 Open Access Article
Open Access ArticleCu(II)-catalyzed C–N coupling of 2-aminobenzothiazoles with boronic acids at room temperature†
Sankaran Radhika,
Aravind Chandravarkar and
Gopinathan Anilkumar
and
Gopinathan Anilkumar *
*
School of Chemical Sciences, Mahatma Gandhi University, P D Hills P O, Kottayam, Kerala, India 686560. E-mail: anilgi1@yahoo.com; anil@mgu.ac.in
First published on 8th June 2023
Abstract
A Cu(II)-catalyzed, effective C–N coupling of 2-aminobenzothiazoles with boronic acids in acetonitrile under open vessel chemistry was achieved. This protocol demonstrates the N-arylation of 2-aminobenzothiazoles with a broad range of differently substituted phenylboronic acids at room temperature and accomplishes moderate to excellent yields of the desired products. Under the optimized condition, phenylboronic acids bearing halogen at the para and meta positions were found to be more fruitful.
1. Introduction
N-Arylated heterocyclic compounds are significant organic structural frameworks in various natural products1 and drugs2 and they also serve as precursors in aromatic heterocyclic synthesis.3 2-Aminobenzothiazole derivatives have extensive applications in pharmaceutical chemistry due to their beneficial medicinal properties like anticancer,4 antidiabetic,5 antitubercular6 etc. and hence have generated inquisitiveness among researchers towards their synthesis. N-Substituted 2-aminobenzothiazoles containing molecules are employed as drugs (Scheme 1), for instance R116010,7 Frentizole8 and Naphthyridone HM13N,9 have been realized as a good inhibitor of trans-retinoic acid metabolism, as an immunosuppressive and as an efficient anti-HIV agent, respectively.Copper catalyzed coupling strategies for carbon–heteroatom bond formation have emerged as an alternative to palladium chemistry, since they are less toxic, cheap, abundant and able to make milder reaction conditions. The comprehensive elaboration of copper based C–N coupling has grown from Ullmann's10 and Goldberg's11 chemistry, but which needed drastic reaction conditions-stoichiometric amount of copper and temperature more than 200 °C. Later, Buchwald et al. reported the copper-catalyzed C–N coupling of amides with aryl halides using diamine ligands12 whereas Chan and Lam disclosed copper mediated coupling of aryl boronic acid with nitrogen nucleophiles at room temperature (RT) under air with promising functional group tolerance.13 The classical Chan–Lam coupling has faced some drawbacks such as requirement of stoichiometric quantity of copper catalyst, long reaction time, use of chlorinated solvent, narrow substrate scope, requirement of substrates with prefunctionalization and higher possibility for the presence of side products which make the purification of targeted products difficult. These downsides may provide some challenges for large-scale productions in industry. Therefore, a large group of researchers have tried to solve these problems and achieved substantial improvement in expanding copper catalyzed Chan–Lam coupling14 to deliver several methodologies for the formation of C–N bond, under relatively mild reaction conditions and with extended substrate scope.15 These methods used different types of copper-based catalytic systems like Cu(OAc)2/amine bases or inorganic bases, Cu(OAc)2/ligand/base, Cu2O, [Cu(OH)·TMEDA]2Cl2, CuCl, CuCl/base, CuCl2·2H2O/ligand/base, CuOTf/ligand etc. to afford N-arylated compounds. Within these, C–N bond fabrication of heterocycles via Chan–Lam coupling was found effective on utilizing Cu(OAc)2 in DCE for C–N coupling of 2-amino nitrogen containing heterocycles,14 Cu(OAc)2/DMAP/KI in DME for the C–N coupling of bidentate amides,16 Cu(OAc)2 in methanol and Cu(OAc)2/CsOPiv in DMF systems for the N-arylation of aminoazoles,17 CuCl2·2H2O/2,2′-bipyridine/K2CO3 in toluene or DMF system for the three-component amination involving boronic acids, cyanamides and amines as substrates,18 Cu2O in DMSO for the amination of tetrazoles,19 CuOTf/1,10-phenanthroline in DMSO for the amination of nitrogen having heterocycles with tautomerism,20 Cu(OAc)2/CsCO3 in DMF for the amination of 2-aminoheteroarenes,21 Cu(OAc)2·H2O in DCM for the amination of aminoindazoles,22 Cu(OAc)2/decanoic acid/2,6-lutidine in toluene23 for the amination of 2-alkynylanilines, [Cu(OH)TMEDA]2Cl2/K2CO3 in DCM for the amination of tetrazoles24 etc.
