Open Access Article
Open Access ArticleMagneto–structural maps and bridged-ligand effect for dichloro-bridged dinuclear copper(II) complexes: a theoretical perspective†
Shuchang Luo *abc,
Xianwei Shenab,
Peng Gaoab,
Ting Tuab and
Xiaoyuan Sun*ab
*abc,
Xianwei Shenab,
Peng Gaoab,
Ting Tuab and
Xiaoyuan Sun*ab
aCollege of Chemical Engineering, Guizhou University of Engineering Science, Bijie 551700, People's Republic of China
bThe Coal Chemical Engineering, 2011 Collaborative Innovation Center of Guizhou Province, Bijie 551700, People's Republic of China. E-mail: lsc840927@126.com; 253433534@qq.com
cGuizhou Province Key Laboratory of Ecological Protection and Restoration of Typical Plateau Wetlands, Bijie 551700, People's Republic of China
First published on 20th April 2023
Abstract
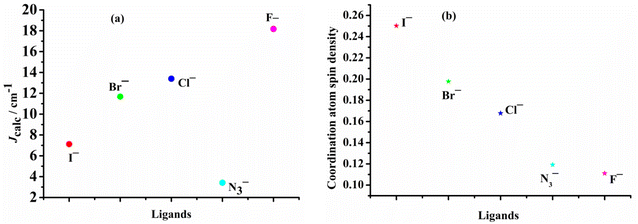
Theoretical understanding of magneto–structural correlations in dichloro-bridged dicopper(II) complexes can guide the design of magnetic materials having broad-scale applications. However, previous reports suggest these correlations are complicated and unclear. To clarify possible correlations, magnetic coupling constants (Jcalc) of variants of a representative {Cu–(μ-Cl)2–Cu} complex A were calculated through BS-DFT. The variation of the Cu–(μ-Cl)–Cu angle (α), Cu⋯Cu distance (R0), and Cu–Cl–Cu–Cl dihedral angle (τ) followed by structural optimization and calculation of the magnetic coupling constant (Jcalc) revealed several trends. Jcalc increased linearly with R0 and τ, and initially increased and then decreased with α. Further, bridging ligand effects on Jcalc for dicopper(II) complexes were evaluated through BS-DFT; the results revealed that Jcalc increased with increasing ligand field strength (I− < Br− < Cl− < N3− < F−). Furthermore, a linear relationship was found between the spin density of the bridging ligand and Jcalc.
Introduction
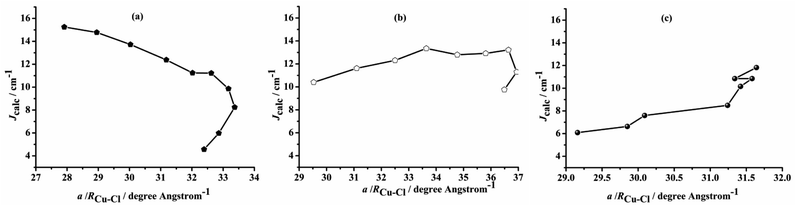
Ligands may tune the structures of biocompatible organometallic magnets, facilitating enzymatic catalysis.1 Bimetallic complexes featuring organic/inorganic bridging ligands catalyze enzymatic or synthetic reactions under mild conditions; however, catalytic structure–property relationships for magnetic interactions between two paramagnetic metal centers require multidisciplinary elucidation, spanning materials science, chemistry, and physics.2,3 Deconvoluting the roles of metals and ligands in the stereoelectronic and magnetic properties of bimetallic active sites would illuminate bimetallic catalyst design principles or enzymatic evolutionary origins. For example, dinuclear Cu complexes are essential for living systems, as oxygen carriers or in active sites for hemocyanin, tyrosinase, Cu oxidase, among others.4–7 Furthermore, synthetic dinuclear paramagnetic Cu(II) chloride complexes that contain {Cu–(μ-Cl)2–Cu} fragments have found spintronic, sewage treatment, and magnetic applications.8–29 While magnetic properties of these complexes feature in their function, analyses of these systems and various synthetic dinuclear Cu(II) complexes have yet to establish structure–magnetic relationships.6,7 Specifically, relationships between exchange interactions between paramagnetic Cu centers, summarized by the magnetic exchange coupling constant (J), and structural parameters for these complexes are poorly understood.25,26 Relevant structural parameters may include the Cu–(μ-Cl)–Cu bond angle (α), Cu–Cl distance (RCu–Cl) or the Cu⋯Cu distance (R0). Notably, at least one pair of non-equivalent RCu–Cl distances are inherent to these complexes, imparting complexity to understanding the magnetic interactions.30–46 Fortunately, these structure–magnetic relationships may be evaluated computationally; Cu(II) complexes are relatively small, Cu(II) paramagnetic centers have minimum spin quantum number (S = 1/2) and may be isotropic.Buvaylo et al.25 and Sikdar et al.26 summarized the experimental correlations among J and α, RCu–Cl, R0 and α/RCu–Cl for paramagnetic {Cu–(μ-X)2–Cu} (X = halide) complexes but found no clear correlations. However, the {Cu–(μ-Cl)2–Cu} complexes they surveyed featured divergent RCu–Cl values (>0.5 Å), and α/RCu–Cl insufficiently describing magneto–structural correlations of {Cu–(μ-Cl)2–Cu} complexes (Table S1 and Fig. S1–S3†). Furthermore, the differences in organic ligands affect bonding, and therefore α, RCu–Cl, R0, making correlations unclear. In addition, when non-chloride halides are used as bridging ligands, participatory ligand p/d orbitals may convolute magnetic exchange interactions.20,21,34 Meanwhile, Ruiz et al.11 studied the relationship between the magnetic coupling constant and the structure of dimeric copper complexes bridged by different halogen ligands and ligands using dichloro-bridged binuclear copper model compounds. Their results showed that when the non-bridging ligand atom was a nitrogen atom, the paramagnetic centers tended to ferromagnetically couple. In chlorine-bridged dimers,16 the ferromagnetic coupling decreases with the increase in the Cu–Cl–Cu bridging angle, while the magnetic coupling constant gradually decreases. However, their studies on the magneto–structural relationship of dichloro-bridged binuclear copper compounds did not correlate parameters such as R0 and α/R0 with the magnetic coupling constants found in the existing experimental data.
In this study, to further explore the magneto–structural relationship in dichloro-bridged binuclear copper complexes, we examined the relationship between these structural parameters and magnetic coupling constants from the aspects of possible structural parameters such as α, R0, Cu–Cl–Cu–Cl dihedral angle (τ), and α/RCu–Cl. Using DFT, we systematically perturbated structural parameters for a representative {Cu–(μ-Cl)2–Cu} complex and calculated the resultant magnetic exchange coupling constants (Jcalc) to gain insight into coupling mechanisms and identify possible correlations. We chose [Cu(PyTn)Cl2]2 (complex A; CCDC: 259315, PyTn = 2-(pyrazol-1-yl)-2-thiazoline), synthesized by Bernalte-García et al.19 Our aim is to effectively describe the magnetic properties of dinuclear copper complexes through theoretical methods and to provide a useful reference for the experimental design and regulation of single-molecule magnets.
Computational details
Complex A is isotropic and low spin (S = 1/2). The Heisenberg–Dirac–van Vleck Hamiltonian (eqn (1)) may express the interaction between two paramagnetic Cu(II) centers:47–50| Ĥ = −2JŜ1·Ŝ2 | (1) |
In this study we estimated Jcalc using a broken-symmetry density functional theory (BS-DFT) method proposed by Noodleman et al.47,48 that is effective for estimating J for various dinuclear and polynuclear complexes.49–67 Detailed technical discussion regarding Jcalc via BS-DFT for dinuclear and trinuclear complexes is available.49,51–54,63–67 Calculations were performed using ORCA 5.0.3 software68,69 using the def2-TZVPP basis set.70,71 Structural optimizations were performed by the M06-2X method.72–74 To optimize the integral calculation speed, we used a resolution of the identity (RI) approximation with a tight self-consistent field (SCF) convergence.75 Magnetic structure calculations were completed in three steps. Complex A was given altered average bond angles (α), Cu⋯Cu distances (R0) and halide ligands (X = Cl, Br, I) to obtain α/RCu–X; complex models were obtained for the role of ligand variation. Then, α or R0 was fixed to partially optimize the model complex. Finally, using the partially optimized structure, the single-point energies of the high-spin state |1/2, 1/2〉 (HS, Cu(II) all spin up, S = 1) and the symmetry-broken state |1/2, −1/2〉 (BS, Cu(1) spin up, Cu(2) spin down, S = 0) were calculated. Eqn (2) was used to obtain Jcalc.76,77
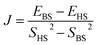 | (2) |
Results and discussion
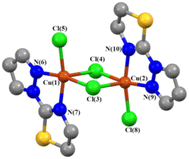
Fig. 1 displays the structure (molecular structures were rendered using VMD software79) of complex A, featuring two paramagnetic Cu(II) centers bridged by two chloride ligands. The Cu–Cl distances are distinct (R(Cu1–Cl3) = R(Cu2–Cl4) = 2.218 Å; R(Cu1–Cl4) = R(Cu2–Cl3) = 2.906 Å), but α for Cu1–Cl3–Cu2 and Cu1–Cl4–Cu2 are equal (88.58°). To interrogate magnetic properties, complex A was optimized at the M06-2X/def2-TZVPP level of theory (Table S2†). Optimization yielded partially averaged RCu–Cl (R(Cu1–Cl3) = R(Cu2–Cl4) = 2.319 Å; R(Cu1–Cl4) = R(Cu2–Cl3) = 2.670 Å) and reduced α for Cu1–Cl3–Cu2 and Cu1–Cl4–Cu2 (85.26°).Evaluation of magnetic coupling constant
Jcalc for the optimized structure of complex A was calculated using BS-DFT, with a zeroth-order canonical approximation (ZORA)80 for consideration of relativistic effects in the ZORA-def2-TZVPP basis set.68,69 The generalized gradient approximation (GGA) and hybrid functional theory (HDFT) were used to investigate the influence of the calculation method on Jcalc (Table S3†). Both methods yielded positive Jcalc, consistent with the measured ferromagnetic interaction. The range for Jcalc using various HDFT methods (O3LYP,81 B3P86,82,83 B3PW91,83,84 B3LYP,83,85 B3LYP*,86 X3LYP,85,87 B1P86,82,83 PBE0,88,89 B1LYP83,85) was relatively narrow (2.45–7.14 cm−1) but does not include the experimental value (Jexpt; Jexpt = 13.73 cm−1). GGA methods (OLYP,81 PBE,89 BP86,82,83 PW91,84 BLYP,82,85 XLYP87) yielded a range of Jcalc (11.63–15.57 cm−1) more consistent with Jexpt. However, the PBE functional yielded most similar Jcalc (12.98 cm−1), implying its suitability for determining Jcalc of complex A.The influence of basis set on Jcalc, was considered by measuring Jcalc of complex A using the PBE functional with various basis sets (ZORA-def2-QZVPP, ZORA-def2-TZVPP, ZORA-def2-TZVP (-f), ZORA-def2-SVP;68,69 Table S4†). These Jcalc were similar (12.54–13.57 cm−1), indicating that the basis sets have little effect on Jcalc of complex A. Despite the ZORA-def2-SVP basis set yielding a Jcalc (13.57 cm−1), the basis set size is insufficient to ensure this was not accidental. Jcalc for ZORA-def2-TZVP (-f) and ZORA-def2-TZVP are identical (13.40 cm−1), but ZORA-def2-TZVP has greater stability and a lower calculated total energy. Accordingly, the PBE/ZORA-def2-TZVP level of theory was used for evaluating magneto–structural correlations for complex A.
Molecular magnetic orbital
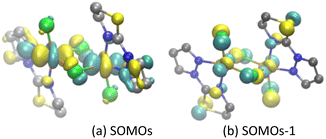
Molecular magnetic orbitals of complex A were analyzed to understand the exchange interaction between Cu(II) centers.57,76,90 Here, the molecular magnetic orbitals of the BS state of the unpaired electron localized on Cu(II) are defined as the local magnetic orbitals (LMOs), and the singly occupied magnetic orbitals of the HS state (SOMOs) are defined as the molecular magnetic orbitals.57,76,90It can be seen from the LMOs diagram that in complex A (Fig. 2), the unpaired electron of Cu(1) and Cu(2) is primarily localized within the 3dx2−y2 orbital (Fig. 2a and b), while the LMO is mixed with a component of the 3dz2 orbital. The p orbitals of the Cl and N atoms of the ligands also participate in LMO formation. The main contributions of the SOMOs and SOMOs−1 molecular magnetic orbitals of complex A come from the 3dx2−y2/3dz2 orbital of the Cu(II) center, the p orbital of the bridging chloride ligand, and the molecular orbitals of the terminal ligand (PyTn) (Fig. 3a and b).
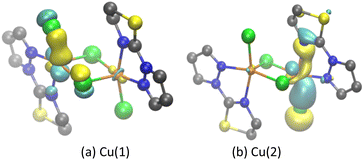 | ||
| Fig. 2 Local molecular magnetic orbitals in BS state of complex A (isovalue: 0.05; yellow (+); cyan (−); (a) the paramagnetic center is Cu(1); (b) the paramagnetic center is Cu(2)). | ||
Mulliken spin population analysis
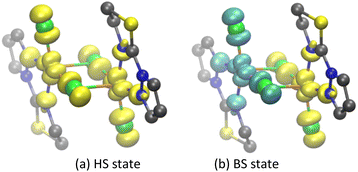
The spin population of complex A was analyzed to evaluate spin densities, which reflect the interaction mechanism between Cu(II) centers.90 This is a spin delocalization mechanism when the paramagnetic centers Cu(1)/Cu(2) and its coordinated atom have the same spin density sign.90 If the spin density sign is opposite, it is a spin polarization mechanism.57,76,90 The spin densities of complex A in the HS and BS states were calculated at the PBE/ZORA-def2-TZVP level of theory to further explore the magnetic coupling mechanism between Cu(II) centers (Table S5† and Fig. 4); positive and yellow represent α spin; negative and cyan represent β spin. Spin delocalization was observed among Cu(1)/Cu(2) and the coordinated N and Cl atoms for both HS and BS states. In the BS state, Cu(1) and Cu(2) each bear spin density from their unpaired electron (−0.49341e and 0.49342e, respectively), delocalized to the coordinated N and Cl atoms, including the bridging Cl(3) (−0.16786e) and Cl(4) (0.16792e) and terminal Cl(5) (−0.18370e), Cl(8) (0.18363e); N(6) (−0.06792e), N(7) (−0.0 06776e), N(9) (0.06793e) and N(10) (0.06776e) atoms. In the BS state, the distribution of spin electrons in each atomic orbital (Table S6†) shows 3d orbitals contain the greatest proportion of spin density (e.g., Cu(1); 3d, −0.50894e; 4s, 0.00989e; 4p, 0.00566e). The spin densities of the bridging chloride ligands (−0.18370e and 0.18363e) are primarily centered in p orbitals (−0.18206e and 0.18200e, respectively). Furthermore, natural bond orbital (NBO) analysis of complex A shows76,91 electron configurations of [core]4s(0.42)3d(9.45)4p(0.03)4d(0.01) for Cu(II), as opposed to [core]3s(1.97)3p(5.66)3d(0.01) for bridging chloride ligands. These data suggest delocalization of Cu(II) spin density by the bridging chlorides, which increases their charge density, leading to electron feedback toward Cu(II).Magneto–structural correlation
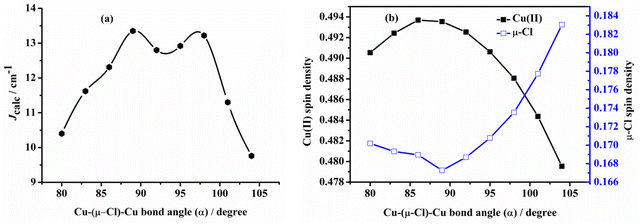
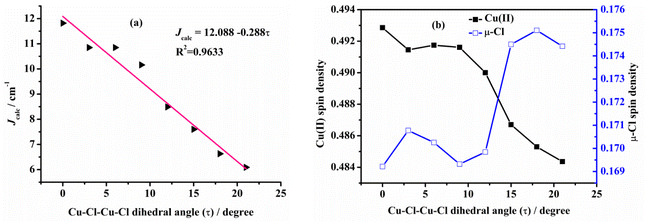
Ruiz et al.60 have studied the magneto–structural correlation of the halo-bridged dinuclear Cu(II) complexes, indicating that in chlorine-bridged complexes, the magnetic coupling constant decreases as the Cu–μ2-Cl–Cu bond angle increases. Similarly, in symmetric units, the magnetic coupling constant first increases and then decreases as the twist angle (τ) of the Cu–N–N–N plane coordinated with the terminal group and the Cu–X–Cu–X (X = F, Cl, Br) plane coordinated with the diaspora ligand increases.However, analysis of the magneto–structural correlation of the dichloro-bridged dinuclear Cu(II) complex is incomplete. To obtain magneto–structural correlations for {Cu–(μ-Cl)2–Cu} complexes, we varied the Cu–(μ-Cl)–Cu angle (α), Cu–Cl–Cu–Cl dihedral angle (τ) and Cu⋯Cu distance (R0) of the complex. The structures were optimized at the M06-2X/def2-TZVPP level of theory with fixed α and R0 to obtain Jcalc at the PBE/ZORA-def2-TZVP level of theory.
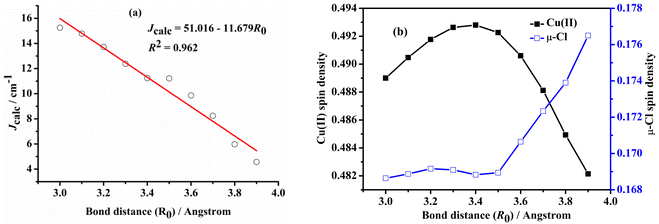
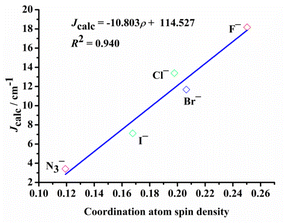
Examining the R0 distance and α revealed that Jcalc decreases gradually with the increase in the Mulliken spin density of the bridging ligand. We studied the Mulliken spin density of the bridged ligands for dinuclear copper(II) complexes bridged by various ligands (Fig. 9b) and found that in the five complexes, the increase in the ligand field strength leads to an increase in the splitting energy of the d orbital of the Cu(II) ion, thereby increasing the energy of the singly occupied orbital of the Cu(II) ion. The number of electrons transferred from the Cu(II) ion to the coordinating atom of the bridging ligand decreases, decreasing the bridging spin density. This may weaken the orbital interaction between the Cu(II) ions and the coordinating atom, decrease the antiferromagnetic contribution, and increase Jcalc. In model II, the difference in the calculated magnetic coupling constant with the change in the ligand is consistent with that of model I. Fig. 10 depicts the linear relationship between the bridging spin density and Jcalc. The magnetic properties of dinuclear Cu(II) complexes with bridging halide ligands can be predicted by calculating the spin densities of the coordinating atoms of the bridging ligands. However, their magnetic coupling constants are not correlated with the Mulliken spin density of the coordinated atoms (Fig. S6†).
Conclusions
In this study, we demonstrated magnetic correlations for Jcalc and the structural parameters α, R0 and τ for {Cu–(μ-Cl)2–Cu} complexes. Variation of α and the R0 followed by structural optimization and calculation of the magnetic coupling constant (Jcalc) revealed that Jcalc increased linearly with R0 and τ, and initially increased and then decreased with α. We also studied ligand effect on Jcalc of paramagnetic dinuclear Cu(II) complexes and found Jcalc increased with the increase of the ligand field strength of the selected ligands I− < Br− < Cl− < N3− < F−. The linear correlation between Jcalc and the spin density of the bridging ligand may predict the magnetic properties of dinuclear copper(II) complexes and facilitate efficient advances in materials design.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Professor Li-hua Gan (Southwest University) for his help with calculations. This work was supported by the Guizhou Education Department Youth Science and Technology Talents Growth Project (grant number KY[2020]158), the Bijie Coal and Phosphorus Chemical Engineering Technology Center (grant number bi ke he zi [2015]01), the Joint Foundation Project of Bijie City Science and Technology Bureau and Guizhou University of Engineering Science (No. bi ke lian he G(2023)41), the Guizhou Province Key Laboratory of Ecological Protection and Restoration of Typical Plateau Wetlands (grant number [2020]2002), and the Undergraduate Innovative and Entrepreneurial Training Program (grant numbers 202110668039, 202110668045, and 202110668042).References
- I. A. Koval, P. Gamez, C. Belle, K. Selmeczi and J. Reedijk, Chem. Soc. Rev., 2006, 35, 814–840 RSC.
- G. R. Moore, Z. X. Huang, C. G. S. Eley, H. A. Barker, G. Williams, M. N. Robinson and R. J. P. Williams, Faraday Discuss. Chem. Soc., 1982, 74, 311–329 RSC.
- S. J. Lippard, Science, 1986, 233, 992 CrossRef CAS PubMed.
- E. I. Solomon, M. J. Baldwin and M. D. Lowery, Chem. Rev., 1992, 92, 521–542 CrossRef CAS.
- C. Remenyi, R. Reviakine and M. Kaupp, J. Phys. Chem. B, 2007, 111, 8290–8304 CrossRef CAS PubMed.
- D. Žilić, B. Rakvin, D. Milić, D. Pajić, I. Đilović, M. Cametti and Z. Džolić, Dalton Trans., 2014, 43, 11877–11887 RSC.
- O. Kahn, Acc. Chem. Res., 2000, 33, 647–657 CrossRef CAS PubMed.
- I.-E. Boulguemh, A. Beghidja, L. Khattabi, J. Long and C. Beghidja, Inorg. Chim. Acta, 2020, 507, 119519 CrossRef CAS.
- A. M. Schuitema, A. F. Stassen, W. L. Driessen and J. Reedijk, Inorg. Chim. Acta, 2002, 337, 48–52 CrossRef CAS.
- E. Gungor and H. Kara, Inorg. Chim. Acta, 2012, 384, 137–142 CrossRef CAS.
- A. Rodríguez-Fortea, P. Alemany, S. Alvarez and E. Ruiz, Inorg. Chem., 2002, 41, 3769–3778 CrossRef PubMed.
- J. F. Torres, N. J. Bello-Vieda, M. A. Macías, A. Muñoz-Castro, C. Rojas-Dotti, J. Martínez-Lillo and J. Hurtado, Eur. J. Inorg. Chem., 2018, 3644–3651 CrossRef CAS.
- S. Choubey, S. Roy, S. Chattopadhayay, K. Bhar, J. Ribas, M. Monfort and B. K. Ghosh, Polyhedron, 2015, 89, 39–44 CrossRef CAS.
- I. Banerjee, P. N. Samanta, K. K. Das, R. Ababei, M. Kalisz, A. Girard, C. Mathonière, M. Nethaji, R. Clérac and M. Ali, Dalton Trans., 2013, 42, 1879–1892 RSC.
- M. Kato, K. Hida, T. Fujihara and A. Nagasawa, Eur. J. Inorg. Chem., 2011, 495–502 CrossRef CAS.
- S. Mandal, F. Lloret and R. Mukherjee, Inorg. Chim. Acta, 2009, 362, 27–37 CrossRef CAS.
- A. D. Ivanova, Y. V. Grigoriev, V. Y. Komarov, T. S. Sukhikh, A. S. Bogomyakov, A. N. Lavrov, L. A. Sheludyakova and L. G. Lavrenova, Inorg. Chim. Acta, 2021, 524, 120452 CrossRef CAS.
- E. S. Barskaya, A. V. Rzheutskiy, A. A. Moiseeva, V. A. Tafeenko, N. V. Zyk and E. K. Beloglazkina, Mendeleev Commun., 2019, 29, 444–446 CrossRef CAS.
- A. Bernalte-García, A. M. Lozano-Vila, F. Luna-Giles and R. Pedrero-Marín, Polyhedron, 2006, 25, 1399–1407 CrossRef.
- A. D. Richardson, T. J. Zirkman, M. T. Kebede, C. P. Landee, M. Rademeyer and M. M. Turnbull, Polyhedron, 2018, 147, 106–119 CrossRef CAS.
- S. Coetzee, M. M. Turnbull, C. P. Landee, J. J. Novoa, M. Deumal, S. Vela and M. Rademeyer, Polyhedron, 2020, 185, 114603 CrossRef CAS.
- M. Malik, A. Świtlicka, A. Bieńko, U. K. Komarnicka, D. C. Bieńko, S. Kozieł, A. Kyzioł, T. Mazur and B. Machura, RSC Adv., 2022, 12, 27648–27665 RSC.
- S. V. Voitekhovich, M. M. Degtyarik, A. S. Lyakhov, L. S. Ivashkevich, J. Klose, B. Kersting and O. A. Ivashkevich, Z. Anorg. Allg. Chem., 2020, 646, 1331–1335 CrossRef CAS.
- S. N. Herringer, C. P. Landee, M. M. Turnbull, J. Ribas-Ariño, J. J. Novoa, M. Polson and J. L. Wikaira, Inorg. Chem., 2017, 56, 5441–5454 CrossRef CAS PubMed.
- E. A. Buvaylo, V. N. Kokozay, V. G. Makhankova, A. K. Melnyk, M. Korabik, M. Witwicki, B. W. Skelton and O. Y. Vassilyeva, Eur. J. Inorg. Chem., 2018, 2018, 1603–1619 CrossRef CAS.
- Y. Sikdar, R. Modak, D. Bose, S. Banerjee, D. Bieńko, W. Zierkiewicz, A. Bieńko, K. D. Das Saha and S. Goswami, Dalton Trans., 2015, 44, 8876–8888 RSC.
- D. Žilić, D. Maity, M. Cetina, K. Molčanov, Z. Džolić and M. Herak, ChemPhysChem, 2017, 18, 2397–2408 CrossRef PubMed.
- P. Torres-García, F. Luna-Giles, Á. Bernalte-García, C. Platas-Iglesias, D. Esteban-Gómez and E. Viñuelas-Zahínos, New J. Chem., 2017, 41, 8818–8827 RSC.
- R. S. Freitas, W. A. Alves and A. Paduan-Filho, Phys. Rev. B, 2017, 95, 184426 CrossRef.
- W. E. Marsh, W. E. Hatfield and D. J. Hodgson, Inorg. Chem., 1982, 21, 2679–2684 CrossRef CAS.
- W. E. Marsh, K. C. Patel, W. E. Hatfield and D. J. Hodgson, Inorg. Chem., 1983, 22, 511–515 CrossRef CAS.
- M. Rodríguez, A. Llobet, M. Corbella, A. E. Martell and J. Reibenspies, Inorg. Chem., 1999, 38, 2328–2334 CrossRef.
- R. Kapoor, A. Kataria, P. Venugopalan, P. Kapoor, M. Corbella, M. Rodríguez, I. Romero and A. Llobet, Inorg. Chem., 2004, 43, 6699–6706 CrossRef CAS PubMed.
- R. Li, B. Moubaraki, K. S. Murray and S. Brooker, Dalton Trans., 2008, 43, 6014–6022 RSC.
- S. Koohzad, H. Golchoubian and Z. Jagličić, Inorg. Chim. Acta, 2018, 473, 60–69 CrossRef CAS.
- Y.-Y. Kou, J.-L. Tian, D.-D. Li, H. Liu, W. Gu and S.-P. Yan, J. Coord. Chem., 2009, 62, 2182–2192 CrossRef CAS.
- F. Yraola, F. Albericio, M. Corbella and M. Royo, Inorg. Chim. Acta, 2008, 361, 2455–2461 CrossRef CAS.
- X. Bu, M. Du, L. Zhang, Z. Shang, R. Zhang and M. Shionoya, J. Chem. Soc., Dalton Trans., 2001, 5, 729–735 RSC.
- X.-H. Bu, M. Du, Z.-L. Shang, L. Zhang, Q.-H. Zhao, R.-H. Zhang and M. Shionoya, Eur. J. Inorg. Chem., 2001, 2001, 1551–1558 CrossRef.
- J. N. Roedel, R. Bobka, B. Neumann, B. Weber, P. Mayer and I.-P. Lorenz, Z. Anorg. Allg. Chem., 2007, 633, 1171–1177 CrossRef CAS.
- R. Singh, F. Lloret and R. Mukherjee, Z. Anorg. Allg. Chem., 2014, 640, 1086–1094 CrossRef CAS.
- D. D. Swank, G. F. Needham and R. D. Willett, Inorg. Chem., 1979, 18, 761–765 CrossRef CAS.
- W. A. Alves, R. H. A. Santos, A. Paduan-Filho, C. C. Becerra, A. C. Borin and A. M. D. C. Ferreira, Inorg. Chim. Acta, 2004, 357, 2269–2278 CrossRef CAS.
- S.-L. Ma, X.-X. Sun, S. Gao, C.-M. Qi, H.-B. Huang and W.-X. Zhu, Eur. J. Inorg. Chem., 2007, 846–851 CrossRef CAS.
- M. Du, Y.-M. Guo, X.-H. Bu, J. Ribas and M. Monfort, New J. Chem., 2002, 26, 645–650 RSC.
- M. Du, Y.-M. Guo, X.-H. Bu, J. Ribas and M. Monfort, New J. Chem., 2002, 26, 939–945 RSC.
- L. Noodleman, J. Chem. Phys., 1981, 74, 5737–5743 CrossRef CAS.
- L. Noodleman and E. R. Davidson, Chem. Phys., 1986, 109, 131–143 CrossRef.
- S. C. Luo, D. Nie, Z. Li, X. Y. Sun, L. Hu and X. Y. Liu, Polyhedron, 2020, 182, 114506 CrossRef CAS.
- S. C. Luo, W. H. Xiao, X. Y. Sun and P. F. Zheng, Polyhedron, 2021, 205, 115312 CrossRef CAS.
- S. C. Luo, H. J. An, B. Z. Lou, B. Zeng and H. Liu, Polyhedron, 2022, 223, 115983 CrossRef CAS.
- Y.-M. Sun, C.-B. Liu, X.-J. Lin and S.-W. Bi, New J. Chem., 2004, 28, 270–274 RSC.
- T. Gupta and G. Rajaraman, Chem. Commun., 2016, 52, 8972–9008 RSC.
- S. K. Singh and G. Rajaraman, Dalton Trans., 2013, 42, 3623–3630 RSC.
- S. K. Singh, N. K. Tibrewal and G. Rajaraman, Dalton Trans., 2011, 40, 10897–10906 RSC.
- T. Gupta, T. Rajeshkumar and G. Rajaraman, Phys. Chem. Chem. Phys., 2014, 16, 14568–14577 RSC.
- S. C. Luo, L. Y. Zhang, X. D. Zhai, X. H. Yang and X. Y. Sun, Chem. Phys. Lett., 2023, 811, 140241 CrossRef CAS.
- S. C. Luo, X. Y. Sun, B. Zeng and P. F. Zheng, Polyhedron, 2021, 194, 114955 CrossRef CAS.
- E. Ruiz, J. Cano, S. Alvarez and P. Alemany, J. Comput. Chem., 1999, 20, 1391–1400 CrossRef CAS.
- E. Ruiz, P. Alemany, S. Alvarez and J. Cano, J. Am. Chem. Soc., 1997, 119, 1297–1303 CrossRef CAS.
- E. Ruiz, J. Cano, S. Alvarez, A. Caneschi and D. Gatteschi, J. Am. Chem. Soc., 2003, 125, 6791–6794 CrossRef CAS PubMed.
- G. A. Craig, G. Velmurugan, C. Wilson, R. Valiente, G. Rajaraman and M. Murrie, Inorg. Chem., 2019, 58, 13815–13825 CrossRef CAS PubMed.
- S. K. Singh, T. Rajeshkumar, V. Chandrasekhar and G. Rajaraman, Polyhedron, 2013, 66, 81–86 CrossRef CAS.
- G. Rajaraman, F. Totti, A. Bencini, A. Caneschi, R. Sessoli and D. Gatteschi, Dalton Trans., 2009, 17, 3153–3161 RSC.
- S. K. Singh, M. F. Beg and G. Rajaraman, Chem. - Eur. J., 2016, 22, 672–680 CrossRef CAS PubMed.
- S. K. Singh, K. R. Vignesh, V. Archana and G. Rajaraman, Dalton Trans., 2016, 45, 8201–8214 RSC.
- J. M. Frost, R. J. Stirling, S. Sanz, N. Vyas, G. S. Nichol, G. Rajaraman and E. K. Brechin, Dalton Trans., 2015, 44, 10177–10187 RSC.
- F. Neese, ORCA-an Ab Initio, Density Functional and Semiempirical Program Package, 5.0.3, University of Bonn, Bonn, Germany, 2022 Search PubMed.
- F. Neese, F. Wennmohs, U. Becker and C. Riplinger, J. Chem. Phys., 2020, 152, 224108 CrossRef CAS PubMed.
- G. Singh, S. Gamboa, M. Orio, D. A. Pantazis and M. Roemelt, Theor. Chem. Acc., 2021, 140, 139 Search PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- M. Roemelt, V. Krewald and D. A. Pantazis, J. Chem. Theory Comput., 2018, 14, 166–179 CrossRef CAS PubMed.
- K. Pierloot, Q. M. Phung and A. Ghosh, Inorg. Chem., 2020, 59, 11493–11502 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, J. Chem. Phys., 2006, 125, 194101 CrossRef PubMed.
- S. C. Luo, H. Mei, X. Y. Sun and P. F. Zheng, J. Mol. Graphics Modell., 2020, 97, 107562 CrossRef CAS PubMed.
- T. Soda, Y. Kitagawa, T. Onishi, Y. Takano, Y. Shigeta, H. Nagao, Y. Yoshioka and K. Yamaguchi, Chem. Phys. Lett., 2000, 319, 223–230 CrossRef CAS.
- S. C. Luo, Y. Y. Su, Y. L. Wang and X. Y. Sun, Comput. Theor. Chem., 2022, 1207, 113524 CrossRef CAS.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- E. V. Lenthe, E. J. Baerends and J. G. Snijders, J. Chem. Phys., 1993, 99, 4597–4610 CrossRef.
- J. Baker and P. Pulay, J. Comput. Chem., 2003, 24, 1184–1191 CrossRef CAS PubMed.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822–8824 CrossRef PubMed.
- A. D. Becke, Phys. Rev. A Gen. Phys., 1988, 38, 3098–3100 CrossRef CAS PubMed.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 6671–6687 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- M. Reiher, O. Salomon and B. A. Hess, Theor. Chem. Acc., 2001, 107, 48–55 Search PubMed.
- X. Xu and W. A. Goddard III, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 2673–2677 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS PubMed.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- S. C. Luo, C. Xu, R. Zhang and X. Y. Sun, Chem. Phys. Lett., 2021, 784, 139102 CrossRef CAS.
- E. D. Glendening, C. R. Landis and F. Weinhold, J. Comput. Chem., 2013, 34, 1429–1437 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00585b |
| This journal is © The Royal Society of Chemistry 2023 |