Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of new N-(aminocycloalkylene)amino acid compounds using chiral triflate esters with N-Boc-aminopyrrolidines and N-Boc-aminopiperidines†
Gita Matulevičiūtė ab,
Neringa Kleizienė
ab,
Neringa Kleizienė a,
Greta Račkauskienėa,
Vytas Martynaitis
a,
Greta Račkauskienėa,
Vytas Martynaitis b,
Aurimas Bieliauskas
b,
Aurimas Bieliauskas a,
Urtė Šachlevičiūtė
a,
Urtė Šachlevičiūtė a,
Rokas Jankauskasa,
Martynas R. Bartkusa,
Frank A. Sløkc and
Algirdas Šačkus*ab
a,
Rokas Jankauskasa,
Martynas R. Bartkusa,
Frank A. Sløkc and
Algirdas Šačkus*ab
aInstitute of Synthetic Chemistry, Kaunas University of Technology, K. Baršausko g. 59, Kaunas LT-51423, Lithuania. E-mail: algirdas.sackus@ktu.lt
bDepartment of Organic Chemistry, Kaunas University of Technology, Radvilėnų pl. 19, Kaunas LT-50254, Lithuania
cVipergen ApS, Gammel Kongevej 23A, Copenhagen V DK-1610, Denmark
First published on 18th July 2023
Abstract
In this study, we prepared a series of new N-(aminocycloalkylene)amino acid derivatives for use in chiral building blocks. The method was based on the conversion of enantiopure α-hydroxy acid esters into the corresponding chiral triflate esters, which were displaced by a nucleophilic substitution SN2 reaction with aminopyrrolidine and aminopiperidine derivatives, and the inversion of the configuration to give methyl 2-[(Boc-amino)cycloamin-1-yl]alkanoates with good yield and high enantiomeric and diastereomeric purity. Synthesized 2-[(Boc-amino)piperidin-1-yl]propanoates combined with ethyl L-phenylalaninate gave new chiral N-Boc- and N-nosyl-dipeptides containing a piperidine moiety. The structures were elucidated by 1H-, 13C-, and 15N-NMR spectroscopy, high-resolution mass spectrometry, and X-ray crystallography analyses.
Introduction
N-(ω-Aminoalkylene)amino acids have many interesting biological activities and play important roles in medicinal chemistry and drug discovery for the pharmaceutical industry.1–4 In biochemistry, N-(ω-aminoalkylene)amino acids, such as N-(2-aminoethyl)glycine I, are used for the synthesis of peptide nucleic acids (PNAs) (Fig. 1).5–8N-(ω-Aminoalkylene)amino acids can be synthesized in various ways: alkylation, reductive amination, and the Mitsunobu reaction.9 In the alkylation reaction, a molecule of ethylenediamine or its mono N-protected derivative reacts with a molecule of an α-halocarboxylic acid to form an N-(ω-aminoalkylene)amino acid molecule. Byt and Gilon reported a method for the alkylation synthesis of N-(ω-aminoalkylene)amino acids via the reaction of alkylenediamine (NH2(CH2)nNH2, n = 2, 3, 6) with α-haloacetic acid at 25 °C for 48 h.10 This experiment had a yield of 53–72%, but used a large excess of alkylenediamine. When optically active α-halogenocarboxylic acids were used as alkylating agents with ethylenediamine, a SN2 nucleophilic substitution occurred with the consequent inversion of configuration. For example, (S)-2-[(2-aminoethyl)amino]-4-methylpentanoic acid (II) was synthesized using ethylenediamine with (R)-2-chloro-4-methylpentanoic acid. However, the target compound gave a poor yield of 36% due to the undesired formation of the corresponding 2,3-dehydro carboxylic acid in the mixture.
It is widely known that the bromo derivative of α-haloacetic acid provides a higher yield during the nucleophilic substitution reaction because of its leaving group ability compared to that of the chloroacetic acid derivative.9 Feagin et al. reported the preparation of benzyl 2-{[2-(Boc-amino)ethyl]amino}acetate by reacting N-Boc-ethylenediamine and ethyl bromoacetate in the presence of triethylamine in acetonitrile at 70–80 °C for 100 min with a good yield of 72%.11 Sugiyama et al. reported that chiral diamine, (S)-Cbz-HNCH2CH(CH3)NH2, reacted with ethyl bromoacetate and yielded the target chiral compound III in the presence of potassium carbonate in DCM at room temperature, but undesired N,N-dialkylated compounds were observed in the mixture as well.12 Sherer and Brugger reported the details of the reaction of tert-butyl N-(azetidin-3-yl)carbamate with ethyl 2-bromopropanoate in the presence of triethylamine in DCM at room temperature for 13 h, a sequence which provided ethyl 2-[3-(N-Boc-amino)azetidin-l-y])propanoate IV at a yield of 44%.13 The compound IV block was used to prepare polycyclic Toll-like receptor (TLR) antagonists useful in the treatment of immune disorders.14
The two most commonly used direct reductive amination methods are described below. The first method utilizes hydride reducing agents, particularly sodium cyanoborohydride – NaBH3CN.15 Manna et al. reported that the treatment of (2S)-2-(N-Boc-amino)propanal with ethyl glycinate hydrochloride in methanol gave the corresponding imine which, after the addition of NaBH3CN and acetic acid, gave the final chiral glycine ester derivative V with a good yield 68%.16 The second method is catalytic hydrogenation with platinum, palladium, or nickel catalysts.17 For example, N-(2-aminoethyl)glycine (I) was prepared by the reaction of diaminoethane with glyoxylic acid monohydrate and over Pd/C in ethanol under hydrogen gas at atm. pressure and room temperature.18 Dueholm and co-workers reported that the treatment of N-Boc-aminoaldehyde with methylglycinate hydrochloride in a solvent containing KOAc and a catalyst Pd/C under a hydrogen atmosphere afforded methyl-N-(2-Boc-aminoethyl)glycinate (71%).19 The difficulty of obtaining amino aldehydes as compounds has been noted, as has their instability.20
Falkevich et al. reported on the development and synthesis of N-(ω-aminoalkylene)amino acid derivatives from N-Boc-β-amino alcohols with N-o-nitrobenzenesulfonyl-protected (o-NBS-protected) amino acid esters using the Mitsunobu reaction.21 For example, the treatment of (2S)-2-(methylamino)propan-1-ol with o-NBS-Gly-OEt under Mitsunobu conditions followed, by deprotection with thiophenol, yielded the chiral glycine ester derivative V.16 Sabu et al. used the Mitsunobu reaction for the prepared N-(2-aminoethyl)glycine derivative VI containing the diglyme moiety.22
Herein, we report the design and preparation of methyl 2-[(N-Boc-amino)cycloaminyl]alkanoates from chiral triflate esters with chiral 3-Boc-aminopyrrolidine, 3-Boc-aminopiperidine and achiral 4-Boc-aminopiperidine. Such amino acid derivatives offer valuable properties as isosteres, new conformationally restricted chiral amino acids, and building blocks that can be used as potentially biologically active substances and peptides.23–27
Results and discussion
The synthetic strategy of methyl 2-[(Boc-amino)cycloaminyl]alkanoates is outlined in Scheme 1. The starting (R)- and (S)-2-hydroxy acid esters 1a–c used in this study are commercially available or were prepared from their acid form (see Experimental section). The synthetic sequence was started by transforming α-hydroxy carboxylates (R)-1a and (S)-1a into the triflate esters, methyl(2R)- and (2S)-2-[(trifluoromethanesulfonyl)oxy]propanoates (R)-2a and (S)-2a, using trifluoromethanesulfonic anhydride and pyridine in DCM.28 A triflate group is an excellent leaving group used in nucleophilic substitution reactions and has been shown to be significantly superior to other leaving groups in the Walden inversion, where the inversion of a stereogenic centre in a chiral molecule takes place.29,30 It is known that the reaction of enantiopure α-halocarboxylic acid esters with amines is accompanied by extensive racemization of α-amino esters; with α-methanesulfonyloxy and α-toluenesulfonyloxy carboxylic acid derivatives, both racemization and elimination products are formed due to the drastic conditions.31 Therefore, triflate esters with primary and secondary amines are known to give N-substituted α-aminocarboxylates in the SN2 reaction, resulting in good chemical as well as optical yields. Another advantage of triflate esters is that they can be generated in situ and used subsequently without isolation.32 According to Effenberger et al. ethyl (S)-2-hydroxypropionate was converted to a triflate ester and then treatment of the triflate ester with N-benzyl-N-methylamine in dichloromethane at 0–20 °C supplied ethyl-N-benzyl-N-methyl-D-alaninate (yield, 96%);33 Nilsson et al. reported that methyl(S)-3-(benzyloxy)-2-[(trifluoromethanesulfonyl)oxy]propanoate with 1-methylpiperazine maintained at −40 °C in toluene containing DIPEA produced methyl(R)-3-(benzyloxy)-2-(4-methylpiperazin-1-yl)propanoates (yield, 66%; ee 96%).34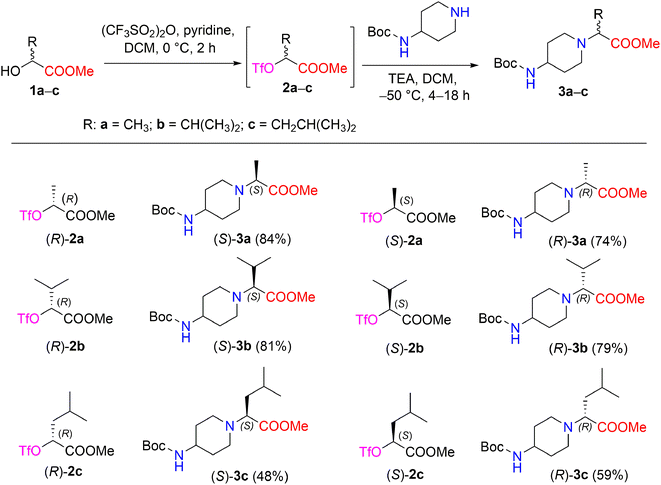 | ||
| Scheme 1 Enantiomers (S)- and (R)-3a–c synthesized from chiral triflates (S)- and (R)-2a–c with 4-Boc-aminopiperidine. *Yields are the overall yields of two steps. | ||
In our work, the reaction of chiral triflate esters (R)-2a or (S)-2a with 4-Boc-aminopiperidine in the presence of TEA in DCM at −50 °C led to the formation of the enantiomeric pure 2-[(Boc-amino)piperidinyl]propanoates, (S)-3a in a 84% yield, and (R)-3a in a 74% yield. The structural assignment of compounds (S)-3a and (R)-3a was readily deduced via detailed spectral data analysis. The IR spectrum of (S)-3a contained characteristic absorption bands, such as 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, ester) and 1681 (C
O, ester) and 1681 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, Boc) cm−1. The 1H NMR spectrum of compound (S)-3a revealed a characteristic resonance for the protons of the COOCH3 group, which appeared as a singlet at δ 3.68 ppm, and the methyl protons of the Boc-group, which appeared as a singlet at δ 1.42 ppm, whereas the methyl protons of the –CHC
O, Boc) cm−1. The 1H NMR spectrum of compound (S)-3a revealed a characteristic resonance for the protons of the COOCH3 group, which appeared as a singlet at δ 3.68 ppm, and the methyl protons of the Boc-group, which appeared as a singlet at δ 1.42 ppm, whereas the methyl protons of the –CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3 moiety yielded a doublet at δ 1.27 (J = 7.1 Hz) ppm. In the 13С NMR spectrum of compound (S)-3a, the signals of the piperidine carbons С-3,5 (δ 33.0 ppm), C-4,6 (δ 47.9 ppm), and C-2 (δ 49.5 ppm), as well as the signals of carbonyl carbons of esters –COOCH3 (δ 173.6 ppm) and –COO(CH3)3 (δ 155.2 ppm), were detected and distinguished based on long-range correlation data obtained through an 1H–13C HMBC experiment. The IR spectrum and 1H-, 13C-, and 15N NMR spectra of compound (R)-3a showed that the corresponding spectral data are identical to those of compound (S)-3a. Furthermore, the reaction conditions for the synthesis of compounds (S)-3a and (R)-3a were applied to the synthesis of other 2-[(Boc-amino)piperidinyl]alkanoates such as (S)-3b,c and (R)-3b,c (Scheme 1). In nucleophilic substitution reactions with the chiral triflate esters (R)-2b,c and (S)-2b,c, 4-Boc-aminopiperidine afforded compounds 3b,c in yields of 48–81%. The structures of the newly synthesized chiral Boc-amino ester derivatives (S)-3a–c and (R)-3a–c were described and confirmed by IR, NMR spectroscopy, and high-resolution mass spectrometry (ESI in Fig. S4–S25†).
3 moiety yielded a doublet at δ 1.27 (J = 7.1 Hz) ppm. In the 13С NMR spectrum of compound (S)-3a, the signals of the piperidine carbons С-3,5 (δ 33.0 ppm), C-4,6 (δ 47.9 ppm), and C-2 (δ 49.5 ppm), as well as the signals of carbonyl carbons of esters –COOCH3 (δ 173.6 ppm) and –COO(CH3)3 (δ 155.2 ppm), were detected and distinguished based on long-range correlation data obtained through an 1H–13C HMBC experiment. The IR spectrum and 1H-, 13C-, and 15N NMR spectra of compound (R)-3a showed that the corresponding spectral data are identical to those of compound (S)-3a. Furthermore, the reaction conditions for the synthesis of compounds (S)-3a and (R)-3a were applied to the synthesis of other 2-[(Boc-amino)piperidinyl]alkanoates such as (S)-3b,c and (R)-3b,c (Scheme 1). In nucleophilic substitution reactions with the chiral triflate esters (R)-2b,c and (S)-2b,c, 4-Boc-aminopiperidine afforded compounds 3b,c in yields of 48–81%. The structures of the newly synthesized chiral Boc-amino ester derivatives (S)-3a–c and (R)-3a–c were described and confirmed by IR, NMR spectroscopy, and high-resolution mass spectrometry (ESI in Fig. S4–S25†).
The synthesized compounds 3a–c exhibited optical activity. The corresponding (S)- and (R)-enantiomers rotated the plane-polarized light in equal amounts but in opposite directions; specific rotations [α]20D for the solution of compounds (S)-3a and (R)-3a in methanol were −19.4° and 19.5°, respectively. Furthermore, enantiomers (S)-3b and (R)-3b, which contained an isopropyl group, afforded a [α]20D = 34.4° (MeOH) and [α]20D = −34.6° (MeOH), respectively. Similarly, [α]20D for the solution of compounds (S)-3c and (R)-3c, which contained a 1-isobutyl group, were −19.2° (MeOH) and 19.4° (MeOH), respectively.
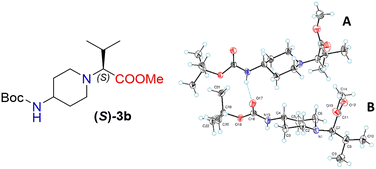
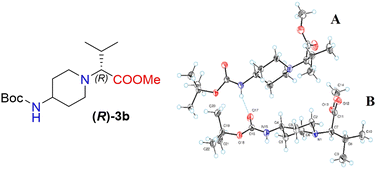
Moreover, X-ray crystallography data confirmed the absolute structure of enantiomers (S)-3b (ref. 35) (Fig. 2) and (R)-3b (ref. 36) (Fig. 3). A single crystal of compounds (S)-3b and (R)-3b was prepared from acetonitrile for X-ray diffraction analysis. The asymmetric unit of (R)-3b consists of two rotameric forms (A and B) (Fig. 3). Both rotamers represent 1,4-trans-disubstituted piperidine in chair conformation, and the substituents are in equatorial positions. The (R)-valinate fragment is orientated so that methoxycarbonylic fragment is directed parallel to the axial C–H bonds of the piperidine ring. One of the methyls from valine is orientated anti-parallel to the methoxycarbonylic fragment.
Owing to possible rotation about the C–C(O) bond, methoxycarbonylic fragments in the A and B rotamers are orientated differently: the dihedral angle O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–C–H in rotamer A is 14.80°, and the same angle in rotamer B is 169.02°. N–H and C
C–C–H in rotamer A is 14.80°, and the same angle in rotamer B is 169.02°. N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from the tert-butoxycarbonylamino fragment of molecule (R)-3b are in E conformation, and they are orientated parallel to the axial C–H bonds of the piperidine ring. Rotamers A and B in the asymmetric unit are both held by three hydrogen bonds (C(55)–H(55a)⋯O(17) 2.825 Å, 131.75°; N(65)–H(65)⋯O(17) 2.042 Å, 159.55°; and C(20)–H(20b)⋯O(68) 2.84 Å, 136.5°).
O bonds from the tert-butoxycarbonylamino fragment of molecule (R)-3b are in E conformation, and they are orientated parallel to the axial C–H bonds of the piperidine ring. Rotamers A and B in the asymmetric unit are both held by three hydrogen bonds (C(55)–H(55a)⋯O(17) 2.825 Å, 131.75°; N(65)–H(65)⋯O(17) 2.042 Å, 159.55°; and C(20)–H(20b)⋯O(68) 2.84 Å, 136.5°).
The crystals of (S)-3b are fully enantiomorphous to the crystals of (R)-3b (Fig. 2). This means that the crystals of (S)-3b and (R)-3b are related, like the left hand is to the right hand. For such crystals, the crystal structures are identical (the same lattice symmetry, equal cell parameters, etc.), except that the molecules of (S)-3b and (R)-3b are enantiomers.
