Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in the application of magnetic nanocatalysts in multicomponent reactions
Hojat Veisi *a,
Mozhgan Pirhayatib,
Pourya Mohammadia,
Taiebeh Tamoradic,
Saba Hemmatia and
Bikash Karmakar*d
*a,
Mozhgan Pirhayatib,
Pourya Mohammadia,
Taiebeh Tamoradic,
Saba Hemmatia and
Bikash Karmakar*d
aDepartment of Chemistry, Payame Noor University, Tehran, Iran. E-mail: hojatveisi@yahoo.com; hojatveisi@pnu.ac.ir
bDepartment of Applied Chemistry, Faculty of Science, Malayer University, Malayer, Iran
cProduction Technology Research Institute-ACECR, Ahvaz, Iran
dDepartment of Chemistry, Gobardanga Hindu College, 24-Parganas (North), India. E-mail: bkarmakar@ghcollege.ac.in
First published on 10th July 2023
Abstract
Recently, the preparation and applications of magnetic nanostructures have attracted increasing attention in nanocatalysis studies, and magnetic nanoparticle (MNP) functionalized catalysts have been applied in important reactions such as Suzuki–Miyaura and Heck couplings. The modified nanocomposites demonstrate significant catalytic efficiency and excellent benefits in the context of catalyst recovery methods. This review discusses the recent modified magnetic nanocomposites in the field of catalytic applications along with the synthetic processes that are usually employed.
1. Introduction
Multicomponent reactions (MCRs) attract considerable attention in medicinal and organic chemistry owing to several advantages including their potential toward the production of desirable products with great atom economy. The reaction of three or more reactants in one step without the isolation of any intermediate and MCRs is an extremely useful method for the synthesis of complex organic compounds by easily available reactants.1–9 Synthetic researchers have repeatedly applied MCRs as a simple tool to construct a variety of molecules from bi-functional precursors that react sequentially in an intramolecular method.10The challenge to create concise, conceptually fine, and new synthetic ways for multicomponent reactions has been a growing driving force in both industry and academia. Among the types of synthetic procedures, current studies have concentrated on establishing catalytic procedures from readily available starting materials under moderate reaction conditions.
A key part of “green chemistry” is the catalyst. Therefore, a stable and green catalyst must possess particular properties, such as great activity, low preparation cost, high selectivity, effective recovery, great stability, and excellent recyclability.11
Traditional catalysts can be classified into heterogeneous and homogeneous. The advantages of homogeneous catalysts include high selectivity and activity and available mechanistic investigations, leading to the optimization of catalysts. However, the problem of separation of these nanocatalysts from reaction media significantly limits their utilization in industry, particularly in the pharmaceutical and drug industry, due to the effect of metal pollution concerning metal-catalyzed production. The features, including the ease of handling, isolation, recycling, and being environmentally friendly, enable the application of heterogeneous catalysts to be more favorable. However, the activity of heterogeneous catalysts is generally less than that of homogeneous ones, owing to the smaller size of the interaction between the compounds and the surface of the catalyst.12–18
Therefore, it is necessary that a catalyst system, in addition to high selectivity and activity, can readily be isolated from the reaction media. This purpose can be achieved with nanocatalysts. Nano-sized catalysts have a high surface-to-volume ratio that is a suitable alternative to common catalysts.19,20 However, when the active site size is decreased to the nanoscale, the surface free energy rises considerably. This effect increases the aggregation of the particles into small clusters and demotes the catalytic performance. Moreover, separation and recovery of these catalysts become hard with decreasing size to nanoscale; in most cases, isolation by usual filtration can even become impossible.21–24
To resolve these problems, the application of magnetic nanoparticles (MNPs) seems to be the most reasonable solution.25–30 Their unique paramagnetic nature and intrinsic insolubility in most solvents let their easy and effective isolation from the reaction mixture by using a magnet. The catalysts can be consequently reutilized in another cycle. Recycling and reuse are two essential properties of magnetic nanocatalysts.31–33 Moreover, suitable surface-modification MNPs may be applied to prevent the aggregation of these nanoparticles, leading to stable, highly dispersed active particles. Due to these benefits, these functionalized nanoparticles have found broad applications in different fields, including magnetic resonance imaging,34,35 biomedicine,36–39 and hetero-catalysis.40–47 Hence, current improvements in magnetically recoverable catalysts (MRCs) have propelled their broad application in oxidation,48–50 hydrogenation,51,52 coupling reactions,53–55 cycloaddition,56,57 acylation,58,59 photocatalysis,60–62 etc.
In recent years, several types of magnetic nanocatalysts have been used for the progress of multi-component reactions. In this review, we provide an overview of various kinds of magnetic nanocatalysts in MCRs and the main advantages of the reported methods.
2. Nano metal oxides (NMOs)
Between various nanocatalysts, nanocrystalline metal oxides are widely used in multi-component reactions. Among the metal oxide nanocatalysts, iron oxides (FeO, Fe2O3, and Fe3O4) are the most encouraging catalysts due to their ease of handling, recovery with an external magnet, high catalytic activities, in addition to the Lewis acid nature of iron that also catalyzes some of the organic reactions.63–75Zhang et al.76 published an effective three-component condensation of aldehydes, isatoic anhydride, and amines in the presence of Fe3O4 under room temperature for a one-pot preparation of 2,3-dihydroquinazolin-4(1H)-ones. Utilizing this method, aromatic amines were observed to be efficient compounds and provided the products in high yields, and the reaction progressed smoothly when aliphatic amines were used (Scheme 1a). Additionally, the catalyst without a decrease in catalytic performance could be reutilized for up to five continuous cycles.
Reddy et al.77 used Fe3O4 nanoparticles for the preparation of α-aminophosphonates from the condensation of amines, aldehydes, and diethyl phosphate under solvent-free conditions. This reaction is appropriate for a large number of aldehydes, including aliphatic or aromatic, which react well and has excellent yields. Further, the catalyst was reutilized for ten runs without a considerable decrease in catalytic performance (Scheme 1b).
An effective procedure for the one-pot preparation of dihydropyrimidinones (thiones) in the present nanocatalyst was studied by Nasr-Esfahani et al.78 Results demonstrated that various types of aromatic aldehydes reacted well with other reagents to obtain the dihydropyrimidinone derivatives in solvent-free conditions with high yields. It is worth mentioning that aromatic aldehydes reacted in a lesser time compared with aliphatic aldehydes (Scheme 1c). The nanocatalyst could be recovered and reused effectively from the reaction mixture for at least four cycles without a noticeable loss in its catalytic efficiency.
Zolfigol, Khazae, Kolvari, et al.79 recently studied the preparation of Hantzsch 1,4-dihydropyridines compounds in the presence of free nano-Fe2O3 as a Lewis-acid catalyst. A large number of aliphatic, aromatic, and heteroaromatic aldehydes were exposed to react with β-keto compounds and ammonium acetate with good yields under moderate conditions. This eco-friendly nanocatalyst could be directly reutilized without any deactivation even after separation from the reaction mixture (Scheme 2a).
 | ||
| Scheme 2 Synthesis of 1,4-dihydropyridines using Fe2O3 NPs at 90 °C (a) and reaction used for the synthesis of pyrimidine-5-carbonitrile derivatives at 100 °C (b). | ||
The Rostamizadeh group80 reported the one-pot synthesis of 4-amino-6-aryl-2-phenyl pyrimidine-5-carbonitrile derivatives via the three-component reaction of an aldehyde, benzamidine hydrochloride, and malononitrile in the presence of Fe3O4 magnetic nanoparticles (Scheme 2b). The products were synthesized with high yields in 1–1.5 h in solvent-free conditions. Under these conditions, aromatic aldehydes with electron-donor and electron-withdrawing substituents exhibited remarkable reactivity in this method. Additionally, these chemicals were appraised for biological performance and demonstrated antibacterial performance related to the reference penicillin.
3. Ferrites of MNPs catalysis
Other forms of iron oxide, spinel ferrites (MFe2O4), have also attracted much attention to MNPs catalysis due to unique properties such as environmental stability and ferrimagnetism. In MFe2O4 compounds, M is a transition element, such as Cu, Zn, Mn, Ni, and Co.Dandia et al.81 showed that CuFe2O4 is a powerful and magnetic nanocatalyst for the one-pot preparation of spirohexahydropyrimidines through aromatic amines, formaldehyde, and ketones (Scheme 3a). The reaction was extended to several aromatic amines, and CuFe2O4 catalytic performance remained unchanged during five cycles, showing the effectiveness and “green” aspect of this nanocatalyst.
The catalytic property of a magnetic CuFe2O4 nanocomposite in the synthesis of spirooxindole via new multicomponent reactions was investigated by Ghahremanzadeh's group.82 The one-pot preparation of spirooxindole-fused heterocycles was achieved by cyanomethanes, cyclic 1,3-dicarbonyl derivatives, and isatins as reactants in the presence of a catalyst in H2O as the reaction solvent, obtaining spirooxindoles in 81% to 97% yields (Scheme 3b). In the primary examination, the reaction of malononitrile, 3-hydroxy-1H-phenalen-1-one, and isatin was performed in the presence of CuFe2O4 (10 mol%) at refluxing temperature for 0.5 h, and the intended spirooxindole was separated in 90% yield. It is important to note that after the first run, copper ferrite nanoparticles as a catalyst could be recovered and reutilized for four continuous cycles without a remarkable decrease in yield.
Pradhan and co-workers83 studied the preparation of dihydropyrano[2,3-c]pyrazole derivatives in one-pot at moderate reaction conditions and in high yields using CuFe2O4 nanoparticles as an effective nanocatalyst. The four-component reaction of a broad diversity of hydrazine derivatives, dialkyl acetylene dicarboxylates, ethyl acetoacetate, and alkyl nitrile derivatives, such as ethyl cyanoacetate and malononitrile, provided the desirable dihydropyrano[2,3-c]pyrazoles in excellent efficiency (Scheme 3c).
Also, CuFe2O4 nanoparticles were used for the synthesis of pyrano[3,2-c]coumarin derivatives in one-pot at moderate reaction conditions in the aqueous media and in high yields.83 The reaction progresses through the MCR's of dialkyl acetylene dicarboxylates, 4-hydroxycoumarin, and ethyl cyanoacetate or malononitrile (Scheme 4a).
Catalytic recovery capability was investigated for the preparation of dihydropyrano[2,3-c]pyrazole. The results demonstrated that the nanocatalyst could be recovered in at least six cycles without a considerable decrease in efficiency.
CuFe2O4 magnetic nanoparticles were synthesized by Das et al.84 and used for the one-pot multi-component synthesis of 4H-chromene derivatives at moderate reaction conditions in aqueous media with high yields. The chemical reaction progresses through MCR's of cyclohexane-1,3-dione or dimedone, dialkyl acetylene dicarboxylates, and ethyl cyanoacetate or malononitrile (Scheme 4b).
Khazaei and colleagues85 reported the one-pot preparation of pyrano[2,3-d]pyrimidines via the three-component condensation of malononitrile, 1,3-diethyl barbituric acid, and aromatic aldehydes in the presence of heterogeneous ZnFe2O4 nanoparticles in solvent-free conditions (Scheme 4c). ZnFe2O4 nanoparticles as a Lewis acid (with the Fe3+ in Fe2O3) and the basic compound (related to the O2− in ZnO) can catalyze this reaction. This procedure provides favorable products in good yields and in almost quick reaction times; it is noteworthy that aldehydes bearing electron-releasing groups increased the reaction times.
Pramanik and colleagues86 prepared a dual Lewis acid-base combined catalyst, i.e., the ZnFe2O4 nanoparticles. For comparison of the catalytic activity of these catalysts, preparation of functionalized tetrahydrospiro[indoline-3,2′-quinoline] derivatives via the reaction of dialkyl acetylene dicarboxylates, arylamines, cyclohexane-1,3-diones, and isatin derivatives in aqueous media at ambient temperature was performed, providing products in high yields (Scheme 4d).
Ni–Zn ferrites are the most useable magnetic nanoparticles because they have high Curie temperature, high saturation magnetization, chemical stability, and high permeability.87
Khazaei et al.88 studied a simple and effective process for the one-pot preparation of 2,4,5-triaryl substituted imidazoles using a three-component reaction in the presence of Ni0.5Zn0.5Fe2O4 as a reusable and heterogeneous nanocatalyst under solvent-free conditions (Scheme 5a). This method has several benefits, such as good yields, green reaction conditions, quick reaction times, easy handling, and proficiency of the catalyst. The magnetic Ni0.5Zn0.5Fe2O4 nanocatalyst was isolated from the reaction media via a magnet. The prepared nanocatalyst was recovered seven times in the subsequent reactions without any significant loss in the yield.
Khodabakhshi et al.89 reported a new application of a bimetal magnetic catalyst, i.e., copper ferrite nanoparticles were used to prepare several new spirooxindoles by a one-pot three-component reaction including isatin with malononitrile and Michael donors (Scheme 5b).
In addition, this nanocatalyst was decanted from the reaction mixture using a magnet and effectively reutilized for subsequent cycles and retained its high performance in the fourth reaction run.
Rawat's group90 synthesized CuO@Fe2O3 nanocatalyst for C1-alkynylation of tetrahydroisoquinoline (THIQ) through A3 coupling among THIQ, alkynes, and aldehydes and its decarboxylative procedures via replacement of alkynes with phenyl propionic acid (Scheme 5c). The described catalytic method was found to be magnetically recoverable 5 times without a remarkable decrease in its performance, proposing a low E-factor and great atom economy.
4. Modification of MNPs
A common problem of MNPs is the fast aggregation to large clusters and the subsequent decrease of the unique features correlated with catalytic reactions due to their large surface energy, small interparticle distances, and the presence of van der Waals forces. To resolve this difficulty, MNPs modification utilizing appropriate stabilizer ligands or coating substances (such as small molecules, polymers, silica, ionic liquids, carbon, and metal or metal oxide nanoparticles) has been confirmed as the best solution now. Thus, the modified systems present active groups or reaction units for noncovalent or covalently grafting the active catalytic sites onto the covered MNPs to produce magnetically recyclable nanocatalysts.4.1. Stabilizing ligands for modification of MNPs
MNPs-supported binuclear Cu(II)–β-cyclodextrin was readily synthesized via the addition of copper sulfate to the β-cyclodextrin in NaOH solution by Kaboudin and colleagues91,92 (Scheme 6a).This catalyst was suitable for the preparation of 1,4-disubstituted 1,2,3-triazoles via one-pot in situ azidation of arylboronic acids and the subsequent click cyclization in aqueous solution in the air at room temperature (Scheme 6b). The results of the studies were successfully applied in the catalytic system producing high yields. In addition, Fe3O4–β-CD–Cu2 could be recycled by using a magnet and efficiently reutilized for 4 catalytic runs without a significant decrease in catalytic activity.
Heydari et al.93 synthesized Fe3O4–proline MNPs without any supplemental linkers, and the catalytic efficiency of this nanocatalyst was considered in the preparation of chromene derivatives. Chromene derivatives are prepared through condensation of 2-hydroxynaphthalene-1,4-dione or 4-hydroxycoumarin, malononitrile, and aryl aldehyde in the presence of Fe3O4–proline nanoparticles at room temperature in good yields (Scheme 7a). Several derivatives of functionalized chromene were prepared under ambient conditions in high yields. The recoverability study showed that the application of a magnet provided separation of the nanocatalyst that was reutilized for a minimum of four runs without a decrease in performance and any iron leaching.
Safari and co-workers94 studied the synthesis of chitosan-coated Fe3O4 MNPs as renewable and heterogeneous magnetic biocatalysts and investigated their catalytic activity in the one-pot synthesis of 2,4,5-trisubstituted imidazoles through condensation of benzil derivatives, ammonium acetate, and aryl aldehydes in ethanol (Scheme 7b). The results showed good-to-high yields of the corresponding imidazoles. This heterogeneous bio-polymer catalyst was separated easily using a magnet, and the reusability of the synthesized nanocatalyst was favorably considered for six runs with only a very low decrease in catalytic performance.
In 2016, Veisi et al.95 additionally studied the synthesis of a new magnetic organocatalyst system, i.e., 3,4-dihydroxypyridine supported on nano-Fe3O4 (Fe3O4/Py) (Scheme 8a).
 | ||
| Scheme 8 Preparation of Fe3O4/Py nanocatalyst (a) and a new nanocatalyst for one-pot synthesis of pyrazolo[3,4-b]pyridines and pyrano[2,3-d]pyrimidines (b). | ||
This efficient nanocatalyst exhibited excellent catalytic performance in the domino condensation of different aromatic aldehydes, 5-methylpyrazol-3-amine and Meldrum's acid in moderate conditions and in ethanol as a solvent. Furthermore, the prepared nanocatalyst was found to be environmentally friendly with good catalytic activity for a three-component condensation of aromatic aldehydes with malononitrile and barbituric acid to the one-pot synthesis of 7-amino-2,4-dioxo-5-phenyl-2,3,4,5-tetrahydro-1H-pyrano[2,3-d]pyrimidine-6-carbonitriles (Scheme 8b). Moreover, noteworthy properties of this new catalyst method were the fast (within 15 s) and effective isolation of the nanocatalyst (100%) with a suitable external magnet, which minimized the loss of nanocatalyst during isolation and it could be reutilized for up to five runs (with insignificant Pd leaching) without notable loss in catalytic efficiency.
The synthesis of Cu-modified MWCNT-GAA@Fe3O4 nanocatalyst was reported by Shaabani et al.96 Cu/MWCNT-GAA@Fe3O4 demonstrated excellent catalytic performance for the preparation of 1,2,3-triazoles towards azide–alkyne 1,3-dipolar cycloaddition reactions and for the synthesis of bis(indolyl)methanes using condensation reaction in the presence of H2O as a green solvent (Scheme 9). The nanocatalyst was recyclable up to four cycles without much decrease in catalytic efficiency; the Cu leaching of the catalyst was very insignificant based on the FAAS analysis.
 | ||
| Scheme 9 Synthesis of 1,2,3-triazole and bis(indolyl)methane derivatives using a Cu/MWCNT-GAA@Fe3O4 catalyst in the presence of H2O as a green solvent. | ||
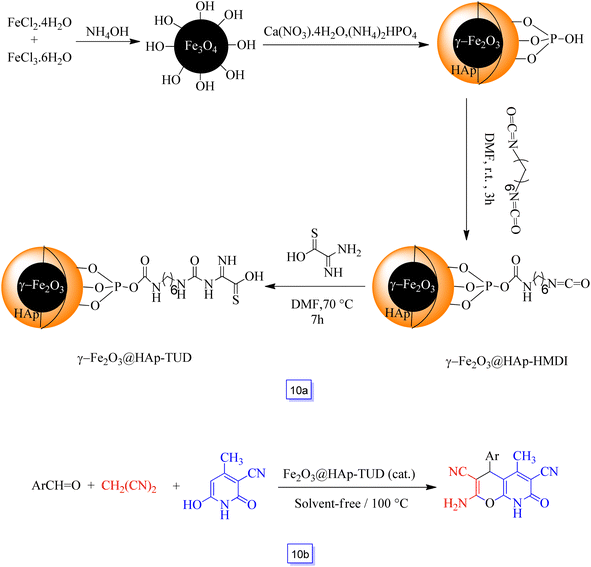
The synthesis of core–shell γ-Fe2O3@HAp-TUD, i.e., thiourea dioxide-grafted hydroxyapatite-encapsulated hybrid nanoparticles, was reported by Azarifar and Ghaemi97 (Scheme 10a).
The catalytic activity of this newly prepared acidic nanocatalyst was considered via the one-pot three-component reaction between malononitrile, several substituted aldehydes, and 3-cyano-6-hydroxy-4-methyl-pyridin-2(1H)-one to provide the respective pyrano[2,3-b]pyridines in good yields (82–98%) in solvent-free conditions (Scheme 10b). It could be reused for seven cycles without any considerable decrease in catalytic efficiency. The aldehydes bearing electron-donating groups in comparison to electron-withdrawing ones, commonly react in longer times.
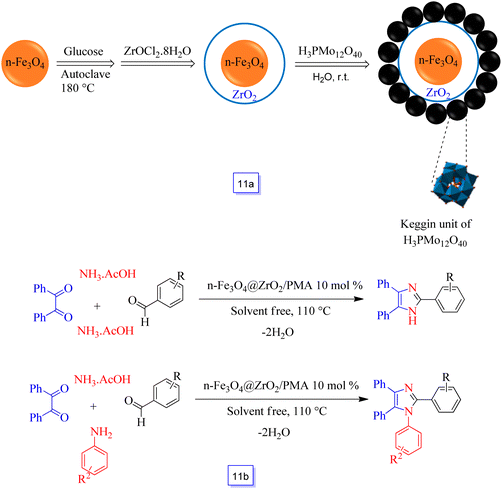
Kolvari et al.98 developed a heterogeneous nanosized acidic nanocatalyst called Fe3O4@ZrO2 supported PMA (or n-Fe3O4@ZrO2/PMA) so that the core–shell Fe3O4@ZrO2 surface was immobilized phosphomolybdic acid (H3PMo12O40) (Scheme 11a). This acidic nanocatalyst was used in several-component reactions for the one-pot preparation of multi-substituted imidazoles and for the preparation of 2,4,5-trisubstituted imidazoles from the reaction of benzil, aryl aldehydes, and NH4OAc, and also for the synthesis of 1,2,4,5-tetrasubstituted imidazoles from the condensation of aryl aldehydes, benzil, amines, and NH4OAc in solvent-free conditions (Scheme 11b).
It is noteworthy that the acidity content of the as-synthesized nanocatalyst demonstrated higher active sites related to homogeneous ones using potentiometric titration with n-butylamine. It is necessary to determine that this catalyst could be efficiently reutilized in the same reaction conditions for five continuous runs without a significant decrease in catalytic efficiency.
4.2. Nano porous systems
The favorite inorganic coating material for MNPs is silica due to its easy linking. Most of them were prepared in an organic solvent via hydrophobic capping agents that caused dispersibility in nonaqueous solvents but negligible dispersion in aqueous media. SiO2 as a covering shell increases biocompatibility and the water solubility of MNPs. The surface of SiO2 consists of two types of functional groups, [Si–O–Si and Si–OH], which provides functionalization with various functional groups. The functionalization of this surface using required reagents and linkers results in the production of a solid acidic catalyst.Ali Maleki and co-workers99 also developed a novel protocol from ready and simple accessible precursors such as a 1,2-diamine, a cyclic or linear ketone, and an isocyanide for the one-pot multicomponent preparation of diazepine derivatives in the presence of Fe3O4/SiO2 as a magnetically recyclable nanocatalyst (Scheme 12a). In this method 4,5,6,7-tetrahydro-1H-1,4-diazepine-5-carboxamide derivatives and 2,3,4,5-tetrahydro-1H-1,5-benzodiazepine-2-carboxamide derivatives were synthesized with excellent yields and mild reaction conditions.
Kiasat and co-workers100 prepared a core–shell structure from sulfuric acid functionalized silica-coated magnetite nanostructure (Fe3O4@SiO2 sulfuric acid) (Scheme 12b) and investigated its catalytic activity for solid acid catalysts in the preparation of pyrazolo[1,2-b]phthalazine-diones and indazolo[2,1-b]phthalazine-triones by a one-pot and three-component reaction of aromatic aldehydes, phthalhydrazide, and linear or cyclic 1,3-diketones in solvent-free conditions. The advantages of this approach are a cleaner reaction, an easy procedure, application of a re-utilizable catalyst (for six runs), simple handling, and a multi-component reaction.
Recyclable Fe3O4–immobilized copper(I) was readily prepared by Xiong et al.101 and they directly modified Fe3O4 MNPs with (3-aminopropyl)-trimethoxysilane and [3-(2-aminoethylamino)propyl]trimethoxysilane through a post-grafting process, followed by complexation with CuBr to produce MNPs-CuBr (1) and MNPs-CuBr (2), respectively (Scheme 13a). This catalyst was applied in one-pot multi-component azide–alkyne cycloaddition (CuAAC) synthesis from the reaction of sodium azide and benzyl chloride with phenylacetylene under microwave irradiation in the aqueous medium. As discussed in the presented research, microwave irradiation could significantly decrease the reaction time and concurrently improve the yields in comparison with common protocols. The first results of the investigation displayed that MNPs-CuBr (1) was a more effective catalyst than MNPs-CuBr (2), several halides, and terminal alkynes and confirmed its versatility in the presence MNPs-CuBr (1). Most of the 1,4-disubstituted 1,2,3-triazoles were separated in good to high efficiency with high selectivity. Moreover, the magnetically isolated nanocatalyst MNPs-CuBr (1) was simply recycled and reutilized for about seven runs with an inconsiderable loss of catalytic performance.
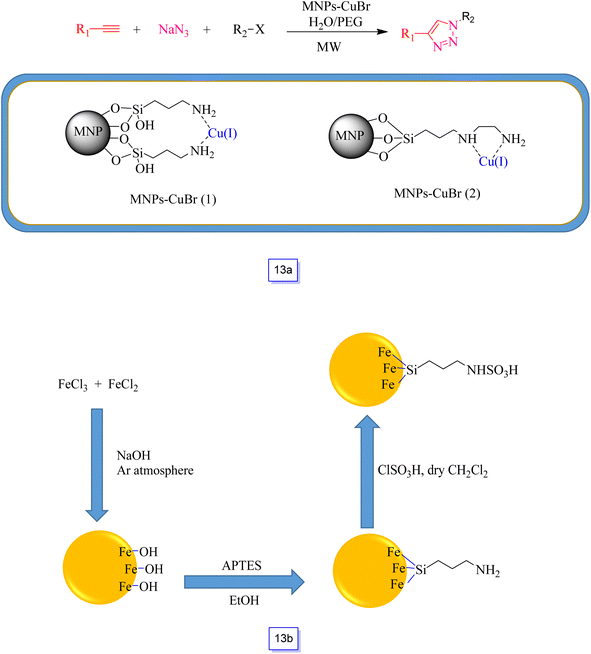 | ||
| Scheme 13 MNP-CuBr-catalyzed one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles (a) and preparation steps for fabricating sulfamic acid-functionalized magnetic Fe3O4 nanoparticles (b). | ||
Safari and colleagues102 reported that on an amino-functionalized Fe3O4 nanoparticle surface, chlorosulfuric acid could be immobilized. The preparation of this material includes the operation of Fe3O4 with 3-aminopropyltriethoxysilane (APTES) through a silanization reaction, followed by the reaction with chlorosulfuric acid (Scheme 13b).
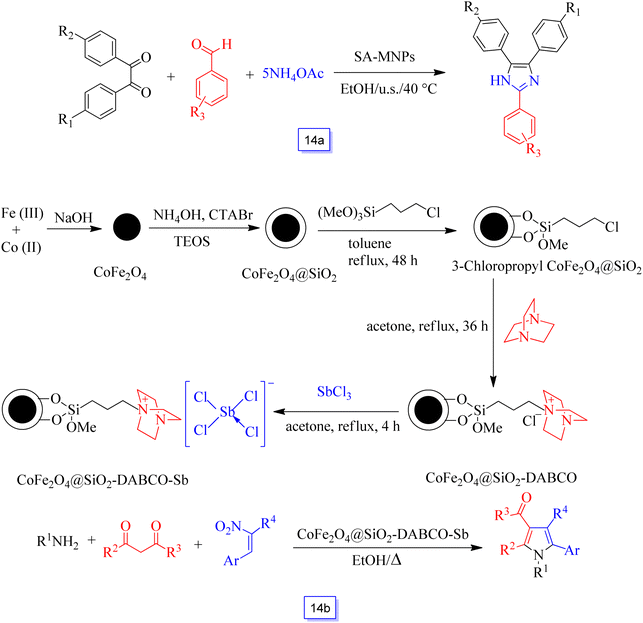
This acid catalyst (SA-MNPs) was then tested for the one-pot synthesis of 2,4,5-trisubstituted imidazoles through a three-component condensation of aldehyde and NH4OAc with 1,2-diketone under sonication (Scheme 14a). This new heterogeneous catalyst also has the capability to sustain a broad type of substitution in the reagents. This nanocatalyst was recycled from the reaction mixture using a magnet, and the recovered catalyst could be applied in five continuous cycles without any remarkable decrease in efficiency.
Multi-substituted pyrroles were synthesized in the presence of CoFe2O4 magnetic nanoparticle-supported Sb ([CoFe2O4@SiO2-DABCO-Sb]) as an effective nanocatalyst (Scheme 15) by Li et al.103 4-H-Pyrans were prepared by the reaction of amines, nitroolefins, and 1,3-dicarbonyl compounds in good yields in short times. Furthermore, a variety of structurally diverse salicylaldehydes reacted favorably with active nucleophilic reagents to produce favorable coumarins in good to excellent yields. This CoFe2O4 nanoparticle-supported Sb catalyst was recycled after five continuous runs without a decrease in its catalytic performance.
Zhang and co-workers104 prepared a CoFe2O4 magnetic nanoparticle-supported molybdenum catalyst ([CoFe2O4@SiO2-PrNH2-Mo(acac)2]), which demonstrated a promising catalytic performance for the one-pot synthesis of multi-substituted pyrroles via reaction of the four-component reaction of amines, aldehydes, nitromethane, and 1,3-dicarbonyl compounds (Scheme 15a). This catalyst could be readily recycled by using a magnet and reutilized five cycles without a notable decrease in activity.38
Recent studies have shown that magnetic nanostructures can act as unique supports for ionic liquids (ILs) owing to their excellent stability, simple synthesis and high applicability, large surface area and simple isolation, and low toxicity and low cost.105
Zolfigol et al.105 demonstrated the stabilization of ionic liquid on silica coated on Fe3O4 surface {Fe3O4@SiO2@(CH2)3Im}C(CN)3 as a new heterogeneous catalyst for the preparation of polyhydroquinoline derivatives using the condensation of dimedone, ethyl acetoacetate, ammonium acetate, and a wide range of aryl aldehydes under green, mild, and solvent-free conditions (Scheme 15b and c).
All starting materials (aryl aldehydes such as those bearing electron-withdrawing and electron-donating groups and halogens) reacted with each other using catalyst MNPs@IL to produce the intended products in good yields in shorter reaction times. The nanocatalyst was reutilized for eight cycles. The obtained results show that the catalytic efficiency of this catalyst was recovered without any significant variations in the performance.
Maleki et al.106 reported that MNP-urea nanostructure could be applied as a new magnetic nanocatalyst for the preparation of substituted imidazoles (Scheme 16a). The compounds were prepared in high yield through the three-component reaction of benzoin or benzil and differently substituted aldehydes with ammonium acetate in the one-pot procedure to produce the intended imidazoles in the presence of Fe3O4/SiO2–urea nanocatalyst under refluxing conditions (Scheme 16b). To study the recyclability of this catalyst, after the end of the reaction, the nanocatalyst was easily isolated from the reaction mixture, washed, air-dried, and reused for a minimum of five runs. The reusability of the catalyst without significant loss in catalytic activity shows excellent and practical recoverability.
The chemical immobilization of Preyssler heteropolyacid (H14 [NaP5W30O110]) onto the Fe3O4 nanoparticles surface modified with guanidine-propyl-trimethoxysilane linker as an organic–inorganic hybrid nanocatalyst (Fe@Si-Gu-Prs) was achieved by Eshghi and co-workers107 and successfully used for the synthesis tetrahydropyridine. Therefore, the reaction of amines, aldehydes, and ethyl acetoacetate at ambient temperature and under solvent-free conditions provided excellent performance (Scheme 16c). A wide range of amines and aldehydes with substituents, either electron-withdrawing or electron-donating groups, favorably reacted via this procedure, and products were obtained in high yields (higher than 90%) following short reaction times. The nanocatalyst was recovered and reused for 5 cycles without any loss of catalytic efficiency.
Shaterian and Moradi108 reported a simple procedure for the one-pot preparation of 7-amino-1,3-dioxo-1,2,3,5-tetrahydropyrazolo[1,2-a][1,2,4]triazole derivatives from the three-component reaction of 4-phenylurazole, aryl aldehydes, and malononitrile by using (3-aminopropyl)-triethoxysilane supported on Fe3O4 surface as a nanocatalyst (Scheme 17a). In addition, the nanocatalyst could be recovered several times without any appreciable loss in the yield.
Saberi et al.109 represented the stabilization of glucose onto nanoparticles of Fe3O4–silica coated@functionalized(3-aminopropyl)triethoxysilane to immobilize copper salts (Scheme 17b). They studied its utilization as a new magnetic nanocatalyst in the one-pot synthesis of 1,3-dipolar cycloaddition of phenylacetylene to azides in H2O, as a green solvent, through a three-component reaction of alkyl halides, alkynes, and sodium azide (Scheme 17c). The main advantage of this approach is the use of glucose as an efficient and green ligand that causes the catalyst to disperse in water by forming hydrogen bonds. Next, the catalyst was almost completely separated using an external magnet, and it was applied for seven further runs without loss in catalytic activity.
Fe3O4@SiO2/collagen as a new magnetic nanocatalyst for the preparation of benzothiazole and benzimidazole derivatives in ethanol was studied by Ghafuri and coworkers110 (Scheme 18a). The synthesized Fe3O4@SiO2 was connected to collagen for the preparation of Fe3O4@SiO2/collagen. Collagen in Fe3O4@SiO2/collagen nanocatalyst has various functional groups that can create hydrogen bonds with protic compounds. Moreover, organic chains of collagen fibers can react with aprotic parts of organic compounds. This property has a principal role in the reaction times and yields in interaction with collagen existing in the nanocatalyst.111–113
One of the most important features of this nanocomposite is its unique properties and excellent selectivity (Scheme 18b). These nanocatalysts were magnetically isolated from the reaction media and reused for several continuous runs without a significant decrease in catalytic efficiency.
Monadi et al.114 reported a new procedure for the covalent attachment of a molybdenum Schiff base complex on the surface of silica covered with nanoparticles (Fe3O4@SiO2) (Scheme 19a).
 | ||
| Scheme 19 Preparation of Fe3O4@SiO2@Mo-Schiff base (a), and synthesis of 2-amino-4H-benzo[h]chromenes in the presence of Fe3O4@SiO2@Mo-Schiff base at 125 °C under solvent free conditions (b). | ||
In addition, the catalytic activity of Fe3O4@SiO2@Mo-Schiff base was investigated via three-component reactions between various substituted aldehydes, 1-naphthol, and malononitrile in one-pot synthesis to afford the corresponding 2-amino-4H-benzo[h]chromenes using Fe3O4@SiO2@Mo-Schiff base nanocatalyst under solvent-free and moderate conditions in good yields (Scheme 19b). After the reaction was completed, the nanocatalyst was collected from the reaction media by an external magnetic field. The nanoparticles were separated and washed with water and ethanol several times and reused for four cycles without a significant decrease in catalytic performance.
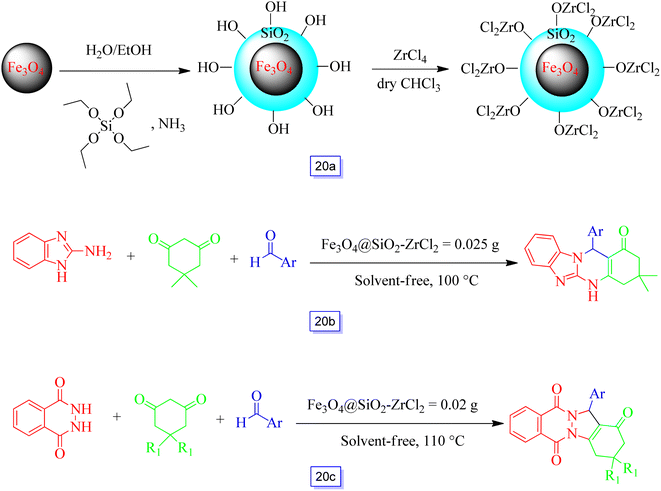
Shirini and colleagues115 prepared an environmentally beneficial and re-utilizable solid acid catalyst by immobilizing ZrCl2 onto silica-coated ferrite nanoparticles (Scheme 20a) for the efficient promotion of this nanocatalyst in solvent-free conditions for the preparation of tetrahydrobenzimidazo[2,1-b]quinazolin-1(2H)-ones and 2H-indazolo[2,1-b]phthalazine-triones (Scheme 20b and c). The prepared nanocatalyst was separated by magnetic isolation and reutilized for eight runs with just a small loss of performance, meaning that the procedure was practical and provided good yields.
Manouchehr Mamaghani et al.116 reported a new HAp-encapsulated γ-Fe2O3-supported, as a dual acidic heterogeneous, re-utilizable, and very effective (Scheme 21a) catalyst. Its application for the one-pot synthesis of 3-pyranylindole and benzoxanthenone derivatives through three-component reactions (Scheme 21b). In this procedure, the utilization of the nanocatalyst presented a beneficial, green, and fast process to fabricate products in shorter reaction times (4–20 min) and good yields (87–96%). The paramagnetic property of this nanocatalyst enabled an easy, facile handling and simple procedure for the isolation of the catalyst by employing an external magnetic field, and it could be applied in 8 runs without a considerable decrease in catalytic performance.
Mohammad Ali Zolfigol et al.117 prepared a new and task-specific Schiff base ligand connected to 2-aminoethyl dihydrogen phosphate as a spacer immobilized on the Fe3O4 nanoparticle surface (Scheme 22a). The resulting nanostructure was successfully applied as a Pd-supported nanocatalyst for Sonogashira and Mizoroki–Heck reactions (Scheme 22b). Therefore, this is the first study of the preparation and uses of Fe3O4@O2PO2(CH2)2NH2 nanoparticles as an appropriate spacer for the synthesis of an adjustable Schiff base ligand and its corresponding Pd complex.
Hamid Reza Shaterian et al.118 successfully prepared a new magnetic and super acidic nanostructure, i.e., γ-Fe2O3@SiO2 functionalized with the dendrimer sulfonic acid as a new reusable and heterogeneous nanocatalyst (Scheme 23a). They have investigated the catalytic efficiency of the catalyst for efficient, facile, and one-pot preparation of 2-hydroxy-1,4-naphthoquinone derivatives through a three-component reaction of aromatic aldehydes, 2-hydroxynaphthalene-1,4-dione, and aniline derivatives (Scheme 23b). The advantages of this study are excellent yields, waste-free, low reaction time, room temperature, solvent-free, and mild conditions.
Ramin Ghorbani-Vaghei et al.119 prepared a novel magnetic catalyst via the reaction of silanol groups (Si–OH), on the Fe3O4@SiO2 nanoparticles surface, with (3-chloropropyl) triethoxysilane followed by hexamethylenetetramine (HMTA) and then chlorosulfonic acid (Scheme 24a). Its catalytic performance was studied in the preparation of pyranopyrazole compounds (Scheme 24b). The products were produced in good to excellent yields within quick times under quite green conditions. Therefore, due to the simple and inexpensive method for the synthesis of this nanocatalyst and several other features, including high catalytic performance, easy isolation by applying a magnetic field, and good recoverability, it may have bright prospects in organic synthesis.
 | ||
| Scheme 24 Preparation of Fe3O4@SiO2-HMTA-SO3H MNPs (a), and preparation of pyranopyrazole derivatives in the presence of Fe3O4@SiO2-HMTA-SO3H at room temperature under solvent free conditions (b). | ||
Ramin Ghorbani-Vaghei et al.120 reported the immobilization of 7-aminonaphthalene-1,3-disulfonic acid (ANDSA) on Fe3O4@SiO2 nanostructure surface (Fe3O4@SiO2@propyl-ANDSA) (Scheme 25a). Then, its catalytic performance was studied in the preparation of derivatives of tetrahydrobenzo[h]tetrazolo[5,1-b]quinazolines and tetrahydrotetrazolo[1,5-a]quinazolines using the one-pot reaction of 5-aminotetrazole, aldehydes, and dimedone or 6-methoxy-3,4-dihyronaphthalen-1(2H)-one in H2O/EtOH at 100 °C (Scheme 25b). High product yields, simple purification, short reaction times, recoverability of the nanocatalyst, and simple procedure are the several advantages. There are two SO3H groups in the catalyst, which presented efficient acidic sites, resulting in the high performance of the nanocatalyst.
 | ||
| Scheme 25 Stepwise synthesis pathway of Fe3O4@SiO2@propyl-ANDSA catalyst (a), and one-pot synthesis of tetrahydrotetrazolo[1,5-a]quinazolines and tetrahydrobenzo[h]tetrazolo[5,1-b]quinazolines (b). | ||
Also, Ramin Ghorbani-Vaghei et al.,121 using the nanocatalyst mentioned above (Scheme 25a), described a new, easy, effective, one-pot three-component method. This procedure prepared the substituted pyrimido[4,5-d]pyrimidines through the reaction of isothiocyanate, N,N-dimethyl-6-amino uracil, and aromatic aldehydes using water as a green solvent and without applying any other toxic organic agents (Scheme 26a). In comparison with other methods, using these hybrid inorganic–organic heterogeneous catalysts can assist in obtaining a green approach, high catalytic efficiency, simple recovery with a magnetic field, and quick reaction times. Ghorbani-Vaghei et al.122 prepared 7-aminonaphthalene-1,3-disulfonic acid immobilized on Fe3O4 nanoparticle surface as a nanocatalyst for the one-pot and multi-component synthesis of substituted 3-pyrrolin-2-ones without the usage of any toxic organic compounds (Scheme 26b). Excellent catalytic efficiency, simple recovery, and ability to be reutilized several times without considerable loss of its catalytic performance using a magnetic field are the environmentally-friendly features of this catalytic method.
Arash Ghorbani-Choghamarani et al.123 reported Fe3O4–Schiff base of Cu(II) (Scheme 27a) as a reusable and heterogeneous nanocatalyst for the fast and effective synthesis of several 2,3-dihydroquinazolin-4(1H)-one derivatives from the condensation of two-component reaction of aldehyde and 2-aminobenzamide (Scheme 27b). This procedure is easy, green, and inexpensive. Isolation and recovery can also be efficiently performed with magnetic separation of the Fe3O4 nanoparticles.
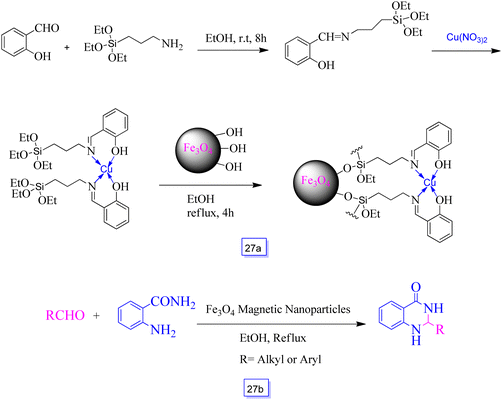 | ||
| Scheme 27 Synthesis of Fe3O4–Schiff base of Cu(II) (a) and synthesis of 2,3-dihydroquinazolin-4(1H)-ones in the presence of Fe3O4 magnetic nanoparticles and ethanol solvent at reflux conditions (b). | ||
Ramin Ghorbani-Vaghei et al.124 reported three separate types of catalysts, i.e., poly(N,N′-dibromo-N-ethylbenzene-1,3-disulfonamide) [PBBS], N,N,N′,N′-tetrabromobenzene-1,3-disulfonamide [TBBDA] (Scheme 28a), and a combination of TBBDA and Fe3O4 nanoparticles functionalized with 4-amino-pyridine supported on silica surface (MNPs@SiO2-Pr-AP) (Scheme 28b). This catalyst was used for the preparation of 2-amino-4-aryl thiazole derivatives via the reaction of thiourea and substituted acetophenones (Scheme 28c). The experiments exhibited that the application of TBBDA along with MNPs@SiO2-Pr-AP provides high yields of the products in the quickest reaction time.
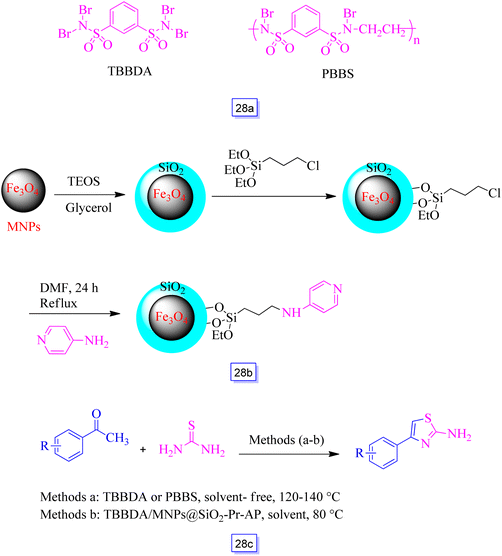 | ||
| Scheme 28 The synthetic route of the nanocatalyst and the structure of TBBDA and PBBS (a), synthetic route of nanocatalyst (b), and one-pot synthesis of 2-amino-4-arylthiazole derivatives (c). | ||
Ramin Ghorbani-Vaghei et al.125 reported Fe3O4 functionalized with piperidinium benzene-1,3-disulfonate as an easy, green, and effective nanocatalyst for the one-pot preparation of pyrano[2,3-c]pyrazole derivatives obtained from the four component reaction between malononitrile, aryl aldehydes, ethyl acetoacetate, and hydrazine hydrate in water at 60 °C (Scheme 29). The Fe3O4@SiO2 nanoparticle supported IL was prepared. This procedure provided benefits such as less reaction time, clean reaction, simple purification, good to high yields, and easily recyclable catalyst.
5. Conclusion
During the past few decades, studies on various kinds of magnetic nanostructured catalysts and their utilization in different organic reactions have achieved many successes. Nanocatalysis with magnetic reusability is a quickly growing field in the context of the high requirements for the progress of green chemistry. Many magnetically recyclable catalysts were utilized in various reactions, including Sonogashira, Heck, Suzuki, Hiyama, hydrogenation, alkyne–azide cycloaddition, oxidation, reduction, epoxidation of alkenes, arylation, alkylation, Fenton-like reaction, and multicomponent “one-pot” synthesis. To avoid aggregation and obtain grafting catalyst varieties on prepared MNPs, functionalization and modification of MNPs with immobilizing ligands or encapsulating/coating substances (such as silica, small molecules, carbon, polymers, mesoporous materials, ionic liquids, carbon nanotubes, and graphene) are necessary. The advantages of using these MNPs as catalysts include good to excellent yields of the products, simple work-up procedure, quick reaction times, and recyclability of the nanocatalysts in most cases. This particular review only covers a segment of the applications of magnetic nanomaterials as efficient catalysts in the field of different multicomponent reactions. Nevertheless, they have tremendous potential in several other fields, including biomedical therapeutics and biotechnology. In the future, we will be focusing on unveiling the bio applications of these diverse magnetic nanomaterials.Conflicts of interest
There are no conflicts to declare.References
- R. W. Armstrong, A. P. Cmbs, P. A. Tempest, S. D. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123–131 CrossRef CAS.
- M. D. Burke and S. L. Schreiber, Angew. Chem., Int. Ed., 2004, 43, 46–58 CrossRef PubMed.
- (a) M. Ghorbani, S. Noura, M. Oftadeh, M. A. Zolfigol and M. Haji Soleimani, J. Mol. Liq., 2015, 212, 291 CrossRef CAS; (b) W. Szymanski, M. Zwolinska and R. Ostaszewski, Tetrahedron, 2007, 63, 7647 CrossRef CAS.
- (a) A. Azarifar, R. Nejat-Yami and D. Azarifar, J. Iran. Chem. Soc., 2013, 10, 297 CrossRef CAS; (b) D. Azarifar, S. M. Khatami and Z. Najminezhad, J. Iran. Chem. Soc., 2014, 11, 587 CrossRef CAS.
- A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef.
- J. Zhu and H. Bienaymé, Multicomponent Reactions, John Wiley & Sons Inc., New York, 2006 Search PubMed.
- A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed.
- G. H. Posner, Chem. Rev., 1986, 86, 831 CrossRef CAS.
- R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123 CrossRef CAS.
- (a) A. Dömling and I. Ugi, Multicomponent reactions with isocyanides, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef; (b) A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (c) C. I. Herrerias, X. Yao, Z. Li and C. Li, Reactions of C-H bonds in water, Chem. Rev., 2007, 107, 2546 CrossRef CAS PubMed.
- S. B. Gawande, P. S. Brancoa and R. S. Varma, ChemSusChem, 2012, 5, 65 CrossRef PubMed.
- J. Wang, S. A. Lee, H. W. Jang and M. Shokouhimehr, Langmuir, 2022, 38, 9064–9072 CrossRef CAS PubMed.
- K. Zhang, J. Kim, K. O. Kirlikovali, J. Wang, T. H. Lee, S. Y. Kim, R. S. Varma, H. W. Jang, O. K. Farha and M. Shokouhimehr, Mol. Catal., 2022, 528, 112459 CrossRef CAS.
- M. Nasrollahzadeh, N. Motahharifar, M. Sajjadi, A. Naserimanesh and M. Shokouhimehr, Inorg. Chem. Commun., 2022, 136, 109135 CrossRef CAS.
- H. Alamgholiloo, N. N. Pesyan, R. Mohammadi, S. Rostamnia and M. Shokouhimehr, J. Environ. Chem. Eng., 2021, 9, 105486 CrossRef CAS.
- M. Shokouhimehr, K. Hong, T. H. Lee and C. W. Moon, Green Chem., 2018, 20, 3809–3817 RSC.
- K. H. Choi, M. Shokouhimehr and Y. E. Sung, Bull. Korean Chem. Soc., 2013, 34, 1477–1480 CrossRef CAS.
- J. Wang, W. S. Cheon, J. Y. Lee, W. Yan and S. Jung, Dalton Trans., 2023, 52, 3567–3574 RSC.
- Nanoparticles and Catalysis, ed. D. Astruc, Wiley-VCH, Weinheim, 2008 Search PubMed.
- G. A. Somorjai, H. Frei and J. Y. Park, J. Am. Chem. Soc., 2009, 131, 16589 CrossRef CAS PubMed.
- M. Benaglia, Recoverable and Recyclable Catalysts, John Wiley & Sons, Chichester, 2009 Search PubMed.
- S. Wittmann, A. Sh€atz, R. N. Grass, W. J. Stark and O. Reiser, Angew. Chem., Int. Ed., 2010, 49, 1867 CrossRef CAS PubMed.
- C. Coperet, M. Chabanas, R. P. Saint-Arroman and J. M. Basset, Angew. Chem., Int. Ed., 2003, 42, 156 CrossRef CAS PubMed.
- J.-M. Basset, C. Coperet, D. Soulivong, M. Taoufik and J. Thivolle-Cazat, Acc. Chem. Res., 2010, 43, 323 CrossRef CAS PubMed.
- A.-H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- Y. Zhu, L. P. Stubbs, F. Ho, R. Liu, C. P. Ship, J. A. Maguire and N. S. Hosmane, ChemCatChem, 2010, 2, 365 CrossRef CAS.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed.
- L. M. Rossi, M. A. S. Garcia and L. L. R. Vono, J. Braz. Chem. Soc., 2012, 23, 1959 CAS.
- R. B. N. Baig and R. S. Varma, Chem. Commun., 2013, 49, 752 RSC.
- V. Polshettiwar and R. S. Varma, Green Chem., 2010, 12, 743 RSC.
- S. Shylesh, V. Sch€unemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428 CrossRef CAS PubMed.
- A. H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS PubMed.
- N. Lee and T. Hyeon, Chem. Soc. Rev., 2012, 41, 2575–2589 RSC.
- D. Le Sage, K. Arai, D. R. Glenn, S. J. DeVience, L. M. Pham, L. Rahn-Lee, M. D. Lukin, A. Yacoby, A. Komeili and R. L. Walsworth, Nature, 2013, 496, 486–489 CrossRef CAS PubMed.
- P. Moroz, S. K. Jones and B. N. Gray, Int. J. Hyperthermia, 2002, 18, 267–284 CrossRef CAS PubMed.
- A. Tomitaka, T. Yamada and Y. Takemura, J. Nanomater., 2012, 2012, 1–5 CrossRef.
- Q. Zhang, J. Tong, H. Chen, L. Jiang, H. Zhu, X. Zhu, H. Yu, J. Liu and B. Liu, J. Nanosci. Nanotechnol., 2012, 12, 127–131 CrossRef PubMed.
- C. Binns, in Nanostructured Materials for Magnetoelectronics, ed. B. Aktaş and F. Mikailzade, Springer, Berlin, Heidelberg, 2013, ch. 8, vol. 175, pp. 197–215 Search PubMed.
- S. Shylesh, V. Schünemann and W. R. Thiel, Angew. Chem., Int. Ed., 2010, 49, 3428–3459 CrossRef CAS PubMed.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- A. Dhakshinamoorthy, S. Navalon, M. Alvaro and H. Garcia, ChemSusChem, 2012, 5, 46–64 CrossRef CAS PubMed.
- C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412–434 CrossRef CAS.
- D. Zhang, C. Zhou, Z. Sun, L.-Z. Wu, C.-H. Tung and T. Zhang, Nanoscale, 2012, 4, 6244–6255 RSC.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J.-M. Basset, Chem. Rev., 2011, 111, 3036–3075 CrossRef CAS PubMed.
- R. B. N. Baig and R. S. Varma, Chem. Commun., 2013, 49, 752–770 RSC.
- H.-J. Xu, X. Wan, Y. Geng and X.-L. Xu, Curr. Org. Chem., 2013, 17, 1034–1050 CrossRef CAS.
- S. Byun, J. Chung, Y. Jang, J. Kwon, T. Hyeon and B. M. Kim, RSC Adv., 2013, 3, 16296–16299 RSC.
- Y.-H. Liu, J. Deng, J.-W. Gao and Z.-H. Zhang, Adv. Synth. Catal., 2012, 354, 441–447 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, Org. Biomol. Chem., 2009, 7, 37–40 RSC.
- A.-H. Lu, W. Schmidt, N. Matoussevitch, H. Bönnemann, B. Spliethoff, B. Tesche, E. Bill, W. Kiefer and F. Schüth, Angew. Chem., 2004, 116, 4403–4406 CrossRef.
- B. Panella, A. Vargas and A. Baiker, J. Catal., 2009, 261, 88–93 CrossRef CAS.
- R. Arundhathi, D. Damodara, P. R. Likhar, M. L. Kantam, P. Saravanan, T. Magdaleno and S. H. Kwon, Adv. Synth. Catal., 2011, 353, 1591–1600 CrossRef CAS.
- J. Liu, X. Peng, W. Sun, Y. Zhao and C. Xia, Org. Lett., 2008, 10, 3933–3936 CrossRef CAS PubMed.
- R. Luque, B. Baruwati and R. S. Varma, Green Chem., 2010, 12, 1540–1543 RSC.
- R. B. Nasir Baig and R. S. Varma, Green Chem., 2012, 14, 625–632 RSC.
- D. Wang, L. Salmon, J. Ruiz and D. Astruc, Chem. Commun., 2013, 49, 6956–6958 RSC.
- S. J. Hoseini, H. Nasrabadi, M. Azizi, A. S. Beni and R. Khalifeh, Synth. Commun., 2012, 43, 1683–1691 CrossRef.
- R. Parella, M. Naveen, A. Kumar and S. A. Babu, Tetrahedron Lett., 2013, 54, 1738–1742 CrossRef CAS.
- J. S. Chen, C. Chen, J. Liu, R. Xu, S. Z. Qiao and X. W. Lou, Chem. Commun., 2011, 47, 2631–2633 RSC.
- G. Li and L. Mao, RSC Adv., 2012, 2, 5108–5111 RSC.
- S. Xuan, W. Jiang, X. Gong, Y. Hu and Z. Chen, J. Phys. Chem. C, 2008, 113, 553–558 CrossRef.
- (a) A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS PubMed; (b) C. Che, W. Z. Li, S. Y. Lin, J. W. Chen, J. Zheng, J. C. Wu, Q. X. Zheng, G. Q. Zhang, Z. Yang and B. W. Jiang, Chem. Commun., 2009, 5990 RSC; (c) X. J. Wu, R. Jiang, B. Wu, X. M. Su, X. P. Xu and S. J. Ji, Adv. Synth. Catal., 2009, 351, 3150 CrossRef CAS.
- (a) G. Chouhan, D. Wang and H. Alper, Chem. Commun., 2007, 4809 RSC; (b) J. Ge, Q. Zhang, T. Zhang and Y. Yin, Angew. Chem., Int. Ed., 2008, 47, 8924 CrossRef CAS PubMed; (c) D.-H. Zhang, G.-D. Li, J.-X. Lia and J.-S. Chen, Chem. Commun., 2008, 3414 RSC.
- (a) V. Polshettiwar, B. Baruwati and R. S. Varma, Chem. Commun., 2009, 1837 RSC; (b) V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC; (c) V. Polshettiwar and R. S. Varma, Chem.–Eur. J., 2009, 15, 1582 CrossRef CAS PubMed.
- (a) Y. Zhanga, Z. Lia, W. Suna and C. Xia, Catal. Commun., 2008, 10, 237 CrossRef; (b) M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735 RSC; (c) L. M. Rossi, F. P. Silva, L. L. R. Vono, P. K. Kiyohara, E. L. Duarte, R. Itri, R. Landers and G. Machado, Green Chem., 2007, 9, 379 RSC.
- (a) W. I. Gilbert, J. Turkevich and E. S. Wallis, J. Org. Chem., 1938, 3, 611 CrossRef; (b) L. S. Glebov, G. A. Kliger, T. P. Popova, V. E. Shiryaeva, V. P. Ryzhikov, E. V. Marchevskaya, O. A. Lesik, S. M. Loktev and V. G. Beryezkin, J. Mol. Catal. A: Chem., 1986, 35, 335 CrossRef CAS.
- (a) Q. Liu, W. Ma, R. He and Z. Mu, Catal. Today, 2005, 106, 52 CrossRef CAS; (b) A. SchPle, U. Nieken, O. Shekhah, W. Ranke, R. Schlçgl and G. Kolios, Phys. Chem. Chem. Phys., 2007, 9, 3619 RSC.
- (a) J. Liang, Q. Zhang, H. Wu, G. Meng, Q. Tang and Y. Wang, Catal. Commun., 2004, 5, 665 CrossRef CAS; (b) D. Guin, B. Baruwati and S. V. Manorama, J. Mol. Catal. A: Chem., 2005, 242, 26 CrossRef CAS.
- (a) M. M. Mojtahedi, M. S. Abaee and T. Alishiri, Tetrahedron Lett., 2009, 50, 2322 CrossRef CAS; (b) B. Sreedhar, A. S. Kumar and P. S. Reddy, Tetrahedron Lett., 2009, 50, 2322 CrossRef.
- (a) T. Q. Zeng, W.-W. Chen, C. M. Cirtiu, A. Moores, G. Song and C.-J. Li, Green Chem., 2010, 12, 570 RSC; (b) Z.-H. Zhang, H.-Y. Lu, S.-H. Yang and J.-W. Gao, J. Comb. Chem., 2010, 12, 643 CrossRef CAS PubMed.
- L. Lartigue, K. Oumzil, Y. Guari, J. Larionova, C. Guerin, J. L. Montero, V. Barragan- Montero, C. Sangregorio, A. Caneschi, C. Innocenti, T. Kalaivani, P. Arosio and A. Lascialfari, Org. Lett., 2009, 11, 2992 CrossRef CAS PubMed.
- M. M. Mojtahedi, M. S. Abaee and M. Eghtedari, Appl. Organomet. Chem., 2008, 22, 529 CrossRef CAS.
- V. Polshettiwar, R. Luque, A. Fihri, H. Zhu, M. Bouhrara and J. M. Basset, Chem. Rev., 2011, 111, 3036 CrossRef CAS PubMed.
- H. Firouzabadi, N. Iranpoor, M. Gholinejad and S. J. Hoseini, Adv. Synth. Catal., 2011, 353, 125 CrossRef CAS.
- Z.-H. Zhang, H.-Y. Lu, S.-H. Yang and J.-W. Gao, J. Comb. Chem., 2010, 12, 643 CrossRef CAS PubMed.
- B. V. Subba Reddy, A. Siva Krishna, A. V. Ganesh and G. G. K. S. Narayana Kumar, Tetrahedron Lett., 2011, 52, 1359 CrossRef.
- M. Nasr-Esfahani, S. J. Hoseini and F. Mohammadi, Chin. J. Catal., 2011, 32, 1484 CrossRef CAS.
- N. Koukabi, E. Kolvari, A. Khazaei, M. A. Zolfigol, B. Shirmardi-Shaghasemi and H. R. Khavasi, Chem. Commun., 2011, 47, 9230 RSC.
- S. Rostamizadeh, M. Nojavan, R. Aryan, H. Sadeghian and M. Davoodnejad, Chin. Chem. Lett., 2013, 24, 629–632 CrossRef CAS.
- A. Dandia, A. K. Jain and S. Sharma, RSC Adv., 2013, 3, 2924 RSC.
- A. Bazgir, G. Hosseini and R. Ghahremanzadeh, ACS Comb. Sci., 2013, 15, 530 CrossRef CAS PubMed.
- K. Pradhan, S. Paul and A. R. Das, Catal. Sci. Technol., 2014, 4, 822–831 RSC.
- K. Pradhan, S. Paul and A. R. Das, Catal. Sci. Technol., 2014, 4, 822–831 RSC.
- A. Khazaei, A. Ranjbaran, F. Abbasi, M. Khazaeia and A. R. Moosavi-Zareb, RSC Adv., 2015, 5, 13643 RSC.
- K. Debnath and A. Pramanik, Tetrahedron Lett., 2015, 56, 1654–1660 CrossRef CAS.
- (a) A. Goldman, Modern Ferrite Technology, Van Nostrand Reinhold, New York, 1990 Search PubMed; (b) H. T. Jiang, X. F. Wang and C. L. Yu, et al., Mater. Manuf. Processes, 2010, 25, 1489–1493 CrossRef CAS; (c) S. Dey, R. Gomes, R. Mondal, S. K. Dey, P. Dasgupta, A. Poddar, V. R. Reddy, A. Bhaumik and S. Kumar, RSC Adv., 2015, 5, 78508–78518 RSC.
- A. Khazaei, H. A. Alavi, A. Ranjbarana and A. R. Moosavi, RSC Adv., 2016, 6, 78881 RSC.
- M. Baghernejad, S. Khodabakhshi and S. Tajikb, New J. Chem., 2016, 40, 2704–2709 RSC.
- U. Gulati, S. Rawat, C. Rajesh and D. S. Rawat, New J. Chem., 2017, 41, 8341 RSC.
- B. Kaboudin, Y. Abedia and T. Yokomatsu, Org. Biomol. Chem., 2012, 10, 4543 RSC.
- B. Kaboudin, R. Mostafalua and T. Yokomatsu, Green Chem., 2013, 15, 2266 RSC.
- K. Azizi and A. Heydari, RSC Adv., 2014, 4, 6508 RSC.
- Z. Zarnegar and J. Safari, RSC Adv., 2014, 4, 20932–20939 RSC.
- M. Pirhayati, A. Kakanejadifard and H. Veisi, Appl. Organomet. Chem., 2016, 30(12), 1004–1008 CrossRef CAS.
- A. Shaabani, R. Afshari, S. E. Hooshmand, A. T. Tabatabaei and F. Hajishaabanha, RSC Adv., 2016, 6, 18113 RSC.
- D. Azarifar and M. Ghaemi, Appl. Organomet. Chem., 2017, e3834 CrossRef.
- S. Zolfagharinia, E. Kolvari, N. Koukabi and M. M. Hosseini, Arabian J. Chem., 2017, 13, 227–241 CrossRef.
- A. Maleki, Tetrahedron, 2012, 68, 7827–7833 CrossRef CAS.
- A. R. Kiasat and J. Davarpanah, J. Mol. Catal. A: Chem., 2013, 373, 46–54 CrossRef CAS.
- X. Xiong and L. Cai, Catal. Sci. Technol., 2013, 3, 1301 RSC.
- J. Safari and Z. Zarnegar, Ultrason. Sonochem., 2013, 20, 740–746 CrossRef CAS PubMed.
- B. L. Li, H. C. Hu, L. P. Mo and Z. H. Zhang, RSC Adv., 2014, 4, 12929–12943 RSC.
- B. L. Li, M. Zhang, H. C. Hu, X. Du and Z. H. Zhang, New J. Chem., 2014, 38, 2435–2442 RSC.
- M. Ali Zolfigol and M. Yarie, RSC Adv., 2015, 5, 103617 RSC.
- A. Maleki, Z. Alrezvani and S. Maleki, Catal. Commun., 2015, 69, 29–33 CrossRef CAS.
- H. Eshghi, A. Khojastehnezhad, F. Moeinpour, S. Rezaeian, M. Bakavolia, M. Teymouri, A. Rostami and K. Haghbeen, Tetrahedron, 2015, 71, 436–444 CrossRef CAS.
- H. R. Shaterian and F. Moradi, Res. Chem. Intermed., 2015, 41, 223–229 CrossRef CAS.
- F. M. Moghaddam, V. Saberi, S. Kalhor and S. E. Ayati, RSC Adv., 2016, 6, 80234 RSC.
- H. Ghafuri, E. Esmaili and M. Talebi, C. R. Chim., 2016, 19, 942–950 CrossRef CAS.
- M. Talebi and H. Ghafuri, Ind. Eng. Chem. Res., 2016, 55, 2970–2982 CrossRef CAS.
- Y.-S. Lee, Y.-H. Cho, S. Lee, J.-K. Bin, J. Yang, G. Chae and C.-H. Cheon, Tetrahedron, 2015, 71, 532–538 CrossRef CAS.
- K. Bahrami, M. M. Khodaei and F. Naali, J. Org. Chem., 2008, 73, 6835–6837 CrossRef CAS PubMed.
- N. Divsalar, N. Monadi and M. Tajbaksh, J. Nanostruct., 2016, 6(4), 312–321 CAS.
- F. Kamali and F. Shirini, New J. Chem., 2017, 41, 11778 RSC.
- L. Kheirkhah, M. Mamaghani, A. Yahyazadeh and N. Mahmoodi, Appl. Organomet. Chem., 2018, 32, e4072 CrossRef.
- M. Aghayee, M. A. Zolfigol, H. Keypour, M. Yarie and L. Mohammadi, Appl. Organomet. Chem., 2016, 30(8), 612–618 CrossRef CAS.
- F. Mollazehi and H. R. Shaterian, Appl. Organomet. Chem., 2017, 32, e4183 CrossRef.
- R. Ghorbani-Vaghei and V. Izadkhah, Appl. Organomet. Chem., 2018, 32(2), e4025 Search PubMed.
- R. Ghorbani-Vaghei, S. Alavini and N. Sarmast, Appl. Organomet. Chem., 2018, 32(2), e4038 Search PubMed.
- R. Ghorbani-Vaghei and N. Sarmast, Appl. Organomet. Chem., 2018, 32(2), e4003 Search PubMed.
- R. Ghorbani-Vaghei, N. Sarmast and J. Mahmoodi, Appl. Organomet. Chem., 2017, 31(8), e3681 CrossRef.
- A. Ghorbani-Choghamarani, Z. Darvishnejad and M. Norouzi, Appl. Organomet. Chem., 2015, 29, 707–711 CrossRef CAS.
- R. Ghorbani-Vaghei, S. Alavinia, Z. Merati and V. Izadkhah, Appl. Organomet. Chem., 2018, 32(3), e4127 CrossRef.
- R. Ghorbani-Vaghei, J. Mahmoodi, A. Shahriari and Y. Maghbooli, Appl. Organomet. Chem., 2017, 31(12), e3816 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |