Open Access Article
Open Access ArticleA recent overview of surfactant–drug interactions and their importance
Dilli Ram Pokhrel
 ad,
Manish Kumar Sah
ad,
Manish Kumar Sah
 b,
Bibaran Gautam
b,
Bibaran Gautam
 a,
Hriday Kumar Basak
a,
Hriday Kumar Basak
 cd,
Ajaya Bhattarai
cd,
Ajaya Bhattarai
 *be and
Abhik Chatterjee
*be and
Abhik Chatterjee
 *d
*d
aDepartment of Chemistry, Damak Multiple Campus, Damak, Jhapa 57217, Nepal
bDepartment of Chemistry, Mahendra Morang Adarsh Multiple Campus, Tribhuvan University, Biratnagar 56613, Nepal. E-mail: ajaya.bhattarai@mmamc.tu.edu.np
cDepartment of Chemistry, Government General Degree College at Kushmandi, Dakshin Dinajpur, West Bengal-733121, India
dDepartment of Chemistry, Raiganj University, Uttar Dinajpur, West Bengal-733134, India. E-mail: abhikchemistry@gmail.com
eDepartment of Chemistry, Indian Institute of Technology, Madras 600036, India
First published on 12th June 2023
Abstract
This review focuses on the self-aggregation properties of different drugs, as well as on their interaction with anionic, cationic, and gemini surfactants. The interaction of drugs with surfactants has been reviewed concerning conductivity, surface tension, viscosity, density, and UV-Vis spectrophotometric measurements, and their relation with critical micelle concentration (CMC), cloud point, and binding constant. The conductivity measurement technique is used for the micellization of ionic surfactants. Cloud point studies can be used for the non-ionic, and also for certain ionic surfactants. Usually, surface tension studies are mostly employed for non-ionic surfactants. The degree of dissociation that is determined is used to evaluate thermodynamic parameters of micellization at various temperatures. The effect of external parameters like temperature, salt, solvent, pH, etc., is discussed for thermodynamics parameters using recent experimental works on drug-surfactant interactions. Consequences of drug–surfactant interaction, condition of drugs during interaction with surfactants, and applications of drug–surfactant interaction are being generalized which reflects current and future potential uses of drug–surfactant interactions.
1. Introduction
Surfactants are widely used chemical compounds that act as cleansing agents due to the formation of micelles. Surfactants are amphiphiles, surface-active agents that change interfacial properties and help to remove a phase from solid surfaces. Also, surfactants are used in the production and processing of foods, pharmaceuticals, agrochemicals, laundry, petroleum, mineral ores, fuel additives, paints, adhesives, and photographic films.1 The interaction of drugs with surfactants has been studied in the last few decades.2 Due to the hydrophobic nature of many kinds of drugs, their bio-availability is lower. The application of surfactants during the formulation of the drug increases its bioavailability.3 Micelles formation is an important phenomenon of surfactants which is due to the spherical aggregation of its molecules balancing the interactions of polar and non-polar parts. The concentration of surfactant above which micelles are formed is called critical micelle concentration (CMC).4 The formation of micelle facilitates the solubilisation of hydrophobic drugs and hence increases their bioavailability.5Any substance (other than food) that is used to prevent, diagnose, treat, or relieve symptoms of a disease or abnormal condition is called a drug. Drugs can also affect how the brain and the rest of bodywork and cause changes in mood, awareness, thoughts, feelings, or behaviour.7
The solubility of poorly aqueous soluble drugs can enhance by different methods like the use of surfactants, preparation of salts of drugs, the use of polymers, preparation of drug nanoparticles etc.8 Among them surfactants are effective members that can be used for stabilizing drugs, and delivery of drug and enhancement of drug solubility in aqueous media.9,10 Solubilization of drugs using surfactants effectively decreases the contact of the drug with inactivating agents like enzymes and hence reduces the side effects of drugs. Therefore, there is great significance of the micelle aggregate of amphiphile to dissolve the water-insoluble medicines to reduce toxicity, and increase bio-availability.11 Due to the large surface area, surfactant micelle can be added toward chemical reactions to increase the rate of reaction rate termed as catalyst.12,13 The micelle structure is concerned with the bio-membrane arrangement.14,15 It is enthusiasm for every researcher to observe the interaction of bio-membrane & drug and other materials of body fluid. Since, the study is also concerned with the behavior of interaction of surfactant and drug in the presence of varieties of solvents, electrolytes, salt, etc.16 Additionally, surfactants can be used in drug delivery systems to increase the solubility and bioavailability of drug species and to decrease the degradation rate of drug molecules by interacting micelles with drug molecules either through their outer surface or through their interior part.17,18 Surfactant protects drug molecules from adverse effects of the biological environment as they can stay in the human body for a longer period.19,20 To develop an effective drug delivery system, transdermal penetration of drugs, treatment of respiratory distress syndrome (RDS) and to design new drugs, it is necessary to focus the study on the aggregation behavior of amphiphilic drugs, their interaction with the micelles of surfactants, and the effect of several factors like pH, temperature, additives, solvent, ionic strength, etc.21,22 According to a literature survey, surface tension, viscosity, density, conductivity, spectroscopic analysis, CMC values, and binding and distribution properties of surfactants are responsible for effective interaction with drug entities.23 Among the different kinds of surfactants, non-ionic surfactants are found to be less toxic and eco-friendly. These surfactants consist of polar heads which are not electrically charged and are soluble in water due to the formation of hydrogen bonding.24 The structure and classification of various surfactants are given in Fig. 1. Numerous drugs can form micelles-like surfactants in an aqueous or non-aqueous medium. In high doses, such drugs can associate with different parts of the body which may cause a harmful effect. To reduce the such effect, micellar solubilisation using surfactants is the most effective method for hydrophobic drugs because it decreases the contact of drugs with enzymes like inactivating species and hence reduces the side effects of drugs.25 The worm-like micelles of biocompatible or biodegradable surfactants have wide applications in drug delivery systems.26 Surfactants play a critical role in the formation of colloidal-sized micelles in solution, which has particular significance in pharmacy due to their ability to enhance the solubility of sparingly soluble drugs in water & finally increases the bioavailability of drugs. From the literature survey, it has been found that lower doses of the surfactant increased absorption of the drug by altering the permeability of the membrane but higher doses decreased absorption due to the unavailability of the drug molecules entrapped in the micelles. This review gives information about the interaction between drugs and surfactants to explain the solution properties like conductivity, surface tension, viscosity, density, and spectroscopic analysis. The discussed drugs and surfactants along with their structure are listed in Tables 1 and 2.
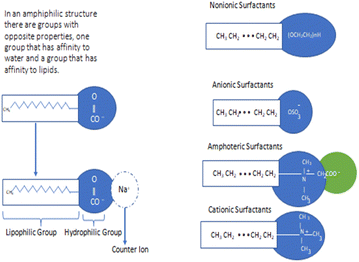 | ||
| Fig. 1 Structure and classification of surfactants. This figure is reproduced from ref. 6 with permission from Elsevier, Copyright 2017. | ||
| SN | Name of compounds | Structure | Ref. |
|---|---|---|---|
| 1. | Moxifloxacin hydrochloride | 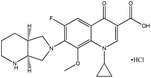 |
2 |
| 2. | Cefepime | 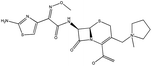 |
11 |
| 3. | Ciprofloxacin hydrochloride | 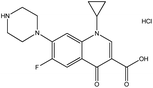 |
28 |
| 4. | Levofloxacin | 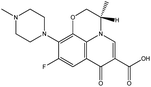 |
29 |
| 5. | Promethazine hydrochloride | 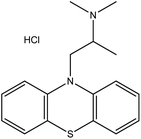 |
30 |
| 6. | Cephalexin monohydrate | 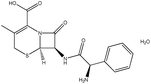 |
31 |
| 7. | Lomefloxacin hydrochloride | 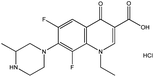 |
32 |
| 8. | Rifampicin | 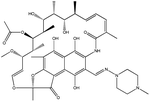 |
35 |
| 9. | Paracetamol | 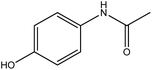 |
36 |
| 10. | Diphen hydramine hydrochloride | 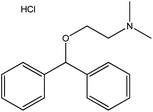 |
37 |
| 11. | Tetracaine hydrochloride | 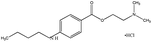 |
47 |
| 12. | Routine trihydrate | 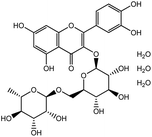 |
48 |
| 13. | Sparfloxacin | 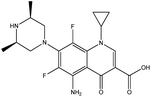 |
49 |
| 14. | Ceftriaxone sodium trihydrate | 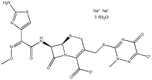 |
54 |
| 15. | Cefadroxil monohydrate | 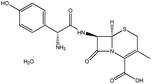 |
55 |
| 16. | Promethazine hydrochloride | 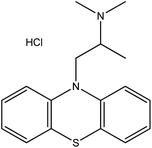 |
30 |
| 17. | Metformin hydrochloride | 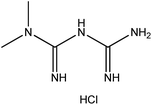 |
61 |
| 18. | Cefixime trihydrate | 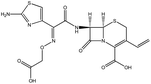 |
67 |
| 19. | Ibuprofen |  |
70 |
| 20. | Amitriptyline hydrochloride |  |
75 |
| 21. | Terazosin |  |
83 |
| 22. | Carvedilol phosphate |  |
85 |
| 23. | Itraconazole | 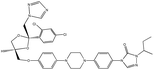 |
87 |
| 24. | Isoniazid | 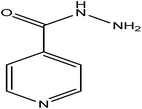 |
90 |
| 25. | Mitoxantrone | 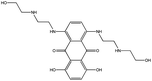 |
92 |
| SN | Name of surfactants | Structure of surfactants | Ref. |
|---|---|---|---|
| 1. | Cetylpyridinium chloride (CPC) | 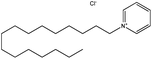 |
66 |
| 2. | Sodium dodecyl sulfate (SDS) |  |
29 |
| 3. | Cetyltrimethylammonium bromide (CTAB) | 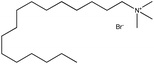 |
31 |
| 4. | Tetradecyltrimethylammonium bromide (TTAB) | 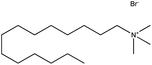 |
30 |
| 5. | Sodium lauroyl sarcosinate (SLS) | 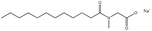 |
35 |
| 6. | Triton X-100 | 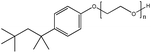 |
39 |
| 7. | Brij 35 | 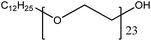 |
|
| 8. | Dodecyltrimethylammonium bromide (DTAB) | 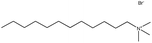 |
39 |
| 9. | Dioctyl sodium sulfosuccinate (AOT) | 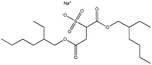 |
47 |
It also deals with the effect of external parameters like temperature, salt, solvent, and pH on drugs–surfactant interaction. The positive aspects of drug–surfactant interaction are to increase its bioavailability, easily solubilise, etc. which has been explained above as well. There is not much literature about its negative aspects whereas spectroscopic study suggests its limited interaction below cloud point only within specific temperature.27
2. Characterization of drugs–surfactants interactions
Surfactants can improve pharmaceutical physicochemical properties. Understanding drug-surfactant interactions plays a key role in enhancing therapeutic efficacy and their application in pharmaceuticals and other industries.Drug–surfactant interactions are influenced by a number of factors, including surfactant concentration, surfactant type, and protein structure. There are numerous characterisation methods or approaches that show the interactions between drugs and surfactants. Different strategies are covered in the subsections and the most popular ones are explained here.
