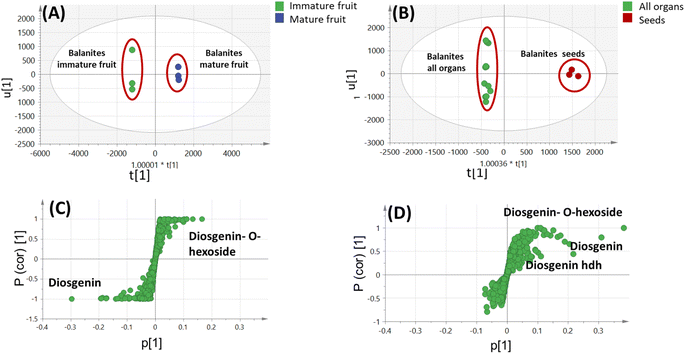
OPLS model exhibited Q2 = 0.97, indicating strong model predictability and total variance coverage of 99% (R2 = 0.99). The respective loading S-plot (Fig. S2B†) revealed that trigonelline was the major distinctive metabolite in immature fruit, whereas isorhamnetin was the major distinct marker in the mature fruit sample, though with a non-significant p-value of 0.18.
3.4. Metabolites profiling of Balanites via UHPLC-ESI-QTOF-MS/MS
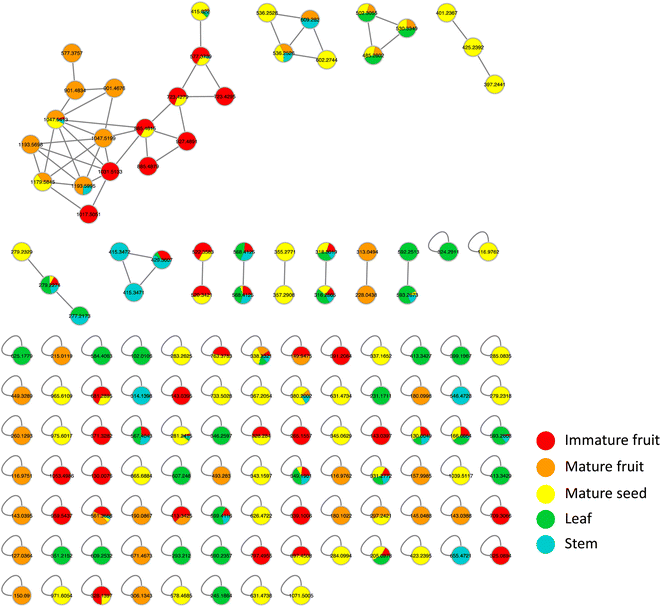
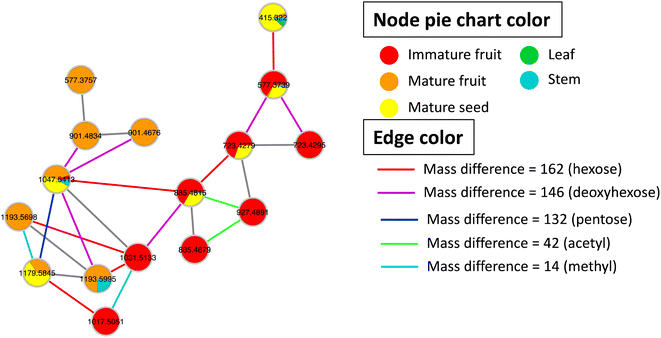
Metabolites profiling of the different B. aegyptiaca parts was carried out via UHPLC-ESI-QTOF-MS/MS (Fig. S3†), in an attempt to identify the distribution of secondary metabolites. Data were further processed employing the feature-based molecular networking (FBMN) approach and web-based tool GNPS. The output of data processing using MZmine 2 included a total of 133 features after removing features corresponding to methanol blank, in-source fragments, and sodium adducts. GNPS-FBMN workflow was performed generating a molecular network where structurally-related metabolites were linked together based on the similarity of their MS/MS fragmentation. The generated molecular network (Fig. 2) showed 45 features comprising 12 spectral families in addition to 88 singleton features. As listed in Table 3, 39 metabolites were identified belonging to different chemical classes including steroidal saponins, oxylipids, phenolic amides, and flavonoids. Visual analysis of MS/MS data via molecular networking enabled the annotation of metabolites, together with highlighting differential features between dissimilar samples that are different parts of B. aegyptiaca. The node pie chart color reflected the relative abundance of features in different parts.| No. | Rt (min) | Compound name | m/z | Molecular formula | Mass error (ppm) | MS2 product ions | IFa | MFa | MSa | La | STa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a IF: immature fruit; MF: mature fruit; MS: mature seed; L: leaf; ST: stem. | |||||||||||
| Steroidal saponins | |||||||||||
| 1 | 6.58 | Diosgenin | 415.322 | C27H42O3+ | 3.2 | 397.3114, 379.2969, 271.2062, 253.1950 | — | √ | √ | √ | √ |
| 2 | 5.49 | Diosgenin-26-hexoside | 577.3757 | C33H53O8+ | 3.8 | 415.3216, 271.2064, 253.1959 | — | √ | — | — | — |
| 3 | 6.58 | Diosgenin-3-hexoside | 577.3739 | C33H53O8+ | 0.7 | 433.2453, 271.1976, 253.1870 | √ | √ | √ | — | √ |
| 4 | 6.59 | Diosgenin hd | 723.4279 | C39H63O12+ | 3.3 | 579.3007, 577.3590, 433.2454, 415.3092, 397.2987, 271.1978, 253.1870 | √ | — | √ | — | — |
| 5 | 7.34 | Diosgenin hd isomer | 723.4295 | C39H63O12+ | −2.6 | 579.3166, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 332 332![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 488, 415.3213, 271.2047, 253.1945 488, 415.3213, 271.2047, 253.1945 |
√ | — | — | — | — |
| 6 | 6.47 | Diosgenin hdh | 885.4879 | C45H73O17+ | 4.1 | 741.3710, 739.4310, 723.4351, 577.3663, 415.3200, 309.1176, 271.2092, 253.1960 | √ | — | — | — | — |
| 7 | 6.80 | Diosgenin hdh | 885.4815 | C45H73O17+ | −3.1 | 741.3469, 723.4102, 577.3587, 415.3086, 309.1081, 271.1972, 253.1871 | √ | — | √ | — | — |
| 8 | 7.25 | Diosgenin hdha | 927.4891 | C47H75O18+ | −6.1 | 783.3533, 781.4193, 577.3549, 415.3093, 351.1160, 271.1974, 253.1870 | √ | — | — | — | — |
| 9 | 3.92 | Diosgenin hhh | 901.4834 | C45H73O18+ | 4.7 | 739.4308, 595.3138, 577.3756, 433.2610, 415.3220, 271.2064, 253.1967 | — | √ | — | — | — |
| 10 | 4.68 | Diosgenin hhh isomer | 901.4824 | C45H73O18+ | 3.6 | 739.4279, 577.3749, 415.3217, 271.2061, 253.1952 | — | √ | — | — | — |
| 11 | 6.55 | Diosgenin hdhd | 1031.5432 | C51H83O21+ | 1.0 | 885.4841, 723.4326, 577.3740, 415.3215 | √ | — | — | — | — |
| 12 | 6.59 | Diosgenin hdhp | 1017.5322 | C50H81O21+ | 5.6 | 871.4772, 723.4346, 577.3757, 415.3224 | √ | — | — | — | — |
| 13 | 3.87 | Diosgenin hdhh | 1047.5413 | C51H83O22+ | 4.0 | 885.4874, 723.4327, 577.3743, 415.3219, 271.2061, 253.1966 | √ | √ | √ | — | √ |
| 14 | 3.85 | Diosgenin hdhhp | 1179.5845 | C56H90O26+ | 4.4 | 1017.5343, 885.4865, 723.4343, 577.3751, 415.3216, 271.2047 | — | √ | √ | — | — |
| 15 | 3.83 | Diosgenin hdhhd | 1193.5995 | C57H93O26+ | 3.8 | 1031.5431, 885.4876, 723.4333, 577.3747, 415.3214, 271.2057, 253.1945 | — | √ | — | — | √ |
| 16 | 4.58 | Diosgenin hdhhd isomer | 1193.5615 | High mass error | — | √ | — | — | — | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| N-Containing metabolites | |||||||||||
| 17 | 0.82 | Xanthosine | 285.0835 | C10H13N4O6+ | 1.9 | 153.0406, 133.0492, 115.0385 | — | — | √ | — | — |
| 18 | 1.51 | Tryptophan | 205.0978 | C11H13N2O2+ | 3.1 | 188.0708, 146.0603, 118.0654 | √ | — | √ | √ | — |
| 19 | 11.75 | Pheophorbide A | 593.2608 | C39H37N4O5+ | High mass error | 533.2387 | √ | — | — | √ | √ |
| 20 | 12.09 | Pheophorbide A isomer | 593.2778 | C39H37N4O5+ | 3.3 | 533.2563 | — | — | √ | √ | √ |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Phenolics | |||||||||||
| 21 | 3.14 | Narcissin | 625.1779 | C28H33O16+ | 2.5 | 479.1154, 317.0659, 129.0546 | |||||
| 22 | 3.98 | Feruloyltyramine | 314.1398 | C18H20NO4+ | 3.5 | 177.0542, 145.0279, 121.0648 | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||
| Fatty acids and lipids | |||||||||||
| 23 | 12.88 | Sphingolipid | 568.4125 | C39H59NO5P+ | −0.07 | 476.3528, 430.3099, 338.2504 | √ | √ | √ | √ | √ |
| 24 | 13.49 | Sphingolipid | 568.4125 | C39H59NO5P+ | −0.07 | 476.3512, 430.3104, 338.2507 | √ | — | — | √ | √ |
| 25 | 0.84 | 2-Methylideneglutaric acid | 145.0488 | C6H9O4+ | −5.1 | 127.0389, 104.0082 | — | √ | — | — | — |
