Open Access Article
Open Access ArticleA palladium(0)–threonine complex immobilized on the surface of magnetic mesocellular foam: an efficient, stable, and magnetically separable nanocatalyst for Suzuki, Stille, and Heck cross-coupling reactions†
Zeinab Shirvandia,
Arash Ghorbani-Choghamarani *b and
Amin Rostami*a
*b and
Amin Rostami*a
aDepartment of Chemistry, Faculty of Science, University of Kurdistan, 66177-15175, Sanandaj, Iran. E-mail: a.rostami@uok.ac.ir
bDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, 6517838683 Hamedan, Iran. E-mail: a.ghorbani@basu.ac.ir; arashghch58@yahoo.com
First published on 12th June 2023
Abstract
In this study, a new palladium nanocatalyst was supported on L-threonine functionalized magnetic mesocellular silica foams (MMCF@Thr-Pd) and was characterized by FT-IR, XRD, BET, SEM, EDS, VSM, TGA, ICP-OES and elemental mapping techniques. The obtained MMCF@Thr-Pd performance can show excellent catalytic activity for Stille, Suzuki, and Heck coupling reactions, and the corresponding products were obtained with high yields. More importantly, the efficient and stable MMCF@Thr-Pd nanocatalyst was recovered by applying an external magnetic field and reused for at least five consecutive runs without a change in the catalytic activity.
Introduction
The carbon–carbon bond formation reaction is one of the basic and necessary reactions in organic synthesis, the pharmaceutical industry, and in current research.1,2 These reactions exist in the procurement of natural products, polymers, drugs, and pharmaceuticals compounds, herbicides, and industrially important materials.3–5 It is well known that Suzuki, Stille, and Heck reactions are efficient techniques for C–C bond formation owing to their good tolerance to different functional groups.6,7 Among the noble metals, palladium-based complexes are the most beneficial catalysts for carbon–carbon cross-coupling in organic transformations. The application of cross-coupling reactions depends on the activity and stability of the palladium complexes.8–10 The most representative palladium catalysts are palladium salts or homogenous complexes coordinated with various phosphine ligands, providing excellent catalytic activity.11–13 However, nothing is perfect, and this kind of homogeneous catalysis suffers from serious drawbacks, which limit their applications to a certain extent.14,15 The most important inconvenience found in homogeneous catalyst deals with the fact that the catalyst cannot be easily separated from the reaction mixture and reused.16–18 This also means that the desired isolated products are usually contaminated with heavy metal, which irremediably leads to negative environmental effects and increases the total cost of catalyzed synthesis.19,20 Additionally, commonly used palladium complexes and catalysts are expensive and not easily recyclable. To solve these issues, the heterogenization of homogeneous catalysts, especially heavy metal complexes, and expensive palladium nanoparticles on the surface of the organic and inorganic solid hosts, has been developed.21–23 Different supports such as metal oxides24 zeolites25 carbon materials26 and mesoporous silica27 are used for the immobilization of palladium nanoparticles. Among various supporters, magnetic nanoparticles have attracted great attention due to their inherent magnetic properties, low toxicity, easy synthesis, environmental-friendly, easy separation, and reusability in consecutive reactions.28,29 However, magnetic nanoparticles suffer from limited participation of available active sites, severe catalyst leaching, poor long-time dispersibility, and stability, which may reason new difficulties including low catalytic activity and selectivity.30,31 This defect can be minimized by using mesocellular silica foam (MCF), a mesoporous material consisting of spherical cells and windows. Their structure has large spherical cell pores (20–42 nm) interconnected with small cylindrical window pores (8–22 nm) to form a continuous three-dimensional (3D) porous structure.32,33 Because MCF silica nanostructure has a high surface area (500–1000 m2 g−1), large pore size, and large pore volume, a large number of active sites can be created on the inner walls of mesocellular foam channels by surface modification. Thus, MCF silica material is an appropriate candidate for use as support for catalysts, as drug delivery carriers, and as the stationary phase in chromatography.34 However, the problems in separation and pollution of the products by small particles of residual mesoporous silica catalysts severely limit their applications economically, catalytic, and industrial processes. Consequently, researchers have sought alternative catalytic systems that offer similar activity and selectivity, but are easier to separate and reuse.35,36 Thus, mesoporous magnetic nanocomposite has emerged as a family of new functional nanoparticles in recent years. Mesoporous magnetic nanocomposite has attracted wide attention owing to the combination of the prominent properties of mesoporous materials (high surface area and large pore size) and properties of magnetic nanoparticles (magnetic separation capability) in drug-targeted delivery, bioseparation, and new-generated catalysts.37,38L-Threonine (Thr) is an essential amino acid produced by yeasts on an industrial scale and has an extensive range of commercial applications, such as a food additive and agricultural feed supplement. Its structure consists of a carboxyl group, an α-amino group, and a side chain containing a hydroxyl group, which makes it a polar and uncharged amino acid. The N–H group of L-threonine, which may form a hydrogen bond with the O–H groups on a mesoporous magnetic surface, can help accelerate the immobilization process by being beside the carboxylic acid group.39 Also, functional groups on its side chains remain available to coordinate metal ions and form the final complex. For this reason, the amino acid L-threonine was used as a green, inexpensive, and efficient promoter for the synthesis of palladium catalysts.
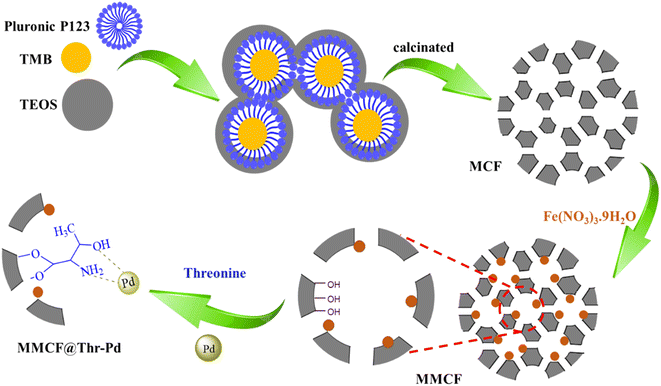
In the present work, γ-Fe2O3 magnetic nanoparticles were encapsulated using the convenient synthesis methodology into mesoporous channels of the MCF silica materials to form mesoporous magnetic nanocomposite and were used as the support to prepare palladium nanocatalyst (MMCF@Thr-Pd) (Scheme 1). Then MMCF@Thr-Pd was identified using different analytical techniques and its catalytic activity was studied as an effective, green, and recyclable nanocatalyst for carbon–carbon bond formation through Suzuki, Stille, and Heck reactions under mild and phosphine-free conditions.