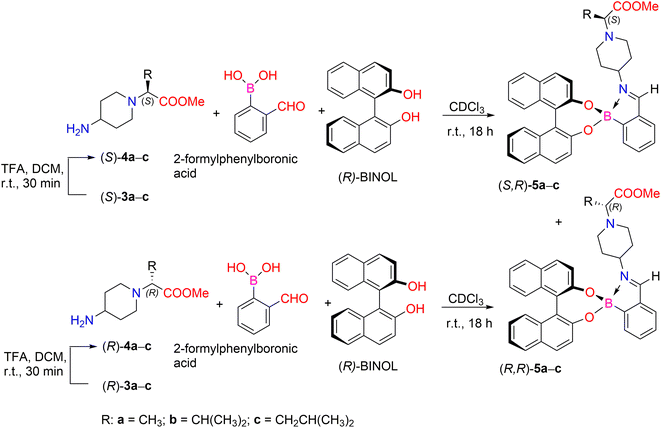
We also tried to analyze the enantiomers (S)-3a–c and (R)-3a–c and their unprotected forms by chiral HPLC analysis. Various attempts were made using different enantioselective HPLC columns, but this method proved unsuccessful. Then, we proceeded to determine the enantiomeric purity of our compounds using NMR methods. Many NMR spectroscopic techniques rely on chiral auxiliaries such as chiral derivatization agents, chiral lanthanide shift reagents, metal complexes, and chiral solvating agents.37,38 Fuertes et al. developed a simple chiral derivatization protocol for the enantiopure determination of chiral primary amines using 1H NMR spectroscopic analysis. The method involves the condensation of amines with 2-formylphenylboronic acid (2-FPBA) and (S)-1,1′-bi-2-naphthol ((S)-BINOL). This method allows a mixture of diastereomeric derivatives to be obtained, the ratio of which can be determined by integrating the resonances in their 1H NMR spectra, which makes it easy to determine the enantiopurity of the starting amine.39
In our case, the synthetic strategy to determine enantiomeric excess (ee) for amines containing remote stereogenic centers is based on the formation of iminoboronate ester complexes (Scheme 2). Deprotection of the N-Boc group from compounds 3a–c was carried out in the presence of TFA, followed by base workup using Cs2CO3 in order to generate free primary amines 4a–c. Furthermore, the reaction of chiral primary amines (S)-4a–c and (R)-4a–c with 2-FPBA and stereodefined (R)-BINOL in CDCl3 with 4 Å molecular sieves for 18 h at room temperature afforded a mixture of diastereomeric iminoboronate ester complexes (S,R)-5a–c and (R,R)-6a–c. The diastereomeric ratio (dr) was determined by comparing the integration ratios of distinct protons in their 1H NMR spectra, thus allowing indirect determination of the enantiopurity of their parent amines 4a–c (Table 1).
| Entry | Compound | dra | ǀΔδǀb (ppm) | Compound | eee (%) |
|---|---|---|---|---|---|
a dr of pairs of iminoboronate esters 5a–c was determined by 1H NMR (700 MHz, CDCl3) spectroscopic analysis from crude sample.b Chemical shift differences (Δδ) of the corresponding protons a, b resonances of pairs of iminoboronate esters (S,R)-5a–c and (R,R)-5a–c in the 1H NMR spectra from reaction of amines 4a–c with 2-FPBA and (R)-BINOL.c –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) CH3.d –COOCH3.e Determined by 1H NMR spectroscopic analysis of the iminoboronate esters (S,R)-5a–c and (R,R)-5a–c. CH3.d –COOCH3.e Determined by 1H NMR spectroscopic analysis of the iminoboronate esters (S,R)-5a–c and (R,R)-5a–c. |
|||||
| 1 | (S,R)-5a | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.03c | (S)-4a | 100 |
| 2 | (R,R)-5a | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.03c | (R)-4a | 100 |
| 3 | (S,R)-5b | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.02d | (S)-4b | 92 |
| 4 | (R,R)-5b | 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.02d | (R)-4b | 88 |
| 5 | (S,R)-5c | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
0.01d | (S)-4c | 86 |
| 6 | (R,R)-5c | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.01d | (R)-4c | 100 |
Analysis of the 1H NMR spectra of the iminoboronate ester complexes (S,R)-5b and (R,R)-5b revealed a characteristic resonance of the methyl protons of the esteric group (COOCH3), which appeared in their 1H NMR spectra as a singlet at δ 3.56 ppm and δ 3.54 ppm (chemical shift difference, Δδ, between methyl protons of COOCH3 is 0.02 ppm), respectively (Fig. 4). NMR analysis showed that the dr of the iminoboronate ester complex (S,R)-5b was 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, whereas the dr of (R,R)-5b was 94
4, whereas the dr of (R,R)-5b was 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. Furthermore, this diastereomeric ratio is expected to be in quantitative agreement with the enantiomeric ratio of chiral amines (S)-4b and (R)-4b. Thus allowing us to conclude that their ee are 92% and 88%, respectively. Analysis of the 1H NMR spectra of each derivatization reaction revealed the presence of at least one pair of resolved diastereomeric resonances in each case, whose integrals could be used to determine indirectly the enantiopurity of their parent amine 4. The 1H NMR spectrum of the pair of diastereomers (S,R)-5c and (R,R)-5c showed characteristic methyl proton resonances of the ester group (COOCH3) at δ 3.57 ppm and δ 3.56 ppm, respectively. In this case, the products (S,R)-5c and (R,R)-5c were obtained with 93
6. Furthermore, this diastereomeric ratio is expected to be in quantitative agreement with the enantiomeric ratio of chiral amines (S)-4b and (R)-4b. Thus allowing us to conclude that their ee are 92% and 88%, respectively. Analysis of the 1H NMR spectra of each derivatization reaction revealed the presence of at least one pair of resolved diastereomeric resonances in each case, whose integrals could be used to determine indirectly the enantiopurity of their parent amine 4. The 1H NMR spectrum of the pair of diastereomers (S,R)-5c and (R,R)-5c showed characteristic methyl proton resonances of the ester group (COOCH3) at δ 3.57 ppm and δ 3.56 ppm, respectively. In this case, the products (S,R)-5c and (R,R)-5c were obtained with 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr (86% ee for (S)-4c) and 100
7 dr (86% ee for (S)-4c) and 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 dr (100% ee for (R)-4c), respectively. However, the 1H NMR spectra of the corresponding products, (S,R)-5a and (R,R)-5a, showed that the methyl ester group protons overlapped and resonated at δ 3.62 ppm. Therefore, the determination of diastereomers (S,R)-5a and (R,R)-5a according to the diastereomeric ratio by integration of their 1H NMR spectra showed the distinct resonances of the proton from the –C
0 dr (100% ee for (R)-4c), respectively. However, the 1H NMR spectra of the corresponding products, (S,R)-5a and (R,R)-5a, showed that the methyl ester group protons overlapped and resonated at δ 3.62 ppm. Therefore, the determination of diastereomers (S,R)-5a and (R,R)-5a according to the diastereomeric ratio by integration of their 1H NMR spectra showed the distinct resonances of the proton from the –C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3 moiety. The –C
CH3 moiety. The –C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3 fragment gave quadruplets at δ 3.21 (J = 7.0 Hz) and δ 3.18 (J = 7.0 Hz) ppm, respectively. The investigation of synthesized iminoboronate ester complexes (S,R)-5a and (R,R)-5a produced 1H NMR spectra with a diastereomeric ratio of 100
CH3 fragment gave quadruplets at δ 3.21 (J = 7.0 Hz) and δ 3.18 (J = 7.0 Hz) ppm, respectively. The investigation of synthesized iminoboronate ester complexes (S,R)-5a and (R,R)-5a produced 1H NMR spectra with a diastereomeric ratio of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (100% ee for (S)-4a and (R)-4a) for both complexes.
0 (100% ee for (S)-4a and (R)-4a) for both complexes.
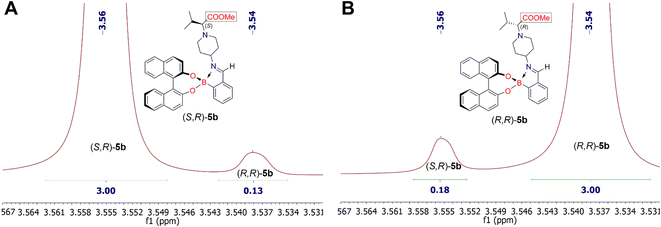 | ||
| Fig. 4 Fragments of 1H NMR spectra of synthesized diastereomeric iminoboronate ester complexes (S,R)-5b (part A) and (R,R)-5b (part B). | ||
In addition, deprotection of the N-Boc group from compounds 3a–c potentially had no influence on changing the enantiomeric ratio for obtained amines 4a–c. Therefore, the synthesis of enantiomers 3a–c (Scheme 1), as described above, is highly enantioselective with no or limited epimerization.
The prepared enantiomers, (S)-3a and (R)-3a, are potential synthons in the synthesis of small peptides. Synthetic small peptides, including heterocyclic dipeptides, are thus attractive agents and targets for therapies and diagnostics.40–42 For example, Pavadai et al. reported synthesis of a piperidin-4-one derivative containing dipeptide as an acetyl cholinesterase and β-secretase inhibitor;43 Blaszczyk et al. synthesized new piperidine dipeptides exhibiting arginase inhibition, with high intracellular activity that could be of use in the treatment of cancer.44,45
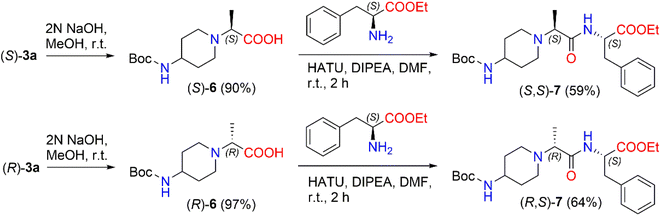
In the present work, we prepared 2-(Boc-amino)piperidine dipeptides (S,S)-7 and (R,S)-7 by coupling enantiomers (S)-3a and (R)-3a with L-phenylalanine (Scheme 3). First, the ester (S)-3a was hydrolyzed with 2 N NaOH in methanol to afford acid (S)-6 in a yield of 90%. After that, the reaction of 2-[(Boc-amino)piperidinyl]propionic acid (S)-6 with 1-[bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium-3-oxid hexafluorophosphate (HATU) was carried out in the presence of DIPEA in a polar aprotic solvent, such as DMF, at room temperature to form the corresponding active ester. HATU has proven to be a highly reactive peptide coupling reagent, free from by-product formation and product racemization compared to other commonly used coupling reagents.46 In our case, the corresponding active ester was coupled with L-phenylalanine ethyl ester hydrochloride to produce N-Boc-dipeptide (S,S)-7 in a 59% yield. The same method was used to synthesize N-Boc-dipeptide (R,S)-7 from acid (R)-6 with L-phenylalanine. The formation of N-Boc-dipeptides (S,S)-7 and (R,S)-7 was established by NMR analysis. The reaction produced (S,S)-7 in a diastereomeric ratio of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and (R,S)-7 in a diastereomeric ratio of 90
6 and (R,S)-7 in a diastereomeric ratio of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (ESI in Fig. S38–S43†). The 1H NMR spectrum of (S,S)-7 revealed a quadruplet of –C
10 (ESI in Fig. S38–S43†). The 1H NMR spectrum of (S,S)-7 revealed a quadruplet of –C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3 proton at δ 3.01 (J = 7.0 Hz) ppm and the signals of the –CHC
CH3 proton at δ 3.01 (J = 7.0 Hz) ppm and the signals of the –CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph protons split to multiplets and detected at δ 3.05–3.10 ppm and δ 3.17–3.22 ppm. In the case of the 1H NMR spectrum of (R,S)-7, a quadruplet of –C
2Ph protons split to multiplets and detected at δ 3.05–3.10 ppm and δ 3.17–3.22 ppm. In the case of the 1H NMR spectrum of (R,S)-7, a quadruplet of –C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3 proton was registered at δ 3.06 (J = 7.1 Hz) ppm, whereas the signals of –CHC
CH3 proton was registered at δ 3.06 (J = 7.1 Hz) ppm, whereas the signals of –CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2Ph protons were observed as a doublet at δ 3.13 (J = 6.1 Hz) ppm.
2Ph protons were observed as a doublet at δ 3.13 (J = 6.1 Hz) ppm.
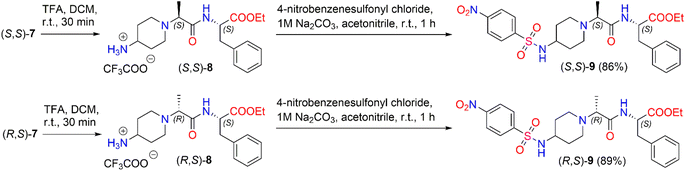
We also investigated the transformation of N-Boc-dipeptides (S,S)-7 and (R,S)-7 to N-nosyl-dipeptides (S,S)-9 and (R,S)-9 (Scheme 4). The use of a p-nitrobenzenesulfonyl (nosyl) group to protect the amino functional group is of great importance for obtaining N-methylated amino acids and peptides.47 Moreover, sulfonamides play a significant role in medicine as antibiotics, antithyroid agents, and antitumor drugs.48,49 For instance, Murthy et al. reported a series of novel benzhydryl piperazine-coupled nitrobenzenesulfonamide hybrids as agents which showed excellent anti-tuberculosis activity,50 and Ugwuja et al. developed and synthesized new peptide-derived antimalaria and antimicrobial agents bearing a sulfonamide moiety.51
To remove the protecting Boc-group,52 dipeptide (S,S)-7 was dissolved in DCM and then TFA was added under stirring at room temperature for 30 min. After the removal of the solvent in vacuo, the corresponding trifluoroacetate (S,S)-8 was used directly in the next step without further purification. The deblocked product, (S,S)-8, was coupled with 4-nitrobenzenesulfonyl chloride in the presence of 1 M Na2CO3 in acetonitrile to obtain the corresponding diastereomeric N-nosyl-dipeptide (S,S)-9 in a yield of 86%. The same reaction conditions as above were applied to synthesize N-nosyl-dipeptide (R,S)-9 in a yield of 89%. 1H NMR spectroscopy of the products confirmed the structure of the corresponding N-nosyl-dipeptides, (S,S)-9 and (R,S)-9; in particular, the 1H NMR spectrum showed a doublet signal of –CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3 protons at δ 1.08 (J = 7.0 Hz) ppm and δ 1.12 (J = 7.0 Hz) ppm for (S,S)-9 and (R,S)-9, respectively. The reaction gave the corresponding product, (S,S)-9, in a dr of 94
3 protons at δ 1.08 (J = 7.0 Hz) ppm and δ 1.12 (J = 7.0 Hz) ppm for (S,S)-9 and (R,S)-9, respectively. The reaction gave the corresponding product, (S,S)-9, in a dr of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, whereas product (R,S)-9 was obtained in a dr of 90
6, whereas product (R,S)-9 was obtained in a dr of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (ESI in Fig. S44–S53†).
10 (ESI in Fig. S44–S53†).
We then performed a nucleophilic substitution reaction with triflate esters (R)-2a–c and (S)-2a–c, chiral 3-Boc-aminopiperidine and 3-Boc-aminopyrrolidine, to obtain diastereomers 10a–c and 11a–c, respectively (Scheme 5). Optimization of the nucleophilic substitution conditions was undertaken for determination of diastereomeric selectivity, choosing triflate esters (R)-2b and (S)-2b as enantiomeric pair. Then, nucleophilic substitution with (R)-3-Boc-aminopiperidine was carried out at different temperatures, such as room temperature, −30 °C, and −50 °C, and the 1H NMR spectral data of the crude samples of products (2S,3R)-10b and (2R,3R)-10b were analyzed (Table 2). The 1H NMR spectra of (2S,3R)-10b and (2R,3R)-10b revealed characteristic resonance for the doublet signal of the proton of –C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2 at δ 2.67 (J = 10.8 Hz) ppm and δ 2.71 (J = 10.8 Hz) ppm, respectively. In our study, the poorest stereoselectivity was observed when the reaction mixture was stirred at room temperature − 75
CH(CH3)2 at δ 2.67 (J = 10.8 Hz) ppm and δ 2.71 (J = 10.8 Hz) ppm, respectively. In our study, the poorest stereoselectivity was observed when the reaction mixture was stirred at room temperature − 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 dr for (2S,3R)-10b and 78
25 dr for (2S,3R)-10b and 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 dr for (2R,3R)-10b. Furthermore, when the reaction was carried out at −30 °C, the resulting diastereomeric ratios were 87
22 dr for (2R,3R)-10b. Furthermore, when the reaction was carried out at −30 °C, the resulting diastereomeric ratios were 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 dr and 93
13 dr and 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr, respectively, for (2S,3R)-10b and (2R,3R)-10b. Moreover, carrying out the reaction at −50 °C yielded a high stereoselectivity (for (2S,3R)-10b, it was 94
7 dr, respectively, for (2S,3R)-10b and (2R,3R)-10b. Moreover, carrying out the reaction at −50 °C yielded a high stereoselectivity (for (2S,3R)-10b, it was 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 dr, and for (2R,3R)-10b, it was 100
6 dr, and for (2R,3R)-10b, it was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 dr) and a good yield (for (2S,3R)-10b, yield was 86%, and for (2R,3R)-10b yield was 83%).
0 dr) and a good yield (for (2S,3R)-10b, yield was 86%, and for (2R,3R)-10b yield was 83%).
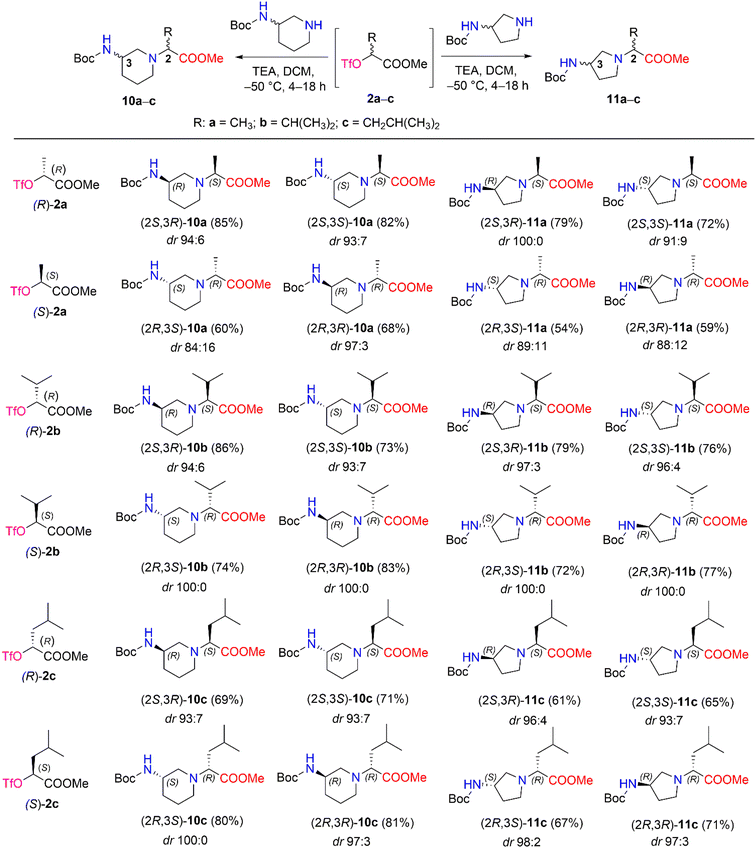 | ||
| Scheme 5 Diastereomers 10a–c and 11a–c synthesized from chiral triflate esters (S)- and (R)-2a–c. *Yields are the overall yields of two steps. | ||
The obtained optimal conditions for the stereoselective synthesis of (2S,3R)-10b and (2R,3R)-10b were applied to the synthesis of other diastereomers, 10a–c and 11a–c (Scheme 5). 3-Boc-aminopiperidine in nucleophilic substitution reactions with chiral triflate esters (R)-2a–c and (S)-2a–c afforded diastereomers 10a–c in yields of 60–86%. Moreover, the synthetic conditions were used to carry out the corresponding reactions with another cyclic amine, such as 3-Boc-aminopyrrolidine. As a result, diastereomers 11a–c were obtained in 54–79% yields. The dr of aminopiperidine and aminopyrrolidine derivatives 10a–c (84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 ≤ dr ≤ 100
16 ≤ dr ≤ 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) and 11a–c (88
0) and 11a–c (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 ≤ dr ≤ 100
12 ≤ dr ≤ 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) were determined by their 1H NMR spectra after purification with column chromatography (Scheme 5) (ESI in Fig. S54–S134†).
0) were determined by their 1H NMR spectra after purification with column chromatography (Scheme 5) (ESI in Fig. S54–S134†).