| 26 | 7.79 | Stearidonic acid | 277.2173 | C18H29O2+ | 3.9 | 235.1691, 221.1574, 207.1386, 149.1319, 135.1168, 121.1007 | — | — | — | √ | √ |
| 27 | 9.34 | Octadecatrienoic acid | 279.2318 | C18H31O2+ | −0.2 | 261.2231, 243.2109, 165.1266, 147.1159 | — | — | √ | — | — |
| 28 | 8.43 | Octadecatrienoic acid isomer | 279.2318 | C18H31O2+ | −0.2 | 250.4211, 243.2113, 218.9202, 173.1314, 135.1160, 109.1009 | — | — | √ | — | — |
| 29 | 12.05 | Octadecenoic acid | 283.2625 | C18H35O2+ | −2.3 | 247.2463, 201.2660, 163.1493, 149.1329, 135.1170, 109.1023 | — | — | √ | — | — |
| 30 | 6.05 | Dehydrophytosphingosine | 316.2865 | C18H38NO3+ | 5.9 | 298.2716, 280.2650, 250.2534, 147.1166, 119.0840 | √ | — | √ | √ | √ |
| 31 | 6.33 | Phytosphingosine | 318.3019 | C18H40NO3+ | 5.1 | 300.2909, 282.2797, 264.2682, 252.2690 | √ | — | √ | √ | √ |
| 32 | 9.49 | Anandamide | 324.2911 | C20H38NO2+ | 4.3 | 306.2844, 227.8998 | — | — | — | √ | — |
| 33 | 6.53 | N-Palmitoyl alanine | 328.2840 | C19H38NO3 | −1.9 | 310.2755, 298.2737, 280.2647, 263.2365, 242.9088, 195.1409, 151.1485 | √ | — | √ | — | — |
| 34 | 3.93 | Octacosahexaenoic acid | 413.3425 | C28H45O2+ | 1.9 | 395.3305, 217.1591, 199.1485 | √ | √ | — | — | — |
| 35 | 4.83 | Octacosahexaenoic acid isomer | 413.3427 | C28H45O2+ | 3.1 | 395.3325, 327.2679 | — | — | — | √ | — |
| 36 | 4.57 | Octacosahexaenoic acid isomer | 413.3429 | C28H45O2+ | 3.6 | 395.3309, 217.1581, 199.1487 | — | — | — | √ | — |
| 37 | 7.57 | Phospahtidyl choline (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/0 2/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
520.3421 | C26H51NO7P+ | 4.5 | 502.3300, 184.0732, 104.1069 | √ | — | √ | — | — |
| 38 | 8.24 | Phospahtidyl choline (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18 0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
522.3583 | C26H53NO7P+ | 5.5 | 504.3471, 184.0735, 104.1071 | √ | — | √ | — | — |
| 39 | 13.10 | 2-Deoxyecdysone | 449.3289 | C27H45O5+ | 6.1 | 431.3203, 253.1142, 187.0693 | — | √ | — | — | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/0
2/0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) (520.3421, C26H51NO7P+), and phosphatidyl choline (0
0) (520.3421, C26H51NO7P+), and phosphatidyl choline (0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (522.3583, C26H53NO7P+). Phosphatidyl choline is a glycerophospholipid with anti-inflammatory action in arthritis and ulcerative colitis41 and supports the health benefits of immature fruit and seeds. Phosphatidylcholine (PC) is an important cell membrane component that is critical for cell structure and membrane stability maintenance, in addition to being a plant growth regulator.42 Phosphatidylcholine application was also found to enhance homeostasis against salt stress.43 In contrast, few reports are found in the literature on lysophospholipids except for their role as priming agents of the plant immune system and resistance against pathogens.44 A detailed comparison between phospholipids and lysophospholipids regarding plants' effects is yet to be reported.
1) (522.3583, C26H53NO7P+). Phosphatidyl choline is a glycerophospholipid with anti-inflammatory action in arthritis and ulcerative colitis41 and supports the health benefits of immature fruit and seeds. Phosphatidylcholine (PC) is an important cell membrane component that is critical for cell structure and membrane stability maintenance, in addition to being a plant growth regulator.42 Phosphatidylcholine application was also found to enhance homeostasis against salt stress.43 In contrast, few reports are found in the literature on lysophospholipids except for their role as priming agents of the plant immune system and resistance against pathogens.44 A detailed comparison between phospholipids and lysophospholipids regarding plants' effects is yet to be reported.3.5 Multivariate data analysis of Balanites UPLC-MS dataset
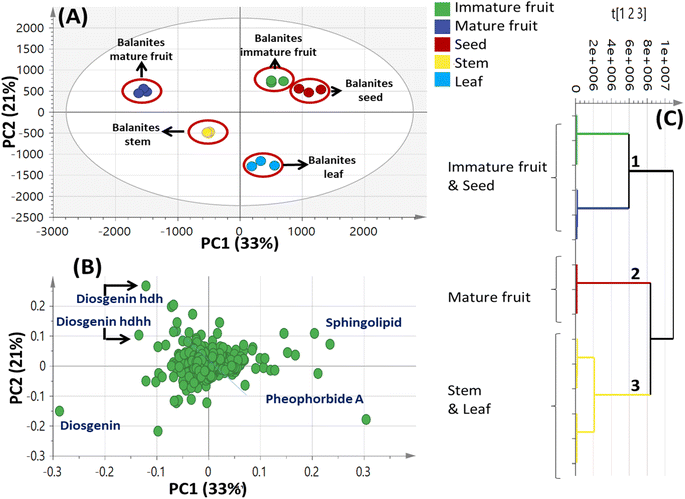 | ||
| Fig. 4 Unsupervised multivariate data analyses of Balanites parts metabolite profiles via UPLC-MS. (A) Principal component analysis (PCA) score plot of PC1 vs. PC2. (B) Loading plot for PC1 and PC2 showing mass peaks and their assignments. (C) Hierarchical cluster analysis (HCA). The metabolites assignment is summarized in Table 2. The model is colored according to marked groups. | ||
3.6. GC-MS profiling of metabolites in B. aegyptiaca extract
Although NMR provided insight into the major primary metabolites, GC-MS is more sensitive for the identification of less abundant metabolites not detected using NMR. GC-MS post-silylation analysis was employed to provide a holistic profile of primary metabolites from B. aegyptiaca parts. A total of 135 metabolites (Table 3 and Fig. 6A) were identified belonging to sugars (mono- and disaccharides), sugar alcohols, sugar acid, amino acids, fatty acids, esters, organic acids, and alcohols, in addition to a few nitrogenous compounds (Fig. 6B).Sugar alcohols were detected at relatively high levels in B. aegyptiaca parts ranging from 24.9 to 35.6 mg g−1 with the highest level in unripe fruits (35.6 mg g−1) followed by leaves and ripe fruits at ca. 30 mg g−1. Sugar alcohol is a low-calorie sweetener without any effect on blood glucose level as they are not absorbed from the intestine and is widely used as a sweetener for diabetic patients.49 D-Pinitol (peak 129) was detected as the major sugar alcohol in B. aegyptiaca parts at the highest level in the unripe fruits at 28.96 mg g−1 followed by leaves at 26.1 mg g−1 posing unripe fruit as low-calorie source sugar. D-Pinitol, a polyol derivative is reported for its antidiabetic properties as it exerts insulin-like hypoglycemic effect50 and is likely to augment trigonelline antidiabetic action in Balanites.
