Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An ionic liquid- and PEO-based ternary polymer electrolyte for lithium metal batteries: an advanced processing solvent-free approach for solid electrolyte processing†
Lukas Herbers a,
Verena Küpers
a,
Verena Küpers a,
Martin Winterab and
Peter Bieker
a,
Martin Winterab and
Peter Bieker *b
*b
aMEET Battery Research Center, Institute of Physical Chemistry, University of Münster, 48149 Münster, Germany
bHelmholtz-Institute Münster (HIMS), IEK-12, Forschungszentrum Jülich GmbH, 48149 Münster, Germany. E-mail: peter.bieker@uni-muenster.de
First published on 14th June 2023
Abstract
A processing solvent-free manufacturing process for cross-linked ternary solid polymer electrolytes (TSPEs) is presented. Ternary electrolytes (PEODA, Pyr14TFSI, LiTFSI) with high ionic conductivities of >1 mS cm−1 are obtained. It is shown that an increased LiTFSI content in the formulation (10 wt% to 30 wt%) decreases the risk of short-circuits by HSAL significantly. The practical areal capacity increases by more than a factor of 20 from 0.42 mA h cm−2 to 8.80 mA h cm−2 before a short-circuit occurs. With increasing Pyr14TFSI content, the temperature dependency of the ionic conductivity changes from Vogel–Fulcher–Tammann to Arrhenius behavior, leading to activation energies for the ion conduction of 0.23 eV. In addition, high Coulombic efficiencies of 93% in Cu‖Li cells and limiting current densities of 0.46 mA cm−2 in Li‖Li cells were obtained. Due to a temperature stability of >300 °C the electrolyte guarantees high safety in a broad window of conditions. In LFP‖Li cells, a high discharge capacity of 150 mA h g−1 after 100 cycles at 60 °C was achieved.
Introduction
Lithium (Li) metal has become the focus of extensive research in the past years.1 Its high gravimetric capacity of 3860 mA h g−1 and low standard reduction potential of −3.04 V vs. standard hydrogen electrode (SHE) make it an ideal choice for a negative electrode active material in high energy density batteries.2 Nonetheless, the application of Li metal has major challenges.3 The decomposition of the electrolyte, the formation of the Solid Electrolyte Interphase (SEI) and the accumulation of inhomogeneous Li metal deposits, respectively ‘high surface area lithium (HSAL)’ and ‘dead Li’ cannot only result in active lithium loss (ALL) but also safety concerns.4–6 In particular, the risk of short-circuits by HSAL and the high reactivity of liquid flammable electrolytes with HSAL can be hazardous.7,8 To overcome these safety concerns, solid electrolytes (SE) and electrolytes based on ionic liquids are considered as safer alternatives.9–11Solid electrolytes are often divided into the categories of inorganic solid electrolytes (ISEs), solid polymer electrolytes (SPEs) and composite polymer-inorganic electrolytes (CPIEs).12 The most studied polymer for Li-based batteries is poly ethylene oxide (PEO) due to its ether-functional groups combined with an alkyl-segment.13 The ether groups interact with Li-ions, granting a great Li salt solubility as well as good ion transport properties.13 Despite the benefit in safety of PEO electrolytes, they usually suffer from a low room temperature (RT) ionic conductivity.14 In comparison, several ISEs provide sufficient ionic conductivity. Nonetheless, ISEs can have challenges on their own, for instance a narrow electrochemical stability window, an expensive synthesis or poor electrode|electrolyte compatibility due to a low mechanical flexibility.15,16 Therefore, hybrids of different types of electrolytes are useful to balance the requirements of processability, ionic conductivity, safety and reversibility of Li metal electrodeposition and -dissolution.17,18 ISEs, SPEs and/or liquid electrolytes can therefore be combined to form CPIEs, gel and pseudo-solid electrolytes. The combination of polymers with RT ionic liquids (IL) and Li salts results in so called ternary solid polymer electrolytes (TSPE), with significantly higher ionic conductivities compared to SPEs and greater mechanical flexibility than ISEs.19,20 Moreover, while more expensive than organic solvent-based electrolytes, the low vapor pressure and high thermal stability of ionic liquids increases the safety compared to liquid organic electrolytes.21
Nonetheless, despite the higher performance by improved ionic conductivity compared to SPEs, TSPEs also show major challenges which need to be addressed to make them applicable in Li metal full cells. As shown by Zhang et al. a PEO-based TSPE (PEOref) with a molar ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (EO
2 (EO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiTFSI (lithium bis(trifluoromethanesulfonyl)imide)
LiTFSI (lithium bis(trifluoromethanesulfonyl)imide)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pyr14TFSI (1-butyl-1-methyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide)) suffers from low mechanical stability (elastic modulus: 0.3 MPa at 20 °C).20,22 This low mechanical strength results in a limited suppression of Li metal HSAL, which penetrates through the electrolyte leading to a cell short-circuit. In this work the ratio of polymer, Li salt and IL is balanced to benefit from the high ionic conductivity provided by the IL but at the same time enable short-circuit prevention by the high mechanical strength of Li salt and polymer. Furthermore, different from commonly used electrolyte processing by use of processing solvent-based casting or hot pressing of solids, which is expensive, time-consuming and in some cases even hazardous, in this work, only liquid or soluble precursors without any processing solvents are used. For this purpose, long chain solid PEO is replaced by PEODA (polyethylene oxide diacrylate), a small chain and therefore liquid SPE. Thus, the energy-consuming removal of the solvents during processing of solid CPIEs and TSPEs after solvent casting is not necessary. Also, in contrast to hot pressing, no elevated temperatures are needed, because the electrolyte can be processed under RT, making the presented electrolyte processing more time- and energy-efficient. During processing, the acrylate end groups of the PEODA are cross-linked by ultraviolet (UV) light induced radical cross-linking process, which leads to a fast phase transition from liquid to solid, enabling the combination of the processability of a liquid with the safety of a solid electrolyte. The ternary electrolyte composition is optimized towards a high conductivity and to meet the safety concerns regarding inhomogeneous Li electrodeposition and -dissolution, temperature stability and high voltage stability. Finally, the Li metal electrodeposition/-dissolution behavior in Li‖Li, Cu‖Li and lithium iron phosphate (LFP)‖Li cells and the electrochemical performance and limitations of the electrolyte in symmetric Li‖Li cells are determined.
Pyr14TFSI (1-butyl-1-methyl-pyrrolidinium bis(trifluoromethylsulfonyl)imide)) suffers from low mechanical stability (elastic modulus: 0.3 MPa at 20 °C).20,22 This low mechanical strength results in a limited suppression of Li metal HSAL, which penetrates through the electrolyte leading to a cell short-circuit. In this work the ratio of polymer, Li salt and IL is balanced to benefit from the high ionic conductivity provided by the IL but at the same time enable short-circuit prevention by the high mechanical strength of Li salt and polymer. Furthermore, different from commonly used electrolyte processing by use of processing solvent-based casting or hot pressing of solids, which is expensive, time-consuming and in some cases even hazardous, in this work, only liquid or soluble precursors without any processing solvents are used. For this purpose, long chain solid PEO is replaced by PEODA (polyethylene oxide diacrylate), a small chain and therefore liquid SPE. Thus, the energy-consuming removal of the solvents during processing of solid CPIEs and TSPEs after solvent casting is not necessary. Also, in contrast to hot pressing, no elevated temperatures are needed, because the electrolyte can be processed under RT, making the presented electrolyte processing more time- and energy-efficient. During processing, the acrylate end groups of the PEODA are cross-linked by ultraviolet (UV) light induced radical cross-linking process, which leads to a fast phase transition from liquid to solid, enabling the combination of the processability of a liquid with the safety of a solid electrolyte. The ternary electrolyte composition is optimized towards a high conductivity and to meet the safety concerns regarding inhomogeneous Li electrodeposition and -dissolution, temperature stability and high voltage stability. Finally, the Li metal electrodeposition/-dissolution behavior in Li‖Li, Cu‖Li and lithium iron phosphate (LFP)‖Li cells and the electrochemical performance and limitations of the electrolyte in symmetric Li‖Li cells are determined.
Experimental
Chemicals
PEODA (Sigma-Aldrich, purity ≤100%, Mn = 700) was stored over activated molecular sieve 3 Å for at least seven days before use (water content: 20 ± 3 ppm by Karl-Fischer titration). Benzophenone (BP) (Merck, purity = 99%) was used as received. Pyr14TFSI (Solvionic, purity = 99.9%) was dried for 48 hours at 110 °C under vacuum followed by 48 hours at 110 °C under high-vacuum <10−7 mbar. LiTFSI (TCI, purity > 98%) was dried for at least 48 hours at 110 °C under vacuum followed by 48 hours at 110 °C under high-vacuum <10−7 mbar. LFP sheets (LiFePO4, NANOMYTE® BE-60E, experimental capacity ≥170 mA h g−1, NEI Corporation) were dried for 48 hours at 100 °C under vacuum.Preparation of electrolyte films
Electrolyte films were prepared in a dry room. The electrolyte “PxILySz” was named according to its mass ratios of x PEODA “Px”, y of the Pyr14TFSI “ILy” and z of LiTFSI “Sz”, see Table 1. Different x, y, z mass ratios were mixed with BP (1 wt% of the polymer content). Afterwards, the liquid mixture was filled into a polytetrafluoroethylene (PTFE) mold with two different mold heights of 250 ± 25 μm or 500 ± 50 μm and covered by a siliconized biaxial-oriented polyethylene terephthalate (boPET) foil. The electrolyte was placed in an UVACUBE 100 with a 100 W lamp (Dr Höhnle AG) and was irradiated by UV light for 10 min. The final electrolyte thicknesses were validated by a layer thickness gauge (Mitutoyo ABSOLUTE) after cross-linking. PEOref was prepared as described elsewhere.20,22| Abbreviation | Component | Chemical |
|---|---|---|
| P | Polymer | PEODA |
| IL | Ionic liquid | Pyr14TFSI |
| S | Salt | LiTFSI |
The 851 Titrando Karl Fischer Coulometer (Metrohm, Herisau, Switzerland) was calibrated with a threefold measurement of a 100 mg L−1 water standard for quantification. Each electrolyte was measured three times with an injection of 1 g. Instrument control, data acquisition and data evaluation were performed using tiamo™ 2.4 (Metrohm).23
The temperature stabilities of the electrolytes were studied using thermogravimetric analysis (TGA, A Q5000IR by TA instruments) with a heat rate of 10 K min−1 from 20 °C to 600 °C.
Fourier-transform infrared spectroscopy (FT-IR) measurements were performed on a BRUKER ALPHA II. The samples were placed onto the attenuated total reflection (ATR) crystal and a wavenumber range from 4000 cm−1 to 400 cm−1 was measured.
For electrochemical measurements round electrolyte discs of 15 mm diameter and 250 μm thickness were assembled in Li‖Li, Cu‖Li, stainless steel (SST)‖Li, LFP‖Li and SST‖SST CR2032 two-electrode coin cells in a dry room. Temperature dependent ionic conductivity profiles were measured by impedance spectroscopy in SST‖SST cells on a Novocontrol Alpha Analyzer. An amplitude of 10 mV, a frequency range from 0.1 Hz to 10 MHz and a temperature interval from 0 °C to 80 °C in 10 °C steps were applied. Galvanostatic polarization tests (0.1 mA cm−2, 0.5 mA h cm−2, 50 cycles) with Li‖Li cells, galvanostatic discharge tests (‚short-circuit tests‘, 0.1 mA cm−2) with Li‖Li cells, galvanostatic polarization tests (0.05 mA cm−2, 100 cycles, 2.5 V to 4.0 V) with LFP‖Li cells and galvanostatic polarization tests (0.1 mA cm−2, 0.1 mA h cm−2, 200 cycles, cutoff voltage ± 1 V) with Cu‖Li cells were performed on a MACCOR battery cycler (MACCOR Series 4000) at 60 °C. Linear sweep voltammetry tests (0.5 mV s−1, from OCV (open-circuit voltage) to 6 V) in SST‖Li cells, charge pulse analysis tests (1.2 mA cm−2, 1.4 mA cm−2, 1.6 mA cm−2, 1.8 mA cm−2, 2.0 mA cm−2 and 2.2 mA cm−2, cutoff voltage 1 V, rest time between charge pulses 3 h) in Li‖Li cells and transference number measurements (10 mHz to 1 MHz, ± 10 mV, 20 ± 1 mV) in Li‖Li cells were performed on a VMP potentiostat (Bio-152 Logic) at 60 °C. A triple measurement set was performed for each technique.
Results and discussion
Processability
The processing of electrolytes of different composition is schematically given in Fig. 1. The mixture of precursors PEODA, Pyr14TFSI and LiTFSI results in a homogeneous liquid phase. PEODA is the key component enabling the fully liquid precursor as well as the solid final state of the electrolyte. Due to its small chain length, it is liquid at RT, making it a solvent-like component for LiTFSI. The ethylene oxide groups strongly interact with Li+ ions and dissolve LiTFSI even at high weight ratios of 40% in contrast to pure Pyr14TFSI-LiTFSI mixtures, which solidify at high LiTFSI contents. While the inner molecular chains (–CH2–CH2–O–)x are beneficial for the liquid state, the end groups (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CO–O–R) of PEODA can lead to a phase formation of the electrolyte from liquid to solid. The acrylate groups at the end of the polymer chains contain double bonds that are cross-linked by radical induced polymerization.24 The full cross-linking mechanism is given in Fig. 11 in the ESI.†
CH–CO–O–R) of PEODA can lead to a phase formation of the electrolyte from liquid to solid. The acrylate groups at the end of the polymer chains contain double bonds that are cross-linked by radical induced polymerization.24 The full cross-linking mechanism is given in Fig. 11 in the ESI.†
 | ||
| Fig. 1 Processing solvent-free preparation ternary electrolyte manufacturing (liquid precursor, molding, cross-linking, solid electrolyte). | ||
The cross-linking process is verified by FT-IR analysis. As shown in Fig. 2a, the non-cross-linked PEODA (blue curve) has a band in the absorption spectra at a wavenumber of 1636 cm−1, which is caused by the valence vibration of the carbon–carbon double bond (v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)). With increasing progress of the radical chain reaction, the peaks representing double bonds decrease, allowing to monitor the determination of the cross-linking process. As is seen from Fig. 2a no v(C
C)). With increasing progress of the radical chain reaction, the peaks representing double bonds decrease, allowing to monitor the determination of the cross-linking process. As is seen from Fig. 2a no v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) peak is observed even after 1 min of irradiation (cyan curve), indicating that despite the low weight percentage of photo initiator, the cross-linking is completed after 1 min, compare Fig. 12 in the ESI.† After 10 min of exposure to UV radiation (black curve) no further changes in the IR spectra are observed. To guarantee a fully cross-linked state even at lower polymer content, all electrolytes were cross-linked for an excessive time of 10 min. A spectra of the electrolyte P2IL5S3 (brown curve) after 10 min of UV radiation, as well as the spectra of the pure Pyr14TFSI (orange curve) and pure LiTFSI (red curve) used for electrolyte mixing, are shown in Fig. 2b. No v(C
C) peak is observed even after 1 min of irradiation (cyan curve), indicating that despite the low weight percentage of photo initiator, the cross-linking is completed after 1 min, compare Fig. 12 in the ESI.† After 10 min of exposure to UV radiation (black curve) no further changes in the IR spectra are observed. To guarantee a fully cross-linked state even at lower polymer content, all electrolytes were cross-linked for an excessive time of 10 min. A spectra of the electrolyte P2IL5S3 (brown curve) after 10 min of UV radiation, as well as the spectra of the pure Pyr14TFSI (orange curve) and pure LiTFSI (red curve) used for electrolyte mixing, are shown in Fig. 2b. No v(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) peak is observed in the P2IL5S3 spectra. The resulting films are transparent, homogeneous, non-porous and elastic (elastic modulus: 3.2 MPa). (see Fig. 1 and 13 in the ESI†).
C) peak is observed in the P2IL5S3 spectra. The resulting films are transparent, homogeneous, non-porous and elastic (elastic modulus: 3.2 MPa). (see Fig. 1 and 13 in the ESI†).
 | ||
| Fig. 2 Absorbance spectra of (a) Pyr14TFSI, LiTFSI and P2IL5S3 cross-linked for 10 min and (b) PEODA with 1 wt% of BP not cross-linked, cross-linked for 1 min and cross-linked for 10 min. | ||
Ionic conductivity
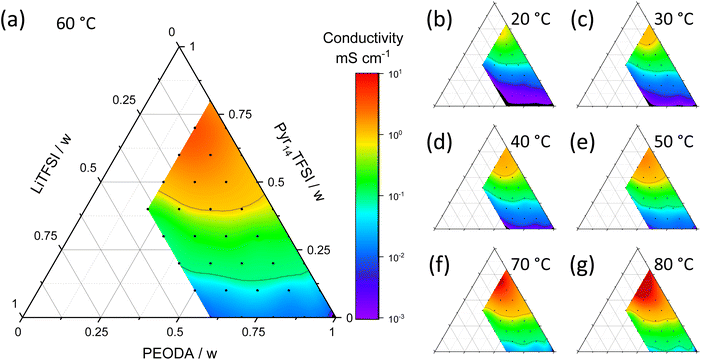
The ternary diagrams of various mass ratios of Pyr14TFSI, LiTFSI and PEODA at different temperatures are shown in Fig. 3. The ionic conductivities from 10−3 mS cm−1 (purple) to 101 mS cm−1 (red) are plotted over the composition in weight fraction. Seven temperatures from 20 °C to 80 °C are shown. Due to viscosity limitations the weight fraction of LiTFSI is restricted to a maximum of 0.4. The polymer content is kept between 0.2 and 1.0 to enable sufficient mechanical stability of the electrolyte.An ionic conductivity of ≥1 mS cm−1 is considered as a benchmark in literature.17,25 This benchmark is not reached for a temperature of 20 °C independent of the mass ratios of the ternary electrolyte, see Fig. 3b, but at slightly elevated temperature of 30 °C (Fig. 3c) for high Pyr14TFSI fractions. An ionic conductivity of ≥1 mS cm−1 is achieved for smaller fractions of Pyr14TFSI when increasing the temperature from 40 °C to 60 °C (Fig. 3a, d and e). When increasing the temperature even further to 70 °C and 80 °C, ionic conductivities of 10 mS cm−1 and higher are reached, Fig. 3f and g.
In order to provide a high ionic conductivity for a broad variety of mass ratios, a temperature of 60 °C (Fig. 3a) is chosen for further analysis. The conductivity range at this temperature differs by several orders of magnitude. In order to understand this behavior, the ionic conductivity σ of an electrolyte mixture can be viewed as a function of number density n, charge q and mobility μ of ions, see eqn (1).13
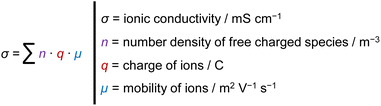 | (1) |
The highest conductivities are achieved with high Pyr14TFSI content. Samples with a Pyr14TFSI content of ≥50 wt% show conductivities greater than 1 mS cm−1, while electrolyte samples with high PEODA as well as high LiTFSI content show comparatively low conductivities. Pyr14TFSI is very ion conductive because the liquid state of the molten salt results in a high mobility μ of charge carriers.26 Exchanging PEODA by Pyr14TFSI elevates the number density n and the overall amount of charges because the concentration of single charge ions like Pyr14+ and TFSI− is increased. In comparison to Pyr14TFSI and LiTFSI, PEODA is non-ionic and therefore decreases the number density of free charged species n and the charge q when increased. Furthermore, when PEODA is cross-linked, it turns from a liquid into a solid. This additionally decreases the ionic conductivity by reducing the overall mobility μ. To achieve conductivities as high as possible, PEODA is therefore reduced in amount to a minimum of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt%. Nonetheless, PEODA is necessary because it is the key component enabling a liquid state as well as the solvent-free phase transition from liquid to solid as described earlier. An increased LiTFSI content improves the ion conductivity only to some extent. While the interaction of ethylene oxide from PEODA and Li+ promotes the LiTFSI dissociation and thereby the number density of free charged species n, the formation of ion clusters at high LiTFSI concentrations decreases the ionic conductivity.27,28 Thus [Li(TFSI)n](n−1)−-complexes are formed, leading to a lower mobility μ.29 These effects lead to an optimal low LiTFSI content of 10 wt%. With a PEODA content of 20 wt% the ionic conductivity is 6.8 mS cm−1 for sample P2IL7S1. When the LiTFSI content is increased, the ionic conductivity is decreased (P2IL6S2: 4.5 mS cm−1; P2IL5S3: 1.3 mS cm−1; P2IL4S4: 0.4 mS cm−1). In conclusion, low PEODA-, high Pyr14TFSI- and moderate LiTFSI-contents are optimal for high ionic conductivity.
wt%. Nonetheless, PEODA is necessary because it is the key component enabling a liquid state as well as the solvent-free phase transition from liquid to solid as described earlier. An increased LiTFSI content improves the ion conductivity only to some extent. While the interaction of ethylene oxide from PEODA and Li+ promotes the LiTFSI dissociation and thereby the number density of free charged species n, the formation of ion clusters at high LiTFSI concentrations decreases the ionic conductivity.27,28 Thus [Li(TFSI)n](n−1)−-complexes are formed, leading to a lower mobility μ.29 These effects lead to an optimal low LiTFSI content of 10 wt%. With a PEODA content of 20 wt% the ionic conductivity is 6.8 mS cm−1 for sample P2IL7S1. When the LiTFSI content is increased, the ionic conductivity is decreased (P2IL6S2: 4.5 mS cm−1; P2IL5S3: 1.3 mS cm−1; P2IL4S4: 0.4 mS cm−1). In conclusion, low PEODA-, high Pyr14TFSI- and moderate LiTFSI-contents are optimal for high ionic conductivity.
The ionic conductivity behavior over the inverse of temperature can give insights into the phase behavior as well as the temperature dependency of ion conduction in electrolytes (Fig. 4).13
In Fig. 4a, the plots at high polymer ratios of ≥40 wt% show a non-linear curved trend of the ionic conductivity (logarithmic scaling) over the inverse temperature, which represents typical VFT (Vogel–Fulcher–Tammann) behavior.13 Therefore, the temperature dependent ionic conductivity is described by the VFT equation (eqn (2)). The VFT equation was originally postulated to describe the viscosity behavior of amorphous polymers above the glass transition temperature TG. Because the viscosity and ion mobility can depend on each other, the VFT equation is widely used in polymer electrolyte research to describe the temperature dependency of the ionic conductivity.30 The ion transport is described by the activation energy for ionic conduction EA, the ideal glass transition temperature T0 also known as Vogel temperature (usually about 50 K below TG), the Boltzmann constant kB and the pre-exponential factor σ0.
 | (2) |
As shown from Fig. 4a–d, this behavior is valid for all compositions with a solid content (PEODA and LiTFSI) ≥50 wt%. The characteristic bending of the logarithmic scaled conductivity over the inverse temperature is caused by the reduction of the denominator T by T0 in the exponent of eqn (2). In Fig. 4d a steeper slope is seen from P2IL4S4 to P6IL0S4. This highlights an increased temperature influence when the liquid content (Pyr14TFSI) is exchanged by PEODA. This is explained by the strong temperature dependency of the segmental motions in polymers, which facilitate ion conduction.15 Due to the VFT behavior, an amorphous state without phase transitions is assumed in the measured temperature range. This is verified by DSC e.g., for P2IL5S3 (see ESI, Fig. 14†).
With increasing Pyr14TFSI and decreasing PEODA amounts, a transition from a VFT (sample P9IL0S1) to a linear behavior (sample P2IL7S1) occurs for LiTFSI shares of ≤30 wt% (Fig. 4a–c). The linearity of the logarithmically scaled ionic conductivity over the inverse temperature is observed when T0 ≪ T. The ionic conductivities of these electrolytes follow the Arrhenius equation in which T0 is not considered, see eqn (3).
 | (3) |
This behavior is typical for high Li salt or high ionic liquid content polymer electrolytes called PISE (polymer-in-salt electrolyte) or iongels.13,31,32 For P2IL7S1 (Fig. 4a, red) and P2IL5S3 (Fig. 4c, red) the activation energy EA was derived by fitting the values according to the Arrhenius equation (see ESI, Fig. 15†). Two major differences are visible when comparing the Ionic conductivities plotted over inverse temperature for P2IL7S1 and P2IL5S3. First the values for P2IL7S1 show higher conductivities as a result of the higher ion mobility μ discussed previously, which is expressed by a higher σ0 in the Arrhenius equation. Second the slope of the ionic conductivities plotted over inverse temperature is lower for P2IL7S1 compared to P2IL5S3, which is expressed by a lower EA in the Arrhenius equation. The activation energies are 0.18 eV and 0.23 eV for P2IL7S1 and P2IL5S3, respectively. The values are in agreement with comparable electrolytes from literature.33
 | (5) |
In summary, an Arrhenius type conductivity with low temperature dependency is achieved for electrolytes with high Pyr14TFSI portion. This behavior is favorable compared to VFT-like properties, in which the conductivity drops faster with decreasing temperature. Therefore, Li metal batteries containing electrolytes with high Pyr14TFSI content can be run in a wider temperature range.
Safety and stability
Apart from high ionic conductivity, electrolytes have to match the safety expectations for Li metal batteries. Especially the formation of HSAL, for example Li dendrite growth through the separator/electrolyte, cause a conductive connection of the electrodes and lead to a cell failure by short-circuit. To investigate the ability of the electrolytes of preventing short-circuits by dendrites single discharge polarization tests are performed, see Fig. 5. A rapid voltage drop indicates a short-circuit. For a symmetric Li‖Li cell with P2IL7S1, a short-circuit occurs after 0.42 mA h cm−2, which is too low to be used in practice. But for the Li‖Li cell using an electrolyte with increased LiTFSI content like P2IL5S3 a short-circuit occurs after deposition of 8.80 mA h cm−2. The capacity is increased by a factor of 20 making it applicable in practice. By partially exchanging Pyr14TFSI with LiTFSI, a trade off is made. On the one hand, an increased solid content (LiTFSI or PEODA) reduces the ionic conductivity as discussed above. On the other hand, a reduced liquid content (Pyr14TFSI) improves the mechanical stability of the electrolyte into a more rigid electrolyte, which reduce the risk of short-circuits caused by dendrites.34 The reason for increasing the solid content by LiTFSI instead by PEODA is discussed in the ESI (Fig. 16†).The oxidative stability of the electrolyte P2IL5S3 is studied by linear sweep voltammetry with a sweep rate of 0.5 mV s−1 (OCV to 6 V) in a SST‖Li setup (Fig. 5b). A major current increase is observed for voltages >5.0 V. The current exceeds a value of 10 μA at 5.03 V and increases rapidly from >5.5 V. Therefore accoding to LSV the electrolyte is suitable to operate within the voltage range of common cathode materials like LFP with a average charge–discharge voltage of 3.4 V – 3.8 V vs. Li|Li+.25,35,36 Nonetheless, as shown by Jung et al. the applicability of an electrolyte towards a cathode material is not only determined by the high voltage stability of an electrolyte, but also by the chemistry of the cathode materials.37,38 As mentioned in their work additionally to the electrochemical oxidation pathway of the electrolyte, also the chemical oxidation has to be considered. Due to oxygen release of the NMC622 cathode material at high rates of discharge, which is excellerated at increased temperatures, the overall stability of the system can be limited to voltages <5 V vs. Li|Li+. Furthermore, as reviewed by Cabañero et al. the high voltage stability of the electrolyte is effected by the chemical reactivity of the cathode aswell as its surface area.39 In case of TSPEs the oxidation of PEO or PEODA at the cathode interface can limit the electrolyte stability and the application towards NMC622.35,40,41 Therefore, additionally to the LSV measurement, the effect of cycling towards LFP and NMC622 are shown in Fig. 10 and 19 in the ESI.† In order to apply NMC cathodes the TSPE composition has to be modified, which is of interest in an upcoming work.
The temperature stability of the electrolyte is measured by TGA over a temperature range from 20 °C to 600 °C. At ≥300 °C the decomposition of PEODA is observed.42 From 360 °C on, LiTFSI and Pyr14TFSI decompose.42–44 In summary, an overall high temperature stability of 300 °C is reached, which is comparable to PEO-Pyr14TFSI-LiTFSI-based electrolytes reported in literature.44
The optimized TSPE composition of P2IL5S3 is shown in Fig. 6. In summary, the 20 wt% of PEODA enable the liquid to solid phase transition, the 50 wt% of Pyr14TFSI boost the ionic conductivity compared to IL-free TSPEs and the 30 wt% LiTFSI improve the short-circuit prevention.
Electrochemical performance
To reveal electrochemical performance and limitations of the electrolyte, the transference number t+ is measured by the Bruce–Vincent method.45 A small constant voltage (ΔV) of 20 mV ± 1 mV is applied to a Li|P2IL5L3|Li cell. The current drops from the initial current (I0) until a constant concentration gradient of ions over the electrolyte is achieved and a lower steady state current (ISS) is reached. In the ideal case, the steady state current ISS is divided by the initial current I0 results in the t+, see eqn (4).
 | (4) |
For solid electrolytes, overvoltages resulting from the interfacial/interphasial (I&I) resistance must be considered, as well. The extended equation with correction terms for the I&I resistance with the I&I resistance before polarization R0 and the I&I resistance under steady state RSS is shown in eqn (5).
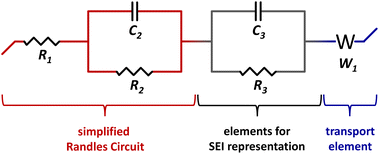
I&I resistances are determined by electrochemical impedance spectroscopy. The impedance spectra are fitted by a representative circuit built of the elements: resistor, capacitor and transport (Warburg) element. The simplified Randles Circuit represents an ideal symmetric cell without diffusion and is extended to use it for fitting the Nyquist plots, see inset Fig. 8a. R1 is the electrolyte resistance. The parallel connection of the R2/C2 element represents the Li|electrolyte interface. C2 is the capacitive behavior of the ionic double layer at the Li|electrolyte interface and R2 represents the resistance for charge-transfer at the Li|electrolyte interface. A transport (Warburg) element (W1) for semi-infinite diffusion is used representing Li-ion diffusion at low frequencies. Because the R1 + (R2/C2) + W1 circuit does not take into account non-ideal phenomenons like the formation of an additional interphase by a SEI a second R3/C3 element is added.46 The equivalent circuit R1 + (R2/C2) + (R3/C3) + W1 is shown in Fig. 7.
The current response of a Li| P2IL5L3|Li cell at a constant voltage of 19.2 mV is shown in Fig. 8a (I0 = 0.2493 mA, ISS = 0.0254 mA). To determine the t+ by the Bruce–Vincent method, impedance spectra are measured before and after polarization. The inset shows the Nyquist plots before and after polarization fitted by the equivalent circuit of Fig. 7 (R0 = 28.97 Ω, RSS = 29.25 Ω). An average t+ of 0.063 ± 0.004 is determined. The t+ highlights the formation of [Li(TFSI)n](n−1)− complexes at high LiTFSI content, which, on one hand, decreases Li-ion mobility and reduces t+, but on the other hand, results in a great prevention of dendrite growth, as discussed before. The value is comparable to other Pyr14TFSI-based systems form literature with a t+ of 0.09.47
A charge-pulse method was applied to investigate the limiting current density.48 The resulting voltage over time plots for charge-pulses of 1.2 mA cm−2 to 2.2 mA cm−2 are shown in Fig. 8b. The cutoff voltage (1 V) is reached faster with increasing current density, indicating a faster depletion of Li+ in the electrolyte at the Li metal|electrolyte interface. As described by Wetjen et al., the depletion time and the current density are used to derive the inverse charge density over current density, see Fig. 8c. By linear fitting the limiting current density is obtained from the x-axis intercept. The resulting limiting current density is 0.46 ± 0.03 mA cm−2. This high limiting current density highlights that despite the low t+ an electrolyte capable of cycling at sufficient rates has been synthesized.
Reversibility of Li metal electrodeposition and -dissolution
The Coulombic efficiency of Li metal electrodeposition and -dissolution is studied by cycling a Cu‖Li cell.49 During electrodeposition/dissolution of Li metal on/from the Cu surface, active Li is lost by the formation of dead Li or side reactions causing a Coulombic efficiency <100% such as SEI formation. In a Cu‖Li cell with the electrolyte P2IL5S3, a Coulombic efficiency of 58% is determined in the first cycle (Fig. 9a). With increasing cycle number, the Coulombic efficiency increases to 93% where it stabilizes after the 90th cycle. In comparison to PEOref from literature the Coulombic efficiency of P2IL5L3 in Cu‖Li cells is higher as well as more stable.22 The homogeneity of Li metal deposition is further evaluated by using a laser scanning microscope, see Fig. 18.†The stability of Li cycling is studied in a Li‖Li cell. The voltage over time profile is shown in Fig. 9b. The high overvoltage in the first cycle (−0.14 V, 0.12 V) is reported to be caused by the native SEI of Li2O and Li2CO3 on the Li metal surface.50 After the first three cycles, the overvoltage drops to ±0.09 V due to the deposition of fresh Li metal without a thick SEI layer, as well as an presumably increased Li surface area.8 Because of the accumulation of dead Li, the Li-ion diffusion through the I&I of the Li metal anode becomes more resistive at later cycles leading to the overvoltage slightly increasing to ±0.12 V after 500 h.51
Additionally, the stability of Li cycling as well as the Coulombic efficiency of Li metal electrodeposition and -dissolution is verified in LFP‖Li cells with P2IL5S3 electrolyte, see Fig. 10. Due to flexibility of the electrolyte the rough cathode surface is contacted well by the P2IL5L3 electrolyte, see Fig. 17 in the ESI.† The Coulombic efficiency of LFP|P2IL5S3|Li cells is 99% in the first cycle and drops to an average of 94% after 10 cycles at which it remains stable, see Fig. 10a. The Coulombic efficiency is slightly higher than in Cu‖Li cells (93%) which is possibly caused by a higher Li loss of highly reactive HSAL on the Cu surface. During cycling, the specific capacity remains at a high level, it starts at 169 mA h g−1 which is close to the maximum theoretical capacity of LFP. At the 100th, cycle 88% (150 mA h g−1) of the initial capacity is maintained, see Fig. 10a and b. During cycling, the overvoltage increases slightly which can be explained by the SEI and dead lithium formation discussed previously.
Finally, the P2IL5S3 is compared to a PEO-based electrolyte PEOref from literature, see Table 2.20,22 As shown in this work by using PEODA instead of PEO the electrolyte can be processed without elevated temperature or the application of processing solvents. Furthermore, the higher Li salt content of P2IL5S3 (30%) compared to PEOref (18%) enables a higher capacity utilization (short-circuit prevention). Due to the smaller polymer chains P2IL5S3 (3.2 MPa) is more rigid compared to PEOref (0.3 MPa) providing a more stable film during application.
| Name | Polymer type | Compositiona | Capacity utilization | Processing without temperature or solvents | Elastic modulus |
|---|---|---|---|---|---|
| a Without BP. | |||||
| P2IL5S3 | Liquid PEODA (Mn: 700) | 20 wt% PEODA | 8.8 mA h cm−2 | Yes | 3.2 MPa |
| 50 wt% IL | |||||
| 30 wt% Li salt | |||||
| PEOref | Solid PEO (Mn: 4 mio) | 28 wt% PEO | 1.6 mA h cm−2 | No | 0.3 MPa |
| 54 wt% IL | |||||
| 18 wt% Li salt | |||||
Conclusion
In this study, a ternary electrolyte (containing polymer, ionic liquid and Li salt) was studied. An alternative electrolyte processing without processing solvents or hot pressing, but taking advantage from a liquid to solid phase transition was enabled. The electrolyte system was sequentially optimized in relation to ionic conductivity and safety. The resulting electrolyte has a high ionic conductivity of >1 mS cm−1 at 60 °C and is able to prevent short-circuits due to dendrite growth when containing high salt contents. The electrolyte has a sufficient limiting current density of 0.46 mA cm−2 as well as a high thermal stability of >300 °C.The unique processing at RT and without processing solvents presented in this work paves the way for a new research direction towards ternary electrolytes. Different from gel or solvated processing, the liquid electrolyte can directly be coated and solidified on electrodes allowing for an improved contact towards the electrode surface, thin film applications and fast processing. Also, different coatings for anode and cathode, that allow for electrolyte compositions matching the requirements, could be applied.
Author contributions
Lukas HerbersA performed the synthesis of electrolytes, cell assembly, electrochemical measurements and evaluation of data. The DSC and TGA measurements were performed by Debbie BerghusA. The KF measurements were performed by Lea-Sophie KemperA. Martin WinterA,B and Peter BiekerB created the concept of work and supervised the work. Lukas HerbersA wrote the manuscript through contributions of Verena KüpersA, Peter BiekerB and Martin WinterA,B.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Ministry for Culture and Science of North Rhine Westphalia (Germany) for funding this work within the International Graduate School for Battery Chemistry, Characterization, Analysis, Recycling and Application (BACCARA).References
- Z. Cheng, T. Liu, B. Zhao, F. Shen, H. Jin and X. Han, Recent advances in organic–inorganic composite solid electrolytes for all-solid-state lithium batteries, Energy Storage Mater, 2021, 34, 388–416 CrossRef.
- Daniel, C. and Besenhard, J. O., Handbook of battery materials, 2., completely revised and enlarged edition, reprinted, Wiley-VCH, Weinheim, 2012 Search PubMed.
- M. Winter, B. Barnett and K. Xu, Before Li Ion Batteries, Chem. Rev., 2018, 118, 11433–11456 CrossRef CAS PubMed.
- J. Janek and W. G. Zeier, A solid future for battery development, Nat. Energy, 2016, 1, 16141 CrossRef.
- Z. Jiang, Q. Han, S. Wang and H. Wang, Reducing the Interfacial Resistance in All-Solid-State Lithium Batteries Based on Oxide Ceramic Electrolytes, ChemElectroChem, 2019, 6, 2970–2983 CrossRef CAS.
- X. Wang, W. Mai, X. Guan, Q. Liu, W. Tu, W. Li, F. Kang and B. Li, Recent Advances of Electroplating Additives Enabling Lithium Metal Anodes to Applicable Battery Techniques, Energy Environ. Mater., 2021, 4, 284–292 CrossRef CAS.
- B. S. Vishnugopi, A. Verma and P. P. Mukherjee, Morphology-Safety Implications of Interfacial Evolution in Lithium Metal Anodes, J. Phys. Chem. C, 2020, 124, 16784–16795 CrossRef CAS.
- G. Bieker, M. Winter and P. Bieker, Electrochemical in situ investigations of SEI and dendrite formation on the lithium metal anode, Phys. Chem. Chem. Phys., 2015, 17, 8670–8679 RSC.
- A. Manthiram, X. Yu and S. Wang, Lithium battery chemistries enabled by solid-state electrolytes, Nat. Rev. Mater., 2017, 2, 16103 CrossRef CAS.
- W. Zhao, J. Yi, P. He and H. Zhou, Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives, Electrochem. Energy Rev., 2019, 2, 574–605 CrossRef CAS.
- D.-H. Liu, Z. Bai, M. Li, A. Yu, D. Luo, W. Liu, L. Yang, J. Lu, K. Amine and Z. Chen, Developing high safety Li-metal anodes for future high-energy Li-metal batteries: strategies and perspectives, Chem. Soc. Rev., 2020, 49, 5407–5445 RSC.
- J. Wu, L. Yuan, W. Zhang, Z. Li, X. Xie and Y. Huang, Reducing the thickness of solid-state electrolyte membranes for high-energy lithium batteries, Energy Environ. Sci., 2021, 14, 12–36 RSC.
- J. Mindemark, M. J. Lacey, T. Bowden and D. Brandell, Beyond PEO—Alternative host materials for Li + -conducting solid polymer electrolytes, Prog. Polym. Sci., 2018, 81, 114–143 CrossRef CAS.
- Q. Yu, K. Jiang, C. Yu, X. Chen, C. Zhang, Y. Yao, B. Jiang and H. Long, Recent progress of composite solid polymer electrolytes for all-solid-state lithium metal batteries, Chin. Chem. Lett., 2021, 32, 2659–2678 CrossRef CAS.
- L. Li, H. Duan, J. Li, L. Zhang, Y. Deng and G. Chen, Toward High Performance All-Solid-State Lithium Batteries with High-Voltage Cathode Materials: Design Strategies for Solid Electrolytes, Cathode Interfaces, and Composite Electrodes, Adv. Energy Mater., 2021, 11, 2003154 CrossRef CAS.
- Z. Wu, Z. Xie, A. Yoshida, Z. Wang, X. Hao, A. Abudula and G. Guan, Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review, Renewable Sustainable Energy Rev., 2019, 109, 367–385 CrossRef CAS.
- S. Qian, H. Chen, Z. Wu, D. Li, X. Liu, Y. Tang and S. Zhang, Designing Ceramic/Polymer Composite as Highly Ionic Conductive Solid-State Electrolytes, Batteries Supercaps, 2021, 4, 39–59 CrossRef CAS.
- J. R. Nair, L. Imholt, G. Brunklaus and M. Winter, Lithium Metal Polymer Electrolyte Batteries: Opportunities and Challenges, Electrochem. Soc. Interface, 2019, 28, 55–61 CrossRef CAS.
- J. Li, F. Li, L. Zhang, H. Zhang, U. Lassi and X. Ji, Recent applications of ionic liquids in quasi-solid-state lithium metal batteries, Green Chemical Engineering, 2021, 2, 253–265 CrossRef.
- G. T. Kim, G. B. Appetecchi, M. Carewska, M. Joost, A. Balducci, M. Winter and S. Passerini, UV cross-linked, lithium-conducting ternary polymer electrolytes containing ionic liquids, J. Power Sources, 2010, 195, 6130–6137 CrossRef CAS.
- L. M. McGrath and J. F. Rohan, Pyrrolidinium Containing Ionic Liquid Electrolytes for Li-Based Batteries, Molecules, 2020, 25, 6002 CrossRef CAS PubMed.
- M. Zhang, A. L. Gui, W. Sun, J. Becking, O. Riedel, X. He, D. Berghus, V. Siozios, D. Zhou and T. Placke, et al., High Capacity Utilization of Li Metal Anodes by Application of Celgard Separator-Reinforced Ternary Polymer Electrolyte, J. Electrochem. Soc., 2019, 166, A2142–A2150 CrossRef CAS.
- J. Henschel, J. M. Dressler, M. Winter and S. Nowak, Reaction Product Analyses of the Most Active “Inactive” Material in Lithium-Ion Batteries—The Electrolyte. I: Themal Stress and Marker Molecules, Chem. Mater., 2019, 31, 9970–9976 CrossRef CAS.
- X. Zeng, L. Dong, J. Fu, L. Chen, J. Zhou, P. Zong, G. Liu and L. Shi, Enhanced interfacial stability with a novel boron-centered crosslinked hybrid polymer gel electrolytes for lithium metal batteries, Chem. Eng. J., 2022, 428, 131100 CrossRef CAS.
- A. Ahniyaz, I. de Meatza, A. Kvasha, O. Garcia-Calvo, I. Ahmed, M. F. Sgroi, M. Giuliano, M. Dotoli, M.-A. Dumitrescu and M. Jahn, et al., Progress in solid-state high voltage lithium-ion battery electrolytes, Adv. Appl. Energy, 2021, 4, 100070 CrossRef CAS.
- A. K. Tripathi, Ionic liquid–based solid electrolytes (ionogels) for application in rechargeable lithium battery, Mater. Today Energy, 2021, 20, 100643 CrossRef CAS.
- P. M. Bayley, G. H. Lane, L. J. Lyons, D. R. MacFarlane and M. Forsyth, Undoing Lithium Ion Association in Ionic Liquids through the Complexation by Oligoethers, J. Phys. Chem. C, 2010, 114, 20569–20576 CrossRef CAS.
- T. Umecky, Y. Saito, Y. Okumura, S. Maeda and T. Sakai, Ionization condition of lithium ionic liquid electrolytes under the solvation effect of liquid and solid solvents, J. Phys. Chem. B, 2008, 112, 3357–3364 CrossRef CAS PubMed.
- S. Kim, M. Lee, C. Park, A. Park, S. Kwon, J. Cho, S. Kim, S. Rho and W. B. Lee, Molecular dynamics study on lithium-ion transport in PEO branched nanopores with PYR14TFSI ionic liquid, Battery Energy, 2022, 1, 20210013 CrossRef CAS.
- K. M. Diederichsen, H. G. Buss and B. D. McCloskey, The Compensation Effect in the Vogel–Tammann–Fulcher (VTF) Equation for Polymer-Based Electrolytes, Macromolecules, 2017, 50, 3831–3840 CrossRef CAS.
- D. Aidoud, D. Guy-Bouyssou, D. Guyomard, J. Le Bideau and B. Lestriez, Photo-Polymerized Organic Host Network of Ionogels for Lithium Batteries: Effects of Mesh Size and of Ethylene Oxide Content, ECS Trans., 2018, 86, 163–178 CrossRef CAS.
- L. Chen and L.-Z. Fan, Dendrite-free Li metal deposition in all-solid-state lithium sulfur batteries with polymer-in-salt polysiloxane electrolyte, Energy Storage Mater, 2018, 15, 37–45 CrossRef.
- M. Montanino, M. Moreno, F. Alessandrini, G. B. Appetecchi, S. Passerini, Q. Zhou and W. A. Henderson, Physical and electrochemical properties of binary ionic liquid mixtures: (1−x) PYR14TFSI–(x) PYR14IM14, Electrochim. Acta, 2012, 60, 163–169 CrossRef CAS.
- C. Zhu, Y. Ning, Y. Jiang, G. Li and Q. Pan, Double-Network Polymer Electrolytes with Ionic Liquids for Lithium Metal Batteries, Polymers, 2022, 14 Search PubMed.
- X. Yang, M. Jiang, X. Gao, D. Bao, Q. Sun, N. Holmes, H. Duan, S. Mukherjee, K. Adair and C. Zhao, et al., Determining the limiting factor of the electrochemical stability window for PEO-based solid polymer electrolytes: main chain or terminal –OH group?, Energy Environ. Sci., 2020, 13, 1318–1325 RSC.
- Y. Zheng, N. Xu, S. Chen, Y. Liao, G. Zhong, Z. Zhang and Y. Yang, Construction of a Stable LiNi0.8Co0.1Mn0.1O2 (NCM811) Cathode Interface by a Multifunctional Organosilicon Electrolyte Additive, ACS Appl. Energy Mater., 2020, 3, 2837–2845 CrossRef CAS.
- R. Jung, P. Strobl, F. Maglia, C. Stinner and H. A. Gasteiger, Temperature Dependence of Oxygen Release from LiNi0.6Mn0.2Co0.2O2 (NMC622) Cathode Materials for Li-Ion Batteries, J. Electrochem. Soc., 2018, 165, A2869–A2879 CrossRef CAS.
- R. Jung, M. Metzger, F. Maglia, C. Stinner and H. A. Gasteiger, Oxygen Release and Its Effect on the Cycling Stability of LiNixMnyCozO2 (NMC) Cathode Materials for Li-Ion Batteries, J. Electrochem. Soc., 2017, 164, A1361–A1377 CrossRef CAS.
- M. A. Cabañero Martínez, N. Boaretto, A. J. Naylor, F. Alcaide, G. D. Salian, F. Palombarini, E. Ayerbe, M. Borras and M. Casas-Cabanas, Are Polymer-Based Electrolytes Ready for High-Voltage Lithium Battery Applications? An Overview of Degradation Mechanisms and Battery Performance, Adv. Energy Mater., 2022, 12, 2201264 CrossRef.
- K. Nie, X. Wang, J. Qiu, Y. Wang, Q. Yang, J. Xu, X. Yu, H. Li, X. Huang and L. Chen, Increasing Poly(ethylene oxide) Stability to 4.5 V by Surface Coating of the Cathode, ACS Energy Lett., 2020, 5, 826–832 CrossRef CAS.
- Y.-C. Jung, M.-S. Park, D.-H. Kim, M. Ue, A. Eftekhari and D.-W. Kim, Room-Temperature Performance of Poly(Ethylene Ether Carbonate)-Based Solid Polymer Electrolytes for All-Solid-State Lithium Batteries, Sci. Rep., 2017, 7, 17482 CrossRef PubMed.
- S. Li, Y.-M. Chen, W. Liang, Y. Shao, K. Liu, Z. Nikolov and Y. Zhu, A Superionic Conductive, Electrochemically Stable Dual-Salt Polymer Electrolyte, Joule, 2018, 2, 1838–1856 CrossRef CAS.
- M. Nádherná, J. Reiter, J. Moškon and R. Dominko, Lithium bis(fluorosulfonyl)imide–PYR14TFSI ionic liquid electrolyte compatible with graphite, J. Power Sources, 2011, 196, 7700–7706 CrossRef.
- M. Joost, G. T. Kim, M. Winter and S. Passerini, Phase stability of Li-ion conductive, ternary solid polymer electrolytes, Electrochim. Acta, 2013, 113, 181–185 CrossRef CAS.
- J. Evans, C. A. Vincent and P. G. Bruce, Electrochemical measurement of transference numbers in polymer electrolytes, Polymer, 1987, 28, 2324–2328 CrossRef CAS.
- A. Barai, K. Uddin, M. Dubarry, L. Somerville, A. McGordon, P. Jennings and I. Bloom, A comparison of methodologies for the non-invasive characterisation of commercial Li-ion cells, Prog. Energy Combust. Sci., 2019, 72, 1–31 CrossRef.
- P. R. Chinnam, V. Chatare, S. Chereddy, R. Mantravadi, M. Gau, J. Schwab and S. L. Wunder, Multi-ionic lithium salts increase lithium ion transference numbers in ionic liquid gel separators, J. Mater. Chem. A, 2016, 4, 14380–14391 RSC.
- M. Wetjen, G.-T. Kim, M. Joost, M. Winter and S. Passerini, Temperature dependence of electrochemical properties of cross-linked poly(ethylene oxide)-lithium bis(trifluoromethanesulfonyl)imide-N-butyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide solid polymer electrolytes for lithium batteries, Electrochim. Acta, 2013, 87, 779–787 CrossRef CAS.
- B. D. Adams, J. Zheng, X. Ren, W. Xu and J.-G. Zhang, Accurate Determination of Coulombic Efficiency for Lithium Metal Anodes and Lithium Metal Batteries, Adv. Energy Mater., 2018, 8, 1702097 CrossRef.
- J. Wellmann, J.-P. Brinkmann, B. Wankmiller, K. Neuhaus, U. Rodehorst, M. R. Hansen, M. Winter and E. Paillard, Effective Solid Electrolyte Interphase Formation on Lithium Metal Anodes by Mechanochemical Modification, ACS Appl. Mater. Interfaces, 2021, 13, 34227–34237 CrossRef CAS PubMed.
- K.-H. Chen, K. N. Wood, E. Kazyak, W. S. LePage, A. L. Davis, A. J. Sanchez and N. P. Dasgupta, Dead lithium: mass transport effects on voltage, capacity, and failure of lithium metal anodes, J. Mater. Chem. A, 2017, 5, 11671–11681 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02488a |
| This journal is © The Royal Society of Chemistry 2023 |