Open Access Article
Open Access ArticleNovel hydrophilic bistriazolyl-phenanthroline ligands with improved solubility and performance in An/Ln separations†
Yong Qiang Wan a,
Huaixin Hao
a,
Huaixin Hao b,
Lu Yu
b,
Lu Yu a,
Zhi Peng Wang*b and
Pavle Mocilac
a,
Zhi Peng Wang*b and
Pavle Mocilac *a
*a
aSchool of Nuclear Science and Technology, Lanzhou University, Lanzhou, China. E-mail: pavlem@lzu.edu.cn; Tel: +86 18719828130
bInstitute of Nuclear and New Energy Technology, Tsinghua University, Beijing, China. E-mail: wangzhipeng@mail.tsinghua.edu.cn; Tel: +86 18111567906
First published on 20th July 2023
Abstract
Two novel bistriazolyl-phenanthroline (BTrzPhen) ligands, bearing benzene-sulphonate (DS-BTrzPhen) and amino-acidic (DAA-BTrzPhen) hydrophilizing moieties were developed and found to be more soluble in aqueous acidic media with improved selectivity for Am(III) over Eu(III) in solvent extraction studies having SFEu/Am values reaching >300. The remarkable activities of both ligands suggest that BTrzPhen ligands are generally still worth exploring and improving.
Introduction
In the recent decade there has been significant development in the area of novel hydrophilic ligands for the innovative SANEX process (i-SANEX),1 currently the most attractive option for the industrial hydrometallurgical separations of minor actinides (An) from lanthanides (Ln) during the advanced reprocessing of nuclear high-level waste (HLW).2–4 These include sulfonated versions of proven heterocyclic ligands such as SO3-Ph-BTP,5 DS-BTBP, TS-BTBP, TS-BTPhen6 and DS-Ph-DAPhen7 as well as CHON compliant bistriazolyl-pyridines (PyTri ligands),8 bistriazolyl-bipyridines (BPTD)9 and bis-triazolyl-phenanthrolines (BTrzPhens).10Development of tetradentate BTrzPhen10 ligands was inspired by the equivalent tridentate water-soluble PyTri ligands.8 In fact, it was an attempt to emulate successful evolution from tridentate BTP ligands11 to the rigid and more selective tetradentate BTBP12 and BTPhen13 ligands that managed to overcome many drawbacks of tridentate BTP ligands. Unfortunately, the combination of triazolyl and phenanthroline moieties proved much less soluble in nitric acid solutions than the parent tridentate PyTri ligands: to be dissolved in aqueous media hydroxy-alkyl-BTrzPhen ligands required acidity of at least 0.33 M HNO3 while their SFEu/Am values were not larger than 47 with relatively high DAm values.10 Higher acidities diminished selectivity toward americium ion, while lower acidities decreased solubility of the BTrzPhen ligands. Therefore, it was obvious that some other variants of BTrzPhen ligands were required to ensure higher solubility in aqueous acidic solution.
In the meantime, successful attempts to develop other hydrophilic BTrzPhen ligands were lacking. On the other hand, there was the case of hydrophobic EH-BTzPhen ligand with bistriazolyl-phenanthroline molecular structure soluble in organic solvents, found to be effective in combination with 2-bromohexanoic acid synergist when 1-octanol was used as a diluent (SFEu/Am > 200).14 Additionally, hydrophilic bistriazolyl-bipyridines were developed: one study describes synthesis of water soluble bistriazolyl-bipyridines15 but so far there was no record of any solvent extraction studies performed using these ligands. Another study is describing bis-hydroxyl-bistriazolyl-bipyridines.9
In the light of these findings it is valid to raise a question: are BTrzPhen ligands truly promising or are they inherently inferior to other more established ligands?6–9 Theoretical studies by DFT modelling and calculations have determined thermodynamical parameters of BTrzPhen selectivity towards minor actinides,16 however, some of DFT theoretical studies suggest inherent flaws in the design of BTrzPhen ligands due to relatively smaller overall impact of triazolyl moieties.17 On the other hand, there is a possibility that the low solubility of the BTrzPhen complexing moiety in aqueous media is the key drawback responsible for their unsatisfactory performance, while better hydrophilizing terminal functional groups could mitigate this drawback. If BTrzPhen ligands prefer lower acidities, then new BTrzPhen ligands that are more soluble in mild nitric acid solutions should be developed; it should be investigated if increase of solubility of BTrzPhen ligands in mild acidic media also improves D and SF parameters rendering these ligands worth of further exploration.
In order to test this hypothesis, we have decided to design, synthesise, and test new BTrzPhen ligands having sulphonate or amino acidic hydrophilizing moieties. The first ligand is disodium 2,9-bis[1-(2-(4-sulfonatophenyl)ethyl)-1H-1,2,3-triazol-4-yl]-1,10-phenanthroline (DS-BTrzPhen, ligand 1, Fig. 1 and 2). As in the case of other similar water-soluble extractants sulphonate group is an important hydrophilizing moiety: introduction of sulphonate groups was the same straightforward strategy for many other hydrophilic ligands.5–7 It is important to note that all the ligands with sulphonate groups are not CHON compliant, and this may present an obstacle for some types of technological processes. Nevertheless, our aim is to investigate and uncover the efficiency of the BTrzPhen complexing moiety when fully soluble regardless of possible future applications. Therefore sulphonate-bearing ligand could be a good option for an experimental ligand since it can improve solubility in aqueous media.