Moreover, myo-inositol (peaks 133 and 135), an insulin sensitizer sugar alcohol was likewise detected in parts though at much lower levels ca. 1.3–2.9 mg g−1. Glycerol, a simple polyol with applications in pharmaceutical, cosmetic, and food industries51 was detected (peak 126) at considerable levels in ripe fruits and leaves at 4–5 mg g−1 and accounting for fruit sweetness. Such evidence-based results provide new insight into B. aegyptiaca fruit as antidiabetics.
Sugar acids were detected in all B. aegyptiaca parts ranging from 1 to 5 with higher abundance in ripe fruits at 4.7 mg g−1. Ribonic acid (peak 121) and glucaric acid (peaks 123 and 124) were the major sugar acids. D-Glucaric acid is used as a food additive as well as in the pharmaceutical industry and dietary supplements.52
3.7. Multivariate data analysis of Balanites GC-MS dataset
| Peak no. | Average Rt | Average RI | Metabolite name | Class | Leaf | Ripe fruit | Seed | Stem | Unripe fruit |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.132 | 1061.73 | Lactic acid, (2TMS) | Acid | 0.95 ± 0.06 | 1.47 ± 0.08 | 0.94 ± 0.14 | 1.10 ± 0.52 | 2.85 ± 0.74 |
| 2 | 5.34 | 1074.65 | Glycolic acid, (2TMS) | Acid | 0.20 ± 0.03 | 0.88 ± 0.10 | 0.29 ± 0.04 | 0.24 ± 0.04 | 1.37 ± 0.59 |
| 3 | 5.528 | 1086.36 | Pyruvic acid, (2TMS) | Acid | 0.49 ± 0.04 | 0.05 ± 0.05 | 0.16 ± 0.05 | 0.44 ± 0.14 | 0.21 ± 0.09 |
| 4 | 6.366 | 1138.42 | β-Lactic acid, (2TMS) | Acid | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 5 | 6.591 | 1152.82 | 3-Hydroxybutyric acid, (2TMS) | Acid | 0.03 ± 0.00 | 0.22 ± 0.06 | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.00 |
| 6 | 7.269 | 1194.52 | Malonic acid, (2TMS) | Acid | 0.03 ± 0.00 | 0.69 ± 0.10 | 0.18 ± 0.07 | 0.03 ± 0.00 | 0.25 ± 0.07 |
| 7 | 7.744 | 1227.75 | 2-Hydroxyisocaproic acid, (2TMS) | Acid | 0.03 ± 0.01 | 0.09 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.04 ± 0.01 |
| 8 | 8.236 | 1262.75 | Itaconic acid, (2TMS) | Acid | 0.22 ± 0.04 | 0.03 ± 0.00 | 0.06 ± 0.03 | 0.04 ± 0.00 | 0.11 ± 0.01 |
| 9 | 8.333 | 1270.13 | Phosphoric acid, (3TMS) | Acid | 0.15 ± 0.03 | 9.83 ± 3.73 | 7.26 ± 1.71 | 0.93 ± 0.12 | 9.05 ± 1.11 |
| 10 | 8.808 | 1304.17 | Succinic acid, (2TMS) | Acid | 0.83 ± 0.16 | 1.20 ± 0.34 | 0.53 ± 0.05 | 0.98 ± 0.22 | 1.37 ± 0.75 |
| 11 | 9.142 | 1328.09 | Glyceric acid, (3TMS) | Acid | 5.86 ± 0.69 | 0.43 ± 0.34 | 1.58 ± 0.30 | 6.27 ± 2.05 | 1.96 ± 1.06 |
| 12 | 9.248 | 1335.65 | γ-Hydroxybutyric acid, (2TMS) | Acid | 0.01 ± 0.00 | 0.06 ± 0.02 | 0.04 ± 0.00 | 0.02 ± 0.00 | 0.14 ± 0.09 |
| 13 | 10.244 | 1408.52 | 3-Deoxytetronic acid, (tri-TMS) | Acid | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.11 ± 0.04 |
| 14 | 10.742 | 1448.23 | 4-Hydroxyvaleric acid, (2TMS) | Acid | 0.08 ± 0.01 | 0.07 ± 0.00 | 0.07 ± 0.00 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| 15 | 10.868 | 1458.31 | Ketomalonic acid hydrate,(TMS) | Acid | 0.07 ± 0.02 | 0.02 ± 0.03 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.07 ± 0.05 |
| 16 | 11.205 | 1485.51 | Malic acid, (3TMS) | Acid | 1.25 ± 0.38 | 9.79 ± 3.42 | 4.49 ± 2.17 | 3.80 ± 0.91 | 11.40 ± 2.64 |
| 17 | 14.413 | 1760.63 | Citric acid, (4TMS) | Acid | 0.01 ± 0.00 | 0.07 ± 0.02 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.03 ± 0.00 |
| Total acids | 10.25 ± 1.49 | 24.94 ± 8.33 | 15.75 ± 4.60 | 14.06 ± 4.07 | 29.12 ± 7.28 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 18 | 5.003 | 1053.69 | 1,3-Propanediol, (2TMS) | Alcohol | 0.39 ± 0.05 | 0.40 ± 0.05 | 0.34 ± 0.13 | 0.37 ± 0.06 | 0.39 ± 0.06 |
| 19 | 6.101 | 1121.97 | Nonanol, (TMS) | Alcohol | 0.07 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| 20 | 11.097 | 1476.75 | Glycerol, (3TMS) | Alcohol | 0.02 ± 0.01 | 0.18 ± 0.05 | 0.07 ± 0.07 | 0.11 ± 0.03 | 0.12 ± 0.03 |
| Total alcohols | 0.48 ± 0.08 | 0.58 ± 0.10 | 0.41 ± 0.21 | 0.50 ± 0.10 | 0.53 ± 0.10 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 21 | 5.483 | 1083.21 | Valine, (TMS) | Amino acid | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 22 | 5.76 | 1100.75 | Alanine, (2TMS) | Amino acid | 1.01 ± 0.32 | 0.12 ± 0.03 | 0.77 ± 0.12 | 0.33 ± 0.04 | 0.84 ± 0.25 |
| 23 | 6.514 | 1147.64 | L-Leucine, (TMS) | Amino acid | 0.08 ± 0.05 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.10 ± 0.01 |
| 24 | 6.685 | 1158.24 | L-Proline, (TMS) | Amino acid | 0.01 ± 0.00 | 0.08 ± 0.02 | 0.02 ± 0.01 | 0.00 ± 0.00 | 0.05 ± 0.05 |
| 25 | 6.82 | 1166.97 | Isoleucine, TMS | Amino acid | 0.03 ± 0.02 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 |
| 26 | 7.057 | 1181.45 | Methacryloyl glycine, (2TMS) | Amino acid | 0.06 ± 0.01 | 0.10 ± 0.03 | 0.11 ± 0.03 | 0.06 ± 0.01 | 0.36 ± 0.15 |
| 27 | 7.349 | 1199.49 | Tiglylglycine, (TMS) | Amino acid | 0.15 ± 0.04 | 0.09 ± 0.07 | 0.09 ± 0.02 | 0.14 ± 0.03 | 0.19 ± 0.04 |
| 28 | 7.394 | 1202.53 | Glycine 3TMS isomer | Amino acid | 0.08 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.00 | 0.08 ± 0.01 | 0.06 ± 0.01 |
| 29 | 7.464 | 1207.65 | Valine, (2TMS) | Amino acid | 1.62 ± 0.40 | 0.03 ± 0.00 | 0.62 ± 0.11 | 0.35 ± 0.08 | 0.70 ± 0.12 |
| 30 | 7.702 | 1224.79 | γ-Hydroxybutyric acid, (2TMS) | Amino acid | 0.42 ± 0.05 | 0.28 ± 0.01 | 0.25 ± 0.01 | 0.26 ± 0.07 | 0.25 ± 0.06 |
| 31 | 8.276 | 1265.93 | L-Leucine, (TMS) | Amino acid | 3.09 ± 0.73 | 0.06 ± 0.01 | 0.99 ± 0.17 | 0.75 ± 0.18 | 1.48 ± 0.26 |
| 32 | 8.59 | 1288.51 | Isoleucine, (2TMS) | Amino acid | 1.01 ± 0.23 | 0.03 ± 0.01 | 0.41 ± 0.08 | 0.24 ± 0.06 | 0.50 ± 0.09 |
| 33 | 8.62 | 1290.67 | Proline, (di-TMS) | Amino acid | 0.04 ± 0.01 | 3.75 ± 2.57 | 0.32 ± 0.10 | 0.03 ± 0.01 | 0.66 ± 0.89 |
| 34 | 8.768 | 1301.24 | Glycine, (3TMS) | Amino acid | 0.05 ± 0.02 | 0.01 ± 0.00 | 0.07 ± 0.03 | 0.02 ± 0.01 | 0.09 ± 0.02 |
| 35 | 9.54 | 1356.67 | Serine, (3TMS) | Amino acid | 0.39 ± 0.11 | 0.03 ± 0.01 | 0.37 ± 0.10 | 0.16 ± 0.04 | 0.31 ± 0.07 |
| 36 | 9.803 | 1375.56 | N-Methylleucine, (2TMS) | Amino acid | 0.48 ± 0.17 | 0.14 ± 0.01 | 0.06 ± 0.02 | 0.05 ± 0.01 | 0.19 ± 0.06 |
| 37 | 9.912 | 1383.36 | L-Threonine, (3TMS) | Amino acid | 0.39 ± 0.10 | 0.03 ± 0.01 | 0.26 ± 0.08 | 0.13 ± 0.04 | 0.36 ± 0.04 |
| 38 | 10.376 | 1418.66 | β-Alanine, (3TMS) | Amino acid | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.03 ± 0.00 |
| 39 | 10.73 | 1447.39 | Pyroglutamic acid, (2TMS) | Amino acid | 0.09 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.04 ± 0.01 |
| 40 | 11.011 | 1470.14 | Aspartic acid, (3TMS) | Amino acid | 0.05 ± 0.03 | 0.01 ± 0.00 | 0.03 ± 0.02 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 41 | 11.574 | 1515.36 | Pyroglutamic acid, (2TMS) | Amino acid | 0.52 ± 0.13 | 0.10 ± 0.01 | 0.40 ± 0.05 | 0.14 ± 0.04 | 0.27 ± 0.46 |
| 42 | 11.594 | 1516.86 | L-Aspartic acid, (3TMS) | Amino acid | 0.09 ± 0.03 | 0.03 ± 0.01 | 0.51 ± 0.13 | 0.11 ± 0.02 | 0.60 ± 0.05 |
| 43 | 12.241 | 1568.96 | α-Hydroxyglutaric acid, (3TMS) | Amino acid | 0.01 ± 0.00 | 0.06 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 44 | 12.333 | 1577.06 | Valine, (bis-TBDMS) | Amino acid | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 45 | 12.693 | 1606.01 | L-Aspartic acid, (3TMS) | Amino acid | 0.03 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.03 | 0.05 ± 0.01 | 0.13 ± 0.03 |
| 46 | 12.768 | 1612.69 | Glutamic acid, (3TMS) | Amino acid | 0.07 ± 0.04 | 0.03 ± 0.00 | 0.18 ± 0.12 | 0.04 ± 0.01 | 0.43 ± 0.14 |
| 47 | 12.863 | 1621.43 | Phenylalanine, (2TMS) | Amino acid | 0.05 ± 0.02 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 | 0.01 ± 0.00 |
| 48 | 12.951 | 1629.15 | Asparagine, (3TMS) | Amino acid | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.01 |
| 49 | 13.139 | 1646.12 | Valine, (bis-TBDMS) | Amino acid | 0.14 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 |
| 50 | 13.368 | 1666.55 | L-Asparagine, (2TMS) | Amino acid | 0.07 ± 0.02 | 0.01 ± 0.00 | 0.05 ± 0.01 | 0.01 ± 0.00 | 0.07 ± 0.01 |
| 51 | 14.232 | 1744.4 | Isoleucine, (2TBDMS) | Amino acid | 0.03 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 52 | 16.212 | 1935.02 | Tyrosine, (3TMS) | Amino acid | 1.26 ± 0.40 | 0.02 ± 0.01 | 0.35 ± 0.11 | 0.23 ± 0.06 | 0.83 ± 0.14 |
| Total amino acids | 11.41 ± 3.02 | 5.29 ± 2.88 | 6.17 ± 1.38 | 3.42 ± 0.78 | 8.70 ± 3.00 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 53 | 5.649 | 1093.87 | 2-Ethylhexanoic acid, (TMS) | Fatty acid/ester | 0.15 ± 0.02 | 0.14 ± 0.00 | 0.16 ± 0.02 | 0.16 ± 0.01 | 0.14 ± 0.02 |
| 54 | 11.406 | 1501.46 | Methyl octanoate, (TMS) | Fatty acid/ester | 0.25 ± 0.03 | 0.26 ± 0.03 | 0.27 ± 0.02 | 0.28 ± 0.04 | 0.26 ± 0.04 |
| 55 | 13.007 | 1634.29 | Lauric acid, (TMS) | Fatty acid/ester | 0.24 ± 0.11 | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.14 ± 0.03 | 0.10 ± 0.01 |
| 56 | 17.072 | 2022.33 | Palmitic acid, (TMS) | Fatty acid/ester | 13.00 ± 3.19 | 5.24 ± 0.55 | 6.97 ± 1.18 | 9.05 ± 2.67 | 7.22 ± 0.78 |
| 57 | 18.596 | 2187.59 | Linoleic acid, (TMS) | Fatty acid/ester | 5.62 ± 2.06 | 1.65 ± 1.05 | 3.08 ± 0.96 | 3.06 ± 0.96 | 3.63 ± 0.18 |
| 58 | 18.641 | 2192.65 | Oleic acid, (TMS) | Fatty acid/ester | 4.54 ± 1.36 | 1.60 ± 1.07 | 1.68 ± 0.34 | 3.46 ± 1.04 | 1.94 ± 0.49 |
| 59 | 18.655 | 2194.97 | α-Linolenic acid, (TMS) | Fatty acid/ester | 10.48 ± 2.49 | 0.67 ± 0.43 | 1.14 ± 0.18 | 3.05 ± 0.90 | 2.19 ± 0.51 |
| 60 | 18.85 | 2216.82 | Stearic acid, (TMS) | Fatty acid/ester | 12.06 ± 2.65 | 4.96 ± 0.54 | 6.17 ± 0.59 | 7.39 ± 1.59 | 7.09 ± 0.19 |
| 61 | 20.391 | 2398.63 | Octadecenoic acid | Fatty acid/ester | 0.34 ± 0.07 | 0.10 ± 0.05 | 0.33 ± 0.22 | 0.14 ± 0.03 | 0.73 ± 0.30 |
| 62 | 21.34 | 2519.98 | Heneicosanoic acid, (TBMS) | Fatty acid/ester | 15.09 ± 1.97 | 11.95 ± 0.69 | 10.24 ± 4.81 | 13.61 ± 3.01 | 12.71 ± 2.87 |
| 63 | 21.717 | 2568.34 | 1-Monopalmitin, (TMS) | Fatty acid/ester | 2.99 ± 1.12 | 1.13 ± 1.26 | 2.70 ± 1.72 | 3.21 ± 2.58 | 3.51 ± 4.15 |
| 64 | 22.155 | 2626.69 | Octadecenoic acid, methyl ester, (3TMS) | Fatty acid/ester | 1.24 ± 0.32 | 0.09 ± 0.02 | 0.14 ± 0.05 | 0.17 ± 0.05 | 0.11 ± 0.04 |
| Total fatty acid/ester | 65.99 ± 15.38 | 27.90 ± 5.72 | 32.95 ± 10.10 | 43.71 ± 12.91 | 39.64 ± 9.56 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 65 | 7.245 | 1193 | 2-Ketobutyric acid, enol, (2TMS) | Nitrogenous | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.00 |
| 66 | 8.196 | 1260.18 | Ethanolamine, (2TMS) | Nitrogenous | 0.34 ± 0.11 | 0.04 ± 0.00 | 0.09 ± 0.03 | 0.13 ± 0.04 | 0.11 ± 0.03 |
| 67 | 8.498 | 1281.81 | Nicotinic acid, (TMS) | Nitrogenous | 0.09 ± 0.02 | 0.15 ± 0.02 | 0.12 ± 0.04 | 0.06 ± 0.00 | 0.22 ± 0.06 |
| 68 | 8.919 | 1312.2 | Picolinic acid, (TMS) | Nitrogenous | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 69 | 9.203 | 1332.45 | Uracil, (2TMS) | Nitrogenous | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.03 |
| 70 | 9.982 | 1388.34 | Ethanolamine, (2TMS) | Nitrogenous | 0.16 ± 0.02 | 0.14 ± 0.01 | 0.16 ± 0.01 | 0.17 ± 0.03 | 0.15 ± 0.02 |
| 71 | 11.348 | 1497 | Trigonelline, (TMS) | Nitrogenous | 0.02 ± 0.01 | 0.08 ± 0.03 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.11 ± 0.08 |
| 72 | 11.68 | 1523.69 | γ-Aminobutyric acid, (3TMS) | Nitrogenous | 0.86 ± 0.21 | 0.02 ± 0.00 | 0.54 ± 0.13 | 0.48 ± 0.14 | 0.57 ± 0.14 |
| 73 | 15.452 | 1859.65 | Adenine, (2TMS) | Nitrogenous | 0.27 ± 0.07 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.09 ± 0.02 | 0.12 ± 0.03 |
| Total nitrogenous | 1.78 ± 0.44 | 0.55 ± 0.08 | 1.06 ± 0.24 | 0.99 ± 0.25 | 1.35 ± 0.38 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 74 | 12.459 | 1586.48 | D-Psicose, (5TMS) | Sugar | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 75 | 12.843 | 1619.48 | D-Arabinose, (4TMS) | Sugar | 2.35 ± 0.78 | 0.63 ± 0.32 | 0.70 ± 0.21 | 0.87 ± 0.24 | 1.18 ± 0.17 |
| 76 | 12.985 | 1632.39 | D-Mannose, (5TMS) | Sugar | 0.19 ± 0.15 | 0.22 ± 0.16 | 0.18 ± 0.05 | 0.05 ± 0.01 | 0.14 ± 0.06 |
| 77 | 12.996 | 1633.26 | L-Rhamnopyranose, (4TMS) | Sugar | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.05 ± 0.02 |
| 78 | 13.045 | 1637.56 | D-Ribofuranose, (4TMS) (isomer 1) | Sugar | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.03 ± 0.01 |
| 79 | 13.202 | 1651.78 | D-Arabinopyranose, (4TMS) (isomer 1) | Sugar | 1.05 ± 0.52 | 1.18 ± 0.70 | 0.43 ± 0.20 | 0.87 ± 0.28 | 0.39 ± 0.08 |
| 80 | 13.414 | 1670.86 | D-Arabinopyranose, (4TMS) (isomer 2) | Sugar | 0.06 ± 0.02 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 |
| 81 | 13.511 | 1678.9 | D-Ribofuranose, (4TMS) (isomer 2) | Sugar | 0.21 ± 0.10 | 0.20 ± 0.13 | 0.09 ± 0.03 | 0.16 ± 0.06 | 0.10 ± 0.01 |
| 82 | 13.672 | 1693.95 | Xylulose, (4TMS) | Sugar | 0.13 ± 0.01 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.13 ± 0.02 | 0.11 ± 0.01 |
| 83 | 13.706 | 1696.07 | D-Ribofuranose, (4TMS) (isomer 2) | Sugar | 0.08 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.01 |
| 84 | 13.745 | 1700.46 | L-Rhamnopyranose, (4TMS) | Sugar | 0.14 ± 0.09 | 0.14 ± 0.08 | 0.14 ± 0.05 | 0.06 ± 0.01 | 0.17 ± 0.08 |
| 85 | 13.8 | 1705.54 | Arabose, (TMS) | Sugar | 0.18 ± 0.07 | 0.01 ± 0.00 | 0.03 ± 0.03 | 0.04 ± 0.01 | 0.06 ± 0.01 |
| 86 | 13.915 | 1715.65 | β-L-Fucopyranose, (4TMS) | Sugar | 0.18 ± 0.08 | 0.21 ± 0.04 | 0.17 ± 0.10 | 0.28 ± 0.09 | 0.12 ± 0.04 |
| 87 | 13.928 | 1718.25 | L-Rhamnopyranose, (4TMS) | Sugar | 0.07 ± 0.02 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| 88 | 14.072 | 1729.69 | Ribitol, (5TMS) | Sugar | 0.06 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 |
| 89 | 14.313 | 1751.58 | D-(+)-Galactose, (5TMS) (isomer 2) | Sugar | 0.21 ± 0.08 | 0.34 ± 0.00 | 0.17 ± 0.06 | 1.13 ± 0.31 | 0.41 ± 0.10 |
| 90 | 14.483 | 1766.87 | α-D-Mannopyranose, (5TMS) | Sugar | 0.12 ± 0.08 | 0.23 ± 0.03 | 0.19 ± 0.11 | 0.28 ± 0.09 | 0.09 ± 0.03 |
| 91 | 14.522 | 1770.36 | D-(−)-Ribofuranose, (4TMS) (isomer 2) | Sugar | 0.29 ± 0.13 | 0.20 ± 0.01 | 0.36 ± 0.07 | 0.27 ± 0.05 | 0.29 ± 0.02 |
| 92 | 14.591 | 1776.49 | L-Fucitol, (5TMS) | Sugar | 0.11 ± 0.04 | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.02 ± 0.00 | 0.05 ± 0.01 |
| 93 | 14.912 | 1806.01 | L-Mannopyranose, 6-deoxy, (4TMS) | Sugar | 2.73 ± 0.70 | 0.46 ± 0.18 | 0.71 ± 0.17 | 0.14 ± 0.04 | 1.88 ± 0.77 |
| 94 | 14.939 | 1808.5 | D-tagatofuranose, (5TMS) (isomer 1) | Sugar | 0.12 ± 0.04 | 0.25 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.15 ± 0.06 |
| 95 | 14.987 | 1813.47 | D-Fructofuranose, (5TMS) (isomer 1) | Sugar | 0.72 ± 0.23 | 29.51 ± 2.90 | 3.83 ± 1.39 | 3.18 ± 1.31 | 3.57 ± 0.23 |
| 96 | 15.073 | 1821.98 | D-Tagatofuranose, (5TMS) (isomer 2) | Sugar | 2.18 ± 0.63 | 52.51 ± 3.72 | 9.55 ± 3.28 | 8.30 ± 2.69 | 9.39 ± 0.33 |
| 97 | 15.148 | 1829.44 | D-Fructopyranose, (5TMS) (isomer 1) | Sugar | 4.03 ± 1.03 | 66.22 ± 21.41 | 11.25 ± 3.00 | 8.98 ± 1.72 | 4.96 ± 0.97 |
| 98 | 15.388 | 1853.33 | β-Galactofuranose, (5TMS) | Sugar | 0.21 ± 0.09 | 4.14 ± 0.71 | 0.69 ± 0.40 | 0.39 ± 0.21 | 1.93 ± 0.81 |
| 99 | 15.408 | 1855.29 | D-Talofuranose, (5TMS) (isomer 2) | Sugar | 0.09 ± 0.04 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.10 ± 0.03 |
| 100 | 15.477 | 1862.79 | Sorbose, (5TMS) | Sugar | 0.13 ± 0.04 | 2.73 ± 0.49 | 0.22 ± 0.07 | 0.18 ± 0.05 | 0.23 ± 0.01 |
| 101 | 15.554 | 1869.76 | D-Galactopyranose, (5TMS) (isomer 1) | Sugar | 1.99 ± 0.69 | 0.69 ± 0.17 | 0.79 ± 0.43 | 1.13 ± 0.29 | 0.27 ± 0.02 |
| 102 | 15.852 | 1899.29 | D-Psicose, (5TMS) | Sugar | 0.18 ± 0.13 | 5.78 ± 1.91 | 0.47 ± 0.17 | 0.37 ± 0.08 | 0.38 ± 0.12 |
| 103 | 15.896 | 1903.64 | β-D-Mannopyranose, (5TMS) | Sugar | 8.29 ± 2.34 | 93.50 ± 20.08 | 22.90 ± 8.01 | 13.59 ± 2.99 | 26.62 ± 1.69 |
| 104 | 16.008 | 1914.77 | D-Glucose, 5TMS | Sugar | 3.51 ± 1.21 | 0.59 ± 0.05 | 1.48 ± 0.75 | 1.94 ± 0.57 | 0.83 ± 0.08 |
| 105 | 16.03 | 1917.07 | α-D-Talopyranose, (5TMS) | Sugar | 0.44 ± 0.16 | 0.01 ± 0.00 | 0.09 ± 0.04 | 0.07 ± 0.03 | 0.26 ± 0.05 |
| 106 | 16.751 | 1988.52 | β-D-Glucopyranose, (TMS) | Sugar | 10.04 ± 2.78 | 102.49 ± 19.64 | 27.94 ± 8.80 | 15.66 ± 3.52 | 33.71 ± 2.69 |
| 107 | 17.275 | 2044.39 | D-glucose, (5TMS) | Sugar | 0.38 ± 0.19 | 0.13 ± 0.02 | 0.05 ± 0.02 | 0.06 ± 0.01 | 0.09 ± 0.02 |
| 108 | 18.74 | 2203.7 | Maltose, (8TMS) (isomer 1) | Sugar | 0.13 ± 0.04 | 1.15 ± 0.76 | 0.51 ± 0.38 | 0.08 ± 0.02 | 1.64 ± 1.20 |
| 109 | 20.09 | 2363.31 | D-(+)-Cellobiose, (8TMS) | Sugar | 0.05 ± 0.03 | 0.16 ± 0.07 | 0.10 ± 0.03 | 0.02 ± 0.01 | 0.22 ± 0.10 |
| 110 | 22.502 | 2674 | Sucrose, (5TMS) | Sugar | 0.69 ± 0.25 | 24.51 ± 15.70 | 54.96 ± 37.49 | 8.79 ± 2.21 | 113.87 ± 25.37 |
| 111 | 23.262 | 2779.72 | D-Trehalose, (8TMS) | Sugar | 0.03 ± 0.02 | 1.42 ± 0.58 | 0.07 ± 0.02 | 0.02 ± 0.01 | 0.09 ± 0.01 |
| 112 | 23.389 | 2796.3 | 3-α-Mannobiose, (8TMS) (isomer 2) | Sugar | 0.26 ± 0.08 | 0.39 ± 0.06 | 0.26 ± 0.19 | 0.11 ± 0.02 | 0.19 ± 0.07 |
| 113 | 23.561 | 2813.35 | Maltose, (8TMS) (isomer 1) | Sugar | 1.31 ± 0.76 | 0.45 ± 0.05 | 0.17 ± 0.07 | 0.55 ± 0.15 | 0.47 ± 0.14 |
| 114 | 23.668 | 2823.39 | D-Cellobiose, (8TMS) (isomer 1) | Sugar | 1.12 ± 0.60 | 0.58 ± 0.03 | 0.22 ± 0.10 | 0.57 ± 0.15 | 0.78 ± 0.20 |
| 115 | 23.986 | 2852.92 | 3-α-Mannobiose, (8TMS) (isomer 1) | Sugar | 0.16 ± 0.05 | 0.10 ± 0.01 | 0.06 ± 0.03 | 0.03 ± 0.00 | 0.17 ± 0.08 |
| Total sugar | 44.25 ± 14.37 | 391.44 ± 90.10 | 139.13 ± 65.81 | 68.56 ± 17.28 | 205.13 ± 35.71 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 116 | 9.746 | 1372.06 | D-Erythronic acid γ-lactone, (2TMS) | Sugar acid | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 117 | 11.974 | 1547.38 | Erythronic acid, (4TMS) | Sugar acid | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 |
| 118 | 12.182 | 1563.71 | L-Threonic acid, (TMS) | Sugar acid | 0.12 ± 0.02 | 0.56 ± 0.17 | 0.15 ± 0.06 | 0.56 ± 0.17 | 0.29 ± 0.05 |
| 119 | 12.563 | 1595.96 | Xylonic acid, D-lactone, (TMS) | Sugar acid | 0.02 ± 0.01 | 0.30 ± 0.13 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.00 |
| 120 | 14.646 | 1781.63 | Ribonic acid, (5TMS) | Sugar acid | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 |
| 121 | 15.774 | 1891.56 | Ribonic acid, (5TMS) | Sugar acid | 0.59 ± 0.19 | 1.38 ± 0.39 | 0.62 ± 0.08 | 0.56 ± 0.11 | 0.98 ± 0.18 |
| 122 | 16.657 | 1979.89 | Gulonic acid, 1,4-lactone, (4TMS) | Sugar acid | 0.08 ± 0.02 | 0.86 ± 0.03 | 0.12 ± 0.05 | 0.13 ± 0.03 | 0.26 ± 0.04 |
| 123 | 17.003 | 2014.75 | Glucaric acid, (6TMS) | Sugar acid | 0.10 ± 0.01 | 0.08 ± 0.01 | 0.07 ± 0.03 | 0.08 ± 0.02 | 0.09 ± 0.01 |
| 124 | 17.162 | 2031.79 | Glucaric acid, (6TMS) | Sugar acid | 0.23 ± 0.13 | 1.04 ± 0.07 | 0.15 ± 0.05 | 0.64 ± 0.23 | 0.90 ± 0.07 |
| 125 | 20.254 | 2382.97 | D-Glucuronic acid, (5TMS) | Sugar acid | 0.13 ± 0.07 | 0.44 ± 0.07 | 0.15 ± 0.08 | 0.06 ± 0.02 | 0.34 ± 0.27 |
| Total sugar acid | 1.35 ± 0.48 | 4.72 ± 0.88 | 1.33 ± 0.37 | 2.09 ± 0.60 | 2.95 ± 0.63 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| 126 | 8.339 | 1270.44 | Glycerol, (tris-TMS) | Sugar alcohol | 4.20 ± 0.86 | 5.18 ± 1.41 | 2.67 ± 0.39 | 2.75 ± 0.57 | 2.02 ± 0.12 |
| 127 | 11.52 | 1511.02 | Erythritol, (4TMS) | Sugar alcohol | 0.05 ± 0.03 | 0.08 ± 0.01 | 0.06 ± 0.02 | 0.01 ± 0.01 | 0.15 ± 0.09 |
| 128 | 11.938 | 1543.53 | Pentitol, (4TMS) | Sugar alcohol | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 129 | 15.305 | 1844.96 | D-Pinitol, (5TMS) | Sugar alcohol | 26.06 ± 4.12 | 18.19 ± 3.76 | 17.63 ± 2.70 | 19.20 ± 2.64 | 28.96 ± 3.59 |
| 130 | 16.275 | 1941.21 | Sorbitol, (6TMS) | Sugar alcohol | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| 131 | 16.309 | 1944.64 | D-Mannitol, (6TMS) | Sugar alcohol | 0.12 ± 0.07 | 0.12 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.02 | 0.10 ± 0.01 |
| 132 | 16.453 | 1959.06 | D-Pinitol isomer, (5TMS) | Sugar alcohol | 0.57 ± 0.23 | 0.53 ± 0.04 | 0.19 ± 0.06 | 0.18 ± 0.05 | 0.52 ± 0.11 |
| 133 | 16.544 | 1968.3 | Myoinositol, (TMS) | Sugar alcohol | 0.50 ± 0.15 | 2.44 ± 0.02 | 1.23 ± 0.56 | 0.48 ± 0.12 | 1.46 ± 0.27 |
| 134 | 16.964 | 2010.43 | D-Pinitol isomer, (5TMS) | Sugar alcohol | 0.40 ± 0.15 | 0.29 ± 0.01 | 0.20 ± 0.07 | 0.12 ± 0.03 | 0.44 ± 0.11 |
| 135 | 17.824 | 2104.18 | Myoinositol, (TMS) | Sugar alcohol | 0.88 ± 0.36 | 1.51 ± 0.40 | 2.48 ± 0.37 | 2.07 ± 0.51 | 1.93 ± 0.45 |
| Total sugar alcohol | 32.82 ± 5.98 | 28.36 ± 5.68 | 24.54 ± 4.19 | 24.91 ± 3.95 | 35.62 ± 4.74 | ||||
3.8. Comparison of 1H NMR, GC-MS and LC-MS multivariate PCA analysis of Balanites parts
By comparing the classification potential of the different profiling techniques in Balanites viz. LC-MS and 1H NMR from their respective PCA results revealed that the PCA score plot derived from LC-MS data (Fig. 4A) showed tighter clustering of data than that in whole region NMR spectra (Fig. 1A). With 1H NMR-based PCA, obvious segregation between Balanites leaf and stem from other parts could be observed alongside PC1. In contrast, PCA of LC-MS derived dataset showed a clear segregation pattern of the 5 parts along the 4 quadrants with seeds being most close to immature fruit metabolome clustering together and in agreement with NMR results. 1H NMR (Fig. 1E) revealed trigonelline being most enriched in immature fruit providing the highest level of the key alkaloid, whereas isorhamnetin was more associated with leaf leading to its segregation. While, the LC-MS loading plot (Fig. 4B) revealed that lipids viz. sphingolipid as well as a nitrogenous metabolite, pheophorbide A were more enriched in immature fruit, seed, and leaf parts. Steroidal saponins viz. diosgenin hdh and diosgenin hdhh major bioactive class in Balanites were found to be associated with Balanites mature fruit, and suggestive that fruit is the richest in diosgenin conjugates. Both NMR and LC-MS techniques were equally effective in classifying the Balanites parts and revealing different markers due to their different detection capacities. Owing to its better peak resolution and higher sensitivity, GC-MS can present another potential tool for profiling primary metabolites. The unsupervised PCA model (Fig. 7B) showed clear discrimination of ripe fruits from all other 4 parts and was in agreement with both NMR and LC-MS results.3.9. Cytotoxic activity of Balanites' different parts
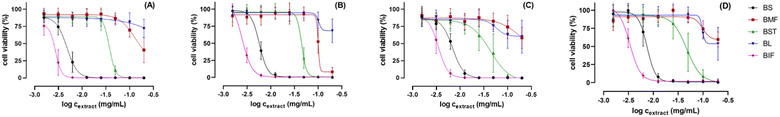