Materials and methods
General information
All starting materials were purchased from commercial suppliers and were used as received. (R)-1,1′-Bi-2-naphthol ((R)-BINOL), 99% (ee: 99%); (+)-methyl-D-lactate, 98% (ee: 96%); and (−)-methyl L-lactate, 98% (ee: 97%) were purchased from Sigma-Aldrich. (R)-2-Hydroxy-3-methylbutyric acid, 97%; (S)-2-hydroxy-3-methylbutyric acid, 98%; (R)-2-hydroxy-4-methylpentanoic acid, 97%; and (S)-2-hydroxy-4-methylpentanoic acid, 97% were purchased from Combi-Blocks. Methylation of (R)- and (S)-hydroxy acids was carried out with dimethyl sulfate in the presence of potassium carbonate in acetone according to the described method.53 Flash column chromatography was performed on silica gel 60 Å (230–400 μm, Merck KGaA, Darmstadt, Germany). Thin-layer chromatography was carried out on silica gel plates (Merck Kieselgel 60 F254) and visualized by UV light (254 nm). Melting points were determined on a Büchi M-565 melting point apparatus and were uncorrected. The IR spectra were recorded on a Bruker Vertex 70v FT-IR spectrometer (Bruker Optik GmbH, Ettlingen, Germany) using neat samples and are reported in the frequency of absorption (cm−1). Mass spectra were obtained on a Shimadzu LCMS-2020 (ESI+) spectrometer (Shimadzu Corporation, Kyoto, Japan). High-resolution mass spectra were measured on a Bruker MicrOTOF-Q III (ESI+), or on a Bruker maXis 4G (ESI+) spectrometer (Bruker Daltonik GmbH, Bremen, Germany). Optical rotation data were recorded on a UniPol L SCHMIDT + HAENSCH polarimeter (concentration of compound (g/100 mL) was included in calculations automatically (Windaus-Labortechnik GmbH & Co. KG, Clausthal-Zellerfeld, Germany)). HPLC analysis was carried out on a Shimadzu LC-2030C apparatus with CHIRAL ART Amylose-SA (100 × 4.6 mm I.D.; S-3 μm; chiral selector amylose tris(3,5-dimethylphenylcarbamate)) and CHIRAL ART Cellulose-SB (100 × 4.6 mm I.D.; S-3 μm; chiral selector cellulose tris(3,5-dimethylphenylcarbamate)) (YMC, Shimadzu USA Manufacturing, Inc., Canby, OR, USA). Single crystals were investigated on a Rigaku, XtaLAB Synergy, Dualflex, HyPix diffractometer. The crystals were kept at 150.0(1) K during data collection. Using Olex2, the structure was solved with the ShelXT structure solution program using intrinsic phasing and refined with the olex2.refine refinement package using Gauss–Newton minimisation. The 1H-, 13C-, and 15N-NMR spectra were recorded in CDCl3 solutions at 25 °C on a Bruker Avance III 700 (700 MHz for 1H, 176 MHz for 13C, 71 MHz for 15N, Bruker BioSpin AG, Fallanden, Switzerland) spectrometer equipped with a 5 mm TCI 1H–13C/15N/D z-gradient cryoprobe. The chemical shifts (δ), expressed in ppm, were relative to tetramethylsilane (TMS). The 15N-NMR spectra were referenced to neat, external nitromethane (coaxial capillary). Full and unambiguous assignment of the 1H-, 13C-, and 15N-NMR resonances was achieved using a combination of standard NMR spectroscopic techniques such as DEPT, COSY, gs-HSQC, and gs-HMBC experiments. NMR spectra and HRMS data for all new compounds are provided in the ESI.†Methylation of (R)- and (S)-hydroxy acids (1b, 1c)
Me2SO4 (1.5 equiv.) was added to a solution of the corresponding chiral hydroxy acid (2 g) and K2CO3 (3 equiv.) in acetone (0.3 M). The reaction mixture was stirred under reflux for 5 hours. After removal of the solvent in vacuo, the residue was dissolved in water (15 mL) and washed with EtOAc (2 × 15 mL) and brine (15 mL). The extracts were combined and dried over sodium sulfate, filtered, and concentrated. The crude product was purified by flash chromatography (SiO2, eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to produce the desired compounds (1b–c).
5, v/v) to produce the desired compounds (1b–c).
![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.01 (d, J = 6.9 Hz, 3H, CHCH(C
3)2), 1.01 (d, J = 6.9 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 2.02–2.09 (m, 1H, CHC
3)2), 2.02–2.09 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.57 (s, 1H, OH), 3.78 (s, 3H, OCH3), 4.04 (d, J = 3.6 Hz, 1H, C
(CH3)2), 2.57 (s, 1H, OH), 3.78 (s, 3H, OCH3), 4.04 (d, J = 3.6 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2). 13C-NMR (176 MHz, CDCl3): δ 16.1 (CHCH(
CH(CH3)2). 13C-NMR (176 MHz, CDCl3): δ 16.1 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 18.9 (CHCH(
H3)2), 18.9 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 32.3 (CH
H3)2), 32.3 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 52.5 (OCH3), 75.2 (
H(CH3)2), 52.5 (OCH3), 75.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)2), 175.5 (
HCH(CH3)2), 175.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 3464 (O–H), 2967, 1729 (C
OOCH3). IR (FT-IR, νmax, cm−1): 3464 (O–H), 2967, 1729 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1027. HRMS (ESI+) for C6H12NaO3 ([M + Na]+) calcd 155.0679, found 155.0680.
O), 1027. HRMS (ESI+) for C6H12NaO3 ([M + Na]+) calcd 155.0679, found 155.0680.Synthesis of triflates (2a–c)
A solution of the corresponding ester (1a–c) (1 equiv.) and pyridine (1.2 equiv.) in DCM (0.1 M) was cooled to 0 °C and stirred for 5 min under an argon atmosphere. Then, trifluoromethanesulfonic anhydride (1.2 equiv.) was added dropwise, and the reaction mixture was stirred for 2 hours. The resulting solution was quenched with water (10 mL), and the aqueous layer was separated and extracted with DCM (2 × 15 mL) and brine (15 mL). The organic layer was dried with anhydrous sodium sulfate, filtered, and then concentrated under reduced pressure. Crude product (2a–c) was directly used in the next step without further purification.Synthesis of alkanoates (3a–c, 10a–c and 11a–c)
Methyl(2S)-2-{4-[(tert-butoxycarbonyl)amino]piperidin-1-yl}propanoate ((S)-3a). Method A. Compound (R)-2a was coupled with 4-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (S)-3a as white crystals. Yield 509 mg (84%), mp 76–78 °C, [α]20D = −19.4° (c 0.89, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.27 (d, J = 7.1 Hz, 3H, CHC
5, v/v) to provide compound (S)-3a as white crystals. Yield 509 mg (84%), mp 76–78 °C, [α]20D = −19.4° (c 0.89, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.27 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.35–1.47 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.87–1.94 (m, 2H, Pip 3,5-H), 2.28 (td, J = 11.5 Hz, 2.6 Hz, 1H, Pip 6-H), 2.35 (td, J = 11.4 Hz, 2.6 Hz, 1H, Pip 2-H), 2.79–2.86 (m, 2H, Pip 2,6-H), 3.27 (q, J = 7.0 Hz, 1H, C
3), 1.35–1.47 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.87–1.94 (m, 2H, Pip 3,5-H), 2.28 (td, J = 11.5 Hz, 2.6 Hz, 1H, Pip 6-H), 2.35 (td, J = 11.4 Hz, 2.6 Hz, 1H, Pip 2-H), 2.79–2.86 (m, 2H, Pip 2,6-H), 3.27 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.39–3.50 (m, 1H, Pip 4-H), 3.68 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.0 (CH
CH3), 3.39–3.50 (m, 1H, Pip 4-H), 3.68 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 33.0 (Pip 3,5-C), 47.9 (Pip 4,6-C), 49.5 (Pip 2-C), 51.5 (OCH3), 62.8 (
H3)3), 33.0 (Pip 3,5-C), 47.9 (Pip 4,6-C), 49.5 (Pip 2-C), 51.5 (OCH3), 62.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.3 (
HCH3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.2 (
(CH3)3), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.6 (
OOC(CH3)3), 173.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −330.3 (Pip), −285.3 (NH). IR (FT-IR, νmax, cm−1): 2947, 2811, 1728 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −330.3 (Pip), −285.3 (NH). IR (FT-IR, νmax, cm−1): 2947, 2811, 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1681 (C
O), 1681 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1172, 1047, 885. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287. 1965, found 287.1968.
O), 1172, 1047, 885. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287. 1965, found 287.1968.
Methyl(2R)-2-{4-[(tert-butoxycarbonyl)amino]piperidin-1-yl}propanoate ((R)-3a). Method A. Compound (S)-2a was coupled with 4-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (R)-3a as yellowish crystals. Yield 449 mg (74%), mp 76–78 °C, [α]20D = 19.5° (c 0.86, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.27 (d, J = 7.1 Hz, 3H, CHC
7, v/v) to provide compound (R)-3a as yellowish crystals. Yield 449 mg (74%), mp 76–78 °C, [α]20D = 19.5° (c 0.86, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.27 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.36–1.46 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.88–1.94 (m, 2H, Pip 3,5-H), 2.28 (td, J = 11.5 Hz, 2.6 Hz, 1H, Pip 6-H), 2.35 (td, J = 11.4 Hz, 2.6 Hz, 1H, Pip 2-H), 2.79–2.86 (m, 2H, Pip 2,6-H), 3.28 (q, J = 7.0 Hz, 1H, C
3), 1.36–1.46 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.88–1.94 (m, 2H, Pip 3,5-H), 2.28 (td, J = 11.5 Hz, 2.6 Hz, 1H, Pip 6-H), 2.35 (td, J = 11.4 Hz, 2.6 Hz, 1H, Pip 2-H), 2.79–2.86 (m, 2H, Pip 2,6-H), 3.28 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.40–3.50 (m, 1H, Pip 4-H), 3.68 (s, 3H, OCH3), 4.43 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.0 (CH
CH3), 3.40–3.50 (m, 1H, Pip 4-H), 3.68 (s, 3H, OCH3), 4.43 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 33.0 (Pip 3,5-C), 47.9 (Pip 4,6-C), 49.5 (Pip 2-C), 51.5 (OCH3), 62.8 (
H3)3), 33.0 (Pip 3,5-C), 47.9 (Pip 4,6-C), 49.5 (Pip 2-C), 51.5 (OCH3), 62.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.3 (
HCH3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.2 (
(CH3)3), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.6 (
OOC(CH3)3), 173.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −330.3 (Pip), −285.3 (NH). IR (FT-IR, νmax, cm−1): 2946, 2811, 1728 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −330.3 (Pip), −285.3 (NH). IR (FT-IR, νmax, cm−1): 2946, 2811, 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1680 (C
O), 1680 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1170, 1046, 884. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1967.
O), 1170, 1046, 884. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1967.
Methyl(2S)-2-{4-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-3-methylbutanoate ((S)-3b). Method B. Compound (R)-2b was coupled with 4-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (S)-3b as white crystals. Yield 482 mg (81%), mp 97–98 °C, [α]20D = 34.4° (c 0.91, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.6 Hz, 3H, CHCH(C
5, v/v) to provide compound (S)-3b as white crystals. Yield 482 mg (81%), mp 97–98 °C, [α]20D = 34.4° (c 0.91, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.6 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.93 (d, J = 6.6 Hz, 3H, CHCH(C
3)2), 0.93 (d, J = 6.6 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.27–1.33 (m, 1H, Pip 3-H), 1.36–1.47 (m, 1H, Pip 5-H), 1.42 (s, 9H, C(CH3)3), 1.85–1.92 (m, 2H, Pip 3,5-H), 1.98–2.04 (m, 1H, CHC
3)2), 1.27–1.33 (m, 1H, Pip 3-H), 1.36–1.47 (m, 1H, Pip 5-H), 1.42 (s, 9H, C(CH3)3), 1.85–1.92 (m, 2H, Pip 3,5-H), 1.98–2.04 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.15–2.21 (m, 1H, Pip 6-H), 2.32–2.39 (m, 1H, Pip 2-H), 2.66–2.73 (m, 1H, Pip 6-H), 2.71 (d, J = 10.5 Hz, 1H, C
(CH3)2), 2.15–2.21 (m, 1H, Pip 6-H), 2.32–2.39 (m, 1H, Pip 2-H), 2.66–2.73 (m, 1H, Pip 6-H), 2.71 (d, J = 10.5 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 2.76–2.81 (m, 1H, Pip 2-H), 3.35–3.48 (m, 1H, Pip 4-H), 3.67 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(
CH(CH3)2), 2.76–2.81 (m, 1H, Pip 2-H), 3.35–3.48 (m, 1H, Pip 4-H), 3.67 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 19.8 (CHCH(
H3)2), 19.8 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 27.0 (CH
H3)2), 27.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 33.2 (Pip 5-C), 33.4 (Pip 3-C), 45.8 (Pip 6-C), 48.1 (Pip 4-C), 50.7 (OCH3), 51.1 (Pip 2-C), 74.5 (
H3)3), 33.2 (Pip 5-C), 33.4 (Pip 3-C), 45.8 (Pip 6-C), 48.1 (Pip 4-C), 50.7 (OCH3), 51.1 (Pip 2-C), 74.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.3 (
HCH(CH3)3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.2 (
(CH3)3), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.1 (
OOC(CH3)3), 172.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2967, 2824, 1726 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2967, 2824, 1726 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1680 (C
O), 1680 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163, 1006, 773. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2281.
O), 1163, 1006, 773. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2281.
Methyl(2R)-2-{4-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-3-methylbutanoate ((R)-3b). Method B. Compound (S)-2b was coupled with 4-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (R)-3b as white crystals. Yield 470 mg (79%), mp 98–100 °C, [α]20D = −34.6° (c 1.29, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.5 Hz, 3H, CHCH(C
5, v/v) to provide compound (R)-3b as white crystals. Yield 470 mg (79%), mp 98–100 °C, [α]20D = −34.6° (c 1.29, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.5 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.93 (d, J = 6.7 Hz, 3H, CHCH(C
3)2), 0.93 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.26–1.34 (m, 1H, Pip 3-H), 1.38–1.46 (m, 1H, Pip 5-H), 1.42 (s, 9H, C(CH3)3), 1.84–1.91 (m, 2H, Pip 3,5-H), 1.97–2.04 (m, 1H, CHC
3)2), 1.26–1.34 (m, 1H, Pip 3-H), 1.38–1.46 (m, 1H, Pip 5-H), 1.42 (s, 9H, C(CH3)3), 1.84–1.91 (m, 2H, Pip 3,5-H), 1.97–2.04 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.15–2.22 (m, 1H, Pip 6-H), 2.32–2.40 (m, 1H, Pip 2-H), 2.66–2.74 (m, 1H, Pip 6-H), 2.71 (d, J = 10.5 Hz, 1H, C
(CH3)2), 2.15–2.22 (m, 1H, Pip 6-H), 2.32–2.40 (m, 1H, Pip 2-H), 2.66–2.74 (m, 1H, Pip 6-H), 2.71 (d, J = 10.5 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 2.76–2.81 (m, 1H, Pip 2-H), 3.34–3.50 (m, 1H, Pip 4-H), 3.67 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(
CH(CH3)2), 2.76–2.81 (m, 1H, Pip 2-H), 3.34–3.50 (m, 1H, Pip 4-H), 3.67 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 19.8 (CHCH(
H3)2), 19.8 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 27.0 (CH
H3)2), 27.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 33.2 (Pip 5-C), 33.4 (Pip 3-C), 45.9 (Pip 6-C), 48.1 (Pip 4-C), 50.7 (OCH3), 51.1 (Pip 2-C), 74.5 (
H3)3), 33.2 (Pip 5-C), 33.4 (Pip 3-C), 45.9 (Pip 6-C), 48.1 (Pip 4-C), 50.7 (OCH3), 51.1 (Pip 2-C), 74.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.3 (
HCH(CH3)3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.2 (
(CH3)3), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.1 (
OOC(CH3)3), 172.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2967, 2824, 1727 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2967, 2824, 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1680 (C
O), 1680 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163, 1006, 773. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2279.
O), 1163, 1006, 773. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2279.
Methyl(2S)-2-{4-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-4-methylpentanoate ((S)-3c). Method B. Compound (R)-2c was coupled with 4-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) to provide compound (S)-3c as white crystals. Yield 283 mg (48%), mp 77–79 °C, [α]20D = −19.2° (c 0.98, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.87 (d, J = 6.1 Hz, 3H, CHCH2CH(C
9, v/v) to provide compound (S)-3c as white crystals. Yield 283 mg (48%), mp 77–79 °C, [α]20D = −19.2° (c 0.98, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.87 (d, J = 6.1 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.90 (d, J = 6.2 Hz, 3H, CHCH2CH(C
3)2), 0.90 (d, J = 6.2 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.27–1.35 (m, 1H, CHC
3)2), 1.27–1.35 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.35–1.53 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.54–1.61 (m, 2H, CHCH2C
2CH(CH3)2), 1.35–1.53 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.54–1.61 (m, 2H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2 and CHC
(CH3)2 and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.84–1.94 (m, 2H, Pip 3,5-H), 2.25–2.30 (m, 1H, Pip 6-H), 2.39–2.46 (m, 1H, Pip 2-H), 2.72–2.86 (m, 2H, Pip 2,6-H), 3.21–3.28 (m, 1H, C
2CH(CH3)2), 1.84–1.94 (m, 2H, Pip 3,5-H), 2.25–2.30 (m, 1H, Pip 6-H), 2.39–2.46 (m, 1H, Pip 2-H), 2.72–2.86 (m, 2H, Pip 2,6-H), 3.21–3.28 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.38–3.50 (m, 1H, Pip 4-H), 3.67 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.7 (CHCH2CH(
CH2CH(CH3)2), 3.38–3.50 (m, 1H, Pip 4-H), 3.67 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.7 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.2 (CHCH2
H3)2), 25.2 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 33.1 (Pip 5-C), 33.3 (Pip 3-C), 38.5 (CH
H3)3), 33.1 (Pip 5-C), 33.3 (Pip 3-C), 38.5 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 46.4 (Pip 6-C), 48.0 (Pip 4-C), 50.6 (Pip 2-C), 51.1 (OCH3), 65.7 (
H2CH(CH3)2), 46.4 (Pip 6-C), 48.0 (Pip 4-C), 50.6 (Pip 2-C), 51.1 (OCH3), 65.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.3 (
HCH2CH(CH3)2), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.2 (
(CH3)3), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.0 (
OOC(CH3)3), 173.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2869, 1734 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2869, 1734 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1677 (C
O), 1677 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1162, 1004, 750. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2433.
O), 1162, 1004, 750. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2433.
Methyl(2R)-2-{4-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-4-methylpentanoate ((R)-3c). Method B. Compound (S)-2c was coupled with 4-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) to provide compound (R)-3c as white crystals. Yield 348 mg (59%), mp 79–81 °C, [α]20D = 19.4° (c 1.06, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.87 (d, J = 6.2 Hz, 3H, CHCH2CH(C
9, v/v) to provide compound (R)-3c as white crystals. Yield 348 mg (59%), mp 79–81 °C, [α]20D = 19.4° (c 1.06, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.87 (d, J = 6.2 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.90 (d, J = 6.3 Hz, 3H, CHCH2CH(C
3)2), 0.90 (d, J = 6.3 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.26–1.34 (m, 1H, CHC
3)2), 1.26–1.34 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.35–1.52 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.53–1.61 (m, 2H, CHCH2C
2CH(CH3)2), 1.35–1.52 (m, 2H, Pip 3,5-H), 1.42 (s, 9H, C(CH3)3), 1.53–1.61 (m, 2H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2 and CHC
(CH3)2 and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.85–1.92 (m, 2H, Pip 3,5-H), 2.22–2.31 (m, 1H, Pip 6-H), 2.38–2.47 (m, 1H, Pip 2-H), 2.73–2.85 (m, 2H, Pip 2,6-H), 3.20–3.28 (m, 1H, C
2CH(CH3)2), 1.85–1.92 (m, 2H, Pip 3,5-H), 2.22–2.31 (m, 1H, Pip 6-H), 2.38–2.47 (m, 1H, Pip 2-H), 2.73–2.85 (m, 2H, Pip 2,6-H), 3.20–3.28 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.38–3.47 (m, 1H, Pip 4-H), 3.66 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.7 (CHCH2CH(
CH2CH(CH3)2), 3.38–3.47 (m, 1H, Pip 4-H), 3.66 (s, 3H, OCH3), 4.42 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.7 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.2 (CHCH2
H3)2), 25.2 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 33.1 (Pip 5-C), 33.3 (Pip 3-C), 38.5 (CH
H3)3), 33.1 (Pip 5-C), 33.3 (Pip 3-C), 38.5 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 46.4 (Pip 6-C), 48.0 (Pip 4-C), 50.6 (Pip 2-C), 51.1 (OCH3), 65.7 (
H2CH(CH3)2), 46.4 (Pip 6-C), 48.0 (Pip 4-C), 50.6 (Pip 2-C), 51.1 (OCH3), 65.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.3 (
HCH2CH(CH3)2), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.2 (
(CH3)3), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.0 (
OOC(CH3)3), 173.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2869, 1734 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2869, 1734 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1678 (C
O), 1678 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1162, 1004, 750. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2434.
O), 1162, 1004, 750. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2434.