The detailed optimization studies and substrate scope exploration of Chan–Lam coupling of 2-aminobenzothiazoles under copper catalytic system was not reported. Motivated from the above mentioned approaches, we were enthusiastic to study the catalytic system for the C–N bond formation of 2-aminobenzothiazoles with aryl boronic acids. As an extension to our curiosity in transition metal catalysis,25 herein we considered the Chan–Lam type coupling of 2-aminobenzothiazoles with arylboronic acid substrates under copper catalysis at room temperature.
2. Results and discussion
We have opted 2-aminobenzothiazole (1) and phenylboronic acid (2a) as our model substrates to optimize the reaction condition. Initially, the reaction of 1 and 2a was executed using 10 mol% of Cu(OAc)2 as catalyst, 20 mol% of 1,10-phenanthroline as ligand and 2 equiv. of K3PO4 as base in DME at RT for 24 hours under open flak method. To our delight, the anticipated product N-phenylbenzo[d]thiazol-2-amine (3a) was noticed in 62% yield (Scheme 2, Table 1, entry 2). Encouraged from this result, we accomplished different optimizations for the C–N coupling of 2-aminobenzothiazoles with various boronic acids and are discussed in Table 1. | ||
| Scheme 2 Preliminary reaction on Cu(II)-catalyzed C–N coupling of 2-aminobenzothiazole with phenylboronic acid. | ||
| S. no. | Cu salts | Ligands | Bases | Solvents | Temp (°C) | Time (h) | Yield (%) of 3aa,b |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 2-aminobenzothiazole (1 equiv.), phenylboronic acid (1.2 equiv.), Cu salts (10 mol%), ligands (20 mol%), bases (2 equiv.), solvents (2 mL), reaction time: 18–30 h, temperature: RT-65 °C, air. Open vessel.b Isolated yield. nd = not detected.c Absence of ligands.d Absence of bases.e 1 equiv. of TBAB used. | |||||||
| 1 | CuBr | L1 | K3PO4 | DME | RT | 24 | 28 |
| 2 | Cu(OAc)2 | L1 | K3PO4 | DME | RT | 24 | 62 |
| 3 | CuBr2 | L1 | K3PO4 | DME | RT | 24 | 51 |
| 4 | — | L1 | K3PO4 | DME | RT | 24 | nd |
| 5 | Cu(OAc)2 | L2 | K3PO4 | DME | RT | 24 | 60 |
| 6 | Cu(OAc)2 | L3 | K3PO4 | DME | RT | 24 | 10 |
| 7 | Cu(OAc)2 | — | K3PO4 | DME | RT | 24 | Tracesc |
| 8 | Cu(OAc)2 | L1 | Cs2CO3 | DME | RT | 24 | 56 |
| 9 | Cu(OAc)2 | L1 | (CH3)3N | DME | RT | 24 | 20 |
| 10 | Cu(OAc)2 | L1 | C6H13N | DME | RT | 24 | 36 |
| 11 | Cu(OAc)2 | L1 | — | DME | RT | 24 | 18d |
| 12 | Cu(OAc)2 | L1 | K3PO4 | CH3CN | RT | 24 | 76 |
| 13 | Cu(OAc)2 | L1 | K3PO4 | DCE | RT | 24 | 70 |
| 14 | Cu(OAc)2 | L1 | K3PO4 | C6H4Cl2 | RT | 24 | 70 |
| 15 | Cu(OAc)2 | L1 | K3PO4 | Water | RT | 24 | 20e |
| 16 | Cu(OAc)2 | L1 | K3PO4 | t-BuOH | RT | 24 | 16 |
| 17 | Cu(OAc)2 | L1 | K3PO4 | MeOH | RT | 24 | 20 |
| 18 | Cu(OAc)2 | L1 | K3PO4 | CH3CN | 45 | 24 | 35 |
| 19 | Cu(OAc)2 | L1 | K3PO4 | CH3CN | 65 | 24 | 10 |
| 20 | Cu(OAc)2 | L1 | K3PO4 | CH3CN | RT | 18 | 61 |
| 21 | Cu(OAc)2 | L1 | K3PO4 | CH3CN | RT | 30 | 73 |
2.1 The choice of Cu-formed catalytic system
A blank test revealed that copper as a catalyst was indispensable to attain a reasonable result (Table 1, entry 4). So, we have studied the effect of different copper salts in the reaction as a catalyst. Cu(II) salts provided higher yields compared to Cu(I) salts, Cu(OAc)2 showed the best catalytic activity with 62% yield of the expected product (Table 1, entry 2).Subsequently, the influence of an array of different ligands, including N,N-type, N,O-type, O,O-type and phosphine ligands, were evaluated. N,N-planar ligands were found to be appropriate for the reaction (Table 1, entries 2 & 5) whereas trans-ligands disfavoured (Table 1, entry 6). 1,10-Phenanthroline was the ligand which offered the maximum outcome, and thus selected as the optimized ligand (Table 1, entry 2). Only traces of the required product 3a was found for the experiment in the absence of ligand (Table 1, entry 7).
Later, the optimization of bases indicated that inorganic bases like potassium phosphate (Table 1, entry 2) displayed good results compared to the organic bases like triethyl amine and cyclohexylamine (Table 1, entries 9 & 10 respectively). A base-free condition was also carried out, but the yield was poor (Table 1, entry 11).
2.2 Catalytic properties of Cu(OAc)2/1,10-phenanthroline/K3PO4 system
The impact of various solvents in the reaction was examined then. The replacement of DME with acetonitrile as solvent, improved the yield to 76% (Table 1, entry 12). Good yields were obtained with chlorinated solvents like DCE and dichlorobenzene also (Table 1, entries 13 & 14 respectively). But polar greener solvents like water, higher and lower alcohols were not applicable for the reaction because of their deprived yields (Table 1, entries 15–17). Since acetonitrile is well known for making coordination bonds with copper complexes, the increased rate of the reaction with the same may be due to its capability for coordination with Cu(OAc)2/1,10-phenanthroline/K3PO4 catalytic system.An appreciable depreciation in the yields of 3a to 35% and 10% were noted, when the temperatures were elevated from RT to 45 °C and 65 °C respectively (Table 1, entries 18 & 19). The evaporation of solvents at these temperatures perhaps led to the non-availability of oxygen in the reaction. The duration of the reaction was then shortened to 18 hours and prolonged to 30 hours, which acquired the targeted products in 61% and 73% respectively (Table 1, entries 20 & 21). The significant decrease of yield in the previous situation could be ascribed to the reduced reaction rate for the lowered duration supported by the appearance of unreacted substrates in the reaction mixture. The unaltered yield for the extended duration proved that 24 hours of the reaction is sufficient to form 3a with maximum optimized yield.
The amount of copper catalyst was then reduced to 5 mol%, which offered 68% of 3a (Table 2, entry 1). No enhancement in the yield of 3a was observed when the amount of copper was raised to 15 mol% (Table 2, entry 3). Decreasing the quantity of 1,10-phenanthroline to 10 mol% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of catalyst and ligand) did alter the yield appreciably to 63% (Table 2, entry 4). An additional trial using 1.5 equiv. of base gave similar yield of the reaction in comparison to that using 2 equiv. of base (Table 2, entry 5). Considerable increase in the yield was not detected, when the quantity of boronic acid was increased to 1.6 equiv. (Table 2, entry 7). Reactions carried out under excess of oxygen and in the presence of inert atmosphere (nitrogen) did not show drastic difference in the yield of the product (Table 2, entries 6 & 8). Thus, we have optimized the reaction condition, which utilized 10 mol% of Cu(OAc)2, 20 mol% of 1,10-phenanthroline and 1.5 equiv. of K3PO4 in acetonitrile at RT for 24 hours for the coupling between 2-aminobenzothiazole and phenylboronic acid.
1 ratio of catalyst and ligand) did alter the yield appreciably to 63% (Table 2, entry 4). An additional trial using 1.5 equiv. of base gave similar yield of the reaction in comparison to that using 2 equiv. of base (Table 2, entry 5). Considerable increase in the yield was not detected, when the quantity of boronic acid was increased to 1.6 equiv. (Table 2, entry 7). Reactions carried out under excess of oxygen and in the presence of inert atmosphere (nitrogen) did not show drastic difference in the yield of the product (Table 2, entries 6 & 8). Thus, we have optimized the reaction condition, which utilized 10 mol% of Cu(OAc)2, 20 mol% of 1,10-phenanthroline and 1.5 equiv. of K3PO4 in acetonitrile at RT for 24 hours for the coupling between 2-aminobenzothiazole and phenylboronic acid.
