Open Access Article
Open Access ArticleSpherical Fe7S8@rGO nanoflowers as electrodes with high electrocatalytic performance in dye-sensitized solar cells†
Xiaoyu Wanga,
Wen Wanga,
Jixin Yaoc,
Qingxiao Zhanga,
Xin Gaoa,
Changcheng Lina,
Qun Yangb,
Xueqin Zuob,
Shaowei Jin *ab and
Guang Li
*ab and
Guang Li *a
*a
aSchool of Materials Science and Engineering, Anhui Key Laboratory of Information Materials and Devices, Institute of Physical Science and Information Technology, Anhui University, Hefei 230601, People's Republic of China. E-mail: liguang1971@ahu.edu.cn
bSchool of Physics and Optoelectronic Engineering, Anhui University, Hefei 230601, People's Republic of China. E-mail: jinsw@mail.ustc.edu.cn
cAnhui Province Key Laboratory of Simulation and Design for Electronic Information System, Universities Joint Key Laboratory of Photoelectric Detection Science and Technology in Anhui Province, Hefei Normal University, Hefei, 230601, People's Republic of China
First published on 9th June 2023
Abstract
Dye-sensitized solar cells (DSSCs) can directly convert solar energy into electricity, and have aroused great research interest from researchers. Here, the spherical Fe7S8@rGO nanocomposites were expediently fabricated by facile methods, and applied in DSSCs as counter electrodes (CEs). The morphological features show the porous structure of Fe7S8@rGO, and it is beneficial to enhance the permeability of ions. Reduced graphene oxide (rGO) has a large specific surface area and good electrical conductivity, shortening the electron transfer path. The presence of rGO promotes the catalytic reduction of I3− ions to I− ions and reduces the charge transfer resistance (Rct). The experimental findings show that the power conversion efficiency (PCE) of Fe7S8@rGO as CEs for DSSCs can reach 8.40% (20 wt% for rGO), significantly higher than Fe7S8 (7.60%) and Pt (7.69%). Therefore, Fe7S8@rGO nanocomposite is expected to be an efficient and cost-effective CE material for DSSCs.
1. Introduction
The overconsumption of minerals results in environmental degradation and energy crisis, thus more and more scientists are working on clean, renewable energy sources in order to overcome these problems.1,2 Solar energy is a natural and renewable energy that can alleviate the need for clean energy. Dye-sensitized solar cells (DSSCs) convert radiant energy into electrical energy by simulating photosynthesis in plants.3,4 This energy conversion device has aroused considerable interest from scientists recently because of its simple design, low manufacturing cost and environmental friendliness. A general DSSC contains a TiO2 photoanode, a counter electrode (CE) and an electrolyte consisting of a redox medium, such as iodine/triiodide (I−/I3−).3–6 The counter electrode plays an instrumental part in the catalytic reduction process of I3−. Platinum (Pt) is a traditional CE material owing to its high electrocatalytic properties and favourable electrical behaviour.7 But, the high price of Pt limits its commercial use as a counter-electrode.7,8 Therefore, it is of great relevance to explore a counter electrode material with abundant resources and superior performance to replace the precious metal, in order to promote the wide application of DSSCs.9–11To date, many catalytically active materials have attracted the interest of researchers. Transition metal sulfides (TMSs) and their complexes have also attracted attention due to their unique electrical properties and excellent electrochemical reactivity.12,13 One promising catalytic material is iron sulphide. Iron sulphides are widely distributed and readily available in nature, and Fe7S8 has attracted the attention of scientists because of its high iron content, which is beneficial for electrochemical catalysis.13–15 For example, Y. J. Zhang et al. reported dual-carbon-confined Fe7S8 materials as lithium battery anodes with excellent rate performance and stability.16 However, the poor electrical conductivity of sulfides limits their development in the field of catalysis. Graphene has become an increasingly important material for nanotechnology research owing to its good electrical conductivity and excellent stability in electrochemical environments.14 For example, J. Yao et al. reported the construction of NHCS/NiS/rGO nanocomposites by immobilizing NiS nanosheets on the surface of NHCS and then encapsulating them with rGO, which the PCE of NHCS/NiS/rGO CE in DSSCs is 9.32%.17 TMSs combined with rGO, carbon nanotubes and other carbonaceous materials can improve the stability and electrochemical activity of TMSs.14,18 Therefore, the effective coupling of rGO with sulfide would be a promising option to greatly facilitate I3− reduction and improve PCE.
Herein, Fe7S8 nanospheres were prepared by hydrothermal method combined with sulfidation; and then compounded with different amounts of rGO to obtain Fe7S8@rGO-x (x = 10, 15, 20, 25, 30, 40 wt%; denotes rGO as a percentage by mass of Fe7S8) composites. Characterization tests show the porous structure of Fe7S8@rGO. The abundant nanopores enhance the contact area of the I3− ions and provide more reactive sites for the reduction of the I3− ions. Photocurrent density voltage (J–V) test indicated that Fe7S8@rGO-20 wt% CE had the best power conversion efficiency (PCE) among all samples (8.40%). It's higher than the Fe7S8 (7.60%), Fe7S8@rGO-10 wt% (7.82%), Fe7S8@rGO-15 wt% (8.20%), Fe7S8@rGO-25 wt% (8.25%), Fe7S8@rGO-30 wt% (7.81%), Fe7S8@rGO-40 wt% (7.49%) and the conventional Pt CE (7.69%). Test results for all samples are also presented in the ESI.† The presence of rGO enhances the specific surface area and is more favorable for charge transfer. These results suggest that compounding iron-based sulfides with carbon materials is a promising option.
2. Experimental section
2.1. Materials
Ferric nitrate nonahydrate (Fe(NO3)3·9H2O), glycerol, isopropyl alcohol, absolute ethanol, polyethylene glycol (PEG 20000), nitric acid (HNO3), sulfuric acid (H2SO4), potassium permanganate (KMnO4), hydrogen peroxide (H2O2), sulphur powder and graphite powder were bought from Macklin. Lithium iodide (LiI), lithium perchlorate (LiClO4) and tetrabutyl ammonium iodide, 4-tert-butyl pyridine, acetonitrile, were obtained from Aladdin. All the chemical-grade agents were commercially purchased without further purification.2.2. Synthesis of Fe7S8 microspheres
Typically, 7.5 mL of glycerol was mixed into 52.5 mL of isopropanol and stirred for 30 min using a magnetic stirring station. Glycerol has a high solubility for many compounds and prevents unwanted chemical reactions from occurring in solution. IPA has a high solubility for esters. Subsequently, 0.5 mmol of Fe(NO3)3·9H2O was added to the above solution at once, and then magnetic stirring was continued for 1 h to obtain an orange solution. Transfer the mixture to the reaction kettle and hold at 160 °C for 10 h. In addition, 140 °C and 180 °C were tested, but the results were not satisfactory. After the reaction was complete, the reaction kettle was cooled to room temperature overnight, the samples were washed 5 to 6 times alternately with anhydrous ethanol and deionized water, and the yellow-green precipitate was obtained after centrifugation at 4000 rpm and dried under vacuum in an oven at 60 °C for 12 h to obtain the Fe-glycerate precursors. After that, 20 mg of sulfur powder was weighed in a quartz boat and placed near the inlet end of the tube furnace, and then a quartz boat containing 100 mg of iron glycerate precursor was placed at the outlet end, and the sample was continuously burned in nitrogen at 500 °C for 2 h at a temperature gradient of 3 °C min−1. Finally, we obtained Fe7S8 nanomaterials.2.3. Preparation of Fe7S8@rGO-x nanocomposite
The Fe7S8@rGO-x nanocomposites were obtained by a facile hydrothermal route. A flow chart for the production of Fe7S8@rGO-x is shown in Fig. 1a. First, GO (10 mg, the weight percentage is 20 wt%) were taken in four 100 mL beakers, all of which were added with 30 mL of anhydrous ethanol and sonicated for 4 hours. Subsequently, 50 mg of Fe7S8 was added to the above four solutions, stirred continuously overnight, and then transferred to a Teflon autoclave and held at 160 °C for 8 h. Cool naturally to room temperature after the reaction, washed and centrifuged, and dried in an oven at 60 °C for 12 h to obtain Fe7S8@rGO-20 wt%. Other different components are represented in the ESI.†2.4. The manufacture of DSSCs
In general, the DSSC is made up of a dye-sensitized photoanode TiO2, a counter electrode with a liquid electrolyte. First, the commercially available TiO2 was placed in N719 dye and sensitized at room temperature at the dark level for 20 h. The sensitized TiO2 was removed, rinsed with ethanol, allowed to dry naturally, and then the transparent adhesive tape was applied to both ends of the TiO2. The photoanode TiO2 and CE were held together, and injected electrolytes to fill the void between them. The electrolyte consists of 0.6 mol 1-propy1-2,3-dimethylimidazolium iodide, 0.5 M LiI, 0.05 mol I2, and 0.5 M 4-tert-butyl pyridine. The CEs include Fe7S8, Fe7S8@rGO-x and commercially available Pt. It has to be noted that all equipment other than CEs was obtained from the commercial market. The preparation of the electrodes and the instrumentation for the characterization are shown in the ESI.†3. Result and discussion
3.1. Morphology and structure
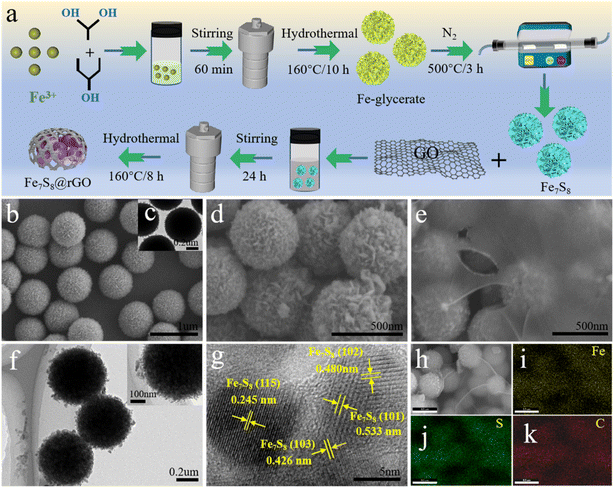
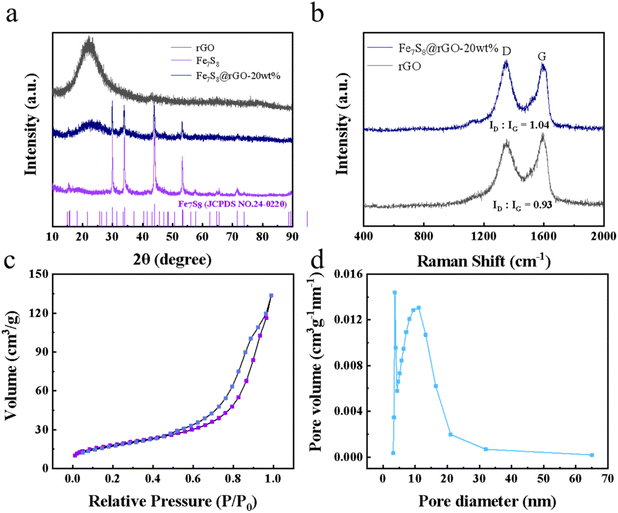
The microstructure of samples can be clearly observed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The SEM image of homogeneous solid Fe-glycerate nanospheres is shown in Fig. 1b. The surface of the prepared Fe-glycerate microspheres contains a large number of nanosheets, which are uniformly distributed with about 500 nm in size. Fig. 1c shows that the Fe-glycerate precursor is a solid sphere with tiny nanosheets interspersed on its surface. The Fe7S8 obtained after sulphation is illustrated in Fig. 1d, and it's also noted that the nanospheres after vulcanization and sintering still maintain their basic morphology without deformation, indicating that the samples exhibit good morphological stability. Meanwhile, it can also be found that the number of nanosheets on the surface of Fe7S8 is more abundant, which allows it to provide a larger specific surface area, which can enable an increased exposure of the active site. It is noteworthy that Fe7S8 spherical nanoflowers are encapsulated inside graphene and are shown in Fig. 1e, where the Fe7S8 and rGO are coupled to each other to form fast channels for electron transfer, in addition to the fact that this encapsulated structure makes Fe7S8 less susceptible to corrosion. As can be seen by TEM in Fig. 1f, the surface of the nanospheres was rougher. In addition, Fig. 1g presents the high-resolution TEM (HRTEM) image of the Fe7S8@rGO-20 wt% composite. Fig. 1g clearly shows multiple lattice fringes with spacings of 0.245 nm, 0.426 nm, 0.480 nm, and 0.533 nm corresponding to the (115), (103), (102), and (101) crystal planes of Fe7S8, respectively, which indicates the good crystallinity of the as-prepared sample. To further identify the existence of Fe, S and C elements in Fe7S8@rGO-20 wt% composites, the distribution of each component was analyzed by elemental mapping. As depicted in Fig. 1h–k, the presence and uniform distribution of Fe, S and C can be found in the energy dispersive X-ray (EDX) mapping.The crystal structure and composition of the prepared materials were determined by X-ray diffraction (XRD) analysis. As exhibited in Fig. 2a, from the XRD pattern of Fe7S8@rGO-20 wt%, a broad diffraction peak near 23.8° can be noted. This diffraction peak corresponds to the (002) crystal plane of carbon, which indicates the highly crystalline structure of rGO.19 Meanwhile, typical diffraction peaks at around 30.0°, 33.9°, 53.4°, 57.6°, 64.8°, and 71.6°, which correspond to the (200), (203), (305), (209), (403) and (406) crystal planes of Fe7S8 (JCPDF, 24-0220), respectively.20,21 The main diffraction peak of the Fe7S8@rGO-20 wt% composite is the same as pure Fe7S8, with a slight weakening of the peak intensity due to the introduction of graphene.
To further confirm the presence of graphene oxide in Fe7S8@rGO-20 wt% composites, it was characterized by Raman spectroscopy with an excitation wavelength of 532 nm. The Raman spectra of rGO and Fe7S8@rGO-20 wt% are displayed in Fig. 2b. In the Raman spectrum, there are two Raman peaks at 1348 cm−1 (D band) and 1580 cm−1 (G band) due to the disordered carbon atoms and the sp2− bonded carbon.22,23 The ratio ID![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IG is commonly applied to measure internal defects in a material, with higher ratios indicating a greater number of defective sites inside the material.10,17,24 It can be observed in the figure that the ID/IG of Fe7S8@rGO-20 wt% is 1.04, while the ID/IG of rGO is 0.93, indicating that the Fe7S8@rGO-20 wt% composite has more defects, which would be more conducive to the catalytic reaction.
IG is commonly applied to measure internal defects in a material, with higher ratios indicating a greater number of defective sites inside the material.10,17,24 It can be observed in the figure that the ID/IG of Fe7S8@rGO-20 wt% is 1.04, while the ID/IG of rGO is 0.93, indicating that the Fe7S8@rGO-20 wt% composite has more defects, which would be more conducive to the catalytic reaction.
Additionally, the specific surface area and pore size of Fe7S8@rGO-20 wt% were examined by N2 adsorption–desorption isotherm. The test results are displayed in Fig. 2c and d. The specific surface area of Fe7S8@rGO-20 wt% can be obtained as 64.9 m2 g−1 based on the calculation of Brunauer–Emmett–Teller (BET). Using the Barrett–Joyner–Halenda (BJH) method to analyze the pore size, the average pore size of Fe7S8@rGO-20 wt% composites is about 12.7 nm. The larger surface area can expand the contact surface with the electrolyte to increase the number of active sites, and the larger pore size will shorten the diffusion path of ions. The porous structure of Fe7S8@rGO-20 wt% ensures better penetration of the electrolyte and further promotes the diffusion of redox pairs, expanding the possibility of efficient transport between electrons and ions, which is beneficial for improving the electrochemical performance of the material.
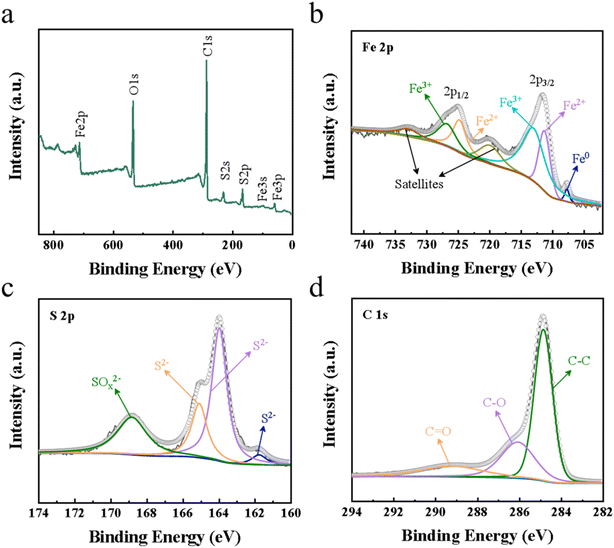
The chemical state of the characteristic elements on the Fe7S8@rGO-20 wt% surface was surveyed by X-ray photoelectron spectroscopy (XPS). Fig. 3a shows the total spectrum of Fe7S8@rGO-20 wt%, from which it can be noted that the complex is composed of Fe, S, C and O. The spectra of Fe 2p, S 2p and C 1s in Fe7S8@rGO-20 wt% are shown in Fig. 3b–d, respectively. In the spectrum of Fe 2p, the presence of three chemical states of Fe can be clearly revealed. The binding energies at 711.1 eV and 724.7 eV are attributed to the presence of Fe2+ species, while the binding energies at 712.9 eV and 726.7 eV are due to the presence of Fe3+ in the material.22–26 The binding energy at 707.6 eV corresponds to the metal Fe0, which may be formed due to the reduction of iron ions by carbon during hydrothermal processes. In addition, two oscillating satellite peaks at 719.8 eV and 732.8 eV can be expected. The S 2p spectrum is given in Fig. 3c. The peaks at 161.7 eV, 163.9 eV and 165.0 eV correspond to S2−, while the broad peak at 168.8 eV is attributed to SOx2−, which is caused by the oxidation of S2− when the specimen is exposed to air.23 For the C 1s spectrum of Fe7S8@rGO-20 wt% composite, the binding energy locate at 284.8 eV, 286.1 eV and 289.2 eV correspond to carbon shell C–C, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.23,26
O, respectively.23,26
3.2. Electrochemical and photovoltaic characterizations
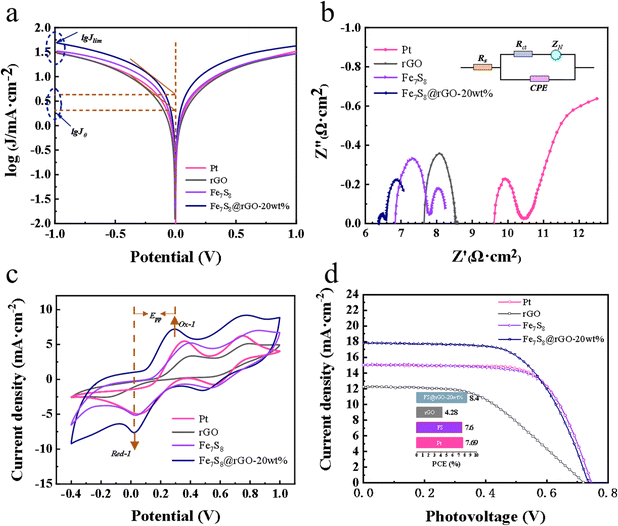
To investigate the transport kinetics of the material, Tafel polarization measurements were made using self-assembled symmetric cells with two identical electrodes (CE/electrolyte/CE).27 Tafel polarization consists of two basic indicators: the exchange current density (J0) and the limiting diffusion current density (Jlim). log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J0 can be acquired through the crossover point of the cathode branch and zero voltage. And the intersection of the cathode branch and the vertical pole in the Tafel curve can be seen as log
J0 can be acquired through the crossover point of the cathode branch and zero voltage. And the intersection of the cathode branch and the vertical pole in the Tafel curve can be seen as log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Jlim. J0 is an accurate indicator of electrochemical reactivity and a large J0 corresponds to a high catalytic performance of CE for the reduction of I3−. Jlim is positively correlated with the diffusion coefficient D. The Tafel curves for the different CEs are presented in Fig. 4a. J0 and Jlim for each sample are obtained from the curves as shown in Table 1. It is worth remarking that CE configured with Fe7S8@rGO-20 wt% reached higher log
Jlim. J0 is an accurate indicator of electrochemical reactivity and a large J0 corresponds to a high catalytic performance of CE for the reduction of I3−. Jlim is positively correlated with the diffusion coefficient D. The Tafel curves for the different CEs are presented in Fig. 4a. J0 and Jlim for each sample are obtained from the curves as shown in Table 1. It is worth remarking that CE configured with Fe7S8@rGO-20 wt% reached higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J0 (0.64 mA cm−2) and log
J0 (0.64 mA cm−2) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Jlim (1.68 mA cm−2), much higher than Pt (0.38 mA cm−2, 1.49 mA cm−2). The results indicated that Fe7S8@rGO-20 wt% has better catalytic activity for the reduction of I3−.
Jlim (1.68 mA cm−2), much higher than Pt (0.38 mA cm−2, 1.49 mA cm−2). The results indicated that Fe7S8@rGO-20 wt% has better catalytic activity for the reduction of I3−.
 | ||
| Fig. 4 (a) Tafel polarization curves, (b) Nyquist plots, (c) CV curves, (d) J–V curves of Pt, rGO, Fe7S8, and Fe7S8@rGO-20 wt% CEs. | ||
| CEs | Rs (Ω cm2) | Rct (Ω cm2) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) J0 (mA cm−2) J0 (mA cm−2) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Jlim (mA cm−2) Jlim (mA cm−2) |
|---|---|---|---|---|
| rGO | 7.64 | 0.44 | 0.30 | 1.47 |
| Fe7S8 | 6.84 | 0.48 | 0.48 | 1.52 |
| Fe7S8@rGO-20 wt% | 6.38 | 0.08 | 0.64 | 1.68 |
| Pt | 9.59 | 0.43 | 0.38 | 1.49 |
Electrochemical impedance spectrum (EIS) is also another measure for catalytic activity at counter electrodes, which like the Tafel polarization needs to be tested with a simulated symmetric cell. As shown in Fig. 4b, a typical impedance spectrum generally consists of two approximate semicircles. The intercept on the horizontal axis in the high-frequency region represents the series resistance (Rs), which is related to the electrical conductivity of the material. The first semicircle in the high-frequency region represents the impedance (Rct) of the charge transfer between the electrode and electrolyte interface and the other semicircle represents the diffusion impedance (ZN). The corresponding Rs and Rct for each sample are summarized in Table 1. The smallest Rs for Fe7S8@rGO-20 wt% is about 6.38 Ω cm2, which is lower than Pt (9.59 Ω cm2), rGO (7.64 Ω cm2), Fe7S8 (6.84 Ω cm2). The sequence of the Rct values is Fe7S8@rGO-20 wt% < Pt < rGO < Fe7S8 CEs, which means that the introduction of rGO can improve the conductivity. Through comparison, it is found that Fe7S8@rGO-20 wt% possesses the smallest transfer impedance, proving that it has the best catalytic activity. This is because the porous structure of Fe7S8 enhances the penetration of electrolyte ions, thus facilitating the diffusion of I3−, and the synergistic effect between graphene and Fe7S8 promotes the rapid transfer of electrons.
To investigate the photovoltaic performance of each material, comparative tests were conducted and the J–V curves for samples are shown in Fig. 4d. The specific data on photovoltaic (PV) parameters such as open-circuit voltage (Voc), fill factor (FF), short-circuit current density (Jsc) acquired from the tests are clearly shown in Table 2, and after obtaining these parameters the corresponding PCE can be calculated by the following eqn (1):
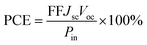 | (1) |
| CEs | Epp (V) | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| rGO | 0.59 ± 0.01 | 12.25 | 0.725 | 48.13 | 4.28 |
| Fe7S8 | 0.36 ± 0.01 | 15.05 | 0.745 | 67.75 | 7.60 |
| Fe7S8@rGO-20 wt% | 0.27 ± 0.01 | 17.83 | 0.740 | 63.64 | 8.40 |
| Pt | 0.33 ± 0.01 | 15.01 | 0.745 | 68.82 | 7.69 |
The difference of PCE mainly comes from the photocurrent density (Jsc), and the change of Jsc is mainly due to the difference of the catalytic ability of the electrode, so the electrocatalytic reduction of I3− on the CE surface is a rate-determining step in DSSCs.28–31 Impressively, the Fe7S8@rGO-20 wt%-based CE reached PCE (8.40%), which is higher than Fe7S8 (7.60%), rGO (4.28%), and Pt (7.69%). The PCE of Fe7S8 CE was significantly smaller than that of Pt CE, but the PCE of the sample was effectively improved after being combined with rGO. It indicates that the PCE of Fe7S8@rGO-x is influenced by the rGO.
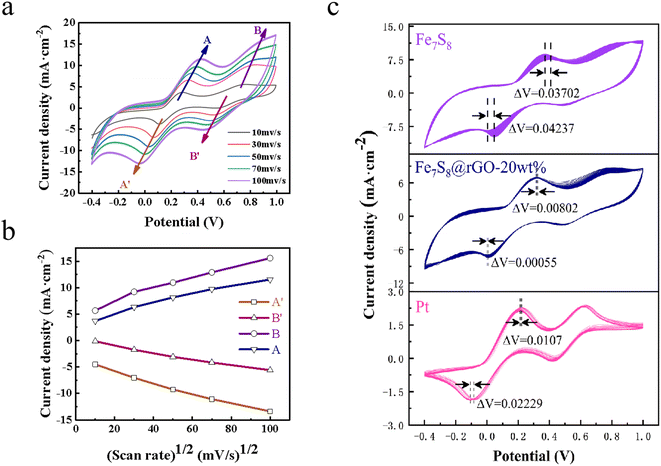
The catalytic ability of various CEs to reduce I3− was further investigated using cyclic voltammetry tests and the CV curves as shown in Fig. 4d were obtained. Two pairs of oxidation–reduction peaks are visible in the graph for each individual curve, the horizontal axis spacing of the first pair of oxidation–reduction peaks (marked as Ox1 and Red1) being Epp.32,33 A smaller Epp value means that the oxidation and reduction reactions of CV alternate more quickly and smoothly, which in turn improves the catalytic properties of the material for the reduction of I3−.32 The coupled peaks Ox1 and Red1 could be described by eqn (2) and (3):
| 3I− − 2e− → I−3 | (2) |
| I−3 + 2e− → 3I− | (3) |
The specific values are listed in Table 2. The graph clearly shows that the Epp of Fe7S8@rGO CE is significantly smaller than that of Pt CE. Fe7S8@rGO-20 wt% has the smallest Epp of about 0.27 V, which is higher than Pt (0.33 V), rGO (0.59 V) and Fe7S8 (0.36 V). In summary, the lowest Epp implies that Fe7S8@rGO-20 wt% has excellent catalytic reduction activity for I3− and can be used as a superior performing CE among DSSCs.
To research the correlation between peak current density and sweep speed in the CV curves, plots of Fe7S8@rGO-20 wt% were measured at different scan rates. From Fig. 5a, it can be concluded that as the electrochemical polarization increases with increasing sweep speed, the overpotential increases and the reversibility decreases. As illustrated in Fig. 5b, the peak current density is related to the square root of the sweep rate, suggesting that Fe7S8@rGO-20 wt% is only reacting with the redox medium, while electron diffusion at the interface between the two is limited.34 Furthermore, stability is also a key parameter to be considered when judging electrode materials. To assess the stability of the samples in the electrolyte, the CV tests were conducted on Fe7S8, Fe7S8@rGO-20 wt% and Pt for 25 consecutive cycles at a scan rate of 25 mV s−1. As can be seen in Fig. 5c, the current density of Fe7S8@rGO-20 wt% did not decrease significantly during cycling, the peak spacing shifts were much smaller than those of Pt and Fe7S8, and the first pair of redox peaks of Fe7S8@rGO-20 wt% had the best overlap. The presence of rGO reduces the corrosive effect of the electrolyte and helps to improve the electrochemical stability of the material. Thus Fe7S8@rGO-20 wt% maintains good electrochemical stability as CE for DSSCs. Fe7S8@rGO-20 wt% may be a promising electrode material in DSSCs.
 | ||
| Fig. 5 (a) CV curves of Fe7S8@rGO-20 wt% CE at different scan rates, (b) peak current densities curve, (c) continuous CV test curves of different CE. | ||
4. Conclusions
In summary, we have successfully synthesized porous Fe7S8@rGO-x composites, and investigated their photovoltaic and electrochemical properties. The experimental results show that Fe7S8@rGO nanomaterials as CEs perform better than Pt in DSSCs. The PCE of the DSSCs assembled by Fe7S8@rGO-20 wt% can reach 8.40%, which is better than that of Pt (7.69%). The excellent performance is on the one hand due to the abundant nanopores and large specific surface area, which enhances the contact area with I3− and provides more active sites for charge transfer and reduction of I3−. On the other hand, the hybridization of Fe7S8 with rGO facilitates charge transfer and reaction kinetics. And cycle stability tests indicate that the electrochemical stability of the Fe7S8@rGO-20 wt% composite is also superior to that of Pt. This is because the presence of rGO reduces the corrosion of the sample by the electrolyte. Such excellent properties indicate that Fe7S8@rGO composite may be a very promising CE material. The research also offers a hopeful idea for effectively improving the performance of DSSCs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2017YFA0403503), the National Natural Science Foundation of China (11674001), the Key Projects of Natural Science Research in Universities of Anhui Province (KJ2020A0018), the Key Natural Science Research Program of Anhui Educational Committee (KJ2018ZD001), 2021 Postgraduate Research Project in Universities – Transition Metal Compounds in Dye Sensitized Solar Cells, Bi-Directional Application of Oxygen Precipitation Reaction (Y020410004).References
- C. Zhao, N. Li, R. Zhang, Z. Zhu, J. Lin, K. Zhang and C. Zhao, ACS Appl. Mater. Interfaces, 2019, 11, 47858–47867 CrossRef CAS PubMed.
- H. Fu, Z. Li, Z. Liu and Z. Wang, Sustainability, 2018, 10, 2488 CrossRef.
- C. Zhang, L. Deng, P. Zhang, X. Ren, Y. Li and T. He, Electrochim. Acta, 2017, 229, 229–238 CrossRef CAS.
- B. Kilic, S. Turkdogan, O. C. Ozer, M. Asgin, O. Bayrakli, G. Surucu, A. Astam and D. Ekinci, Mater. Lett., 2016, 185, 584–587 CrossRef CAS.
- J. Qiu, D. He, R. Zhao, B. Sun, H. Ji, N. Zhang, Y. Li, X. Lu and C. Wang, J. Colloid Interface Sci., 2018, 522, 95–103 CrossRef CAS PubMed.
- W. Zhang, R. Zhu, L. Ke, X. Liu, B. Liu and S. Ramakrishna, Small, 2010, 6, 2176–2182 CrossRef CAS PubMed.
- Q. Wang, Y. Wang, P. Wei, X. Liu and L. Duan, Appl. Surf. Sci., 2019, 494, 1–7 CrossRef CAS.
- A. Sarkar, S. Bera and A. K. Chakraborty, Sol. Energy, 2020, 208, 139–149 CrossRef CAS.
- S. Lu, M. Chen, Y. Wang, R. Li, J. Lin and X. Zhang, Sol. Energy, 2021, 220, 788–795 CrossRef CAS.
- Q. Yang, X. Zuo, J. Yao, K. Zhang, H. Zhang, M. W. Khan, W. Wang, H. Tang, M. Wu, G. Li and S. Jin, J. Electroanal. Chem., 2019, 844, 34–42 CrossRef CAS.
- K. Xiong, G. Li, C. Jin and S. Jin, Mater. Lett., 2016, 164, 609–612 CrossRef CAS.
- J. Ma, W. Shen and F. Yu, J. Power Sources, 2017, 351, 58–66 CrossRef CAS.
- A. Sarkar, A. K. Chakraborty and S. Bera, Sol. Energy Mater. Sol. Cells, 2018, 182, 314–320 CrossRef CAS.
- R. Kang, S. Li, B. Zou, X. Liu, Y. Zhao, J. Qiu, G. Li, F. Qiao and J. Lian, J. Alloys Compd., 2021, 865, 158824 CrossRef CAS.
- N. Cheng, X. Chen, L. Zhang and Z. Liu, J. Energy Chem., 2021, 54, 604–611 CrossRef CAS.
- Y. J. Zhang, W. Chang, J. Qu, S. M. Hao, Q. Y. Ji, Z. G. Jiang and Z. Z. Yu, Chemistry, 2018, 24, 17339–17344 CrossRef CAS PubMed.
- J. Yao, W. Wang, X. Zuo, Q. Yang, M. W. Khan, M. Wu, H. Tang, S. Jin and G. Li, Appl. Catal., B, 2019, 256, 117857 CrossRef CAS.
- Z. Zhao, X. Teng, Q. Xiong, H. Chi, Y. Yuan, H. Qin and Z. Ji, Sustainable Mater. Technol., 2021, 29, e00313 CrossRef CAS.
- B. Liu, F. Zhang, Q. Wu, J. Wang, W. Li, L. Dong and Y. Yin, Mater. Chem. Phys., 2015, 151, 60–65 CrossRef CAS.
- M. J. Choi, J. Kim, J. K. Yoo, S. Yim, J. Jeon and Y. S. Jung, Small, 2018, 14, 1–6 Search PubMed.
- Y. Wang, Z. Wen, C. C. Wang, C. C. Yang and Q. Jiang, Small, 2021, 17, 1–10 Search PubMed.
- L. Shi, D. Li, J. Yu, H. Liu, Y. Zhao, H. Xin, Y. Lin, C. Lin, C. Li and C. Zhu, J. Mater. Chem. A, 2018, 6, 7967–7976 RSC.
- F. Jiang, Q. Wang, R. Du, X. Yan and Y. Zhou, Chem. Phys. Lett., 2018, 706, 273–279 CrossRef CAS.
- F. Du, X. Zuo, Q. Yang, G. Li, Z. Ding, M. Wu, Y. Ma and K. Zhu, J. Mater. Chem. C, 2016, 4, 10323–10328 RSC.
- Y. He, Y. Xu, J. Li, Z. Xu, Z. Zhang, J. Sun, M. Zhang, X. Zhu and X. Zhou, Energy Fuels, 2021, 35, 3490–3496 CrossRef CAS.
- Q. Zhang, J. Liao, M. Liao, J. Dai, H. Ge, T. Duan and W. Yao, Appl. Surf. Sci., 2019, 473, 799–806 CrossRef CAS.
- J. Yao, K. Zhang, W. Wang, X. Zuo, Q. Yang, H. Tang, M. Wu and G. Li, ACS Appl. Mater. Interfaces, 2018, 10, 19564–19572 CrossRef CAS PubMed.
- I. T. Chiu, C. T. Li, C. P. Lee, P. Y. Chen, Y. H. Tseng, R. Vittal and K. C. Ho, Nano Energy, 2016, 22, 594–606 CrossRef CAS.
- M. Gurulakshmi, A. Meenakshamma, K. Susmitha, N. Charanadhar, V. V. S. S. Srikanth, S. Narendra Babu, Y. P. Venkata Subbaiah, K. Venkateswarlu and M. Raghavender, Sol. Energy, 2019, 193, 568–575 CrossRef CAS.
- S. Yun, A. Hagfeldt and T. Ma, Adv. Mater., 2014, 26, 6210–6237 CrossRef CAS PubMed.
- A. Listorti, B. O'Regan and J. R. Durrant, Chem. Mater., 2011, 23, 3381–3399 CrossRef CAS.
- L. Shao, X. Qian, H. Li, C. Xu and L. Hou, Chem. Eng. J., 2017, 315, 562–572 CrossRef CAS.
- W. Wang, X. Zuo, Q. Yang, Q. Yang, H. Tang, H. Zhang and G. Li, Appl. Catal., B, 2022, 300, 120726 CrossRef CAS.
- W. Hou, Y. Xiao, G. Han and H. Zhou, Electrochim. Acta, 2016, 190, 720–728 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02457a |
| This journal is © The Royal Society of Chemistry 2023 |