The second ligand is 2,9-bis[1-(3-amino-3-carboxyl-propyl)-1H-1,2,3-triazol-4-yl]-1,10-phenanthroline, (DAA-BTrzPhen, ligand 2, Fig. 1 and 2). Comparing to the ligand 1 the ligand 2 bearing amino-acidic hydrophilizing terminal moieties is CHON compliant. Despite general rejection of amino group as hydrophilizing moiety for the An/Ln extracting ligands we have decided to develop BTrzPhen ligand hydrophilized with amino-acid groups: while they will ensure good solubility across range of pH values the amino-acid groups are two C atoms away from the complexing moiety of the molecule and we have assumed that this could minimise antagonizing effect of positively charged amino group.
Results and discussion
Synthetic procedures
Both ligands 1 (DS-BTrzPhen) and 2 (DAA-BTrzPhen) were synthesised (Fig. 2) using the same strategy as for the previous BTrzPhen10 ligands with CuAAC reaction as the final step. Commercially available 2,9-dimethyl-1,10-phenathroline was oxidized into 2,9-dicarbaldehyde-1,10-phenathroline (1) by using selenium dioxide13,18 and then Seyferth–Gilbert homologation by using Ohira–Bestmann reagent (2) turned it into 2,9-diethynyl-1,10-phenanthroline (3).10 Commercially available 4-(2-bromoethyl) benzene sulfonate was reacted with sodium azide and converted into 4-(2-azidoethyl) benzene sulfonate (4).19 The final reaction was the CuAAC reaction of the compounds 3 and 4. It was performed using similar conditions as in the literature for BTrzPhen ligands![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) complex of Cu(II) with TBTA (tris(benzyltriazolylmethyl)amine) served as a catalyst while the solvent was CH2Cl2/H2O biphasic system.10 After the workup the product was purified by using reverse phase chromatography with C-18 silica as stationary phase and 30% of MeOH in water as mobile phase. Isolated yield was 70% and the product was off-white powder. Detailed description of the synthesis and characterisation data is given in the ESI.†
complex of Cu(II) with TBTA (tris(benzyltriazolylmethyl)amine) served as a catalyst while the solvent was CH2Cl2/H2O biphasic system.10 After the workup the product was purified by using reverse phase chromatography with C-18 silica as stationary phase and 30% of MeOH in water as mobile phase. Isolated yield was 70% and the product was off-white powder. Detailed description of the synthesis and characterisation data is given in the ESI.†
The azide for the second ligand was L-azidohomoalanine (L-AHA, compound 8). Although this compound is commercially available it is prohibitively expensive for the required quantity. Therefore, we decided to synthesise it by following a similar route as described in the protocol20 for L-AHA. It started with reduction of commercially available N-Boc-O-Bn-L-aspartic acid into N-Boc-O-Bn-L-Homoserinate (5) by using NaBH4 after activation with iso-butyl chloroformate, and then proceeded with tosylation of the free OH group with tosyl chloride to obtain compound 6 and then azidation was achieved by the reaction of sodium azide with tosyl group to obtain benzyl 4-azido-2-((tert-butoxycarbonyl)amino)butanoate (7). Deprotections were achieved in two steps by removing benzyl group with LiOH and Boc group by HCl in dioxane thus obtaining L-AHA hydrochloride (8). The CuAAC reaction to obtain ligand 2 between compounds 3 and 8 was identical as for the ligand 1 using Cu(II)-TBTA complex, sodium ascorbate and CH2Cl2/H2O biphasic system. After the workup purification was achieved by using reverse phase chromatography on C-18 silica. The isolated yield was 88%. Detailed descriptions of all synthetic procedures as well as all characterisation data and spectra (1H-NMR, 13C-NMR, MS, HRMS and IR) are given in ESI.†
Solubility of ligands
Ligand 1 was found to be quite soluble in plain neutral water while some turbidity appears in certain concentrations of nitric acid aqueous solutions ranging from 0.1 to 0.3 M. This is consistent with observations of other sulphonated ligands6 as well as with BTrzPhen ligands10 which showed not to be soluble in aqueous acidic solutions below concentrations of 0.33 M. Despite observed turbidity solvent extraction studies were still viable. On the other hand, due to the presence of amino-acidic groups the ligand 2 showed excellent solubility across the range of different pH values and acidities, yet it was insufficiently soluble in the plain neutral water. Solubility of the ligand 2 quickly increases as pH is falling, but for the preparation of the stock solution (33 mM) higher acidity was required (0.68 M).Solvent extraction studies
Ability of the ligands 1 and 2 to selectively separate minor actinides from lanthanides were tested in i-SANEX conditions using trace activities of 241Am, 152Eu and 154Eu: the ligands were dissolved in aqueous solution spiked with 241Am and 152/154Eu nitrates and extracted with organic phase containing mixture of kerosene and 1-octanol (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) as well as 0.2 M of TODGA. Therefore, ligands 1 and 2 were effectively masking agents for the extraction with TODGA. Standard concentration of the ligands was 10 mM while the aqueous phase was containing additional 0.5 M of NaNO3 and various concentration of nitric acid. Aqueous and organic phases were mixed in a plastic vial, shaken by using a vortex oscillator at constant 20 °C, then centrifuged to completely separate the two phases. Samples of aqueous and organic phase were then taken out, and activities counted (in cmp) using LSC in different channels, which can differentiate 241Am and 152/154Eu counts. Ratio of counts in two phases were then interpreted as extraction parameters DAm and DEu respectively, while SFEu/Am was calculated as DEu/DAm ratio.
5) as well as 0.2 M of TODGA. Therefore, ligands 1 and 2 were effectively masking agents for the extraction with TODGA. Standard concentration of the ligands was 10 mM while the aqueous phase was containing additional 0.5 M of NaNO3 and various concentration of nitric acid. Aqueous and organic phases were mixed in a plastic vial, shaken by using a vortex oscillator at constant 20 °C, then centrifuged to completely separate the two phases. Samples of aqueous and organic phase were then taken out, and activities counted (in cmp) using LSC in different channels, which can differentiate 241Am and 152/154Eu counts. Ratio of counts in two phases were then interpreted as extraction parameters DAm and DEu respectively, while SFEu/Am was calculated as DEu/DAm ratio.
Both ligands 1 and 2 were tested for the three sets of solvent extraction studies: acidity studies (constant ligand concentration while increasing the concentration of nitric acid), studies of extraction kinetics (change of extraction parameters over the time intervals), and ligand concentration studies (varying the ligand concentration while keeping the concentration of nitric acid constant). In addition, studies of thermodynamics of solvent extractions using ligand 1 and ligand 2 were undertaken by performing solvent extraction studies at different temperatures ranging from 283 to 313 K.
The results of the studies are given in the Fig. 3–10. Experimental details and protocols as well as the tabulated results are in the ESI.†
 | ||
| Fig. 3 DAm, DEu and SFEu/Am for the ligand 1 at 0, 1, 5, 10, 50 and 100 mM of HNO3 and constant 10 mM of ligand concentration. | ||
Fig. 3 shows results of the acidity studies for the ligand 1 (DS-BTrzPhen). Despite very good solubility in neutral pH and the presence of NaNO3 the ligand 1 has no significant ability to selectively extract Am3+ ions in neutral pH. In mild acidic conditions (0.001 to 0.01 M) SFEu/Am values are significantly increasing but both distribution ratios DAm and DEu are falling significantly. Only at the acidity of 0.05 M DEu comes significantly above 1 (19) which means that majority of Eu ions are in the organic phase, while DAm is sufficiently low (0.06) and overall SFEu/Am is at around 300. This result is very significant and suggest that 0.05 M is the optimal acidity for this type of ligands. Further increase of acidity brings two negative trends. Firstly, SFEu/Am values decrease as both the DEu, and DAm are growing but DAm grows much more significantly. Secondly, solubility of the ligand is deteriorating, and turbidity appears. Further increase of acidity towards 0.25 M is detrimental to solubility: significant turbidity occurs. The reason for this phenomenon could be that from 0.05 M to higher acidities protonation of benzene-sulphonate moiety occurs, and molecule effectively becomes neutral, but this neutrality is causing precipitation of the ligand. Similar phenomenon was observed in other sulphonated ligands.6 Further increase of acidity above 0.5 M can remove turbidity, but it is assumed that above 0.5 M acidity extraction selectivity towards actinide ions quickly deteriorates as seen in previous BTrzPhens that were tested at higher acidities.10 Because of this phenomenon DS-BTrzPhen is not expected to find industrial applications: we need to develop similar BTrzPhen ligands of even better solubility by increasing acidity of the terminal moieties.