| S. no. | Cu(OAc)2 (mol%) | 1,10-Phenanthroline (mol%) | Yield (%) of 3aa,b |
|---|---|---|---|
| a Reaction conditions: 2-aminobenzothiazole (1 equiv.), phenylboronic acid (1.2 equiv.), Cu(OAc)2 (5–15 mol%), 1,10-phenanthroline (10–30 mol%), K3PO4 (2 equiv.), acetonitrile (2 mL), reaction time: 24 h, temperature: RT, air. Open vessel.b Isolated yield.c 1.5 equiv. of K3PO4.d Excess of O2.e 1.6 equiv. of phenylboronic acid.f In N2 atmosphere. | |||
| 1 | 5 | 10 | 68 |
| 2 | 10 | 20 | 76 |
| 3 | 15 | 30 | 60 |
| 4 | 10 | 10 | 63 |
| 5 | 10 | 20 | 75c |
| 6 | 10 | 20 | 36d |
| 7 | 10 | 20 | 77e |
| 8 | 10 | 20 | 19f |
2.3 Efficiency of Cu(OAc)2/1,10-phenanthroline/K3PO4 system
With the inspiring results in the optimized condition, we moved to the substrate scope studies by changing different phenylboronic acid substituents with 2- aminobenzothiazole derivatives and the observations are assembled in Scheme 3 and 4. A broad range of substituted phenylboronic acids underwent C–N coupling with 2-aminobenzothiazole (1) and afforded the corresponding N-arylated products in moderate to excellent yields (Scheme 3). Para and meta substituted halogen derivatives of phenylboronic acids provided the desired products in excellent yields with 1 (Scheme 3, 3e–g). Electron-withdrawing group (EWG) containing substrates of phenylboronic acids having 3-nitro and 4-CF3 substitution (Scheme 3, 3b and 3c) also reacted successfully and delivered products in good yields. The coupling of 3-CF3 substituted phenylboronic acid with 1 furnished the targeted product in moderate yield (Scheme 3, 3d). This may be explained on the basis of the reasonable nucleophilicity of the aromatic ring with EWG to follow transmetallation and reductive elimination in the mechanism. The EWG enhancing the yield of the reaction can be attributed mainly to the optimal formation of the complex C via transmetallation as well as the fast transformation of complex D to the targeted product. Fascinatingly, substrates containing electron-releasing substituents such as 2-methyl and 4-methoxy phenylboronic acids also coupled well affording 49% and 45% yield of the product respectively (Scheme 3, 3j and 3k). This methodology was also extended to ortho substituted halogen derivatives of phenylboronic acids, which afforded moderate yields, presumably due of steric reasons (Scheme 3, 3h and 3i).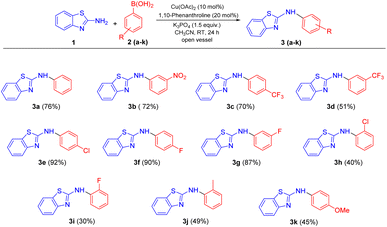 | ||
| Scheme 3 Substrate scope of the reaction between substituted phenylboronic acids and 2-aminobenzothiazole. | ||
To investigate the generality of the present coupling strategy, 6-methyl 2-aminobenzothiazole (4) was reacted with several phenylboronic acids (Scheme 4). 67% yield was obtained when phenylboronic acid was used as the coupling partner with 4 (Scheme 4, 6a). 4-Cl, 4-F and 3-F substituted boronic acids tolerated well and yielded the corresponding products with 89%, 86% and 80% respectively (Scheme 4, 6d, 6e and 6f). Electron deficient phenylboronic acids also afforded the coupling products in good yields (Scheme 4, 6b and 6c). The anticipated N-arylation of para-methoxy phenylboronic acid was detected in 41% of the yield (Scheme 4, 6g).
The reactivity of 2-aminothiazole with para-chlorophenylboronic acid was also tried, which offered only 28% of the respective coupled product (Scheme 5, 8a).
Based on the experimental studies and reported literature works,26 we have suggested a possible mechanism for the C–N coupling approach (Scheme 6). The formation of an octahedral copper complex A from copper acetate and 1,10-phenanthroline could be considered as the first step. Acetate replacement of complex A with 2-aminobenzothiazole generates complex B. Transmetallation of boronic acid with complex B produces complex C, which undergoes oxidative addition to form a copper(III) complex D. D on reductive elimination furnishes the N-arylated product. Copper(I) complex E formed during this step experiences oxidation by O2 and thus regenerates complex A. There is also a possibility for the solvent (acetonitrile) to coordinate with the copper27 complexes mostly by the replacement of acetate ions, but this will be in equilibrium. Supposedly, K3PO4 base is found to be strong thermodynamically in aprotic solvents;28 hence the rate of deprotonation from NH2 group in 1 (or 4) may be feasible by K3PO4 in acetonitrile.
