Open Access Article
Open Access ArticleRecent developments, applications and challenges for carbon quantum dots as a photosynthesis enhancer in agriculture
Yamuna A/P Chowmasundarama,
Tong Ling Tan *a,
Rosimah Nulitc,
Mashitah Jusohd and
Suraya Abdul Rashid*ab
*a,
Rosimah Nulitc,
Mashitah Jusohd and
Suraya Abdul Rashid*ab
aInstitute of Nanoscience and Nanotechnology, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. E-mail: yamunaa2707@gmail.com; tongling@upm.edu.my; suraya_ar@upm.edu.my
bDepartment of Chemical and Environmental Engineering, Faculty of Engineering, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia
cDepartment of Biology, Faculty Science, Universiti Putra Malaysia, 43400 UPM Serdang, Selangor, Malaysia. E-mail: rosimahn@upm.edu.my
dDepartment of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia, 43400, Selangor, Malaysia. E-mail: mashitahj@upm.edu.my
First published on 23rd August 2023
Abstract
Since the world's population is expanding, mankind may be faced with a huge dilemma in the future, which is food scarcity. The situation can be mitigated by employing sustainable cutting-edge agricultural methods to maintain the food supply chain. In recent years, carbon quantum dots (CQD), a member of the well-known carbon-based nanomaterials family, have given rise to a new generation of technologies that have the potential to revolutionise horticulture and agriculture research. CQD has drawn much attention from the research community in agriculture owing to their remarkable properties such as good photoluminescence behaviour, high biocompatibility, photo-induced electron transfer, low cost, and low toxicity. These unique properties have led CQD to become a promising material to increase plant growth and yield in the agriculture field. This review paper highlights the recent advances of CQD application in plant growth and photosynthesis rate at different concentrations, with a focus on CQD uptake and translocation, as well as electron transfer mechanism. The toxicity and biocompatibility studies of CQD, as well as industrial scale applications of CQD for agriculture are discussed. Finally, the current challenges of the present and future perspectives in this agriculture research are presented.
1. Introduction
The population of the world is approximately 7.9 billion people in 2022 and it will most likely be near to 9.73 billion in 2064.1 The World Factbook estimates that the global population is growing at a rate of 1.06% yearly. As a result, there will be a considerable increase in demand for survival food, which will become an essential requirement. Planting crops has been one of the most evolved civilizations in human history. It is a field that has been studied and practised for thousands of years. Agriculture is progressing every decade as our planet's age increases. The future viability of sustainable agriculture, on the other hand, is still up for debate. The term “sustainable” refers to how long agriculture can be long-lasting and advanced enough to produce high yield end production as the world's population grows.2 Crop production needs to be advanced in order to supply enough food in the future. To maintain a continuous food chain, a strategic approach in the agriculture field should be planned properly to accelerate the yield of food production in a shorter period.Nanomaterials (NM) are one of the most cutting-edge human achievements and are expected to be a solution for future food supply concerns. Numerous discoveries have been made about various NM by researchers. Due to their diverse abilities to react differently in the environment, various types have been employed in a variety of applications, including pharmaceuticals, semiconductors, electronic devices, paints, catalysts, and solar cells.3–5 Some NM research work with regards to agriculture revolve around plant stress reduction, disease prevention, plant growth increment, and fertilizer.6–12 Metal and metal oxide NM are among the materials that have recently captured the attention of researchers in the agriculture field. For example, TiO2, ZnO, Mg, Ag, Cu, Al, and Zn NM have been used to enhance plant growth.13–23 However, metal and metal oxide NM have a number of drawbacks, one of which is their toxicity.24,25 Although these NM may show improved plant growth and yield, the majority of metal and metal oxide NM are hazardous to plants at high doses and are believed to pose a concern to the environment.26 The toxicity of these metal and metal oxide NM is not only harmful to the environment but will also cause huge side effects to the consumers who ingest them for long periods.27,28 For example, it has been reported in 182 studies that zinc oxide NM has adverse effects on the cardiovascular, neurological system, alimentary canal, reproductive system, and respiratory systems of the human body system.29 Several chemical approaches to produce metal oxide NM have also been reported to include harmful substances such as H2S, toxic material, and metallic precursors.30,31 Other than that, the cost of the precursor is very expensive and has limitations in scaling up,32 which would not make it a sustainable approach for future agriculture.
Besides metals and metal oxides, non-metallics are also another class of NM. Non-metallic nanomaterials (NM), such as silicon (Si), sulphur (S), selenium (Se), and carbon-based NM, have been extensively studied. They positively regulate plant growth and development when applied to the plants. It has been found that Si, S, and Se nanomaterials (NM) have improved disease resistance and plant stress tolerance against both abiotic and biotic stressors.33–36 The main limitations of Si and S NM is higher manufacturing costs, which restrict scalability for increased productivity.37–39 S NM has constraints due to its incapacity to synthesize hydrophilic and stable aqueous solutions.40 Due to the abundance of carbon in nature, carbon-based nanomaterials are more accessible and frequently more affordable41 than other non-metallic NM.
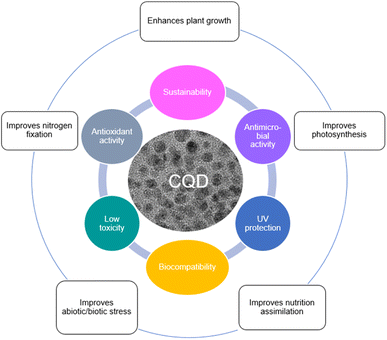
The most commonly investigated carbon-based NM is zero-dimensional (0D) carbon quantum dots (CQD, CD, or C-dot), which typically have a particle size of less than 10 nm.42–45 CQD has been one of the most widely studied NM since their discovery in 2004 because it is more biocompatible with plants and environmentally friendly.46–48 CQD also has a number of remarkable properties that make it one of the most promising materials to emerge in the agricultural area in the last century. Biocompatibility, low toxicity, antioxidant activity, UV protection, antimicrobial activity, and sustainability are the properties of CQD that make it more special than the rest.45,49–53 These characteristics enhance their environmental friendliness and capacity to lessen reliance on finite resources. The chemically inert features of CQD mean it is particularly unreactive and safe to use in the environment, and these special properties of CQD could provide an eco-friendly material with the lowest risk to the environment. It also means that CQD applied in agriculture is a sustainable approach particularly in terms of improving crops photosynthesis rate and plant growth, and enabling the production of sufficient food for future generations.
Recently, the impact of CQD application in plant systems have been reviewed.54 Fig. 1 illustrates the properties of CQD and their effects on plants. However, the field is rapidly expanding and there is a need for a review that covers recent developments. Since most reports are related to lab scale trials, the more practical aspects of CQD applications on plants at the industrial scale become important to consider. This article firstly describes the uptake and translocation of CQD in plants utilizing apoplastic or symplastic strategies. Then it covers a review on the application methods of CQD on plants, and the effects of CQD on plant growth and photosynthesis rate. This is followed by the electron transfer mechanism of CQD assisted photosynthesis. The toxicity and biocompatibility studies of CQD from different carbon precursors are reviewed and summarized. The feasibility of industrial scale applications of CQD for agriculture, in terms of practicality, scalability, safety, and cost compared to conventional fertilizers are discussed. Finally, the limitations in the current literature and highlighted topics worthy of future investigation are also assessed, which hopefully will provide important elicitation for future researchers in this field.
2. Uptake and translocation of carbon quantum dot (CQD) in plants
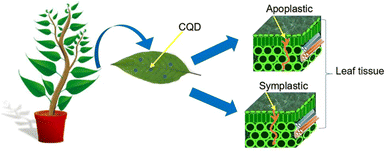
Uptake refers to the uptake of particles from the environment into the plant via root hair or foliar.55–57 Translocation is defined as the movement of particles from one region of the plant to another such as from the root section to the shoot and vice versa.58,59 CQD enters the plant by penetrating via the plant cell wall, cell membrane, epidermis cells, mesophyll cells and cell nucleus.60–63 The rigid and tough structure of the cell wall is made up of two layers; primary cell wall and secondary cell wall.64–66 The primary cell wall is made up of pectin, hemicellulose, and cellulose, while the secondary cell wall is a complex structure rich in lignin, which acts as a wall reinforcer.67,68 Cell wall pores are less than 10 nm in diameter, sometimes could have maximum diameter of 20 nm.69 When the pectin layer between two cells are degraded and cellulose-microfibrils are revealed, the diameters of cell wall pores are less than 30–40 nm with a maximum value of 60 nm.69 Meanwhile, the cell membrane is the second outermost layer of the plant, situated between the cell wall and the cytoplasm.70 This thin and fragile layer has a thickness of 5 to 10 nm, depending on the type of cell.71 The smaller pore distribution of the plant cell wall permits nano-sized CQD to penetrate both the plant and the cell membrane. The CQD enter the plant cytoplasm after passing through the cell membrane. Plant epidermis and mesophyll cells have also been discovered to be penetrated by CQD.62,72 Epidermis is the outermost layer of protoderm derived cells that covers sections of plants such as leaf, root, stem, fruit, flower and seed.73 The epidermis, in conjunction with the waxy cuticle, serves as a protective layer for the plants, shielding it from mechanical damage, water loss, and infection.74In general, there are two epidermis layers on the leaf: the upper epidermis and the lower epidermis.75 Mesophyll cells can be found in the leaf's two epidermal intercellular spaces, which are made up of chlorenchyma cells and irregularly shaped spongy parenchyma cells.76,77 Since chlorenchyma cells are densely packed with chloroplast, this mesophyll cell is frequently alluded as specialized photosynthetic tissue.78,79 Therefore, after reaching the mesophyll cell, the CQD is expected to enter the chloroplast, a green photosynthetic pigment rich in chlorophyll. According to Li et all, CQD can also penetrate the plant cell nucleus.60 Cell nucleus is one of the highly specialized organelles that is located in the cytoplasm fluid.80 It plays a key role in plants by storing hereditary material, such as deoxyribonucleic acid (DNA) and coordinating cell activities such as secondary metabolism, reproduction, protein synthesis and growth.81 After the CQD has successfully penetrated the plant, it will hold together with the plant cell wall with the aid of covalent and ionic bonds. The CQD attach either to the lignin which is one part of the cell wall via the carbon covalent bond or to the cellulose through a hydroxyl bond.82,83 The CQD translocation of various plants regions performs a particular pathway that can be apoplastic or symplastic which are entirely influenced by transpiration pull and diffusion coefficient.84 The translocation of CQD occurs via the intercellular space and the cell wall of the plants which is referred to as the apoplastic pathway.85–87 The symplastic pathway involves CQD moving through the cytosol via plasmodesmata channels that extend across neighbouring cells.86,88 CQD penetrates the leaf tissue via apoplastic and symplastic pathway as summarized in Fig. 2.
There are few methods that can be carried out to determine the presence of the CQD. Confocal laser scanning microscopy is the most common method for assessing the uptake of NMs by plant, as evidenced by several research articles. As shown in Fig. 3(i), CQD uptake by mung bean was detected in the vascular bundle with red fluorescence after a 160 min incubation time in the solution.72 Furthermore, green colour fluorescence was detected on each section of the CQD treated wheat plant, although no green fluorescence was observed in the control, indicating that the CQD was able to penetrate the plant cell and be excited under a certain wavelength.89 The red and green fluorescence is the emission of the light exhibited by the CQD under a wavelength. Confocal imaging clearly show that the CQD luminescence signals were primarily localized in the vascular system in the longitudinal sections of root and stem, which is supported by Fig. 3(ii).72
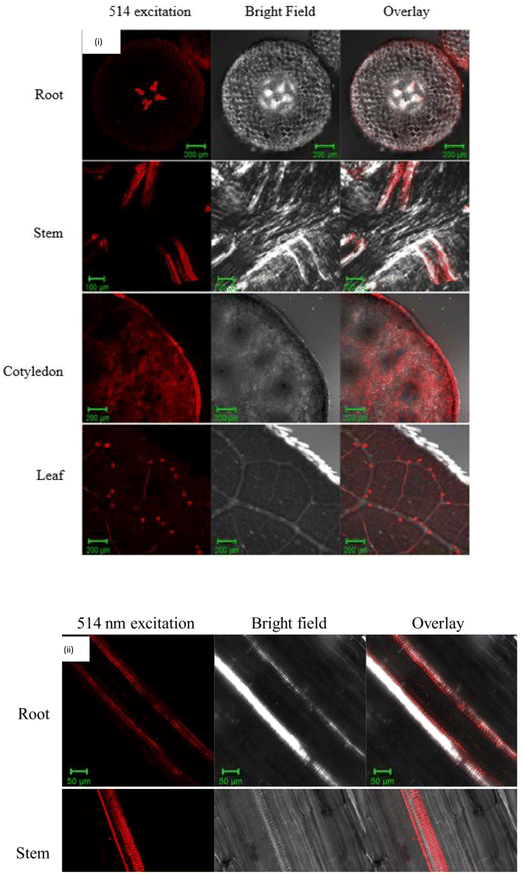 | ||
| Fig. 3 (i)The laser scanning microscopy (LSM) images of CQD uptake into the mung bean root, stem, cotyledon, and leaf. (ii) Laser scanning microscopy (LSM) images of longitudinal sections from root and stem treated with CQD. (Reproduced from ref. 67 with permission from the American Chemical Society, Copyright © 2016). | ||
3. Methods of applying CQD on plants
CQD can be applied to plants in two ways: by seed and root soaking or foliar spraying method. The seed soaking approach involves soaking the seeds in the CQD solution for a few days before transplanting them to the soil or transferring them to hydroponics systems.89,90 This seed soaking method can also be known as seed primitive.91 Sometimes, the young seedling's roots are soaked in the CQD solution to allow the uptake of the CQD into the vascular bundle of the roots which later eventually translocate via the apoplastic or symplastic process.92,93 The root soaking method allows the plants to choose the uptake of CQD via the root entry pathway such as via lateral roots, root hairs, and root tips.84 Foliar spraying, on the other hand, is a technique of application that involves spraying the CQD solution directly into the plant leaves.94 This approach was employed starting from the seedling stage. Foliar spraying allows CQD to enter the plant via foliar entry points such as stomata, cuticles, wounds, and hydathodes.84 Most recent papers have introduced the CQD using the foliar spraying method to the plant leaves.95–100 Both approaches, however, can reach the respective organelle for further electron transfer exchange. In addition, a recent publication has taken a new approach apart from these two methods, focusing on soil application.101 The method involves mixing CQD with soil before the seeds germinated.The soaking approach enters through the root, making it slower than the other procedure. The CQD can be delivered directly to the leaf part, which is in charge of plant photosynthesis, making the spraying approach a direct and quick process. The spraying method is more effective than the soaking method in terms of effectiveness. Soaking is effective for tasks that require the liquid to penetrate deeply into the leaf section. When compared to spraying, it may not give as exact or controlled liquid dispersion. A more equal and controlled application of the liquid is possible with spraying. Coating or dispersing substances over the leaf's surface enables greater precision and can be changed to obtain the appropriate coverage. When comparing the two approaches, spraying is the most appropriate approach for applying CQD to plants. Table 1 lists the various methods of CQD applications to plants. Based on Table 1, CQD is applied to different plant sections such as seed, root and leaves. The duration of CQD exposed varied depending on the researchers; some exposed the plant to the CQD for a longer period while some exposed it for a shorter period. In CQD-exposed plants, root length, shoot length, plant biomass, photosynthesis rate, and gene expression all increased.
| No | Type of plants | Plant section | Methods of application | Exposure duration (days) | Observation | References |
|---|---|---|---|---|---|---|
| 1 | Melon & wheat | Seed | Soaking | 2,6 & 9 | Increased root & shoot length | 89 |
| 2 | Arabidopsis thaliana | Roots | Soaking | 5 & 13 | Increased plant biomass & root length | 63 |
| 3 | Morus alba | Roots | Soaking | 15 & 35 | Increased root number & length | 93 |
| 4 | Rice & maize | Leaves | Foliar spraying | 2 | Increased photosynthesis rate | 95 |
| 5 | Arabidopsis thaliana | Leaves | Foliar spraying | 1 | Increased antioxidant responses | 96 |
| 6 | Maize | Leaves | Foliar spraying | 7 | Increased plant growth and photosynthesis rate | 97 |
| 7 | Soybean | Leaves | Foliar spraying | 7 | Increased yield, nitrogen content and protein content | 98 |
| 8 | Maize | Leaves | Foliar spraying | 7 | Increased root exudates | 99 |
| 9 | Maize | Leaves | Foliar spraying | 8 | Increased plant growth and photosynthesis rate | 100 |
| 10 | Soybean | Root | Soil application | — | Increased root exudates | 101 |
4. Effects of CQD on plant growth and photosynthesis rate
The physical traits of the plant are a common indicator of treatment efficacy. Plant height, root length, shoot length, fresh biomass and dry biomass are some of the commonly measured and collected physical data since the start of agriculture research. The number of leaves, surface area, root fresh weight and shoot fresh weight are only a few examples. The fact that treated plants have a higher physical characteristic than control plants shows that the CQD is effective in promoting the plant growth. Each data can be collected using different methods. Plant height, root and shoot length are measured with a simple measuring method that involves using a ruler, while plant biomass is measured with a weighting balance. The fresh biomass is the weight of the plant obtained directly after harvest, in which the plant weight and moisture content are determined. The plant dry biomass is the weight of the plant after it has been dried and there is no more water content left within the plant's cell.There are few examples of CQD impact on plants' physical traits. Fig. 4(i) shows that the CQD treated Rome lettuce plants yielded more, with biomass increasing by 48.09% compared to the control plant.102 When CQD is sprayed, maize plant height and grain weight both increase by 20.9% and 39.6%, respectively.95 A study by Swift et al. revealed that the wheat treated CQD enhanced shoot biomass, grain yield and seed production by 17%, 18% and 12%, respectively.103 Mung beans treated with CQD have been shown to promote root length by 29.9%, root vigour by 36.1%, stem length by 18.3%, fresh biomass by 14.9% and moisture content by 54.5%.104 When mung beans are treated with CQD, their root length, shoot length, fresh biomass, root vigour and moisture content all increase, as illustrated in Fig. 4(ii).72,104,105 Other than that, spirulina-based CQD increases the seedling biomass, seedling length and root length by about 48.1%, 120.2% and 105.3%, respectively.106 Because of its higher fluorescence quantum yield, nitrogen-doped CQD or amine terminated CQD (N-CQD) have lately been exploited in various studies.46,48,107 The 5 valence electrons of the nitrogen atom are balanced by covalently bonding with carbon atoms to form N-CQD.108,109 N-CQD appears to have a favourable effect on plant growth and development, according to some N-CQD studies.110,111 In terms of effectively stimulating plant growth, it has the same potential as CQD nanomaterial.
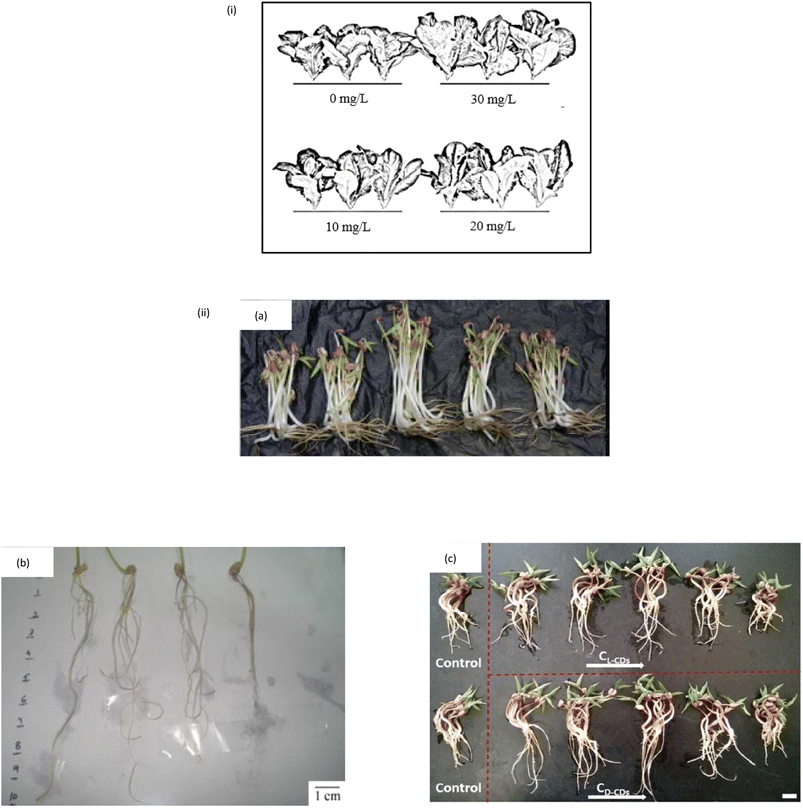 | ||
| Fig. 4 (i) The effect of CQD on the yield and morphology of lettuce plant (reproduced from ref. 92 with permission from the American Chemical Society, Copyright © 2017). (ii) The CQD effect on the yield and morphology of mung bean (a) (Reproduced from ref. 62 with permission from the American Chemical Society, Copyright © 2016). (b and c) (Reproduced from ref. 81 with permission from the Shweta Tripathi et al., Copyright © 2014). | ||
After being treated with N-CQD, the relative yield of all the soybean, tomato, eggplant, capsicums, watermelon, radish, celery and cabbage plants increases by 20%.110 Furthermore, it has been demonstrated that maize plants treated with N-CQD increase the fresh biomass of shoot and root by 24.03% and 34.56%, respectively. In a similar paper, it was demonstrated that N-CQD increased the dry biomass of the shoot and root of maize plants by 72.30% and 55.75%, respectively.101 Another study found that maize plants yielded more, with fresh biomass increasing by 50.6% in the roots and 62.1% in the shoots. Additionally, it also showed that maize plants' dry biomass increased by 37.5% in the roots and 29.2% in the shoots.99 Wang and his group similarly treated N-CQD on maize seedlings. After treatment, the fresh and dry biomass levels are similarly much greater. This investigation was conducted under drought-stress conditions. As a result, the fresh biomass of the maize plant has increased by 360% in the shoots and by 224.5% in the roots. In addition, the dry biomass increased by 63.3% in the shoots and 230.8% in the roots compared to the control plant.100 Cerium doped CQD was tested on wheat at various concentrations, and reported that the 25 ppm increases the root number by 45%, root length by 57%, leaf length by 28% and plant height by 46%.112
In addition to assessing the plant's physical properties, plant photosynthesis parameters are also important indicators to prove that CQD treatment is effective. The photosynthesis parameters measurement, in general, are used for expressing the plant photosynthesis rate. Examples of the photosynthesis parameters measurements are net photosynthesis rate, stomatal conductance, rubisco activity, carbohydrate content, and chlorophyll content.7,105,113,114 The impact of CQD on plant development have been evaluated and compared to that of control plants, with the majority of the research focusing on photosynthetic enhancement. Stomatal conductance (gl) is a measurement of the amount of stomatal opening in plants and is used to determine the amount of water in the plant.115 The rate of CO2 penetrating or water vapour departing via stomata is evaluated with a porometer. CQD treated plants, for example, had higher stomatal conductance than control plants.102 When rice and maize plants were treated with CQD, their stomatal conductance increased by up to 56% and 18%, respectively.95
Photosynthesis is also determined by rubisco activity. Rubisco activase is a component of the rubisco activity, which is responsible for the CO2 assimilation of the plant.116 The higher the rubisco activity of the plant, the higher the photosynthesis rate.117,118 The rice plant treated with CQD shows a significantly increased in rubisco activity of about 42%.60 According to a research, the CQD increased rubisco activity by 30.9%.104 In a study, CQD exposure increased the rubisco enzyme by approximately 20.5% as compared to control.119 The photosynthetic rate is also determined by measuring carbohydrate and chlorophyll content. Carbohydrate is a glucose compound that is formed at the end of the photosynthesis process; the faster photosynthesis rate leads to higher carbohydrate production.91,120 Based on the research papers, CQD could enhance the carbohydrate content of mung bean by up to 22% when compared to untreated plants.104,119 Meanwhile, chlorophyll concentration is the pigment that captures light photons; the higher the chlorophyll content, the greater the electron transport which consequently increases photosynthesis rate.79,121 It has been reported that mung bean chlorophyll content increased by 14.8% as compared to the control.104 It is also reported that CQD can increase the resistance to disease.60 CQD has the ability to penetrate the plant nucleus and loosen its DNA structure. This improves the thionin expression gene which will eventually increase the plant's resistance to disease.60
Other indicators of plant photosynthesis parameters include net assimilation, transpiration rate, Hill Reaction, 2,6-Dichlorophenolindophenol (DCPIP) reduction, hydrogen peroxide (H2O2) content, oxygen evolution, ferricyanide reduction, nicotinamide–adenine dinucleotide phosphate (NAPDH) formation, adenosine triphosphate (ATP) formation, photosystem I (PSI) rate, photosystem II (PSII) rate, electron transfer rate of PSI (ETRI) and electron transfer rate of PSII (ETRII).9,16,104,111,118 Many researches have indicated that CQD treatment on plants has a positive effect in terms of improved photosynthesis when compared to the control. For instance, Li group mixed CQD with extracted spinach chloroplast and discovered that CQD/chloroplast reduces DCPIP by 28% and generates 2.8 times more ATP than control. When compared to chloroplasts that were not treated with CQD, CQD treated chloroplasts demonstrated a maximum increase of 25% in electron transport rate at 7 ppm.122 It has been claimed that the CQD increases carbohydrate content by 21.9%, PSI rate by 10.4%, and ETRI by 8.8%.104 In a study, N-CQD exposure increased the carbohydrate content by approximately 35.4% in shoot and 113.6% in root compared to control plant.99 Recent research showed that CQD has improved the soybean plant's nitrogen content by 13.2% in shoots and 30.5% in roots.98 It also has been reported that nitrogen content improved in the shoots and roots of soybean plants by 18.5% and 14.8%, respectively.101
N-CQD-treated maize plant had improved its nitrogen content by 33.5%.99 Apart from the protein, fatty, and amino acid contents were studied. According to a study, it has been claimed that protein, fatty, and amino acid contents increased by 3.4%, 6.9%, and 17.3%, respectively.101 Furthermore, another study has claimed that protein content improved by 3.7% while amino acid content increased by 257.5% in the shoot and 57.5% in the root, respectively.98 Lipid peroxidation of the treated maize plant's root section had noteworthy higher values than control by 1.65 times, while the H2O2 content is 6.5 times higher than control.123 In comparison to the control at the optimal CQD concentration of 54.2 ppm, N-CQD treated mung bean showed a maximum of 26% higher reduction of DCPIP.111 According to a study, the N-CQD increased the net photosynthesis rate of maize plants by 21.51%.97 In a study, N-CQD exposure improved the net photosynthesis rate by approximately 206.8% as compared to the control.119 It also has been reported that the maize plant photosynthesis rate increased by 122.9% as compared to the control.99 The rate of photosynthesis was reported to be 28.6% higher in another study.100 The CQD effects on plant growth and photosynthesis parameters are summarized in Table 2.
| No | Type of CQD | Source of CQD | Plant types | Treated plant part | Concentration (ppm) | Effect on plant growth and photosynthesis parameters | Cytotoxicity | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | CQD | Citric acid & urea | Maize | Seed | 250, 500, 1000, 2000 | • Increase H2O2 content (6.5 times) | Not provided | 123 |
| • Increase lipid peroxidation (1.65 times) | ||||||||
| • Increase antioxidant enzymes activities | ||||||||
| • Reduce shoot & root fresh weight | ||||||||
| 2 | CQD | Hydrogen peroxide solution oxidation & sodium borohydride reduction and glucose | Rice plant | Seed | 280, 560, 1120, 2240 | • Increase moisture level, seedling length, root length & carbohydrate content | Yes (biochemistry & hematology analysis) | 60 |
| • Increase rice yield (14.8%) | ||||||||
| • Increase rubisco activity (42%) | ||||||||
| • Increase resistance to disease | ||||||||
| 3 | Amine terminated CQD | Ascorbic acid | Mung bean | Chloroplast | 10, 50, 54.2, 75, 100, 200 | • Increase hill activity (26%) | Yes (MTT assay) | 111 |
| • Increase ferricyanide reduction (74.3%) | ||||||||
| • Increase electron transfer rate (93%) | ||||||||
| • Increase oxygen evolution, ATP formation & NADPH formation | ||||||||
| 4 | CQD | Ammonia | Mung bean | Seed | 20, 40, 120 | • Increase root length (29.9%) | Not provided | 104 |
| • Increase root vigor (36.1%) | ||||||||
| • Increase stem length (18.3%) | ||||||||
| • Increase fresh biomass (14.9%) | ||||||||
| • Increase moisture level (54.5%) | ||||||||
| • Increase mass loss (34.9%) | ||||||||
| • Increase carbohydrate content (21.9%) | ||||||||
| • Increase PSI (10.4%) | ||||||||
| • Increase ETRI (8.8%) | ||||||||
| • Increase chlorophyll (14.8%) | ||||||||
| • Increase Rubisco activity (30.9%) | ||||||||
| 5 | CQD | Rapeseed pollen | Rome lettuce | Root | 10, 20, 30 | • Increase biomass (48.09%) | Not provided | 102 |
| • Increase growth rate (41.98%) | ||||||||
| • Increase leaf area & leaf number | ||||||||
| • Increase nitrate content, transpiration rate & stomatal conductance | ||||||||
| 6 | Nitrogen doped CQD | Graphite rod | Arabidopsis thaliana & Trifolium repens L. | Seed | 280, 560, 1120, 2240 | • Increase germination rate (15.6%) | Yes (MTT assay) | 110 |
| • Increase root length (247%) | ||||||||
| • Increase rubisco activity (42%) | ||||||||
| • Increase carbohydrate content & metal ion absorption | ||||||||
| Bean seed | 560 | • Increase moisture level & root length | ||||||
| Soybean, tomato, eggplant, capsicums, watermelon, radish, celery, cabbage | 560 | • Increase yield (20%) | ||||||
| 7 | CQD | Empty fruit bunch (EFB) biochar | Rice | Leave | 150 | • Increase plant height (4.8%) | Yes (MTT assay & embryos assay) | 95 |
| • Increase grain weight (5.1%) | ||||||||
| • Increase assimilation rate (56%) | ||||||||
| • Increase stomatal conductance (56%) | ||||||||
| Maize | • Increase plant height (20.9%) | |||||||
| • Increase grain weight (39.6%) | ||||||||
| • Increase assimilation rate (16%) | ||||||||
| • Increase stomatal conductance (18%) | ||||||||
| 8 | CQD | Citric acid & cysteine (cys) | Mung bean | Seed | 10, 50, 100, 500, 1000 | • Increase root vigor (8.4%) | Yes (MTT assay) | 105 |
| • Increase RuBisCO enzyme (20.5%) | ||||||||
| • Improve water absorption capacity | ||||||||
| • Increase carbohydrate content (22.5%) | ||||||||
| 9 | (i) CQD-PAA | Citric acid & ethylenediamine | Pumpkin | Root | 100, 200, 400, 800 | (i) CQD-PAA | Not provided | 119 |
| • Slightly increase fresh weight | ||||||||
| • Increase root length | ||||||||
| • Increase lipid peroxidation (60%) | ||||||||
| (ii) CQD-PEI | • Increase antioxidant enzyme activity | |||||||
| (ii) CQD-PEI | ||||||||
| • Decrease fresh weight (25%) | ||||||||
| • Increase root length | ||||||||
| • Increase lipid peroxidation (60%) | ||||||||
| • Increase antioxidant enzyme activity | ||||||||
| 10 | CQD | Phenylenediamine | Mung bean | Seed | 100, 400, 700, 1000 | • Increase stem length, root length, fresh weight & moisture content | Not provided | 72 |
| 11 | CQD | Sugar | Wheat | Root | N/A | • Increase assimilation, stomatal conductance, reactive oxygen species | Not provided | 103 |
| • Increase grain yield (18%) | ||||||||
| • Increase seed number (12%) | ||||||||
| • Increase shoot dry biomass (17%) | ||||||||
| 12 | Spirulina-based CQD | Spirulina | Lentil | Seed | 50, 100, 200, 500 | • Increase seedling biomass (48.1%) | Not provided | 106 |
| • Increase seedling length (120.2%) | ||||||||
| • Increase root length (105.3%) | ||||||||
| 13 | Nitrogen and sulphur co-doped CQD | Coal | Stevia rebaudiana | Leave | 75, 150 | • Increase biomass yield of shoot, root and plant | Yes (cell viability assay & genotoxicity assay) | 124 |
| • Increase plant height | ||||||||
| • Increase length of leaves | ||||||||
| • Increase protein | ||||||||
| 14 | Cerium doped CQD | Citric acid, urea, acetic acid cerium acetate hydrate, thiobarbituric acid (TBA), and guaiacol | Wheat | Leave | 10, 25, 50, 100, 200, 400 | • Increase root number (45%) | Not provided | 112 |
| • Increase root length (57%) | ||||||||
| • Increase leaf length (28%) | ||||||||
| • Increase plant height (46%) | ||||||||
| • Increase chlorophyll content (51%) | ||||||||
| • Increase peroxidase activity (76%) | ||||||||
| • Decrease malondialdehyde content (68%) | ||||||||
| 15 | Nitrogen doped CQD | Citric acid | Maize | Leave | 1, 5, 10, 50 | • Increased net photosynthesis rate (21.51%) | Yes (cellular toxicity) | 97 |
| • Increased carbohydrate content (66.43% in roots and 42.03% in shoots) | ||||||||
| • Increased fresh weight (24.03% in roots and 34.56% in shoots) | ||||||||
| • Increased dry weight (72.30% in roots and 55.75% in shoots) | ||||||||
| 16 | CQD | Citric acid | Soybean | Leave | 5 | • Increased nitrogen content (13.2% in shoot and 30.5% in root) | Not provided | 98 |
| • Increased amino acids content (257.5% in shoot and 57.5% in root) | ||||||||
| • Increased plant yield (21.5%) | ||||||||
| • Increased protein content (3.7%) | ||||||||
| 17 | CQD | Citric acid | Soybean | Root | 1, 5, 10, 50 | • Increased nitrogenase activity (8.6%) | Not provided | 101 |
| • Increased nitrogen content (18.5% in shoot and 14.8% in root) | ||||||||
| • The expression of GmNRT, GmAMT, GmLB, and GmAQP genes in roots were upregulated by 1.2-, 1.8-, 2.7-, and 2.3-fold respectively | ||||||||
| • Enhanced nitrogen transport and water uptake | ||||||||
| • Increased protein content (3.4%) | ||||||||
| • Increased fatty acids content (6.9%) | ||||||||
| • Increased amino acids content (17.3%) | ||||||||
| 18 | Nitrogen doped CQD | Citric acid | Maize | — | 5 | • Decreased reactive oxygen species (ROS) accumulation | Not provided | 125 |
| • Increased the net photosynthesis rate (206.8%) | ||||||||
| • Increased abscisic acid (6.9%) and proline (36.3%) in roots | ||||||||
| 19 | Nitrogen doped CQD | Citric acid | Maize | Leave | 5 | • Increased root exudates succinic acid (14.5 folds), pyruvic acid (10.0 folds), and betaine (11.8 folds) | Not provided | 99 |
| • Increased pseudomonas, sphingomonas, nitrospira, and conocybe by 344.4%, 233.3%, 126.2%, and 122.6% | ||||||||
| • Increased nitrogen (33.5%) | ||||||||
| • Increased phosphorus (16.8%) | ||||||||
| • Increased plant water uptake (37.2%) | ||||||||
| • Increased net photosynthesis rate (122.9%) | ||||||||
| • Increased carbohydrate content (35.4% in shoot and 113.6% in root) | ||||||||
| • Increased fresh weight (62.1% in shoot and 50.6% in root) | ||||||||
| • Increased dry weight (29.2% in shoot and 37.5% in root) | ||||||||
| 20 | Nitrogen doped CQD | Citric acid | Maize | Leave | 5 | • Increased net photosynthesis rate (28.6%) | Yes (cellular toxicity) | 100 |
| • Increased fresh weight (360% in shoot and 224.5% in root) | ||||||||
| • Increased dry weight (63.3% in shoot and 230.8% in root) | ||||||||
| • Increased superoxide dismutase activity (26.7%) | ||||||||
| • Reduced malondialdehyde enzyme activity (18.9%) | ||||||||
| • Increased psbA gene expression (81.7-fold) |
According to Table 2, most of the research work confirmed that CQD is able to boost up the plant growth and photosynthesis parameters when compared to the control. Mung bean is one of the most commonly employed plants in research because of its quick-growing phase, which takes about a week to fully develop. Other plants studied included maize, rice, wheat, Rome lettuce, pumpkin, Arabidopsis thaliana, Trifolium repens L., soybean, tomato, eggplant, capsicums, watermelon, radish, celery, and cabbage. Different concentrations of synthesized CQD were tested on various plants. The concentrations applied on the plants range from 10 to 2000 ppm. The most common CQD concentration tested on plants is 100 ppm and less research work are considered at a larger CQD concentration of more than 800 ppm. The range of concentrations that were chosen by researchers varies; some use a large range from 10 ppm to 1000 ppm while others use a smaller range from 3 ppm to 9 ppm. The treatment which resulted in significant growth in plant height, shoot length fresh biomass and dry biomass is considered as the optimum concentration. In the studies, higher than optimum CQD concentration tends to inhibit plant growth. As a result, the optimum concentration level of CQD applied on plants is one of the most important factors to be considered. This is because higher concentrations of CQD has the potential to be toxic which will affect the plant growth.9
On white clover and thale cress, for example, five different concentrations of CQD were tested: 280, 560, 1120, 2240 ppm. The optimum concentration for this experiment was 560 ppm, however, plant growth is inhibited at 1120 ppm and 2240 ppm, indicating that a higher concentration is not always the best treatment for excellent plant development.110 Zhang and his group have placed mung bean seeds in Petri dishes containing various CQD concentrations. The obtained data indicated that the mung bean growth increases from 10 to 100 ppm, confirming that 100 ppm is the most optimum concentration while 1000 ppm is an inhibitor.105 Nevertheless, the optimal concentration varies depending on the type of plant. According to the Li group, among the other treatments (100, 400, 700, and 1000 ppm) that induce higher mung bean plant growth, 400 ppm is the optimal concentration.72 Another paper that conducted the same research work on the mung bean reported that 20 ppm is the optimum concentration meanwhile 120 ppm is the mung bean inhibitor.104 A lot of studies of CQD treatment were conducted on mung beans, however, the optimum concentration is varied. There might be few reasons for the differences. The first is the precursor that is used to produce CQD are different and different synthetic approaches such as pyrolysis, microwave-assisted, laser ablation, pyrolysis, wet oxidation, ultrasound, arc discharge, hydrothermal synthesis, etc. are applied.47,92 Different synthetic approaches and different starting materials have been published in a review study on CQD which results in different properties of the CQD.126,127 Furthermore, the surrounding temperature, humidity level, location, light intensity, and the section of plant chosen to expose the CQD all affect the plant's ability to grow.
5. Electron transfer mechanism of CQD assisted photosynthesis
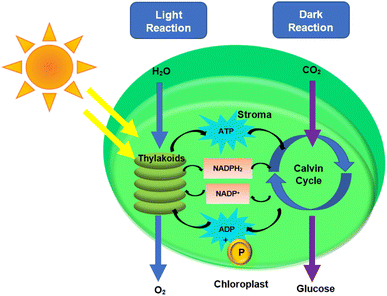
Electron transfer is a major aspect of the plant mechanism that demonstrates the flow of electrons from the sunlight to the end product of the plant photosynthesis. The photosynthetic mechanism in plants is commonly thought to work in two non-exclusive modes: linear and cyclic electron flows.128 During photosynthesis, electrons from water will be exchanged to NADP+, resulting in a photoinduced linear electron flow (LEF).128 The adenosine triphosphate (ATP) synthase utilizes the proton through process constrain delivered by electron-coupled proton translocation to make ATP over the thylakoid layer.129 Cyclic electron transfer involves two protons transported into the thylakoid lumen when electrons from PSI are returned to the Cyt b6/f complex. PSI requires roughly four photons to make an additional two ATP.130 Two types of reactions are involved in photosynthesis which includes light reaction and dark reaction.131 The light reaction takes place in the thylakoid, while the dark reaction takes place in the stroma.132,133 The light reaction requires light, whereas the dark reaction does not. Photons from the sunlight react with water to form ATP, NADPH, and oxygen (O2) in the light reaction.134 The Calvin–Benson–Bassham cycle, also known as the dark reaction utilizes photoproduced adenosine triphosphate (ATP) and nicotinamide–adenine dinucleotide phosphate (NADPH) for CO2 fixation.131,135 The equation of light reaction, dark reaction and overall reaction are summarized below.131 Fig. 5 depicts both the light and dark reaction pathways.Light reactions:
| 2H2O + light → O2 + 4H+ + 4e− | (1) |
Dark reactions:
| CO2 + 4H+ + 4e− → CH2O + H2O | (2) |
Overall:
| H2O + light + CO2 → CH2O + O2 | (3) |
Due to the photoluminescent properties of CQD, this photosynthesis process allows for photon absorption and emission. The plant's real antenna pigment which is chlorophyll can only harvest photons from visible sunlight with a short wavelength range and pass the captured energy to the reaction centre.18,136 The photons that are absorbed instantly by the artificial antennae enable the electron to be transferred quickly within the PSI and PSII, allowing the electron to have energies that are higher than average speed. When the amount of photon absorption is greater, the electron transport process within the PSI and PSII will be faster, affecting the photosynthesis rate.18,137 It has been reported that artificial antennae, CQD, efficiently pass photon energies to the plant's reaction centre after harvesting a large number of photons from the UV, visible, and near infrared spectra.138
The reaction centre has its own harvesting system. When CQD cooperates with the reaction centre, massive energy is absorbed, and the CQD will easily conquer the reaction centre if the right design is produced.138 Asides from its ability to absorb light energy, the CQD also tends to play a role as an electron donor, enhancing plant photosynthesis. The overall pathway of the CQD that transfers electrons to the chloroplast has been reported.105 Because CQD and chloroplast both absorbed light in the same wavelength range, a single wavelength might excite both; accelerating electron transfer from carbon dots to chloroplast and chloroplast to the photosystem.121 The massive electron transferred from CQD to chloroplast could be explained by the near relation of the donor and acceptor, which occurs during CQD adsorption on the high area of the chloroplast that generates a light-harvesting complex. The electrons are then transmitting to PSII and PSI, boosting the total electron transport chain (ETC) in photosynthesis, as shown in Fig. 6. As a result, by increasing water splitting to O2, NADP reduction, and ATP production, the complete light cycle pathway in PSI and PSII is prolonged.95,111
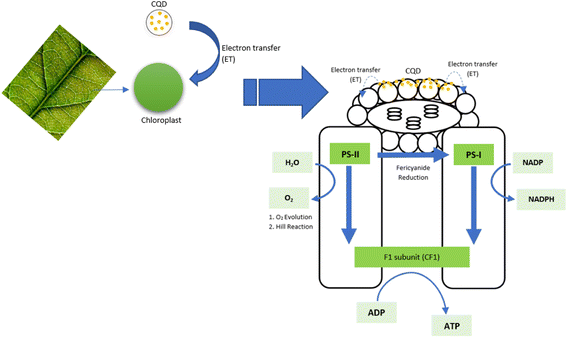 | ||
| Fig. 6 The schematic pathway of electron transfer (reproduced from ref. 111 with permission from the Royal Society of Chemistry, Copyright © 2014). | ||
The fluorescence intensity measurement is utilised to demonstrate that the electron transfer mechanism of the plant is enhanced by the application of CQD. PSI produces a fluorescence peak at a wavelength more than 700 nm. However, PSII produces a fluorescence peak in the region of far-red to the near-infrared at range of 650–780 nm.139,140 The CQD, extracted chloroplast from the plant, and the mixture of CQD/chloroplast fluorescence intensity were measured at various excitation wavelengths. It was found that the CQD peak was detected at 440–450 nm, the chloroplast peak was detected at 650–780 nm, and the CQD/chloroplast peak was detected in both ranges, as shown in Fig. 7.111 It can be stated that the CQD tends to emit electrons at higher state at the wavelength of 400–450 nm while noticeably, the chloroplast tends to emit electrons at higher state at the wavelength of 650–780 nm at the same level of PSII.141–144 At 400–450 nm, the combined CQD/chloroplast peak is lower than the CQD peak, while at 650–780 nm, the peak is higher than the chloroplast peak. This demonstrates that the CQD is hypothesized to have a resonant energy transfer with chloroplast as electron donors, causing its fluorescence property to be lower than the pure CQD. Since the electron are transferred to the chloroplast, it has a higher energy, causing it to produce a higher fluorescence intensity peak compared to the pure chloroplast itself.95,111
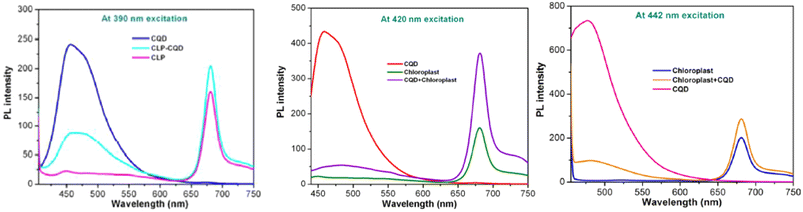 | ||
| Fig. 7 PL spectrum of CQD, chloroplast and CQD conjugated CLP at 390, 420, 442 nm excitation wavelength. (Reproduced from ref. 111 with permission from the Royal Society of Chemistry, Copyright © 2014). | ||
The 2,6-dichlorophenolindophenol (DCPIP) reduction method, ferricyanide reduction assay and PL lifetime measurement are also other ways for proving electron transfer between CQD and chloroplast. For DCPIP reduction method, the Hill reaction was used to examine the photosynthetic performance of CQD/chloroplast complexes by measuring the reduction rate of DCPIP. The DCPIP reduction test can be used to assess the PSII activity of the chloroplast due to the highlighted property of the DCPIP, which has a high electronegativity that is transferred from PSII to PSI throughout the chloroplast reaction mechanism.145 It has been reported that the DCPIP reduction of CQD/chloroplast was higher than the chloroplast which indicates that the amount of oxygen evolved is also higher. Therefore, the electron transfer mechanism of CQD/chloroplast is higher too.111,122 Moreover, the rate of electron transfer within the CQD/chloroplast can be demonstrated by conducting the ferricyanide reduction assay which necessitates the photophosphorylation activity. Extracted chloroplast releases oxygen in the existence of ferricyanide. Electron that transfer from PSII are utilizes for the ferricyanide reduction.146 As a result, the CQD/chloroplast tends to have a higher ferricyanide reduction when compared with the pure chloroplast itself. Besides that, the PL lifetime measurement is another method to measure electron transfer. The electron transfer between the CQD and the chloroplast occurs quickly at the time scale of a nanosecond. It is not surprising that the CQD has a substantially longer lifetime than the CQD/chloroplast complex. After the mixing, the CQD/chloroplast lifetime decreased significantly to 0.08 ns, whereas the CQD lifetime was previously measured at 4.16 ns.111 When the excitation energy approaches the PSII centre, the transfer of excitation energy proceeds at a nanosecond to a femtosecond.95
6. Toxicity and biocompatibility studies of CQD
Nanomaterials safety is a highly important consideration if it is to be used in agriculture and is highly material specific and dose dependent. As a growing number of researchers discovered eco-friendly ways to develop biocompatible CQD, the usage of CQD for agriculture has increased. Other types of earlier carbon nanoparticles, such as graphene quantum dots for example, were previously prepared via traditional chemical oxidation procedures, which make use of harsh acids and chemicals. These substances were not considered to be biocompatible and could only be used for applications that did not involve direct contact with living organisms. Since methods of production will affect the purity of the end product, not all CQD are created equally and precautions need to be taken to assess biocompatibility. Even trace substances from any chemicals used during the production process can influence cytotoxicity tests, and it will be difficult and expensive to completely remove any harsh chemicals from the finished product.CQD is known for its excellent biocompatibility because there are fewer chances of negative reactions occurring by interacting with cells since CQDs' carbon-based nature is comparable similar to the organic elements found in biological systems. The most useful test to determine whether a CQD is biocompatible and has low toxicity in the environment as well as for consumers is by conducting cytotoxicity testing. It is a biological assessment and screening test that uses tissue cells in vitro to determine how CQD affects cell growth, reproduction, and morphology.147 Since CQD is applied in agriculture, where crops are used as a food source for humans, it is critical to conduct a vitro cytotoxicity test, which is commonly done using the MTT assay.147 MTT assay is also known as cell viability assay.148 The MTT assay is a colorimetric test that is used to determine the vitality of all cells.149
The toxicity level of the CQD can be determined by performing an in vivo embryo toxicity test on either zebrafish embryos or larvae embryos. Zebrafish are an important model organism for toxicity evaluations since they share more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 genes with humans, which accounts for around 70% of the human genome.150 This similarity in genetic makeup enables zebrafish to exhibit comparable physiological responses to many human drugs, further supporting their utility in studying potential toxic effects.151 A group conducted a zebrafish toxicity test on CQD at the concentration of 1000 ppm and reported that the CQD concentration used for plants is non-toxic and biocompatible.95 Kang et al. used fluorescence-based techniques to analyse the absorption, distribution, metabolism, and excretion of CQD in zebrafish embryos. The results of their investigation showed that CQD did not accumulate within zebrafish embryos and had no negative consequences on their developmental processes, indicating that CQD interference was not present in this particular model organism.152 Few other papers also mentioned the non-toxic and biocompatibility of CQD.153–155 Fig. 8 shows the toxicological study of CQD in developing zebrafish embryos.
000 genes with humans, which accounts for around 70% of the human genome.150 This similarity in genetic makeup enables zebrafish to exhibit comparable physiological responses to many human drugs, further supporting their utility in studying potential toxic effects.151 A group conducted a zebrafish toxicity test on CQD at the concentration of 1000 ppm and reported that the CQD concentration used for plants is non-toxic and biocompatible.95 Kang et al. used fluorescence-based techniques to analyse the absorption, distribution, metabolism, and excretion of CQD in zebrafish embryos. The results of their investigation showed that CQD did not accumulate within zebrafish embryos and had no negative consequences on their developmental processes, indicating that CQD interference was not present in this particular model organism.152 Few other papers also mentioned the non-toxic and biocompatibility of CQD.153–155 Fig. 8 shows the toxicological study of CQD in developing zebrafish embryos.
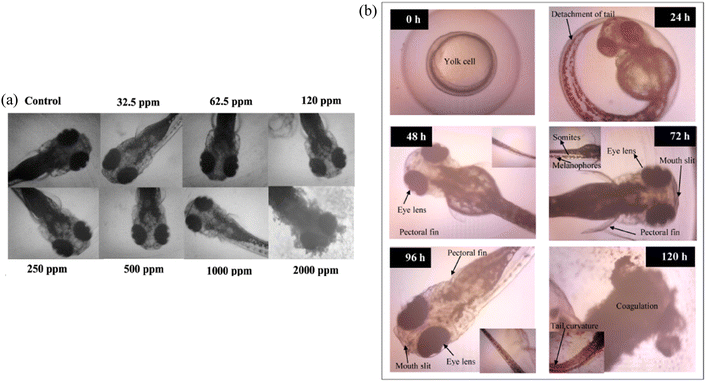 | ||
| Fig. 8 Toxicological study of (a) different concentrations of CQD on zebrafish larvae at 120 hpe; (b) effects of exposure to 2000 ppm CQD in developing zebrafish embryos. (Reproduced from ref. 95 Copyright © 2016 with permission from the Elsevier Masson SAS. All right reserved.) | ||
Other than cytotoxicity tests, the genotoxicity test is used to determine whether the CQD causes damage to the DNA or chromosome.156 A research team conducted a genotoxicity test on their CQD, and reported that no damage to the DNA was found after being tested with CQD at a concentration of 200 ppm after 24 hours of exposure.124 Researchers that conducted cytotoxicity and genotoxicity tests as part of their work are also listed in Table 2. Furthermore, some researchers have also studied CQD toxicity using the cellular toxicity method by exposing the CQD to tobacco plant cells.97,100 Studies have shown that CQD in different cell lines had only minor negative impacts on cell viability, proliferation, and general cellular functioning. Animal models used in vivo research, such as mice or zebrafish, have demonstrated that CQD can be well tolerated with little toxic or negative side effects.110,155
In recent years, scientists have started to report the widespread presence of naturally occurring carbon nanoparticles like CQD in food. According to a previous report as early as 2012, amorphous carbon nanoparticles have been found in a variety of carbohydrate-based food caramels that have been heated during preparation.157 All heating, roasting and grilling have the tendency to create food-bourne nanoparticles. In a particular study, CQD were isolated from grilled pike eels, which have been eaten for thousands of years.158 The isolated CQD were scarcely toxic to MC3T3-E1 cells in contrast to the control group. The report also highlighted other work which extracted or synthesized CQD from various foods barely showed any toxicity in vitro. Most of these traditional foods have been consumed by people for thousands of years and thus not considered harmful. Nevertheless, the extraction of carbon nanoparticles from food, however, may potentially result in the introduction of some toxicity. For instance, a previous study found that human mesenchymal stem cells experience metabolic stress when exposed to carbon nanostructures extracted from bread products are extracted.159 On closer inspection, the extraction method utilizes chemicals such as dichloromethane and it is believed that even traces of this chemical can affect cytotoxicity tests.
Note that oral toxicity testing is very much less reported in literature by scientists that work in the area of CQD applications for plants possibly due to the need for certain expertise and dedicated external lab services. In the future, oral toxicity could be another necessary test as CQD for plants and agriculture transitions from the lab to real field applications. Nonetheless, the therapeutic potential of CQD for humans have been thoroughly studied and reviewed.160 Excretion is considered a prerequisite for viable nanotherapeutics and it is generally known that CQD, when below 6 nm, can be rapidly excreted through urine, implying that it does not bioaccumulate in the body. In summary, although CQD are generally considered biocompatible and non-toxic at the concentrations used for applications onto plants, not all CQD are created equal. Only CQD obtained from processing which is green, scalable and economical will have the potential to be applied at the industrial scale, and considered safe for human consumption.
7. Industrial scale applications of CQD for agriculture
Traditional chemical fertilizers typically involve industrial processes that produce specific compounds namely nitrogen in the form of ammonia, phosphorus in the form of phosphoric acid and potassium in the form of potassium chloride. Ammonia is produced via the Haber-Bosch process, whilst phosphorus and potassium are usually mined from natural deposits. The capacity of these chemical plants lie on average of 10 million metric tons per year. The Haber-Bosch process for example involves industrial scale air separation unit, sour gas unit, acid gas removal unit and ammonia synthesis unit. It involves catalysed reactions at temperatures of 400° to 650° and pressures from 200 to 400 atmosphere. Despite the use of expensive heavy machinery in the processing of fertilizers, the cost of conventional fertilizers is relatively low due to economies of scale.Meanwhile, the preparation of CQD can be performed using either top-down (electrochemical, laser ablation, arch discharge and hydrothermal/solvothermal extraction) or bottom-up methods (thermal breakdown, microwave synthesis, pyrolysis/carbonization, and hydrothermal/solvothermal processes).40,41 Some of these processes especially hydrothermal and solvothermal processes have high potential to be scalable and economical. Although most reports still revolve around lab scale production capacities, it is not difficult to imagine the relative simplicity and scalability of most hydrothermal and solvothermal extraction or synthesis processes for CQD production. Depending on the production methods and economies of scale, it can be foreseen that CQD prices should be within the price range of typical agriculture inputs such as fertilizers and pesticides. Although CQD is not a replacement for fertilizers and pesticides, the potential benefits of using CQD photosynthesis enhancers towards increasing crop yields would make it a worthwhile investment for farmers.
In some circumstances, the result of over-fertilization may be detrimental to crops,161 not to mention harmful for the environment. A potential advantage of CQD as a photosynthesis enhancer could lead to healthier crops which are able to optimize the uptake of nutrients. To avoid over-fertilization, the correlation between CQD as photosynthesis enhancer and fertilizer consumption on plant development must be investigated. A modest amount of fertilizer might sometimes be sufficient to help the plant grow healthier and provide a higher yield. As a result, the fertilization cost in agriculture can be reduced. It might be possible to reduce costs even further if the CQD's precursors were derived from various food and agriculture waste such as onion peel, papaya waste, peanut shell, lychee seed, green tea, empty fruit bunch (EFB), and palm kernel shell (PKS).162–169 Companies that can generate biocompatible CQD using environmentally friendly, scalable, and cost-effective technologies will have a huge advantage when trying to penetrate into the agrichemicals market.
At the industrial level, the application of CQD as a foliar spray is simply accomplished by employing standard spraying techniques, either spraying manually or using tractors or crop dusters. The viability of employing CQD in farming is dose dependent and material specific. CQD is frequently used in agriculture to increase photosynthesis at relatively low concentrations, usually in the low hundred ppm range, and is generally regarded as non-toxic. Although no long-term impacts of CQD towards the environment have been documented in the literature, it is likely that they will become part of the biome. It is unlikely for CQD to survive pristinely in the environment; they will agglomerate and lose photostability and become inert compounds of the earth and water bodies. To assess the viability, effectiveness, and potential difficulties related to large-scale CQD deployment in the agricultural sector, extensive studies and field testing are required. CQD are considered to be environmentally benign if natural carbon sources are used as the precursor material.170 The green pathway for the synthesis of carbon dots is a highly favoured strategy because it will also reduce environmental pollution in the form of solid waste and be inert to the environment.171
When it comes to large-scale implementation of CQD onto crops, one of the most significant challenges that most farmers confront is the struggle to achieve precision farming. The importance of precision farming is to optimize agricultural production with quality by precisely measuring the biological and physical data of crops.172 However, monitoring vegetation indicators that show plant growth, detecting disease, or evaluating fruit maturity is a crucial aspect in obtaining good quality fruit or vegetables. Technological breakthroughs are transforming agriculture, with future scenarios including the possibility for farmers to remotely control their fields. Hyperspectral imaging is a non-destructive remote sensing technology that collect data precisely via satellite.173 It can determine the crop's physiology and condition as it grows; as well as providing useful information such as crop yield, disease, drought stress, and nutrient status.174 By measuring their reflecting characteristics throughout a variety of narrow spectral bands, hyperspectral imaging can be used to capture real-time images and as a rapid analytic tool for crop monitoring in real-time.175,176 Therefore, suitable vegetation indices can be determined from hyperspectral imaging to represent growth and yield of CQD treated crop. As a result, adopting remote sensing to help farmers overcome their challenges would pave the way for future sustainable agriculture.
Plant nutrition is also a key factor in growth and yield. However, there are still gaps in our understanding of the nutrition of CQD-treated plants. Plants treated with CQD might have different nutrition than plants that have not been treated. The quality of plant nutrition is not only determined by their total sugar, total protein, and ascorbic acid levels. The presences of various vitamins (vitamins A, B, D, and E), minerals and fibre all play an important role in the nutritional value of vegetables.177 Due to enhanced photosynthesis afforded by CQD, it is very likely for CQD-treated crops to have higher nutritional values. Therefore, it is necessary to evaluate the nutritional composition, minerals and vitamins contents, as well as antioxidant contents and activity of CQD treated crops.
8. Limitations and future demand
The advantages of using CQD, one of the most biocompatible among NM and environmentally friendly in the agriculture field have been established through many studies. The varying levels of optimum CQD concentrations in different studies indicate that the CQD can have different properties due to different synthetic approaches and precursors. Thus, every type of CQD has to be tested accordingly to determine the optimum concentration that allows for the best plant growth and yield. According to some research, relatively high concentrations of CQD are good for plant growth and photosynthesis. Therefore, it is imperative to ensure that studies on the cytotoxicity and genotoxicity of the optimum CQD concentration is carried out to ensure safe produce for consumers. It is also important to continue toxicity studies using oral tests in order to better understand CQD's mechanism of action on the living organism. It is worth noting that the FDA has reviewed and approved some nanotechnology based products in the food industry.178 As a result, the lower toxicity of NM application on food makes it potentially safe to be consumed.To date, there is still a lack of knowledge concerning the electron transfer mechanisms of CQD/chloroplast on different types of crops. At the moment it is assumed that the mechanism is similar for all C3 plants and C4 plants respectively. CQD has only been tested on a relatively few plants so far, and the electron transfer rate may vary depending on the plant types. As a result, the electron transfer mechanism in different types of plants should be investigated. Aside from that, no research has been done on how long CQD will stay active in the leaves following application. Few researchers validated the presence of CQD in leaves using a variety of methods after it had been applied, but there is no information on CQD longevity. Furthermore, most of the reported research on the effect of CQD as a photosynthesis enhancer is limited towards physiological effects like height, length and weight of plants. There is still gap in the literature to correlate physiological effects to photosynthesis effects like net assimilation rate, stomatal conductance, transpiration rate and intrinsic water use efficiency. Studies on the effects of CQD towards photosynthesis enhancement and the actual light assimilation mechanism in terms of chlorophyll fluorescence parameter (Fv/FM) and the quantum yield of PSII (PhiPSII) have also yet to be addressed. Increasing our understanding of how CQD affects both plant physiology and photosynthesis enhancement will help us to design optimum application schedules for different crops according to crop types, crop cycles.
In terms of indoor farming which is considered in some cities the farming of the future, the effect of CQD as a photosynthesis enhancer under different light spectra is worth to be investigated. The impact of different light wavelengths, light intensities and photo-duration periods have huge implications for indoor farming. This is because the largest contributor to the operational cost of indoor farms is the energy required for lighting. The grow light for indoor plants is available at a different wavelength. Each wavelength of light has a different effect on plant development. Red light, for example, is used specifically for fruiting and flowering plants, whereas blue light is utilised for vegetable plant.179,180 Moreover, light intensity can be categorized into low light (LL) and high light (HL).181 Certain types of plants require HL for optimal plant growth, whereas for some plant types, LL is sufficient for them to grow well. When compared to HL, LL uses less electricity. If the CQD treated plant can collect the same number of photons from the LL as the HL, it is more energy-efficient to use LL instead of the HL, which consumes more power.181 More energy can also be saved if CQD-treated plants require less photo-duration period to grow just as well as untreated plants. Therefore, more research on CQD-treated plants at different light wavelengths, light intensities and photo-duration periods is required in order to increase output and profitability of indoor farms, while still being at an acceptable cost for consumers.
9. Conclusion
In conclusion, CQD is the most promising nanomaterial in this new era, which has potential to disrupt the agriculture industry. CQD aids the plant by providing artificial photosynthesis support, which increases the photosynthesis rate, leading to increased plant growth and yield. The rate of photosynthesis is linked to the physical characteristics of the plant. Plant height, biomass, moisture content, leaf area, seed number, and leaf number are all improved by CQD. CQD also contributes to increased photosynthetic rates by raising the stomatal conductance, Rubisco activity, Hill Reaction, DCIPC, H2O2 content, oxygen evolution, DCPIP reduction, ferricyanide reduction, oxygen uptake, NAPDH formation, ATP formation, PSI rate, PSII rate, ETRI and ETRII and so on. CQD helps plants receive photons from light and increase the electron transfer chain, which allows them to photosynthesize at a faster rate. As a result, the plant's glucose level rises, which promotes plant growth and yield.Although CQD is generally regarded as biocompatible and non-toxic, the correct dosage and how the CQD was produced is paramount in determining the safety for agriculture application and the environment. The production of CQD has the potential to be scaled up to the same industrial levels as fertilizers, with costs comparable to normal agriculture inputs such as fertilizers and pesticides. There are still more studies to be done especially in terms of correlating plant physiology with photosynthesis parameters such as net assimilation rate, stomatal conductance, transpiration rate and intrinsic water use efficiency. Studies on the effects of CQD towards photosynthesis enhancement and the actual light assimilation mechanism in terms of chlorophyll fluorescence parameter (Fv/FM) and the quantum yield of PSII (PhiPSII) have also yet to be addressed. Increasing our understanding of how CQD affects both plant physiology and photosynthesis enhancement will help us to design optimum application schedules for different crops according to crop types, crop cycles. In terms of indoor farming, which is considered in some cities the farming of the future, more research on CQD-treated plants at different light wavelengths, light intensities and photo-duration periods is required in order to increase output and profitability of indoor farms, while still being at an acceptable cost for consumers. All of this knowledge will be a game changer for the application of CQD as a photosynthesis enhancer in agriculture.
Author contributions
Y. C. prepared the manuscript together with T. L. T., and technical support from S. A. R., R. N. and M. J.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors gratefully acknowledge the financial support from Universiti Putra Malaysia for the Fundamental Research Grant Scheme (FRGS/1/2019/TK05/UPM/02/8: 5540329).References
- S. E. Vollset, et al., Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study, Lancet, 2020, 1285–1306, DOI:10.1016/S0140-6736(2030677-2).
- T. Kuhlman and J. Farrington, What is sustainability?, Sustainability, 2010, 2(11), 3436–3448, DOI:10.3390/su2113436.
- A. Salman Ali, Application of Nanomaterials in Environmental Improvement, Nanotechnol. Environ., 2020, 1–20, DOI:10.5772/intechopen.91438.
- R. Tala-ighil, Nanomaterials in Solar Cells, 2016 Search PubMed.
- M. K. Nayak, J. Singh, B. Singh, S. Soni, V. S. Pandey and S. Tyagi, Introduction to semiconductor nanomaterial and its optical and electronics properties, Elsevier Inc., 2017 Search PubMed.
- C. Kole, D. S. Kumar and M. V. Khodakovskaya, Effects of Nanoparticles on Plant Growth and Development, Plant Nanotechnol. Princ. Pract., 2016, 1–383, DOI:10.1007/978-3-319-42154-4.
- Y. Liu, L. Yue, C. Wang, X. Zhu, Z. Wang and B. Xing, Photosynthetic response mechanisms in typical C3 and C4 plants upon La2O3 nanoparticle exposure, Environ. Sci. Nano, 2020, 7(1), 81–92, 10.1039/c9en00992b.
- P. Mathur and S. Roy, Nanosilica facilitates silica uptake, growth and stress tolerance in plants, Plant Physiol. Biochem., 2020, 157, 114–127, DOI:10.1016/j.plaphy.2020.10.011.
- K. Banerjee, P. Pramanik, A. Maity, D. C. Joshi, S. H. Wani and P. Krishnan, Methods of Using Nanomaterials to Plant Systems and Their Delivery to Plants (Mode of Entry, Uptake, Translocation, Accumulation, Biotransformation and Barriers), in Advances in Phytonanotechnology, Elsevier Inc., 2019, pp. 123–152 Search PubMed.
- Y. Krishnan, et al., Co-composting of palm empty fruit bunch and palm oil mill effluent: microbial diversity and potential mitigation of greenhouse gas emission, J. Clean. Prod., 2017, 146, 94–100, DOI:10.1016/j.jclepro.2016.08.118.
- R. Das, R. Bandyopadhyay and P. Pramanik, Carbon quantum dots from natural resource: a review, Mater. Today Chem., 2018, 8, 96–109, DOI:10.1016/j.mtchem.2018.03.003.
- S. Das, A. Mukherjee, G. Sengupta and V. K. Singh, Overview of nanomaterials synthesis methods, characterization techniques and effect on seed germination, in Nano-Materials as Photocatalysts for Degradation of Environmental Pollutants: Challenges and Possibilities, Elsevier Inc., 2019, pp. 371–401 Search PubMed.
- R. Kumari, A. Sahai and N. Goswami, Effect of nitrogen doping on structural and optical properties of ZnO nanoparticles, Prog. Nat. Sci. Mater. Int., 2015, 25(4), 300–309, DOI:10.1016/j.pnsc.2015.08.003.
- F. Gao, et al., Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase?, BioMetals, 2008, 21(2), 211–217, DOI:10.1007/s10534-007-9110-y.
- N. Karak, Fundamentals of Nanomaterials and Polymer Nanocomposites, in Nanomaterials and Polymer Nanocomposites, Elsevier Inc., 2019, pp. 1–45 Search PubMed.
- P. Li, et al., Insight into the interaction between Fe-based nanomaterials and maize (Zea mays) plants at metabolic level, Sci. Total Environ., 2020, 738, 139795, DOI:10.1016/j.scitotenv.2020.139795.
- L. Nemček, M. Šebesta, M. Urík, M. Bujdoš, E. Dobročka and I. Vávra, Impact of bulk ZnO, ZnO nanoparticles and dissolved zn on early growth stages of barley—a pot experiment, Plants, 2020, 9(10), 1–14, DOI:10.3390/plants9101365.
- S. Mingyu, W. Xiao, L. Chao and Q. Chunxiang, Promotion of Energy Transfer and Oxygen Evolution in Spinach Photosystem II by Nano-anatase TiO2, Biol. Trace Elem. Res., 2007, 119(2), 183–192, DOI:10.1007/s12011-007-0065-1.
- A. Gandhi and S. Wu, Strong Deep-Level-Emission Photoluminescence in NiO Nanoparticles, Nanomaterials, 2017, 7(8), 231, DOI:10.3390/nano7080231.
- A. N. U. Haq, et al., Impact of zinc oxide nanoflowers on growth dynamics and physio-biochemical response of Triticum aestivum, Toxicol. Environ. Chem., 2020, 1–26, DOI:10.1080/02772248.2020.1837133.
- M. V. Khodakovskaya, et al., Carbon nanotubes as plant growth regulators: effects on tomato growth, reproductive system, and soil microbial community, Small, 2013, 9(1), 115–123, DOI:10.1002/smll.201201225.
- P. Aqaei, W. Weisany, M. Diyanat, J. Razmi and P. C. Struik, Response of maize (Zea mays L.) to potassium nano-silica application under drought stress, J. Plant Nutr., 2020, 43(9), 1205–1216, DOI:10.1080/01904167.2020.1727508.
- J. Zhou, et al., An electrochemical avenue to blue luminescent nanocrystals from multiwalled carbon nanotubes (MWCNTs), J. Am. Chem. Soc., 2007, 129(4), 744–745, DOI:10.1021/ja0669070.
- P. G. Jamkhande, N. W. Ghule, A. H. Bamer and M. G. Kalaskar, Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications, J. Drug Deliv. Sci. Technol., 2019, 53, 1–11, DOI:10.1016/j.jddst.2019.101174.
- A. Mitra and G. De, Sol-Gel Synthesis of Metal Nanoparticle Incorporated Oxide Films on Glass, Glas. Nanocomposites Synth. Prop. Appl., 2016, 145–163, DOI:10.1016/B978-0-323-39309-6.00006-7.
- A. Rastogi, et al., Impact of metal and metal oxide nanoparticles on plant: a critical review, Front. Chem., 2017, 5, 1–16, DOI:10.3389/fchem.2017.00078.
- B. A. Jamuna and R. V. Ravishankar, Environmental Risk, Human Health, and Toxic Effects of Nanoparticles, Nanomater. Environ. Prot., 2015, 523–535, DOI:10.1002/9781118845530.ch31.
- A. Alengebawy, S. T. Abdelkhalek, S. R. Qureshi and M. Q. Wang, Heavy metals and pesticides toxicity in agricultural soil and plants: ecological risks and human health implications, Toxics, 2021, 9(3), 1–34, DOI:10.3390/toxics9030042.
- S. Keerthana and A. Kumar, Potential risks and benefits of zinc oxide nanoparticles: a systematic review, Crit. Rev. Toxicol., 2020, 50(1), 47–71, DOI:10.1080/10408444.2020.1726282.
- S. Sabir, M. Arshad and S. K. Chaudhari, Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications, Sci. World J., 2014, 2014, 925494, DOI:10.1155/2014/925494.
- G. Raghavendra, Cell Viability Studies of Green Synthesised ZnO Nanoparticles for Antibacterial Properties, Am. J. Mater. Synth. Process., 2017, 2(5), 56–60, DOI:10.11648/j.ajmsp.20170205.11.
- A. B. Seabra and N. Durán, Nanotoxicology of metal oxide nanoparticles, Metals, 2015, 5(2), 934–975, DOI:10.3390/met5020934.
- N. Fan, et al., Nanosilicon alters oxidative stress and defence reactions in plants: a meta-analysis, mechanism and perspective, Environ. Sci. Nano, 2022, 9(10), 3742–3755 RSC.
- Z. Xiao, et al., Silicon Nanodots Increase Plant Resistance against Herbivores by Simultaneously Activating Physical and Chemical Defenses, ACS Nano, 2023, 17(3), 3107–3118, DOI:10.1021/acsnano.2c12070.
- X. Cao, et al., Elemental Sulfur Nanoparticles Enhance Disease Resistance in Tomatoes, ACS Nano, 2021, 15(7), 11817–11827, DOI:10.1021/acsnano.1c02917.
- S. Tolerance, A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance, Plants, 2023, 12, 1–24 Search PubMed.
- Y. Li, C. Yao, C. Wang, P. Yang, R. Wu and L. Fei, An approach through steam to form sulfur nanoparticles for lithium sulfur batteries, Electrochem. Commun., 2021, 125, 107010, DOI:10.1016/j.elecom.2021.107010.
- M. Suleiman, A. Al Ali, A. Hussein, B. Hammouti and T. B. Hadda, Sulfur Nanoparticles: Synthesis, Characterizations and their Applications, J. Mater. Environmn. Sci., 2013, 4(6), 1029–1033 CAS.
- T. Hao, et al., Emerging Applications of Silica Nanoparticles as Multifunctional Modifiers for High Performance Polyester Composites, Nanomaterials, 2021, 11(11), 2810 CrossRef CAS PubMed.
- R. Priyadarshi, Z. Riahi, J.-W. Rhim and J. T. Kim, Comparison of physicochemical and functional properties of elemental sulfur, sulfur nanoparticles, and sulfur quantum dots for sustainable biological applications, Mater. Today Sustain., 2022, 20, 100243, DOI:10.1016/j.mtsust.2022.100243.
- A. D. Goswami, D. H. Trivedi, N. L. Jadhav and D. V. Pinjari, Sustainable and green synthesis of carbon nanomaterials: a review, J. Environ. Chem. Eng., 2021, 9(5), 106118, DOI:10.1016/j.jece.2021.106118.
- S. Cong and Z. Zhao, Carbon Quantum Dots: A Component of Efficient Visible Light Photocatalysts, in Visible-Light Photocatalysis of Carbon-Based Materials, 2018 Search PubMed.
- M. Farshbaf, S. Davaran, F. Rahimi, N. Annabi, R. Salehi and A. Akbarzadeh, Carbon quantum dots: recent progresses on synthesis, surface modification and applications, Artif. Cells, Nanomedicine Biotechnol., 2018, 46(7), 1331–1348, DOI:10.1080/21691401.2017.1377725.
- M. Budimir, S. Szunerits, Z. Markovic and R. Boukherroub, Nanoscale materials for the treatment of water contaminated by bacteria and viruses, in Nanomaterials for Sustainable Energy and Environmental Remediation, Elsevier Inc., 2020, vol. 3, pp. 261–305 Search PubMed.
- Y. Wang and A. Hu, Carbon quantum dots: synthesis, properties and applications, J. Mater. Chem. C, 2014, 2(34), 6921–6939, 10.1039/c4tc00988f.
- A. Tan, G. Yang and X. Wan, Ultra-high quantum yield nitrogen-doped carbon quantum dots and their versatile application in fluorescence sensing, bioimaging and anti-counterfeiting, Spectrochim. Acta Mol. Biomol. Spectrosc., 2021, 253, 119583, DOI:10.1016/j.saa.2021.119583.
- X. Wang, Y. Feng, P. Dong and J. Huang, A Mini Review on Carbon Quantum Dots: Preparation, Properties, and Electrocatalytic Application, Front. Chem., 2019, 7(671), 1–9, DOI:10.3389/fchem.2019.00671.
- M. Jorns and D. Pappas, A review of fluorescent carbon dots, their synthesis, physical and chemical characteristics, and applications, Nanomaterials, 2021, 11(6), 1448, DOI:10.3390/nano11061448.
- J. Sharma and P. Y. Dave, Carbon Dots: Zero Dimensional Fluorescent Material, J. Nanomater. Mol. Nanotechnol., 2020, 10, 1–10 CAS.
- P. Kaur and G. Verma, Converting fruit waste into carbon dots for bioimaging applications, Mater. Today Sustain., 2022, 18, 100137, DOI:10.1016/j.mtsust.2022.100137.
- B. Guo, et al., The role of fluorescent carbon dots in crops: mechanism and applications, Smart Mater., 2022, 3(2), 208–225, DOI:10.1002/smm2.1111.
- K. Woo, Y. Min, S. Young, J. Young and H. Kim, One-pot synthesis of UV-protective carbon nanodots from sea cauliflower (Leathesia difformis), Electron. J. Biotechnol., 2022, 56, 22–30, DOI:10.1016/j.ejbt.2021.12.004.
- S. Manzoor, A. H. Dar, K. K. Dash, V. K. Pandey, S. Srivastava, I. Bashir and S. A. Khan, Carbon dots applications for development of sustainable technologies for food safety: a comprehensive review, Appl. Food Res., 2023, 3, 100263, DOI:10.1016/j.afres.2023.100263.
- Y. Li, et al., A review on the effects of carbon dots in plant systems, Mater. Chem. Front., 2020, 4(2), 437–448, 10.1039/c9qm00614a.
- B. M. Görlach and K. H. Mühling, Phosphate foliar application increases biomass and P concentration in P deficient maize, J. Plant Nutr. Soil Sci., 2021, 184(3), 360–370, DOI:10.1002/jpln.202000460.
- R. E. Karamanos, Nutrient Uptake and Metabolism in Crops, Prairie Soils Crop. J., 2013, vol. 6, pp. pp. 52–63, Online, available: http://www.cfi.ca/_documents/uploads/elibrary/d161_NU_W_01[1].pdf Search PubMed.
- M. Badruzzaman, J. Pinzon, J. Oppenheimer and J. G. Jacangelo, Sources of nutrients impacting surface waters in Florida: a review, J. Environ. Manage., 2012, 109, 80–92, DOI:10.1016/j.jenvman.2012.04.040.
- C. A. Atkins and P. M. C. Smith, Translocation in legumes: assimilates, nutrients, and signaling molecules, Plant Physiol., 2007, 144(2), 550–561, DOI:10.1104/pp.107.098046.
- K. J. Oparka and R. Turgeon, Sieve elements and companion cells - Traffic control centers of the phloem, Plant Cell, 1999, 11(4), 739–750, DOI:10.1105/tpc.11.4.739.
- H. Li, et al., Impacts of carbon dots on rice plants: boosting the growth and improving the disease resistance, ACS Appl. Bio Mater., 2018, 1(3), 663–672, DOI:10.1021/acsabm.8b00345.
- M. Khodakovskaya, et al., Carbon Nanotubes are Able to Penetrate Plant Seed Coat and Dramatically Affect Seed Germination and Plant Growth, ACS Nano, 2009, 3(10), 3221–3227 CrossRef CAS PubMed.
- B. M. Görlach, A. Sagervanshi, J. N. Henningsen, B. Pitann and K. H. Mühling, Uptake, subcellular distribution, and translocation of foliar-applied phosphorus: short-term effects on ion relations in deficient young maize plants, Plant Physiol. Biochem., 2021, 166, 677–688, DOI:10.1016/j.plaphy.2021.06.028.
- Q. Chen, et al., Impacts of surface chemistry of functional carbon nanodots on the plant growth, Ecotoxicol. Environ. Saf., 2020, 206, 1–8 Search PubMed.
- H. Höfte and A. Voxeur, Plant cell walls, Current BiologyElsevier, 2017, 27(17), R865–R870 CrossRef PubMed.
- A. J. Bidhendi and A. Geitmann, Relating the mechanics of the primary plant cell wall to morphogenesis, J. Exp. Bot., 2016, 67(2), 449–461, DOI:10.1093/jxb/erv535.
- K. Houston, M. R. Tucker, J. Chowdhury, N. Shirley and A. Little, The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions, Front. Plant Sci., 2016, 7(AUG2016), 1–18, DOI:10.3389/fpls.2016.00984.
- Y. Zhang, S. Xu, F. Ji, Y. Hu, Z. Gu and B. Xu, Plant cell wall hydrolysis process reveals structure–activity relationships, Plant Methods, 2020, 16(1), 1–10, DOI:10.1186/s13007-020-00691-5.
- G. Banerjee, J. S. Scott-Craig and J. D. Walton, Improving enzymes for biomass conversion: a basic research perspective, Bioenergy Res., 2010, 3(1), 82–92, DOI:10.1007/s12155-009-9067-5.
- M. C. McCann, B. Wells and K. Roberts, Direct visualization of cross-links in the primary plant cell wall, J. Cell Sci., 1990, 96(2), 323–334 CrossRef.
- G. Dallner, The structure of the cell membrane in the cancer cell, Lakartidningen, 1980, 77(30–31), 2617–2621, DOI:10.1016/B978-0-12-119259-4.50006-6.
- V. Heinrich, K. Ritchie, N. Mohandas and E. Evans, Elastic thickness compressibilty of the red cell membrane, Biophys. J., 2001, 81(3), 1452–1463, DOI:10.1016/S0006-3495(01)75800-6.
- W. Li, Y. Zheng, H. Zhang, Z. Liu and W. Su, Phytotoxicity, uptake and translocation of fluorescent carbon dots in mung bean plants, ACS Appl. Mater. Interfaces, 2016, 8(31), 1–29, DOI:10.1021/acsami.6b07268.
- D. O. Robinson and A. H. K. Roeder, Themes and variations in cell type patterning in the plant epidermis, Curr. Opin. Genet. Dev., 2015, 32, 55–65, DOI:10.1016/j.gde.2015.01.008.
- R. Crang, S. Lyons-Sobaski and R. Wise, Plant Anatomy: A Concept-Based Approach to the Structure of Seed Plants, Springer, 2018 Search PubMed.
- L. Panawala, Difference Between Upper and Lower Epidermis, Pediaa, 2017 Search PubMed.
- E. M. Yahia, A. Carrillo-López, G. M. Barrera, H. Suzán-Azpiri and M. Q. Bolaños, Photosynthesis, Elsevier Inc., 2018 Search PubMed.
- S. G. Pallardy, The Woody Plant Body, Physiol. Woody Plants, 2008, 9–38, DOI:10.1016/b978-012088765-1.50003-8.
- S. Torre, MORPHOLOGY AND ANATOMY|Leaves, Encycl. Rose Sci., 2003, 497–504, DOI:10.5040/9781350068407.0006.
- E. M. Yahia, A. Carrillo-López, G. M. Barrera, H. Suzán-Azpiri and M. Q. Bolaños, Photosynthesis, in Postharvest Physiology and Biochemistry of Fruits and Vegetables, Elsevier Inc., 2018, pp. 47–72 Search PubMed.
- L. Deslandes and S. Rivas, The plant cell nucleus, Plant Signal. Behav., 2011, 6(1), 42–48, DOI:10.4161/psb.6.1.13978.
- B. Petrovská, M. Šebela and J. Doležel, Inside a plant nucleus: discovering the proteins, J. Exp. Bot., 2015, 66(6), 1627–1640, DOI:10.1093/jxb/erv041.
- C. Pang and Y. Gong, Current status and future prospects of semiconductor quantum dots in botany, J. Agric. Food Chem., 2019, 67(27), 7561–7568, DOI:10.1021/acs.jafc.9b00730.
- D. Djikanović, et al., Interaction of the CdSe quantum dots with plant cell walls, Colloids Surf. B Biointerfaces, 2012, 91, 41–47, DOI:10.1016/j.colsurfb.2011.10.032.
- S. Ali, A. Mehmood and N. Khan, Uptake, Translocation, and Consequences of Nanomaterials on Plant Growth and Stress Adaptation, J. Nanomater., 2021, 1–17 Search PubMed.
- E. Steudle, Water uptake by plant roots: an integration of views, Plant Soil, 2000, 226(1), 45–56, DOI:10.1023/A:1026439226716.
- M. Zarebanadkouki, P. Trtik, F. Hayat, A. Carminati and A. Kaestner, Root water uptake and its pathways across the root: quantification at the cellular scale, Sci. Rep., 2019, 9(1), 1–11, DOI:10.1038/s41598-019-49528-9.
- Y. X. Kim, K. Ranathunge, S. Lee, Y. Lee, D. Lee and J. Sung, Composite transport model and water and solute transport across plant roots: an update, Front. Plant Sci., 2018, 9, 1–9, DOI:10.3389/fpls.2018.00193.
- P. J. White, The pathways of calcium movement to the xylem, J. Exp. Bot., 2001, 52(358), 891–899, DOI:10.1093/jexbot/52.358.891.
- L. Ding, X. Wang, J. Li, J. Huang and Z. Li, Synthesis of Fluorescent Carbon Quantum Dots and Their Application in the Plant Cell Imaging, J. Wuhan Univ. Technol.-Materials Sci. Ed., 2018, 33(6), 1546–1550, DOI:10.1007/s11595-018-2004-8.
- S. Tripathi and S. Sarkar, Influence of water soluble carbon dots on the growth of wheat plant, Nanoscience, 2014, 5(5), 609–616, DOI:10.1007/s13204-014-0355-9.
- H. M. M. Abdel-Aziz, M. N. A. Hasaneen and A. M. Omer, Impact of engineered nanomaterials either alone or loaded with NPK on growth and productivity of French bean plants: seed priming vs foliar application, South Afr. J. Bot., 2019, 125, 102–108, DOI:10.1016/j.sajb.2019.07.005.
- A. Sciortino, A. Cannizzo and F. Messina, Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response, J. Carbon Res., 2018, 4(67), 1–35, DOI:10.3390/c4040067.
- R. D. Waghmare, A. H. Gore, P. V. Anbhule, D. Sohn and G. B. Kolekar, Dataset on the shooting and rooting ability of Morus alba using waste tea residue derived carbon dots as an alternative of growth plant stimulator, Data Brief, 2020, 29, 1–7, DOI:10.1016/j.dib.2020.105345.
- B. Patil and H. Chetan, Foliar fertilization of nutrients, Marumegh Kisaan E Patrika, 2016, 3(1), 49–53 Search PubMed.
- T. L. Tan, N. A. Zulkifli, A. S. K. Zaman, M. binti Jusoh, M. N. Yaapar and S. A. Rashid, Impact of photoluminescent carbon quantum dots on photosynthesis efficiency of rice and corn crops, Plant Physiol. Biochem., 2021, 162, 737–751, DOI:10.1016/j.plaphy.2021.03.031.
- M. Kolackova, et al., Antioxidant, gene expression and metabolomics fingerprint analysis of Arabidopsis thaliana treated by foliar spraying of ZnSe quantum dots and their growth inhibition of Agrobacterium tumefaciens, J. Hazard. Mater., 2019, 365, 932–941, DOI:10.1016/j.jhazmat.2018.11.065.
- C. Wang, H. Yang, F. Chen, L. Yue, Z. Wang and B. Xing, Nitrogen-Doped Carbon Dots Increased Light Conversion and Electron Supply to Improve the Corn Photosystem and Yield, Environ. Sci. Technol., 2021, 55(18), 12317–12325, DOI:10.1021/acs.est.1c01876.
- Y. Ji, et al., Carbon dots promoted soybean photosynthesis and amino acid biosynthesis under drought stress: reactive oxygen species scavenging and nitrogen metabolism, Sci. Total Environ., 2023, 856, 159125, DOI:10.1016/j.scitotenv.2022.159125.
- H. Yang, et al., Foliar carbon dot amendment modulates carbohydrate metabolism, rhizospheric properties and drought tolerance in maize seedling, Sci. Total Environ., 2022, 809, 151105, DOI:10.1016/j.scitotenv.2021.151105.
- C. Wang, et al., Regulation Mechanisms of Nitrogen-Doped Carbon Dots in Enhanced Maize Photosynthesis under Drought Stress, ACS Agric. Sci. Technol., 2023, 3, 181–189, DOI:10.1021/acsagscitech.2c00286.
- C. Wang, et al., Carbon Dots Improve Nitrogen Bioavailability Quality of Soybeans under Drought Stress, ACS Nano, 2022, 16, 12415–12424, DOI:10.1021/acsnano.2c03591.
- Y. Zheng, et al., Bioimaging Application and Growth-Promoting Behavior of Carbon Dots from Pollen on Hydroponically Cultivated Rome Lettuce, ACS Omega, 2017, 2(7), 3958–3965, DOI:10.1021/acsomega.7b00657.
- T. A. Swift, et al., Photosynthesis and crop productivity are enhanced by glucose- functionalized carbon dots, New Phytol., 2020, 229(2), 738–790, DOI:10.1111/nph.16886.
- H. Wang, et al., Carbon dots promote the growth and photosynthesis of mung bean sprouts, Carbon, 2018, 136, 94–102, DOI:10.1016/j.carbon.2018.04.051.
- M. Zhang, et al., One-step hydrothermal synthesis of chiral carbon dots and their effects on mung bean plant growth, Nanoscale, 2018, 10(26), 12734–12742, 10.1039/C8NR01644E.
- L. D. Agnol, R. M. Neves, M. Maraschin, S. Moura, H. L. Ornaghi Jr, F. T. G. Dias and O. Bianchi, Green synthesis of Spirulina-based carbon dots for stimulating agricultural plant growth, Sustain. Mater. Technol., 2021, 30, e00347, DOI:10.1016/j.susmat.2021.e00347.
- X. Tang, et al., Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples, Bioact. Mater., 2021, 6(6), 1541–1554, DOI:10.1016/j.bioactmat.2020.11.006.
- W. Liu, et al., Preparation of nitrogen-doped carbon dots with a high fluorescence quantum yield for the highly sensitive detection of Cu2+ ions, drawing anti-counterfeit patterns and imaging live cells, New Carbon Mater., 2019, 34(4), 390–402, DOI:10.1016/s1872-5805(19)30024-1.
- Y. Dong, R. Wang, G. Li, C. Chen, Y. Chi and G. Chen, Polyamine-functionalized carbon quantum dots as fluorescent probes for selective and sensitive detection of copper ions, Anal. Chem., 2012, 84(14), 6220–6224, DOI:10.1021/ac3012126.
- H. Li, et al., Enhanced RuBisCO activity and promoted dicotyledons growth with degradable carbon dots, Nano Res., 2019, 12(7), 1–9, DOI:10.1007/s12274-019-2397-5.
- S. Chandra, et al., High throughput electron transfer from carbon dots to chloroplast: a rationale of enhanced photosynthesis, Nanoscale, 2014, 6(7), 3647–3655, 10.1039/C3NR06079A.
- Y. Gong and Z. Dong, Transfer, transportation, and accumulation of cerium-doped carbon quantum dots: promoting growth and development in wheat, Ecotoxicol. Environ. Saf., 2021, 226, 112852, DOI:10.1016/j.ecoenv.2021.112852.
- M. Okamura, T. Hirose, Y. Hashida and R. Ohsugi, Suppression of starch accumulation in ‘ sugar leaves ’ of rice affects plant productivity under field conditions, Plant Prod. Sci., 2016, 20(1), 102–110, DOI:10.1080/1343943X.2016.1259958.
- O. Shalaby and M. Ramadan, Effect of Seed Soaking and Foliar Spraying With Selenium on the Growth and Yield of Lettuce Under Saline Stress, Egypt. J. Desert Res., 2017, 67(2), 251–263, DOI:10.21608/ejdr.2017.7137.
- C. Giménez, M. Gallardo and R. B. Thompson, Plant–Water Relations, Ref. Modul. Earth Syst. Environ. Sci., 2013,(May), 1–8, DOI:10.1016/b978-0-12-409548-9.05257-x.
- J. A. Perdomo, S. Capó-Bauçà, E. Carmo-Silva and J. Galmés, Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit, Front. Plant Sci., 2017, 8, 1–15, DOI:10.3389/fpls.2017.00490.
- L. H. Gunn, E. M. Avila, R. Birch and S. M. Whitney, The dependency of red Rubisco on its cognate activase for enhancing plant photosynthesis and growth, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(41), 25890–25896, DOI:10.1073/pnas.2011641117.
- A. Khan, et al., Validation of an Enzyme-Driven Model Explaining Photosynthetic Rate Responses to Limited Nitrogen in Crop Plants, Front. Plant Sci., 2020, 11, 1–14, DOI:10.3389/fpls.2020.533341.
- K. Qian, H. Guo, G. Chen, C. Ma and B. Xing, Distribution of different surface modified carbon dots in pumpkin seedlings, Sci. Rep., 2018, 1–9, DOI:10.1038/s41598-018-26167-0.
- M. Thompson, D. Gamage, N. Hirotsu, A. Martin and S. Seneweera, Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: a perspective on root sugar sensing and hormonal crosstalk, Front. Physiol., 2017, 8, 1–13, DOI:10.3389/fphys.2017.00578.
- H. Wang, et al., Carbon dots promote the growth and photosynthesis of mung bean sprouts, Carbon, 2018, 136, 94–102, DOI:10.1016/j.carbon.2018.04.051.
- W. Li, S. Wu, H. Zhang, X. Zhang, J. Zhuang and C. Hu, Enhanced Biological Photosynthetic Efficiency Using Light- Harvesting Engineering with Dual-Emissive Carbon Dots, Adv. Funct. Mater., 2018, 1–11, DOI:10.1002/adfm.201804004.
- J. Chen, et al., The effect and fate of water-soluble carbon nanodots in maize (Zea mays L.) The effect and fate of water-soluble carbon nanodots in maize, Nanotoxicology, 2016, 10(6), 818–828, DOI:10.3109/17435390.2015.1133864.
- M. Saikia, A. Singh, A. Dihingia, P. Khare, J. Kalita and B. K. Saikia, Scalable production, cell toxicity assessment, and plant growth promotion activities of carbon quantum dots derived from low-quality coal feedstock, Chem. Eng. J., 2021, 433(2), 133633, DOI:10.1016/j.cej.2021.133633.
- C. Wang, et al., Physiological and molecular level understanding of advanced carbon dots to enhance maize drought tolerance: modulation of photosynthesis and signaling molecules, Environ. Sci. Nano, 2022, 9(10), 3821–3832, 10.1039/D2EN00176D.
- A. Sharma and J. Das, Small molecules derived carbon dots: synthesis and applications in sensing, catalysis, imaging and biomedicine, J. Nanobiotechnol., 2019, 17(92), 1–24, DOI:10.1186/s12951-019-0525-8.
- P. Zuo, X. Lu, Z. Sun, Y. Guo and H. He, A review on syntheses, properties, characterization and bioanalytical applications of fluorescent carbon dots, Microchim. Acta, 2016, 183(2), 519–542, DOI:10.1007/s00604-015-1705-3.
- P. Sétif, G. Shimakawa, A. Krieger-Liszkay and C. Miyake, Identification of the electron donor to flavodiiron proteins in Synechocystis sp. PCC 6803 by in vivo spectroscopy, Biochim. Biophys. Acta Bioenerg., 2020, 1861(10), 148256, DOI:10.1016/j.bbabio.2020.148256.
- R. Höhner, A. Aboukila, H. H. Kunz and K. Venema, Proton gradients and proton-dependent transport processes in the chloroplast, Front. Plant Sci., 2016, 7(FEB2016), 1–7, DOI:10.3389/fpls.2016.00218.
- X. G. Zhu, S. P. Long and D. R. Ort, Improving photosynthetic efficiency for greater yield, Annu. Rev. Plant Biol., 2010, 61, 235–261, DOI:10.1146/annurev-arplant-042809-112206.
- M. P. Johnson, Photosynthesis, Essays Biochem., 2016, 60(3), 255–273, DOI:10.1042/EBC20160016.
- E. A. Lysenko, A. A. Klaus, A. V. Kartashov and V. V. Kusnetsov, Distribution of Cd and other cations between the stroma and thylakoids: a quantitative approach to the search for Cd targets in chloroplasts, Photosynth. Res., 2018, 337–358, DOI:10.1007/s11120-018-0528-6.
- M. P. Johnson and E. Wientjes, The relevance of dynamic thylakoid organisation to photosynthetic regulation, Biochim. Biophys. Acta Bioenerg., 2020, 1861(4), 1–11, DOI:10.1016/j.bbabio.2019.06.011.
- H. B. Yildiz, E. Cevik and B. B. Carbas, Nanotechnology for biological photovoltaics; industrial applications of nanomaterials, Elsevier Inc., 2019 Search PubMed.
- Y. Okegawa, L. Basso, T. Shikanai and K. Motohashi, Cyclic electron transport around PSI contributes to photosynthetic induction with thioredoxin f, Plant Physiol., 2020, 184(3), 1291–1302, DOI:10.1104/pp.20.00741.
- G. D. Scholes, G. R. Fleming, A. Olaya-Castro and R. Van Grondelle, Lessons from nature about solar light harvesting, Nat. Chem., 2011, 3(10), 763–774, DOI:10.1038/nchem.1145.
- J. P. Giraldo, et al., Plant nanobionics approach to augment photosynthesis and biochemical sensing, Nat. Mater., 2014, 13(4), 400–408, DOI:10.1038/nmat3890.
- I. Nabiev, et al., Fluorescent Quantum Dots as Artificial Antennas for Enhanced Light Harvesting and Energy Transfer to Photosynthetic Reaction Centers, Angew. Chem., Int. Ed., 2010, 49(40), 7217–7221, DOI:10.1002/anie.201003067.
- N. Zamzam, et al., Femtosecond visible transient absorption spectroscopy of chlorophyll-f-containing photosystem II, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(37), 23158–23164, DOI:10.1073/pnas.2006016117.
- H. Mattila, et al., Action spectrum of the redox state of the plastoquinone pool defines its function in plant acclimation, Plant J., 2020, 1088–1104, DOI:10.1111/tpj.14983.
- F. Paquin, J. Rivnay, A. Salleo, N. Stingelin and C. Silva, Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors, J. Mater. Chem. C, 2015, 10715–10722, 10.1039/b000000x.
- M. O. Alas and R. Genc, An investigation into the role of macromolecules of different polarity as passivating agent on the physical, chemical and structural properties of fluorescent carbon nanodots, J. Nanoparticle Res., 2017, 19(5), 1–15, DOI:10.1007/s11051-017-3863-1.
- M. J. Wu, et al., All Organic Label-like Copper(II) Ions Fluorescent Film Sensors with High Sensitivity and Stretchability, ACS Sensors, 2018, 3(1), 99–105, DOI:10.1021/acssensors.7b00635.
- C. W. A. do Nascimento and M. C. Marques, Metabolic alterations and X-ray chlorophyll fluorescence for the early detection of lead stress in castor bean (Ricinus communis) plants, Acta Sci. Agron., 2018, 40(1), 1–26, DOI:10.4025/actasciagron.v40i1.39392.
- F. Kopnov, I. Cohen-Ofri and D. Noy, Electron Transport between Photosystem II and Photosystem I Encapsulated in Sol-Gel Glasses, Angew. Chem., 2011, 123(51), 12555–12558, DOI:10.1002/ange.201106293.
- J. E. Sireci, A. Plotner, R. Barr and F. L. Crane, Selective thiol inhibition of ferricyanide reduction in photosystem II of spinach chloroplasts, Biochem. Biophys. Res. Commun., 1978, 85(3), 976–982, DOI:10.1016/0006-291X(78)90639-3.
- M. Jedrzejczak-Silicka and E. Mijowska, General Cytotoxicity and Its Application in Nanomaterial Analysis, Cytotoxicity, IntechOpen, London, UK, 2018, pp. 177–205, DOI:10.5772/intechopen.72578.
- D. Karakaş, F. Ari and E. Ulukaya, The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts, Turkish J. Biol., 2017, 41(6), 919–925, DOI:10.3906/biy-1703-104.
- S. D. Mahajan, et al., Nanoparticle-mediated targeted delivery of antiretrovirals to the brain, Elsevier Inc., 1st edn, 2012, vol. 509 Search PubMed.
- K. Howe et al., The zebrafish reference genome sequence and its relationship to the human genome,” Letter, pp. 1–6, 2013, doi: DOI:10.1038/nature12111.
- M. L. Kent and J. L. S. S. S. C. E. Al-samarrie, Review of diseases and health management in zebrafish Danio rerio (Hamilton 1822) in research facilities, J. Fish Dis., 2020, 1–14, DOI:10.1111/jfd.13165.
- Y. Kang, Y. Li, Y. Fang, Y. Xu, X. Wei and X. Yin, Carbon Quantum Dots for Zebrafish Fluorescence Imaging, Sci. Rep., 2015, 5(1), 1–12, DOI:10.1038/srep11835.
- E. Umar, M. Ikram, J. Haider, W. Nabgan, A. Haider, M. Imran and G. Nazir, A state-of-the-art review on carbon quantum dots: prospective, advances, zebrafish biocompatibility and bioimaging in vivo and bibliometric analysis, Sustain. Mater. Technol., 2022, 35, e00529 Search PubMed.
- C.-Y. Chung, Y.-J. Chen, C.-H. Kang, H.-Y. Lin, C.-C. Huang and H.-J. Lin, Toxic or Not Toxic, That Is the Carbon Quantum Dot’ s Question: A Comprehensive Evaluation with Zebrafish Embryo, Eleutheroembryo, and Adult Models, Polymers, 2021, 13(1598), 1–14 Search PubMed.
- W. Liu, et al., Zebrafish: a promising model for evaluating the toxicity of carbon dot-based nanomaterials, ACS Appl. Mater. Interfaces, 2020, 12(43), 49012–49020 CrossRef CAS PubMed.
- D. H. Phillips and V. M. Arlt, Genotoxicity: damage to DNA and its consequences, Mol. Clin. Environ. Toxicol., 2009, 1, 87–110, DOI:10.1007/978-3-7643-8336-7_4.
- M. P. Sk, A. Jaiswal, A. Paul, S. S. Ghosh and A. Chattopadhyay, Presence of Amorphous Carbon Nanoparticles in Food Caramels, Sci. Rep., 2012, 2(1), 1–5, DOI:10.1038/srep00383.
- J. Bi, Y. Li, H. Wang, Y. Song and S. Cong, Physicochemical properties and cytotoxicity of carbon dots in grilled fish, New J. Chem., 2017, 41, 8490–8496, 10.1039/C7NJ02163A.
- A. M. Al-hadi, V. S. Periasamy, J. Athinarayanan and A. A. Alshatwi, The presence of carbon nanostructures in bakery products induces metabolic stress in human mesenchymal stem cells through CYP1A and p53 gene expression, Environ. Toxicol. Pharmacol., 2016, 41, 103–112, DOI:10.1016/j.etap.2015.11.012.
- A. Truskewycz, et al., Carbon Dot Therapeutic Platforms: Administration, Distribution, Metabolism, Excretion, Toxicity, and Therapeutic Potential, Small, 2022, 18, 1–24, DOI:10.1002/smll.202106342.
- M. Yousaf, et al., Effects of fertilization on crop production and nutrient-supplying capacity under rice-oilseed rape rotation system, Sci. Rep., 2017, 7(1), 1–9, DOI:10.1038/s41598-017-01412-0.
- M. Xue, M. Zou, J. Zhao, Z. Zhanab and S. Zhao, Green preparation of fluorescent carbon dots from lychee seed and its application for selective detection of methylene blue and imaging in living cells, J. Mater. Chem. B, 2015, 1–7, 10.1039/b000000x.
- H. S. Shahraki and A. Ahmad, Synthesis, characterization of carbon dots from onion peel and their application as absorbent and anticancer activity, Inorg. Chem. Commun., 2023, 150, 110514, DOI:10.1016/j.inoche.2023.110514.
- H. Laddha, P. Yadav, M. Sharma, M. Agarwal and R. Gupta, Waste to value transformation: converting Carica papaya seeds into green fluorescent carbon dots for simultaneous selective detection and degradation of tetracycline hydrochloride in water, Environ. Res., 2023, 227, 115820, DOI:10.1016/j.envres.2023.115820.
- I. Xue, Z. Zhan, M. Zou, L. Zhanga and S. Zhao, Green synthesis of stable and biocompatible fluorescent carbon dots from peanut shell for multicolor living cell imaging, New J. Chem., 2015, 40(2), 1698–1703, 10.1039/C5NJ02181B.
- F. Wen, P. Li, Y. Zhang, H. Zhong, H. Yan and W. Su, Preparation, characterization of green tea carbon quantum dots/curcumin antioxidant and antibacterial nanocomposites, J. Mol. Struct., 2023, 1273, 134247, DOI:10.1016/j.molstruc.2022.134247.
- N. Jamaludin, T. Tan, A. Zaman, A. Sadrolhosseinin and S. Rashid, Acid-Free Hydrothermal-Extraction and Molecular Structure of Carbon Quantum Dots Derived from Empty Fruit Bunch Biochar, Materials, 2020, 13(15), 1–22 CrossRef PubMed.
- X. J. Lee, L. Y. Lee, S. Gan, S. Thangalazhy-Gopakumar and H. K. Ng, Biochar potential evaluation of palm oil wastes through slow pyrolysis: thermochemical characterization and pyrolytic kinetic studies, Bioresour. Technol., 2017, 236, 155–163, DOI:10.1016/j.biortech.2017.03.105.
- W. L. Ang, et al., Microwave - assisted conversion of palm kernel shell biomass waste to photoluminescent carbon dots, Sci. Rep., 2020, 1–15, DOI:10.1038/s41598-020-78322-1.
- L. P. Magagula, C. M. Masemola, M. As, Z. N. Tetana, N. Moloto and E. C. Linganiso, Lignocellulosic Biomass Waste-Derived Cellulose Nanocrystals and Carbon Nanomaterials: A Review, Int. J. Mol. Sci., 2022 Search PubMed.
- L. J. Desmond, A. N. Phan and P. Gentile, Critical overview on the green synthesis of carbon quantum dots and their application for cancer therapy, Environ. Sci. Nano, 2021, 8, 848–862, 10.1039/d1en00017a.
- R. Bongiovanni and J. Lowenberg-Deboer, Precision agriculture and sustainability, Precis. Agric., 2004, 5(4), 359–387, DOI:10.1023/B:PRAG.0000040806.39604.aa.
- A. Benelli, C. Cevoli and A. Fabbri, In-field hyperspectral imaging: an overview on the ground-based applications in agriculture, J. Agric. Eng., 2020, 51(3), 129–139, DOI:10.4081/jae.2020.1030.
- D. Caballero, R. Calvini and J. M. Amigo, Hyperspectral imaging in crop fields: precision agriculture, in Data Handling in Science and Technology, Elsevier, 2019, vol. 32, pp. 453–473, DOI:10.1016/B978-0-444-63977-6.00018-3.
- A. Faltynkova, G. Johnsen and M. Wagner, Hyperspectral imaging as an emerging tool to analyze microplastics: a systematic review and recommendations for future development, Microplast. Nanoplast., 2021, 1(1), 1–19, DOI:10.1186/s43591-021-00014-y.
- B. Zhang, W. Yang, L. Gao and D. Chen, Real-time target detection in hyperspectral images based on spatial-spectral information extraction, EURASIP J. Adv. Signal Process., 2012, 2012(1), 1–15, DOI:10.1186/1687-6180-2012-142.
- N. W. Albert, D. H. Lewis, H. Zhang, L. J. Irving, P. E. Jameson and K. M. Davies, Light-induced vegetative anthocyanin pigmentation in Petunia, J. Exp. Bot., 2009, 60(7), 2191–2202, DOI:10.1093/jxb/erp097.
- A. Patri, Nanotechnology Research at NCTR, U.S Food & Drug Administration, 2020, https://www.fda.gov/about-fda/nctr-research-focus-areas/nanotechnology#:%7E:text=Nanotechnology%20Research%20at%20NCTR,%20and%20detect%20the%20nanoscale%20materials Search PubMed.
- S. W. Hogewoning, G. Trouwborst, H. Maljaars, H. Poorter, W. van Ieperen and J. Harbinson, Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light, J. Exp. Bot., 2010, 61(11), 3107–3117, DOI:10.1093/jxb/erq132.
- S. Demotes-Mainard, et al., Plant responses to red and far-red lights, applications in horticulture, Environ. Exp. Bot., 2016, 121, 4–21, DOI:10.1016/j.envexpbot.2015.05.010.
- T. Schumann, S. Paul, M. Melzer, P. Dörmann and P. Jahns, Plant growth under natural light conditions provides highly flexible short-term acclimation properties toward high light stress, Front. Plant Sci., 2017, 8(May), 1–18, DOI:10.3389/fpls.2017.00681.
| This journal is © The Royal Society of Chemistry 2023 |