Result and discussion
Catalyst characterizations
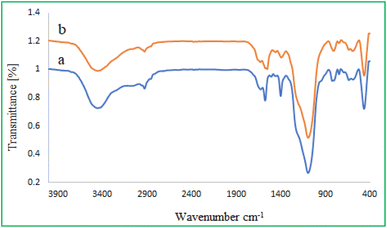
Fig. 1 displays the FT-IR spectrum of (a) MMCF, (b) M-MCF@Thr, and (c) MMCF@Thr-Pd. In all figures, the symmetric and asymmetric vibrations of Si–O–Si bonds at 468, 808, and 1087 cm−1 and the stretching vibration of Fe–O bonds at 584 and 638 cm−1 reveal the embedding of MNP nanoparticles into MCF. Also, the absorption band around 3460 cm−1 corresponds to the O–H stretching vibrations and is visible in all spectra. In Fig. 1b, two prominent bands at 2858 and 2927 cm−1 are observed, which are assigned to the aliphatic C–H stretching of amino acid L-threonine. The presence of absorption bands at 1396 cm−1 can correspond to C–N stretching vibrations, 1692 cm−1 and 1575 cm−1 are associated with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H vibrations, which confirm the successful attachment of the amino acid L-threonine on the surface of MMCF. In the case of Fig. 1c, we noted that the N–H stretching vibration bands of –NH2 shifted to a lower wave number (1575 cm−1 to 1550 cm−1). The above is indicated that the bonding interactions are formed between the functional groups of the amino acid L-threonine with palladium nanoparticles.
O and N–H vibrations, which confirm the successful attachment of the amino acid L-threonine on the surface of MMCF. In the case of Fig. 1c, we noted that the N–H stretching vibration bands of –NH2 shifted to a lower wave number (1575 cm−1 to 1550 cm−1). The above is indicated that the bonding interactions are formed between the functional groups of the amino acid L-threonine with palladium nanoparticles.
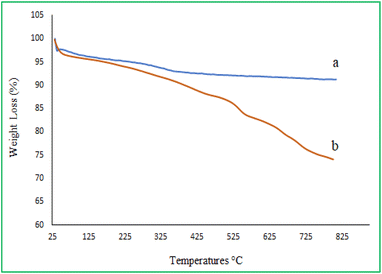
Thermal stability of (a) MMCF and (b) MMCF@ThrPd was determined using TGA analysis. As shown in Fig. 2a, the MMCF shows a 2.3% weight loss at around 200 °C due to the evaporation of physically adsorbed moisture. Also, between 200 and 500 °C, M-MCF loses about 3.5% of its weight due to the condensation process between the surface Si–OH groups. The thermogram of the MMCF@Thr-Pd shows two stages of weight loss (Fig. 2b). The weight loss (2%) below 200 °C may be due to the removal of moisture and organic solvent present on the surface of the MMCF@Thr-Pd nanocatalyst. The other weight loss (8.2%) between 200 and 655 °C may be due to the breakdown of L-threonine bonds and the degradation of the complex Pd coated on the surface of M-MCF nanoparticles. Thus, the above results show that the MMCF@Thr-Pd nanocatalyst has high thermal stability and is suitable for use in most organic reactions.
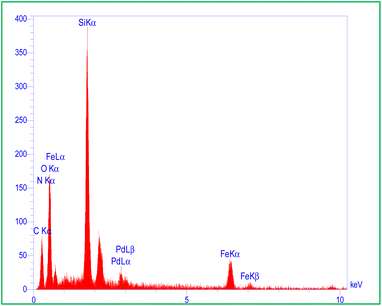
The elemental compositions of MMCF@Thr-Pd were determined by energy-dispersive X-ray (EDS) spectrum (Fig. 3) and their distributions were confirmed by the EDS mappings (Fig. 4). As shown in Fig. 3, the EDS analysis of MMCF@Thr-Pd nanoparticles verified the presence of Fe, C, Si, O, N, and Pd elements. Additionally, a homogeneous distribution of the elements was indicated by EDS mapping of MMCF@Thr-Pd (Fig. 4). Additionally, the amount of palladium loading on MMCF nanoparticles was determined by ICP-OES, which was found to be 1.59 mmol g−1.
The structure of the MMCF@Thr-Pd nanocatalyst was surveyed by scanning electron microscopy (SEM). SEM image of the MMCF@Thr-Pd nanocatalyst is presented in Fig. 5. As shown in the SEM images, the M-MCF@Thr-Pd nanoparticles have a uniform quasi-spherical morphology with diameters of about 15–25 nm.
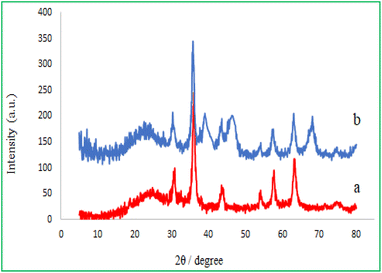
The high-angle XRD patterns of the M-MCF particles and MMCF@Thr-Pd nanocatalyst were recorded, and their diagrams are shown in Fig. 6. A broad peak in the range of 16–28 degrees was detected in both samples, which were assigned to amorphous silica. In addition, both samples show six diffraction peaks identical with 2θ = 30.3°, 35.8°, 43.4°, 53.8°, 57.3° and 63.0°, which correspond to the crystalline phase of γ-Fe2O3. This result means that the magnetic nanoparticles have been successfully placed within the MCF pore.40 In the XRD spectrum of MMCF@Thr-Pd nanocatalyst (Fig. 6b), the presence of all peaks related to γ-Fe2O3 nanoparticles confirmed that the coating of the palladium complex did not change the phase of γ-Fe2O3 nanoparticles. Also, three new peaks at 2θ = 40.1°, 46.6°, and 68.0° corresponding to Pd crystal plates were observed in the MMCF@Thr-Pd nanocatalyst spectrum,41 indicating that the Pd(0) complex was successfully immobilized on the surface of MMCF nanoparticles.