In the Fig. 4 the results of the acidity study for the ligand 2 (DAA-BTrzPhen) are shown. What is very obvious is that DAA-BTrzPhen works better at lower acidities with its DEu being significantly above 1 and higher than it was in the case of DS-BTrzPhen, although overall SFEu/Am is lower. However, at 0.05 M of acidity DAA-BTrzPhen is significantly less efficient than DS-BTrzPhen![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEu is almost the same while DAm is significantly higher (more than 3 times) and overall SFEu/Am is just 84 comparing to ≈300 for DS-BTrzPhen. Such low SFEu/Am suggests that DAA-BTrzPhen is at the limits of applicability for an industrial extraction process. As acidity is increasing DEu and DAm are quickly increasing rendering the ligand inefficient. This drop in efficiency is consistent with what was seen before for BTrzPhen ligands10 and well as other similar ligands: acidity higher than 0.05 M is causing significant protonation of the ligands and prevents An/Ln ions to be coordinated and kept in the aqueous phase. Why DAA-BTrzPhen shows higher D and lower SF values comparing to DS-BTrzPhen even at the same acidity? One possible explanation can be overall positive charge of the molecule even though the terminal aminoacidic moieties are at the very end of the molecules and possibly highly hydrated and surrounded with nitrate counterions. This is suggesting a rule that any highly efficient ligand for An/Ln separations needs to be either negatively charged or neutral, positive charge is not recommended. Another possible explanation is presence of carboxylic groups themselves: carboxylates, although protonated at low pH, can easily get deprotonated by coordination to a metal ion and it is known that carboxylates have huge affinity for hard metal ions such as lanthanides and actinides.
DEu is almost the same while DAm is significantly higher (more than 3 times) and overall SFEu/Am is just 84 comparing to ≈300 for DS-BTrzPhen. Such low SFEu/Am suggests that DAA-BTrzPhen is at the limits of applicability for an industrial extraction process. As acidity is increasing DEu and DAm are quickly increasing rendering the ligand inefficient. This drop in efficiency is consistent with what was seen before for BTrzPhen ligands10 and well as other similar ligands: acidity higher than 0.05 M is causing significant protonation of the ligands and prevents An/Ln ions to be coordinated and kept in the aqueous phase. Why DAA-BTrzPhen shows higher D and lower SF values comparing to DS-BTrzPhen even at the same acidity? One possible explanation can be overall positive charge of the molecule even though the terminal aminoacidic moieties are at the very end of the molecules and possibly highly hydrated and surrounded with nitrate counterions. This is suggesting a rule that any highly efficient ligand for An/Ln separations needs to be either negatively charged or neutral, positive charge is not recommended. Another possible explanation is presence of carboxylic groups themselves: carboxylates, although protonated at low pH, can easily get deprotonated by coordination to a metal ion and it is known that carboxylates have huge affinity for hard metal ions such as lanthanides and actinides.
 | ||
| Fig. 4 DAm, DEu and SFEu/Am for the ligand 2 at 5, 10, 50, 100, and 500 mM of HNO3 and constant 10 mM of ligand concentration. | ||
Despite the lower efficiency DAA-BTrzPhen ligand is highly soluble in a wide range of acidities and has no turbidity problems as seen in DS-BTrzPhen.
Fig. 5 is showing the study of kinetics, influence of contact time on extraction parameters of the ligand 1 (DS-BTrzPhen). Here it is not fully clear if the plateau has really been reached after 30 minutes since a tendency of DAm to grow seems to be still detectable. Nevertheless, the overall trend does suggest stabilisation after 30 minutes, however this slow kinetics it is not ideal for the practical applications. The results of the kinetics study of the ligand 2 (DAA-BTrzPhen) are given in the Fig. 6. The near flatness of the line suggests that in the case of DAA-BTrzPhen equilibrium is reached very fast within one minute. This is in a very contrast to DS-BTrzPhen where at least 15–30 minutes is required.
 | ||
| Fig. 5 DAm, DEu and SFEu/Am for the ligand 1 at 1, 2, 5, 10, 15 and 30 minutes of contact time, 50 mM of nitric acids and 10 mM of ligand 1. | ||
 | ||
| Fig. 6 DAm, DEu and SFEu/Am for the ligand 2 at 1, 2, 5, 10, 15 and 30 minutes of contact time, 50 mM of nitric acids and 10 mM of the ligand 2. | ||
Next, Fig. 7 is showing the influence of DS-BTrzPhen ligand concentration on extraction parameters. Due to the problems with solubility we decided to additionally investigate extraction at concentrations of ligands lower than the standard ones (1 and 2.5 mM). All the tests were conducted with acidity being 0.05 M of nitric acid. The results are suggesting that most of the concentrations parameters are constant from low concentration to 10 mM, while further increase of ligands concentration favours aqueous complexation and both DAm and DEu are falling, while SF keeps being high. Overall concentrations of 10–15 mM seem to be the optimal one.
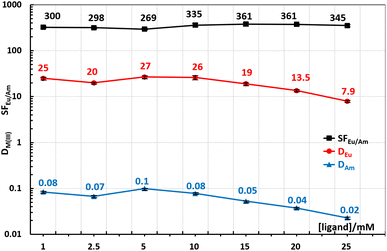 | ||
| Fig. 7 DAm, DEu and SFEu/Am for the ligand 1 increase of ligand concentration from 1 mM to 25 mM and constant 0.05 M of HNO3. | ||
Fig. 8 describes the dependence of the concentration of DAA-BTrzPhen ligand of extraction efficiency. As the concentration of the ligands is increasing SFEu/Am is also increasing while DEu and DAm are decreasing. Yet, DAm is decreasing faster than DEu effectively brining the SFEu/Am to 132 which is the highest value achieved for DAA-BTrzPhen ligand at 20 °C. Decrease of D and increase of SFEu/Am values is expected: the increase of the ligand 2 certainly forces the higher affinity actinide ion to stay in water solution better than the lower affinity lanthanide. This is happening despite possibly lower stability constants of DAA-BTrzPhen comparing to DS-BTrzPhen. Nevertheless, this result is very encouraging since achieved SFEu/Am value of 132 is close to those of PyTri and significantly higher than in the previously10 published BTrzPhen ligands.
 | ||
| Fig. 8 DAm, DEu and SFEu/Am for the ligand 2 increase of ligand concentration from 5 mM to 25 mM and constant 0.05 M of HNO3. | ||
Finally, the effect of the variable temperature on D and SFEu/Am are presented in the Fig. 9 (for ligand 1) and 10 (for ligand 2). As expected, distribution ratios for both metals in the case of both ligands are steadily decreasing revealing exothermic nature of the extraction process. Different from similar tests21 where only one ligand is used in our case we have a biphasic extraction system with two ligands, each in its own preferable phase (TODGA and BTrzPhens). Therefore, the extraction process and its thermodynamic parameters are the interplay between the two ligands and cannot be contributed to the specific ligand. The thermodynamic parameters such as ΔH can be derived by using van't Hoff equation and are reflecting the whole extraction system. In order to determine ΔH we used the method from the literature:21 diagrams in the Fig. 9 and 10 were rearranged to express the x-axis as 1000/T (in mK−1) and y-axis as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DM as shown in the Fig. 11 and 12.
DM as shown in the Fig. 11 and 12.
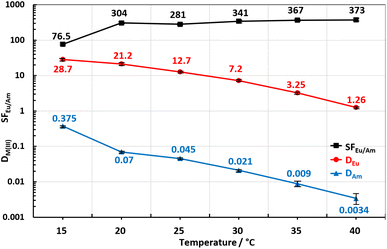 | ||
| Fig. 9 Effect of temperature increase on DAm, DEu and SFEu/Am for the ligand 1 at constant ligands concentration of 10 mM and acidity of 50 mM of HNO3. | ||
 | ||
| Fig. 10 Effect of temperature increase on DAm, DEu and SFEu/Am for the ligand 2 at constant ligands concentration of 10 mM and acidity of 50 mM of HNO3. | ||
 | ||
Fig. 11 Effect of temperature on DM for the ligand 1: plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DM vs. 1000/T for Eu(III) and Am(III). DM vs. 1000/T for Eu(III) and Am(III). | ||
 | ||
Fig. 12 Effect of temperature on DM for the ligand 2: plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DM vs. 1000/T for Eu(III) and Am(III). DM vs. 1000/T for Eu(III) and Am(III). | ||
With the assumption that the heat capacity of the extraction system is constant within the studied temperature range, the relationships of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DM vs. (1000/T) are nearly linear and the slope values of the trending line equations can be used to calculate ΔH by using eqn (9) from the literature.21 According to this method, it can be calculated that for the DS-BTrzPhen/TODGA system ΔHAm is −130.8 kJ mol−1, while for ΔHEu it is −93.1 kJ mol−1. Also, for the DAA-BTrzPhen/TODGA system ΔHAm is −111.9 kJ mol−1, while ΔHEu is −92.4 kJ mol−1. Although these values for ΔH should be taken cautiously, it is clear that for DAA-BTrzPhen ligand ΔHAm value is lower than the one for DS-BTrzPhen, while two ΔHEu values are almost the same.
DM vs. (1000/T) are nearly linear and the slope values of the trending line equations can be used to calculate ΔH by using eqn (9) from the literature.21 According to this method, it can be calculated that for the DS-BTrzPhen/TODGA system ΔHAm is −130.8 kJ mol−1, while for ΔHEu it is −93.1 kJ mol−1. Also, for the DAA-BTrzPhen/TODGA system ΔHAm is −111.9 kJ mol−1, while ΔHEu is −92.4 kJ mol−1. Although these values for ΔH should be taken cautiously, it is clear that for DAA-BTrzPhen ligand ΔHAm value is lower than the one for DS-BTrzPhen, while two ΔHEu values are almost the same.
Calculation of other thermodynamic parameters, ΔG and ΔS cannot be achieved usings the method described in the literature21 due to inherently more complex nature of our extraction process.