3. Conclusions
In summary, Cu(II)/1,10-phenanthroline based catalytic system was established for the N-arylation of 2-aminobenzothiazoles with substituted phenylboronic acids at room temperature using acetonitrile as the solvent. This protocol is useful to synthesize a variety of N-arylated 2-aminobenzothiazole derivatives in moderate to excellent yields under simple and mild reaction conditions. Also, this method exhibited good functional group tolerance and particularly, proved to be highly efficient for the coupling with meta and para substituted halogen derivatives of phenylboronic acids. One of the chief benefits of our strategy include the use of less harmful acetonitrile as the reaction solvent at room temperature, though several Chan-Lam type C–N coupling reactions were reported in noxious organic solvents like DMF, THF etc. and chlorinated solvents like DCE at higher temperatures. Usually, addition of oxidants are required for Chan-Lam conditions at higher temperatures. Open flask chemistry made easier to monitor the development of the reaction, arrange the experiments, control the procedures and facilitates the substrates to react without adding oxidants. The optimized condition at room temperature with low copper loading, absence of additives like oxidant, favourable functional group compatibility are the other key advantages of our protocol. However, there are reports available in which researchers have performed their reactions under ligand- and base-free conditions.14 Since N-arylated heterocycles are unavoidable structural scaffolds in several drugs and natural products, novel discoveries in their synthetic routes might rise confidence among researchers in the scientific community.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SR thanks the Kerala State Council for Science, Technology and Environment (KSCSTE), Trivandrum for a research fellowship. G. A. thanks the EVONIK Industries, Germany for financial support (ECRP 2016 dated 6.10.2016). G. A. thanks the Kerala State Council for Science Technology and Environment (KSCSTE), Trivandrum, India for a research grant (Order No. 341/2013/KSCSTE dated 15.03.2013).References
- R. G. S. Berlinck and M. H. Kossuga, Modern Alkaloids, Wiley-VCH Verleg GmbH & Co. KGaA, 2007, 305 Search PubMed.
- A. Bikker, M. Brooijmans, A. Wissner and T. S. Mansour, J. Med. Chem., 2009, 52, 1493 CrossRef PubMed.
- M. A. McGowan, C. Z. McAvoy and S. L. Buchwald, Org. Lett., 2012, 14, 3800 CrossRef CAS PubMed.
- (a) D. A. Ibrahim, D. S. Lasheen and M. Y. Zaky, Bioorg. Med. Chem., 2015, 23, 4989 CrossRef CAS PubMed; (b) T. L. Dadmal, K. Appalanaidu, R. M. Kumbhare, T. Mondal, M. J. Ramaiah and M. P. Bhadra, New J. Chem., 2018, 42, 15546 RSC; (c) F. Sultana, M. A. Saifi, R. Syed, G. S. Mani, S. P. Shaik, E. G. Osas, C. Godugu, S. Shahjahanb and A. Kamal, New J. Chem., 2019, 43, 7150 RSC.
- (a) G. Sadhasivam and K. Kulanthai, J. Chem. Pharm. Res., 2015, 7, 425 CAS; (b) K. Harrouche, J.-F. Renard, N. Bouider, P. Tullio, E. Goffin, P. Lebrun, G. Faury, B. Pirotte and S. Khelili, Eur. J. Med. Chem., 2016, 115, 352 CrossRef CAS PubMed.
- (a) F. M. Shaikh, N. B. Patel, G. Sanna, B. Busonera, P. L. Colla and D. P. Rajani, Med. Chem. Res., 2015, 24, 3129 CrossRef CAS; (b) S. Radhika, M. B. Aleena and G. Anilkumar, J. Catal., 2022, 416, 233 CrossRef CAS.
- J. Van Heudsen, R. Van Ginckel, H. Bruwiere, P. Moelans, B. Janssen, W. Floren, B. J. Van der Leede, J. van Dun, G. Sanz, M. Venet, L. Dillen, C. Van Hove, G. Willemsens, M. Janicot and W. Wouters, Br. J. Cancer, 2002, 86, 605 CrossRef PubMed.
- M. Scheetz, D. Carlson and M. Schinitsky, Infect. Immun., 1977, 15, 145 CrossRef CAS PubMed.
- S. Massari, D. Daelemans, M. L. Barreca, A. Knezevich, S. Sabatini, V. Cecchetti, A. Marcello, C. Pannecouque and O. Tabarrini, J. Med. Chem., 2010, 53, 641 CrossRef CAS PubMed.
- F. Ullmann, Ber. Dtsch. Chem. Ges., 1903, 36, 2382 CrossRef.
- I. Goldberg, Ber. Dtsch. Chem. Ges., 1906, 39, 1691 CrossRef.
- (a) A. Klapars, J. C. Antilla, X. H. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2001, 123, 7727 CrossRef CAS PubMed; (b) A. Klapars, X. H. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421 CrossRef CAS PubMed.
- (a) D. M. T. Chan, K. L. Monaco, R.-P. Wang and M. P. Winters, Tetrahedron Lett., 1998, 39, 2933 CrossRef CAS; (b) P. Y. S. Lam, C. G. Clark, S. Saubern, J. Adams, M. P. Winters, D. M. T. Chan and A. Combs, Tetrahedron Lett., 1998, 39, 2941 CrossRef CAS.
- D. N. Rao, Sk. Rasheed, S. Aravinda, R. A. Vishwakarma and P. Das, RSC Adv., 2013, 3, 11472–11475 RSC.
- (a) A. Vijayan, D. N. Rao, K. V. Radhakrishnan, P. Y. S. Lam and P. Das, Synthesis, 2021, 53, 805 CrossRef CAS; (b) J.-Q. Chen, J.-H. Li and Z.-B. Dong, Adv. Synth. Catal., 2020, 362, 3311 CrossRef CAS; (c) M. J. West, J. W. B. Fyfe, J. C. Vantourout and A. J. B. Watson, Chem. Rev., 2019, 119, 12491 CrossRef CAS PubMed.
- H. Sahoo, S. Mukherjee, G. S. Grandhi, J. Selvakumar and M. Baidya, J. Org. Chem., 2017, 82, 2764 CrossRef CAS PubMed.
- D. N. Rao, S. Rasheed, R. A. Vishwakarma and P. Das, Chem. Commun., 2014, 50, 12911 RSC.
- J. Li and L. Neuville, Org. Lett., 2013, 15, 6124 CrossRef CAS PubMed.
- C. Y. Liu, Y. Li, J. Y. Ding, D. W. Dong and F. S. Han, Chem.–Eur. J., 2014, 20, 2373 CrossRef CAS PubMed.
- K. A. Kumar, P. Kannaboina, C. K. Jaladanki, P. V. Bharatam and P. Das, ChemistrySelect, 2016, 3, 601 CrossRef.
- S. Rasheed, D. N. Rao and P. Das, J. Org. Chem., 2015, 80, 9321 CrossRef CAS PubMed.
- K. A. Kumar, P. Kannaboina, D. K. Dhaked, R. A. Vishwakarma, P. V. Bharatam and P. Das, Org. Biomol. Chem., 2015, 13, 1481 RSC.
- J. Gao, Y. Shao, J. Zhu, J. Zhu, H. Mao, X. Wang and X. Lv, J. Org. Chem., 2014, 79, 9000 CrossRef CAS PubMed.
- T. Onaka, H. Umemoto, Y. Miki, A. Nakamura and T. Maegawa, J. Org. Chem., 2014, 79, 6703 CrossRef CAS PubMed.
- (a) S. Saranya, S. Radhika and G. Anilkumar, ChemistrySelect, 2021, 6, 6847 CrossRef CAS; (b) S. Radhika, T. Aneeja, R. M. Philip and G. Anilkumar, Appl. Organomet. Chem., 2021, 35, e6217 CrossRef CAS; (c) A. M. Thomas, S. Asha, K. S. Sindhu and G. Anilkumar, Tetrahedron Lett., 2015, 56, 6560 CrossRef CAS.
- D. N. Rao, Sk. Rasheed, K. A. Kumar, A. S. Reddy and P. Das, Adv. Synth. Catal., 2016, 358, 2126 CrossRef CAS.
- J. C. Vantourout, H. N. Miras, A. Isidro-Llobet, S. Sproules and A. J. B. Watson, J. Am. Chem. Soc., 2017, 139, 4769 CrossRef CAS PubMed.
- A. Klapars, X. Huang and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 7421 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: A comprehensive report on the experimental approaches, procedures and materials employed etc. were included in the ESI. The characterization data with spectral details (1H, 13C NMR) of the prepared compounds are also comprised in this section. See DOI: https://doi.org/10.1039/d3ra02979d |
| This journal is © The Royal Society of Chemistry 2023 |