The magnetic measurements of the MMCF and MMCF@Thr-Pd were examined by a vibrating sample magnetometer (VSM) at room temperature in an applied magnetic field of up to 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe, and the magnetization curves are shown in Fig. 7. These samples show superparamagnetic behavior and have a saturation magnetization of 25.44 emu g−1 and 23.26 emu g−1, respectively. As can be seen, the saturation magnetization values in the MMCF@Thr-Pd nanocatalyst are reduced compared to the M-MCF nanoparticles. This is due to the loading of L-threonine and Pd nanoparticles on the surface of the M-MCF support.
000 Oe, and the magnetization curves are shown in Fig. 7. These samples show superparamagnetic behavior and have a saturation magnetization of 25.44 emu g−1 and 23.26 emu g−1, respectively. As can be seen, the saturation magnetization values in the MMCF@Thr-Pd nanocatalyst are reduced compared to the M-MCF nanoparticles. This is due to the loading of L-threonine and Pd nanoparticles on the surface of the M-MCF support.
The textural properties of the MMCF@Thr-Pd nanocatalyst studied using N2 adsorption/desorption analysis are reported in Table 1 and compared to a pure M-MCF. The N2 adsorption–desorption isotherms are shown in Fig. 8. As observed, the isotherms exhibit a type IV behavior, with a hysteresis loop, that is characteristic of mesoporous materials.42 The surface area, pore volume, window size, and cell size of M-MCF were 285.95 m2 g−1, 1.01 cm3 g−1, 15.01, and 25.85 nm, respectively. After the modification of MMCF with Pd complexes, the amount of N2 adsorbed decreased significantly, and accordingly, the surface area, pore volume, window size, and cell size changed to 168.89 m2 g−1, 0.57 cm3 g−1, 14.40, and 22.61 nm, respectively. These results confirm a fine coating of the Pd complex inside the magnetic mesocellular foams.
| Sample | BET surface area (m2 g−1) | Pore diameter by BJH method (nm) | Pore volume (cm3 g−1) | |
|---|---|---|---|---|
| Window (nm) | Cell (nm) | |||
| M-MCF | 285.95 | 15.01 | 25.85 | 1.01 |
| MMCF@Thr-Pd | 168.89 | 14.40 | 22.61 | 0.57 |
Catalytic studies
The catalytic property of the MMCF@Thr-Pd nanocatalyst was initially examined in the Suzuki cross-coupling reaction. The reaction of iodobenzene with phenylboronic acid in the presence of MMCF@Thr-Pd nanocatalyst was selected as the model reaction for optimization of different reaction conditions including base, solvent types, amount of catalyst, and temperature. As shown in Table 2, the model reaction was first tested using different solvents, among which EtOH was more appropriate for the Suzuki cross-coupling reaction and gave the highest yield (Table 2, entries 2–7). Then, different bases such as NaOH, KOH, Et3N, K2CO3, and Na2CO3 were screened for their effect on the reaction (Table 2, entries 8–11); it was found that K2CO3 with the yield is 95% more effective. Next, the effect of the catalyst amount was examined. With increasing the amount of catalyst, the yield of the desired product does not increase, but by decreasing the amount of MMCF@Thr-Pd nanocatalyst (Table 2, entries 9–13), the yield of the desired product decreases. Finally, the effect of different temperatures was investigated, and the results showed that 60 °C was more appropriate than other temperatures (Table 2, entries 14, 15). Next, we evaluated the Suzuki coupling reaction of iodobenzene with phenylboronic acid in the presence of the unsupported palladium complex (L-threonine ligand with Pd(0) ions: Thr-Pd) under optimized conditions (Table 2, entry 2). When the model reaction was carried out under homogeneous conditions, the product yield was 60% after 20 min (Table 2, entry 16). While for the MMCF@Thr-Pd heterogeneous catalyst under the same conditions, the product yield is up to 95% (Table 2, entry 2). According to these results, the large surface area and pores of the MMCF support facilitate the diffusion of reactants in the pores of the MMCF support to interact with the catalytic centers. On the other hand, the heterogeneous palladium complex with MMCF magnetic nanocomposite is an easy and efficient way to separate and recycle the palladium complex from the reaction mixture by an external magnetic field. Also, the model reaction was performed using MMCF@Pd nanoparticles under ligand-free and similar reaction conditions (Table 2, entry 17). In this case, the desired product yield is reduced to 45%. This result shows that the catalytic activity of the nanocatalyst is reduced in the absence of a ligand. The ligand is coordinated with palladium to activate the catalytic sites.| Entry | Catalyst (mmol) | Solvent | Base (3 mmol) | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: iodobenzene (1 mmol), phenylboronic acid (1 mmol), base (3 mmol), catalyst and solvent (2 mL).b Isolated yield.c Complex Thr-Pd as the catalyst.d MMCF@Pd as the catalyst. | ||||||
| 1 | — | EtOH | K2CO3 | 60 | 10 h | N.R |
| 2 | 0.0079 | EtOH | K2CO3 | 60 | 20 | 95 |
| 3 | 0.0079 | DMF | K2CO3 | 60 | 20 | 48 |
| 4 | 0.0079 | PEG200 | K2CO3 | 60 | 20 | 88 |
| 5 | 0.0079 | DMSO | K2CO3 | 60 | 20 | 40 |
| 6 | 0.0079 | H2O | K2CO3 | 60 | 20 | 70 |
| 7 | 0.0079 | Dioxane | K2CO3 | 60 | 20 | 38 |
| 8 | 0.0079 | EtOH | KOH | 60 | 20 | 88 |
| 9 | 0.0079 | EtOH | NaOH | 60 | 20 | 72 |
| 10 | 0.0079 | EtOH | Et3N | 60 | 20 | 45 |
| 11 | 0.0079 | EtOH | Na2CO3 | 60 | 20 | 78 |
| 12 | 0.0047 | EtOH | K2CO3 | 60 | 20 | 80 |
| 13 | 0.0111 | EtOH | K2CO3 | 60 | 20 | 96 |
| 14 | 0.0079 | EtOH | K2CO3 | 80 | 20 | 96 |
| 15 | 0.0079 | EtOH | K2CO3 | 40 | 20 | 58 |
| 16c | 0.0079 | EtOH | K2CO3 | 60 | 20 | 60 |
| 17d | 0.0079 | EtOH | K2CO3 | 60 | 20 | 45 |
With the optimized reactions conditions in hand (Table 2, entry 2), the substrate scope of the aryl halides and phenylboronic acid was studied in the presence of K2CO3 and 0.0079 mmol of MMCF@Thr-Pd in EtOH at 60 °C, and the results are summarized in Table 3. Different aryl halides containing electron-withdrawing and electron-donating groups reacted efficiently with phenylboronic acid, and excellent isolated yields and high TOF values were obtained.
| Entry | Ar–X | Product | Time (min) | Yielda,b (%) | TOF (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halides (1 mmol), phenylboronic acid (1 mmol), K2CO3 (3 mmol), M-MCF@Thr-Pd (0.0079 mmol), EtOH (2 mL) and 60 °C.b Isolated yield. | |||||
| 1 |  |
 |
20 | 95 | 360.75 |
| 2 |  |
 |
35 | 91 | 197.46 |
| 3 |  |
 |
180 | 86 | 36.28 |
| 4 |  |
 |
45 | 93 | 156.96 |
| 5 |  |
 |
80 | 93 | 88.29 |
| 6 |  |
 |
50 | 91 | 138.22 |
| 7 |  |
 |
35 | 95 | 206.14 |
| 8 |  |
 |
50 | 92 | 139.74 |
| 9 |  |
 |
50 | 90 | 136.70 |
| 10 |  |
 |
70 | 93 | 100.90 |
| 11 |  |
 |
80 | 92 | 87.34 |
| 12 |  |
 |
35 | 96 | 208.31 |
| 13 |  |
 |
90 | 92 | 77.63 |
| 14 |  |
 |
100 | 88 | 66.83 |
| 15 |  |
 |
180 | 75 | 31.64 |
Having demonstrated that the MMCF@Thr-Pd nanocatalyst was very effective for the Suzuki reaction, its activity was also examined in the Stille coupling reaction of various aryl halides with triphenyltin chloride. The reaction between iodobenzene and triphenyltin chloride was selected as a model coupling reaction. The model reaction was performed using different bases, such as K2CO3, Na2CO3, NaOH, KOH, and Et3N, and a series of solvents, such as EtOH, H2O, DMF, DMSO, PEG-200, and dioxane at the specific temperatures (Table 4, entries 5–13). In addition, the effect of the amount of catalyst on Stille coupling was also tested (Table 4, entries 2–4).
| Entry | Catalyst (mmol) | Solvent | Base (3 mmol) | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: iodobenzene (1 mmol), triphenyltin chloride (0.5 mmol), base (3 mmol), catalyst and solvent (2 mL).b Isolated yield. | ||||||
| 1 | — | PEG200 | K2CO3 | 80 | 10 h | N.R |
| 2 | 0.0111 | PEG200 | K2CO3 | 80 | 40 | 95 |
| 3 | 0.0079 | PEG200 | K2CO3 | 80 | 40 | 95 |
| 4 | 0.0047 | PEG200 | K2CO3 | 80 | 40 | 86 |
| 5 | 0.0079 | DMF | K2CO3 | 80 | 40 | 65 |
| 6 | 0.0079 | EtOH | K2CO3 | 80 | 40 | 78 |
| 7 | 0.0079 | DMSO | K2CO3 | 80 | 40 | 70 |
| 8 | 0.0079 | H2O | K2CO3 | 80 | 40 | 68 |
| 9 | 0.0079 | Dioxane | K2CO3 | 80 | 40 | 20 |
| 10 | 0.0079 | PEG200 | KOH | 80 | 40 | 70 |
| 11 | 0.0079 | PEG200 | NaOH | 80 | 40 | 64 |
| 12 | 0.0079 | PEG200 | Et3N | 80 | 40 | 55 |
| 13 | 0.0079 | PEG200 | Na2CO3 | 80 | 40 | 85 |
| 14 | 0.0079 | PEG200 | K2CO3 | 100 | 40 | 96 |
| 15 | 0.0079 | PEG200 | K2CO3 | 60 | 40 | 56 |
The optimal conditions of the Stille coupling reaction were presented in Table 4, entry 3. Based on the optimized reaction conditions, the generality of Stille coupling reactions between iodobenzene and triphenyltin chloride was examined in the presence of K2CO3 and 0.0079 mmol of MMCF@Thr-Pd at 80 °C in PEG-200, and the results are listed in Table 5.
| Entry | Ar–X | Product | Time (min) | Yielda,b (%) | TOF (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl halides (1 mmol), triphenyltin chloride (0.5 mmol), K2CO3 (3 mmol), M-MCF@Thr-Pd (0.0079 mmol), PEG200 (2 mL) and 80 °C.b Isolated yield. | |||||
| 1 |  |
 |
40 | 95 | 180.37 |
| 2 |  |
 |
120 | 90 | 56.96 |
| 3 |  |
 |
240 | 76 | 24.05 |
| 4 |  |
 |
60 | 92 | 116.45 |
| 5 |  |
 |
80 | 88 | 83.54 |
| 6 |  |
 |
90 | 90 | 75.94 |
| 7 |  |
 |
35 | 90 | 195.29 |
| 8 |  |
 |
55 | 89 | 122.89 |
| 9 |  |
 |
70 | 88 | 95.47 |
| 9 |  |
 |
90 | 85 | 71.72 |
| 11 |  |
 |
130 | 88 | 51.41 |
| 12 |  |
 |
65 | 90 | 105.16 |
| 13 |  |
 |
120 | 90 | 56.96 |
| 14 |  |
 |
150 | 88 | 44.55 |
| 15 |  |
 |
240 | 70 | 22.15 |
According to the above satisfactory results of Suzuki and Stille reactions, the activity of MMCF@Thr-Pd nanocatalyst for Heck reaction was also evaluated. The reaction between iodobenzene and n-butyl acrylate was chosen as a model reaction to determine optimal reaction conditions such as solvents, nature of the base, amount of the catalyst, and temperature (Table 6). Experimental results showed that the use of Na2CO3 as base and PEG-200 as solvent at 100 °C for 30 min gave the best result for the Heck reaction of iodobenzene and n-butyl acrylate (96%, Table 6, entry 3).
| Entry | Catalyst (mmol) | Solvent | Base (3 mmol) | Temp. (°C) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: iodobenzene (1 mmol), n-butyl acrylate (1.2 mmol), base (3 mmol), catalyst and solvent (2 mL).b Isolated yield. | ||||||
| 1 | — | PEG200 | Na2CO3 | 100 | 10 h | N.R |
| 2 | 0.0159 | PEG200 | Na2CO3 | 100 | 30 | 96 |
| 3 | 0.0127 | PEG200 | Na2CO3 | 100 | 30 | 96 |
| 4 | 0.0079 | PEG200 | Na2CO3 | 100 | 30 | 78 |
| 5 | 0.0047 | PEG200 | Na2CO3 | 100 | 30 | 54 |
| 6 | 0.0127 | DMF | Na2CO3 | 100 | 30 | 93 |
| 7 | 0.0127 | DMSO | Na2CO3 | 100 | 30 | 80 |
| 8 | 0.0127 | Dioxane | Na2CO3 | 100 | 30 | 30 |
| 9 | 0.0127 | PEG200 | KOH | 80 | 30 | 55 |
| 10 | 0.0127 | PEG200 | NaOH | 80 | 30 | 60 |
| 11 | 0.0127 | PEG200 | Et3N | 80 | 30 | 74 |
| 12 | 0.0127 | PEG200 | K2CO3 | 80 | 30 | 88 |
| 13 | 0.0127 | PEG200 | Na2CO3 | 80 | 30 | 65 |
| 14 | 0.0127 | PEG200 | Na2CO3 | 60 | 30 | 97 |
Using the optimal reaction conditions (Table 6, entry 3)., the scope of this catalytic system in the Heck reaction was extended to the reaction of n-butyl acrylate, acrylonitrile and phenyl styrene with aryl halides having various electron-withdrawing and electron-donating substituents. As shown in Table 7, aryl iodides and activated aryl bromides reacted well and generated the desired products in good to excellent yields (65–96%) and high TOF. It should be mentioned that when chlorobenzene was used as the substrate, a good yield was obtained (70%) (Table 7, entry 3).
| Entry | Ar–X | Alkene | Product | Time (min) | Yield (%)a,b | TOF (h−1) |
|---|---|---|---|---|---|---|
| a Reaction conditions: aryl halides (1 mmol), alkene (1.2 mmol), Na2CO3 (3 mmol), M-MCF@Thr-Pd (0.0127 mmol), PEG200 (2 mL) and 100 °C.b Isolated yield. | ||||||
| 1 |  |
Butyl acrylate |  |
30 | 96 | 151.18 |
| 2 |  |
Butyl acrylate |  |
60 | 87 | 68.50 |
| 3 |  |
Butyl acrylate |  |
240 | 70 | 13.77 |
| 4 |  |
Butyl acrylate |  |
35 | 95 | 128.23 |
| 5 |  |
Butyl acrylate |  |
50 | 88 | 83.14 |
| 6 |  |
Butyl acrylate |  |
50 | 91 | 85.98 |
| 7 |  |
Butyl acrylate |  |
30 | 90 | 141.73 |
| 8 |  |
Butyl acrylate |  |
60 | 88 | 69.29 |
| 9 |  |
Butyl acrylate |  |
40 | 85 | 100.39 |
| 10 |  |
Butyl acrylate |  |
75 | 92 | 57.95 |
| 11 |  |
Butyl acrylate |  |
70 | 90 | 60.74 |
| 12 |  |
Butyl acrylate |  |
110 | 85 | 36.50 |
| 13 |  |
Acrylonitrile |  |
180 | 90 | 23.62 |
| 14 |  |
Acrylonitrile |  |
270 | 85 | 14.87 |
| 15 |  |
Acrylonitrile |  |
330 | 78 | 11.16 |
| 16 |  |
Phenyl styrene |  |
360 | 85 | 11.15 |
| 17 |  |
Phenyl styrene |  |
600 | 75 | 5.90 |
| 18 |  |
Phenyl styrene |  |
600 | 63 | 4.96 |
Reusability of the catalyst
An inherent advantage of heterogeneous catalysts over homogeneous catalysts, which is also economically feasible, is the reusability of expensive catalysts. Thus, the recyclability of this heterogeneous Pd catalyst was investigated in Suzuki, Stille, and Heck cross-coupling reactions and is shown in Fig. 9. The model reaction of iodobenzene with phenylboronic acid (Suzuki reaction), iodobenzene with triphenyltin chloride (Stille reaction), and iodobenzene with n-butyl acrylate (Heck reaction) was carried out under optimized reaction conditions. To reuse the catalyst in the next step, after the completion of the reaction, the palladium catalyst was separated easily using a simple magnetic magnet, washed with ethyl acetate, and dried under a vacuum. As the results showed (Fig. 9), the palladium catalyst was recycled and reused up to five times without a significant decrease in its catalytic activity.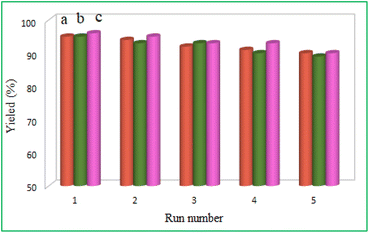 | ||
| Fig. 9 Reusability of the MMCF@Thr-Pd nanocatalyst in (a) Suzuki reaction, (b) Stille reaction, and (c) Heck reaction. | ||
The recovered catalyst that was used in the Suzuki reaction was identified by FT-IR spectrum (Fig. 10), VSM curve (Fig. 11) and SEM images (Fig. 12). The results showed that the recycled catalyst is similar to the fresh catalyst, which indicates the chemical stability of the MMCF@Thr-Pd nanocatalyst after recycling.
Hot filtration study
To investigate the heterogeneous nature of MMCF@Thr-Pd nanocatalyst, the reaction of n-butyl acrylate with iodobenzene was carried out under optimal conditions. After half of the reaction time (15 min), the reaction stopped and only 58% of the related product was obtained. The reaction was repeated and the catalyst was removed with a magnetic magnet at half of the reaction time, and the reaction mixture was allowed to react in the absence of the catalyst for another 15 min, and the reaction yield was 65%. During this period, no increase in product yield was observed and only 7% of the reaction of n-butyl acrylate with iodobenzene was obtained under conditions without palladium catalyst for another 15 min.Comparison of the catalyst
As a comparison, we investigated the catalytic activity of MMCF@Thr-Pd nanocatalyst compared to other Pd-based catalysts reported in the literature for Suzuki and Heck cross-coupling reactions. In Table 8, the results of the reaction of iodobenzene with phenylboronic (Table 8, entries 1–6) and the reaction of iodobenzene with n-butyl acrylate (Table 8, entries 7–11) are presented. As can be seen, the MMCF@Thr-Pd nanocatalyst showed higher reaction yields in lower reaction times compared to previously reported catalysts. Thermal stability, low cost, non-toxic, and easy magnetic separation are the other advantages of the new MMCF@Thr-Pd nanocatalyst.| Entry | Catalyst | Reaction condition | Time (min) | Yielda (%) | Ref |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | CA/Pd(0) (0.5–2.0 mol%) | Iodobenzene (1 mmol), phenylboronic acid (1 mmol), K2CO3 (2 mmol), water (10 mL), 100 °C | 120 | 94 | 43 |
| 2 | PdCl2 (0.05 mol%) | Iodobenzene (2 mmol), phenylboronic acid (2.4 mmol), L5 (0.1 mol%), Cs2CO3 (4 mmol), DMF (3 mL) | 120 | 95 | 44 |
| 3 | Pd/Au NPs (4.0 mol%) | Iodobenzene (1 mmol), Ar0B(OH)2 (1.1 mmol), K2CO3 (2 mmol), EtOH/H2O (25 mL), N2 atm, 80 °C | 24 h | 88 | 45 |
| 4 | PANI-Pd (0.022 mmol) | Iodobenzene (0.98 mmol), K2CO3 (1.96 mmol), dioxane–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), phenylboronic acid (1.17 mmol), 95 °C 1), phenylboronic acid (1.17 mmol), 95 °C |
240 | 91 | 46 |
| 5 | LDH-Pd(0) (0.03 g) | Iodobenzene (1 mmol), phenylboronic acid (1.5 mmol), K2CO3 (3 mmol), 1,4-dioxane–water (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) = 10 mL, 80 °C 1) = 10 mL, 80 °C |
600 | 96 | 47 |
| 6 | M-MCF@Thr-Pd (0.0079 mmol) | Iodobenzene (1 mmol), phenylboronic acid (1 mmol), K2CO3 (3 mmol), water (10 mL), 100 °C | 40 | 95 | This work |
| 7 | SiO2@Fe3O4-Pd (0.0050 mmol) | Iodobenzene (0.5 mmol), n-butyl acrylate (0.6 mmol), K2CO3 (1 mmol), EtOH (2 mL), 60 °C | 460 | 97 | 48 |
| 8 | Pd-Py-MCM-41 (3.12 mol%) | Iodobenzene (1 mmol), n-butyl acrylate (1.2 mmol), K2CO3 (3 mmol), DMF, 120 °C | 240 | 94 | 49 |
| 9 | MNP@NHC-Pd (0.56 mol%) | Iodobenzene (0.2 mmol), n-butyl acrylate (0.2 mmol), NaHCO3 (0.2 mmol), DMF (1 mL), 120 °C | 22 h | 86 | 50 |
| 10 | PdAS-V (5 × 10−5 mol equiv.) | Iodobenzene (1 mmol), n-butyl acrylate (1.5 mmol), Et3N (1.5 mol equiv.), toluene, 100 °C | 20 h | 98 | 51 |
| 11 | M-MCF@Thr-Pd (0.0127 mmol) | Iodobenzene (1 mmol), n-butyl acrylate (1.2 mmol), Na2CO3 (3 mmol), PEG-200 (2 mL), 100 °C | 30 | 96 | This work |
Conclusion
In this work, an L-threonine amino acid-modified MMCF magnetic nanocomposite, MMCF@Thr, was successfully synthesized and used as a solid support for the immobilization of palladium nanoparticles, MMCF@Thr-Pd. The nanocatalyst was fully characterized by various instrumental techniques and the high catalytic activities of this novel magnetic nanocomposite were investigated in Suzuki, Stille, and Heck cross-coupling reactions. All products were obtained in significant yields and appropriate TOF values. The performance of the catalyst was almost completely maintained during the reuse process and was easily reused at least five times. In addition, the new magnetic catalyst has high stability and is environmentally friendly.Experimental
MMCF preparation
Mesostructured cellular foam (MCF) silica, was prepared according to the reported procedure.52,53 Pluronic P123 (2 g) was added to a solution of hydrochloric acid (75 mL, 1.6 M) at room temperature. The solution was stirred by magnetic stirring until completely dissolved. Then, NH4F (23 mg) and TMB (2 g) were added to the solution. After stirring at 40 °C for 45 min, TEOS (4.4 g) was added to the mixture and the stirring continued at 40 °C for 20 h. The milky solution was transferred into an autoclave at 100 °C for 20 h. After that, the product was filtered and it was washed several times with deionized water, and dried in a vacuum at 50 °C. The solid product was calcined for 5 h at 550 °C to yield the white MCF silica.Magnetic γ-Fe2O3 nanoparticles were incorporated into the pores of mesocellular silica foams through modified procedures.42,54 For this purpose, Fe(NO3)3·9H2O (1.34 g) dissolved in methanol was added to foam silica (1 g). After the mixture was dried in an oven at 85 °C, propionic acid was added at 90 °C for 3 h to form the iron propionate complex. Then, the solid product was calcined for 30 min at 300 °C to produce MMCF.
Modification MMCF with Pd(0)–threonine complex
According to the similar procedure,40,55 the obtained MMCF (1 g) was well dispersed in deionized water (40 mL) under ultrasonication for 20 min. Afterward, L-threonine (0.39 g, 3.0 mmol) was added and stirred under reflux for 48 h under an nitrogen atmosphere. The product was then obtained by an external magnet, washed with water and ethanol, and dried at 50 °C for 12 h to give MMCF@Thr nanoparticles. Next, the MMCF@Thr (1 g) was ultrasonicated in 35 mL of ethanol, and Pd(OAc)2 (0.5 g) was added to the reaction mixture under the N2 atmosphere then refluxed for 20 h. Then 3.0 mmol (0.11 g) of NaBH4 was added to the reaction mixture and further stirred for 2 h to reduce palladium(II) to palladium(0). Finally, the obtained MMCF@Thr-Pd was collected with a magnet, washed with ethanol, and dried at 50 °C.General procedure for Suzuki reaction
Aryl halide (1.0 mmol), phenylboronic acid (1.0 mmol), MMCF@Thr-Pd nanocatalyst (5 mg, 0.0079 mmol), and K2CO3 (3.0 mmol) were combined in a glass flask containing 2 mL of ethanol without degassing. The mixture was magnetically stirred at 60 °C. The end of the reaction was monitored by TLC. After completion of the reaction, the MMCF@Thr-Pd nanocatalyst was separated by applying a magnetic field and extracted with water and ethyl acetate (3 × 10 mL). The combined organic layers were dried over anhydrous Na2SO4. The desired products were obtained in excellent yields.General procedure for Stille reaction
To a round-bottomed flask, PEG-200 (2 mL), K2CO3 (3.0 mmol), aryl halide (1.0 mmol), triphenyltin chloride (0.5 mmol), and MMCF@Thr-Pd nanocatalyst (5 mg, 0.0079 mmol) were added without degassing and stirred at 80 °C. After completion of the reaction (monitored by TLC), the MMCF@Thr-Pd nanocatalyst was separated with a magnet, and the product was extracted with water and ethyl acetate (3 × 10 mL). The combined organic phases were dried and concentrated to give the corresponding products in good to excellent yields.General procedure for Heck reaction
Alkene (1.2 mmol), aryl halide (1.0 mmol), Na2CO3 (3.0 mmol), the synthesized MMCF@Thr-Pd nanocatalyst (8 mg, 0.0127 mmol), and 2 mL of PEG-200 were added into a round-bottomed flask without degassing. The resulting mixture was mechanically stirred at 100 °C. TLC analysis was used to evaluate the reaction progress. After completion, the MMCF@Thr-Pd nanocatalyst was removed by an external magnet. The resultant organic phase was washed with distilled water and extracted with ethyl acetate (3 × 10 mL). The organic phase was dried over anhydrous Na2SO4. The desired products were obtained in good to excellent yields.Selected spectral data
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of this research by the University of Kurdistan and Bu-Ali Sina University.Notes and references
- M. Busch, M. D. Wodrich and C. Corminboeuf, ACS Catal., 2017, 7, 5643–5653 CrossRef CAS.
- A. Biffis, P. Centomo, A. Del Zotto and M. Zecca, Chem. Rev., 2018, 118, 2249–2295 CrossRef CAS PubMed.
- S. M. McAfee, J. S. McCahill, C. M. Macaulay, A. D. Hendsbee and G. C. Welch, RSC Adv., 2015, 5, 26097–26106 RSC.
- S. Kumari, S. Layek and D. D. Pathak, New J. Chem., 2017, 41, 5595–5604 RSC.
- A. Ogata, C. Furukawa, K. Sakurai, H. Iba, Y. Kitade and Y. Ueno, Bioorg. Med. Chem. Lett., 2010, 20, 7299–7302 CrossRef CAS.
- J. M. Richardson and C. W. Jones, J. Catal., 2007, 251, 80–93 CrossRef CAS.
- S. Jagtap, Catalysts, 2017, 7, 267 CrossRef.
- K. Zhan, P. Lu, J. Dong and X. Hou, Chin. Chem. Lett., 2020, 31, 1630–1634 CrossRef CAS.
- L. Jin, J. Qian, N. Sun, B. Hu, Z. Shen and X. Hu, Chem. Commun., 2018, 54, 5752–5755 RSC.
- H.-J. Ai, C.-X. Cai, X. Qi, J.-B. Peng, F. Zheng and X.-F. Wu, Tetrahedron Lett., 2017, 58, 3846–3850 CrossRef CAS.
- F. Rajabi and W. R. Thiel, Adv. Synth. Catal., 2014, 356, 1873–1877 CrossRef CAS.
- Y. Liu and X. Bai, Appl. Organomet. Chem., 2017, 31, e3561 CrossRef.
- B. Zhang, Z. Ye, M. Qin, Q. Wang, Y. Du, C. Qi and L. Shao, J. Appl. Polym. Sci., 2021, 138, 49666 CrossRef CAS.
- Q. Du, W. Zhang, H. Ma, J. Zheng, B. Zhou and Y. Li, Tetrahedron, 2012, 68, 3577–3584 CrossRef CAS.
- X. Chen, D. Qian, G. Xu, H. Xu, J. Dai and Y. Du, Colloids Surf., A, 2019, 573, 67–72 CrossRef CAS.
- M. Lamblin, L. Nassar-Hardy, J. C. Hierso, E. Fouquet and F. X. Felpin, Adv. Synth. Catal., 2010, 352, 33–79 CrossRef CAS.
- R. Ma, P. Yang and F. Bian, New J. Chem., 2018, 42, 4748–4756 RSC.
- B. Agrahari, S. Layek, R. Ganguly and D. D. Pathak, Inorg. Chim. Acta, 2018, 471, 345–354 CrossRef CAS.
- K. R. Balinge, A. G. Khiratkar and P. R. Bhagat, J. Organomet. Chem., 2018, 854, 131–139 CrossRef CAS.
- M. Nasrollahzadeh, N. Motahharifar, F. Ghorbannezhad, N. S. S. Bidgoli, T. Baran and R. S. Varma, Mol. Catal., 2020, 480, 110645 CrossRef CAS.
- A. Ohtaka, M. Kawase, S. Aihara, Y. Miyamoto, A. Terada, K. Nakamura, G. Hamasaka, Y. Uozumi, T. Shinagawa and O. Shimomura, ACS Omega, 2018, 3, 10066–10073 CrossRef CAS PubMed.
- Y. Wang, C. Lu, G. Yang, Z. Chen and J. Nie, React. Funct. Polym., 2017, 110, 38–46 CrossRef CAS.
- A. A. Ibrahim, A. Lin, M. S. Adly and M. S. El-Shall, J. Catal., 2020, 385, 194–203 CrossRef CAS.
- M. Hosseini-Sarvari, Z. Razmi and M. M. Doroodmand, Appl. Catal., A, 2014, 475, 477–486 CrossRef CAS.
- C. Liu, C. Cao, J. Liu, X. Wang, Y. Zhu and W. Song, J. Mater. Chem., 2017, 5, 17464–17469 RSC.
- R. Yu, R. Liu, J. Deng, M. Ran, N. Wang, W. Chu, Z. He, Z. Du, C. Jiang and W. Sun, Catal. Sci. Technol., 2018, 8, 1423–1434 RSC.
- S. Molaei and M. Ghadermazi, Microporous Mesoporous Mater., 2021, 319, 110990 CrossRef CAS.
- H. A. Elazab, A. R. Siamaki, S. Moussa, B. F. Gupton and M. S. El-Shall, Appl. Catal., A, 2015, 491, 58–69 CrossRef CAS.
- A. Ghorbani-Choghamarani and M. Norouzi, Appl. Organomet. Chem., 2016, 30, 140–147 CrossRef CAS.
- Q. Yue, J. Sun, Y. Kang and Y. Deng, Angew. Chem., 2020, 132, 15936–15949 CrossRef.
- F. Yang, X. Hu, S. Gao, X. Liu, S. Zhou and Y. Kong, J. Alloys Compd., 2020, 842, 155817 CrossRef CAS.
- L. Wei, Y. Zhao, Y. Zhang, C. Liu, J. Hong, Q. Xiao, H. Xiong and J. Li, ChemCatChem, 2017, 9, 3895–3903 CrossRef CAS.
- C. Xin, Y. Ren, Z. Zhang, L. Liu, X. Wang and J. Yang, ACS Omega, 2021, 6, 7739–7745 CrossRef CAS PubMed.
- K. Grzelak, M. Ziolek and M. Trejda, Catal. Commun., 2020, 142, 106045 CrossRef CAS.
- B. Zuo, Q. Deng, H. Shao, B. Cao, Y. Fan, W. Li and M. Huang, ACS Appl. Nano Mater., 2021, 4, 1831–1840 CrossRef CAS.
- A. Jha, C. R. Patil, A. C. Garade and C. V. Rode, Ind. Eng. Chem. Res., 2013, 52, 9803–9811 CrossRef CAS.
- M. T. P. da Silva, J. Villarroel-Rocha, C. F. Toncón-Leal, F. F. Barbosa, M. O. Miranda, M. A. M. Torres, K. Sapag, S. B. Pergher and T. P. Braga, Microporous Mesoporous Mater., 2021, 310, 110582 CrossRef CAS.
- S. Zhou, C. Song, W. Kong, B. Wang and Y. Kong, Appl. Surf. Sci., 2020, 527, 146853 CrossRef CAS.
- C. Petit, Y. Kim, S.-K. Lee, J. Brown, E. Larsen, D. R. Ronning, J.-W. Suh and C.-M. Kang, ACS Omega, 2018, 3, 1178–1186 CrossRef CAS PubMed.
- Z. Shirvandi, A. Rostami and A. Ghorbani-Choghamarani, Nanoscale Adv., 2022, 4, 2208–2223 RSC.
- M. Hajjami and Z. Shirvandi, J. Iran. Chem. Soc., 2020, 17, 1059–1072 CrossRef CAS.
- D. Lee, J. Lee, H. Lee, S. Jin, T. Hyeon and B. M. Kim, Adv. Synth. Catal., 2006, 348, 41–46 CrossRef CAS.
- V. W. Faria, D. G. Oliveira, M. H. Kurz, F. F. Gonçalves, C. W. Scheeren and G. R. Rosa, RSC Adv., 2014, 4, 13446–13452 RSC.
- S. J. Sabounchei and A. Hashemi, Inorg. Chem. Commun., 2014, 47, 123–127 CrossRef CAS.
- M. Nasrollahzadeh, A. Azarian, M. Maham and A. Ehsani, J. Ind. Eng. Chem., 2015, 21, 746–748 CrossRef CAS.
- H. A. Patel, A. L. Patel and A. V. Bedekar, Appl. Organomet. Chem., 2015, 29, 1–6 CrossRef CAS.
- S. Singha, M. Sahoo and K. Parida, Dalton Trans., 2011, 40, 7130–7132 RSC.
- P. Li, L. Wang, L. Zhang and G. W. Wang, Adv. Synth. Catal., 2012, 354, 1307–1318 CrossRef CAS.
- M. Nikoorazm, A. Ghorbani-Choghamarani and A. Jabbari, J. Porous Mater., 2016, 23, 967–975 CrossRef CAS.
- A. Z. Wilczewska and I. Misztalewska, Organometallics, 2014, 33, 5203–5208 CrossRef CAS.
- Y. M. Yamada, K. Takeda, H. Takahashi and S. Ikegami, Tetrahedron Lett., 2003, 44, 2379–2382 CrossRef CAS.
- L. Wei, Y. Zhao, Y. Zhang, C. Liu, J. Hong, H. Xiong and J. Li, J. Catal., 2016, 340, 205–218 CrossRef CAS.
- P. Schmidt-Winkel, W. W. Lukens, D. Zhao, P. Yang, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1999, 121, 254–255 CrossRef CAS.
- Z. Shokri, N. Azimi, S. Moradi and A. Rostami, Appl. Organomet. Chem., 2020, 34, e5899 CrossRef CAS.
- M. Hajjami, Z. Shirvandi and Z. Yousofvand, J. Porous Mater., 2017, 24, 1461–1472 CrossRef CAS.
- O. Diebolt, P. Braunstein, S. P. Nolan and C. S. Cazin, Chem. Commun., 2008, 3190–3192 RSC.
- M. C. Hong, M. C. Choi, Y. W. Chang, Y. Lee, J. Kim and H. Rhee, Adv. Synth. Catal., 2012, 354, 1257–1263 CrossRef CAS.
- A. Ghorbani-Choghamarani, B. Tahmasbi, R. H. Hudson and A. Heidari, Microporous Mesoporous Mater., 2019, 284, 366–377 CrossRef CAS.
- D. Liu, C. Zhang, F. Wang, Z. Huang, N. Zhang, H. Zhou and Y. Kuang, J. Mater. Chem., 2015, 3, 16583–16589 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02721j |
| This journal is © The Royal Society of Chemistry 2023 |