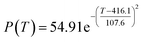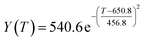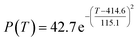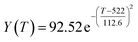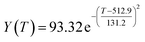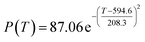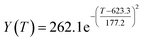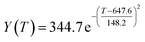Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A mathematical model of catalyst combination design and temperature control in the preparation of C4 olefins through ethanol coupling
Pengxiang Tang a,
Hongting Li
a,
Hongting Li b,
Xiaomei Zhang
b,
Xiaomei Zhang *a and
Xin Sun
*a and
Xin Sun *a
*a
aSchool of Pharmacy, Jilin Medical University, Jilin, Jilin Province 132013, China. E-mail: cxtpxedu@163.com; zirenjlcc@jlmu.edu.cn; sunxin@jlmu.edu.cn
bQueen Mary College, Nanchang University, Nanchang, Jiangxi Province 330000, China
First published on 4th April 2023
Abstract
The preparation of C4 olefins through ethanol catalytic coupling is a crucial area of study. According to the experimental data obtained by a chemical laboratory for different catalysts at different temperatures, three mathematical models were developed to provide insights into the relationships among ethanol conversion rate, C4 olefins selectivity, yield, catalyst combination, and temperature. The first model is a nonlinear fitting function that analyses the relationships among ethanol conversion rate, C4 olefins selectivity, and temperature under varying catalyst combinations. Two-factor analysis of variance was employed to determine the influence of catalyst combinations and temperatures on ethanol conversion rate and C4 olefins selectivity. The second model is a multivariate nonlinear regression model that describes the relationships among the yield of C4 olefins, catalyst combination, and temperature. Finally, an optimization model was derived based on the experimental conditions; it provides a solution for the selection of the optimal catalyst combinations and temperatures to achieve the maximum yield of C4 olefins. This work has significant implications for the field of chemistry and the production of C4 olefins.
Introduction
Ethanol is a clean, easily obtained raw material that chemical industry methods and biological fermentation techniques can produce. Biological fermentation is the popular primary technology and uses corn, sugarcane, and other crops as raw materials to produce ethanol.1 As the technology matures and the scale of use expands, the industrial application of ethanol as a raw material is also increasing. For example, ethanol is a renewable fuel that can be used in engines.2 It can also be used as a coolant in various metal–organic frames and similar applications,3 which has broad prospects for producing high-value-added products, such as C4 olefins, an essential primary chemical raw material. C4 olefins can be obtained through fluid catalytic cracking (FCC) or from byproducts in ethylene cracking reactions. Isobutene can be converted into methyl tert-butyl ether (MTBE) by methanol etherification,4,5 and is one gasoline additive. The reaction mechanism of preparation of C4 olefins by ethanol coupling is the Prince mechanism,6 or aldol condensation mechanism.7The preparation of C4 olefins through ethanol coupling is very complicated, and the mechanism of the reaction must be further studied. In the preparation of C4 olefins through ethanol coupling, it is crucial to control the temperature and catalyst design.8 In an experiment in China, Lv9 designed a Co/SiO2-HAP catalyst with both acid and base activities on the surface that is aimed at the preparation of C4 olefins using ethanol. She studied the optimum conditions for the catalyst charging ratio and reaction temperature in a chemical experiment; her conclusions are consistent with those of this article which employs a mathematical modelling method. Ge10 studied the selective superposition process of mixed C4 olefins using experimental methods and investigated the influence of reaction conditions on the selective superposition of mixed C4 olefins, such as temperature, air speed, and pressure. Through such experiments, it has been concluded that the selectivity of C4 olefins will be significantly reduced if the temperature drops, which supports the findings obtained from the analysis of experimental data in this paper.
However, using experimental data, the mathematical modelling method can be employed to study the quantitative relationship and optimal design in the preparation of C4 olefins through ethanol coupling, which is an interdisciplinary method. Mathematical modelling is widely used in various fields. For example, it has been applied to identify an optimization strategy to improve the performance of microbial fuel cells11 and to assess the risk of airborne transmission of COVID-19.12 Moreover, it has been used for drug discovery and development.13 In examining the preparation of C4 olefins by ethanol coupling, Li et al.14 established the Analytic Hierarchy Process/Entropy Weight Method-Technique for Order Preference by Similarity to Ideal Solution (AHP/EWM-TOPSIS) and built a production-quality C4 olefins assessment system. With the support of the evaluation system, the improved mixed congruence method was used to simulate the production conditions of the preparation of C4 olefins through ethanol coupling and to construct the reverse neural network (BPNN). Then, the optimal scoring production scheme at different temperatures was determined using the mathematical model. Wang et al.15 employed a logistic regression model to analyse the relationship between ethanol temperature and conversion rate with C4 olefins selectivity in C4 olefins preparation through ethanol coupling. The relationship between different catalysts and temperature with the maximum yield of C4 olefins was also examined by constructing a neural network. Zhang et al.16 conducted a two-dimensional visualisation analysis using experimental data on ethanol-coupled C4 olefins and used clustering analysis for different catalyst combinations. Finally, a BPNN was used to calculate the reaction conditions for the maximum yield of ethanol-coupled C4 olefins. However, these studies have all been conducted from a single point of view, giving us an incomplete and unsystematic understanding of the preparation of C4 olefins by ethanol coupling.
Therefore, based on the experimental data collected from the reactions of preparing C4 olefins through ethanol coupling, this paper systematically analysed and solved the four-part problem using mathematical modelling. In the first part, based on the characteristics of the experimental data and on the premise of the unknown reaction mechanism, the relationships among the key components, such as ethanol, C4 olefins, and temperature, were analysed, and different fitting functions were compared. In the second part, a specific constant catalyst combination and reaction temperature were selected to study the data characteristics of specific indexes of the reaction components under different experimental time, which has further explained how the reaction conditions change over time. In the third part, the influence of varying catalyst combinations and temperatures on the critical indexes of ethanol conversion rate and C4 olefins selectivity were analysed using experimental data. In the fourth part, the yield of C4 olefins in the reaction was calculated according to the experimental data, and a multivariate nonlinear model of C4 olefins yield with catalyst and temperature was established. A reasonable optimisation model was established to find the optimum catalyst combination and corresponding temperature under different charging methods.
The general reaction process of preparing C4 olefins through ethanol coupling is as follows:
 | (1) |
A chemical laboratory has conducted several experiments on the preparation of C4 olefins through ethanol coupling. The corresponding experimental data were obtained by changing the experimental conditions of catalyst combination (Co loading, Co/SiO2, HAP loading ratio, ethanol concentration) and temperature. In Experimental Data 1, there are 21 groups of catalyst combinations (14 groups of class A, 7 groups of class B). Each group contains five temperatures and the corresponding ethanol conversion rates, ethylene selectivity, C4 olefins selectivity, acetaldehyde selectivity, carbon number 4–12 fatty alcohol selectivity, methyl benzaldehyde and methyl benzyl alcohol selectivity, and the experimental data for the selectivity of other products. Experimental Data 2 comprises data of unknown catalyst combinations at 350 °C at six time points and contains the ethanol conversion rate, C4 olefins selectivity, and so on. It is of great practical significance to study the influence of changing temporal conditions on C4 olefins selectivity and C4 olefins yield. It is also important to use existing experimental data and results to analyse and explore the reactions of C4 olefins preparation through ethanol coupling.
Experimental design
Data sources
The original experimental data used in this paper are from Question B of the 2021 Higher Education Community Cup National Mathematical Contest in Modelling for College Students;17 charging method I was used in catalyst experiments A1–A14, and charging method II was used in catalyst experiments B1–B7. Some experimental data are shown in Tables 1 and 2, and the parameters used in this paper are presented in Table 3.| Catalyst combination number | Catalyst combination | Temperature (°C) | Ethanol conversion rate (%) | Ethylene selectivity (%) | C4 olefins selectivity (%) | Acetaldehyde selectivity (%) |
|---|---|---|---|---|---|---|
| A1 | 200 mg 1 wt% Co/SiO2-200 mg HAP-ethanol concentration 1.68 ml min−1 | 250 | 2.07 | 1.17 | 34.05 | 2.41 |
| 275 | 5.85 | 1.63 | 37.43 | 1.42 | ||
| 300 | 14.97 | 3.02 | 46.94 | 4.71 | ||
| 325 | 19.68 | 7.97 | 49.70 | 14.69 | ||
| 350 | 36.80 | 12.46 | 47.21 | 18.66 | ||
| A2 | 200 mg 2 wt% Co/SiO2-200 mg HAP-ethanol concentration 1.68 ml min−1 | 250 | 4.60 | 0.61 | 18.07 | 0.94 |
| 275 | 17.20 | 0.51 | 17.28 | 1.43 | ||
| 300 | 38.92 | 0.85 | 19.60 | 2.21 | ||
| 325 | 56.38 | 1.43 | 30.62 | 3.79 | ||
| 350 | 67.88 | 2.76 | 39.10 | 4.20 | ||
| A14 | 33 mg 1 wt% Co/SiO2-67 mg HAP-ethanol concentration 1.68 ml min−1 | 250 | 2.50 | 0.14 | 1.89 | 2.63 |
| 275 | 5.30 | 0.14 | 2.55 | 2.80 | ||
| 300 | 10.2 | 0.25 | 3.61 | 4.07 | ||
| 350 | 24.0 | 1.04 | 10.83 | 6.25 | ||
| 400 | 53.6 | 2.92 | 22.30 | 7.22 | ||
| B1 | 50 mg 1 wt% Co/SiO2-50 mg HAP-ethanol concentration 1.68 ml min−1 | 250 | 1.40 | 0.10 | 6.32 | 5.70 |
| 275 | 3.40 | 0.19 | 8.25 | 4.03 | ||
| 300 | 6.70 | 0.45 | 12.28 | 4.11 | ||
| 350 | 19.3 | 1.22 | 25.97 | 4.40 | ||
| 400 | 43.6 | 3.77 | 41.08 | 4.13 | ||
| B7 | 100 mg 1 wt% Co/SiO2-100 mg HAP-ethanol concentration 0.9 ml min−1 | 250 | 4.40 | 0.13 | 4.08 | 2.04 |
| 275 | 7.90 | 0.15 | 6.62 | 3.49 | ||
| 300 | 11.70 | 0.20 | 12.86 | 6.47 | ||
| 325 | 17.80 | 1.42 | 18.45 | 7.94 | ||
| 350 | 30.20 | 1.53 | 25.05 | 10.30 | ||
| 400 | 69.40 | 2.51 | 38.17 | 13.96 |
| Time (min) | Ethanol conversion rate (%) | Selectivity (%) | ||
|---|---|---|---|---|
| Ethylene selectivity | C4 olefins selectivity | Acetaldehyde selectivity | ||
| 20 | 43.50 | 4.23 | 39.90 | 5.17 |
| 70 | 37.80 | 4.28 | 38.55 | 5.60 |
| 110 | 36.60 | 4.46 | 36.72 | 6.37 |
| 163 | 32.70 | 4.63 | 39.53 | 7.82 |
| 197 | 31.70 | 4.62 | 38.96 | 8.19 |
| 240 | 29.90 | 4.76 | 40.32 | 8.42 |
| 273 | 29.90 | 4.68 | 39.04 | 8.79 |
| Parameter | Explanation |
|---|---|
| T | Temperature |
| t | Time |
| i | Combination number |
| Ai | Catalyst combination number using charging method I in Table 1 |
| Bi | Catalyst combination number using charging method II in Table 1 |
| Y(T) | Ethanol conversion rate, corresponding to a specific catalyst combination and temperature (%) |
| P(T) | C4 olefins selectivity, corresponding to a specific catalyst combination and temperature (%) |
| yI | Yield of C4 olefins in charging method I |
| yII | Yield of C4 olefins in charging method II |
| x1 | Co load |
| x2 | Co/SiO2 |
| x3 | HAP |
| x4 | Amount of ethanol added per minute |
The relationships among ethanol conversion rate, selectivity of C4 olefins, and temperature under each catalyst combination
The relationships among temperature change and selectivity of ethanol conversion rate and C4 olefins are studied in different catalyst combinations. The experimental data in Tables 1 and 2 have been preliminarily analysed using scatterplots. The findings indicate that the temperature changes in different catalyst combinations have some relationships with ethanol conversion rate and C4 olefins selectivity. The curve fitting toolbox (cftool) in MATLAB was used for preliminary data fitting.18 Through comparing the coefficients of determination, R2, and the residuals among various fitting functions, a better fitting function was obtained. Then their correlation was analyzed.19 Next, the data in Table 2 were classified. Since the time data are not uniformly distributed but are complete, spline interpolation was used to supplement the complete time data. The selectivity data were analysed using scatterplots and were processed according to the data trends.Model 1: nonlinear curve fitting of ethanol conversion rate, C4 olefins, and temperature
The original experimental data in Table 1 suggest that the temperature increases from 250 °C in each group of catalysts. There are specific changes in the ethanol conversion rate Y and C4 olefins selectivity P, which were the core elements of the experiment. MATLAB software was used to draw each catalyst combination scatter plot of temperature and ethanol conversion rate. For example, the relationship between temperature T and ethanol conversion rate Y in catalyst group A1 is shown in Fig. 1.The preliminary analysis of the figure indicates a specific relationship between the temperature T of the A1 catalyst and ethanol conversion rate Y; the curve fitting toolbox in MATLAB was used for fitting. In the chemical reaction with an unknown mechanism, the most suitable curve model was selected according to the data distribution in the scatter plot.20 The known values increased in the change of temperature-to-ethanol conversion rate, which accorded with the exponential model. However, the ethanol conversion rate is unlikely to grow explosively, as in an exponential model, and it is unlikely to exceed or equal 100%, so the exponential model was not adopted. At the beginning the trend of ethanol conversion rate increases with the temperature, and then at a certain point of time, it decreases, and it does not change periodically hence.21 Therefore, the relation equation should be obtained by fitting the Gaussian distribution model;22 the same is true for the selectivity of C4 olefins (Fig. 2).
 | ||
| Fig. 2 Fitting function diagram of temperature T and ethanol conversion rate Y in catalyst group A1. | ||
According to the curve fitting, the relationship between temperature T and ethanol conversion rate Y satisfied the equation:
 | (2) |
The residual value is 13.6410, and the coefficient of determination, R2, is 0.9817, indicating an excellent fit.
By comparing the R2 and residual values, the closer R2 is to 1, the better, and the smaller the residual value is, the better. Furthermore, considering the simplicity of the equation, the fitting functions of ethanol conversion rate, C4 olefins selectivity, and temperature under the other catalyst groups (groups A02–A14 and B01–B07) could be obtained, as shown in Table 4.
According to the fitting functions in Table 4, the corresponding values of ethanol conversion rate and C4 olefins conversion at a given temperature under each catalyst combination can be calculated.
| Time (min) | Ethanol conversion rate (%) | Selectivity (%) | ||
|---|---|---|---|---|
| Ethylene selectivity | C4 olefins selectivity | Acetaldehyde selectivity | ||
| 20 | 43.55 | 4.23 | 39.90 | 5.17 |
| 40 | 39.99 | 4.21 | 40.27 | 5.30 |
| 60 | 38.24 | 4.25 | 39.28 | 5.48 |
| 80 | 37.49 | 4.32 | 37.82 | 5.75 |
| 100 | 36.98 | 4.41 | 36.79 | 6.13 |
| 120 | 35.93 | 4.51 | 37.05 | 6.65 |
| 140 | 34.33 | 4.59 | 38.38 | 7.25 |
| 160 | 32.88 | 4.63 | 39.47 | 8.06 |
| 180 | 32.14 | 4.62 | 39.31 | 8.06 |
| 200 | 31.61 | 4.63 | 38.97 | 8.21 |
| 220 | 30.72 | 4.69 | 39.57 | 8.31 |
| 240 | 29.85 | 4.76 | 40.32 | 8.42 |
| 260 | 29.55 | 4.76 | 40.15 | 8.61 |
| 280 | 30.34 | 4.61 | 37.99 | 8.92 |
The results presented in Table 5 suggest that the ethanol conversion rate decreases monotonically with time, and acetaldehyde selectivity increases with time. The other data fluctuate around their means. The grey prediction model GM (1,1)24,25 could describe the relationship between time and ethanol conversion rate. It was used to predict the ethanol conversion rate (%); the results are shown in Fig. 3.
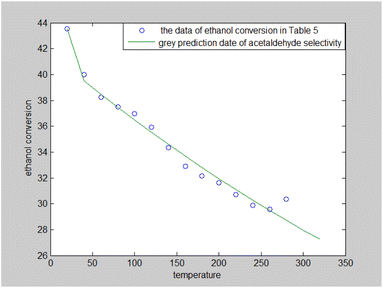 | ||
| Fig. 3 Expectancy map of the grey prediction model for ethanol conversion rate in Table 5. | ||
Fig. 3 illustrates that the ethanol conversion rate decreased with the increase in reaction time, but the rate of decline also decreased over time. It stabilized at about 29% when the reaction time was 260 minutes.
 | ||
| Fig. 4 qq diagram of ethylene selectivity in Table 2. | ||
Based on the above analyses, it is clear that ethylene selectivity and C4 olefins selectivity are weakly correlated with reaction time.
 | (3) |
The data for ethanol conversion rate and catalyst combinations at a uniform temperature were obtained.
 | ||
| Fig. 7 Catalyst combination-temperature-ethanol box diagram. Note: the abscissa represents the catalyst group number, and the ordinate represents the ethanol conversion rate. | ||
The mean, maximum, and minimum values of the ethanol conversion rate differ for the 21 catalyst combinations and corresponding temperatures. Moreover, the ethanol conversion rate in charging method I is higher than that in charging method II, which implies that the ethanol conversion rate may be affected by the catalyst combination, temperature, and charging method. To further verify these observations, a two-factor analysis of variance was conducted.27 The null hypothesis of no relationship was rejected, as catalyst combination and temperature have a significant effect on ethanol conversion rate (p < 0.001).
Additional analyses were conducted to explore the influence of each temperature group on the ethanol conversion rate using the data in Table 6. A box plot of the ethanol conversion rate for six temperature groups was drawn, as shown in Fig. 8.
| Ethanol conversion rate at… | ||||||
|---|---|---|---|---|---|---|
| Catalyst combination | 250 °C | 275 °C | 300 °C | 325 °C | 350 °C | 400 °C |
| A1 | 2.07 | 5.85 | 14.97 | 19.68 | 36.80 | 87.09 |
| A2 | 4.60 | 17.20 | 38.92 | 56.38 | 67.88 | 77.88 |
| A3 | 9.70 | 19.20 | 29.30 | 37.60 | 48.90 | 83.70 |
| A4 | 4.00 | 12.10 | 29.50 | 43.30 | 60.50 | 88.40 |
| A5 | 14.80 | 12.40 | 20.80 | 28.30 | 36.80 | 76.00 |
| A6 | 13.40 | 12.80 | 25.50 | 39.50 | 55.80 | 83.30 |
| A7 | 19.70 | 29.00 | 40.00 | 49.30 | 58.60 | 76.00 |
| A8 | 6.30 | 8.80 | 13.20 | 21.06 | 31.70 | 56.10 |
| A9 | 2.10 | 3.00 | 4.70 | 8.00 | 13.40 | 40.80 |
| A10 | 0.30 | 1.00 | 1.70 | 4.30 | 9.00 | 28.60 |
| A11 | 0.20 | 0.50 | 1.60 | 3.70 | 8.20 | 32.60 |
| A12 | 1.40 | 3.50 | 6.90 | 12.00 | 19.90 | 44.50 |
| A13 | 1.30 | 2.30 | 4.10 | 8.10 | 14.60 | 40.00 |
| A14 | 2.50 | 5.30 | 10.20 | 15.40 | 24.00 | 53.60 |
| B1 | 1.40 | 3.40 | 6.70 | 11.60 | 19.30 | 43.60 |
| B2 | 2.80 | 4.40 | 6.20 | 10.10 | 16.20 | 45.10 |
| B3 | 0.40 | 0.60 | 1.10 | 3.30 | 6.00 | 21.10 |
| B4 | 0.50 | 1.10 | 3.00 | 6.10 | 9.60 | 33.50 |
| B5 | 2.10 | 3.80 | 5.80 | 9.80 | 15.90 | 45.00 |
| B6 | 2.80 | 7.50 | 12.60 | 15.90 | 27.00 | 63.20 |
| B7 | 4.40 | 7.90 | 11.70 | 17.80 | 30.20 | 69.40 |
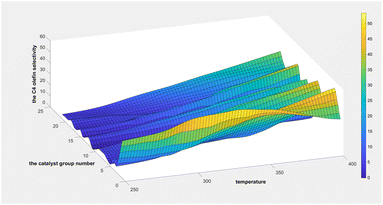
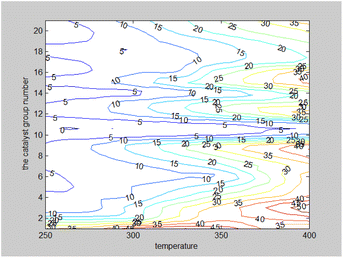
As indicated in Fig. 8, the ethanol conversion rate is the highest when temperature is high (t = 400 °C) and catalyst combination A2 is used. Using two-dimensional interpolation,28 the curves for ethanol conversion rate, catalyst combination, and temperature were obtained (Fig. 9 and 10).
Fig. 9 depicts the surface plot of the ethanol conversion rate with catalyst combination and temperature, while Fig. 10 shows the contour plot of the ethanol conversion rate with catalyst combination and temperature. From these illustrations, it is clear that the ethanol conversion rate was highest when the temperature was 400 °C and the catalyst combination was A1, A3, or A6.
The results of a two-factor analysis of variance indicate that the null hypothesis that catalyst combination and temperature have no significant effects on C4 olefins selectivity should be rejected (p < 0.001 for both).29
As shown in Fig. 12, when the temperature increased, the C4 olefins selectivity also increased. When the maximum temperature was 400 °C, the ethanol conversion rate was highest.
Two-dimensional interpolation was used to create the surface plot (Fig. 13) and contour plot (Fig. 14) for C4 olefins selectivity with catalyst combination and temperature. The results indicate that the selectivity of C4 olefins is higher when the temperature is 400 °C and the catalyst combination is A2 or A3.
Model 2: multivariate nonlinear regression model of C4 olefins yield with catalyst combination and temperature
The C4 olefins yield equation is as follows:| C4 olefins yield = ethanol conversion rate × C4 olefins selectivity | (4) |
Using eqn (4) and available data, the yield of C4 olefins was calculated, as shown in Table 7.
| Yield at… | ||||||
|---|---|---|---|---|---|---|
| Category | 250 °C | 275 °C | 300 °C | 325 °C | 350 °C | 400 °C |
| A1 | 0.70 | 2.19 | 7.03 | 9.78 | 17.37 | 40.81 |
| A2 | 0.83 | 2.97 | 7.63 | 17.26 | 26.54 | 33.83 |
| A3 | 0.53 | 1.55 | 4.98 | 10.79 | 18.03 | 44.73 |
| A4 | 0.39 | 1.04 | 3.16 | 8.18 | 16.48 | 36.28 |
| A5 | 0.29 | 0.83 | 2.11 | 3.93 | 6.90 | 29.06 |
| A6 | 0.44 | 0.91 | 1.83 | 3.52 | 5.94 | 31.11 |
| A7 | 1.13 | 1.91 | 3.53 | 6.77 | 10.92 | 25.28 |
| A8 | 0.35 | 0.75 | 1.82 | 4.29 | 8.21 | 23.24 |
| A9 | 0.11 | 0.29 | 0.76 | 1.89 | 4.16 | 17.15 |
| A10 | 0.01 | 0.02 | 0.04 | 0.12 | 0.30 | 2.94 |
| A11 | 0.00 | 0.01 | 0.03 | 0.11 | 0.36 | 2.58 |
| A12 | 0.09 | 0.28 | 0.78 | 2.01 | 4.43 | 19.83 |
| A13 | 0.07 | 0.18 | 0.52 | 1.47 | 3.43 | 11.18 |
| A14 | 0.05 | 0.14 | 0.37 | 1.11 | 2.60 | 11.96 |
| B1 | 0.09 | 0.28 | 0.83 | 2.22 | 5.01 | 17.91 |
| B2 | 0.09 | 0.22 | 0.58 | 1.63 | 3.70 | 17.47 |
| B3 | 0.01 | 0.03 | 0.09 | 0.25 | 0.47 | 2.91 |
| B4 | 0.04 | 0.07 | 0.15 | 0.50 | 1.26 | 7.18 |
| B5 | 0.09 | 0.19 | 0.46 | 1.14 | 2.43 | 11.62 |
| B6 | 0.12 | 0.36 | 1.11 | 2.55 | 6.06 | 19.28 |
| B7 | 0.18 | 0.52 | 1.50 | 3.28 | 7.57 | 26.49 |
The data were normalized, and a multiple linear regression model was established, with the yield of C4 olefins as the response variable, and with temperature and four catalysts (Co load, Co/SiO2, HAP, ethanol addition per minute) as the predictor variables. The coefficient of determination, R2, of the multiple linear regression30 is only 0.69, which is small, and the optimization results are poor.
Since the units of temperature, Co loading, Co/SiO2, HAP, and ethanol added per minute differ (Table 8), the data for these variables were divided by the corresponding data in the first row in order to remove the units. From previous analyses, multiple interaction effects are known to exist under charging method I, and multiple nonlinear regression was used.33 The model can be written as follows:
| yI = 90.384 − 114.04 × T − 1.6525 × x1 − 136.45 × x3 − 9.2553 × x4 + 52.928 × T2 − 3.1473 × T × x1 + 67.791 × T × x2 − 27.336T × x3 − 7.0734T × x4 − 1.0955 × x12 − 102.06 × x1 × x2 + 104.65 × x1 × x3 + 8.5108 × x1 × x4 − 84.468 × x22 + 153.35 × x2 × x3 + 7.0522 × x3 × x4 + 2.6543 × x42 | (5) |
| C4 olefin yield (y) | Temperature (x1) | Co loading capacity (x2) | Co/SiO2 (x3) | HAP (x4) | Amount of ethanol added per minute (x5) |
|---|---|---|---|---|---|
| 0.04 | 250 | 1 | 25 | 25 | 1.68 |
| 0.07 | 275 | 1 | 25 | 25 | 1.68 |
| 0.15 | 300 | 1 | 25 | 25 | 1.68 |
| 0.50 | 325 | 1 | 25 | 25 | 1.68 |
| 1.26 | 350 | 1 | 25 | 25 | 1.68 |
| 7.18 | 400 | 1 | 25 | 25 | 1.68 |
| 0.09 | 250 | 1 | 50 | 50 | 2.10 |
| 0.19 | 275 | 1 | 50 | 50 | 2.10 |
| 0.46 | 300 | 1 | 50 | 50 | 2.10 |
| 1.14 | 325 | 1 | 50 | 50 | 2.10 |
| 2.43 | 350 | 1 | 50 | 50 | 2.10 |
| 11.62 | 400 | 1 | 50 | 50 | 2.10 |
| 0.12 | 250 | 1 | 75 | 75 | 1.68 |
| 0.36 | 275 | 1 | 75 | 75 | 1.68 |
| 1.11 | 300 | 1 | 75 | 75 | 1.68 |
| 2.55 | 325 | 1 | 75 | 75 | 1.68 |
| 6.06 | 350 | 1 | 75 | 75 | 1.68 |
| 19.28 | 400 | 1 | 75 | 75 | 1.68 |
| 0.18 | 250 | 1 | 100 | 100 | 0.90 |
| 0.52 | 275 | 1 | 100 | 100 | 0.90 |
| 1.50 | 300 | 1 | 100 | 100 | 0.90 |
| 3.28 | 325 | 1 | 100 | 100 | 0.90 |
| 7.57 | 350 | 1 | 100 | 100 | 0.90 |
| 26.49 | 400 | 1 | 100 | 100 | 0.90 |
The R2 value is 0.91, indicating that the interaction effects and data nonlinearity in the reaction have a strong fit; however, the model is very complex, which is not conducive to interpreting the results. Therefore, stepwise regression was carried out to further highlight the model's key factors (Fig. 15).34 The model for the stepwise regression is:
| yI = 77.1798 − 129.936 × T − 2.3408 × x2 + 54.831 × T2 − 0.7725 × T × x1 + 41.1626 × T × x2 | (6) |
The results indicate that R2 is 0.85, further highlighting the key influencing factors and improving the applicability of the model. Moreover, it is concise.
In charging method II, first, based on the results of the multiple linear regression and considering the existence of the interaction effects, group B1 was taken as the benchmark for comparison after removing the units. Complete quadratic polynomial fitting was used to obtain the following model:
| yII = 106.47 − 172.91 × T + 69.481 × T2 + 9.4405 × T × x2 − 6.445 × T × x4 − 3.3272 × x22 + 0.2338 × x2 × x4 + 0.80079 × x42 | (7) |
The results indicate that the R2 value is 0.96.
Furthermore, the model using stepwise regression was as follows:
| yII = 115.486 − 181.657 × T − 11.557 × x2 + 69.48 × T2 + 10.4993 × T × x2 | (8) |
The R2 value is 0.96, and the results are shown in Fig. 16.
Model 3: optimizing the model for C4 olefins yield with catalyst combination and temperature
The optimization model was established using eqn (6) and was constructed as follows:35| maxyI = 77.1798 − 129.936 × T − 2.3408 × x2 + 54.831 × T2 − 0.7725 × T × x1 + 41.1626 × T × x2 | (9) |
The optimized conditions were divided according to the available experimental data:
| s.t T ≥ 1; T ≤ 1.8 | (10) |
| x1 ≥ 0.5; x1 ≤ 5 | (11) |
| x2 ≥ 0.165; x2 ≤ 1 | (12) |
Lingo software was used to identify the optimization solution, and the following results were obtained. Under charging method I, when T = 1.6, x1 = 0.5, and x2 = 1 (i.e. when the temperature was 450 °C, the Co load was 0.5 wt%, and the Co/SiO2 was 200 mg), the maximum yield of C4 olefins was 52%.
When the temperature was below 350 °C, the constraint conditions were changed to identify the optimal solution:36
| T ≥ 1; T ≤ 1.4 | (13) |
| x1 ≥ 0.5; x1 ≤ 5 | (14) |
| x2 ≥ 0.165; x2 ≤ 1 | (15) |
Again using Lingo software, the following results were obtained. When the temperature was lower than 350 °C, T = 1.4, x1 = 0.5, and x2 = 1 (i.e. when the temperature was 350 °C, the Co load was 0.5 wt%, and the Co/SiO2 was 200 mg), the C4 olefins yield was at its maximum of 7.48%.
Using eqn (8), an optimization model was built:
| maxyII = 115.486 − 181.657 × T − 11.557 × x2 + 69.48 × T2 + 10.4993 × T × x2 | (16) |
| s.t T ≥ 1; T ≤ 1.6 | (17) |
| x1 ≥ 1; x1 ≤ 4 | (18) |
Lingo software was again used to identify the optimization solution, and the following results were obtained. Under charging method II, when T = 1.6 and x1 = 4 (i.e. temperature was 400 °C and Co/SiO2 was 100 mg), the C4 olefins yield reached the maximum of 23.67%. When the temperature was below 350 °C and Co/SiO2 was 100 mg, the C4 olefins yield reached the maximum of 9.92%.
Conclusion
In the preparation of C4 olefins through ethanol catalytic coupling, the ethanol conversion rate and C4 olefins selectivity are two core indexes. The results of this study indicate that the fitting function between ethanol conversion rate, C4 olefins selectivity, and temperature under each catalyst combination predicted the values of ethanol conversion rate and C4 olefins selectivity under different temperatures. The two-factor analysis of variance showed that different catalyst combinations and temperatures had significant effects on ethanol conversion rate and selectivity of C4 olefins. However, analysing the test results under a given catalyst combination at 350 °C at different times in an experiment indicated that the ethylene selectivity and C4 olefins selectivity correlate less as reaction time increases. Therefore, the catalyst combination and reaction temperature are mainly considered when analysing the above indexes. To find a certain catalyst combination and temperature that will achieve the highest C4 olefins yield under the same experimental conditions, a multiple nonlinear regression model and stepwise regression model of C4 olefins yield with four catalysts and temperatures were established and the goal function in the optimization model was obtained. Then, constraint conditions were given under laboratory conditions. Finally, the maximum C4 olefins yield was obtained.Through the establishment and analysis of three mathematical models, this research showed that both catalyst combination and reaction temperature would affect the C4 olefins yield. Moreover, the higher the reaction temperature, the higher the yield of C4 olefins. The influence of Co loading and Co/SiO2 on the yield of C4 olefins is greater than that of the other two catalysts. When the minimum of Co loading was 0.5 wt% and the maximum of Co/SiO2 was 200 mg, the yield of C4 olefins was largest. The amount of ethanol added per minute had little effect on the C4 olefins yield.
Based on the experimental data, this paper established a mathematical model and concluded that the higher the reaction temperature, the higher the C4 olefins yield. However, when the reaction temperature is higher than the maximum value of 400 °C in the experimental data, will the C4 olefins yield continue to increase? And when the temperature continually rises, will the four catalysts undergo denaturation? There are insufficient experimental data to answer these questions, both of which need further study.
Data availability
The data in this paper come from Question B of the 2021 Chinese Contemporary Undergraduate Mathematical Contest in Modelling (CUMCM).Author contributions
Conceptualization, methodology, software, validation, formal analysis, data curation, writing—original draft preparation, visualization, P. T.; writing—review and editing, H. L.; supervision, project administration, funding acquisition, X. Z. and X. S. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Problem B Annex of the National College Students Mathematical Modeling Competition of the Higher Education Society Cup for experimental data support. The authors are also grateful to Hang Zhou and Wenhao Fan at the Jilin Medical University for their help. Financial support came from the Jilin Province Higher Education Reform Project (Grant No. JLJY202269980687) and the Jilin Province Vocational Education and Adult Education Teaching Reform Project (Grant No. 2022ZCZ051) of the Department of Education of Jilin Province; this assistance is acknowledged and appreciated.References
- B. Wu, Y. W. Wang, Y. H. Dai, C. Song, Q. L. Zhu, H. Qin, F. R. Tan, H. C. Chen, L. C. Dai, G. Q. Hu and M. X. He, Renewable Sustainable Energy Rev., 2021, 145, 17 Search PubMed.
- A. Z. Mendiburu, C. H. Lauermann, T. C. Hayashi, D. J. Marinos, R. B. R. da Costa, C. J. R. Coronado, J. J. Roberts and J. A. de Carvalho, Energy, 2022, 257, 27 CrossRef.
- T. H. Rupam, K. A. Rocky, M. L. Palash and B. B. Saha, Therm. Sci. Eng. Prog., 2023, 37, 8 Search PubMed.
- M. A. Ershov, D. A. Potanin, S. V. Tarazanov, T. M. M. Abdellatief and V. M. Kapustin, Energy Fuels, 2020, 34, 2816–2823 CrossRef CAS.
- C. P. Nicolaides, C. J. Stotijn, E. R. A. Vanderveen and M. S. Visser, Appl. Catal., A, 1993, 103, 223–232 CrossRef CAS.
- F. F. Madeira, N. S. Gnep, P. Magnoux, S. Maury and N. Cadran, Appl. Catal., A, 2009, 367, 39–46 CrossRef CAS.
- M. D. Jones, C. G. Keir, C. Di Iulio, R. A. M. Robertson, C. V. Williams and D. C. Apperley, Catal. Sci. Technol., 2011, 1, 267–272 RSC.
- F. Yuan, G. H. Zhang, J. Zhu, F. S. Ding, A. F. Zhang, C. S. Song and X. W. Guo, Catal. Today, 2021, 371, 142–149 CrossRef CAS.
- S. Lu, Master's degree, Dalian University of Technology, 2018 Search PubMed.
- Y. Ge, Master's degree, East China University of Science and Technology, 2019 Search PubMed.
- D. A. Jadhav, A. A. Carmona-Martinez, A. D. Chendake, S. Pandit and D. Pant, Bioresour. Technol., 2021, 320, 11 CrossRef PubMed.
- R. Mittal, C. Meneveau and W. Wu, Phys. Fluids, 2020, 32, 15 Search PubMed.
- M. Y. Sun, S. D. Zhao, C. Gilvary, O. Elemento, J. Y. Zhou and F. Wang, Briefings Bioinf., 2020, 21, 919–935 CrossRef PubMed.
- M. H. Li, L. L. Zhao, S. Jin, D. L. Li and J. X. Liu, Heliyon, 2022, 8, 12 Search PubMed.
- S. Wang, Z. H. Jiang, J. Yang, Y. L. Tang and B. Liu, Energy Rep., 2022, 8, 370–376 CrossRef.
- S.-M. Zhang, W.-L. Zhan, H. Hu, Y.-S. Liu and J.-M. Zhu, J. Chem., 2022, 2022, 5324336 Search PubMed.
- C. S. f. I. A. Mathematics, Question B of the 2021 Chinese Contemporary Undergraduate Mathematical Contest in Modeling, http://www.mcm.edu.cn/html_cn/node/4d73a36cc88b35bd4883c276afe39d89.html.
- C. L. Sun, M. Z. Liu and S. H. Ge, Appl. Sci., 2022, 12, 20 Search PubMed.
- X. G. Jiang, S. S. Wei, J. B. Ji, F. F. Liu, P. Li and C. Y. Liu, Artery Res., 2018, 23, 56–62 CrossRef.
- L. Y. Yu, X. Y. Wang, Z. Y. Hou, Z. Y. Du, Y. F. Zeng and Z. Y. Mu, Appl. Sci., 2021, 11, 17 Search PubMed.
- Y. W. Chen and Y. H. Hsu, Catalysts, 2021, 11, 13 Search PubMed.
- L. L. Zhang, S. K. Oh, W. Pedrycz, B. Yang and Y. M. Han, Appl. Soft. Comput., 2021, 112, 16 Search PubMed.
- V. Rodinkova, V. Mokin, T. Vuzh and M. Dratovanyj, Aerobiologia, 2021, 37, 695–706 CrossRef.
- K. Salhein, J. Ashraf and M. Zohdy, Energies, 2021, 14, 12 CrossRef.
- H. Wang, Y. H. Wang and D. D. Wu, Grey Syst., 2022, 12, 293–317 Search PubMed.
- W. Sohn and E. H. Hong, Nucl. Eng. Technol., 2021, 53, 1664–1675 CrossRef CAS.
- D. G. Cloutman and D. C. Jackson, Fish. Manag. Ecol., 2003, 10, 173–177 CrossRef.
- F. Anton, D. Mioc and A. Fournier, Visual Comput., 2001, 17, 134–146 CrossRef.
- S. S. Wu, X. L. Hu, W. B. Zheng, C. C. He, G. C. Zhang, H. Zhang and X. Wang, Bull. Eng. Geol. Environ., 2021, 80, 5405–5421 CrossRef.
- X. F. Xie, T. Wu, M. Zhu, G. J. Jiang, Y. Xu, X. H. Wang and L. J. Pu, Ecol. Indic., 2021, 120, 9 CrossRef.
- S. Oliveira, F. Oehler, J. San-Miguel-Ayanz, A. Camia and J. M. C. Pereira, For. Ecol. Manag., 2012, 275, 117–129 CrossRef.
- V. Shah, S. C. K. Jagupilla, D. A. Vaccari and D. Gebler, Water, 2021, 13, 13 Search PubMed.
- A. T. C. Goh and W. G. Zhang, Eng. Geol., 2014, 170, 1–10 CrossRef.
- S. Radovanovic, M. Milivojevic, B. Stojanovic, S. Obradovic, D. Divac and N. Milivojevic, Appl. Sci., 2022, 12, 20 Search PubMed.
- L. V. Enikeeva, A. G. Faskhutdinov, K. F. Koledina, R. I. Faskhutdinova and I. M. Gubaydullin, React. Kinet. Mech. Catal., 2021, 133, 879–895 CrossRef CAS.
- K. F. Koledina, I. M. Gubaydullin and S. N. Koledin, React. Kinet. Mech. Catal., 2022, 135, 155–167 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(
cos(