Open Access Article
Open Access ArticleEnhancing the biochemical growth of Haematococcus pluvialis by mitigation of broad-spectrum light stress in wastewater cultures†
Megha Mourya‡
a,
Mohd. J. Khan‡a,
Vandana Sirotiya‡ab,
Ankesh Ahirwarab,
Benoit Schoefs b,
Justine Marchandb,
Sunita Varjanicd and
Vandana Vinayak
b,
Justine Marchandb,
Sunita Varjanicd and
Vandana Vinayak *a
*a
aDiatom Nanoengineering and Metabolism Laboratory (DNM), School of Applied Science, Dr. Hari Singh Gour Central University, Sagar, MP 470003, India. E-mail: kapilvinayak@gmail.com
bMetabolism, Bioengineering of Microalgal Metabolism and Applications (MIMMA), Biology of Organisms, Stress, Health and Environment, Le Mans University, IUML - FR 3473 CNRS, Le Mans, France
cSchool of Energy and Environment, City University of Hong Kong, Tat Chee Avenue, Kowloon, 999077, Hong Kong
dSustainability Cluster, School of Engineering, University of Petroleum and Energy Studies, Dehradun-248 007, Uttarakhand, India
First published on 12th June 2023
Abstract
In this study, the microalgae Haematococcus pluvialis were cultivated in wastewater inoculated into low-density polypropylene plastic air pillows (LDPE-PAPs) under a light stress. The cells were irradiated to different light stresses using white LED lights (WLs) as the control, and broad-spectrum lights (BLs) as a test for the period of 32 days. It was observed that the inoculum (70 × 102 mL−1 cells) of H. pluvialis algal cells increased almost 30 and 40 times in WL and BL, respectively, at day 32 coherent to its biomass productivity. Higher lipid concentration of up to 36.85 μg mL−1 was observed in BL irradiated cells compared to 13.215 μg L−1 dry weight of biomass in WL. The chlorophyll ‘a’ content was 2.6 times greater in BL (3.46 μg mL−1) compared to that in WL (1.32 μg mL−1) with total carotenoids being about 1.5 times greater in BL compared to WL on day 32. The yield of red pigment 'Astaxanthin' was about 27% greater in BL than in WL. The presence, of different carotenoids including astaxanthin was also confirmed by HPLC, whereas fatty acid methyl esters (FAMEs) were confirmed by GC-MS. This study further confirmed that wastewater alongwith with light stress is suitable for the biochemical growth of H. pluvialis with good biomass yield as well as carotenoid accumulation. Additionally there was 46% reduction in chemical oxygen demand (COD) in a far more efficient manner when cultured in recycled LDPE-PAP. Such type of cultivation of H. pluvialis made the overall process economical and suitable for upscaling to produce value-added products such as lipids, pigments, biomass, and biofuel for commercial applications.
1. Introduction
Microalgae such as H. pluvialis are a fount of an extensive collection of natural products including carotenoids, astaxanthin, lipids, carbohydrates, and proteins.1–4 Their biomass is capable to build up a notable quantity of these compounds and can be used in nutraceutical, pharmaceutical, and cosmetics industries besides being a reservoir of biofuel.4–6 They produce astaxanthin, which is known as the ‘king of ketocarotenoids’ and has stormed the commercial market with its price shooting to 2.57 billion US$ in 2025 which can reach up to 3.4 billion US$ by 2027.1,7–9 However, the factors that play a role in controlling the growth of H. pluvialis, metabolism, and biochemical composition for producing value-added products are temperature, pH, light intensity, and nutrient availability.10 Among these environmental factors, light has been acknowledged as an essential factor for microalgal growth as it is one of the key aspects that regulate the quantity of accessible energy for photosynthesis and its impact on growth.10,11 Microalgae have emitted adaptable techniques in reaction to a strong light environment.12 There are many statements on the combined effects of high light intensity on biomass production and biomass composition; thus, it is essential to evaluate the interactive outcome of light on the growth and biochemical composition of microalgae.13 This study was focused on analysing the growth and metabolic level under different light intensities in H. pluvialis, which is one of the richest sources of pigments and lipids.14,15 Two different artificial light stresses were used to compare the growth of H. pluvialis cells in synthetic wastewater (SWW) exposed to WL (white light) and the BL (broad spectrum light) combination of blue and red LED lights.16 Additionally, the novelty of this study is that it has also focused on plastic waste management as well as wastewater management.17 Wastewater has been used as a growth medium for the algal cells as they are rich in nitrates and phosphates and recycling them has overall reduced the pollution.18 In earlier studies, the treatment of wastewater by microalgae using other techniques such as microbial fuel cells has been demonstrated and have been very effective in removing pollutants.19–21 On the offset, recycling discarded plastic waste has given rise to bubble farming wherein microalgal farming is performed in plastic bubble wraps.22 Low-density polypropylene plastic bubble wraps (LDPE-PAP), which are discarded plastic waste filled with air are used as packing material in the transportation of goods. The use of LDPE-PAP for the purpose of cultivating microalgae (H. pluvialis) offers a more sustainable approach for growing microalgae at a large scale in comparison to open and closed photobioreactors, which are not cost-effective processes and techniques. In earlier reports, it has been shown that the microalgae (diatoms) when cultivated in conical flasks sealed with LDPE-PAP, were well adapted for gaseous exchange without any loss of water from its media thus simulating a closed economical photobioreactor.23 Thus, this time, inspired by previous results, microalgae (H. pluvialis) cells were cultivated in bigger plastic air pillows with SWW instead of standard nutrient media under BL and WL and their biochemical parameters were analyzed and compared. Thus, the overall aim of the present study was to recycle discarded plastic waste by using it in algal cultivation with bubble wrap. This method not only reduces plastic disposal and contamination risks but it also helps to minimize cultivation costs. Additionally, the bubble wrap prevents evaporation and can be reused for further cultivation cycles and can be resealed if it becomes damaged. This means that the reduction in the loss of water and nutrients during the cultivation process makes LDPE-PAP a sustainable and cost-effective solution for recycling plastic and wastewater waste while promoting eco-friendly cultivation practices. While the primary objective of our research is to examine this innovative approach, we also included an evaluation of different light sources, including broad-spectrum light, to provide additional insights into optimizing the cultivation of H. pluvialis for value added compounds.2. Materials and methods
2.1. Microalgae strain and culture medium
H. pluvialis cultures were obtained from laboratory-maintained cultures grown in BG-11 media and later optimised for growth in SWW. The SWW was used to promote the growth of microalgal cells as per standard laboratory protocol reported elsewhere24 with few modifications rich in, C12H22O11, NaHCO3, NH4Cl, K2HPO4, KH2PO4, MgSO4·7H2O, FeSO4·7H2O, CaCl2·2H2O, CH3COONa, and trace metal solution at concentrations of 2.70 g L−1, 4.50 g L−1, 0.95 g L−1, 0.08 g L−1, 0.03 g L−1, 0.19 g L−1, 0.06 g L−1, 0.75 g L−1, 3.84 g L−1 and 1.5 mL−1, respectively, in 1 L of distilled water.25 The stock of trace metal solutions however, contained mainly FeSO4·7H2O = ¼ 10 mg L−1, NiSO4 6H2O = ¼ 0.50 mg L−1, MnCl2·4H2O = 0.50 mg L−1, ZnSO4·7H2O = 0.10 mg L−1, H3BO3 = 0.10 mg L−1, CoCl2·26H2O = 50 μg L−1, CuSO4·5H2O ¼ = 5 μg L−1, and (NH4)6Mo7O24·4H2O = 50 μg L−1.26The total COD of SWW initially was 41.71 mg L−1. The cultures were maintained at a temperature of 25 ± 1 °C under different light conditions viz.; WL of light intensity 5 klx as the control and BL popularly known as LED grow lights of light intensity 5 klx (combination of blue and red) as a test. Each light source had the power of 20 W irradiated for fixed light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark regime time period of 16
dark regime time period of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h for 32 days. LDPE-PAPs of capacity 300 mL, were used as a mini photobioreactor instead of regular conical flasks or bottles with plugs. Each LDPE-PAP was filled with 180 mL of SWW using a U-100 injection syringe. Approximately, 70 × 102 mL−1 cells of H. pluvialis in their green stage were inoculated in the LDPE-PAP and sealed as discussed elsewhere.23 The control and test samples were tested for their growth and biochemical composition pattern on day 0 (day of inoculation), day 2, 4, 8, 16, and 32 in triplicates and analysed biochemically at every stage of the experiment. The control and test microalgal samples in LDPE-PAP were further compared for their growth pattern via optical density at 750 nm with a standard conical flask inoculated with same parameters kept under the same conditions but sealed with a cotton plug for 32 days to establish variation if any in the growth pattern of microalgal cells in a conical flask and those grown in LDPE-PAP. However, parameters such as the rate of the loss of water content in the conical flask and LDPE-PAP have been previously reported.27
8 h for 32 days. LDPE-PAPs of capacity 300 mL, were used as a mini photobioreactor instead of regular conical flasks or bottles with plugs. Each LDPE-PAP was filled with 180 mL of SWW using a U-100 injection syringe. Approximately, 70 × 102 mL−1 cells of H. pluvialis in their green stage were inoculated in the LDPE-PAP and sealed as discussed elsewhere.23 The control and test samples were tested for their growth and biochemical composition pattern on day 0 (day of inoculation), day 2, 4, 8, 16, and 32 in triplicates and analysed biochemically at every stage of the experiment. The control and test microalgal samples in LDPE-PAP were further compared for their growth pattern via optical density at 750 nm with a standard conical flask inoculated with same parameters kept under the same conditions but sealed with a cotton plug for 32 days to establish variation if any in the growth pattern of microalgal cells in a conical flask and those grown in LDPE-PAP. However, parameters such as the rate of the loss of water content in the conical flask and LDPE-PAP have been previously reported.27
2.2 Cell count and biomass
Light plays a important role in controlling not only the cell density of microalgal cells but light sources like blue light favours pigment formation.28 The cell density of H. pluvialis cells was estimated by counting the cells in a Neubauer counting chamber29 under a compound light microscope (Olympus CH20i, Japan) on days 0, 2, 4, 8, 16, and 32 for both WL and BL. Biomass productivity was for control and test cells was calculated in a 15 mL pre-weighed centrifuge tube which was centrifuged at 4000 rpm for 5 to 10 min for pellet formation and dried subsequently at 40 °C and again weighed. To determine the weight of microalgal biomass the following formula was used as shown in eqn (1).| Total weight of biomass = weight of tube with sample − weight of empty tube | (1) |
2.3 Chlorophyll estimation
Different formulas were used for the calculation of different chlorophylls and carotenoids as shown in eqn (2)–(5).
| Chlorophyll ‘a’ (Ca) = (13.36 × A664.2 − 5.19 × A648.6) | (2) |
| Chlorophyll ‘b’ (Cb) = (27.43 × A648.6 − 8.12 × A664.2) | (3) |
| Chlorophyll ‘a + b’ (Ca + Cb) = [5.24 × A664.2 + 22.24 × A648.6] | (4) |
| Carotenoids C (x + c) = (1000 × A470 − 2.13Ca − 97.64Cb)/209 | (5) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) ratio. The samples were incubated for 5 min at 70 °C and again, centrifuged at 4000 rpm for 5 min. Thereafter, the supernatant was discarded to remove chlorophyll and the pellet was washed 3 times with Milli Q water followed by the addition of 5 mL DMSO till the pellet become colourless.31 The amount of astaxanthin was calculated as per the slope equation obtained via standard plot of pure astaxanthin purchased from Sigma (SML0982, Aldrich, USA) at 490 nm spectrophotometrically.
1) ratio. The samples were incubated for 5 min at 70 °C and again, centrifuged at 4000 rpm for 5 min. Thereafter, the supernatant was discarded to remove chlorophyll and the pellet was washed 3 times with Milli Q water followed by the addition of 5 mL DMSO till the pellet become colourless.31 The amount of astaxanthin was calculated as per the slope equation obtained via standard plot of pure astaxanthin purchased from Sigma (SML0982, Aldrich, USA) at 490 nm spectrophotometrically.2.4 Lipid extraction and estimation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 v/v/v as per the protocol suggested by Bligh and Dyer.32 Samples were vigorously stirred in a vortex for 10 minutes then centrifuged at 4000 rpm for 5 min and kept at 37 °C temperature for about 1–2 hours. Two separate layers appeared comprising the lower organic layer having oil and the upper aqueous layer containing the cell debris. The oil-rich lower organic layer was pipetted out into a pre-weighed microcentrifuge tube. The percentage of lipids was determined using the following formula as shown in eqn (6).
0.8 v/v/v as per the protocol suggested by Bligh and Dyer.32 Samples were vigorously stirred in a vortex for 10 minutes then centrifuged at 4000 rpm for 5 min and kept at 37 °C temperature for about 1–2 hours. Two separate layers appeared comprising the lower organic layer having oil and the upper aqueous layer containing the cell debris. The oil-rich lower organic layer was pipetted out into a pre-weighed microcentrifuge tube. The percentage of lipids was determined using the following formula as shown in eqn (6).
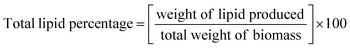 | (6) |
3. Results and discussion
3.1. Cell count and biomass productivity
H. pluvialis cells grown in LDPE-PAP under WL and BL irradiation for a period of 32 days showed that on the day of inoculation, cell count was 7 × 102 mL−1 for both WL and BL, which increased up to 223 × 103 cells per mL and 510 × 103 cells per mL, respectively, on day 32 (Fig. 1A). Likewise, on the inoculation day, the dry cell weight of biomass was 1 μg L−1 for WL and BL. Biomass productivity increased up to 23 μg L−1 and 26 μg L−1 for WL and BL, respectively, on day 32. Although the experiment was conducted for 32 days, cell division reached its maximum around 8 days, as shown in Fig. 1, and remained approximately similar throughout the 32 days period. It was evident that the cell size may have increased despite the limited cell division. This increase could be due to the depletion of a nitrogen source inside the cells. Additionally, the accumulation of biomass could also be linked to the thickening of cell walls and increased diameter of H. pluvialis during the culture period.36 This indicated that the cell growth in the SWW inoculated in recycled LDPE-PAP followed the standard growth patterns and showed healthy quasi-exponential growth patterns of cells on different days since inoculation. A similar growth pattern was observed for the same number of microalgal cells in a conical flask sealed with a cotton plug in a flask under white light, which showed that the growth of microalgal cells was similar to that for the cells grown in SWW in LDPE-PAP. This showed that the LDPE-PAP allowed sufficient gas exchange and WL favoured growth in a similar manner as in a conical flask sealed with a cotton plug (Fig. S1†). However, LDPE-PAP is still preferred as it is economical, recyclable, prevents water loss and contamination, and is a cheap way of upscaling large scale cultivation.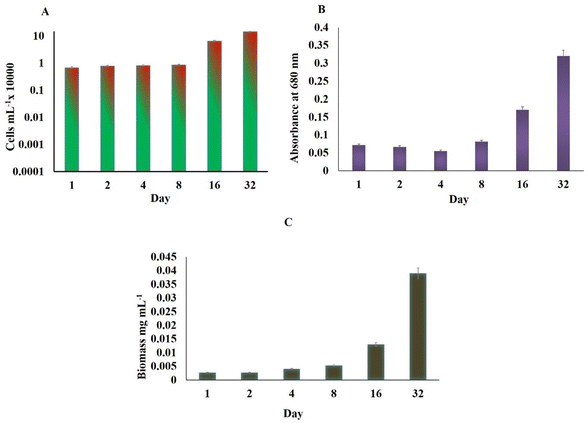 | ||
| Fig. 1 (A) Cell count, (B) absorbance, and (C) biomass in Haematococcus pluvialis after different days of growth on being irradiated to white light and broad spectrum light. | ||
On the other hand, SWW used as inoculum media for cultivating H. pluvialis showed that the total COD of SWW initially was found to be 41.71 mg L−1, which decreased to 22.49 mg L−1 on day 32, showing that there was 46% COD removal efficiency, mainly for nitrates and ammonium salts since microalgae took their nitrogen requirements mainly from the ammonium salts from SWW in the present case.37 The exponential growth of H. pluvialis cells in SWW in LDPE under WL and BL stress could be a step up to cultivate H. pluvialis cells in real-time wastewater at large scales. However, using real wastewater as nutrient media for microalgae, it is necessary to pre-treat the wastewater to get rid of harmful microbes and bacteria so that the lipids, astaxanthin, and other rich pigments can be utilised commercially at an economical scale.38 Studies have shown the growth of H. pluvialis cells in cassava water,39 diluted piggery wastewater,40 and potato wastewater pre-treated with acidification or methanation showing about 51.3–75.8% COD removal efficiency and cells reaching an exponential phase in 7 days.41 In this study however, since the experiment was performed on a laboratory scale, cultivation of microalgae using LDPE-PAP should be tested at the industrial level, which is advantageous due to the easy availability of air pillows which are generally discarded plastic waste material. Henceforth these palstic air pillows/LDPE-PAP can be used again and again for the cultivation of microalga in presence of artificial or natural lights. The results further demonstrated that enough gas exchange occurs through the LDPE bubble wrap, which supported the microalgal growth without any loss of water and any nutrient uptake. Since, in WL and BL, the cell density increased up to 223 × 103 cells per mL and 510 × 103 cells per mL, respectively on day 32 thus showing that LDPE may not only be a good alternative for cultivation of microalgae but also a cost-effective means of bridging the operational cost gap between costly photobioreactors, which thus raise the price of by-products such as biofuels, pigments and other value added compounds.
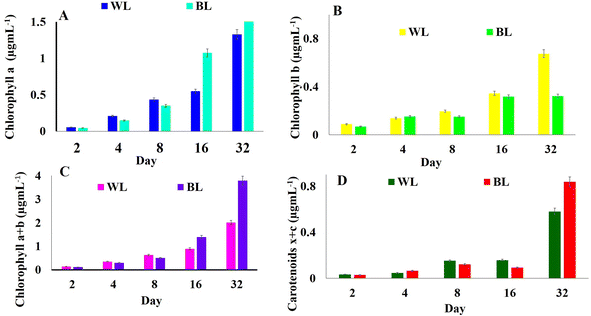
3.2 Chlorophyll content and total carotenoids
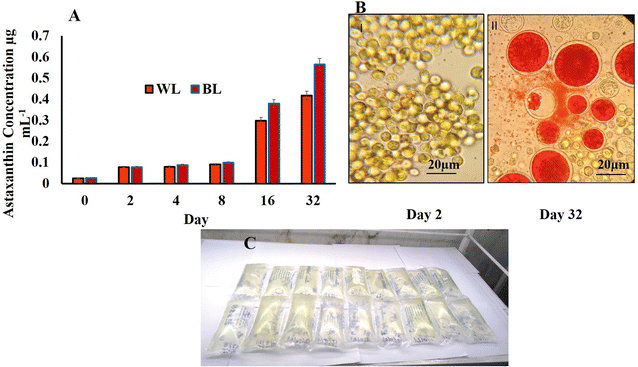
The astaxanthin content started showing a slight increase on 4th day of both WL and BL treatments. This could have been probably due to the sodium acetate in the SWW with samples irradiated to BL stress showing 26% more astaxanthin (0.417 μg mL−1) compared to WL (0.564 μg mL−1) on day 32 for 510 × 103 cells per mL (Fig. 3A). During the astaxanthin synthesis stage of the H. pluvialis, the cells start turning red and increased in size, as shown in Fig. 3B inside the LDPE-PAP due to the depletion of nitrates with time (Fig. 3C). It has been seen in studies that H. pluvialis cells cultured in SWW irradiated to BL showed higher astaxanthin production compared to the control sample in WL.41 The potential of utilizing synthetic dairy wastewater (DWW) to cultivate H. pluvialis for producing high-value products, particularly astaxanthin, was found to be 2.1 ± 0.1% of 0.55 ± 0.01 g L−1 biomass.43
The control H. pluvialis samples in WL on day 32 showed a total of 2 major peaks at Rt 7.91 and 6.70 (Fig. 4A and B). Of this, the peak at Rt 7.91 showed pigment compounds with mass spectra of 1119.67 m/z, 530.25 m/z, and 231.25 m/z identified as astaxanthin diester, chlorophyll, and halocynthiaxanthin in accordance with those identified at 1119.00 m/z, 532 m/z, and 233 m/z for astaxanthin diester, chlorophyll, and halocynthiaxanthin respectively.44,45 Another peak at Rt 6.70 corresponded to mass spectra with m/z vales of 233.17, 251.08 and 593.50 representing compounds such as halocynthiaxanthin, (3S,4R,3′R)-4-hydroxyalloxanthin and astacin-C1, respectively, whereas 104.83 m/z represented halocynthiaxanthin and diatoxanthin as reported earlier.44,45
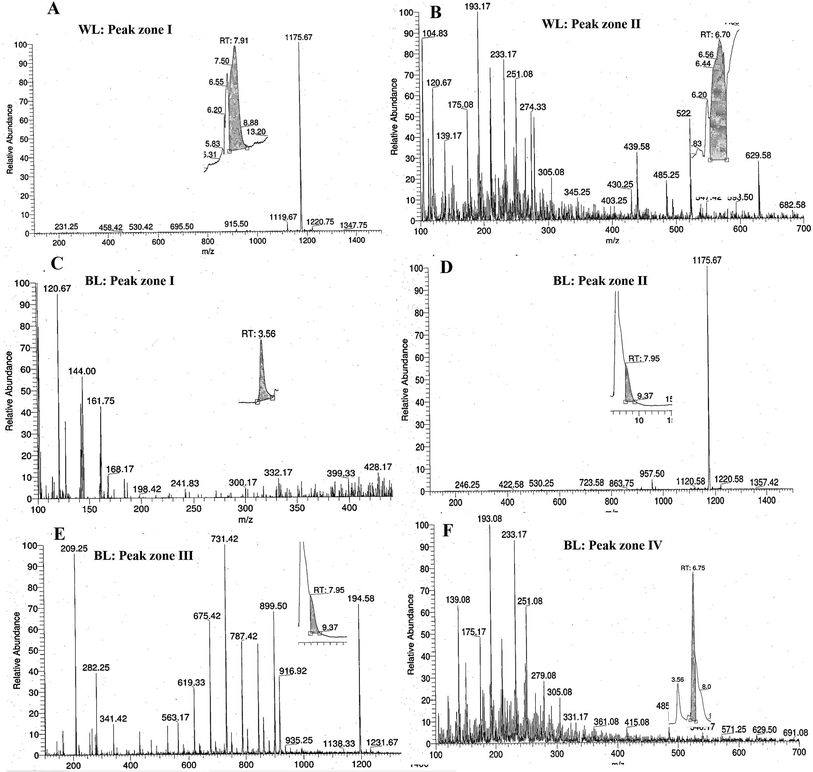 | ||
| Fig. 4 LCMS of pigments extracted from Haematococcus pluvialis on day 32 in different lights (white light (A and B) and broad spectrum light (C–F)). | ||
The test samples of BL stress on day 32 were studied for a total of 4 major peaks (Fig. 4C–F). One such peak was studied at Rt 3.56 which had an m/z value of 120.67 depicting the diatoxanthin, canthaxanthin, and m/z of 144.00 halocynthiaxanthin, which was in accordance with earlier reported studies conducted on Isochrysis galbana, which gives similar compounds at 199 m/z and 147 m/z.45 Also, 495.33 m/z was indicative of violaxanthin, neoxanthin, and 473.33 m/z for antheraxanthin, as reported in similar studies wherein 491.4 m/z was specific for violaxanthin, neoxanthin, and 475.4 m/z for antheraxanthin.46 On the offset, another peak of BL sample at Rt 7.95 ranging from Rt 7.91–8.50 represented mass spectra with compounds of 1120.58 m/z and 957.50 m/z values identified as astaxanthin diester, as reported in a similar study47 for 1120.8 m/z and 955 m/z identified compounds.48 The other major compounds with 863.75 m/z and 530.25 m/z represented astaxanthin monoester and chlorophyll, respectively, as identified in reported literature49 with 863.3 m/z and 532 m/z compounds size, respectively.45 However, the same peak of Rt 7.95 with the different ranges from Rt 7.91 to 8.49 showed compounds such as vulgaxanthin, cholorophyll ‘a’ and (3S,4R,3′R)-4-hydroxyalloxanthin at 209.25 m/z, 899.50 m/z, and 563.17 m/z values, respectively, matching with earlier reported compounds at 209 m/z, 894 m/z, and 563 m/z.45,46 The highest peak evaluated in the BL was at Rt 6.75, which according to their mass spectrum compounds at m/z of 233.17, 251.08, and 540.17 were identified as halocynthiaxanthin, (3S,4R,3′R)-4-hydroxyalloxanthin, all trans β carotene, respectively. Another interesting pigment compound was having m/z of 571.25 and was identified as zeaxanthin and lutein earlier identified at m/z 233, 251, and 538, respectively45 whereas zeaxanthin and lutein were at 569.54 m/z.50
3.3 Lipid accumulation in H. pluvialis cells
H. pluvialis cells just like any other microalgae accumulate lipids, which become more pronounced during the time cell reaches the astaxanthin stage. Herein, the transesterified lipid was estimated and it was found that for the WL sample, it was 1.2 μg mL−1 per 223 × 103 cells per mL and that for BL stress cells it increased to 1.5 μg mL−1 per 510 × 103 cells per mL (Fig. 5A).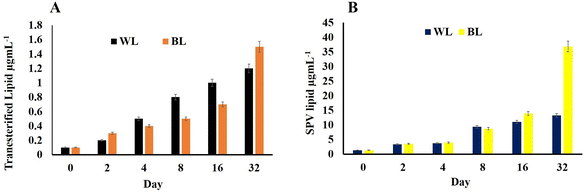 | ||
| Fig. 5 (A) Transesterified lipid concentration and (B) lipid by SPV estimation in Haematococcus pluvialis cells at different days irradiated to white light and broad spectrum light. | ||
The lipid content by SPV was nearly the same in H. pluvialis cells under both light conditions till the 8th day; however, on reaching day 32, it was high in cells exposed to BL stress (36.859 μg L−1) compared to those exposed to WL (13.215 μg L−1) weight of dry biomass (Fig. 5B). It has been already established that SWW along with BL irradiations enhances the biomass productivity in microalgae H. pluvialis while simultaneously enhancing the value-added compounds with high commercial demand viz.; pigments, biofuels, lipids, proteins, polyhydroxyalkanoates and biomass. H. pluvialis is considered a prominent source of lipid production when the growth nutrients start depleting and the cells come under stress making them appear red due to accumulation of red pigment known as astaxanthin. The standard laboratory-prepared wastewater in the present study has proved not only an essential and economical nutrient medium to grow H. pluvialis cells but also resulted in, maintaining the economy of its cultivation on being cultured in LDPE-PAPs.
Therefore, wastewater not only provides essential nitrates, phosphates, and essential macro and micronutrients but also sometimes induces factors responsible for stress in H. pluvialis, resulting in the early arrival of the cyst/stress stage for astaxanthin production. The value-added products harvested from H. pluvialis grown in wastewater in earlier reported studies show that H. pluvialis grew better or comparable in wastewater compared to standard nutrient media meant for its growth in vitro.
3.4 Lipid profiling by gas chromatography mass spectroscopy
The transesterified lipid was scanned for its fatty acid methyl contents by GCMS. Since H. pluvialis is a robust species with the ability to grow under optimal as well as stress conditions.51 As disucssed conditions such as light intensity and nutrient stress are preferred to promote lipid synthesis while simultaneously reducing its multiplication.52 On transesterification the main fatty acids methyl esters (FAME)53 of standard lipid oil viz; linseed oil at Rt 36.60 belonged to C15 and C18 compounds and were found fragmented into pentadecanoic acid methyl ester and oleic acid methyl esters. The fragments of decanoic acid at Rt 5.69 were fragmented to tridecanoic acid methyl ester, 15-methylhexadecanoic acid methyl ester, 14-methylpentadecanoic acid methyl ester, tridecanoic acid methyl ester, methyl tetradecanoate heneicosanoic acid methyl ester, dodecanoic acid methyl ester, decanoic acid methyl ester, undecanoic acid methyl ester, methyl tetradecanoate, tridecanoic acid methyl ester, heptadecanoic acid methyl ester, 2-tridecanoic acid methyl ester, nonanoic acid methyl ester, docosanoic acid methyl ester were among the others compounds, which were identified (Fig. S3†).On day 32 in the control sample, i.e., WL showed Rt at 24.92, 37.77.42.65, and 45.95 while mass spectra showed a major ion at 57 m/z corresponding to methyl ester with various peaks fragmenting till 100 m/z whereas there were other substantial alcohol and ester groups shown at mass spectra peaks above 100 m/z as 105 m/z, 119 m/z,133 m/z,147 m/z,191 m/z, and 317 m/z. The compounds mostly present were butyric acid 2-phenyl-hept-2-yl ester, pentafluoropropionic acid butyl ester, malonic acid ethyl neopentyl ester, carbonic acid butyl ethyl ester, sulfurous acid octadecyl 2-propyl ester, pentadecafluorooctanoic acid octadecyl ester, pentadecafluorooctanoic acid hexadecyl ester, pentadecafluorooctanoic acid pentadecyl ester, and dichloroacetic acid heptadecyl ester (Fig. 6A).
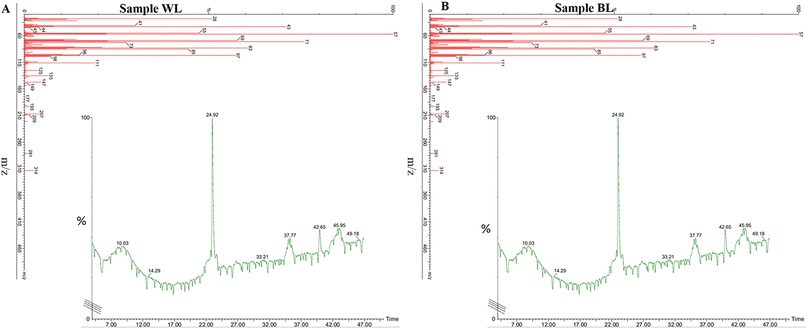 | ||
| Fig. 6 GCMS of biofuel transesterified lipid and fatty acids extracted on day 32 Haematococcus pluvialis cells exposed to (A) white light and (B) broad spectrum light. | ||
On the other hand, in BL, the FAME sample of H. pluvialis showed the presence of Rt peaks at 23.62, 24.33, 24.80, 25.02, 26.58, 27.74, 29.12, 32.40, 34.65, 37.37, 40.87, and 43.56 with mass spectra of major compounds at 57 m/z and various functional groups fragmenting till 100 m/z, various substantial groups corresponding biofuel/FAME were seen at 111 m/z, 125 m/z, 135 m/z, 147 m/z, 149 m/z, 193 m/z, 207 m/z, 209 m/z, 281 m/z, and 314 m/z. The essential carbon groups were C8, C40, C17, C14, C7, and C5 with butyric acid 2-phenyl-hept-2-yl ester, pentafluoropropionic acid butyl ester showing a total of 17 major compounds and mass spectra peaks establishing BL has supported the formation of more FAME compounds (Fig. 6B). These fatty acids and FAME compounds were in concordance with earlier studies.54
Thus, the potential of growing H. pluvialis in wastewater under light stress has yielded different value-added products in a more hassle-free way than that under standard flask cultivation. The H. pluvialis cells have shown a quasi-exponential growth pattern, which is more in broad spectrum light stress covering the majority of red and blue areas. Red light promotes cell growth, while blue light accelerated astaxanthin formation.13,55 Comparing the costs associated with different algae cultivation methods, it is crucial to determine the economic viability of algae-based production. Furthermore, the expenses involved in photobioreactors, including both closed and open ponds, have been a major deterrent, with costs ranging from $3.36 to $8.35 per L of its biomass production.22 However, the economic feasibility of algae-based industries can be improved, by adopting cost-effective techniques such as bubble farming, which will will use recyclable plastics and have the advantage of being a closed system devoid of water loss and contamination. On the offset, scale-up would require larger air pillows, which automatically results in an efficient yield of its biobased products such as astaxanthin, pigments, biomass, and lipid, compared to conventional style.
4. Conclusion
The comparative study of exposing H. pluvialis cells to white light and broad spectrum light stress along with growing them in a double economic reactor made up of plastic bubble air pillows fed with modified synthetic wastewater, has shown a synergistic behaviour to upscale economic cultivation of microalgae for its value-added compounds. The cell density in broad spectrum light stress was almost double that in white light, with a high amount of chlorophyll ‘a’ (1.32 μg mL−1), ‘a + b’ (2.00 μg mL−1), and total carotenoids of about 0.83 μg mL−1, astaxanthin (0.5643 μg mL−1) and lipid (36.859 μg mL−1) per 510 × 103 cells per mL on day 32 after initial inoculation. Hence, broad-spectrum light stress alone or in combination with other stress to H. pluvialis cells in plastic air pillows can be upscaled to bigger air pillows with different types of real-time pre-treated wastewater in future studies for economical astaxanthin, lipid, and biofuel production.Data availability
All data already mentioned in the manuscript.Author contributions
MM: literature review, writing – original draft, data curation; MJK: literature review, writing – original draft, data curation; VS: writing – original draft; AA: data curation; SV: review and editing and VV: conceptualization, supervision, writing – original draft, review, and editing, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
MJK thanks DST Nanomission project for the postdoc fellowship and VS and AA thanks the CEFIPRA Indo-French project for the fellowship. VV thanks DST Nanomission project (SR/NM/NT-1090/2014(G)) and Indo-French Centre for the Promotion of Advanced Research (IFCPAR/CEFIPRA) project number (PPMB-7133/2020) sanctioned to her. VV would also like to thank Prof. Richard Gordon Theoretical Biologist nominated for Nobel Prize 2021 retired from the University of Manitoba for his guidance for bubble farming microalgae at economical scale.References
- Y. Ren, et al., Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook, Bioresour. Technol., 2021, 340, 125736 CrossRef CAS PubMed.
- G. K. S. H. Nishshanka, et al., Haematococcus pluvialis: A potential feedstock for multiple-product biorefining, J. Cleaner Prod., 2022, 131103 CrossRef CAS.
- M. Mourya, et al., Latest trends and developments in microalgae as potential source for biofuels: The case of diatoms, Fuel, 2022, 314, 122738 CrossRef CAS.
- A. Ahirwar, et al., Microalgal drugs: A promising therapeutic reserve for the future, J. Biotechnol., 2022, 349, 32–46 CrossRef CAS PubMed.
- E. J. Olguín, et al., Microalgae-Based Biorefineries: Challenges and Future Trends to Produce Carbohydrate Enriched Biomass, High-Added Value Products and Bioactive Compounds, Biology, 2022, 11(8), 1146 CrossRef PubMed.
- S. Chakraborty, et al., Catalyst in algal refinery: A way towards production of high-quality biofuel, Sustainable Chem. Pharm., 2023, 33, 101092 CrossRef CAS.
- A. Bauer and M. Minceva, Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid–liquid chromatography, RSC Adv., 2019, 9(40), 22779–22789 RSC.
- B. Kim, et al., Cell disruption and astaxanthin extraction from Haematococcus pluvialis: Recent advances, Bioresour. Technol., 2022, 343, 126124 CrossRef CAS PubMed.
- A. Nair, et al., Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties, Mar. Drugs, 2023, 21(3), 176 CrossRef CAS PubMed.
- R. I. Papry, S. Miah and H. Hasegawa, Integrated environmental factor-dependent growth and arsenic biotransformation by aquatic microalgae: A review, Chemosphere, 2022, 135164 CrossRef CAS PubMed.
- V. Sirotiya, et al., Astaxanthin bioaccumulation in microalgae under environmental stress simulated in industrial effluents highlighting prospects of Haematococcus pluvialis: knowledge gaps and prospective approaches, Phytochem. Rev., 2022, 1–26 Search PubMed.
- U. Shankar, et al., Review of the structures and functions of algal photoreceptors to optimize bioproduct production with novel bioreactor designs for strain improvement, Biotechnol. Bioeng., 2022, 119(8), 2031–2045 CrossRef CAS PubMed.
- A. Ahirwar, et al., Light modulates transcriptomic dynamics upregulating astaxanthin accumulation in Haematococcus: A review, Bioresour. Technol., 2021, 340, 125707 CrossRef CAS PubMed.
- S. Ota, et al., Carotenoid dynamics and lipid droplet containing astaxanthin in response to light in the green alga Haematococcus pluvialis, Sci. Rep., 2018, 8(1), 1–10 CAS.
- A. Ahirwar, et al., Nanotechnological approaches to disrupt the rigid cell walled microalgae grown in wastewater for value-added biocompounds: commercial applications, challenges, and breakthrough, Biomass Convers. Biorefin., 2021, 1–26 Search PubMed.
- Y. H. Tan, et al., Assessment of Domestic Wastewaters as Potential Growth Media for Chlorella vulgaris and Haematococcus pluvialis, Pertanika J. Sci. Technol., 2022, 30(1), 565–580 Search PubMed.
- K. Mostaghimi and J. Behnamian, Waste minimization towards waste management and cleaner production strategies: a literature review, Environ. Dev. Sustain., 2022, 1–48 Search PubMed.
- M. J. Khan, et al., Insights into diatom microalgal farming for treatment of wastewater and pretreatment of algal cells by ultrasonication for value creation, Environ. Res., 2021, 201, 111550 CrossRef CAS PubMed.
- R. Deka, et al., A techno-economic approach for eliminating dye pollutants from industrial effluent employing microalgae through microbial fuel cells: Barriers and perspectives, Environ. Res., 2022, 212, 113454 CrossRef CAS PubMed.
- M. J. Khan, et al., Live diatoms as potential biocatalyst in a microbial fuel cell for harvesting continuous diafuel, carotenoids and bioelectricity, Chemosphere, 2022, 291, 132841 CrossRef CAS PubMed.
- A. Ahirwar, et al., Photosynthetic microbial fuel cell for bioenergy and valuable production: A review of circular bio-economy approach, Algal Res., 2023, 102973 CrossRef.
- R. Gordon, et al., Bubble farming: scalable microcosms for diatom biofuel and the next green revolution, Diatoms, 2019, 583–654 CAS.
- A. Rai, et al., Sustainable treatment of dye wastewater by recycling microalgal and diatom biogenic materials: Biorefinery perspectives, Chemosphere, 2022, 135371 CrossRef CAS PubMed.
- Z. Zhao, et al., Ferric-carbon micro-electrolysis and zeolite reduce CH4 and N2O emissions from the aerated constructed wetland, J. Cleaner Prod., 2022, 342, 130946 CrossRef CAS.
- G. Jadhav and M. Ghangrekar, Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration, Bioresour. Technol., 2009, 100(2), 717–723 CrossRef CAS PubMed.
- M. Ghangrekar, S. Asolekar and S. Joshi, Characteristics of sludge developed under different loading conditions during UASB reactor start-up and granulation, Water Res., 2005, 39(6), 1123–1133 CrossRef CAS PubMed.
- M. J. Khan, et al., Employing newly developed plastic bubble wrap technique for biofuel production from diatoms cultivated in discarded plastic waste, Sci. Total Environ., 2022, 823, 153667 CrossRef CAS PubMed.
- R. Ma, et al., Blue light enhances astaxanthin biosynthesis metabolism and extraction efficiency in Haematococcus pluvialis by inducing haematocyst germination, Algal Res., 2018, 35, 215–222 CrossRef.
- J. G. A. Barbedo, Automatic object counting in Neubauer chambers. 2013 Search PubMed.
- H. K. Lichtenthaler, [34] Chlorophylls and carotenoids: pigments of photosynthetic biomembranes, in Methods in enzymology, Elsevier, 1987, pp. 350–382 Search PubMed.
- N. Dragoş, et al., Astaxanthin production from a new strain of Haematococcus pluvialis grown in batch culture, Ann. Romanian Soc. Cell Biol., 2010, 15(2), 353–361 Search PubMed.
- E. G. Bligh and W. J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, 37(8), 911–917 CrossRef CAS PubMed.
- M. J. Khan, et al., TiO2 doped polydimethylsiloxane (PDMS) and Luffa cylindrica based photocatalytic nanosponge to absorb and desorb oil in diatom solar panels, RSC Adv., 2019, 9(39), 22410–22416 RSC.
- J. Park, et al., Easy and rapid quantification of lipid contents of marine dinoflagellates using the sulpho-phospho-vanillin method, Algae, 2016, 31(4), 391–401 CrossRef CAS.
- S. D. Ríos, et al., Lipid extraction methods from microalgal biomass harvested by two different paths: Screening studies toward biodiesel production, Bioresour. Technol., 2013, 133, 378–388 CrossRef PubMed.
- B. K. Amos, Up Regulation of Heat Shock Protein 70B (HSP70B) and SSA1 in Chlamydomonas Reinhardtii via HSP70A-RBCS2 and PSAD Promoter, M.Sc thesis, University of Kentucky, 2015, pp. 1–63.
- L. Ramanna, et al., The optimization of biomass and lipid yields of Chlorella sorokiniana when using wastewater supplemented with different nitrogen sources, Bioresour. Technol., 2014, 168, 127–135 CrossRef CAS PubMed.
- X. Liu, et al., Efficient removal of nitrogen/phosphorous by mix-cultivation of Haematococcus pluvialis and Simplicillium lanosoniveum in wastewater supplemented with NaHCO3, Biochem. Eng. J., 2022, 182, 108433 CrossRef CAS.
- O. H. C. Rodrigues, et al., Evaluation of astaxanthin biosynthesis by Haematococcus pluvialis grown in culture medium added of cassava wastewater, Int. Biodeterior. Biodegrad., 2021, 163, 105269 CrossRef.
- C. D. Kang, et al., Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater, Biochem. Eng. J., 2006, 31(3), 234–238 CrossRef CAS.
- M. Pan, et al., Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis, Bioresour. Technol., 2021, 326, 124761 CrossRef CAS PubMed.
- A. Kume, T. Akitsu and K. N. Nasahara, Why is chlorophyll b only used in light-harvesting systems?, J. Plant Res., 2018, 131(6), 961–972 CrossRef CAS PubMed.
- G. K. S. H. Nishshanka, et al., Sustainable cultivation of Haematococcus pluvialis and Chromochloris zofingiensis for the production of astaxanthin and co-products, Can. J. Chem. Eng., 2022, 100(10), 2835–2849 CrossRef CAS.
- F. Miao, et al., Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography–atmospheric pressure chemical ionization mass spectrometry, Anal. Biochem., 2006, 352(2), 176–181 CrossRef CAS PubMed.
- M. S. A. Bustamam, et al., Complementary analytical platforms of NMR spectroscopy and LCMS analysis in the metabolite profiling of Isochrysis galbana, Mar. Drugs, 2021, 19(3), 139 CrossRef CAS PubMed.
- H. A. Pantami, et al., Comprehensive GCMS and LC-MS/MS metabolite profiling of chlorella vulgaris, Mar. Drugs, 2020, 18(7), 367 CrossRef CAS PubMed.
- D. E. Breithaupt, Identification and quantification of astaxanthin esters in shrimp (Pandalus borealis) and in a microalga (Haematococcus pluvialis) by liquid chromatography-mass spectrometry using negative ion atmospheric pressure chemical ionization, J. Agric. Food Chem., 2004, 52(12), 3870–3875 CrossRef CAS PubMed.
- R. Frassanito, et al., A new method for the identification and the structural characterisation of carotenoid esters in freshwater microorganisms by liquid chromatography/electrospray ionisation tandem mass spectrometry, Rapid Commun. Mass Spectrom., 2008, 22(22), 3531–3539 CrossRef CAS PubMed.
- R. Gallego, et al., Application of compressed fluid–based extraction and purification procedures to obtain astaxanthin-enriched extracts from Haematococcus pluvialis and characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry, Anal. Bioanal. Chem., 2020, 412(3), 589–599 CrossRef CAS PubMed.
- R. C. Symonds, et al., Carotenoids in the sea urchin Paracentrotus lividus: Occurrence of 9′-cis-echinenone as the dominant carotenoid in gonad colour determination, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2007, 148(4), 432–444 CrossRef PubMed.
- D.-Y. Kim, et al., Cell-wall disruption and lipid/astaxanthin extraction from microalgae: Chlorella and Haematococcus, Bioresour. Technol., 2016, 199, 300–310 CrossRef CAS PubMed.
- K. Li, et al., In vivo kinetics of lipids and astaxanthin evolution in Haematococcus pluvialis mutant under 15% CO2 using Raman microspectroscopy, Bioresour. Technol., 2017, 244, 1439–1444 CrossRef CAS PubMed.
- L. H. Fasolin, R. M. Rodrigues, and R. N. Pereira, Effects of electric fields and electromagnetic wave on food structure and functionality, in Food Structure and Functionality, Elsevier, 2021, pp. 95–113 Search PubMed.
- P. Otero, et al., Identification of optimum fatty acid extraction methods for two different microalgae Phaeodactylum tricornutum and Haematococcus pluvialis for food and biodiesel applications, Anal. Bioanal. Chem., 2017, 409(19), 4659–4667 CrossRef CAS PubMed.
- S. Pereira and A. Otero, Effect of light quality on carotenogenic and non-carotenogenic species of the genus Dunaliella under nitrogen deficiency, Algal Res., 2019, 44, 101725 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01530k |
| ‡ Equally contributed first authors. |
| This journal is © The Royal Society of Chemistry 2023 |