Spectroscopic UV-vis titrations and fluorescence spectroscopy
To further characterise coordination abilities of the ligand 1 and 2 we have undertaken coordination studies by spectroscopic titrations of the ligands 1 and 2 with lanthanide ions (Eu3+ and Tb3+). The aim was to uncover the stability constants for the formation of [ML]3+ and [ML2]3+ complexes and to compare them with stability constants of the similar previously published BTrzPhen ligands.10 The ligand 1 (DS-BTrzPhen) was titrated with Eu(NO3)3 or Tb(NO3)3 in neutral aqueous solution containing Me4NNO3 (I = 0.01 M), while the medium for the titration of the ligand 2 (DAA-BTrzPhen) was additionally acidified with nitric acid (0.028 M) to improve the solubility of the ligand.The resulting spectroscopic data were processed and analysed by HyperQuad22 providing the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β1:1 and log
β1:1 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β1:2 values (Table 1). For the ligand 1 (DS-BTrzPhen) results of the spectroscopic titrations are shown in the Fig. 13 and 14 as well as in the ESI Section 4.1. In the case of both metals spectroscopic titrations of DS-BTrzPhen yield similar results of stability constants with β1:1 being from 7 to 6.6, while β1:1 being from 12 to 12.4. These results are generally consistent with the previous findings for other BTrzPhen ligands10 yet, speciation diagrams (ESI, Section 4.3†) are suggesting much larger dominance of 1
β1:2 values (Table 1). For the ligand 1 (DS-BTrzPhen) results of the spectroscopic titrations are shown in the Fig. 13 and 14 as well as in the ESI Section 4.1. In the case of both metals spectroscopic titrations of DS-BTrzPhen yield similar results of stability constants with β1:1 being from 7 to 6.6, while β1:1 being from 12 to 12.4. These results are generally consistent with the previous findings for other BTrzPhen ligands10 yet, speciation diagrams (ESI, Section 4.3†) are suggesting much larger dominance of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex in solution, while 1
1 complex in solution, while 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex seems to be less present.
2 complex seems to be less present.
On the other hand, our attempts to perform spectroscopic titration with DAA-BTrzPhen (2) produced different results. Firstly, the equivalent titration of DAA-BTrzPhen with Eu(NO3)3 in aqueous solution of nitric acid (0.028 M) produced absorption spectra (Diagram 13 in ESI†) that lacked typical absorbance peak shifts as seen in DS-BTrzPhen titrations as well as in the ligands 8 and 9 from the literature.10 Instead, lack of any significant wavelength shifts suggested just a dilution effect without complex formation, while attempts to process spectra with Hyperquad and find stability constants gave no results. Therefore, another titration was attempted with Eu(OTf)3 in pure water acidified with non-coordinating HClO4 (0.03 M). However, this attempt gave us very similar results (Diagram 14 in ESI†) as the previous attempt. The final attempt was spectroscopic titration with Eu(OTf)3 in a mixture of organic solvents and water (acetonitrile/methanol/water = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of acidified water. The outcome was different as shown in the Diagram 15 yet was still not as expected. This perhaps can be attributed to combination of dilution effect and some other form of interaction different from ligand–metal binding via N-donor atoms. Fitting process did not yield any reliable stability constants. It is not fully understood why titration of DAA-BTrzPhen has repeatedly failed and produced different outcome, yet this is a clear indication that amino-acidic groups do interfere in the complexation process.
1) instead of acidified water. The outcome was different as shown in the Diagram 15 yet was still not as expected. This perhaps can be attributed to combination of dilution effect and some other form of interaction different from ligand–metal binding via N-donor atoms. Fitting process did not yield any reliable stability constants. It is not fully understood why titration of DAA-BTrzPhen has repeatedly failed and produced different outcome, yet this is a clear indication that amino-acidic groups do interfere in the complexation process.
To further examine complexation between the ligands and Eu(III) fluorescence emission spectra of both ligands 1 and 2 were recorded with added 0.5 equivalents of Eu(III). The emission spectra of the ligand 1 were recorded in neat water, while for the ligand 2 were recorded in aqueous solution of HClO4 (0.03 M). Due to technical limitations, we were able to record only emission spectra. Therefore, we decided to use excitation wavelengths of 267 nm and 326 nm that were optimal for the BTrzPhen ligand 8 from the literature.10 Emission spectra for both excitation wavelengths were found to be nearly identical therefore spectra recorded at 326 nm are shown in the Fig. 15 (DS-BTrzPhen) and 16 (DAA-BTrzPhen), while those recorded at 276 nm are shown in the ESI, Section 5.† Additional fluorescence spectra for Eu(OTf)3 in aqueous solution of HClO4 was also recorded for comparison (see ESI, Section 5†).
 | ||
Fig. 15 Fluorescence emission spectrum of DS-BTrzPhen-Eu complex (0.2 mM, molar ration 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in water at excitation wavelength of 326 nm. 1) in water at excitation wavelength of 326 nm. | ||
Emission spectra for both ligands were similar to the one in the literature10 obtained for the BTrzPhen ligand 8. However, ligand 1 was showing the most intense peak in the emission spectrum at 619 nm that is the 5D0 → 7F2 ‘‘hypersensitive transition’’, with relative 595/619 peak intensity ratio 0.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For the ligand 2 (Fig. 16), the 619 peak was relatively weaker with 595/619 peak intensity ratio 0.40
1. For the ligand 2 (Fig. 16), the 619 peak was relatively weaker with 595/619 peak intensity ratio 0.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The weaker intensity of this peak may indicate weaker complexation of Eu(III) ions which is consistent with observations in solvent extraction studies as well as in spectroscopic titration. Yet, the comparison with an emission spectrum of sole Eu(OTf)3 (Diagram 22, Section 5, ESI†) is giving us a firm indication that DAA-BTrzPhen does bind Eu(III) ions. In total, these observations are suggesting that both DS-BTrzPhen and DAA-BTrzPhen ligands are complexing Eu(III) ions via their N-donor ligands – this interaction is producing typical reddish fluorescence of the BTrzPhen-Eu complex.
1. The weaker intensity of this peak may indicate weaker complexation of Eu(III) ions which is consistent with observations in solvent extraction studies as well as in spectroscopic titration. Yet, the comparison with an emission spectrum of sole Eu(OTf)3 (Diagram 22, Section 5, ESI†) is giving us a firm indication that DAA-BTrzPhen does bind Eu(III) ions. In total, these observations are suggesting that both DS-BTrzPhen and DAA-BTrzPhen ligands are complexing Eu(III) ions via their N-donor ligands – this interaction is producing typical reddish fluorescence of the BTrzPhen-Eu complex.
 | ||
Fig. 16 Fluorescence emission spectrum of DAA-BTrzPhen-Eu complex (0.2 mM, molar ration 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in aqueous solution of HClO4 (0.03 M) at excitation wavelength of 326 nm. 1) in aqueous solution of HClO4 (0.03 M) at excitation wavelength of 326 nm. | ||
Conclusions
It total, we have successfully synthesised, characterised, and tested two new BTrzPhen ligands. These are the first new hydrophilic water-soluble ligands of the BTrzPhen family since their introduction, and are showing higher solubility as well as significantly better extraction efficacy and selectivity towards Am(III) versus Eu(III). The highest SFEu/Am value obtained by DS-BTrzPhen was 361 when acidity was 0.05 M and concentration of ligand was 15 or 20 mM. In the case of DAA-BTrzPhen it was 132 which is less than in the case of DS-BTrzPhen and even PyTri ligands, but more than the original two BTrzPhen ligands.10Our results suggests that the optimal pH for BTrzPhen ligands seems to be 0.05 M. Studies at this acidity were not tried for the older BTrzPhen ligands10 since those ligands were not even soluble at that acidity and required at least 0.33 M to be dissolved. However, at higher acidity protonation becomes more significant resulting in the decrease of SFEu/Am values. Deactivation by protonation is especially plausible scenario since SFEu/Am value of DAA-BTrzPhen quickly drops when acidity increases more than 0.05, while at 0.1 M it is just 19, lower than the original BTrzPhen ligands.10
Both ligands, DS-BTrzPhen and DAA-BTrzPhen have two significant drawbacks: despite very high observed SFEu/Am values DS-BTrzPhen is still insufficiently soluble in nitric acid solution and hence cannot be considered as a ligand capable of practical application, while DAA-BTrzPhen is positively charged with adjacent carboxyl moiety, and this is significantly reducing its efficiency in the terms of SF and D so its usefulness in practical applications is on the borderline.
Coordination studies by spectroscopic titrations and fluorescence spectroscopy further revealed differences between ligands 1 and 2. While ligand 1 behaves somewhat like the ligand 8 from the literature10 as shown by successful spectroscopic titrations, obtained stability constants and fluorescence spectra, ligand 2 is controversial. On one hand, we can see firm indications that ligand 2 does bind f-elements as observed from solvent extraction studies and fluorescence spectra, however the failure to produce the expected UV-vis titration spectra and to obtain stability constants suggests that amino-acidic groups do significantly interfere in the coordination process. To properly address this phenomenon more research is desired.
Therefore, this study suggests a key criterion: a good BTrzPhen ligand for i-SANEX needs to be at least neutral or negatively charged to have proper efficiency. This is very hard to achieve if the ligand needs to stay CHON compliant since the choice for hydrophilizing terminal moieties are very limited. To fulfil this criterion a compromise might be necessary.
Nevertheless, both ligands have achieved quite good results suggesting that if the problem of solubility at acidity of 0.05 M is solved while the molecule of the ligand is neutral or negatively charged, we may eventually develop BTrzPhen ligands proper enough for i-SANEX application. These promising results are suggesting that BTrzPhen ligands are worthy of further investigation and development and are compelling us to continue our quest.
Author contributions
Y. Q. W. performed most of the synthetic and purification work as well as characterisation, fluorescence, spectroscopic titrations and contributed to ESI.† H. X. H. and W. Z. P. performed the solvent extraction studies and HRMS analysis, L. Y. contributed to the synthesis of the ligands. P. M. conceptualised idea and designed the ligands, contributed to the synthesis and characterisation, designed the solvent extraction studies, wrote the manuscript, and supervised the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Lanzhou University and NSFS for the funding of the project. We would like to express our special gratitude to Dr Yong Cong for his great help with NMR characterisation, to Professor Kieran Nolan from Dublin City University and to Professor Neil R. Thomas from Nottingham University for giving us the precious synthetic advice. We also wish to thank to Professor Wangsuo Wu from Lanzhou University for bringing our two groups together and establishing our collaboration.Notes and references
- G. Modolo, A. Wilden, P. Kaufholz, D. Bosbach and A. Geist, Prog. Nucl. Energy, 2014, 72, 107–114 CrossRef CAS.
- J. Veliscek-Carolan, J. Hazard. Mater., 2016, 318, 266–281 CrossRef CAS.
- A. Geist and P. J. Panak, Solvent Extr. Ion Exch., 2021, 39(2), 128–151 CrossRef CAS.
- Y. Wang, K. M. Shield and R. J. Abergel, Sep. Purif. Rev., 2023, 1–19 CrossRef.
- A. Geist, U. Mullich, D. Magnusson, P. Kaden, G. Modolo, A. Wilden and T. Zevaco, Solvent Extr. Ion Exch., 2012, 30, 433–444 CrossRef CAS.
- F. W. Lewis, L. M. Harwood, M. J. Hudson, A. Geist, V. N. Kozhevnikov, P. Distler and J. John, Chem. Sci., 2015, 6, 4812–4821 RSC.
- R. Ren, P.-W. Huang, X.-F. Yang, Y. Zou, W.-Q. Tao, S.-L. Yang, Y.-H. Liu, Q.-Y. Wu, L.-Y. Yuan, Z.-F. Chai and W.-Q. Shi, Inorg. Chem., 2021, 60, 357–365 CrossRef.
- E. Macerata, E. Mossini, S. Scaravaggi, M. Mariani, A. Mele, W. Panzeri, N. Boubals, L. Berthon, M.-C. Charbonnel, F. Sansone, A. Arduini and A. Casnati, J. Am. Chem. Soc., 2016, 138, 7232–7235 CrossRef CAS PubMed.
- P. Weßling, M. Maag, G. Baruth, T. Sittel, F. S. Sauerwein, A. Wilden, G. Modolo, A. Geist and P. J. Panak, Inorg. Chem., 2022, 61, 17719–17729 CrossRef PubMed.
- A. C. Edwards, P. Mocilac, A. Geist, L. M. Harwood, C. A. Sharrad, N. A. Burton, R. C. Whitehead and M. A. Denecke, Chem. Commun., 2017, 53, 5001–5004 RSC.
- M. G. B. Drew, D. Guillaneux, M. J. Hudson, P. B. Iveson and C. Madic, Inorg. Chem. Commun., 2001, 4, 462–466 CrossRef CAS.
- M. G. B. Drew, M. R. S. J. Foreman, C. Hill, M. J. Hudson and C. Madic, Inorg. Chem. Commun., 2005, 8, 239–241 CrossRef CAS.
- F. H. Lewis, L. M. Harwood, M. J. Hudson, M. G. B. Drew, J. F. Desreux, G. Vidick, N. Bouslimani, G. Modolo, A. Wilden, M. Sypula, T. H. Vu and J. P. Simonin, J. Am. Chem. Soc., 2011, 133, 13093–13102 CrossRef CAS PubMed.
- P. Zsabka, T. Opsomer, K. Van Hecke, W. Dehaen, A. Wilden, G. Modolo, M. Verwerft, K. Binnemans and T. Cardinaels, Solvent Extr. Ion Exch., 2020, 38, 719–734 CrossRef CAS.
- S. A. Labb, C. J. Masteran, S. G. Albright, B. Ali, H. A. Chapman, Y. J. Cheng, R. M. Cusic, B. N. Hartlove, A. N. Marr, M. Timmons and S. J. Friese, Synlett, 2020, 31, 1384–1388 CrossRef CAS.
- P.-W. Huang, C.-Z. Wang, Q.-Y. Wu, J.-H. Lan, Z.-F. Chai and W.-Q. Shi, Radiochim. Acta, 2020, 108, 517–526 CrossRef CAS.
- Z.-R. Ye, Q.-Y. Wu, C.-Z. Wang, J.-H. Lan, Z.-F. Chai, H.-Q. Wang and W.-Q. Shi, Inorg. Chem., 2022, 61, 6110–6119 CrossRef CAS.
- N. T. Coogan, M. A. Chimes, J. Raftery, P. Mocilac and M. A. Denecke, J. Org. Chem., 2015, 80, 8684–8693 CrossRef CAS PubMed.
- B. P. Gindt, D. G. Abebe, Z. J. Tang, M. B. Lindsey, J. Chen, R. A. Elgammal, T. A. Zawodzinski and T. Fujiwara, J. Mater. Chem. A, 2016, 4, 4288–4295 RSC.
- S. Roth, W. Drewe and N. R. Thomas, Nat. Protoc., 2010, 5, 1967–1973 CrossRef CAS PubMed.
- X.-R. Zhang, Q.-Y. Wu, J.-H. Lan, L.-Y. Yuan, C. Xu, Z.-F. Chai and W.-Q. Shi, Sep. Purif. Technol., 2019, 223, 274–281 CrossRef CAS.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739–1753 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02707d |
| This journal is © The Royal Society of Chemistry 2023 |