Open Access Article
Open Access ArticleBio-oriented synthesis of ibuprofen derivatives for enhancement efficacy in post-operative and chronic inflammatory pain models†
Nisar Zamin Shaha,
Satya Kumar Avula b,
Nasiara Karim
b,
Nasiara Karim *ac,
Nazar Ul Islamd,
Gaber El-Saber Batihae,
Abdullatif Bin Muhsinahf,
Ajmal Khan*b and
Ahmed Al-Harrasi*b
*ac,
Nazar Ul Islamd,
Gaber El-Saber Batihae,
Abdullatif Bin Muhsinahf,
Ajmal Khan*b and
Ahmed Al-Harrasi*b
aDepartment of Pharmacy, University of Malakand, Chakdara, 18800, Khyber Pakhtunkhwa, Pakistan. E-mail: nasiara.karim@hotmail.com
bNatural and Medical Sciences Research Center, University of Nizwa, P. O. Box-33, Birkat Al-Mauz, Postal Code-616, Nizwa, Oman. E-mail: ajmalkhan@unizwa.edu.om; aharrasi@unizwa.edu.om
cDepartment of Pharmacy, University of Peshawar, 25120, Khyber Pakhtunkhwa, Pakistan
dDepartment of Pharmacy, Sarhad University of Science and Information Technology, Peshawar, Pakistan
eDepartment of Pharmacology and Therapeutics, Faculty of Veterinary Medicine, Damanhour University, Da-manhour 22511, AlBeheira, Egypt
fDepartment of Pharmacognosy, College of Pharmacy, King Khalid University, Abha 61441, Saudi Arabia
First published on 21st April 2023
Abstract
The discovery of post-operative, chronic inflammatory pain and any gastroulcerogenic potential using well-established animal models in vivo with new structures, high efficiency, broad-spectrum, and low toxicity has been the focus of medicinal chemists. In the present article, we are reporting the design and synthesis of various derivatives of ibuprofen by modifying the carboxyl group of ibuprofen using three steps reactions; esterification under microwave-irradiation in 10 minutes, hydrazide formation, and finally schiff's base reaction. Microwave-assisted esterification reaction can be employed to quickly explore and increase molecular diversity in synthetic chemistry. All of the newly synthesized compounds (NS1–NS4) were characterized by 1H-, 13C-NMR, and HR-ESI-MS spectroscopy and evaluated for post-operative, chronic inflammatory pain and any gastroulcerogenic potential using well-established animal models in vivo. The synthesized compounds at the tested doses of 100 and 150 mg kg−1 significantly attenuated the incisional-injury induced post-operative pain like condition and, also inhibited the phologistic agent induced inflammatory responses in both the acute and chronic testing paradigms. The gastric histological and biochemical parameters exhibited that the synthesized compounds were devoid of any ulcerogenic potential in comparison to aspirin and ibuprofen. These findings concluded that the synthesized ibuprofen derivatives exhibited profound analgesic, anti-inflammatory properties with reduced ulcerogenic potential and might be considered as effective therapeutic agents to treat pathological conditions associated with pain and inflammation.
1. Introduction
Postoperative pain is a major clinical problem that is associated with severe physiological and psychological outcomes for the sufferer. The symptoms usually persist beyond 2 months past the expected duration of remission.1 Postoperative pain is a physiological response to the stimulation of somatic and visceral pain pathways. The inflammatory mediators including histamine, prostaglandins, bradykinins, serotonin, leukotriene, nitric oxide, neurotrophic, neuropeptide and cytokines, such as TNF-alpha, interleukin-1B get released from their stores. These mediators stimulate the specialized nociceptors and the signals than propagated through specialized fibers into the cerebral cortex through the spino-thalamic or spino-reticular tracts.2The reduction and elimination of pain and discomfort with medications having minimal side-effects tendency is the major goal of postoperative pain management.3 This can be achieved by utilizing a multitude of approaches in the pain management including various medications exerting both anti-inflammatory and antinociceptive effects and thus impacting multiple sites in pain components.2,4 However, the clinical use of these medications is greatly limited by the occurrence of various toxic effects in addition to accompanying reduction in the overall clinical efficacy of medications.3,5 In addition, the pain-relieving medications mainly address the central nervous system component to provide analgesia and they do not have any effect on the inflammatory component of pain. In this regard, addressing the inflammatory response may have the utility to reduce the overall need for opioid analgesics and improve recovery after surgical procedures.
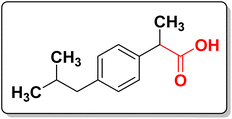
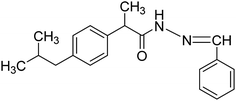
Nonsteroidal anti-inflammatory drugs (NSAIDs) are well-established class of drugs that have long been used to relieve pain and inflammation associated with acute and chronic pain.6 NSAIDs can maintain inhibition of prostaglandin over the course of a prolonged surgery and during the postoperative period. They do not cause tolerance or addiction and therefore their postoperative use reduces the growing concerns related to opioids. NSAIDs containing aryl-propionic acid (ibuprofen (Fig. 1), fenoprofen, ketoprofen, naproxen, etc.) have an important place, as they are the most widely used analgesic drugs in the market.
However, continuing use of NSAIDS for pain suppression are associated with serious GIT ulceration and renal failure along with evidence of diminishing efficacy.7 Therefore, there is a consistent need for research to synthesize new derivatives of NSAIDs that should be safe, effective and provide optimum analgesia for treating postoperative and chronic inflammatory pain conditions. There are numbers of ibuprofen derivatives that have been previously synthesized.8–15 In this study, various ibuprofen derivatives (NS1–NS4) were synthesized, characterized and tested in vivo in the postoperative pain and inflammatory animal models. Thus, to the best of our knowledge, this is the first study that has carried out extensive evaluation of these derivatives for their antinociceptive potential using single repeated dose paradigms of postoperative and inflammatory pain. Furthermore, these compounds were found to be devoid of any ulcerogenic potential that is associated with the currently available drugs.
2. Results and discussion
2.1. Chemistry
3. Biological activity
3.1. Pharmacological studies
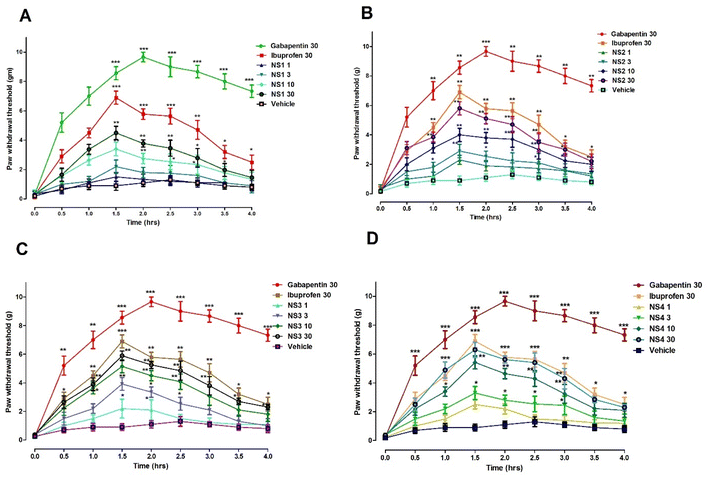
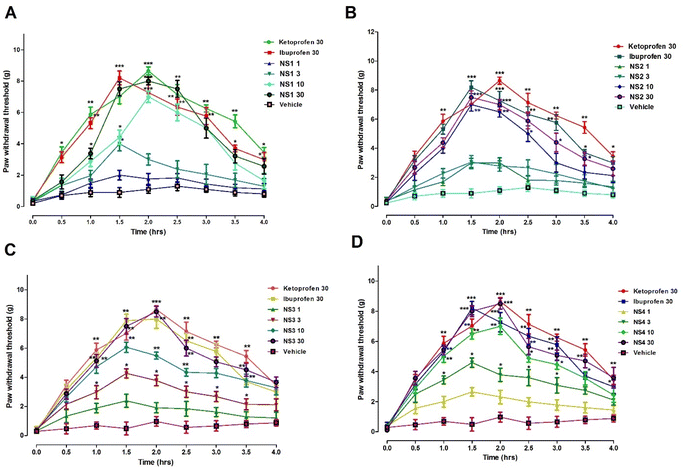
Marked acute post-operative pain suppression effect was produced by hydrazide derivative of ibuprofen at doses of 1, 3, 10 and 30 mg kg−1. A significant effect (F4,40 = 9.034, P < 0.001) in case of ibuprofen and synthesized derivative was observed after 90 min of dose administration. The observed dose dependent paw withdrawal thresholds were noted as 2.300 ± 0.23 g, 2.900 ± 0.31 g, 4.00 ± 0.45 g, 5.800 ± 0.34 g, and 5.8 g at doses of 1, 3, 10 and 30 mg kg−1, respectively. The maximum response was observed at doses of 30 mg kg−1 (P < 0.001) and 10 mg kg−1 (P < 0.01).
While comparing the post-operative anti allodynic effect of benzaldehyde derivative of ibuprofen at doses of 1, 3, 10 and 30 mg kg−1, a dose dependent pain suppression effect was observed (P < 0.001). A significant effect was observed at a dose of 10 mg kg−1 and comparatively potent effect was observed at a dose of 30 mg kg−1 of benzaldehyde derivative of ibuprofen. Overall, the paw withdrawal threshold was noted as 2.200 ± 0.66 g, 3.920 ± 0.45 g, 5.15 ± 0.312 g and 5.890 ± 0.312 g at doses of 1, 3, 10 and 10 mg kg−1, respectively.
The salicylaldehyde synthesized compound of ibuprofen showed significant post-operative pain suppression effect in the acute study at doses of 10 and 30 mg kg−1. Overall, the observed effects were found to be significant (F4,40 = 8.821, P < 0.001). The maximum peak effect was observed 90 min after dose administration. In a dose dependent manner, the paw withdrawal effect was observed as 2.500 ± 0.31 g, 3.300 ± 0.35 g, 5.420 ± 0.56 g and 6.300 ± 0.2 g at doses of 1, 3, 10 and 30 mg kg−1, respectively.
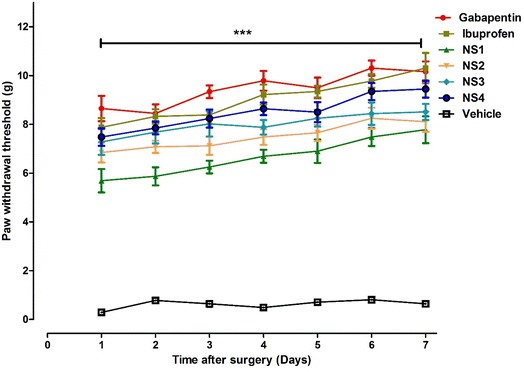
| Days | Gabapentin | Ibuprofen | NS1 | NS2 | NS3 | NS4 | Vehicle |
|---|---|---|---|---|---|---|---|
| a Values are expressed as Mean ± SEM. Data were analyzed using One-way analysis of variance (ANNOVA) followed by Dunnett's test. ***P < 0.001 as compared to the vehicle control group. | |||||||
| 1 | 9.66 ± 0.33*** | 6.90 ± 0.35*** | 4.50 ± 0.24*** | 5.80 ± 0.35*** | 5.89 ± 0.25*** | 6.30 ± 0.25*** | 0.17 ± 0.08*** |
| 2 | 10.01 ± 0.37*** | 7.10 ± 0.28*** | 4.68 ± 0.36*** | 5.85 ± 0.20*** | 5.98 ± 0.41*** | 6.44 ± 0.40*** | 0.64 ± 0.07*** |
| 3 | 10.22 ± 0.26*** | 7.29 ± 0.51*** | 4.80 ± 0.350*** | 6.01 ± 0.31*** | 6.02 ± 0.22*** | 6.51 ± 0.25*** | 0.69 ± 0.14*** |
| 4 | 10.45 ± 0.26*** | 7.22 ± 0.41*** | 4.96 ± 0.32*** | 6.19 ± 0.37*** | 6.36 ± 0.38*** | 6.60 ± 0.24*** | 0.90 ± 0.05*** |
| 5 | 11.34 ± 0.48*** | 7.42 ± 0.22*** | 5.38 ± 0.34*** | 6.11 ± 0.40*** | 6.51 ± 0.36*** | 6.87 ± 0.41*** | 0.70 ± 0.14*** |
| 6 | 11.85 ± 0.34*** | 7.84 ± 0.37*** | 5.590 ± 0.32*** | 6.350 ± 0.42*** | 6.677 ± 0.30*** | 7.250 ± 0.22*** | 0.69 ± 0.18*** |
| 7 | 12.20 ± 0.47*** | 7.77 ± 0.42*** | 5.79 ± 0.34*** | 6.54 ± 0.31*** | 7.10 ± 0.28*** | 7.02 ± 0.34*** | 0.87 ± 0.08*** |
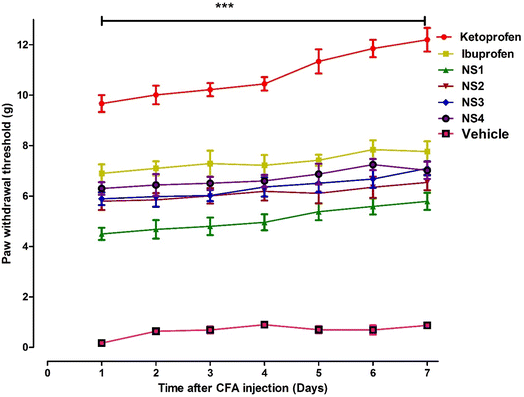
The hydrazide derivative of ibuprofen showed marked dose dependent anti-allodynic effect in the chronic inflammatory pain induced by CFA (F4,40=9.450, P < 0.001) (Fig. 4B). Significant antinociceptive response was observed at doses of 10 mg kg−1 (P < 0.01) and 30 mg kg−1 (P < 0.001). The maximum response was observed at 30 mg kg−1 after 90 min-2 h of dose administration. The effect at 10 and 30 mg kg−1 observed with NS2 was comparable to the standard drugs ibuprofen and ketoprofen at the doses of 30 mg kg−1.
In case of benzaldehyde derivative of ibuprofen (NS3), a significant antinociceptive response (F4,40=13.31, ***P < 0.001) was observed as compared to the vehicle control. The anti-allodynic effect was prominent at doses of 10 mg kg−1 (**P < 0.01) and 30 mg kg−1 (***P < 0.001) with a maximal suppression of CFA-induced pain was noted at the dose of 30 mg kg−1 dose at 1.5–2 h. The antinocipeptive response elicited by NS3 was similar to the ones obtained with the parent compound iburprofen and reference compound ketoprofen at 30 mg kg−1 (Fig. 4C).
A similar significant effect was observed with the salicylaldehyde derivative of ibuprofen (NS4) at doses of 10 and 30 mg kg−1 was observed (F4,40=11.78, P < 0.001) as shown in the Fig. 4D. The effect was more prominent at a dose of 30 mg (P < 0.001). The dose dependent paw withdrawal effect was observed as 2.650 ± 0.44 g, 4.590 ± 0.22 g, 6.66 ± 0.33 g and 7.480 ± 0.55 g at doses of 1, 3, 10 and 30 mg kg−1, respectively. The peak effect was observed after 90 min of dose administration.
| Days | Ketoprofen | Ibuprofen | NS1 | NS2 | NS3 | NS4 | Vehicle |
|---|---|---|---|---|---|---|---|
| a Values are expressed as Mean ± SEM. Data were analyzed using One-way analysis of variance (ANNOVA) followed by Dunnett's test. ***P < 0.001 as compared to the corresponding vehicle control group. | |||||||
| 1 | 8.65 ± 0.52*** | 7.88 ± 0.38*** | 5.69 ± 0.48*** | 6.85 ± 0.41*** | 7.28 ± 0.53*** | 7.48 ± 0.36*** | 0.29 ± 0.05*** |
| 2 | 8.45 ± 0.37*** | 8.32 ± 0.30*** | 5.87 ± 0.37*** | 7.08 ± 0.25*** | 7.68 ± 0.46*** | 7.85 ± 0.26*** | 0.78 ± 0.09*** |
| 3 | 9.34 ± 0.26*** | 8.39 ± 0.18*** | 6.25 ± 0.26*** | 7.12 ± 0.37*** | 8.02 ± 0.52*** | 8.24 ± 0.37*** | 0.64 ± 0.12*** |
| 4 | 9.79 ± 0.40*** | 9.22 ± 0.41*** | 6.69 ± 0.26*** | 7.48 ± 0.32*** | 7.88 ± 0.30*** | 8.64 ± 0.26*** | 0.49 ± 0.10*** |
| 5 | 9.50 ± 0.42*** | 9.35 ± 0.24*** | 6.90 ± 0.48*** | 7.66 ± 0.34*** | 8.25 ± 0.34*** | 8.50 ± 0.41*** | 0.71 ± 0.07*** |
| 6 | 10.31 ± 0.30*** | 9.78 ± 0.28*** | 7.48 ± 0.37*** | 8.25 ± 0.44*** | 8.44 ± 0.46*** | 9.35 ± 0.35*** | 0.81 ± 0.04*** |
| 7 | 10.16 ± 0.42*** | 10.31 ± 0.62*** | 7.78 ± 0.55*** | 8.11 ± 0.42*** | 8.51 ± 0.33*** | 9.45 ± 0.35*** | 0.64 ± 0.11*** |
3.2. Gastric protective effects
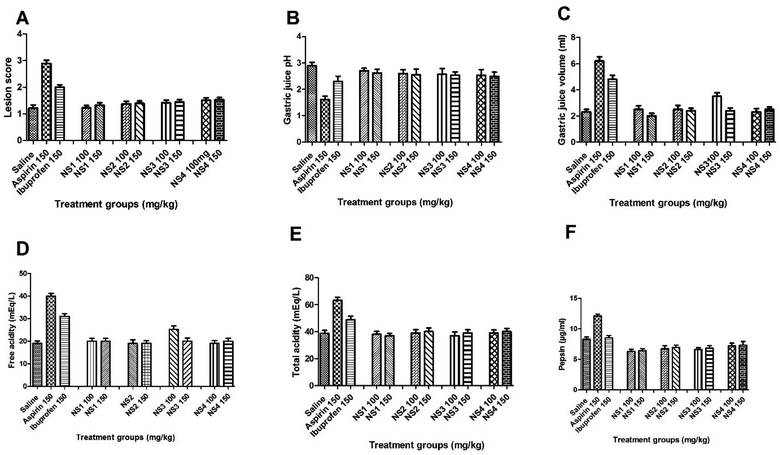
The synthesized derivatives of ibuprofen when administered at the respective doses (100 and 150 mg kg−1) were found to have no significant effect on lesion score, when compared to the negative control group (Fig. 6 and 7). In comparison, the group of animals administered with the aspirin and ibuprofen at a dose of 150 mg kg−1 showed a significant increase (P < 0.001, P < 0.01) in the lesion score (Fig. 6A). The gastric juice pH was remarkable decreased (F10,55=3.839, P < 0.001) up to the level of acidic in case of aspirin (Fig. 6B). No significant decrease in gastric juice pH toward acidic was observed in the case of ibuprofen and the synthesized derivatives. While comparing with saline treated group, a significant increase (F10,55 = 15.68, P < 0.001) in the volume of gastric juice was observed in case of aspirin and ibuprofen (P < 0.001); however, there was no significant remarkable change in gastric juice volume of the synthesized compounds (Fig. 6C). A significant increase (F10,55 = 16.47, P < 0.001) in free acidity was observed in case of aspirin (P < 0.001) and ibuprofen (P < 0.01) at a dose of 150 mg kg−1 (Fig. 6D). No significant increase was observed in the case of the synthesized derivatives. Similarly, the total acidity was also significantly (F5,30 = 13.69, P < 0.001) increased with aspirin (P < 0.001) and ibuprofen (P < 0.05) at a dose of 150 mg kg−1 (Fig. 6E). There was no prominent effect of the synthesized derivatives at respective doses on pepsin total acidity. The pepsin concentration (F5,30 = 6.294, P < 0.001) was significantly increased by aspirin at 150 mg kg−1 (P < 0.001), while no change in pepsin concentration was noted with ibuprofen (150 mg kg−1) and the synthesized compounds at the tested doses of 100 and 150 mg kg−1 (Fig. 6F).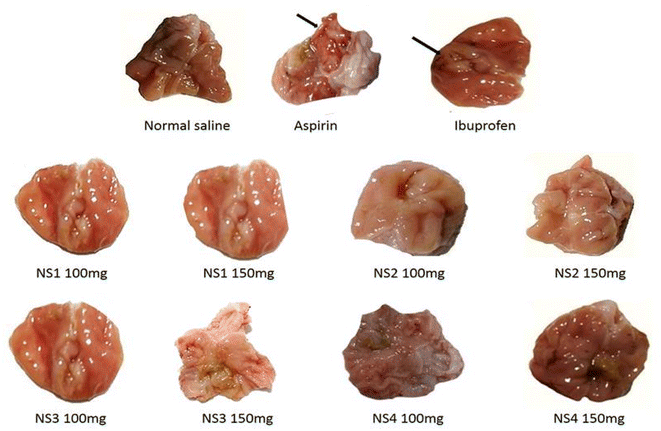 | ||
| Fig. 7 Gross macroscopic appearance of gastric mucosa after administration of ibuprofen and its derivatives. | ||
From the examination of gross morphological changes of stomach, it was observed that the aspirin and ibuprofen treated animals are associated with hemorrhagic streaks, red colorations, or ulcer spots. However, these were not present in the groups of animals treated with the synthesized compounds at doses of 100 and 150 mg kg−1. The observations in these treated groups were like the saline administered group except in the group of compounds I (150 mg kg−1), red coloration of the stomach mucosa was noted.
In the saline group, the gastric mucosa has normal histoarchitecture of the glandular portion of the stomach consisting of superficial mucosa, submucosa and muscularis externa. The histopathological changes in the stomach mucosa of aspirin administered animals group revealed mucosal epithelial cells shredding, heavy congestion of underlying blood vessels with infiltration of lymphocytes. There was superficial ulceration of mucosa as well as deep ulcerative pits extending to the submucosa and muscularis externa. The group of animals administrated with ibuprofen also was observed to have ulcerative mucosa. However, these histopathological ulcerogenic changes were not observed in the groups of animals treated with the 100 and 150 mg kg−1 doses of the synthesized compounds, thus revealing that these compounds were free of any inherent gastric ulcerogenicity.
Postoperative pain remains a major clinical problem and has limited preventive strategies. The antinociceptive agents have been proven to be effective in preventing and relieving this type of pain.16 Inflammation is one of the protective responses of the vascularized tissue of the body. Inflammation may be acute or chronic depending upon cellular and vascular changes that are observed during inflammation. Various drug substances are currently used to suppress postoperative pain and inflammation.17 However, they are associated with serious adverse effects, there is a dire need for the discovery and development of safer and effective alternative agents as the currently available analgesic and anti-inflammatory drugs are associated with severe adverse effects.
The NSAIDs especially the aryl-propionic acid derivatives including ibuprofen are widely utilized for treating pain and inflammation related diseases including rheumatic disease, colic, headache and dysmenorrhea.18,19 Among the NSAIDs, ibuprofen has found a vital place as a main nonselective inhibitor of cyclooxygenase (COX) enzymes (COX-1 and COX-2) with having a free carboxylic group allowing a multitude of structural modification for probable increasing of ibuprofen's efficacy and reduction of its inherent toxicity.20 It was observed that the carboxyl group is responsible for the side-effects associated with NSAIDs in general and ibuprofen in particular including gastric ulceration and hepatic and renal complications.21 Thus attachment of other moieties to the carboxyl group may have prolific effect on the overall pharmacological profile of ibuprofen.22
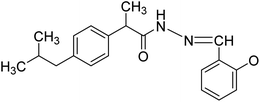
In the current study, the salicylaldehyde was attached as imine moiety with the carboxylic group of ibuprofen (NS4). The attachment of salicylaldehyde bestowed a marked beneficial effectiveness to the ibuprofen as revealed from the significant inhibition of post-operative pain and inflammation. In addition, salicylaldehyde-ibuprofen derivative was also found to be devoid of any gastric ulcerogenicity. Previous studies have shown that salicylaldehyde associated compounds significantly inhibited inflammatory responses23,24 and attenuated both nociceptive and neuropathic pain like conditions.25,26 Moreover, salicylaldehyde related compounds also exhibited remarkable biological activities and having a lesser gastric ulcerogenic propensity.27–29 Similarly, all other derivatives including NS1 (ester), NS2 (hydrazide) and NS3 (benzaldehyde) showed significant post-operative and chronic inflammatory pain suppression effect of ibuprofen derivatives in both acute and chronic studies. The benzaldehyde (NS3) and salicylaldehyde derivatives of ibuprofen were found to be the most efficacious among the compounds studied. Previous studies have also demonstrated that salicylaldehyde functionalized or ibuprofen associated derivatives have afforded significant analgesic and anti-inflammatory properties.30–32 In this study, the ibuprofen derivatives (NS1–NS4) demonstrated significant antinociceptive and anti-inflammatory activities in post-operative pain and anti-inflammatory pain models. Furthermore, these compounds were found to be devoid of any untoward effects traditionally associated with their parent compound and other NSAIDS. Thus, these synthesized derivatives of ibuprofen (NS1–NS4) could be used as potential candidates in alleviating post-operative and inflammatory pain without any inherent gastric ulcerogenecity.
4. Conclusion
In summary, we are reporting the design and synthesis of various derivatives of ibuprofen by modifying the carboxyl group of ibuprofen using three steps reactions; esterification under microwave-irradiation in 10 minutes, hydrazide formation, and finally schiff's base reaction. Microwave-assisted esterification reaction can be employed to quickly explore and increase molecular diversity in synthetic chemistry. All the synthesized compounds (NS1-NS4) were characterized by 1H-, 13C-NMR, and HR-ESI-MS spectroscopy and evaluated for post-operative, chronic inflammatory pain and any gastroulcerogenic potential using well-established animal models in vivo. The above findings suggest that the synthesized ibuprofen derivatives by virtue of their profound analgesic, anti-inflammatory and gastroprotective properties can be used as effective therapeutic agents to treat post-operative and chronic inflammatory conditions.5. Experimental section
5.1. General
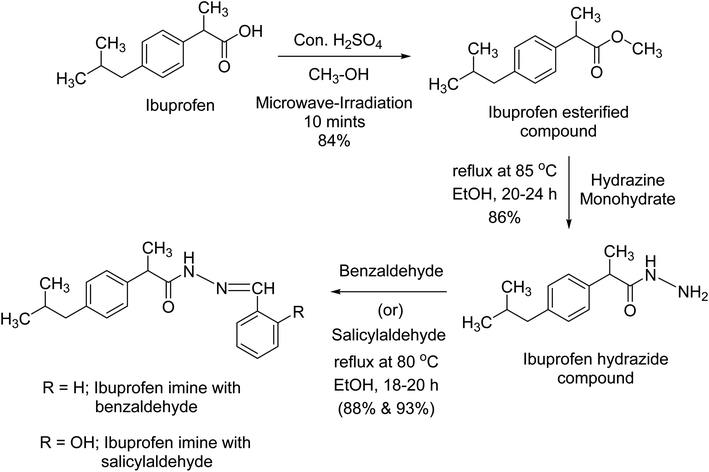
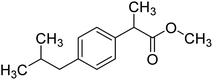
All reagents, chemicals and solvents of analytical grade were purchased from Sigma-Aldrich Co. USA. The TLC plates were purchased from the local market. All other chemicals were of analytical grade and were used without further purification. Melting points were determined on an Electrothermal 9100 melting point apparatus (Weiss-Gallenkamp, Loughborough, UK). Compounds purity were confirmed by Thin Layer Chromatography (TLC) using Kieselgel 60 F254 (Merck). EI-MS was performed on JEOL JMS 600 mass spectrometer. We have performed Elemental analyses of all synthesized compounds with an Elementar-Vario EL instrument. 1H-NMR Bruker signals appeared at 600 MHz in CH3OH-d4 and that of 13C-NMR at 150 MHz in CHCl3-d.45.2. Synthesis of ibuprofen esterified compound (NS1)8,15
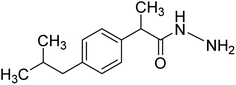
For esterified derivatives synthesis, in a 10 mL microwave glass tube were placed ibuprofen (500 mg) in mmol concentration was added to round bottom flask followed by addition of 20 mL of methanol and 0.6 mL of sulfuric acid with a magnetic stir. The vessel was sealed with a septum and placed into the microwave cavity of the Microwave Synthesis Reactor (Monowave, 300, Anton Paar, Graz, Austria). Microwave-irradiation of 300 W was used, the temperature is ramped from room temperature to 80 °C. Once 80 °C was reached, the reaction mixture was held at this temperature for 10 minutes. After completion of the reaction, the mixture was allowed to cool to room temperature, the reaction vessel was opened, and the contents were poured into a separating funnel. Completion of the reaction was indicated by formation of clear oily esterified layer. The compound was transferred to separating funnel washed and purified with cool water to remove excess sulfuric acid and sodium carbonate followed by addition of sodium bisulphite to remove excess water. Finally, the filtered and dried desired ibuprofen esterified compound (NS1, 84%) in a good yield, was characterized using various spectrometric techniques.5.3. Synthesis of ibuprofen hydrazide compound (NS2)9,11
The hydrazide derivative was synthesized by adding purified ibuprofen esterified compound (NS1) (350 mg) in mmol concentration to reflux condenser followed by addition of calculated mmol concentration of hydrazine monohydrate using ethanol (50 mL) as a solvent. The reaction was refluxed until the completion of the reaction process, confirmed by thin layer chromatography. The appearance of white solid mass indicated the formation of ibuprofen hydrazide compound (NS2, 86%) as a desired product and purified by using distilled water and dried.5.4. Synthesis of ibuprofen imine with benzaldehyde (NS3)10,11 and ibuprofen imine with salicylaldehyde (NS4)12,13
The resulting ibuprofen hydrazide compound (NS2) were reacted with benzaldehyde or salicylaldehyde in specific mole concentration in a reflux condenser using ethanol as a solvent. The reactions were monitored with thin layer chromatography until the completion of the reaction and to confirm the formation of product. On completion, an off-white solid mass appeared which was washed with cooled water, filtered and dried. For crystallization the product was passed through a hot filtration mechanism and the resultant crystals were filtered, dried and characterized.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00, lights off at 20
00, lights off at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00) with free access to food and water. The animals were randomly allocated to the various experimental groups (n = 8). The study was approved by the institutional ethical committee having an approval number of (DAEC/PHARM/2016/17). All the experimental protocols on animals were performed according to the UK Animals (Scientific Procedures) Act 1986.
00) with free access to food and water. The animals were randomly allocated to the various experimental groups (n = 8). The study was approved by the institutional ethical committee having an approval number of (DAEC/PHARM/2016/17). All the experimental protocols on animals were performed according to the UK Animals (Scientific Procedures) Act 1986.5.5. Incisional surgery and pain evaluation
The postoperative pain condition was induced by performing the well-established incisional surgery protocol as previously reported.33 The animals were anesthetized with a mixture of 2% isoflurane and 100% oxygen having a flow rate of 5 L min−1. To induce the pain condition, a 5 mm longitudinal incision was made using a surgical blade on the plantar side of the hind paw. The incisional areas were then sutured while using a 5–0 nylon suture. The animals were then transferred to their cages for recovery. To measure the paw withdrawal threshold underlying mechanical hyperalgesia a series of 20 von Frey filaments (0.008 g/1.65 to 300 g/6.65) were used. All animals were acclimatized for at least 15 minutes before PWT measurement in specially designed cage. Respective filaments were applied in ascending order to the right hind paw of each animal and paw withdraw thresholds were measured in triplicate. Mechanical paw withdraw threshold is defined as the force that cause at least two paw withdraw in three consecutive trial procedure.5.6. Assessment for post-operative pain
Mechanical hyperalgesia was measured 24 h after the surgery for acute experiments. The synthesized compounds were administered through intraperitoneal route in doses of 1, 3, 10 and 30 mg kg−1 24 h of post-operation. A base-line study was performed for at least three days prior to the incisional surgery. Animals were divided into various groups and in the case of each group animals a paw withdraw threshold to a mechanical stimulus was measured. On the planter surface of the right hind paw of each animal that was exposed to test sample a 1 cm longitudinal incision cut was made. In the acute study, paw withdrawal threshold was measured every thirty minutes for four hours after administration of the synthesized compounds. In the repeated dosing study, the synthesized compounds were administered daily at a dose of 30 mg for 7 days and paw withdrawal threshold was measured 2 h after dose administration.5.7. Assessment for inflammatory pain
For inflammatory assessment, the synthesized compounds were administered intraperitoneally in 1, 3, 10 and 30 mg kg−1 doses. The animals were divided into various groups (n = 8). Standard laboratory conditions were followed to habituate the animals for 3 days before administration of complete freund’ adjuvant (CFA). The base-line parameters were measured. A series of calibrated von Frey filaments were used. For induction of inflammation induced mechanical allodynia CFA was injected using saline group as control. The synthesized compounds were injected 1 day post CFA injection and paw withdrawal threshold to a mechanical stimulus was measured after every thirty minutes for four hours. In the repeated dosing study, the synthesized compounds were administered daily for 7 days, and paw withdrawal threshold was measured 2 h after dose administration.5.8. Gastro-protective activity
The synthesized compounds were evaluated for gastroprotective potential using the well-established protocol.34 Briefly saline, reference drug and the synthesized compounds were orally administered to various groups for 6 days. After one hour from the last dose of 12 hours fasted animals, the pyloric ligation procedure was carried out under ketamine induced anesthesia. Food and water were withheld for 4 h to accumulate gastric juice. The stomach was then opened, and gastric contents and tissue were examined for any ulcerogenecity. The gastroprotective effect were evaluated by examining free and total acidity and pepsin concentration.35,36 The gross morphologic changes were recorded by examining photomicrographs of gastric mucosa and the degree of ulceration were graded according to previous reported ulcer scoring method.375.9. Statistical analysis
The data were expressed as mean ± SEM. Data were analyzed by One way or Two ANOVA followed by either Dunnett's test or Bonferroni test where appropriate. A P value less than 0.05 was considered statistically significant.Ethics approval
All animal procedures were approved by the Departmental Animal Ethical Committee, University of Malakand, KPK, and Pakistan (DAEC/PHARM/2016/17) and were conducted according to the UK animal scientific procedure act, 1986.Author contributions
NK, AK and AAH designed the study. NZS, and NK performed the in vivo experiments. NZS, AKA and NUI synthesized and characterized the compound. GEB and ABM analyzed the data. NK, NZS and AK wrote and refined the manuscript. All authors read and approved the manuscript.Conflicts of interest
All authors confirm that this article content has no conflict of interest.Acknowledgements
Dr Nasiara Karim acknowledges funding (National research program for universities; NRPU 20-3425) from the Higher Education Commission of Pakistan for the completion of this research work. The authors also extend their appreciation to the Deanship of scientific research at King Khalid University for funding this work through the Large Groups Project under grant number (RGP. 2/159/43/ 2022).References
- T. J. Gan, J. Pain Res., 2017, 10, 2287–2298 CrossRef CAS PubMed.
- M. K. Stasiowska, S. C. Ng, A. N. Gubbay and R. Cregg, Br. J. Hosp. Med., 2015, 76, 570–575 CrossRef PubMed.
- A. Buvanendran and J. S. Kroin, Curr. Opin. Anaesthesiol., 2009, 22, 588–593 CrossRef PubMed.
- W. Ahmad, I. Khan, M. A. Khan, M. Ahmad, F. Subhan and N. Karim, J. Ethnopharmacol., 2014, 151, 618–623 CrossRef PubMed.
- N. Vadivelu, S. Mitra and D. Narayan, Yale J. Biol. Med., 2010, 83, 11–25 CAS.
- A. Gupta and M. Bah, Curr. Pain Headache Rep., 2016, 20, 62 CrossRef.
- C. Sostres, C. J. Gargallo and A. Lanas, Arthritis Res. Ther., 2013, 15, S3 CrossRef.
- Q. Shi, Y. Jia, H. Wang, S. Li, H. Li, J. Guo, T. Dou, B. Qin and S. You, Enzyme Microb. Technol., 2021, 150, 109880 CrossRef CAS PubMed.
- H. Lgaz and H.-S. Lee, J. Mol. Liq., 2022, 347, 117952 CrossRef CAS.
- Y. El Bakri, S. K. Mohamed, S. Ahmad, M. R. Albayati, S. M. Elgarhy, C. H. Lai and J. T. Mague, J. Biochem. Mol. Toxicol., 2022, 36, e23082 CrossRef CAS PubMed.
- N. H. Naser, A. A. A. Alibeg and A. J. AbdAl-Zahra, Mater. Today: Proc., 2022, 65, 2669–2675 CAS.
- A. M. Abbas, A. Aboelmagd, S. M. Kishk, H. H. Nasrallah, W. C. Boyd, H. Kalil and A. S. Orabi, Molecules, 2022, 27, 7540 CrossRef CAS PubMed.
- Q. Y. Zhang, X. J. Cheng, X. Y. Zhao, L. M. Wu, L. F. Jin and H. J. Zhang, Synlett, 2019, 30, 1334–1338 CrossRef CAS.
- P. A. Halim, H. B. El-Nassan and Y. S. El-Dash, Med. Chem., 2022, 18, 427–443 CrossRef CAS PubMed.
- S.-E. Suh, S.-J. Chen, M. Mandal, I. A. Guzei, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2020, 142, 11388–11393 CrossRef CAS PubMed.
- C. E. Argoff, Pain Pract., 2014, 14, 477–487 Search PubMed.
- V. Garimella and C. Cellini, Clin. Colon Rectal Surg., 2013, 26, 191–196 CrossRef PubMed.
- S. Wongrakpanich, A. Wongrakpanich, K. Melhado and J. Rangaswami, Aging Dis., 2018, 9, 143 CrossRef PubMed.
- I.-M. Vasincu, M. Apotrosoaei, S. Constantin, M. Butnaru, L. Vereştiuc, C.-E. Lupuşoru, F. Buron, S. Routier, D. Lupaşcu and R.-G. Tauşer, BMC Pharmacol. Toxicol., 2021, 22, 1–9 CrossRef PubMed.
- I. M. Vasincu, M. Apotrosoaei, A.-T. Panzariu, F. Buron, S. Routier and L. Profire, Molecules, 2014, 19, 15005–15025 CrossRef PubMed.
- A. Ahmadi, M. Khalili, S. Ahmadian, N. Shahghobadi and B. Nahri-Niknafs, Pharm. Chem. J., 2014, 48, 109–115 CrossRef CAS.
- N. Ullah, Z. Huang, F. Sanaee, A. Rodriguez-Dimitrescu, F. Aldawsari, F. Jamali, A. Bhardwaj, N. U. Islam and C. A. Velázquez-Martínez, J. Enzyme Inhib. Med. Chem., 2016, 31, 1018–1028 CrossRef CAS.
- N. Ahmad, F. Subhan, N. U. Islam, M. Shahid, F. U. Rahman and K. Fawad, Arch. Pharm., 2017, 350, e1600365 CrossRef.
- M. S. Zafar, K. Shahid, G. C. Gobe, R. Yasmin, N. Naseem and M. Shahzad, J. King Saud Univ., Sci., 2022, 34, 101877 CrossRef.
- N. Ahmad, F. Subhan, N. U. Islam, M. Shahid, N. Ullah, R. Ullah, M. Khurram, M. U. Amin, S. Akbar, I. Ullah and R. D. E. Sewell, Naunyn-Schmiedeberg’s Arch. Pharmacol., 2021, 394(10), 2033–2047 CrossRef CAS.
- N. Ahmad, F. Subhan, N. U. Islam, M. Shahid, F. U. Rahman and R. D. Sewell, Eur. J. Pharmacol., 2017, 814, 302–312 CrossRef CAS PubMed.
- K. Rahman, G. Ali, R. Khan, I. Khan, I. Ali, O. F. Mosa, A. Ahmed, M. Ayaz, A. Nawaz and H. A. Murthy, Drug Des., Dev. Ther., 2022, 16, 1143 CrossRef PubMed.
- A. A. Farag, M. F. El Shehry, S. Y. Abbas, S. N. Abd-Alrahman, A. A. Atrees, H. Z. Al-basheer and Y. A. Ammar, Z. Naturforsch. B, 2015, 70, 519–526 CrossRef CAS.
- W. B. Júnior, M. S. Alexandre-Moreira, M. A. Alves, A. Perez-Rebolledo, G. L. Parrilha, E. E. Castellano, O. E. Piro, E. J. Barreiro, L. M. Lima and H. Beraldo, Molecules, 2011, 16, 6902–6915 CrossRef PubMed.
- A. Ahmadi, M. Khalili, Z. Olama, S. Karami and B. Nahri-Niknafs, Mini-Rev. Med. Chem., 2017, 17, 799–804 CrossRef CAS PubMed.
- J. Wang, D. Dai, Q. Qiu, X. Deng, H. Lin, H. Qian and W. Huang, Chem. Biol. Drug Des., 2015, 85, 623–632 CrossRef CAS PubMed.
- H. Rafea, M. S. Farhan and A. A. Fadhil, Syst. Rev. Pharm., 2020, 11, 1851–1856 CAS.
- Q. Zhu, L.-N. Mao, C.-P. Liu, Y.-H. Sun, B. Jiang, W. Zhang and J.-X. Li, Sci. Rep., 2016, 6, 19266 CrossRef CAS PubMed.
- M. Mabrouk, F. Nnawodu, Y. Tanko, F. Dawud and A. Mohammed, Curr. Res. J. Biol. Sci., 2009, 1, 15–19 Search PubMed.
- J. Kore Kakasaheb, V. Shete Rajkumar, J. Patel Apsari and B. Kulkarni Jitendra, Int. J. Res. Pharm. Chem., 2011, 13, 29–31 Search PubMed.
- P. Kalra, A. K. Datusalia and S. Sharma, Int. J. Green Pharm., 2011, 5, 149–154 CrossRef.
- D. K. Patidar, Int. J. Pharma Bio Sci., 2011, 2, 524–529 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra01385e |
| This journal is © The Royal Society of Chemistry 2023 |