Methyl(2S)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}propanoate ((2S,3R)-10a). Method A. Compound (R)-2a was coupled with (R)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3R)-10a as transparent oil in an 85% yield (515 mg) with a dr of 94
5, v/v) to provide compound (2S,3R)-10a as transparent oil in an 85% yield (515 mg) with a dr of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, [α]20D = 14.2° (c 0.77, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.1 Hz, 3H, CHC
6, [α]20D = 14.2° (c 0.77, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.43 (s, 9H, C(CH3)3), 1.47–1.61 (m, 3H, Pip 4,5-H), 1.62–1.71 (m, 1H, Pip 5-H), 2.32–2.47 (m, 2H, Pip 2,6-H), 2.54–2.62 (m, 1H, Pip 6-H), 2.70–2.80 (m, 1H, Pip 2-H), 3.27–3.34 (m, 1H, C
3), 1.43 (s, 9H, C(CH3)3), 1.47–1.61 (m, 3H, Pip 4,5-H), 1.62–1.71 (m, 1H, Pip 5-H), 2.32–2.47 (m, 2H, Pip 2,6-H), 2.54–2.62 (m, 1H, Pip 6-H), 2.70–2.80 (m, 1H, Pip 2-H), 3.27–3.34 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.64–3.72 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 5.05 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 14.3 (CH
CH3), 3.64–3.72 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 5.05 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 14.3 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 22.7 (Pip 5-C), 28.6 (C(
H3), 22.7 (Pip 5-C), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.9 (Pip 4-C), 46.6 (Pip 3-C), 50.0 (Pip 6-C), 51.4 (OCH3), 54.9 (Pip 2-C), 62.5 (
H3)3), 29.9 (Pip 4-C), 46.6 (Pip 3-C), 50.0 (Pip 6-C), 51.4 (OCH3), 54.9 (Pip 2-C), 62.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.1 (
HCH3), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.5 (
OOC(CH3)3), 173.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −337.5 (Pip), −290.0 (NH). IR (FT-IR, νmax, cm−1): 2939, 2858, 1732 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −337.5 (Pip), −290.0 (NH). IR (FT-IR, νmax, cm−1): 2939, 2858, 1732 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1698 (C
O), 1698 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1160, 1050, 780. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1968.
O), 1160, 1050, 780. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1968.
Methyl(2R)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}propanoate ((2R,3S)-10a). Method A. Compound (S)-2a was coupled with (S)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3S)-10a as yellowish oil in an 60% yield (364 mg) with a dr of 84
5, v/v) to provide compound (2R,3S)-10a as yellowish oil in an 60% yield (364 mg) with a dr of 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, [α]20D = −14.4° (c 1.00, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.1 Hz, 3H, CHC
16, [α]20D = −14.4° (c 1.00, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.43 (s, 9H, C(CH3)3), 1.47–1.61 (m, 3H, Pip 4,5-H), 1.63–1.70 (m, 1H, Pip 5-H), 2.35–2.53 (m, 2H, Pip 2,6-H), 2.54–2.63 (m, 1H, Pip 6-H), 2.72–2.82 (m, 1H, Pip 2-H), 3.27–3.34 (m, 1H, C
3), 1.43 (s, 9H, C(CH3)3), 1.47–1.61 (m, 3H, Pip 4,5-H), 1.63–1.70 (m, 1H, Pip 5-H), 2.35–2.53 (m, 2H, Pip 2,6-H), 2.54–2.63 (m, 1H, Pip 6-H), 2.72–2.82 (m, 1H, Pip 2-H), 3.27–3.34 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.62–3.75 (m, 1H, Pip 3-H), 3.68 (s, 3H, OCH3), 5.05 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 14.3 (CH
CH3), 3.62–3.75 (m, 1H, Pip 3-H), 3.68 (s, 3H, OCH3), 5.05 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 14.3 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 22.7 (Pip 5-C), 28.6 (C(
H3), 22.7 (Pip 5-C), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.9 (Pip 4-C), 46.6 (Pip 3-C), 50.0 (Pip 6-C), 51.4 (OCH3), 54.9 (Pip 2-C), 62.5 (
H3)3), 29.9 (Pip 4-C), 46.6 (Pip 3-C), 50.0 (Pip 6-C), 51.4 (OCH3), 54.9 (Pip 2-C), 62.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.1 (
HCH3), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.5 (
OOC(CH3)3), 173.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2940, 2858, 1732 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2940, 2858, 1732 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1699 (C
O), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1161, 1049, 780. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1967.
O), 1161, 1049, 780. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1967.
Methyl(2S)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}propanoate ((2S,3S)-10a). Method A. Compound (R)-2a was coupled with (S)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3S)-10a as white crystals in an 82% yield (497 mg) with a dr of 93
5, v/v) to provide compound (2S,3S)-10a as white crystals in an 82% yield (497 mg) with a dr of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, mp 77–78 °C, [α]20D = −52.8° (c 0.98, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.2 Hz, 3H, CHC
7, mp 77–78 °C, [α]20D = −52.8° (c 0.98, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.2 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.43 (s, 9H, C(CH3)3), 1.46–1.53 (m, 2H, Pip 4,5-H), 1.55–1.62 (m, 1H, Pip 4-H), 1.64–1.72 (m, 1H, Pip 5-H), 2.43–2.57 (m, 3H, Pip 2,6-H), 2.62–2.70 (m, 1H, Pip 2-H), 3.29 (q, J = 7.1 Hz, 1H, C
3), 1.43 (s, 9H, C(CH3)3), 1.46–1.53 (m, 2H, Pip 4,5-H), 1.55–1.62 (m, 1H, Pip 4-H), 1.64–1.72 (m, 1H, Pip 5-H), 2.43–2.57 (m, 3H, Pip 2,6-H), 2.62–2.70 (m, 1H, Pip 2-H), 3.29 (q, J = 7.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.67 (s, 3H, OCH3), 3.69–3.75 (m, 1H, Pip 3-H), 4.96 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.2 (CH
CH3), 3.67 (s, 3H, OCH3), 3.69–3.75 (m, 1H, Pip 3-H), 4.96 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.2 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 22.7 (Pip 5-C), 28.6 (C(
H3), 22.7 (Pip 5-C), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.9 (Pip 4-C), 46.4 (Pip 3-C), 50.3 (Pip 6-C), 51.3 (OCH3), 54.6 (Pip 2-C), 62.5 (
H3)3), 29.9 (Pip 4-C), 46.4 (Pip 3-C), 50.3 (Pip 6-C), 51.3 (OCH3), 54.6 (Pip 2-C), 62.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.1 (
HCH3), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.5 (
OOC(CH3)3), 173.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −336.5 (Pip), −290.2 (NH). IR (FT-IR, νmax, cm−1): 2942, 2844, 1736 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −336.5 (Pip), −290.2 (NH). IR (FT-IR, νmax, cm−1): 2942, 2844, 1736 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1699 (C
O), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1164, 1089, 775. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1967.
O), 1164, 1089, 775. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1967.
Methyl(2R)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}propanoate ((2R,3R)-10a). Method A. Compound (S)-2a was coupled with (R)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3R)-10a as white crystals in an 68% yield (412 mg) with a dr of 97
5, v/v) to provide compound (2R,3R)-10a as white crystals in an 68% yield (412 mg) with a dr of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, mp 77–78 °C, [α]20D = 53.1° (c 0.91, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.1 Hz, 3H, CHC
3, mp 77–78 °C, [α]20D = 53.1° (c 0.91, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.25 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.43 (s, 9H, C(CH3)3), 1.47–1.53 (m, 2H, Pip 4,5-H), 1.55–1.62 (m, 1H, Pip 4-H), 1.64–1.72 (m, 1H, Pip 5-H), 2.36–2.57 (m, 3H, Pip 2,6-H), 2.63–2.71 (m, 1H, Pip 2-H), 3.29 (q, J = 7.1 Hz, 1H, C
3), 1.43 (s, 9H, C(CH3)3), 1.47–1.53 (m, 2H, Pip 4,5-H), 1.55–1.62 (m, 1H, Pip 4-H), 1.64–1.72 (m, 1H, Pip 5-H), 2.36–2.57 (m, 3H, Pip 2,6-H), 2.63–2.71 (m, 1H, Pip 2-H), 3.29 (q, J = 7.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.67 (s, 3H, OCH3), 3.69–3.74 (m, 1H, Pip 3-H), 4.97 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.1 (CH
CH3), 3.67 (s, 3H, OCH3), 3.69–3.74 (m, 1H, Pip 3-H), 4.97 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 15.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 22.7 (Pip 5-C), 28.6 (C(
H3), 22.7 (Pip 5-C), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.9 (Pip 4-C), 46.4 (Pip 3-C), 50.3 (Pip 6-C), 51.3 (OCH3), 54.6 (Pip 2-C), 62.5 (
H3)3), 29.9 (Pip 4-C), 46.4 (Pip 3-C), 50.3 (Pip 6-C), 51.3 (OCH3), 54.6 (Pip 2-C), 62.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.1 (
HCH3), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.5 (
OOC(CH3)3), 173.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −336.4 (Pip), −290.2 (NH). IR (FT-IR, νmax, cm−1): 2941, 2846, 1736 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −336.4 (Pip), −290.2 (NH). IR (FT-IR, νmax, cm−1): 2941, 2846, 1736 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1699 (C
O), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163, 1089, 775. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1968.
O), 1163, 1089, 775. MS m/z (%): 287 ([M + H]+). HRMS (ESI+) for C14H27N2O4 ([M + H]+) calcd 287.1965, found 287.1968.
Methyl(2S)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-3-methylbutanoate ((2S,3R)-10b). Method B. Compound (R)-2b was coupled with (R)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3R)-10b as yellowish oil in an 86% yield (512 mg) with a dr of 94
5, v/v) to provide compound (2S,3R)-10b as yellowish oil in an 86% yield (512 mg) with a dr of 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, [α]20D = 19.0° (c 0.92, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.5 Hz, 3H, CHCH(C
6, [α]20D = 19.0° (c 0.92, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.5 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.98 (d, J = 6.7 Hz, 3H, CHCH(C
3)2), 0.98 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.38–1.50 (m, 1H, Pip 5-H), 1.44 (m, 9H, C(CH3)3), 1.51–1.57 (m, 1H, Pip 4-H), 1.59–1.65 (m, 2H, Pip 4,5-H), 2.03–2.09 (m, 1H, CHC
3)2), 1.38–1.50 (m, 1H, Pip 5-H), 1.44 (m, 9H, C(CH3)3), 1.51–1.57 (m, 1H, Pip 4-H), 1.59–1.65 (m, 2H, Pip 4,5-H), 2.03–2.09 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.21–2.38 (m, 2H, Pip 2,6-H), 2.49–2.58 (m, 1H, Pip 6-H), 2.67 (d, J = 10.8 Hz, 1H, C
(CH3)2), 2.21–2.38 (m, 2H, Pip 2,6-H), 2.49–2.58 (m, 1H, Pip 6-H), 2.67 (d, J = 10.8 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 2.73–2.80 (m, 1H, Pip 2-H), 3.61–3.72 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(
CH(CH3)2), 2.73–2.80 (m, 1H, Pip 2-H), 3.61–3.72 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 20.0 (CHCH(
H3)2), 20.0 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 23.0 (Pip 5-C), 27.0 (CH
H3)2), 23.0 (Pip 5-C), 27.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.4 (Pip 4-C), 47.1 (Pip 3-C), 49.8 (Pip 6-C), 50.7 (OCH3), 55.8 (Pip 2-C), 74.7 (
H3)3), 30.4 (Pip 4-C), 47.1 (Pip 3-C), 49.8 (Pip 6-C), 50.7 (OCH3), 55.8 (Pip 2-C), 74.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.2 (
HCH(CH3)3), 79.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.0 (
OOC(CH3)3), 172.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2937, 2811, 1729 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2937, 2811, 1729 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1711 (C
O), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1161, 1017, 778. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2281.
O), 1161, 1017, 778. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2281.
Methyl(2R)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-3-methylbutanoate ((2R,3S)-10b). Method B. Compound (S)-2b was coupled with (S)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3S)-10b as orange oil in an 74% yield (440 mg) with a dr of 100
5, v/v) to provide compound (2R,3S)-10b as orange oil in an 74% yield (440 mg) with a dr of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, [α]20D = −18.9° (c 0.96, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.6 Hz, 3H, CHCH(C
0, [α]20D = −18.9° (c 0.96, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.84 (d, J = 6.6 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.98 (d, J = 6.7 Hz, 3H, CHCH(C
3)2), 0.98 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.37–1.48 (m, 1H, Pip 5-H), 1.43 (s, 9H, C(CH3)3), 1.51–1.56 (m, 1H, Pip 4-H), 1.58–1.68 (m, 2H, Pip 4,5-H), 2.02–2.08 (m, 1H, CHC
3)2), 1.37–1.48 (m, 1H, Pip 5-H), 1.43 (s, 9H, C(CH3)3), 1.51–1.56 (m, 1H, Pip 4-H), 1.58–1.68 (m, 2H, Pip 4,5-H), 2.02–2.08 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.20–2.39 (m, 2H, Pip 2,6-H), 2.47–2.58 (m, 1H, Pip 6-H), 2.67 (d, J = 10.8 Hz, 1H, C
(CH3)2), 2.20–2.39 (m, 2H, Pip 2,6-H), 2.47–2.58 (m, 1H, Pip 6-H), 2.67 (d, J = 10.8 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 2.72–2.81 (m, 1H, Pip 2-H), 3.60–3.71 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 4.86 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(
CH(CH3)2), 2.72–2.81 (m, 1H, Pip 2-H), 3.60–3.71 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 4.86 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.4 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 20.0 (CHCH(
H3)2), 20.0 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 23.0 (Pip 5-C), 27.0 (CH
H3)2), 23.0 (Pip 5-C), 27.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.4 (Pip 4-C), 47.1 (Pip 3-C), 49.9 (Pip 6-C), 50.7 (OCH3), 55.8 (Pip 2-C), 74.7 (
H3)3), 30.4 (Pip 4-C), 47.1 (Pip 3-C), 49.9 (Pip 6-C), 50.7 (OCH3), 55.8 (Pip 2-C), 74.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.2 (
HCH(CH3)3), 79.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.0 (
OOC(CH3)3), 172.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2937, 2811, 1727 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2937, 2811, 1727 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1711 (C
O), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1161, 1017, 778. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2280.
O), 1161, 1017, 778. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2280.
Methyl(2S)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-3-methylbutanoate ((2S,3S)-10b). Method B. Compound (R)-2b was coupled with (S)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3S)-10b as yellowish oil in an 73% yield (434 mg) with a dr of 93
5, v/v) to provide compound (2S,3S)-10b as yellowish oil in an 73% yield (434 mg) with a dr of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, [α]20D = −90.2° (c 0.92, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.83 (d, J = 6.5 Hz, 3H, CHCH(C
7, [α]20D = −90.2° (c 0.92, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.83 (d, J = 6.5 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.94 (d, J = 6.7 Hz, 3H, CHCH(C
3)2), 0.94 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.37–1.50 (m, 2H, Pip 4,5-H), 1.43 (m, 9H, C(CH3)3), 1.58–1.71 (m, 2H, Pip 4,5-H), 1.96–2.03 (m, 1H, CHC
3)2), 1.37–1.50 (m, 2H, Pip 4,5-H), 1.43 (m, 9H, C(CH3)3), 1.58–1.71 (m, 2H, Pip 4,5-H), 1.96–2.03 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.28–2.51 (m, 3H, Pip 2,6-H), 2.58–2.65 (m, 1H, Pip 2-H), 2.71 (d, J = 10.8 Hz, 1H, C
(CH3)2), 2.28–2.51 (m, 3H, Pip 2,6-H), 2.58–2.65 (m, 1H, Pip 2-H), 2.71 (d, J = 10.8 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 3.67 (m, 3H, OCH3), 3.69–3.75 (m, 1H, Pip 3-H), 4.84 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.3 (CHCH(
CH(CH3)2), 3.67 (m, 3H, OCH3), 3.69–3.75 (m, 1H, Pip 3-H), 4.84 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.3 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 20.0 (CHCH(
H3)2), 20.0 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 23.1 (Pip 5-C), 26.8 (CH
H3)2), 23.1 (Pip 5-C), 26.8 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.3 (Pip 4-C), 46.7 (Pip 3-C), 50.2 (Pip 6-C), 50.7 (OCH3), 55.2 (Pip 2-C), 74.8 (
H3)3), 30.3 (Pip 4-C), 46.7 (Pip 3-C), 50.2 (Pip 6-C), 50.7 (OCH3), 55.2 (Pip 2-C), 74.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.2 (
HCH(CH3)3), 79.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.1 (
OOC(CH3)3), 172.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2963, 2811, 1729 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2963, 2811, 1729 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1712 (C
O), 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1160, 1001, 780. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2280.
O), 1160, 1001, 780. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2280.
Methyl(2R)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-3-methylbutanoate ((2R,3R)-10b). Method B. Compound (S)-2b was coupled with (R)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3R)-10b as yellowish oil in an 83% yield (494 mg) with a dr of 100
5, v/v) to provide compound (2R,3R)-10b as yellowish oil in an 83% yield (494 mg) with a dr of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, [α]20D = 90.4° (c 1.03, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.83 (d, J = 6.5 Hz, 3H, CHCH(C
0, [α]20D = 90.4° (c 1.03, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.83 (d, J = 6.5 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.95 (d, J = 6.6 Hz, 3H, CHCH(C
3)2), 0.95 (d, J = 6.6 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.38–1.49 (m, 2H, Pip 4,5-H), 1.43 (s, 9H, C(CH3)3), 1.61–1.72 (m, 2H, Pip 4,5-H), 1.97–2.03 (m, 1H, CHC
3)2), 1.38–1.49 (m, 2H, Pip 4,5-H), 1.43 (s, 9H, C(CH3)3), 1.61–1.72 (m, 2H, Pip 4,5-H), 1.97–2.03 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.32–2.52 (m, 3H, Pip 2,6-H), 2.58–2.67 (m, 1H, Pip 2-H), 2.71 (d, J = 10.8 Hz, 1H, C
(CH3)2), 2.32–2.52 (m, 3H, Pip 2,6-H), 2.58–2.67 (m, 1H, Pip 2-H), 2.71 (d, J = 10.8 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 3.67 (s, 3H, OCH3), 3.69–3.76 (m, 1H, Pip 3-H), 4.84 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.3 (CHCH(
CH(CH3)2), 3.67 (s, 3H, OCH3), 3.69–3.76 (m, 1H, Pip 3-H), 4.84 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.3 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 20.0 (CHCH(
H3)2), 20.0 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 23.1 (Pip 5-C), 26.8 (CH
H3)2), 23.1 (Pip 5-C), 26.8 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.3 (Pip 4-C), 46.7 (Pip 3-C), 50.3 (Pip 6-C), 50.7 (OCH3), 55.1 (Pip 2-C), 74.8 (
H3)3), 30.3 (Pip 4-C), 46.7 (Pip 3-C), 50.3 (Pip 6-C), 50.7 (OCH3), 55.1 (Pip 2-C), 74.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.2 (
HCH(CH3)3), 79.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.1 (
OOC(CH3)3), 172.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2962, 2811, 1729 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2962, 2811, 1729 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1712 (C
O), 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1160, 1002, 780. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2280.
O), 1160, 1002, 780. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2280.
Methyl(2S)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-4-methylpentanoate ((2S,3R)-10c). Method B. Compound (R)-2c was coupled with (R)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2S,3R)-10c as yellow oil in an 69% yield (409 mg) with a dr of 93
7, v/v) to provide compound (2S,3R)-10c as yellow oil in an 69% yield (409 mg) with a dr of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, [α]20D = 19.2° (c 1.51, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.6 Hz, 3H, CHCH2CH(C
7, [α]20D = 19.2° (c 1.51, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.6 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.92 (d, J = 6.7 Hz, 3H, CHCH2CH(C
3)2), 0.92 (d, J = 6.7 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.41–1.46 (m, 1H, Pip 5-H), 1.43 (s, 9H, C(CH3)3), 1.50–1.58 (m, 3H, Pip 4-H, CHCH2C
3)2), 1.41–1.46 (m, 1H, Pip 5-H), 1.43 (s, 9H, C(CH3)3), 1.50–1.58 (m, 3H, Pip 4-H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2, CHC
(CH3)2, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.59–1.67 (m, 3H, Pip 4,5-H and CHC
2CH(CH3)2), 1.59–1.67 (m, 3H, Pip 4,5-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.31–2.44 (m, 2H, Pip 2,6-H), 2.54–2.62 (m, 1H, Pip 6-H), 2.78–2.86 (m, 1H, Pip 2-H), 3.22 (t, J = 7.7 Hz, 1H, C
2CH(CH3)2), 2.31–2.44 (m, 2H, Pip 2,6-H), 2.54–2.62 (m, 1H, Pip 6-H), 2.78–2.86 (m, 1H, Pip 2-H), 3.22 (t, J = 7.7 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.63–3.69 (m, 1H, Pip 3-H), 3.66 (s, 3H, OCH3), 4.91 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.3 (CHCH2CH(
CH2CH(CH3)2), 3.63–3.69 (m, 1H, Pip 3-H), 3.66 (s, 3H, OCH3), 4.91 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.3 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.9 (Pip 5-C and CHCH2CH(
H3)2), 22.9 (Pip 5-C and CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.1 (CHCH2
H3)2), 25.1 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.2 (Pip 4-C), 38.1 (CH
H3)3), 30.2 (Pip 4-C), 38.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 46.9 (Pip 3-C), 49.6 (Pip 6-C), 51.0 (OCH3), 55.7 (Pip 2-C), 65.6 (
H2CH(CH3)2), 46.9 (Pip 3-C), 49.6 (Pip 6-C), 51.0 (OCH3), 55.7 (Pip 2-C), 65.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.1 (
HCH2CH(CH3)2), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.0 (
OOC(CH3)3), 173.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2867, 1721 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2867, 1721 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1711 (C
O), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1159, 1008, 778. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2439.
O), 1159, 1008, 778. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2439.
Methyl(2R)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-4-methylpentanoate ((2R,3S)-10c). Method B. Compound (S)-2c was coupled with (S)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2R,3S)-10c as transparent oil in an 80% yield (472 mg) with a dr of 100
7, v/v) to provide compound (2R,3S)-10c as transparent oil in an 80% yield (472 mg) with a dr of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, [α]20D = −19.1° (c 1.13, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.6 Hz, 3H, CHCH2CH(C
0, [α]20D = −19.1° (c 1.13, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.6 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.92 (d, J = 6.6 Hz, 3H, CHCH2CH(C
3)2), 0.92 (d, J = 6.6 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.41–1.48 (m, 1H, Pip 5-H), 1.43 (s, 9H, C(CH3)3), 1.49–1.58 (m, 3H, Pip 4-H, CHCH2C
3)2), 1.41–1.48 (m, 1H, Pip 5-H), 1.43 (s, 9H, C(CH3)3), 1.49–1.58 (m, 3H, Pip 4-H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2, CHC
(CH3)2, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.59–1.69 (m, 3H, Pip 4,5-H and CHC
2CH(CH3)2), 1.59–1.69 (m, 3H, Pip 4,5-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.29–2.45 (m, 2H, Pip 2,6-H), 2.55–2.62 (m, 1H, Pip 6-H), 2.78–2.87 (m, 1H, Pip 2-H), 3.22 (t, J = 7.6 Hz, 1H, C
2CH(CH3)2), 2.29–2.45 (m, 2H, Pip 2,6-H), 2.55–2.62 (m, 1H, Pip 6-H), 2.78–2.87 (m, 1H, Pip 2-H), 3.22 (t, J = 7.6 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.63–3.70 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 4.91 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.3 (CHCH2CH(
CH2CH(CH3)2), 3.63–3.70 (m, 1H, Pip 3-H), 3.67 (s, 3H, OCH3), 4.91 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.3 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.9 (Pip 5-C and CHCH2CH(
H3)2), 22.9 (Pip 5-C and CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.1 (CHCH2
H3)2), 25.1 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.2 (Pip 4-C), 38.1 (CH
H3)3), 30.2 (Pip 4-C), 38.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 46.9 (Pip 3-C), 49.6 (Pip 6-C), 51.0 (OCH3), 55.7 (Pip 2-C), 65.6 (
H2CH(CH3)2), 46.9 (Pip 3-C), 49.6 (Pip 6-C), 51.0 (OCH3), 55.7 (Pip 2-C), 65.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.1 (
HCH2CH(CH3)2), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.0 (
OOC(CH3)3), 173.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2869, 1725 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2869, 1725 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1711 (C
O), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1159, 1008, 778. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2438.
O), 1159, 1008, 778. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2438.
Methyl(2S)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-4-methylpentanoate ((2S,3S)-10c). Method B. Compound (R)-2c was coupled with (S)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2S,3S)-10c as white crystals in an 71% yield (419 mg) with a dr of 93
7, v/v) to provide compound (2S,3S)-10c as white crystals in an 71% yield (419 mg) with a dr of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, mp 54–55 °C, [α]20D = −57.6° (c 1.35, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.6 Hz, 3H, CHCH2CH(C
7, mp 54–55 °C, [α]20D = −57.6° (c 1.35, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.6 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.91 (d, J = 6.7 Hz, 3H, CHCH2CH(C
3)2), 0.91 (d, J = 6.7 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42–1.56 (m, 4H, Pip 4,5-H, CHCH2C
3)2), 1.42–1.56 (m, 4H, Pip 4,5-H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2 and CHC
(CH3)2 and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.43 (s, 9H, C(CH3)3), 1.57–1.70 (m, 3H, Pip 4,5-H and CHC
2CH(CH3)2), 1.43 (s, 9H, C(CH3)3), 1.57–1.70 (m, 3H, Pip 4,5-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.42–2.58 (m, 3H, Pip 2,6-H), 2.62–2.69 (m, 1H, Pip 2-H), 3.24 (t, J = 7.7 Hz, 1H, C
2CH(CH3)2), 2.42–2.58 (m, 3H, Pip 2,6-H), 2.62–2.69 (m, 1H, Pip 2-H), 3.24 (t, J = 7.7 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.66 (s, 3H, OCH3), 3.68–3.73 (m, 1H, Pip 3-H), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.4 (CHCH2CH(
CH2CH(CH3)2), 3.66 (s, 3H, OCH3), 3.68–3.73 (m, 1H, Pip 3-H), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.4 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.9 (CHCH2CH(
H3)2), 22.9 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 23.0 (Pip 5-C), 25.0 (CHCH2
H3)2), 23.0 (Pip 5-C), 25.0 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.2 (Pip 4-C), 38.4 (CH
H3)3), 30.2 (Pip 4-C), 38.4 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 46.6 (Pip 3-C), 50.4 (Pip 6-C), 51.0 (OCH3), 54.9 (Pip 2-C), 65.6 (
H2CH(CH3)2), 46.6 (Pip 3-C), 50.4 (Pip 6-C), 51.0 (OCH3), 54.9 (Pip 2-C), 65.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.1 (
HCH2CH(CH3)2), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.0 (
OOC(CH3)3), 173.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2867, 1728 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2867, 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1688 (C
O), 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163, 1011, 781. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2441
O), 1163, 1011, 781. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2441
Methyl(2R)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]piperidin-1-yl}-4-methylpentanoate ((2R,3R)-10c). Method B. Compound (S)-2c was coupled with (R)-3-Boc-aminopiperidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2R,3R)-10c as white crystals in an 81% yield (478 mg) with a dr of 97
7, v/v) to provide compound (2R,3R)-10c as white crystals in an 81% yield (478 mg) with a dr of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, mp 55–57 °C, [α]20D = 57.8° (c 1.16, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.6 Hz, 3H, CHCH2CH(C
3, mp 55–57 °C, [α]20D = 57.8° (c 1.16, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.6 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.91 (d, J = 6.7 Hz, 3H, CHCH2CH(C
3)2), 0.91 (d, J = 6.7 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42–1.56 (m, 4H, Pip 4,5-H, CHCH2C
3)2), 1.42–1.56 (m, 4H, Pip 4,5-H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2 and CHC
(CH3)2 and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.43 (s, 9H, C(CH3)3), 1.57–1.71 (m, 3H, Pip 4,5-H and CHC
2CH(CH3)2), 1.43 (s, 9H, C(CH3)3), 1.57–1.71 (m, 3H, Pip 4,5-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.42–2.58 (m, 3H, Pip 2,6-H), 2.60–2.69 (m, 1H, Pip 2-H), 3.24 (t, J = 7.6 Hz, 1H, C
2CH(CH3)2), 2.42–2.58 (m, 3H, Pip 2,6-H), 2.60–2.69 (m, 1H, Pip 2-H), 3.24 (t, J = 7.6 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.66 (s, 3H, OCH3), 3.68–3.76 (m, 1H, Pip 3-H), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.4 (CHCH2CH(
CH2CH(CH3)2), 3.66 (s, 3H, OCH3), 3.68–3.76 (m, 1H, Pip 3-H), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.4 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.9 (CHCH2CH(
H3)2), 22.9 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 23.0 (Pip 5-C), 25.0 (CHCH2
H3)2), 23.0 (Pip 5-C), 25.0 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.6 (C(
H(CH3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 30.2 (Pip 4-C), 38.4 (CH
H3)3), 30.2 (Pip 4-C), 38.4 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 46.6 (Pip 3-C), 50.4 (Pip 6-C), 51.0 (OCH3), 54.8 (Pip 2-C), 65.6 (
H2CH(CH3)2), 46.6 (Pip 3-C), 50.4 (Pip 6-C), 51.0 (OCH3), 54.8 (Pip 2-C), 65.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.1 (
HCH2CH(CH3)2), 79.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.3 (
(CH3)3), 155.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.0 (
OOC(CH3)3), 173.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2867, 1728 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2953, 2867, 1728 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1688 (C
O), 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163, 1011, 781. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2437.
O), 1163, 1011, 781. MS m/z (%): 329 ([M + H]+). HRMS (ESI+) for C17H33N2O4 ([M + H]+) calcd 329.2435, found 329.2437.
Methyl(2S)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}propanoate ((2S,3R)-11a). Method A. Compound (R)-2a was coupled with (R)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3R)-11a as yellowish oil in an 79% yield (455 mg) with a dr of 100
5, v/v) to provide compound (2S,3R)-11a as yellowish oil in an 79% yield (455 mg) with a dr of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, [α]20D = 5.7° (c 0.86, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.32 (d, J = 7.1 Hz, 3H, CHC
0, [α]20D = 5.7° (c 0.86, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.32 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.41 (s, 9H, C(CH3)3), 1.56–1.67 (m, 1H, Pyr 4-H), 2.13–2.22 (m, 1H, Pyr 4-H), 2.48–2.57 (m, 1H, Pyr 5-H), 2.60–2.68 (m, 1H, Pyr 2-H), 2.78–2.87 (m, 2H, Pyr 2,5-H), 3.24 (q, J = 7.0 Hz, 1H, C
3), 1.41 (s, 9H, C(CH3)3), 1.56–1.67 (m, 1H, Pyr 4-H), 2.13–2.22 (m, 1H, Pyr 4-H), 2.48–2.57 (m, 1H, Pyr 5-H), 2.60–2.68 (m, 1H, Pyr 2-H), 2.78–2.87 (m, 2H, Pyr 2,5-H), 3.24 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.69 (s, 3H, OCH3), 4.09–4.19 (m, 1H, Pyr 3-H), 4.97 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH
CH3), 3.69 (s, 3H, OCH3), 4.09–4.19 (m, 1H, Pyr 3-H), 4.97 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.5 (Pyr 4-C), 49.3 (Pyr 5-C), 49.8 (Pyr 3-C), 51.7 (OCH3), 57.3 (Pyr 2-C), 60.7 (
H3)3), 32.5 (Pyr 4-C), 49.3 (Pyr 5-C), 49.8 (Pyr 3-C), 51.7 (OCH3), 57.3 (Pyr 2-C), 60.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.3 (
HCH3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 174.1 (
OOC(CH3)3), 174.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −329.8 (Pyr), −283.5 (NH). IR (FT-IR, νmax, cm−1): 2976, 2816, 1734 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −329.8 (Pyr), −283.5 (NH). IR (FT-IR, νmax, cm−1): 2976, 2816, 1734 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1709 (C
O), 1709 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1158, 1057, 853. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1809.
O), 1158, 1057, 853. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1809.
Methyl(2R)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}propanoate ((2R,3S)-11a). Method A. Compound (S)-2a was coupled with (S)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v) to provide compound (2R,3S)-11a as yellowish oil in an 54% yield (311 mg) with a dr of 89
6, v/v) to provide compound (2R,3S)-11a as yellowish oil in an 54% yield (311 mg) with a dr of 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, [α]20D = −5.6° (c 0.91, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.31 (d, J = 7.1 Hz, 3H, CHC
11, [α]20D = −5.6° (c 0.91, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.31 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.40 (s, 9H, C(CH3)3), 1.57–1.67 (m, 1H, Pyr 4-H), 2.14–2.21 (m, 1H, Pyr 4-H), 2.49–2.56 (m, 1H, Pyr 5-H), 2.61–2.67 (m, 1H, Pyr 2-H), 2.75–2.87 (m, 2H, Pyr 2,5-H), 3.23 (q, J = 7.0 Hz, 1H, C
3), 1.40 (s, 9H, C(CH3)3), 1.57–1.67 (m, 1H, Pyr 4-H), 2.14–2.21 (m, 1H, Pyr 4-H), 2.49–2.56 (m, 1H, Pyr 5-H), 2.61–2.67 (m, 1H, Pyr 2-H), 2.75–2.87 (m, 2H, Pyr 2,5-H), 3.23 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.68 (s, 3H, OCH3), 4.10–4.19 (m, 1H, Pyr 3-H), 5.01 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH
CH3), 3.68 (s, 3H, OCH3), 4.10–4.19 (m, 1H, Pyr 3-H), 5.01 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.4 (Pyr 4-C), 49.3 (Pyr 5-C), 49.8 (Pyr 3-C), 51.7 (OCH3), 57.2 (Pyr 2-C), 60.8 (
H3)3), 32.4 (Pyr 4-C), 49.3 (Pyr 5-C), 49.8 (Pyr 3-C), 51.7 (OCH3), 57.2 (Pyr 2-C), 60.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.3 (
HCH3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 174.1 (
OOC(CH3)3), 174.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2977, 2817, 1735 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2977, 2817, 1735 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1708 (C
O), 1708 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1158, 1057, 853. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1811.
O), 1158, 1057, 853. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1811.
Methyl(2S)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}propanoate ((2S,3S)-11a). Method A. Compound (R)-2a was coupled with (S)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3S)-11a as yellowish oil in an 72% yield (415 mg) with a dr of 91
5, v/v) to provide compound (2S,3S)-11a as yellowish oil in an 72% yield (415 mg) with a dr of 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, [α]20D = −19.2° (c 0.84, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.33 (d, J = 7.0 Hz, 3H, CHC
9, [α]20D = −19.2° (c 0.84, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.33 (d, J = 7.0 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.41 (s, 9H, C(CH3)3), 1.59–1.65 (m, 1H, Pyr 4-H), 2.16–2.23 (m, 1H, Pyr 4-H), 2.46–2.53 (m, 1H, Pyr 5-H), 2.54–2.61 (m, 1H, Pyr 2-H), 2.80–2.92 (m, 2H, Pyr 2,5-H), 3.23 (q, J = 7.0 Hz, 1H, C
3), 1.41 (s, 9H, C(CH3)3), 1.59–1.65 (m, 1H, Pyr 4-H), 2.16–2.23 (m, 1H, Pyr 4-H), 2.46–2.53 (m, 1H, Pyr 5-H), 2.54–2.61 (m, 1H, Pyr 2-H), 2.80–2.92 (m, 2H, Pyr 2,5-H), 3.23 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.70 (s, 3H, OCH3), 4.13–4.19 (m, 1H, Pyr 3-H), 4.91 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH
CH3), 3.70 (s, 3H, OCH3), 4.13–4.19 (m, 1H, Pyr 3-H), 4.91 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.5 (Pyr 4-C), 49.2 (Pyr 5-C), 49.8 (Pyr 3-C), 51.8 (OCH3), 57.7 (Pyr 2-C), 60.9 (
H3)3), 32.5 (Pyr 4-C), 49.2 (Pyr 5-C), 49.8 (Pyr 3-C), 51.8 (OCH3), 57.7 (Pyr 2-C), 60.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.3 (
HCH3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 174.1 (
OOC(CH3)3), 174.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). 15N-NMR (71 MHz, CDCl3): δ −329.1, −283.5 (NH). IR (FT-IR, νmax, cm−1): 2977, 2815, 1709 (C
OOCH3). 15N-NMR (71 MHz, CDCl3): δ −329.1, −283.5 (NH). IR (FT-IR, νmax, cm−1): 2977, 2815, 1709 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1693 (C
O), 1693 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1160, 1058, 781. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1811.
O), 1160, 1058, 781. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1811.
Methyl(2R)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}propanoate ((2R,3R)-11a). Method A. Compound (S)-2a was coupled with (R)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3R)-11a as yellowish oil in an 59% yield (340 mg) with a dr of 88
5, v/v) to provide compound (2R,3R)-11a as yellowish oil in an 59% yield (340 mg) with a dr of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, [α]20D = 19.3° (c 1.21, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.34 (d, J = 7.0 Hz, 3H, CHC
12, [α]20D = 19.3° (c 1.21, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.34 (d, J = 7.0 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.42 (s, 9H, C(CH3)3), 1.61–1.66 (m, 1H, Pyr 4-H), 2.17–2.23 (m, 1H, Pyr 4-H), 2.48–2.55 (m, 1H, Pyr 5-H), 2.56–2.63 (m, 1H, Pyr 2-H), 2.82–2.93 (m, 2H, Pyr 2,5-H), 3.24 (q, J = 7.0 Hz, 1H, C
3), 1.42 (s, 9H, C(CH3)3), 1.61–1.66 (m, 1H, Pyr 4-H), 2.17–2.23 (m, 1H, Pyr 4-H), 2.48–2.55 (m, 1H, Pyr 5-H), 2.56–2.63 (m, 1H, Pyr 2-H), 2.82–2.93 (m, 2H, Pyr 2,5-H), 3.24 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.70 (s, 3H, OCH3), 4.13–4.22 (m, 1H, Pyr 3-H), 4.92 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH
CH3), 3.70 (s, 3H, OCH3), 4.13–4.22 (m, 1H, Pyr 3-H), 4.92 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 17.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.5 (Pyr 4-C), 49.2 (Pyr 5-C), 49.8 (Pyr 3-C), 51.8 (OCH3), 57.7 (Pyr 2-C), 61.0 (
H3)3), 32.5 (Pyr 4-C), 49.2 (Pyr 5-C), 49.8 (Pyr 3-C), 51.8 (OCH3), 57.7 (Pyr 2-C), 61.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.3 (
HCH3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 174.1 (
OOC(CH3)3), 174.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2977, 2815, 1709 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2977, 2815, 1709 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1693 (C
O), 1693 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1160, 1056, 781. MS m/z (%): 273 ([M + H]+). ]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1808.
O), 1160, 1056, 781. MS m/z (%): 273 ([M + H]+). ]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1808.
Methyl(2S)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-3-methylbutanoate ((2S,3R)-11b). Method B. Compound (R)-2b was coupled with (R)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3R)-11b as yellowish oil in an 79% yield (449 mg) with a dr of 97
5, v/v) to provide compound (2S,3R)-11b as yellowish oil in an 79% yield (449 mg) with a dr of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, [α]20D = 12.1° (c 0.94, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.7 Hz, 3H, CHCH(C
3, [α]20D = 12.1° (c 0.94, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.98 (d, J = 6.8 Hz, 3H, CHCH(C
3)2), 0.98 (d, J = 6.8 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.43 (s, 9H, C(CH3)3), 1.54–1.62 (m, 1H, Pyr 4-H), 1.97–2.04 (m, 1H, CHC
3)2), 1.43 (s, 9H, C(CH3)3), 1.54–1.62 (m, 1H, Pyr 4-H), 1.97–2.04 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.11–2.18 (m, 1H, Pyr 4-H), 2.60–2.70 (m, 2H, Pyr 2,5-H), 2.71–2.83 (m, 2H, Pyr 2,5-H), 2.94 (d, J = 9.1 Hz, 1H, C
(CH3)2), 2.11–2.18 (m, 1H, Pyr 4-H), 2.60–2.70 (m, 2H, Pyr 2,5-H), 2.71–2.83 (m, 2H, Pyr 2,5-H), 2.94 (d, J = 9.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 3.68 (s, 3H, OCH3), 4.06–4.17 (m, 1H, Pyr 3-H), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(
CH(CH3)2), 3.68 (s, 3H, OCH3), 4.06–4.17 (m, 1H, Pyr 3-H), 4.87 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 20.0 (CHCH(
H3)2), 20.0 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 28.6 (C(
H3)2), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.1 (CH
H3)3), 29.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 32.2 (Pyr 4-C), 47.7 (Pyr 5-C), 49.8 (Pyr 3-C), 51.0 (OCH3), 56.7 (Pyr 2-C), 71.0 (
H(CH3)2), 32.2 (Pyr 4-C), 47.7 (Pyr 5-C), 49.8 (Pyr 3-C), 51.0 (OCH3), 56.7 (Pyr 2-C), 71.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.3 (
HCH(CH3)3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.8 (
OOC(CH3)3), 172.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2965, 2815, 1711 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2965, 2815, 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1695 (C
O), 1695 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1159, 1003, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H29N2O4 ([M + H]+) calcd 301.2122, found 301.2125.
O), 1159, 1003, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H29N2O4 ([M + H]+) calcd 301.2122, found 301.2125.
Methyl(2R)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-3-methylbutanoate ((2R,3S)-11b). Method B. Compound (S)-2b was coupled with (S)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3S)-11b as transparent oil in an 72% yield (409 mg) with a dr of 100
5, v/v) to provide compound (2R,3S)-11b as transparent oil in an 72% yield (409 mg) with a dr of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, [α]20D = −11.8° (c 1.12, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.7 Hz, 3H, CHCH(C
0, [α]20D = −11.8° (c 1.12, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.97 (d, J = 6.7 Hz, 3H, CHCH(C
3)2), 0.97 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.53–1.61 (m, 1H, Pyr 4-H), 1.96–2.02 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.53–1.61 (m, 1H, Pyr 4-H), 1.96–2.02 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.10–2.18 (m, 1H, Pyr 4-H), 2.58–2.67 (m, 2H, Pyr 2,5-H), 2.71–2.81 (m, 2H, Pyr 2,5-H), 2.93 (d, J = 9.1 Hz, 1H, C
(CH3)2), 2.10–2.18 (m, 1H, Pyr 4-H), 2.58–2.67 (m, 2H, Pyr 2,5-H), 2.71–2.81 (m, 2H, Pyr 2,5-H), 2.93 (d, J = 9.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 3.67 (s, 3H, OCH3), 4.08–4.14 (m, 1H, Pyr 3-H), 4.86 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(
CH(CH3)2), 3.67 (s, 3H, OCH3), 4.08–4.14 (m, 1H, Pyr 3-H), 4.86 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 19.9 (CHCH(
H3)2), 19.9 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 28.5 (C(
H3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.1 (CH
H3)3), 29.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 32.2 (Pyr 4-C), 47.6 (Pyr 5-C), 49.8 (Pyr 3-C), 50.9 (OCH3), 56.7 (Pyr 2-C), 71.0 (
H(CH3)2), 32.2 (Pyr 4-C), 47.6 (Pyr 5-C), 49.8 (Pyr 3-C), 50.9 (OCH3), 56.7 (Pyr 2-C), 71.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.3 (
HCH(CH3)3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.9 (
OOC(CH3)3), 172.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2965, 2815, 1711 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2965, 2815, 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1694 (C
O), 1694 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1159, 1003, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H29N2O4 ([M + H]+) calcd 301.2122, found 301.2122.
O), 1159, 1003, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H29N2O4 ([M + H]+) calcd 301.2122, found 301.2122.
Methyl(2S)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-3-methylbutanoate ((2S,3S)-11b). Method B. Compound (R)-2b was coupled with (S)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2S,3S)-11b as white crystals in an 76% yield (431 mg) with a dr of 96
5, v/v) to provide compound (2S,3S)-11b as white crystals in an 76% yield (431 mg) with a dr of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, mp 47–49 °C, [α]20D = −24.1° (c 1.00, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.7 Hz, 3H, CHCH(C
4, mp 47–49 °C, [α]20D = −24.1° (c 1.00, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.97 (d, J = 6.8 Hz, 3H, CHCH(C
3)2), 0.97 (d, J = 6.8 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.53–1.61 (m, 1H, Pyr 4-H), 1.94–2.03 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.53–1.61 (m, 1H, Pyr 4-H), 1.94–2.03 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.09–2.18 (m, 1H, Pyr 4-H), 2.43–2.56 (m, 2H, Pyr 2,5-H), 2.82–2.91 (m, 2H, Pyr 2,5-H), 2.94 (d, J = 9.2 Hz, 1H, C
(CH3)2), 2.09–2.18 (m, 1H, Pyr 4-H), 2.43–2.56 (m, 2H, Pyr 2,5-H), 2.82–2.91 (m, 2H, Pyr 2,5-H), 2.94 (d, J = 9.2 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 3.67 (s, 3H, OCH3), 4.07–4.15 (m, 1H, Pyr 3-H), 4.77 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(
CH(CH3)2), 3.67 (s, 3H, OCH3), 4.07–4.15 (m, 1H, Pyr 3-H), 4.77 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 19.9 (CHCH(
H3)2), 19.9 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 28.5 (C(
H3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 28.8 (CH
H3)3), 28.8 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 32.1 (Pyr 4-C), 48.7 (Pyr 5-C), 49.7 (Pyr 3-C), 50.9 (OCH3), 55.7 (Pyr 2-C), 71.0 (
H(CH3)2), 32.1 (Pyr 4-C), 48.7 (Pyr 5-C), 49.7 (Pyr 3-C), 50.9 (OCH3), 55.7 (Pyr 2-C), 71.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.3 (
HCH(CH3)3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.7 (
OOC(CH3)3), 172.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2966, 2875, 1723 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2966, 2875, 1723 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1685 (C
O), 1685 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1149, 1004, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H28N2NaO4 ([M + Na]+) calcd 323.1941, found 323.1940.
O), 1149, 1004, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H28N2NaO4 ([M + Na]+) calcd 323.1941, found 323.1940.
Methyl(2R)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-3-methylbutanoate ((2R,3R)-11b). Method B. Compound (S)-2b was coupled with (R)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to provide compound (2R,3R)-11b as white crystals in an 77% yield (438 mg) with a dr of 100
5, v/v) to provide compound (2R,3R)-11b as white crystals in an 77% yield (438 mg) with a dr of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, mp 49–51 °C, [α]20D = 24.4° (c 0.96, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.7 Hz, 3H, CHCH(C
0, mp 49–51 °C, [α]20D = 24.4° (c 0.96, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.97 (d, J = 6.7 Hz, 3H, CHCH(C
3)2), 0.97 (d, J = 6.7 Hz, 3H, CHCH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.53–1.62 (m, 1H, Pyr 4-H), 1.95–2.03 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.53–1.62 (m, 1H, Pyr 4-H), 1.95–2.03 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2), 2.09–2.17 (m, 1H, Pyr 4-H), 2.43–2.56 (m, 2H, Pyr 2,5-H), 2.81–2.91 (m, 2H, Pyr 2,5-H), 2.94 (d, J = 9.2 Hz, 1H, C
(CH3)2), 2.09–2.17 (m, 1H, Pyr 4-H), 2.43–2.56 (m, 2H, Pyr 2,5-H), 2.81–2.91 (m, 2H, Pyr 2,5-H), 2.94 (d, J = 9.2 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH(CH3)2), 3.67 (s, 3H, OCH3), 4.06–4.17 (m, 1H, Pyr 3-H), 4.78 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(
CH(CH3)2), 3.67 (s, 3H, OCH3), 4.06–4.17 (m, 1H, Pyr 3-H), 4.78 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 19.2 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 19.9 (CHCH(
H3)2), 19.9 (CHCH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 28.5 (C(
H3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 28.8 (CH
H3)3), 28.8 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 32.1 (Pyr 4-C), 48.7 (Pyr 5-C), 49.7 (Pyr 3-C), 50.9 (OCH3), 55.7 (Pyr 2-C), 71.0 (
H(CH3)2), 32.1 (Pyr 4-C), 48.7 (Pyr 5-C), 49.7 (Pyr 3-C), 50.9 (OCH3), 55.7 (Pyr 2-C), 71.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH(CH3)3), 79.3 (
HCH(CH3)3), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 172.6 (
OOC(CH3)3), 172.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2966, 2875, 1723 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2966, 2875, 1723 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1685 (C
O), 1685 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1149, 1004, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H29N2O4 ([M + H]+) calcd 301.2122, found 301.2124.
O), 1149, 1004, 782. MS m/z (%): 301 ([M + H]+). HRMS (ESI+) for C15H29N2O4 ([M + H]+) calcd 301.2122, found 301.2124.
Methyl(2S)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-4-methylpentanoate ((2S,3R)-11c). Method B. Compound (R)-2c was coupled with (R)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2S,3R)-11c as yellow oil in an 61% yield (345 mg) with a dr of 96
7, v/v) to provide compound (2S,3R)-11c as yellow oil in an 61% yield (345 mg) with a dr of 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, [α]20D = 20.1° (c 1.01, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.3 Hz, 3H, CHCH2CH(C
4, [α]20D = 20.1° (c 1.01, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.3 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C
3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.48–1.55 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.48–1.55 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.56–1.64 (m, 3H, CHCH2C
2CH(CH3)2), 1.56–1.64 (m, 3H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2, Pyr 4-H and CHC
(CH3)2, Pyr 4-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.09–2.17 (m, 1H, Pyr 4-H), 2.61–2.71 (m, 2H, Pyr 2,5-H), 2.73–2.89 (m, 2H, Pyr 2,5-H), 3.28–3.37 (m, 1H, C
2CH(CH3)2), 2.09–2.17 (m, 1H, Pyr 4-H), 2.61–2.71 (m, 2H, Pyr 2,5-H), 2.73–2.89 (m, 2H, Pyr 2,5-H), 3.28–3.37 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.07–4.17 (m, 1H, Pyr 3-H), 4.95 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(
CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.07–4.17 (m, 1H, Pyr 3-H), 4.95 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.8 (CHCH2CH(
H3)2), 22.8 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.3 (CHCH2
H3)2), 25.3 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.4 (Pyr 4-C), 40.2 (CH
H3)3), 32.4 (Pyr 4-C), 40.2 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 48.2 (Pyr 5-C), 49.9 (Pyr 3-C), 51.3 (OCH3), 56.5 (Pyr 2-C), 62.9 (
H2CH(CH3)2), 48.2 (Pyr 5-C), 49.9 (Pyr 3-C), 51.3 (OCH3), 56.5 (Pyr 2-C), 62.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.3 (
HCH2CH(CH3)2), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.9 (
OOC(CH3)3), 173.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1722 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1722 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1711 (C
O), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1157, 1077, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2285.
O), 1157, 1077, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2285.
Methyl(2R)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-4-methylpentanoate ((2R,3S)-11c). Method B. Compound (S)-2c was coupled with (S)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2R,3S)-11c as transparent oil in an 67% yield (379 mg) with a dr of 98
7, v/v) to provide compound (2R,3S)-11c as transparent oil in an 67% yield (379 mg) with a dr of 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, [α]20D = −19.9° (c 1.08, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.3 Hz, 3H, CHCH2CH(C
2, [α]20D = −19.9° (c 1.08, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.88 (d, J = 6.3 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C
3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.47–1.55 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.47–1.55 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.56–1.64 (m, 3H, CHCH2C
2CH(CH3)2), 1.56–1.64 (m, 3H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2, Pyr 4-H and CHC
(CH3)2, Pyr 4-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.09–2.19 (m, 1H, Pyr 4-H), 2.60–2.71 (m, 2H, Pyr 2,5-H), 2.73–2.89 (m, 2H, Pyr 2,5-H), 3.29–3.35 (m, 1H, C
2CH(CH3)2), 2.09–2.19 (m, 1H, Pyr 4-H), 2.60–2.71 (m, 2H, Pyr 2,5-H), 2.73–2.89 (m, 2H, Pyr 2,5-H), 3.29–3.35 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.06–4.17 (m, 1H, Pyr 3-H), 4.95 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(
CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.06–4.17 (m, 1H, Pyr 3-H), 4.95 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.8 (CHCH2CH(
H3)2), 22.8 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.3 (CHCH2
H3)2), 25.3 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.4 (Pyr 4-C), 40.2 (CH
H3)3), 32.4 (Pyr 4-C), 40.2 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 48.2 (Pyr 5-C), 49.9 (Pyr 3-C), 51.3 (OCH3), 56.5 (Pyr 2-C), 62.9 (
H2CH(CH3)2), 48.2 (Pyr 5-C), 49.9 (Pyr 3-C), 51.3 (OCH3), 56.5 (Pyr 2-C), 62.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.3 (
HCH2CH(CH3)2), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.9 (
OOC(CH3)3), 173.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1720 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1720 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1711 (C
O), 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1156, 1077, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2284.
O), 1156, 1077, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2284.
Methyl(2S)-2-{(3S)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-4-methylpentanoate ((2S,3S)-11c). Method B. Compound (R)-2c was coupled with (S)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2S,3S)-11c as yellowish oil in an 65% yield (367 mg) with a dr of 93
7, v/v) to provide compound (2S,3S)-11c as yellowish oil in an 65% yield (367 mg) with a dr of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, [α]20D = −11.6° (c 0.94, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.4 Hz, 3H, CHCH2CH(C
7, [α]20D = −11.6° (c 0.94, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.4 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C
3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.49–1.54 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.49–1.54 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.55–1.66 (m, 3H, CHCH2C
2CH(CH3)2), 1.55–1.66 (m, 3H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2, Pyr 4-H and CHC
(CH3)2, Pyr 4-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.09–2.19 (m, 1H, Pyr 4-H), 2.48–2.56 (m, 2H, Pyr 2,5-H), 2.86–2.98 (m, 2H, Pyr 2,5-H), 3.29–3.35 (m, 1H, C
2CH(CH3)2), 2.09–2.19 (m, 1H, Pyr 4-H), 2.48–2.56 (m, 2H, Pyr 2,5-H), 2.86–2.98 (m, 2H, Pyr 2,5-H), 3.29–3.35 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.08–4.19 (m, 1H, Pyr 3-H), 4.83 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(
CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.08–4.19 (m, 1H, Pyr 3-H), 4.83 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.9 (CHCH2CH(
H3)2), 22.9 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.2 (CHCH2
H3)2), 25.2 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.4 (Pyr 4-C), 40.1 (CH
H3)3), 32.4 (Pyr 4-C), 40.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 48.5 (Pyr 5-C), 49.8 (Pyr 3-C), 51.4 (OCH3), 56.5 (Pyr 2-C), 63.0 (
H2CH(CH3)2), 48.5 (Pyr 5-C), 49.8 (Pyr 3-C), 51.4 (OCH3), 56.5 (Pyr 2-C), 63.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.3 (
HCH2CH(CH3)2), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.8 (
OOC(CH3)3), 173.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1711 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1696 (C
O), 1696 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1158, 1008, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2279.
O), 1158, 1008, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2279.
Methyl(2R)-2-{(3R)-3-[(tert-butoxycarbonyl)amino]pyrrolidin-1-yl}-4-methylpentanoate ((2R,3R)-11c). Method B. Compound (S)-2c was coupled with (R)-3-Boc-aminopyrrolidine. The obtained residue was purified by column chromatography (SiO2, eluent: EtOAc/n-hexane, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) to provide compound (2R,3R)-11c as transparent oil in an 71% yield (401 mg) with a dr of 97
7, v/v) to provide compound (2R,3R)-11c as transparent oil in an 71% yield (401 mg) with a dr of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, [α]20D = 11.6° (c 1.01, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.3 Hz, 3H, CHCH2CH(C
3, [α]20D = 11.6° (c 1.01, MeOH). 1H-NMR (700 MHz, CDCl3): δ 0.89 (d, J = 6.3 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C
3)2), 0.91 (d, J = 6.4 Hz, 3H, CHCH2CH(C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3)2), 1.42 (s, 9H, C(CH3)3), 1.49–1.54 (m, 1H, CHC
3)2), 1.42 (s, 9H, C(CH3)3), 1.49–1.54 (m, 1H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 1.55–1.66 (m, 3H, CHCH2C
2CH(CH3)2), 1.55–1.66 (m, 3H, CHCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) (CH3)2, Pyr 4-H and CHC
(CH3)2, Pyr 4-H and CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH(CH3)2), 2.10–2.19 (m, 1H, Pyr 4-H), 2.46–2.51 (m, 2H, Pyr 2,5-H), 2.86–2.98 (m, 2H, Pyr 2,5-H), 3.29–3.36 (m, 1H, C
2CH(CH3)2), 2.10–2.19 (m, 1H, Pyr 4-H), 2.46–2.51 (m, 2H, Pyr 2,5-H), 2.86–2.98 (m, 2H, Pyr 2,5-H), 3.29–3.36 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.09–4.17 (m, 1H, Pyr 3-H), 4.85 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(
CH2CH(CH3)2), 3.68 (s, 3H, OCH3), 4.09–4.17 (m, 1H, Pyr 3-H), 4.85 (s, 1H, NH). 13C-NMR (176 MHz, CDCl3): δ 22.6 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 22.9 (CHCH2CH(
H3)2), 22.9 (CHCH2CH(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)2), 25.2 (CHCH2
H3)2), 25.2 (CHCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H(CH3)2), 28.5 (C(
H(CH3)2), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.4 (Pyr 4-C), 40.1 (CH
H3)3), 32.4 (Pyr 4-C), 40.1 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH(CH3)2), 48.6 (Pyr 5-C), 49.8 (Pyr 3-C), 51.4 (OCH3), 56.5 (Pyr 2-C), 63.0 (
H2CH(CH3)2), 48.6 (Pyr 5-C), 49.8 (Pyr 3-C), 51.4 (OCH3), 56.5 (Pyr 2-C), 63.0 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH2CH(CH3)2), 79.3 (
HCH2CH(CH3)2), 79.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.5 (
(CH3)3), 155.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.8 (
OOC(CH3)3), 173.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1711 (C
OOCH3). IR (FT-IR, νmax, cm−1): 2956, 2870, 1711 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1696 (C
O), 1696 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1158, 1008, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2284.
O), 1158, 1008, 781. MS m/z (%): 315 ([M + H]+). HRMS (ESI+) for C16H31N2O4 ([M + H]+) calcd 315.2278, found 315.2284.
Protocol used for investigation of ee values (diastereomers 5a–c)
Compounds 3a–c (100 mg) were dissolved in DCM (1 mL) and treated with TFA (1 mL). The solutions were stirred at r.t. for 30 min. After removal of the solvent in vacuo, the obtained ammonium salts were neutralized with Cs2CO3 (1 equiv.) in order to generate amines 4a–c, which were directly used in the next step without further purification.2-Formylphenylboronic acid (1 equiv.) was dissolved in CDCl3 (3 mL) and treated with (R)-1,1′-bi-2-naphthol ((R)-BINOL) (1.1 equiv.). The solution was stirred at 40 °C for 30 min. Then, the corresponding amine (4a–c) (1 equiv.) was added to reaction mixture with 4 Å molecular sieves and stirred at r.t. for 18 h. The reaction mixture was transferred to an NMR tube for 1H NMR analysis.
Synthesis of (piperidin-1-yl)propanoic acid (6)
The corresponding ester (3a) (200 mg) was dissolved in MeOH (2 mL) and treated with 2 N NaOH (1 mL). The solution was stirred at r.t. for 2 h. After removal of the solvent in vacuo, the residue was purified by flash chromatography (EtOAc→MeOH).![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.41 (s, 9H, C(CH3)3), 1.91–2.10 (m, 4H, Pip 3,5-H), 2.57–2.73 (m, 1H, Pip 6-H), 2.75–2.84 (m, 1H, Pip 2-H), 3.37–3.54 (m, 3H, Pip 2,6-H and C
3), 1.41 (s, 9H, C(CH3)3), 1.91–2.10 (m, 4H, Pip 3,5-H), 2.57–2.73 (m, 1H, Pip 6-H), 2.75–2.84 (m, 1H, Pip 2-H), 3.37–3.54 (m, 3H, Pip 2,6-H and C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.56–3.67 (m, 1H, Pip 4-H), 4.84–5.01 (m, 2H, NH and COOH). 13C-NMR (176 MHz, CDCl3): δ 13.3 (CH
CH3), 3.56–3.67 (m, 1H, Pip 4-H), 4.84–5.01 (m, 2H, NH and COOH). 13C-NMR (176 MHz, CDCl3): δ 13.3 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.6 (C(
H3), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.7 (Pip 5-C), 29.9 (Pip 3-C), 46.0 (Pip 4-C), 48.3 (Pip 6-C), 51.2 (Pip 2-C), 65.8 (
H3)3), 29.7 (Pip 5-C), 29.9 (Pip 3-C), 46.0 (Pip 4-C), 48.3 (Pip 6-C), 51.2 (Pip 2-C), 65.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.5 (
HCH3), 79.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.6 (
(CH3)3), 155.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.1 (
OOC(CH3)3), 173.1 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOH). IR (FT-IR, νmax, cm−1): 3427, 2977, 1687 (C
OOH). IR (FT-IR, νmax, cm−1): 3427, 2977, 1687 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 (C
O), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1167, 1016, 863. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273,1809, found 273.1805.
O), 1167, 1016, 863. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273,1809, found 273.1805.![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.41 (s, 9H, C(CH3)3), 1.88–2.10 (m, 4H, Pip 3,5-H), 2.58–2.70 (m, 1H, Pip 6-H), 2.73–2.83 (m, 1H, Pip 2-H), 3.33–3.53 (m, 3H, Pip 2,6-H and C
3), 1.41 (s, 9H, C(CH3)3), 1.88–2.10 (m, 4H, Pip 3,5-H), 2.58–2.70 (m, 1H, Pip 6-H), 2.73–2.83 (m, 1H, Pip 2-H), 3.33–3.53 (m, 3H, Pip 2,6-H and C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.55–3.67 (m, 1H, Pip 4-H), 4.94–5.30 (m, 2H, NH and COOH). 13C-NMR (176 MHz, CDCl3): δ 13.3 (CH
CH3), 3.55–3.67 (m, 1H, Pip 4-H), 4.94–5.30 (m, 2H, NH and COOH). 13C-NMR (176 MHz, CDCl3): δ 13.3 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.6 (C(
H3), 28.6 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 29.8 (Pip 5-C), 30.0 (Pip 3-C), 46.0 (Pip 4-C), 48.3 (Pip 6-C), 51.1 (Pip 2-C), 65.8 (
H3)3), 29.8 (Pip 5-C), 30.0 (Pip 3-C), 46.0 (Pip 4-C), 48.3 (Pip 6-C), 51.1 (Pip 2-C), 65.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.5 (
HCH3), 79.5 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 155.6 (
(CH3)3), 155.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 173.3 (
OOC(CH3)3), 173.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOH). IR (FT-IR, νmax, cm−1): 3427, 2978, 1688 (C
OOH). IR (FT-IR, νmax, cm−1): 3427, 2978, 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1615 (C
O), 1615 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1162, 1018, 863. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1809.
O), 1162, 1018, 863. MS m/z (%): 273 ([M + H]+). HRMS (ESI+) for C13H25N2O4 ([M + H]+) calcd 273.1809, found 273.1809.Synthesis of peptides (7)
DIPEA (0.1 mL, 1 equiv.) was added to a mixture of acid 6 (150 mg, 1 equiv.) and HATU (210 mg, 1 equiv.) in DMF (10 mL), and the solution was stirred at r.t. for 5 min. Then L-phenylalanine ethyl ester hydrochloride (127 mg, 1 equiv.) was added to reaction mixture. After 30 min DIPEA (0.2 mL, 2 equiv.) was added to solution and stirred at r.t. for 1 h. Reaction mixture was diluted with EtOAc (10 mL), washed with 1 M KHSO4 (10 mL), 1 M NaHCO3 (10 mL) and brine (10 mL). The organic layer was dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (SiO2, eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v).
3, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, [α]20D = −2.1° (c 0.96, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.09–1.17 (m, 1H, Pip 3-H), 1.12 (d, J = 7.1 Hz, 3H, CHC
6, [α]20D = −2.1° (c 0.96, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.09–1.17 (m, 1H, Pip 3-H), 1.12 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.24–1.33 (m, 1H, Pip 5-H), 1.26 (t, J = 7.2 Hz, 3H, COOCH2C
3), 1.24–1.33 (m, 1H, Pip 5-H), 1.26 (t, J = 7.2 Hz, 3H, COOCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.44 (s, 9H, C(CH3)3), 1.77–1.85 (m, 2H, Pip 3,5-H), 2.05–2.12 (m, 1H, Pip 6-H), 2.24–2.29 (m, 1H, Pip 2-H), 2.43–2.48 (m, 1H, Pip 6-H), 2.61–2.69 (m, 1H, Pip 2-H), 3.01 (q, J = 7.0 Hz, 1H, C
3), 1.44 (s, 9H, C(CH3)3), 1.77–1.85 (m, 2H, Pip 3,5-H), 2.05–2.12 (m, 1H, Pip 6-H), 2.24–2.29 (m, 1H, Pip 2-H), 2.43–2.48 (m, 1H, Pip 6-H), 2.61–2.69 (m, 1H, Pip 2-H), 3.01 (q, J = 7.0 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.05–3.10 (m, 1H, PhC
CH3), 3.05–3.10 (m, 1H, PhC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH), 3.17–3.22 (m, 1H, PhC
2CH), 3.17–3.22 (m, 1H, PhC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH), 3.35–3.43 (m, 1H, Pip 4-H), 4.19 (q, J = 7.2 Hz, 2H, COOC
2CH), 3.35–3.43 (m, 1H, Pip 4-H), 4.19 (q, J = 7.2 Hz, 2H, COOC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3), 4.39 (s, 1H, NHBoc), 4.83 (q, J = 6.9 Hz, 6.1 Hz, 1H, PhCH2C
2CH3), 4.39 (s, 1H, NHBoc), 4.83 (q, J = 6.9 Hz, 6.1 Hz, 1H, PhCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 7.10–7.14 (m, 2H, Ph 2,6-H), 7.21–7.24 (m, 1H, Ph 4-H), 7.25–7.29 (m, 2H, Ph 3,5-H), 7.62 (d, J = 8.1 Hz, 1H, NHCO). 13C-NMR (176 MHz, CDCl3): δ 11.9 (CH
), 7.10–7.14 (m, 2H, Ph 2,6-H), 7.21–7.24 (m, 1H, Ph 4-H), 7.25–7.29 (m, 2H, Ph 3,5-H), 7.62 (d, J = 8.1 Hz, 1H, NHCO). 13C-NMR (176 MHz, CDCl3): δ 11.9 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 14.3 (COOCH2
H3), 14.3 (COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.9 (Pip 3-C), 33.0 (Pip 5-C), 38.0 (Ph
H3)3), 32.9 (Pip 3-C), 33.0 (Pip 5-C), 38.0 (Ph![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH), 46.8 (Pip 6-C), 47.5 (Pip 2-C), 51.1 (Pip 4-C), 52.5 (PhCH2
H2CH), 46.8 (Pip 6-C), 47.5 (Pip 2-C), 51.1 (Pip 4-C), 52.5 (PhCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H), 61.6 (COO
H), 61.6 (COO![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH3), 63.9 (
H2CH3), 63.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.4 (
HCH3), 79.4 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 127.1 (Ph 4-C), 128.6 (Ph 3,5-C), 129.3 (Ph 2,6-C), 136.2 (Ph 1-C), 155.2 (
(CH3)3), 127.1 (Ph 4-C), 128.6 (Ph 3,5-C), 129.3 (Ph 2,6-C), 136.2 (Ph 1-C), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 171.9 (
OOC(CH3)3), 171.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH2CH3), 173.9 (
OOCH2CH3), 173.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) ONH). IR (FT-IR, νmax, cm−1): 3330, 2977, 2936, 1669 (C
ONH). IR (FT-IR, νmax, cm−1): 3330, 2977, 2936, 1669 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1499, 1169, 1021, 700. MS m/z (%): 448 ([M + H]+). HRMS (ESI+) for C24H38N3O5 ([M + H]+) calcd 448.2806, found 448.2809.
O), 1499, 1169, 1021, 700. MS m/z (%): 448 ([M + H]+). HRMS (ESI+) for C24H38N3O5 ([M + H]+) calcd 448.2806, found 448.2809.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, [α]20D = −13.6° (c 1.27, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.11–1.20 (m, 1H, Pip 3-H), 1.17 (d, J = 7.1 Hz, 3H, CHC
10, [α]20D = −13.6° (c 1.27, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.11–1.20 (m, 1H, Pip 3-H), 1.17 (d, J = 7.1 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.22–1.28 (m, 1H, Pip 5-H), 1.24 (t, J = 7.2 Hz, 3H, COOCH2C
3), 1.22–1.28 (m, 1H, Pip 5-H), 1.24 (t, J = 7.2 Hz, 3H, COOCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.45 (s, 9H, C(CH3)3), 1.80–1.86 (m, 2H, Pip 3,5-H), 2.09–2.15 (m, 1H, Pip 2-H), 2.33–2.40 (m, 1H, Pip 6-H), 2.51–2.56 (m, 1H, Pip 6-H), 2.62–2.67 (m, 1H, Pip 2-H), 3.06 (q, J = 7.1 Hz, 1H, C
3), 1.45 (s, 9H, C(CH3)3), 1.80–1.86 (m, 2H, Pip 3,5-H), 2.09–2.15 (m, 1H, Pip 2-H), 2.33–2.40 (m, 1H, Pip 6-H), 2.51–2.56 (m, 1H, Pip 6-H), 2.62–2.67 (m, 1H, Pip 2-H), 3.06 (q, J = 7.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.13 (d, J = 6.1 Hz, 2H, PhC
CH3), 3.13 (d, J = 6.1 Hz, 2H, PhC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH), 3.35–3.44 (m, 1H, Pip 4-H), 4.17 (q, J = 7.2 Hz, 2H, COOC
2CH), 3.35–3.44 (m, 1H, Pip 4-H), 4.17 (q, J = 7.2 Hz, 2H, COOC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3), 4.40 (s, 1H, NHBoc), 4.83 (dt, J = 8.9 Hz, 6.1 Hz, 1H, PhCH2C
2CH3), 4.40 (s, 1H, NHBoc), 4.83 (dt, J = 8.9 Hz, 6.1 Hz, 1H, PhCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 7.12–7.15 (m, 2H, Ph 2,6-H), 7.23–7.27 (m, 1H, Ph 4-H), 7.27–7.31 (m, 2H, Ph 3,5-H), 7.71 (d, J = 8.7 Hz, 1H, NHCO). 13C-NMR (176 MHz, CDCl3): δ 10.7 (CH
), 7.12–7.15 (m, 2H, Ph 2,6-H), 7.23–7.27 (m, 1H, Ph 4-H), 7.27–7.31 (m, 2H, Ph 3,5-H), 7.71 (d, J = 8.7 Hz, 1H, NHCO). 13C-NMR (176 MHz, CDCl3): δ 10.7 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 14.2 (COOCH2
H3), 14.2 (COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 28.5 (C(
H3), 28.5 (C(![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3)3), 32.7 (Pip 3-C), 33.2 (Pip 5-C), 37.9 (Ph
H3)3), 32.7 (Pip 3-C), 33.2 (Pip 5-C), 37.9 (Ph![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH), 46.6 (Pip 2-C), 47.4 (Pip 4-C), 50.9 (Pip 6-C), 52.4 (PhCH2
H2CH), 46.6 (Pip 2-C), 47.4 (Pip 4-C), 50.9 (Pip 6-C), 52.4 (PhCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H), 61.5 (COO
H), 61.5 (COO![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH3), 63.7 (
H2CH3), 63.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 79.4 (
HCH3), 79.4 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) (CH3)3), 127.1 (Ph 4-C), 128.7 (Ph 3,5-C), 129.4 (Ph 2,6-C), 136.3 (Ph 1-C), 155.2 (
(CH3)3), 127.1 (Ph 4-C), 128.7 (Ph 3,5-C), 129.4 (Ph 2,6-C), 136.3 (Ph 1-C), 155.2 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOC(CH3)3), 171.8 (
OOC(CH3)3), 171.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH2CH3), 173.7 (
OOCH2CH3), 173.7 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) ONH). IR (FT-IR, νmax, cm−1): 3332, 2977, 2936, 1671 (C
ONH). IR (FT-IR, νmax, cm−1): 3332, 2977, 2936, 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1498, 1169, 1022, 700. MS m/z (%): 448 ([M + H]+). HRMS (ESI+) for C24H38N3O5 ([M + H]+) calcd 448.2806, found 448.2808.
O), 1498, 1169, 1022, 700. MS m/z (%): 448 ([M + H]+). HRMS (ESI+) for C24H38N3O5 ([M + H]+) calcd 448.2806, found 448.2808.Synthesis of nosyl-peptides (9)
The corresponding N-Boc-dipeptide (100 mg) was dissolved in DCM (1 mL) and treated with TFA (1 mL). The solution was stirred at r.t. for 30 min. After removal of the solvent in vacuo, the obtained amine was used directly in the next step without further purification.4-Nitrobenzenesulfonyl chloride (50 mg, 1 equiv.) was added to a mixture of the corresponding amine (78 mg, 1 equiv.) and 1 M Na2CO3 (1 mL, 4 equiv.) in acetonitrile (15 mL), and the solution was stirred at r.t. for 1 hour. The reaction mixture was diluted with EtOAc (20 mL) and washed with H2O (2 × 15 mL) and brine (15 mL). The organic layer was dried with anhydrous sodium sulfate, filtered, and then concentrated under reduced pressure. The crude product was purified by flash chromatography (SiO2, eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone/n-hexane, 1
acetone/n-hexane, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v).
1, v/v).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, [α]20D = −7.6° (c 0.81, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.08 (d, J = 7.0 Hz, 3H, CHC
6, [α]20D = −7.6° (c 0.81, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.08 (d, J = 7.0 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.20–1.26 (m, 1H, Pip 3-H), 1.24 (t, J = 7.2 Hz, 3H, COOCH2C
3), 1.20–1.26 (m, 1H, Pip 3-H), 1.24 (t, J = 7.2 Hz, 3H, COOCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.34–1.39 (m, 1H, Pip 5-H), 1.63–1.70 (m, 2H, Pip 3,5-H), 1.98–2.05 (m, 1H, Pip 6-H), 2.15–2.20 (m, 1H, Pip 2-H), 2.39–2.44 (m, 1H, Pip 6-H), 2.57–2.65 (m, 1H, Pip 2-H), 2.97 (q, J = 7.1 Hz, 1H, C
3), 1.34–1.39 (m, 1H, Pip 5-H), 1.63–1.70 (m, 2H, Pip 3,5-H), 1.98–2.05 (m, 1H, Pip 6-H), 2.15–2.20 (m, 1H, Pip 2-H), 2.39–2.44 (m, 1H, Pip 6-H), 2.57–2.65 (m, 1H, Pip 2-H), 2.97 (q, J = 7.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.02–3.07 (m, 1H, PhC
CH3), 3.02–3.07 (m, 1H, PhC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH), 3.09–3.15 (m, 1H, Pip 4-H), 3.15–3.21 (m, 1H, PhC
2CH), 3.09–3.15 (m, 1H, Pip 4-H), 3.15–3.21 (m, 1H, PhC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH), 4.16 (q, J = 7.1 Hz, 2H, COOC
2CH), 4.16 (q, J = 7.1 Hz, 2H, COOC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3), 4.77–4.83 (m, 1H, PhCH2C
2CH3), 4.77–4.83 (m, 1H, PhCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 5.16 (d, J = 7.7 Hz, 1H, NHSO2), 7.07–7.10 (m, 2H, Ph 2,6-H), 7.17–7.20 (m, 1H, Ph 4-H), 7.21–7.25 (m, 2H, Ph 3,5-H), 7.47 (d, J = 8.2 Hz, 1H, NHCO), 8.06 (d, J = 8.8 Hz, 2H, PhNO2 2,6-H), 8.35 (d, J = 8.8 Hz, 2H, PhNO2 3,5-H). 13C-NMR (176 MHz, CDCl3): δ 12.0 (CH
), 5.16 (d, J = 7.7 Hz, 1H, NHSO2), 7.07–7.10 (m, 2H, Ph 2,6-H), 7.17–7.20 (m, 1H, Ph 4-H), 7.21–7.25 (m, 2H, Ph 3,5-H), 7.47 (d, J = 8.2 Hz, 1H, NHCO), 8.06 (d, J = 8.8 Hz, 2H, PhNO2 2,6-H), 8.35 (d, J = 8.8 Hz, 2H, PhNO2 3,5-H). 13C-NMR (176 MHz, CDCl3): δ 12.0 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 14.2 (COOCH2
H3), 14.2 (COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 33.4 (Pip 3,5-C), 37.8 (Ph
H3), 33.4 (Pip 3,5-C), 37.8 (Ph![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH), 46.6 (Pip 6-C), 50.5 (Pip 2-C), 51.1 (Pip 4-C), 52.5 (PhCH2
H2CH), 46.6 (Pip 6-C), 50.5 (Pip 2-C), 51.1 (Pip 4-C), 52.5 (PhCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H), 61.6 (COO
H), 61.6 (COO![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH3), 63.8 (
H2CH3), 63.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 124.5 (PhNO2 3,5-C), 127.1 (Ph 4-C), 128.2 (PhNO2 2,6-C), 128.6 (Ph 3,5-C), 129.3 (Ph 2,6-C), 136.1 (Ph 1-C), 147.3 (PhNO2 4-C), 150.1 (PhNO2 1-C), 171.9 (
HCH3), 124.5 (PhNO2 3,5-C), 127.1 (Ph 4-C), 128.2 (PhNO2 2,6-C), 128.6 (Ph 3,5-C), 129.3 (Ph 2,6-C), 136.1 (Ph 1-C), 147.3 (PhNO2 4-C), 150.1 (PhNO2 1-C), 171.9 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH2CH3), 173.6 (
OOCH2CH3), 173.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) ONH). 15N-NMR (71 MHz, CDCl3): δ −332.1 (Pip), −274.5 (NH–SO2), −271.8 (NH–CO), −16.0 (NO2). IR (FT-IR, νmax, cm−1): 3269, 3104, 2927, 1659 (C
ONH). 15N-NMR (71 MHz, CDCl3): δ −332.1 (Pip), −274.5 (NH–SO2), −271.8 (NH–CO), −16.0 (NO2). IR (FT-IR, νmax, cm−1): 3269, 3104, 2927, 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1528 (N–O), 1348 (S
O), 1528 (N–O), 1348 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1163 (C
O), 1163 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1092, 736. MS m/z (%): 533 ([M + H]+). HRMS (ESI+) for C25H33N4O7S ([M + H]+) calcd 533.2064, found 533.2068.
O), 1092, 736. MS m/z (%): 533 ([M + H]+). HRMS (ESI+) for C25H33N4O7S ([M + H]+) calcd 533.2064, found 533.2068.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, [α]20D = −14.2° (c 0.99, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.12 (d, J = 7.0 Hz, 3H, CHC
10, [α]20D = −14.2° (c 0.99, MeOH). 1H-NMR (700 MHz, CDCl3): δ 1.12 (d, J = 7.0 Hz, 3H, CHC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.18–1.26 (m, 1H, Pip 3-H), 1.22 (t, J = 7.2 Hz, 3H, COOCH2C
3), 1.18–1.26 (m, 1H, Pip 3-H), 1.22 (t, J = 7.2 Hz, 3H, COOCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 3), 1.30–1.40 (m, 1H, Pip 5-H), 1.63–1.73 (m, 2H, Pip 3,5-H), 2.00–2.07 (m, 1H, Pip 2-H), 2.25–2.32 (m, 1H, Pip 6-H), 2.47–2.53 (m, 1H, Pip 6-H), 2.56–2.62 (m, 1H, Pip 2-H), 3.04 (q, J = 7.1 Hz, 1H, C
3), 1.30–1.40 (m, 1H, Pip 5-H), 1.63–1.73 (m, 2H, Pip 3,5-H), 2.00–2.07 (m, 1H, Pip 2-H), 2.25–2.32 (m, 1H, Pip 6-H), 2.47–2.53 (m, 1H, Pip 6-H), 2.56–2.62 (m, 1H, Pip 2-H), 3.04 (q, J = 7.1 Hz, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) CH3), 3.08–3.17 (m, 3H, Pip 4-H and PhC
CH3), 3.08–3.17 (m, 3H, Pip 4-H and PhC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH), 4.11–4.18 (m, 2H, COOC
2CH), 4.11–4.18 (m, 2H, COOC![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) 2CH3), 4.80 (dt, J = 8.7 Hz, 6.0 Hz, 1H, PhCH2C
2CH3), 4.80 (dt, J = 8.7 Hz, 6.0 Hz, 1H, PhCH2C![[H with combining low line]](https://www.rsc.org/images/entities/i_char_0048_0332.gif) ), 5.05 (d, J = 7.7 Hz, 1H, NHSO2), 7.07–7.11 (m, 2H, Ph 2,6-H), 7.19–7.23 (m, 1H, Ph 4-H), 7.23–7.27 (m, 2H, Ph 3,5-H), 7.56 (d, J = 8.6 Hz, 1H, NHCO), 8.05 (d, J = 8.8 Hz, 2H, PhNO2 2,6-H), 8.35 (d, J = 8.8 Hz, 2H, PhNO2 3,5-H). 13C-NMR (176 MHz, CDCl3): δ 10.5 (CH
), 5.05 (d, J = 7.7 Hz, 1H, NHSO2), 7.07–7.11 (m, 2H, Ph 2,6-H), 7.19–7.23 (m, 1H, Ph 4-H), 7.23–7.27 (m, 2H, Ph 3,5-H), 7.56 (d, J = 8.6 Hz, 1H, NHCO), 8.05 (d, J = 8.8 Hz, 2H, PhNO2 2,6-H), 8.35 (d, J = 8.8 Hz, 2H, PhNO2 3,5-H). 13C-NMR (176 MHz, CDCl3): δ 10.5 (CH![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 14.2 (COOCH2
H3), 14.2 (COOCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H3), 33.2 (Pip 3-C), 33.7 (Pip 5-C), 37.9 (Ph
H3), 33.2 (Pip 3-C), 33.7 (Pip 5-C), 37.9 (Ph![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH), 46.3 (Pip 2-C), 50.3 (Pip 6-C), 51.0 (Pip 4-C), 52.4 (PhCH2
H2CH), 46.3 (Pip 2-C), 50.3 (Pip 6-C), 51.0 (Pip 4-C), 52.4 (PhCH2![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H), 61.6 (COO
H), 61.6 (COO![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) H2CH3), 63.6 (
H2CH3), 63.6 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) HCH3), 124.5 (PhNO2 3,5-C), 127.1 (Ph 4-C), 128.2 (PhNO2 2,6-C), 128.7 (Ph 3,5-C), 129.4 (Ph 2,6-C), 136.2 (Ph 1-C), 147.3 (PhNO2 4-C), 150.1 (PhNO2 1-C), 171.8 (
HCH3), 124.5 (PhNO2 3,5-C), 127.1 (Ph 4-C), 128.2 (PhNO2 2,6-C), 128.7 (Ph 3,5-C), 129.4 (Ph 2,6-C), 136.2 (Ph 1-C), 147.3 (PhNO2 4-C), 150.1 (PhNO2 1-C), 171.8 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) OOCH2CH3), 173.3 (
OOCH2CH3), 173.3 (![[C with combining low line]](https://www.rsc.org/images/entities/i_char_0043_0332.gif) ONH). 15N-NMR (71 MHz, CDCl3): δ −332.9 (Pip), −274.5 (NH–SO2), −271.3 (NH–CO), −16.0 (NO2). IR (FT-IR, νmax, cm−1): 3328, 3176, 2938, 1657 (C
ONH). 15N-NMR (71 MHz, CDCl3): δ −332.9 (Pip), −274.5 (NH–SO2), −271.3 (NH–CO), −16.0 (NO2). IR (FT-IR, νmax, cm−1): 3328, 3176, 2938, 1657 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1528 (N–O), 1348 (S
O), 1528 (N–O), 1348 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1165 (C
O), 1165 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1091, 734. MS m/z (%): 533 ([M + H]+). HRMS (ESI+) for C25H33N4O7S ([M + H]+) calcd 533.2064, found 533.2066.
O), 1091, 734. MS m/z (%): 533 ([M + H]+). HRMS (ESI+) for C25H33N4O7S ([M + H]+) calcd 533.2064, found 533.2066.Conclusions
In this study, we prepared a series of new heterocyclic analogues of N-(ω-aminoalkylene)amino acid derivatives as chiral building blocks. The method was based on the conversion of enantiopure α-hydroxy acid esters into the corresponding chiral triflate esters, which were displaced in a nucleophilic substitution SN2 reaction with aminopyrrolidine and aminopiperidine derivatives by the inversion of the configuration to produce methyl 2-[(Boc-amino)cycloamin-1-yl]alkanoates with a good yield and with high enantiomeric and diastereomeric purity. The synthesized 2-[(Boc-amino)piperidin-1-yl]proponoates combined with methyl-L-phenylalaninate, produced new chiral N-Boc- and N-nosyl-dipeptides containing a piperidine moiety. The structures were elucidated by 1H-, 13C-, and 15N-NMR spectroscopy, high-resolution mass spectrometry, and X-ray crystallography analysis.Author contributions
Conceptualization, F. A. S. and A. Š.; methodology, F. A. S., A. Š. and N. K.; formal analysis, G. M., A. B. and G. R.; investigation, G. M., U. Š., R. J.; M. R. B. and A. B; resources, F. A. S. and A. Š.; data curation, F. A. S., A. Š. and N. K.; writing—original draft preparation, G. M., V. M. and A. Š.; writing—review and editing, G. M., A. Š., V. M. and F. A. S.; visualization, G. M., A. B., V. M., F. A. S. and A. Š.; supervision, A. Š. and F. A. S.; funding acquisition, F. A. S. and A. Š. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been accomplished with financial support provided by Vipergen ApS company (Copenhagen, Denmark). The authors are grateful to Mr S. Belyakov (Latvian Institute of Organic Synthesis, Riga, Latvia) for performing the X-ray analysis.References
- J. Malcher, J. Wesoly and H. A. R. Bluyssen, Mini. Rev. Med. Chem., 2014, 14, 401–410, DOI:10.2174/1389557514666140428114613.
- M. Metry, N. D. A. Dirda, J. P. Raufman, J. E. Polli and J. P. Y. Kao, Eur. J. Pharm. Sci., 2023, 180, 106335, DOI:10.1016/j.ejps.2022.106335.
- M. Nörrlinger, S. Hafner and T. Ziegler, Beilstein J. Org. Chem., 2016, 12, 1939–1948, DOI:10.3762/bjoc.12.183.
- R. Gambari, Expert Opin. Ther. Targets, 2014, 24, 267–294, DOI:10.1517/13543776.2014.863874.
- P. Wittung, P. E. Nielsen, O. Buchardt, M. Egholm and B. Nordén, Nature, 1994, 368, 561–563, DOI:10.1038/368561a0.
- T. Sugiyama and A. Kittaka, Molecules, 2013, 18, 287–310, DOI:10.3390/molecules18010287.
- P. E. Nielsen and G. Haaima, Chem. Soc. Rev., 1997, 26, 73–78, 10.1039/CS9972600073.
- K. R. Singh, P. Sridevi and R. P. Singh, Eng. Rep., 2020, 2, e12238, DOI:10.1002/eng2.12238.
- K. P. Nandhini, D. Al Shaer, F. Albericio and B. G. de la Torre, Chem. Soc. Rev., 2023, 52, 2764–2789, 10.1039/D2CS00049K.
- G. Byk and C. Gilon, J. Org. Chem., 1992, 57, 5687–5692, DOI:10.1021/jo00047a022.
- T. A. Feagin, N. I. Shah and J. M. Heemstra, J. Nucleic. Acids, 2012, 2012, 354549, DOI:10.1155/2012/354549.
- T. Sugiyama, Y. Imamura, Y. Demizu, M. Kurihara, M. Takano and A. Kittaka, Biol. Med. Chem. Lett., 2011, 21, 7317–7320, DOI:10.1016/j.bmcl.2011.10.017.
- B. A. Sherer and N. Bruger, Patent WO 2017/106607 A1, 2017.
- H. Zheng, P. Wu and P. A. Bonne, Molecules, 2023, 28, 634, DOI:10.3390/molecules28020634.
- F. I. McGonagle, D. S. MacMillan, J. Murray, H. F. Sneddon, C. Jamieson and A. J. B. Watson, Green Chem., 2013, 15, 1159–1165, 10.1039/C3GC40359A.
- A. Manna, S. Rapireddy, G. Sureshkumar and D. H. Ly, Tetrahedron, 2015, 71, 3507–3514, DOI:10.1016/j.tet.2015.03.052.
- P. Mäki-Arvela, I. L. Simakova and D. Yu. Murzin, Catal. Rev. Sci., 2023, 65, 501–568, DOI:10.1080/01614940.2021.1942689.
- G. Breipohl, US Pat.5516937, 1996 Search PubMed.
- K. L. Dueholm, M. Egholm, C. Behrens, L. Christensen, H. F. Hansen, T. Vulpius, K. H. Petersen, R. H. Berg, P. E. Nielsen and O. Buchardt, J. Org. Chem., 1994, 59, 5767–5773, DOI:10.1021/jo00098a042.
- L. Mestrom, P. Bracco and U. Hanefeld, Eur. J. Org. Chem., 2017, 2017, 7019–7025, DOI:10.1002/ejoc.201701213.
- B. Falkiewicz, A. S. Kołodziejczyk, B. Liberek and K. Wiśniewski, Tetrahedron, 2001, 57, 7909–7917, DOI:10.1016/S0040-4020(01)00759-1.
- B. Sahu, I. Sacui, S. Rapireddy, K. J. Zanotti, R. Bahal, B. A. Armitage and D. H. Ly, J. Org. Chem., 2011, 76, 5614–5627, DOI:10.1021/jo200482d.
- J. Liu, J. Han, K. Izawa, T. Sato, S. White, N. A. Meanwell and V. A. Soloshonok, Eur. J. Med. Chem., 2020, 208, 112736, DOI:10.1016/j.ejmech.2020.112736.
- B. A. Chalyk, I. Y. Kandaurova, K. V. Hrebeniuk, O. V. Manoilenko, I. B. Kulik, R. T. Iminov, V. Kubyshkin, A. V. Tverdokhlebov, O. K. Ablialimov and P. K. Mykhailiuk, RSC Adv., 2016, 6, 25713–25723, 10.1039/C6RA02365G.
- J. Bruzgulienė, G. Račkauskienė, A. Bieliauskas, V. Milišiūnaitė, M. Dagilienė, G. Matulevičiūtė, V. Martynaitis, S. Krikštolaitytė, F. A. Sløk and A. Šačkus, Beilstein J. Org. Chem., 2022, 18, 102–109, DOI:10.3762/bjoc.18.11.
- J. Han, J. Escorihuela, S. Fustero, A. Landa, V. A. Soloshonok and A. Sorochinsky, Molecules, 2022, 27, 3797, DOI:10.3390/molecules27123797.
- D. Madsen, C. Azevedo, I. Micco, L. K. Petersen and N. J. V. Hansen, Prog. Med. Chem., 2020, 59, 181–249, DOI:10.1016/bs.pmch.2020.03.001.
- G. M. Elisi, A. Bedini, L. Scalvini, C. Carmi, S. Bartolucci, V. Lucini, F. Scaglione, M. Mor, S. Rivara and G. Spadoni, Molecules, 2020, 25, 4057, DOI:10.3390/molecules25184057.
- B. Dhakal, L. Bohé and D. Crich, J. Org. Chem., 2017, 82, 9263–9269, DOI:10.1021/acs.joc.7b01850.
- P. Kasal and J. Jindřich, ACS Omega, 2022, 7, 20137–20144, DOI:10.1021/acsomega.2c01965.
- F. Effenberger, U. Burkard and J. Willfahrt, Angew. Chem., Int. Ed. Engl., 1983, 22, 65–66, DOI:10.1002/anie.198300652.
- R. W. Feenstra, E. H. M. Stokkingreef, R. J. F. Nivard and H. C. J. Ottenheijm, Tetrahedron, 1988, 44, 5583–5595, DOI:10.1016/s0040-4020(01)86063-4.
- F. Effenberger, U. Burkard and J. Willfahrt, Liebigs Ann. Chem., 1986, 314–333, DOI:10.1002/jlac.198619860213.
- K. M. Nilsson, A. B. M. Astrand, A. I. K. Berggren, J. R. Johansson, M. J. Lepisto, S. P. Kawatkar, Q. Su and J. G. Kettle, JAK1 selective inhibitors, Patent WO 2018/134213Al, 2018.
- CCDC 2168602† contains the supplementary crystallographic data for tert-butyl 1-((S)-1-(methoxycarbonyl)-2-methylpropyl)piperidin-4-ylcarbamate (S)-3c: formula 2(C16H30N2O4); unit cell parameters: (a) 10.0924(2) (b) 12.8242(2) (c) 14.0647(2), space group P21..
- CCDC 2168596† contains the supplementary crystallographic data for tert-butyl 1-((R)-1-(methoxycarbonyl)-2-methylpropyl)piperidin-4-ylcarbamate (R)-3d: formula 2(C16H30N2O4); unit cell parameters: (a) 10.0869(1) (b) 12.8088(2) (c) 14.0693(2), space group P21..
- T. J. Wenzel and C. D. Chisholm, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 59, 1–63, DOI:10.1016/j.pnmrs.2010.07.003.
- P. P. Lankhorst, J. H. J. van Rijn and A. L. L. Duchateau, Molecules, 2018, 23, 1785, DOI:10.3390/molecules23071785.
- Y. P. Fuertes, A. M. Kelly, J. S. Fossey, M. E. Powell, S. D. Bull and T. D. James, Nat. Protoc., 2008, 3, 210–214, DOI:10.1038/nprot.2007.524.
- I. W. Hamley, Chem. Rev., 2017, 117, 14015–14041, DOI:10.1021/acs.chemrev.7b00522.
- S. Kuzmič, M. Hrast and R. Frlan, Acta Chim. Slov., 2022, 69, 261–270, DOI:10.17344/acsi.2021.6673.
- A. Žukauskaitė, A. Moretto, C. Peggion, M. De Zotti, A. Šačkus, F. Formaggio, N. De Kimpe and S. Mangelinckx, Eur. J. Org. Chem., 2014, 2014, 2312–2321, DOI:10.1002/ejoc.201301741.
- P. Pavadai, S. Ramalingam, T. Panneerselvam, S. Kunjiappan, P. Perumal, V. Mani, G. Saravanan, V. Alagarsamy, D. N. Ammunje and J. Chimakurthy, Anti-Infect. Agents, 2020, 18, 160–168, DOI:10.2174/2211352517666190405155505.
- R. Blaszczyk, A. Gzik, B. Borek, M. Dziegielewski, K. Jedrzejczak, J. Nowicka, J. Chrzanowski, J. Brzezinska, A. Golebiowski, J. Olczak, M. M. Grzybowski and J. Peczkowicz-Szyszka, Dipeptide piperidine derivatives, US Pat.2019/0300525A1m 2019 Search PubMed.
- M. M. Grzybowski, P. S. Stańczak, P. Pomper, R. Błaszczyk, B. Borek, A. Gzik, J. Nowicka, K. Jędrzejczak, J. Brzezińska, T. Rejczak, N. C. Güner-Chalimoniuk, A. Kikulska, M. Mlącki, J. Pęczkowicz-Szyszka, J. Olczak, A. Gołębiowski, K. Dzwonek, P. Dobrzański and Z. Zasłona, Cancers, 2022, 14, 3967, DOI:10.3390/cancers14163967.
- E. I. Vrettos, N. Sayyad, E. M. Mavrogiannaki, E. Stylos, A. D. Kostagianni, S. Papas, T. Mavromoustakos, V. Theodorou and A. G. Tzakos, RSC Adv., 2017, 7, 50519–50526, 10.1039/c7ra06655d.
- A. Leggio, M. L. Di Gioia, F. Perri and A. Liguori, Tetrahedron, 2007, 63, 8164–8173, DOI:10.1016/j.tet.2007.05.121.
- M. S. A. El-Gabya, Y. A. Ammarb, M. I. H. El-Qalieia, A. M. Ahmed, M. F. Husseina and F. A. Faraghallya, Egypt. J. Chem., 2020, 63, 5289–5327, DOI:10.21608/EJCHEM.2020.33860.2707.
- M. Remko and C. W. von der Lieth, Biol. Med. Chem., 2004, 12, 53955403, DOI:10.1016/j.bmc.2004.07.049.
- V. S. Murthy, Y. Tamboli, V. S. Krishna, D. Sriram, F. X. Zhang, G. W. Zamponi and V. Vijayakumar, ACS Omega, 2021, 6, 9731–9740, DOI:10.1021/acsomega.1c00369.
- D. I. Ugwuja, U. C. Okoro, S. S. Soman, R. Soni, S. N. Okafor and D. I. Ugwu, J. Enzyme Inhib. Med. Chem., 2019, 34, 1388–1399, DOI:10.1080/14756366.2019.1651313.
- B. F. Lundt, N. L. Johansen, A. Vølund and J. Markussen, Int. J. Pept. Protein Res., 1978, 12, 258–268, DOI:10.1111/j.1399-3011.1978.tb02896.x.
- H. J. Zhang, A. W. Schuppe, S. T. Pan, J. X. Chen, B. R. Wang, T. R. Newhouse and L. Yin, J. Am. Chem. Soc., 2018, 140, 5300–5310, DOI:10.1021/jacs.8b01886.
- K. N. Fanning, A. G. Jamieson and A. Sutherland, Org. Biomol. Chem., 2005, 3, 3749–3756, 10.1039/B510808J.
- S. W. Horgan, T. A. Ayers and T. J. N. Watson, Stereospecific preparation of β-[[(2S)-2-[4,4-dimethyl-3-[[4-[[[(2-methylphenyl)amino]carbonyl]amino]phenyl]methyl]-2,5-dioxo-1-imidazolidinyl]-4-methyl-1-oxopentyl]amino]-(βS)-phenylpropanoic acid, Patent WO2003/000662A2, 2003.
- M. Poterała and J. Plenkiewicz, Tetrahedron: Asymmetry, 2011, 22, 294–299, DOI:10.1016/j.tetasy.2011.01.024.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C, 1H-15N HMBC, 1H-15N HSQC NMR, HRMS, X-ray. CCDC 2168602 and 2168596. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra03060a |
| This journal is © The Royal Society of Chemistry 2023 |