2.1 Conductivity measurement
While the study of the micellization of ionic surfactant, the conductivity measurement is taken which helps to analyze the degree of dissociation which can be applied to obtain thermodynamic parameters of micellization. On the other hand, the non-ionic surfactants reflect phase separation on heating in the post-micellization region. Hence, the conductivity measurement technique is applied for the micellization of ionic surfactants and cloud point measurement for non-ionic surfactants.The study on conductivity, cloud point, and molecular dynamics simulations for the interaction of surfactants with ciprofloxacin hydrochloride (CFH) drug shows that the value of cloud point of Tween-80 (TW-80) surfactant and CFH mixture decreases with increase in CFH concentration. The micelle formation process for the TW-80 + CFH mixture was found to be spontaneous but the clouding phenomenon was non-spontaneous.28
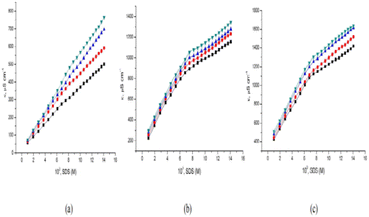
Bhardwaj et al.29 studied the sodium dodecyl sulfate (SDS) and levofloxacin drug interaction in the context of conductance study, CMC, and some thermodynamic parameters at different temperatures. The CMC value was found to be decreased in the presence of a drug. The value of specific conductance increases with an increase in SDS concentration at all temperatures but an increase in the concentration of the drug (levofloxacin) decreases the value of specific conductance (κ). Fig. 2 illustrates the plot depicting the specific conductivity of SDS in the presence of three distinct concentrations of levofloxacin. The presence of the drug causes a decrease in the CMC values when compared to the standard CMC value of SDS in water. The presence of different functional groups (–F– and –COOH–) on levofloxacin results in better interaction and therefore causes earlier micellization. Levofloxacin's added hydrophobicity causes a decrease in the CMC value of SDS. Such decrement of CMC of SDS in an aqueous medium of levofloxacin attributed to hydrogen bonding between the –H (COOH) of the drug & the –O (SO42−) of the SDS moiety, existence of hydrophobic interaction within the hydrophobic tail of the SDS and the hydrophobic group of the drug which contribute toward formation of micellization.
Since, the associate of factors (hydrophobic interaction and H-bonding) dominates the ion–ion (–COO– of drug and O– of SDS) and ion – hydrophilic (–COO– of drug and SO42− of SDS) cooperation during the process of micellization. The increment of CMC with an increment of temperature also affects the disruption of water structure toward the hydrophobic portion of the surfactant molecules which opposes the micelle formation leading to an increase in CMC values.
The effect of additives and temperature on the solution properties of TTAB was studied by Amin et al.30 by conductivity measurement and molecular dynamic simulations. The interaction between TTAB and promethazine hydrochloride (PMH) drug shows that the value of CMC of surfactant decreases with the addition of PMH drug. In this study, the influence of NaCl and Na2SO4 salts was also studied. The result shows that the value of CMC in the presence of sodium salts (CMCNaCl) is greater than that of sodium sulfate (CMCNa2SO4). It is because Na2SO4 easily creates a favorable condition for micellization than NaCl. Since the sulfate ion is a strong kosmotropic ion with high charge density than the chloride ion which has a low charge density & is called a chaotropic ion. Such ion has a higher capacity to break down the water molecule & reduces the aggregation of amphiphile monomers.
The interaction between cephalexin monohydrate (CLM) with the ionic surfactants CTAB & SDS, and non-ionic surfactant Triton X-100 was investigated by Hogue et al.31 by using conductivity measurement & spectroscopic techniques in H2O & H2O + NaCl media. The CMC values were found to decrease non-linearly with increasing concentrations of NaCl for all surfactant–drug mixtures. It was found that the addition of NaCl stabilizes the CLM-supported surfactant micelles. The process of decrement value of CMC with an increment of neutral salt (NaCl) is called the salting-out effect. Also, the CMC value of CLM–CTAB mixture in pure water decreases at the reduced temperature & gradually increases at increment temperature. The CMC values for CLM–SDS mixtures in an aqueous medium decrease with increasing temperature. In the case of ionic surfactants, like CTAB and SDS, they undergo almost complete ionization in solution, and hence specific conductivities (κ) were found to increase gradually as shown in Fig. 3. Since the inclusion of certain surfactants where κ increases to a specific level then distinct breakpoint was observed during the plot of κ versus the concentration of surfactant solution.
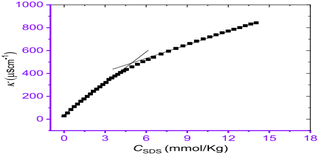 | ||
| Fig. 3 The concentration of SDS vs. specific conductivity (κ) of the CLM + SDS systems. This figure is reproduced from ref. 31 with permission from Elsevier, Copyright 2021. | ||
Conductivity and cloud point studies of the interaction of lomefloxacin hydrochloride (LMFH) drug with anionic surfactant SDS (Fig. 4) and non-ionic surfactant Triton X-100 in electrolyte media were observed in two CMC values for LMFH + SDS mixtures.32 The CMC of SDS increases with increasing LMFH concentration while the CMC values of LMFH & SDS mixtures were decreased by increasing the ionic strength of the electrolyte. Haq et al.33 reported that increment in temperature and concentration of surfactant, the conductivity of the surfactant solution was increased.
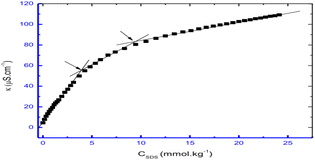 | ||
| Fig. 4 A plot between specific conductivity vs. concentration of SDS in 0.05 mmol kg−1 LMFH. This figure is reproduced from ref. 32 with permission from Elsevier, Copyright 2021. | ||
2.2 Surface tension measurement
If the concentration or temperature of the respective surfactant-based solution is increased then surface tension will be decreased. An increment in temperature influence toward breaking of the H-bond which influence the amphiphile molecule being more hydrophobic.34The interaction between amino acid-based surfactant, sodium lauryl sarcosinate (SLS), and sodium stearyl sarcosinate (SSS) with less water soluble drug rifampicin (RIF) on the physiochemical and rheological properties of methylcellulose (MC) surface active polymer system reveals different results.
The existence of a consistent amount of MC, two breakpoints was seen within surface tension vs. concentration of surfactant in Fig. 5, where the breakpoint at lower surfactant concentration reflects critical aggregation concentration (CAC) whereas the second breakpoint at higher concentration corresponds to CMC.
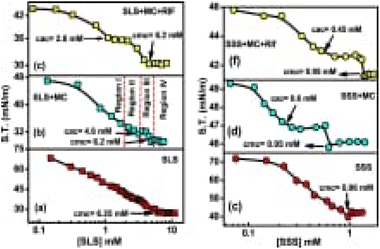 | ||
| Fig. 5 Plot between surface tension vs. concentration of surfactant (a) SLS (b) MC + SLS (c) MC + SLS + RIF (d) SSS (e) MC + SSS and (f) MC + SSS + RIF. This figure is reproduced from ref. 35 with permission from Elsevier, Copyright 2020. | ||
The initial addition of SLS/SSS to an aqueous MC solution reduces the surface tension due to the replacement of MC molecules by surfactant molecules. It is because surfactant molecules are much higher surface active than MC. In the presence of RIF-drug, the surface tension also decreases with the addition of surfactant to the mixture which is due to the replacement of the MC-RIF complex from the air–water interface. It is observed finally that, aggregation of polymeric chain presence at less concentration than CMC within surfactant solution where decrement within the presence of RIF due to dominant hydrophobic interactions.35
The interaction between hydrophilic drugs, paracetamol with the cationic surfactant CTAB in an aqueous media has been investigated by Nabi et al.36 using conductometric, tensiometric, and spectroscopic methods. In the aqueous phase, the surface tension decreases during the interaction between surfactant and drug which is due to the breakdown of hydrogen bonding at the surface and with the increased concentration of amphiphiles. It was found that the surfactant concentration was comparatively greater at the interface as compared to bulk due to adsorption.
The surface tension method was applied to investigate the values of CMC of single and mixed amphiphiles (diphenhydramine hydrochloride).37 It is observed that an increment of surface tension with an increment of amphiphile concentration in a specific condition. The possible value of CMC can be calculated via the breakpoint of γ vs. log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [C] graph. Since decrement of γ at the premicellar region is due to hydrophobic groups of amphiphile being able to have separation from the water and adsorbed at the surface. Since saturation is observed on the surface within amphiphile molecules, an increment within the concentration of amphiphiles forces them to aggregate into a micelle.
[C] graph. Since decrement of γ at the premicellar region is due to hydrophobic groups of amphiphile being able to have separation from the water and adsorbed at the surface. Since saturation is observed on the surface within amphiphile molecules, an increment within the concentration of amphiphiles forces them to aggregate into a micelle.
2.3 Viscosity measurement
The volumetric properties, viscosity coefficients & aggregation behaviour of binary mixtures of 1,8-diazabicyclo[5.4.0]undec-7-enium acetate (DBU-acetate), protic ionic liquid (PIL) with water–ethanol & acetonitrile was studied by Musale et al.38 The relative viscosity data of the DBU-acetate solution was studied by using the Jones–Dole equation for obtaining viscosity coefficients. Fig. 6 shows that variations in the viscosity behaviour of DBU-acetate in water, ethanol & acetonitrile solution were due to ion–dipole interaction between the ions & solvent. There was no significant increase in viscosity value at lower concentrations of PIL solution due to the solvation of ions but in the concentrated solution increasing ion–ion interactions results enhances the value of viscosity.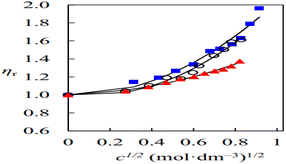 | ||
| Fig. 6 Plot between relative viscosity (ηr) vs. the square root of concentration (c1/2) of [DBUH][OAc] in water, ethanol, and acetonitrile. This figure is reproduced from ref. 38 with permission from Elsevier, Copyright 2020. | ||
Thummer et al.39 investigated the interaction of a tri-block co-polymer with various surfactants (SDS, DTAB, and TX-100) to form hydrophilic co-polymer surfactant complexes in aqueous media. The researchers used several techniques to measure the properties of these complexes, including surface tension, dynamic light scattering, and viscosity measurements at different temperatures in both water and NaCl solutions.