Extracts of Balanites' different parts were further assessed for their in vitro cytotoxic effect against the human prostate cancer cell line (PC3) and the human colorectal cancer cell line (HCT-116) by using MTT and CV assays (see experimental), and in relation to metabolites profiling. Amongst the tested extracts, immature fruit (BIF) and seed (BS) exerted the most potent cytotoxic activity against both tested cell lines in both MTT and CV assays. BIF exhibited the strongest activity with IC50 values determined to be 2.8 and 3.4–3.5 μg mL−1, respectively, followed by (BS) with IC50 values of around 4.8–5.6 and 6.8–7.8 μg mL−1 against PC3 and HCT-116 cells, respectively (Table S1† and Fig. 8). These results are in line with the previously reported anticancer activity of trigonelline56 detected as a major metabolite in BIF, in addition to several known anticancer sphingolipids57 as major peaks in BIF and BS. Balanites stem extract (BST) showed moderate cytotoxicity with IC50 values of 38.3–45.6 and 47.7–48.2 μg mL−1 against PC3 and HCT-116 cells, respectively. In contrast, mature fruit extract (BMF) exhibited only weak activity against PC3 cells with corresponding higher IC50 values of 98.4–112.6 μg mL−1 and was inactive against HCT-116 cells. Balanites leaf extract (BL) was inactive against both cell lines at all tested doses (Table S1† and Fig. 8). Further detailed assays should be employed using isolated compounds to confirm the cytotoxic activity of the different parts Balanites and to identify the most active compound ingredients.4. Conclusions
The current study presented the first comparative multiplex metabolomics-based investigation of B. aegyptiaca parts based on NMR, UPLC-MS, and GC-MS-based platforms coupled with multivariate analyses. NMR fingerprinting analysis led to the identification and quantification of 15 different metabolites including alkaloids, saponins, flavonoids, sugars, and amino and fatty acids. Furthermore, trigonelline and isorhamnetin accounted for BMF and BIF segregation in modeling results using NMR. In contrast, UPLC/MS/MS based on GNPS networking allowed for the annotation of 39 secondary metabolites mostly belonging to steroidal saponins (16), N-containing metabolites (4), phenolics (2), in addition to fatty acids and lipids (17). GC-MS profiling of primary metabolites identified a total of 135 peaks belonging to sugars, fatty acids/esters, amino acids, nitrogenous, and organic acids. Mature fruits were mostly predominated with monosaccharides, whereas disaccharides were more abundant in immature fruits. Leaf and stem were mostly enriched in amino acids and fatty acids. Detection of nicotinic acid, trigonelline as well as the sugar alcohol D-pinitol (first time to be detected) in B. aegyptiaca parts, especially in unripe fruit is suggestive of novel insight into the well-reported antidiabetic effect in Balanites. Moreover, Balanites' immature fruit and seeds exhibited potential efficacy against human prostate cancer (PC3) and human colorectal cancer (HCT-116) cell lines are likely attributed to its richness in trigonelline, diosgenin, and pheophorbide A, in addition to other bioactive metabolites. Nevertheless, it should be highlighted that these are only preliminary findings that will need to be followed by more extensive investigations of B. aegyptiaca organ extracts performing more advanced cancer cell-based in vitro and, ideally, in vivo studies, and identification and research on specific, isolated components.Author contributions
Mohamed A. Farag: conceptualization, methodology, writing – review, and editing. Mostafa H. Baky: investigation, data curation, formal analysis, writing – original draft. Ibrahim Morgan: biological investigation, writing – review and editing. Robert Rennert: biological investigation, writing – review and editing. Andrea Porzel: NMR analysis. Ludger A. Wessjohann: biological investigation, writing – review and editing. Magdy M. El-Sayed: investigation, writing – review and editing. Osama Gomaa: LCMS analysis and data acquisition. Mohamed Reda: GCMS analysis and data acquisition, Nehal S. Ramadan: investigation, data curation, formal analysis, writing – original draft.Conflicts of interest
There is no conflict of interest.References
- M. A. Farag, A. Porzel and L. A. Wessjohann, J. Pharm. Biomed. Anal., 2015, 115, 383–387 CrossRef CAS.
- M. H. Baky, M. T. Badawy, A. F. Bakr, N. M. Hegazi, A. Abdellatif and M. A. Farag, RSC Adv., 2021, 11, 39680–39695 RSC.
- H. N. Murthy, G. G. Yadav, Y. H. Dewir and A. Ibrahim, Plants, 2020, 10, 32 CrossRef PubMed.
- M. A. Farag, A. Porzel and L. A. Wessjohann, J. Pharm. Biomed. Anal., 2015, 115, 383–387 CrossRef CAS PubMed.
- B. P. Chapagain, Y. Yehoshua and Z. Wiesman, Bioresour. Technol., 2009, 100, 1221–1226 CrossRef CAS PubMed.
- M. B. Sagna, A. Diallo, P. S. Sarr, O. Ndiaye, D. Goffner and A. Guisse, Afr. J. Biotechnol., 2014, 13(2), 336–342 CrossRef CAS.
- G. Khamis, A. M. Saleh, T. H. Habeeb, W. N. Hozzein, M. A. Wadaan, J. Papenbrock and H. AbdElgawad, J. Food Biochem., 2020, 44, e13229 CrossRef CAS.
- M. A. Farag, A. R. Khattab, S. Shamma and S. M. J. F. Afifi, Foods, 2021, 10, 728 CrossRef CAS PubMed.
- M. A. Farag, S. E. Khaled, Z. El Gingeehy, S. N. Shamma and A. J. M. Zayed, Metabolites, 2022, 12, 614 CrossRef CAS.
- M. A. Farag, N. S. Ramadan, M. Shorbagi, N. Farag and H. A. Gad, Foods, 2022, 11, 1339 CrossRef CAS PubMed.
- A. M. Otify, A. Serag, A. Porzel, L. A. Wessjohann and M. A. Farag, Food Anal. Methods, 2022, 15, 2095–2106 CrossRef.
- A. Zayed, A. Abdelwareth, T. A. Mohamed, H. A. Fahmy, A. Porzel, L. A. Wessjohann and M. A. J. F. C. Farag, Food Chem., 2022, 373, 131452 CrossRef CAS PubMed.
- I. Y. Younis, M. A. Farag, A. M. Elgamal and E. J. I. C. Mohsen, Ind. Crops Prod., 2023, 197, 116561 CrossRef CAS.
- M. A. Farag, Z. T. A. Shakour, M. M. Elmassry and M. S. J. F. C. Donia, Food Chem., 2022, 371, 131147 CrossRef CAS.
- M. A. Farag, N. M. Hegazi and M. S. J. M. Donia, Metabolomics, 2020, 16, 1–15 CrossRef PubMed.
- T. Pluskal, S. Castillo, A. Villar-Briones and M. Orešič, BMC Bioinform., 2010, 11, 1–11 Search PubMed.
- E. H. Reda, N. M. Hegazi, M. Marzouk, Z. T. A. Shakour, A. M. El-Halawany, E.-S. A. El-Kashoury, T. A. Mohamed, M. A. Ibrahim, K. A. Shams and N. S. J. M. Abdel-Azim, Molecules, 2023, 28, 674 CrossRef CAS.
- M. Ernst, K. B. Kang, A. M. Caraballo-Rodríguez, L.-F. Nothias, J. Wandy, C. Chen, M. Wang, S. Rogers, M. H. Medema and P. C. Dorrestein, Metabolites, 2019, 9, 144 CrossRef CAS PubMed.
- M. A. Farag, A. A. Maamoun, A. Ehrlich, S. Fahmy and L. A. Wesjohann, LWT, 2017, 80, 145–154 CrossRef CAS.
- M. A. Farag, N. S. Ramadan, M. Shorbagi, N. Farag and H. A. J. F. Gad, Foods, 2022, 11, 1339 CrossRef CAS PubMed.
- I. Morgan, L. A. Wessjohann and G. N. J. C. Kaluđerović, Cells, 2022, 11, 168 CrossRef CAS PubMed.
- Y. T. Lam, M. G. Ricardo, R. Rennert, A. Frolov, A. Porzel, W. Brandt, P. Stark, B. Westermann and N. Arnold, Int. J. Mol. Sci., 2021, 22, 12718 CrossRef CAS PubMed.
- I. Ware, K. Franke, H. Hussain, I. Morgan, R. Rennert and L. A. J. M. Wessjohann, Molecules, 2022, 27, 4363 CrossRef CAS.
- M. Feoktistova, P. Geserick and M. Leverkus, Cold Spring Harbor Protoc., 2016, 2016, pdb.prot087379 CrossRef.
- A. M. Otify, A. M. El-Sayed, C. G. Michel and M. A. Farag, Metabolomics, 2019, 15, 1–17 CrossRef CAS PubMed.
- X. Wang, D.-F. Hong, G.-L. Hu, Z.-R. Li, X.-R. Peng, Q.-Q. Shi and M.-H. J. M. Qiu, Molecules, 2021, 26, 4914 CrossRef CAS PubMed.
- H. N. Murthy, G. G. Yadav, Y. H. Dewir and A. J. P. Ibrahim, Plants, 2020, 10, 32 CrossRef PubMed.
- M. Choi, S. Mukherjee and J. W. Yun, Phytother. Res., 2021, 35, 1113–1124 CrossRef CAS PubMed.
- Z. Hu, C. Wang, L. Pan, S. Han, M. Jin, Y. Xiang, L. Zheng, Z. Li, R. Cao and B. Qin, Appl. Microbiol. Biotechnol., 2021, 105, 9333–9342 CrossRef CAS PubMed.
- K. Pazhanichamy, K. Bhuvaneswari, B. Kunthavai, T. Eevera and K. Rajendran, J. Planar Chromatogr.-Modern TLC, 2012, 25, 566–570 CrossRef CAS.
- N. H. Kandil, I. M. Ayoub, S. H. El-Ahmady and S. A. El-Moghazy, Phytochem. Anal., 2022, 33, 155–169 CrossRef CAS PubMed.
- A. S. Zaky, M. Kandeil, M. Abdel-Gabbar, E. M. Fahmy, M. M. Almehmadi, T. M. Ali and O. M. J. P. Ahmed, Pharmaceutics, 2022, 14, 263 CrossRef CAS PubMed.
- G. Gong, Y.-Y. Guan, Z.-L. Zhang, K. Rahman, S.-J. Wang, S. Zhou, X. Luan and H. Zhang, Biomed. Pharmacother., 2020, 128, 110301 CrossRef CAS.
- S. M. Osman, W. A. El Kashak, M. Wink and M. A. El Raey, Pharmacogn. Mag., 2016, 12, S47 CrossRef PubMed.
- J. J. Thelen and J. B. Ohlrogge, Metab. Eng., 2002, 4, 12–21 CrossRef CAS PubMed.
- Q. Du, L. Zhou, M. Li, F. Lyu, J. Liu and Y. J. F. F. Ding, Food Front., 2022, 3, 239–255 CrossRef CAS.
- P. Pontieri, J. Troisi, M. Calcagnile, S. R. Bean, M. Tilley, F. Aramouni, A. Boffa, G. Pepe, P. Campiglia and F. J. F. Del Giudice, Foods, 2022, 11, 436 CrossRef CAS PubMed.
- J. Kim, Y. Jo, D. Cho and D. J. N. C. Ryu, Nat. Commun., 2022, 13, 6554 CrossRef CAS PubMed.
- B. Mousavi, M.-H. Azizi and S. Abbasi, Food Chem. Mol. Sci., 2022, 4, 100104 CrossRef CAS.
- A. Serag, M. H. Baky, S. Döll and M. A. Farag, RSC Adv., 2020, 10, 76–85 RSC.
- H. O. Doğan, O. Şenol, A. Karadağ and S. N. Yıldız, Clin. Nutr. ESPEN, 2022, 50, 124–132 CrossRef.
- A. K. Cowan, Plant Growth Regul., 2006, 48, 97–109 CrossRef CAS.
- M. Sun, X. Liu, H. Gao, B. Zhang, F. Peng and Y. Xiao, Int. J. Mol. Sci., 2022, 23, 2585 CrossRef CAS PubMed.
- R. Völz, J.-Y. Park, W. Harris, S. Hwang and Y.-H. Lee, BMC Biotechnol., 2021, 21, 1–12 CrossRef PubMed.
- J. Boubaker, W. Bhouri, M. Ben Sghaier, K. Ghedira, M. Dijoux Franca and L. Chekir-Ghedira, Cell Proliferation, 2011, 44, 453–461 CrossRef CAS PubMed.
- A. Saide, C. Lauritano and A. J. M. D. Ianora, Mar. Drugs, 2020, 18, 257 CrossRef CAS.
- M. H. Baky, S. N. Shamma, J. Xiao and M. A. Farag, Food Chem., 2022, 383, 132374 CrossRef CAS.
- J. P. Bantle, J. Nutr., 2009, 139, 1263S–1268S CrossRef CAS PubMed.
- N. S. Ramadan, L. A. Wessjohann, A. Mocan, D. C Vodnar, N. H. El-Sayed, S. A. El-Toumy, D. Abdou Mohamed, Z. Abdel Aziz, A. Ehrlich and M. J. M. A. Farag, Molecules, 2020, 25, 2423 CrossRef CAS.
- M. Sánchez-Hidalgo, A. J. León-González, M. Gálvez-Peralta, N. H. González-Mauraza and C. J. P. R. Martin-Cordero, Phytochem. Rev., 2021, 20, 211–224 CrossRef.
- H. Tan, A. A. Aziz and M. Aroua, Renew. Sustainable Energy Rev., 2013, 27, 118–127 CrossRef CAS.
- J. L. Perez, G. Jayaprakasha, K. S. Yoo and B. S. Patil, J. Chromatogr. A, 2008, 1190, 394–397 CrossRef CAS PubMed.
- M. A. Farag, S. E. Khaled, Z. El Gingeehy, S. N. Shamma and A. Zayed, Metabolites, 2022, 12, 614 CrossRef CAS PubMed.
- . Alagawany, S. S. Elnesr, M. R. Farag, R. Tiwari, M. I. Yatoo, K. Karthik, I. Michalak and K. J. V. Q. Dhama, Vet. Quart., 2021, 41, 1–29 CrossRef PubMed.
- O. Yoshinari and K. Igarashi, Curr. Med. Chem., 2010, 17, 2196–2202 CrossRef CAS PubMed.
- N. Mohamadi, F. Sharififar, M. Pournamdari and M. Ansari, J. Diet. Suppl., 2018, 15, 207–222 CrossRef CAS PubMed.
- J. M. Padrón, Curr. Med. Chem., 2006, 13, 755–770 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03141a |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2023 |