One of the techniques employed in the study was dilute solution viscosity measurements, which helped the researchers to obtain information on the hydrodynamic size of micellar aggregates in terms of intrinsic viscosities [η]. To do this, the Huggins equation was used.
Fig. 7 shows the variation of intrinsic viscosities and Huggins constant with the concentration of surfactants. The figure indicates that the intrinsic viscosity values increase as the surfactant concentration increases, regardless of whether the surfactant is ionic or non-ionic. On the other hand, the Huggins constant values show a decreasing trend as the surfactant concentration increases.
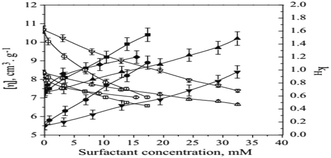 | ||
| Fig. 7 Intrinsic viscosity and Huggins constant versus temperature. This figure is reproduced from ref. 39 with permission from Elsevier, Copyright 2011. | ||
The intrinsic viscosity rate of copolymer-surfactant mixtures is determined by multiple factors, such as the size of the hydrophobic core and hydrophilic shell of the micelles, the number of unimers, the equilibrium constant of micelles ↔ unimer, and the amount of hydrated water in both micelles and unimers, as well as the hydrated water of surfactant micelles. When copolymer and surfactant are mixed, the resulting solution contains surfactant-bound unimers or micelles, and the mixed micelles have the maximum number of surfactant molecules. This leads to an increase in the hydrophilicity of the solution compared to pure copolymer micelles, resulting in high intrinsic viscosities.
Shakeel & Mohmood40 studied the volumetric, viscometric & aggregation properties of sodium valproate (SV) in an aqueous solution & also the interaction of the drug with CTAB. For the viscosity measurement of the SV solution, the Jones–Dole equation was used. The positive value of the Jones–Dole coefficient (J–D coefficient) for SV in an aqueous solution reflects structure promoting effect of the drug on water. While a decrease in the J–D coefficient with a rise in temperature shows the hydrophobicity of the solute. Gibbs free energy for the viscous flow of solution was found to be +ve & larger than the free energy of activation per mole of solvent which indicates the stronger interaction of the drug with solvent molecules in the ground state as compared to that in the transition state. It also represents the destruction & breakage of intermolecular bonds in the solvent structure. The positive value of molar activation enthalpy of viscous flow reflects the endothermic process & transition state within the involvement of breaking of bonds.
2.4 Density measurement
The density can affect the solubility and the stability of the drug-surfactant system, which in turn can affect the efficacy of the drug. At low densities, the drug-surfactant interaction can be weaker due to the increased availability of solvent molecules, leading to a higher degree of drug solubilisation.41 Kaur et al. discuss the effect of surfactant concentration and density on drug release from microemulsions. The authors found that increasing surfactant concentration and density can improve drug release, but beyond a certain point, further increases can lead to a decrease in drug release. The effect of surfactant density on the properties of drug-loaded nanoemulsion was studied by Khan et al.42 The authors found that increasing surfactant density led to increased stability and drug-loading capacity of the nano-emulsion.Sharma et al.43,44 studied the interactions between antibiotics and anti-histaminic drugs with electrolytes under pressure at various temperatures. The result shows that the value of density increases with a rise in the concentration of drugs in aqueous electrolytic solutions while the inverse effect of temperature on density is due to an increase in kinetic energy of the molecules & causes expansion on breaking the structure of water. When CTAB interacts with cefepime the value of either density or speed of sound increases, with an increase in the concentration of the drug monotonically within all temperatures. As per distinct observation, density is inversely proportional to the temperature but directly proportional to concentration at a specific temperature.11 Overall, the effect of density on drug-surfactant interactions can be complex and depends on a variety of factors, including the specific drugs and surfactants involved in the concentration of each component and the environmental conditions.
2.5 Spectroscopic studies
Various spectroscopic techniques are widely used in physical and analytical chemistry to obtain the information regarding detection, quantification, and identification of atoms and molecules.45 Proton nuclear magnetic resonance (1H NMR) helps to find out the structure of a compound by identifying the carbon–hydrogen framework of an organic compound.46 FTIR and mass spectroscopy along with 1H NMR gives total information about the chemical structure of the compound and also determine the exact position of a particular molecule in a compound.47,48 Thapa et al.49 explain the binding nature of the hydrophobic drug, tetracaine hydrochloride (TH) used as an organic counter-ion on ionic surfactants using UV-spectro-photometry. The binding constant (Kb) of TC in AOT & SDS surfactant was measured in the literature 49. The result shows that AOT has a higher Kb value with a double carbon chain while SDS has a single carbon chain with lower values of Kb (Fig. 8).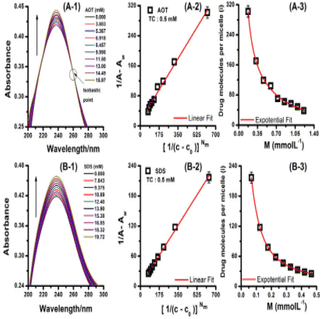 | ||
| Fig. 8 UV spectra of TC in the presence of AOT and SDS are displayed in plots A-1 and B-1, respectively. Plots A-2 and B-2 depict the TC-AOT and TC-SDS systems. The solid red line in these plots represents the linear fitting that was used to calculate Kb from the slope ratio and intercept. In plots A-3 and B-3, the variation of micelle mean occupancy (determined from absorbance data) with micelle concentration is shown for TC-AOT and TC-SDS. The solid red line in these plots represents the exponential fitting that was used to determine the total mean ion occupancy (i0) from the empirical equation. This figure is reproduced from ref. 49 with permission from Elsevier, Copyright 2021. | ||
Abbot and Sharma conducted a study50 to analyze the thermodynamic, acoustic, and spectroscopic parameters of rutin trihydrate with the cationic surfactant CTAB in hydro-ethanolic solvent systems at varying temperatures and concentrations. In the FTIR study, molecular interaction within CTAB & rutin trihydrate was observed within surfactant solution. Additionally, 1H NMR spectroscopic study suggests that the interaction occurred at the less hydrophobic region of CTAB. The spectra reflect the chemical shift of the CTAB molecule in Fig. 9. The resonance peaks at ∼3.26 ppm, ∼1.66 ppm, and 1.42 ppm, 3.04 ppm, 1.24 ppm, and ∼0.85 ppm reflect α, β, and γ –CH2– groups, [N + (CH3)3], bulkier –(CH2)12– group, (–CH3–) group respectively. So, the micellar structure of surfactant (CTAB) contains rutin trihydrate.
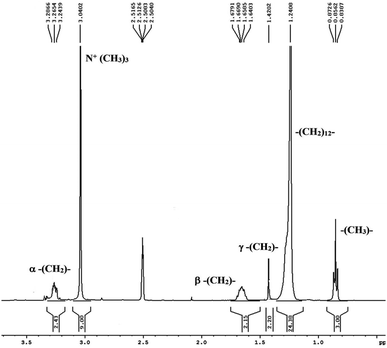 | ||
| Fig. 9 The 1H NMR spectrum of the CTAB molecule. This figure is reproduced from ref. 50 with permission from Elsevier, Copyright 2021. | ||
Sohail et al.51 studied the interaction between sparfloxacin (SPRF) solution & DTAB, & SDS by thermo-acoustic, spectroscopic & electrochemical investigation at various temperatures. The interaction between DTAB cation & SPRF anions reduces their mutual repulsion forces which favor the drug aggregation & hence the absorption of SPRF decreases with DTAB. In contrast, SPRF absorbance initially decreases and then rises with SDS. The self-association of drug molecules supported by the surfactant chain cause an initial decrease in absorbance. In the case of the premicellar region, the increase in absorbance is due to absorb light that is favored by the formation of premicellar aggregates while in the post-micellar region, the increase in absorbance is due to entrapped of drug molecules in the micelles, whose chromophores are directed near the surface & hence they can still absorb light greater than in the aqueous bulk solution.52
3. Effect of external parameters on drugs–surfactants interaction
The relationship between pharmaceuticals and surfactants to external factors has to be thoroughly studied for a variety of biological, pharmacological, and therapeutic purposes. Understanding these sorts of mechanisms and related properties, such as the degree of dissociation (α), critical micelle concentration (CMC), and thermodynamic parameters is critical for understanding the pharmacodynamics and pharmacokinetics of such a system. Since it is well established that variations in temperature, salt, solvent, and pH within human bodies have a substantial impact on the solubility, stability, and bioavailability of pharmaceuticals, thus, it is even more crucial for us to understand how these variables interact with the drug surfactant systems.3.1 Temperature
The value of CMC or XCMC in the CFH + CTAB drug surfactant system decreases as the temperature rises, passes the lowest value at certain temperature, and then begins to grow as the temperature rises further.53 The XCMC vs. T plots showed a nonlinearity pattern consistent with previous observations of nonlinearity or a minimum position in the CMC vs. T plots for several ionic amphiphiles,54 as shown in Fig. 10. The alteration in the type of hydration around the micelles can help explain how the values of CMC change as a result of temperature.55 Positive ΔH values in an aqueous medium for CFH and CTAB drug surfactant systems were found at 298.15 K temperature. However, it changes to a negative value at 303.15 K and higher, and as the temperature rises, the magnitude of these negative values likewise grows. The ΔG and ΔS for the mixed system, on the other hand, are negative and positive for all observed temperatures, as indicated in Table 3.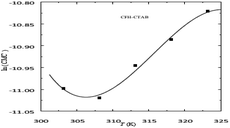 | ||
| Fig. 10 A plot between ln(CMC) vs. temperature for (CFH + CTAB) mixed system. This figure is reproduced from ref. 55 with permission from Elsevier, Copyright 2023. | ||
| System | Temp (K) | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1 K−1) | Ref. |
|---|---|---|---|---|---|
| CFH + CTAB | 298.15 | −48.60 | 3.47 | 174.64 | 53 |
| 303.15 | −49.50 | −9.14 | 140.16 | ||
| 308.15 | −49.10 | −20.94 | 115.38 | ||
| CFM + CTAB | 303.2 | −46.94 | −1.32 | 153.0 | 57 |
| 308.2 | −47.79 | −4.60 | 142.4 | ||
| 313.2 | −47.67 | −7.92 | 129.0 | ||
| CFM + SDS | 293.2 | −32.06 | 7.38 | 129.3 | |
| 303.2 | −31.76 | 5.51 | 121.9 | ||
| 313.2 | −33.46 | 3.36 | 112.4 | ||
| TTAB + PMH + NaCl | 300.55 | −43.75 | −12.52 | 103.89 | 30 |
| 305.55 | −44.18 | −13.73 | 99.65 | ||
| 310.55 | −44.99 | −15.14 | 96.12 | ||
| TTAB + PMH + Na2SO4 | 300.55 | −45.89 | 14.72 | 201.67 | |
| 305.55 | −46.61 | 19.36- | 215.90 | ||
| 310.55 | −45.89 | 23.53 | 223.52 | ||
| TTAB + CFH + NaCl | 295.15 | −42.76 | 8.11 | 172.37 | 69 |
| 300.15 | −43.07 | −0.94 | 140.38 | ||
| 305.15 | −44.91 | −10.68 | 112.15 | ||
| TTAB + CFT | 300.15 | −33.23 | −0.90 | 107.70 | 71 |
| 305.15 | −35.15 | 17.92 | 56.49 | ||
| 310.15 | −36.63 | −36.76 | −0.42 | ||
| TTAB + CFT + EtOH | 300.15 | −36.32 | 12.50 | 162.64 | |
| 305.15 | −36.17 | 12.35 | 159.00 | ||
| 310.15 | −37.01 | 12.51 | 159.66 | ||
| TTAB + LFH | 298.15 | −41.03 | −32.99 | 26.98 | 62 |
| 303.15 | −40.83 | −37.28 | 11.71 | ||
| 308.15 | −42.32 | −43.47 | −37.13 | ||
| TTAB + LFH + EtOH | 298.15 | −38.81 | −55.61 | −56.35 | |
| 303.15 | −39.22 | −55.76 | −54.53 | ||
| 308.15 | −37.83 | −52.30 | −46.96 |
In pure water, it was found that as the temperature was increased for the system of the drug (ceftriaxone sodium trihydrate) and surfactant (cetyltrimethylammonium bromide), the CMC values first increased, peaked, and then tended to decrease as the temperature increased, as shown in Fig. 10. The 20 °C range was wide enough, with the highest temperature being greater than that of body temperature, which is around 318.15 K. According to the pattern in the CMC vs. T plot when the combination is injected into the body, its CMC will be reduced at temperatures above and below 318.15 K (Fig. 11).56 At the beginning, the values of ΔH were first found to be negative (exothermic); however, the sign quickly flipped to positive (endothermic), and those values climbed as the temperature was increased. The value of ΔG was revealed to be negative, but the values of ΔS were found to be positive and it climbed gradually with increasing temperature (Fig. 11).
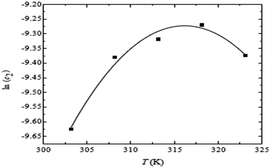 | ||
| Fig. 11 Plot of ln(CMC) versus T for (CFT + CTAB) mixed system containing 0.30 mol L−1 CFT in water. This figure is reproduced from ref. 56 with permission from Elsevier, Copyright 2016. | ||
Akhtar et al. studied two systems and found that the cefadroxil monohydrate drug and cationic surfactant mixed system (CFM + CTAB) had CMC values that first decreased with rising temperature but then continued to rise after reaching a minimum temperature (Tmin). Moreover, as shown in Fig. 12, the drug-anionic surfactant mixed system (CFM + SDS) showed that the CMC values decreased with rising temperature, mostly due to the breakdown of hydrophobic hydration that occurs around the hydrophobic chain of the surfactant.57 In the presence of CFM, positive ΔS values are seen for the mixed system, and the positive values decrease as the temperature increases. The ΔH values in this system are negative, and the negative ΔH values increase as the temperature rises. The (CFM + CTAB) systems' negative ΔH and positive ΔS findings indicate that electrostatic interactions are essential for the accumulation of the drug and the surfactant, in addition to hydrophobic contacts.58 The positive values of ΔS and ΔH for the (CFM + SDS) system decrease as the temperature increases. Thus, throughout the range of temperatures examined, and notably at lower ranges of temperature, the micellization of the (CFM + SDS) system is entropically controlled.59
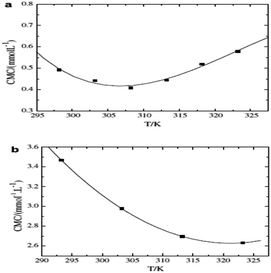 | ||
| Fig. 12 (a) Plot between CMC vs. tempr. for the CFM + CTAB and (b) CFM + SDS in the presence of 5.0 mM NaCl. This figure is reproduced from ref. 57 with permission from Elsevier, Copyright 2008. | ||
The CMC1 values increased gradually with temperature in the aqueous solution of the drug Moxifloxacin hydrochloride (MFH) and surfactant (TTAB) system, but in the case of CMC2, the values increased initially with temperature, peaked, and then decreased as the temperature increased. When the CMC of the drug–surfactant system was obtained in the form of mole fraction, the curve of ln(CMC) versus T was achieved to be nonlinear. With very few exceptions, the values of ΔH and ΔS for the systems were positive at each of the temperatures that were investigated. Although not always, their values declined as the temperature increased gradually. According to the parameters of ΔS and ΔH, the micellization phenomenon is only entropy controlled in drug surfactant systems of MFH-TTAB.2
When the temperature is increased in a CPC + BSA mixed system, the CMC or XCMC value drops to a certain temperature, passes the lowest value, but then gradually climbs as the temperature rises further (Fig. 13).60 All of the examined systems have negative values of ΔG, which shows that micellization events are thermodynamically spontaneous. The values of ΔH1m0 and ΔH2m0 for the drug surfactant system were negative at 298.15, 308.15, and 303.15 K, and the negative values tended to diminish as the temperature climbed from negative to positive (i.e., from exothermic to endothermic process). The data of ΔH and ΔS show that the relationships between the drug and surfactant are largely electrostatic and hydrophobic, with electrostatic interaction dominating at lower temperatures and hydrophobic interaction having a considerable impact at higher temperatures.
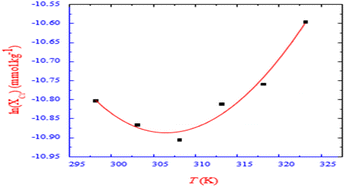 | ||
| Fig. 13 ln (XC1*) with tempr. for CPC + BSA system in the presence of 0.05 mmol kg−1 BSA in an aq. medium at different temperatures. This figure is reproduced from ref. 60 with permission from Elsevier, Copyright 2018. | ||
It could be observed that the CMC vs. T plot is nonlinear for both ionic and non-ionic surfactants. It is essential to understand that in a system containing both drugs and ionic surfactants, the CMC first decreases as temperature increases until it reaches a minimum, at which point it begins to increase as temperature increases further. However, in some circumstances, the value of CMC only rises as the temperature rises. Furthermore, in the case of the mixed system of drug and non-ionic surfactant, it is noted that at first, the CMC value rises with temperature and then falls at higher temperatures.
3.2 Salt
When various salts are present, the resulting CMC for the drug surfactant system is less in magnitude than for an aqueous medium, indicating that the addition of salt encourages the micellization of the drug surfactant system. In the presence of a certain salt concentration, the CMC values of the system exhibit the pattern CMCNaCl > CMCNa2SO4. The CMC values of the sulfate ion (SO42−) decrease more than those of the chloride ion because it is a strong kosmotropic ion with a charge density that is larger than the chloride ion. The obtained ΔG values for every system were negative, demonstrating the thermodynamic spontaneity of the association processes.61 The ΔH values are found to be negative for drug-surfactant systems in sodium chloride solution, suggesting that the process shows exothermic activity.62 However, TTAB and Promethazine hydrochloride (PMH) in Na2SO4 medium produced a positive standard enthalpy change ΔH, indicating that the method exhibits endothermic activity. With electrolytes present, the measured ΔS values for the TTAB + PMH combination are positive (Fig. 14).30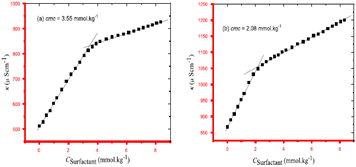 | ||
| Fig. 14 A plot between specific conductance vs. Csurfactant for TTAB + PMH mixture in (a) 3.02 mmol kg−1 NaCl and (b) 3.01 mmol kg−1 Na2SO4 at 310.55 K temperature. This figure is reproduced from ref. 30 with permission from Elsevier, Copyright 2020. | ||
The interactions of sodium dodecyl sulphate (SDS), cetyltrimethylammonium bromide (CTAB), and metformin hydrochloride (MNH) in the presence of NaCl have been investigated using the conductometric technique. The CMC values were dropped in the presence of NaCl, and they dropped even more sharply when the amount of NaCl was raised at a specific temperature.63 This effect is consistent with observations from other systems that include ionic surfactants when NaCl is present.64 The strong electrical repulsion between the ionic surfactant's positively charged head groups was much diminished, which was the main cause of the decline in CMC values.
The interactions between the surfactant cetylpyridinium chloride (CPC) and the drug polyvinyl pyrrolidone (PVP) were examined using the conductivity measurement method at various temperatures and in the presence of salts like NaCl and Na2SO4. In total, three different CMC values for the PVP and CPC mixed system were found, as indicated in Fig. 15. In every instance, it was discovered that the CMC for the PVP and CPC combination was higher in the aqueous solution compared to the presence of NaCl and Na2SO4. The CMC values of the drug surfactant system were found to be greater in an aqueous solution than in the presence of NaCl and Na2SO4 in all circumstances due to the repulsive interactions,65 which increased the diffusion coefficient and reduced the dimension of the micelles.66
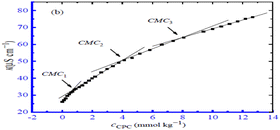 | ||
| Fig. 15 A plot of κ vs. concentration of CPC for the CPC + 0.1% (w/v) PVP mixture in 1.50 mmol kg−1 NaCl solution at 303.15 K. This figure is reproduced from ref. 66 with permission from MDPI, Copyright 2022. | ||
When non-ionic surfactant turbidity in solution becomes apparent to the unaided eye, it is referred to as a cloud. The cloud point (CP) is defined as the temperature at a certain point or even above it where phase separation occurs. Below a particularly critical point, the micelle in the solution remains in a single phase; however, above the CP, there is a decline in the solubility of the drug surfactant mixture in an aqueous solution, and the solution changes into a hazy diffusion. The CP of the cefixime trihydrate (CMT) drug and TW-80 surfactant mixture was reduced when various salts were included, compared to an aqueous media. The literature also reports a more pronounced decrease in CP values when Na2SO4 is present than when NaCl is present.67 The type of electrolyte may be able to regulate this effect of CP value lowering.68
The estimated CP values for Tw 80 + CFH mixed systems at various CFH/Tw 80 contents are profiled in Fig. 16a.28 Similar to this, the pattern of falling CP values in salt solutions was discovered in the series of CP (NaCl), CP (Na2SO4), and CP (Na3PO4) (Fig. 16b).28 The composition of added salt may regulate this impact of reduced CP levels. Additionally, the value of CMC of the surfactant TTAB and drug CFH combination in the sodium chloride solution was lower than those of the aqueous system in terms of magnitude. In surfactant systems, the presence of electrolytes results in lower electrostatic expulsion between charged head groups, which is accomplished by the counter ion particle (Fig. 17).69
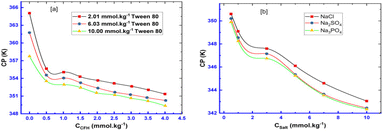 | ||
| Fig. 16 Plot of CP versus (a) CFH concentration for CFH + Tw 80 mixtures containing different Tw 80 concentrations (b) NaCl, Na2SO4 & Na3PO4 concentrations for 2.05 mmol kg−1 CFH + 6.03 mmol kg−1 Tw 80 mixtures. This figure is reproduced from ref. 28 with permission from Elsevier, Copyright 2021. | ||
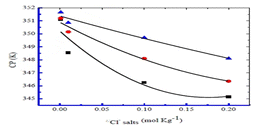 | ||
| Fig. 17 Plot between CP (K) vs. chloride concentrations for NaCl (■), KCl (●), and NH4Cl (▲), in 0.51 mmol kg−1 CMT mixed with 6.05, 6.04, and 6.06 mmol kg−1 TW-80, respectively. This figure is reproduced from ref. 69 with permission from Elsevier, Copyright 2020. | ||
Two mechanisms affect the CMC of the drug–surfactant mixed system. For the first method, the electrolytes change the property of water to act as a solvent, whereas, in the second, the counter ions interact with their head groups to induce the surface of the micelle to bind and/or diffuse the counter ions. In comparison to the aqueous medium, it has been shown that the CMC for the surfactant drug mixed system in an aqueous salt solution is lower. The decrease in CMCs is brought on by the weaker electrostatic interactions among the charged head of the amphiphiles.
3.3 Solvent
When alcohols were present, the CMC values for mixed systems of LFH + TTAB showed larger magnitudes (except ethanol at 298.15 K). Similarly, in the polyols solution, it is observed that the value of CMC for the drug surfactant system was less than those obtained in the absence of polyols.57 This demonstrated that the presence of alcohols harmed the micellization of the mixture, whereas polyols had a positive impact. The ionic surfactant head group experience more repulsion due to the presence of alcohol, which increases the CMC value both while alcohol is present and when the alcohol% increases (Fig. 18).70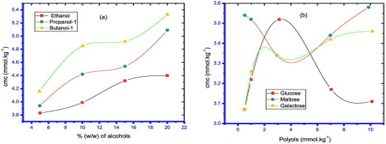 | ||
| Fig. 18 The plots representing the critical micelle concentration (CMC) of the mixed system of TTAB + LFH vs. concentration of (a) alcohols and (b) polyols at 303.15 K. This figure is reproduced from ref. 62 with permission from Elsevier, Copyright 2018. | ||
Examining the impact of alcohols on the interaction of the ceftriaxone sodium trihydrate with the surfactant revealed that adding more ethanol raised the values of CMC of the drug and surfactant combinations. Thus, CFT-TTAB mixture aggregation is prevented by the ethanolic solution. All measured ΔG values were negative for the ethanolic solution (C2H5OH), suggesting that stable aggregation formation is thermodynamically spontaneous. Furthermore, the drug and surfactant mixed system has positive ΔS and ΔH values in the C2H5OH solution, which suggests that the majority of their interactions are hydrophobic (Fig. 19).71
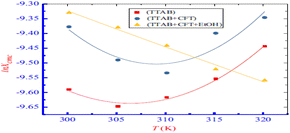 | ||
| Fig. 19 Plot between ln(CMC) with temperature for pure TTAB and drug + TTAB mixed system. This figure is reproduced from ref. 71 with permission from Elsevier, Copyright 2019. | ||
Abdul Rub et al.72 studied the interaction of sodium salt of ibuprofen and sodium taurocholate mixture in different media. Because urea is a strong protein denaturant, its hindrance to micellization properties suggest that drug surfactant mixed system have higher CMC values in urea than in an aqueous solution.73 Additionally, it has been suggested that urea has to stabilize properties, which causes aggregation to occur at higher concentrations.74 According to Dias et al.,75 the addition of urea causes H2O to become even more polar, which causes the urea and water mixture system to solvate polar and ionic groups more effectively than H2O alone. Urea has a stabilizing effect by reducing the repulsive contact between head groups owing to an increase in dielectric constant and better head group screening as a result of bigger dimensions of urea compared to those of water (Fig. 20).
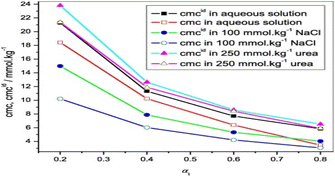 | ||
| Fig. 20 CMC vs. α1 of NaIBU-NaTaC. This figure is reproduced from ref. 72 with permission from Elsevier, Copyright 2018. | ||
Amitriptyline hydrochloride, an antidepressant, and a gemini surfactant (14-E2-14) were studied for their interaction using a variety of methods, including FT-IR, tensiometry, UV-visible spectroscopy, and fluorimetry in different distinct mediums. When compared to the aqueous system, the CMC values of drug surfactant combinations were reduced in the presence of sodium chloride solution, but in the presence of urea, there was an enhancement of CMC. In contrast to the aqueous solution, the Nagg was observed to rise in the presence of salt and fall in the presence of urea, which is because the addition of urea increases the surface area per head group.76 Greater Nagg in all solvents for the drug (AMT) and surfactant (14-E2-14) system proves that micellar growth is a result of a favourable synergistic relationship (Fig. 21).77
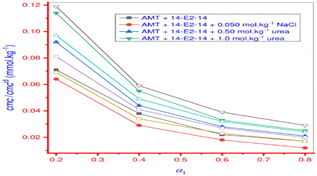 | ||
| Fig. 21 Plot between the change of CMC/CMCid value of AMT + 14-E2-14 mixed system vs. mole fraction (α1) of 14-E214. This figure is reproduced from ref. 77 with permission from PLoS One, Copyright 2020. | ||
The conductivity approach was used at different temperatures to understand the interaction of the drug amitriptyline hydrochloride and surfactant sodium dodecyl sulphate in an aqueous solution as well as in the presence of brine and urea. Urea raises the critical micelles concentration (CMC) because of an increase in the surface charges, but NaCl reduces the CMC value due to the salting-out action of the mixture. Furthermore, the degree of counter ion dissociation (α) value is increased in the involvement of urea, indicating that urea molecules are removing H2O molecules from the hydration sphere; the α values decrease in the availability of inorganic salt, indicating improved aggregation of molecules is a function of increasing charge density (Fig. 22).78
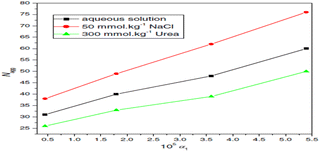 | ||
| Fig. 22 Relationship between the aggregation number (Nagg) and the mole fraction of SDS (α1) in mixed AMT + SDS systems in three distinct media. This figure is reproduced from ref. 78 with permission from Elsevier, Copyright 2016. | ||
The dielectric constant of the resultant EtOH-water mixed medium also decreases with adding EtOH to water, which facilitates an increase in repulsion between the head groups by reducing solvophobic interactions. It takes more heat energy to see the clouding effect as the mole fraction of surfactant rises. It is important to note that a concentration that is substantially higher than the critical micelle concentration (CMC) can still result in micellar development.79 When heated to higher compositions of EtOH above its boiling point, the system exhibits no clouding of AMT; however, at pH 6.7, the clouding is seen in mixed media containing up to 15 wt% of EtOH. This difference in behavior from a prior investigation using different solvents (ethylene glycol and glycerol) can explain by the difference in dielectric constant values.80 In EtOH–water mixed media, the degree of counterion dissociation (α) of AMT increases with % wt. of EtOH. The addition of 10% of GL & 15% of EG raises the value of α slightly but not in a regular trend.81 The critical micelle concentration (CMC) and cloud point (CP) values of the solution rise with the addition of solvents, with ethyl glycol–water (EG–WR) mixed media showing a more dramatic impact than glycerol (GL–WR) mixed media. The increased electrostatic repulsion between the drug-micelle head groups, accomplished by lowering the permittivity of the mixed fluids, is responsible for the increase in values.82
Different types of additives found in the body or utilized in drug formulation might influence drug surfactant interactions, which can affect the drug release rate. Thus, to achieve greater drug release and optimum drug activity, drug interaction with surfactant in the presence of solvent should be understood. The value of CMC of the drug surfactant mixture change according to the additive, based on the interaction of the mixed system with the solvent, and can either support or be a hindrance to micellization.
3.4 pH
The impact of altering the pH to see how terazosin reacts electrochemically, both with and without surfactant, was investigated.83 Terazosin's stated pKa value is 7.1 and is used to treat hypertension84 and alleviate the symptoms of urinary blockage in benign prostatic hyperplasia.85 The interaction of the drug both in the presence and absence of surfactant (sodium dodecyl sulphate) is shown in Fig. 23(a) and (b) respectively, in the pH of the B–R buffer. The highest and lowest oxidation current signals in both solutions were measured at pH 5.0 and pH 9.0, respectively. The fact that the oxidation peak potential is pH-dependent suggests that protonation and deprotonation are involved in the charge transfer process.86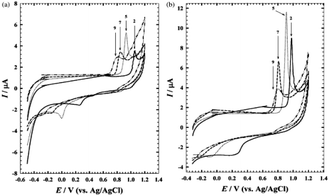 | ||
| Fig. 23 Effect of pH on the response of terazosin at GC electrode. This figure is reproduced from ref. 86 with permission from Elsevier, Copyright 2003. | ||
Chakraborty et al.87 investigated how pH affected the solubilization properties of several surfactants for carvedilol phosphate and found the following was the order of solubility increase at basic pH: CTAB > T80 > SDS > STC whereas in acidic pH, CTAB and T-80 also show a significant solubility improvement. The solubility of anionic surfactants in acidic media was delayed, except for SDS at pH 1.2. It was shown that cationic and non-ionic surfactants were successful in boosting the solubility of carvedilol phosphate, and this mixture may be employed to maintain the in vitro sink state in the primary dissolving medium. Sodium chloride was used to lower the ionic concentration of the media to 0.1 M.88 Fig. 24 shows the impact of the concentration of surfactant on the solubility of carvedilol phosphate at various pH levels.
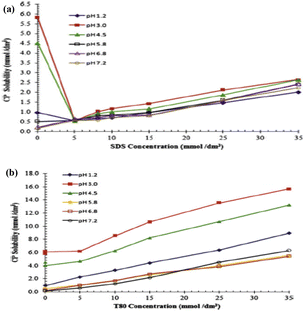 | ||
| Fig. 24 (a)Effect of concentration of SDS on the solubility of carvedilol phosphate in buffers of different pH. (b) Effect of concentration of T-80 on the solubility of carvedilol phosphate in buffers of different pH. This figure is reproduced from ref. 87 with permission from Elsevier, Copyright 2009. | ||
The maximum availability of the drug–surfactant mixture varies depending on the system pH. The appropriate pH range in the production of drug formulations, the physicochemical characteristics of itraconazole, and potential post-administration situations all had a role in the selection of these pH values.89 Itraconazole was significantly more soluble in all anionic surfactant solutions at pH = 3, increasing in the solution containing the surfactant C14SO4Na to a maximum of 3.6 g L−1 containing the surfactant C14SO4Na. The effects of pH on the other types of investigated surfactants, however, were considerably less pronounced: for non-ionic surfactants, itraconazole showed an increase in solubility somewhat at pH = 3 compared to pH = 6.5. There was either no impact or a minor decrease in solubility for the non-ionic surfactants, as shown in the following (Fig. 25).90
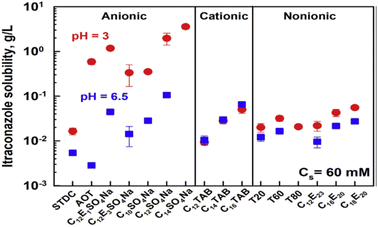 | ||
| Fig. 25 Solubility of itraconazole in surfactant solutions of 60 mM at pH values of 3 (represented by red circles) and 6.5 (represented by blue squares). This figure is reproduced from ref. 90 with permission from Elsevier, Copyright 2020. | ||
Rippie et al. compared the experimentally derived data of solubility and data collected by models at varying pH. Additionally, it became clear that the information projected by the suggested model was even more accurate if the concentration of surfactant was significantly large. At both pH 2.5 and pH 6.3, the solubility of flurbiprofen rises in a straight line in tandem with the added surfactant, as demonstrated in Fig. 26. When the medicine is largely ionized at pH 6.3, as opposed to when it is mostly unionized at pH 2.5, the solubility of the drug increases significantly.91
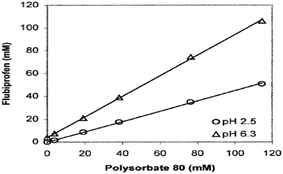 | ||
| Fig. 26 Solubility of flurbiprofen versus polysorbate 80 at different solution pH. This figure is reproduced from ref. 91 with permission from Wiley-Liss, Copyright 2003. | ||
It investigated how introducing surfactant to the drug (isoniazid) containing electrolytes affected the electrochemical reaction. It was found that the pH of the medium played a role in the increment of the current signal, which was brought on by the oxidation process. Particularly at low pH levels, it was found that the addition of anionic surfactant decreases the positive value of the anodic peak potential, whereas SOS exhibits the reverse tendency. Similarly, in the presence of cationic surfactants, specifically at pH 5.0, INH shows a consistent decrease in oxidation peak current. INH could be regarded as a positively charged species at low pH levels. As a result, there will be a greater likelihood of electrostatic contact between the positively charged medication and the negatively adsorbed surfactant coating.92 Furthermore, in the case of non-ionic surfactants, for the whole range of pH, there is a considerable decrease in current, although Triton X-405 shows a slight increase in current at pH 5.0 (Fig. 27).93
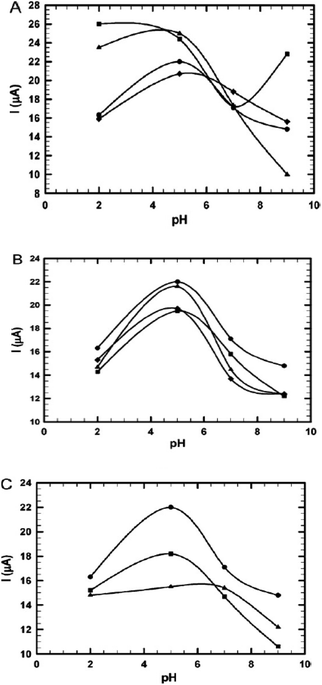 | ||
| Fig. 27 (A) The effect of pH on the oxidation peak current of INH (5 × 10−4 M) in the absence (●) and presence of SDS (■), SDBS (▲), and SOS (♦) (10−4 M). (B) The effect of pH on the oxidation peak current of INH (5 × 10−4 M) in the absence (●) and presence of CTAB (■), TMOB (▲), and CPB () (10−4 M). (C) The influence of pH on the oxidation peak current of INH (5 × 10−4 mol L−1) in the absence (●) and presence of Triton X-405(■) and albumin (▲) (10−4 mol L−1). This figure is reproduced from ref. 93 with permission from Elsevier, Copyright 2011. | ||
Thus, it may be inferred that the pH of the medium has a significant impact on how soluble the drug surfactant combination is. Also, the interaction of different surfactants and drugs with pH varies greatly based on the characteristics of the surfactant. Additionally, the electrolyte's pH had an impact on the values of current and oxidation peak potential, and the addition of surfactants changed these values based on the charge present in the polar group.
4. Applications of drug–surfactant interaction
The passage of the drug into the cellular level isn't easy due to complex tissue structure whereas interaction with surfactant micelles makes a suitable passway for the drug within bio-membranes. Surfactants' ability to self-assemble is a highly effective feature in drug delivery systems, which enhances the solubility and consequently the bioavailability of pharmaceutical products that are poorly soluble in aqueous solutions.94 The interaction of antitumor drugs (mitoxantrone) with anionic (SDS), cationic (CTAB), and non-ionic surfactants (Triton X-100, Tween-20, and Tween-80) reflects the disaggregation of antitumor drugs which has been aggregated to monomers and mitoxantrone is encapsulated in micelles in monomeric form shows antitumor action.95 The interesting thermodynamic result of the interaction of levofloxacin and SDS shows the decrement of CMC which reflects the hydrophobic interaction and spontaneous formation of micelles which is fruitful for pharmaceutical applications.29 The usability of exogenous surfactant is to carry pulmonary therapeutics for having intuitive sense which has lots of evidence that shows the positive approach of drug–surfactant interaction for lung treatment as well as having lots of therapeutic means. Lots of tools of discipline-related drugs, their design, etc. reflect the role of surfactants as a drug carrier within individual means. This makes the pathway toward clinical practices as well which has shown in given Fig. 28.96 In addition to their role in drug delivery systems, surfactants exhibit antimicrobial properties by inhibiting the growth and nourishment of various pathogenic microbes such as bacteria, fungi, algae, viruses, and others. This ensures that pharmaceutical preparations are free from harmful microbes.97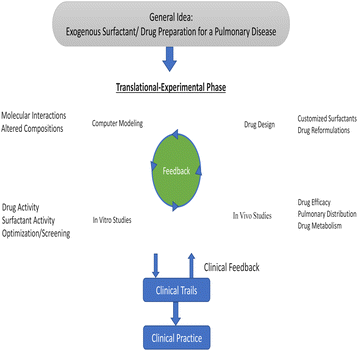 | ||
| Fig. 28 Schematic summary of surfactant–drug development. This figure is reproduced from ref. 96 with permission from Elsevier, Copyright 2019. | ||
In recent days, inhalable nanomedicines' interaction with a pulmonary surfactant is being known which seems to be may alter the pharmacokinetic and pharmacodynamic profiles of nanomedicines which show the needy approach of vivo treatment. Even, future research and development are also concerned about it.98 In recent days, endogenous components for drug formation have highly valued interest in where the interaction of pulmonary surfactant & surfactant proteins (SP) within extra- and intracellular membranes, proteins, or particles. SP plays a vital role in the drug delivery process, drug carrier, and target nanomedicine toward intracellular delivery. Since, SP lies in the lungs, tissues, and other organs present naturally.99 Nanoparticles are being engaged in bio-imaging, diagnosis, and their effect on the lungs and affect the circulatory system. Since PS makes surfactant binds NPs that determine this reflects the interaction of alveolar cells and delivery of NPs to the targeted cells during drug administration. Such binding recruits NP depend upon its behaviour which finally affects its functions or metabolism.100,101 Similarly, diversified application of drug surfactant has been carried out for pharmacological purposes. Here, cutting-edge scientific work on the applications of surfactant-based, polymer-based, and combination surfactant–polymer nanoparticle therapeutic formulations for use in the medical industry and drug delivery. Additionally, the scientific committee focuses on the systems' nature, attributes, physicochemical characteristics, characterization approaches, and pharmacokinetic behavior.102 Respiratory failure is a life-threatening complication of acute respiratory distress syndrome (ARDS). Nanoparticle drug delivery and surfactant-based drug carriers are possible techniques for delivering medicines to the wounded lung in ARDS, although there are no molecularly targeted pharmaceutical medications to prevent or treat ARDS despite several clinical trials.103 Since a variety of circumstances, including the drug's insolubility non a co-solvent, interactions with excipients, physiochemical characteristics, a sudden change in the environment's pH, compatibility issues with a surfactant, etc., can cause drug precipitation. Drug precipitation can take place in two stages: crystal development and nucleation.104 There are several ways to prevent precipitation, including the use of polymers, the inclusion of surfactants, adjusting drug loading and solubilising capacity, altering the environment's pH, etc.105 Nanotechnology-based carriers' capacity to entrap both hydrophilic and lipophilic medicines, improve ocular permeability, prolong residence duration, enhance drug stability, and increase bioavailability is the reason for their development.106 The consequences of the built-in nanocarriers can be predicted using a variety of in vitro, ex vivo, and in vivo characterization techniques.107 In terms of analytical means, mathematical models are being employed for theoretical analysis, which is a crucial gateway to elucidate the interactions present in the mixed systems of poorly soluble medicines and surface-active agents and identify the novel physical features.108 Surfactants are essential for creating and maintaining nano-emulsions. The drugs in emulsions can either be water-soluble or poorly water-soluble and the distribution of hydrophobic drugs in O/W emulsions is greater than in W/O emulsions. Hence, self-emulsifying systems (SES) are used to increase the solubility and bioavailability of poorly soluble drugs for oral delivery, by creating an isotropic mixture of oil, surfactant, and co-solvent.109
4.1 Future prospect
This review focused on the study of parameters like pH, density, conductivity, surface tension, and viscosity of drug & surfactant interaction which is a need of the medical industry in upcoming years. On the other hand, analytical study is a basic point for drug and surfactant development for further applied research. Upcoming studies should focus on cost analysis, technically feasible assessment, and drug engineering for reducing cost but increasing production. To connect experimental data analysis with real-world applications, a pilot project is needed.5. Conclusions
Drug surfactant interaction is a vital study where various parameters have been illustrated within this review. The conductivity and surface tension behave variable with the temperature & concentration with the variation of either drug or surfactant. In the case of drugs & ionic surfactants, the CMC is decreased with increment of temperature whereas CMC rises with temperature in the case of the non-ionic drug. A thermodynamic study has been carried out which shows negative Gibbs free energy and variation in enthalpy and entropy. In comparison to the aqueous medium, it has been shown that the CMC for the surfactant drug mixed system in an aqueous salt solution is lower than the electrolyte one. The value of CMC of the drug surfactant mixture change according to the additive, based on the interaction of the mixed system with the solvent, and can either support or be a hindrance to micellization. The pH of the medium has a significant impact on how soluble is the drug surfactant combination.Abbreviations
| CFH | Ciprofloxacin hydrochloride |
| CTAB | Cetyltrimethylammonium bromide |
| CFT | Ceftriaxone sodium trihydrate |
| CFM | Cefadroxil monohydrate |
| SDS | Sodium dodecyl sulfate |
| MFH | Moxifloxacin hydrochloride |
| TTAB | Tetradecyltrimethylammonium bromide |
| BSA | Biopolymer bovine serum albumin |
| CPC | Cetylpyridinium chloride |
| PMH | Promethazine hydrochloride |
| MNH | Metformin hydrochloride |
| CPC | Cetylpyridinium chloride |
| PVP | Polyvinylpyrrolidone |
| CP | Cloud point |
| Tw 80 | Tween 80 |
| CMT | Cefixime trihydrate |
| LFH | Levofloxacin hemihydrate |
| NaIBU | Sodium salt of ibuprofen |
| NaTaC | Sodium taurocholate |
| AMT | Amitriptyline hydrochloride |
| 14-E2-14 | Ethane-1,2-dial bis(N,N-dimethyl-N-tetradecyl ammonium acetoxy)dichloride |
| SOS | Sodium octyl sulfate |
| SDBS | Sodium dodecyl benzene sulphonate |
| TMOB | Trimethyloctylammonium bromide |
| CPB | Cetylpyridinium bromide |
Author contributions
The manuscript was prepared by Dilli Ram Pokhrel and Manish Kumar Sah. Bibaran Gautam, Hriday Kumar Basak, Ajaya Bhattarai and Abhik Chatterjee edited the manuscript. It was revised several times by Bibaran Gautam, Hriday Kumar Basak, Ajaya Bhattarai and Abhik Chatterjee.Conflicts of interest
There are no conflicts of interest.Acknowledgements
The authors would like to acknowledge Raiganj University, West Bengal, India for providing library access during the writing of this review paper. Ajaya Bhattarai acknowledges TWAS-UNESCO Associateship-ref. 3240321550 for providing opportunities to visit the Department of Chemistry, Indian Institute of Technology Madras, Chennai, India.References
- M. Rahman, S. J. Anwar, M. R. Molla, S. Rana, M. A. Hoque, M. A. Rub, M. A. Khan and D. Kumar, J. Mol. Liq., 2019, 292, 11322 CrossRef.
- S. Aktar, M. Robel Molla, S. Mahbub, M. Abdul Rub, M. A. Hoque and D. M. S. Islam, J. Dispersion Sci. Technol., 2018, 40, 574–586 CrossRef.
- M. A. Hoque, M. M. Rahman, S. Mahbub, M. Hossain, M. A. Khan, M. R. Amin, A. S. Alqahtani, M. Z. Ahmed, M. S. Alqahtani and O. M. Almarfadi, Korean J. Chem. Eng., 2021, 38, 1487–1498 CrossRef CAS.
- M. A. Rahim, S. Mahbub, M. K. Islam, S. M. A. Ahsan, S. Rana, M. A. Rub, A. Khan and M. A. Hoque, J. Surfactants Deterg., 2019, 23, 457–470 CrossRef.
- M. Abdul Rub and D. Kumar, J. Chem. Eng. Data, 2020, 65, 2659–2672 CrossRef CAS.
- K. Sakamoto, R. Y. Lochhead, H. I. Maibach and Y. Yamashita, Cosmetic science and technology: theoretical principles and applications, Elsevier, 2017, 854 Search PubMed.
- R. Sharma, D. Nandni and R. K. Mahajan, Colloids Surf., A, 2014, 451, 107–116 CrossRef CAS.
- T. M. Deshpande, H. Shi, J. Pietryka, S. W. Hoag and A. Medek, Mol. Pharm., 2018, 15, 962–974 CrossRef CAS PubMed.
- M. Abdul Rub, A. KoyaPulikkal, N. Azum and A. M. Asiri, J. Mol. Liq., 2022, 356, 118997 CrossRef CAS.
- J. Lalthlengliani, J. Gurung and A. K. Pulikkal, J. Mol. Liq., 2022, 354, 118823 CrossRef CAS.
- L. Pathania and S. Chauhan, J. Mol. Liq., 2020, 299, 112210 CrossRef CAS.
- J. H. Fendler and E. J. Fendler, Catalysis in micellar and macromolecular systems, Academic Press, New York, 1975 Search PubMed.
- D. Attwood and A. T. Florence, Surfactant systems. Their chemistry, pharmacy and biology, Chapman and Hall, New York, 1983 Search PubMed.
- M. Fresta, S. Guccione, A. R. Beccari, P. M. Furneri and G. Puglisi, Bioorg. Med. Chem., 2002, 10, 3871–3879 CrossRef CAS PubMed.
- J. N. Israelachvili, Intermolecular, and Surface Forces, John Wiley & Sons, New York, 2nd edn, 1995 Search PubMed.
- S. Mahbub, I. Shahriar, M. Iqfath, M. A. Hoque, M. A. Halim, M. A. Khan, M. A. Rub and A. M. Asiri, J. Environ. Chem. Eng., 2019, 7, 103364 CrossRef CAS.
- T. Farías, L. C. de Ménorval, J. Zajac and A. Rivera, Colloids Surf., A, 2009, 345, 51–57 CrossRef.
- S. Fayyaz, S. Ali, N. Khalid, A. Shah and F. Ullah, J. Surfactants Deterg., 2016, 19, 841–848 CrossRef CAS.
- F. A. Itoo, J. M. Mir, N. A. Malik and A. Ali, . King Saud Univ., 2020, 32, 2505–2512 CrossRef.
- K. Akhter, K. Ullah, R. Talat, A. Haider, N. Khalid, F. Ullah and S. Ali, Heliyon, 2019, 5, e01885 CrossRef PubMed.
- S. Schreier, S. V. P. Malheiros and E. de Paula, Biochim. Biophys. Acta, Biomembr., 2000, 1508, 210–234 CrossRef CAS PubMed.
- U. Saha, A. Banerjee and B. Das, J. Mol. Liq., 2020, 309, 113164 CrossRef CAS.
- J. Gurung, J. Anjudikkal and A. K. Pulikkal, J. Mol. Liq., 2020, 318, 114221 CrossRef CAS.
- M. A. Rub, N. Azum, D. Kumar, M. Nadeem Arshad, A. Khan, M. M. Alotaibi and A. M. Asiri, Polymers, 2021, 13, 4025 CrossRef CAS PubMed.
- D. Kumar and M. A. Rub, J. Mol. Liq., 2017, 238, 389–396 CrossRef CAS.
- M. Maswal and A. A. Dar, Colloids Surf., A, 2013, 436, 704–713 CrossRef CAS.
- N. M. Kovalchuk and M. J. H. Simmons, Adv. Colloid Interface Sci., 2023, 312, 102844 CrossRef CAS PubMed.
- M. A. Rahim, S. Mahbub, S. M. A. Ahsan, M. Alam, M. Saha, I. Shahriar, S. Rana, M. A. Halim, M. A. Hoque, D. Kumar and J. M. Khan, J. Mol. Liq., 2021, 322, 114683 CrossRef CAS.
- V. Bhardwaj, T. Bhardwaj, K. Sharma, A. Gupta, S. Chauhan, S. S. Cameotra, S. Sharma, R. Gupta and P. Sharma, RSC Adv., 2014, 4, 24935–24943 RSC.
- M. R. Amin, S. A. Alissa, M. Saha, J. Hossian, I. Shahriar, M. A. Halim, M. A. Hoque, Z. A. Alothman, S. M. Wabaidur and S. E. Kabir, J. Mol. Liq., 2020, 311, 113246 CrossRef CAS.
- M. A. Hoque, M. M. Rahman, M. M. Alam, S. Mahbub, M. A. Khan, D. Kumar, M. D. Albaqami and S. M. Wabaidur, J. Mol. Liq., 2021, 326, 115337 CrossRef CAS.
- S. M. Ali Ahsan, S. Mahbub, M. Ruhul Amin, J. Masood Khan, M. Anamul Hoque, A. Malik, A. Ahmed, M. Z. Ahmed and M. Khalid Anwer, J. Mol. Liq., 2021, 342, 116953 CrossRef.
- Z. U. Haq, N. Rehman, F. Ali, N. M. Khan and H. Ullah, Sains Malays., 2021, 46, 733–741 CrossRef.
- E. Mohajeri and G. D. Noudeh, e-J.Chem., 2012, 9, 2268–2274 CrossRef CAS.
- S. Afzal, M. S. Lone, M. Maswal and A. A. Dar, J. Mol. Liq., 2020, 319, 114353 CrossRef CAS.
- A. Nabi, S. Tasneem, C. G. Jesudason, V. S. Lee and S. B. M. Zain, J. Mol. Liq., 2018, 256, 100–107 CrossRef CAS.
- N. Azum, M. Abdul Rub, A. M. Asiri, H. M. Marwani and M. Akram, J. Saudi Chem. Soc., 2020, 24, 683–692 CrossRef CAS.
- S. P. Musale, P. S. Babalsure and D. D. Pawar, J. Mol. Liq., 2020, 319, 114197 CrossRef CAS.
- A. D. Thummar, N. V. Sastry, G. Verma and P. A. Hassan, Colloids Surf., A, 2011, 386, 54–64 CrossRef CAS.
- M. Shakeel and K. Mahmood, J. Mol. Liq., 2019, 285, 158–164 CrossRef CAS.
- P. Kaur, T. Garg and G. Rath, J. Dispersion Sci. Technol., 2015, 36, 612–625 Search PubMed.
- T. Hayat, M. Waqas, S. A. Shehzad and A. Alsaedi, J. Mol. Liq., 2016, 222, 181–187 CrossRef CAS.
- S. Sharma, K. Kumar and S. Chauhan, Chem. Data Collect., 2021, 34, 100745 CrossRef CAS.
- S. Sharma, K. Singh, S. Chauhan and K. Kumar, J. Mol. Liq., 2021, 342, 117481 CrossRef CAS.
- H. Schulz and M. Baranska, Vib. Spectrosc., 2007, 43, 13–25 CrossRef CAS.
- L. M. Jackman and S. Sternhell, International Series in Organic Chemistry, Elsevier, 2013 Search PubMed.
- N. Subramanian, N. Sundaraganesan and J. Jayabharathi, Spectrochim. Acta, Part A, 2010, 76, 259–269 CrossRef CAS PubMed.
- Y. Li, S. Laurent, L. Esser, L. V. Elst, R. N. Muller, A. B. Lowe, C. Boyer and T. P. Davis, Polym. Chem., 2014, 5, 2592–2601 RSC.
- U. Thapa, M. Kumar, R. Chaudhary, V. Singh, S. Singh and A. Srivastava, J. Mol. Liq., 2021, 335, 116564 CrossRef CAS.
- V. Abbot and P. Sharma, J. Mol. Liq., 2021, 328, 115489 CrossRef CAS.
- M. Sohail, H. M. U. Rahman and M. N. Asghar, J. Mol. Liq., 2021, 340, 117186 CrossRef CAS.
- M. F. Nazar, S. S. Shah and M. A. Khosa, J. Surfactants Deterg., 2010, 13, 529–537 CrossRef CAS.
- M. A. Hoque, M. M. Alam, M. R. Molla, S. Rana, M. A. Rub, M. A. Halim, M. A. Khan and F. Akhtar, Chin. J. Chem. Eng., 2018, 26, 159–167 CrossRef CAS.
- M. A. Hoque, M. A. Khan and M. D. Hossain, J. Chem. Thermodyn., 2013, 60, 71–75 CrossRef CAS.
- P. E. Ohale, C. A. Igwegbe, K. O. Iwuozor, E. C. Emenike, C. C. Obi and A. Białowiec, MethodsX, 2023, 10, 102180 CrossRef CAS PubMed.
- M. Rahman, M. A. Khan, M. A. Rub and M. A. Hoque, J. Mol. Liq., 2016, 223, 716–724 CrossRef CAS.
- F. Akhtar, M. A. Hoque and M. A. Khan, J. Chem. Thermodyn., 2008, 40, 1082–1086 CrossRef CAS.
- R. L. Reeves, R. S. Kaiser and H. W. Mark, J. Colloid Interface Sci., 1973, 45, 396–405 CrossRef CAS.
- S. Blokhina, A. Sharapova, M. Ol’khovich and G. Perlovich, J. Chem. Thermodyn., 2019, 132, 281–288 CrossRef CAS.
- M. A. Hoque, F. Ahmed, M. A. Halim, M. R. Molla, S. Rana, M. A. Rahman and M. A. Rub, J. Mol. Liq., 2018, 260, 121–130 CrossRef CAS.
- S. M. I. H. Sristy, S. Mahbub, M. M. Alam, S. M. Wabaidur, S. Rana, M. A. Hoque and M. A. Rub, J. Mol. Liq., 2019, 284, 12–22 CrossRef CAS.
- M. R. Amin, S. Mahbub, S. Hidayathulla, M. M. Alam, M. A. Hoque and M. A. Rub, J. Mol. Liq., 2018, 269, 417–425 CrossRef CAS.
- M. A. Hoque, M. M. Alam, M. A. Khan, D. Kumar, J. M. Khan, A. Malik, M. Z. Ahmed and A. Ahamed, J. Phys. Org. Chem., 2020, 34 Search PubMed.
- E. Dutkiewicz and A. Jakubowska, Colloid Polym. Sci., 2002, 280, 1009–1014 CrossRef CAS.
- D. Varade, T. Joshi, V. K. Aswal, P. S. Goyal, P. A. Hassan and P. Bahadur, Colloids Surf., A, 2005, 259, 95–101 CrossRef CAS.
- M. F. Ahmed, M. Abdul Rub, M. T. R. Joy, M. R. Molla, N. Azum and M. Anamul Hoque, Gels, 2022, 8, 62 CrossRef CAS PubMed.
- K. Shinoda and H. Takeda, J. Colloid Interface Sci., 1970, 32, 642–646 CrossRef CAS.
- E. Ritter, R. Racheva, S. Storm, S. Müller, T. Ingram and I. Smirnova, J. Chem. Eng. Data, 2016, 61, 1496–1501 CrossRef CAS.
- M. Rahman, M. A. Rub, M. A. Hoque, M. A. Khan and A. M. Asiri, J. Mol. Liq., 2020, 312, 113366 CrossRef CAS.
- B. Kumar, D. Tikariha, K. K. Ghosh and P. Quagliotto, J. Mol. Liq., 2012, 172, 81–87 CrossRef CAS.
- M. Rahman, S. J. Anwar, M. R. Molla, S. Rana, M. A. Hoque, M. A. Rub, M. A. Khan and D. Kumar, J. Mol. Liq., 2019, 292, 111322 CrossRef CAS.
- M. A. Rub, N. Azum, F. Khan and A. M. Asiri, J. Chem. Thermodyn., 2018, 121, 199–210 CrossRef.
- R. Jha and J. C. Ahluwalia, Faraday Trans., 1993, 89, 3465 RSC.
- M. J. Rosen, Surfactants and Interfacial Phenomena, 2004, pp. 303–331 Search PubMed.
- L. G. Dias, F. H. Florenzano, W. F. Reed, M. S. Baptista, S. M. B. Souza, E. B. Alvarez, H. Chaimovich, I. M. Cuccovia, C. L. C. Amaral, C. R. Brasil, L. S. Romsted and M. J. Politi, Langmuir, 2001, 18, 319–324 CrossRef.
- D. J. Jobe, V. C. Reinsborough and S. D. Wetmore, Langmuir, 1995, 11, 2476–2479 CrossRef CAS.
- M. Abdul Rub, PLoS One, 2020, 15, e0241300 CrossRef CAS PubMed.
- F. Khan, M. S. Sheikh, M. A. Rub, N. Azum and A. M. Asiri, J. Mol. Liq., 2016, 222, 1020–1030 CrossRef CAS.
- J. Gurung and A. K. Pulikkal, Chem. Eng. Commun., 2019, 207, 1462–1473 CrossRef.
- J. Gurung and A. K. Pulikkal, J. Chem. Eng. Data, 2018, 63, 3829–3838 CrossRef CAS.
- J. Gurung and A. K. Pulikkal, J. Chem. Eng. Data, 2019, 64, 4493–4500 CrossRef CAS.
- J. Gurung and P. A. Koya, ChemistrySelect, 2017, 2, 9193–9200 CrossRef CAS.
- M. M. Ghoneim, M. A. El Ries, E. Hammam and A. M. Beltagi, Talanta, 2004, 64, 703–710 CrossRef CAS PubMed.
- C. Hu, Q. He, Q. Li and S. Hu, Anal. Sci., 2004, 20, 1049–1054 CrossRef CAS PubMed.
- T. B. Lytle, S. J. Coles and M. A. Waite, J. Clin. Pharm. Ther., 1991, 16, 263–273 CrossRef CAS PubMed.
- N. Atta, S. Darwish, S. Khalil and A. Galal, Talanta, 2007, 72, 1438–1445 CrossRef CAS PubMed.
- S. Chakraborty, D. Shukla, A. Jain, B. Mishra and S. Singh, J. Colloid Interface Sci., 2009, 335, 242–249 CrossRef CAS PubMed.
- S. D. Mithani, V. Bakatselou, C. N. TenHoor and J. B. Dressman, Pharm. Res., 1996, 13, 163–167 CrossRef CAS PubMed.
- J. Peeters, P. Neeskens, J. P. Tollenaere, P. Van Remoortere and M. E. Brewster, J. Pharm. Sci., 2002, 91, 1414–1422 CrossRef CAS PubMed.
- Z. Vinarov, G. Gancheva, N. Burdzhiev and S. Tcholakova, J. Drug Delivery Sci. Technol., 2020, 57, 101688 CrossRef CAS.
- P. Li and L. Zhao, J. Pharm. Sci., 2003, 92, 951–956 CrossRef CAS PubMed.
- H. Zhou, C. Lengsfeld, D. J. Claffey, J. A. Ruth, B. Hybertson, T. W. Randolph, K. Ng and M. C. Manning, J. Pharm. Sci., 2002, 91, 1502–1511 CrossRef CAS PubMed.
- N. F. Atta, A. Galal, F. M. Abu-Attia and S. M. Azab, Electrochim. Acta, 2011, 56, 2510–2517 CrossRef CAS.
- B. Das, B. Kumar, W. Begum, A. Bhattarai, M. H. Mondal and B. Saha, Chem. Afr., 2022, 5, 459–480 CrossRef CAS.
- M. Enache, A. Toader and M. Enache, Molecules, 2016, 21, 1356 CrossRef PubMed.
- B. Baer, L. M. P. Souza, A. S. Pimentel and R. A. W. Veldhuizen, Biochem. Pharmacol., 2019, 164, 64–73 CrossRef CAS PubMed.
- M. Suhail, A. K. Janakiraman, A. Khan, A. Naeem and S. Faisal Badshah, J. Pharm. Pharmacol., 2019, 6, 72–82 Search PubMed.
- X. Pan, Z. Huang and C. Wu, Organ Specific Drug Delivery and Targeting to the Lungs, 2022, 109–114 Search PubMed.
- R. Guagliardo, J. Pérez-Gil, S. De Smedt and K. Raemdonck, J. Controlled Release, 2018, 291, 116–126 CrossRef CAS PubMed.
- C. Garcia-Mouton, A. Hidalgo, A. Cruz and J. Pérez-Gil, Eur. J. Pharm. Biopharm., 2019, 144, 230–243 CrossRef PubMed.
- R. Sheng, Q. Y. Ding, Z. H. Ren, D. N. Li, S. C. Fan, L. L. Cai, X. F. Quan, Y. Wang, M. T. Yi, Y. X. Zhang, Y. X. Cao, H. Wang, J. R. Wang, Q. H. Zhang and Z. B. Qian, J. Mol. Liq., 2021, 334, 116064 CrossRef CAS.
- O. Kontogiannis, D. Selianitis, N. Lagopati, N. Pippa, S. Pispas and M. Gazouli, Pharmaceutics, 2023, 15, 501 CrossRef CAS PubMed.
- Q. Fei, I. Bentley, S. N. Ghadiali and J. A. Englert, Pulm. Pharmacol. Ther., 2023, 79, 102196 CrossRef CAS PubMed.
- S. Ahmed, M. M. Amin and S. Sayed, AAPS PharmSciTech, 2023, 24, 66 CrossRef PubMed.
- S. Ahmed, M. M. Amin and S. Sayed, Drug Delivery, 2023, 30, 2179129 CrossRef PubMed.
- Y. Wu, Q. Tao, J. Xie, L. Lu, X. Xie, Y. Zhang and Y. Jin, Gels, 2023, 9, 292 CrossRef CAS PubMed.
- Y. Liu, G. Yang, Y. Hui, S. Ranaweera and C. Zhao, Small, 2022, 18, 2106580 CrossRef CAS PubMed.
- R. J. Wilson, Y. Li, G. Yang and C.-X. Zhao, Particuology, 2022, 64, 85–97 CrossRef CAS.
- T. C. Nguyen and H. Thai, Vietnam J. Sci. Technol., 2023, 61, 1–26 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |