Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Feruloylmonotropeins: promising natural antioxidants in Paederia scandens†
Nguyen Quang Trung *ab,
Nguyen Thi Thu Thanhc,
Nguyen Thi Hoad,
Adam Mechler
*ab,
Nguyen Thi Thu Thanhc,
Nguyen Thi Hoad,
Adam Mechler e and
Quan V. Vo
e and
Quan V. Vo *d
*d
aThe University of Danang – University of Science and Education, Da Nang 550000, Vietnam. E-mail: nqtrung.quatest2@gmail.com
bQuality Assurance and Testing Center 2, Da Nang 550000, Vietnam
cLe Thanh Phuong High School, An My, Phu Yen 621640, Vietnam
dThe University of Danang – University of Technology and Education, Danang 550000, Vietnam. E-mail: vvquan@ute.udn.vn
eDepartment of Biochemistry and Chemistry, La Trobe University, Victoria 3086, Australia
First published on 20th February 2023
Abstract
Paederia scandens (Lour.) is a widely used medicinal herb in Vietnam, China, India, and Japan for the treatment of a variety of conditions, including toothache, chest pains, piles, and spleen inflammation. There is broad interest in identifying the composition of its extracts and confirming their numerous biological activities, including anti-nociceptive, antiviral, and anticancer properties. Two iridoid glucosides obtained from the MeOH extract of P. scandens, 6′-O-E-feruloylmonotropein (6-FMT) and 10′-O-E-feruloylmonotropein (10-FMT), are potential antioxidants based on their structure. In this study, the hydroperoxyl scavenging activity of 6-FMT and 10-FMT was examined in silico by using density functional theory. These FMTs are predicted to be weak antioxidants in non-polar environments, whereas a good HOO˙ scavenging activity is expected in polar environments (pH = 7.4) with koverall = 3.66 × 107 M−1 s−1 and 9.45 × 106 M−1 s−1, respectively. This activity is better than many common antioxidants such as trolox and nearly equivalent to ascorbic acid and resveratrol. The hydroperoxyl scavenging activity was exerted mainly by the di-anion form of FMTs in water at physiological pH following the single electron transfer mechanism. The results suggest that FMTs are promising natural antioxidants in aqueous physiological environments.
1. Introduction
Paederia scandens (Lour.) (P. scandens) is a species of the Rubiaecae family, which is widely distributed in the southern region of the Korean peninsula, Vietnam, India, China, Japan, the Philippines, and the USA1 P. scandens is a common dietary herb, but it is also a medicinal plant. The roots and aerial parts of this plant are used in traditional medicine to treat toothache, chest pains, piles, inflammation of the spleen, as well as for simple effects as emetic and diuretic.2 Recent works on the constituents and bioactivities of extracts from parts of P. scandens showed that extracts have anti-nociceptive, antiviral, antitumor, and anti-inflammatory activities.3–5 In the extracts several bioactive substances were identified such as iridoid glucosides,1,6,7 volatile oils,8–10 flavonoids,11,12 glucosides,13–15 and quinones.13,16,17Two iridoid glucosides: 6′-O-E-feruloylmonotropein (6-FMT) and 10′-O-E-feruloylmonotropein (10-FMT) (Fig. 1) were isolated from the MeOH extract of P. scandens.6 Recent works also elaborated on antioxidant activity of the P. scandens extracts, showing among others the elimination of hydroxyl free radicals, and antioxidant enzyme activity.18,19 However, there is no tangible link thus far between the presence of iridoid glucosides in the extracts and their antioxidant activity, which is highly likely based on structural characteristics.
In the past few years, numerous studies were conducted on bioactivities of natural compounds using a computational approach.20–25 From the kinetic and thermodynamic parameters, the main mechanistic pathways of radical scavenging, as well as the capacity of radical scavenging activity in different media (gas phase, polar and non-polar solvent at physiological pH) were described.26,27 Those calculated results were useful for confirming the results of the earlier experimental studies on antioxidant activities of natural compounds. In this paper, we use this approach to investigate the antiradical properties of 6-FMT and 10-FMT (Fig. 1) through three main mechanisms: formal hydrogen transfer (FHT), single electron transfer followed by proton transfer (SETPT), and sequential proton loss electron transfer (SPLET).
2. Computational details
Thermochemical parameters: bond dissociation energies (BDEs), ionization energies (IEs) and proton affinities (PAs), together with kinetic parameters including activation energies ΔG‡ (kcal mol−1), tunneling corrections (κ) and rate constants (k) in the gas phase and in physiological environments (water for the aqueous solution and pentyl ethanoate for lipid medium) were calculated by M06-2X functional.28–32 The quantum mechanics based test for overall free radical scavenging activity (QM-ORSA) protocol23,28,33–36 was used for the kinetic calculations.The rate constant (k) was computed following the transition state theory (TST) and 1 M standard state at 298.15 K by the formula (1):27,29,36–41
 | (1) |
Due to the large molecules (∼70 atoms), the thermodynamic data were calculated at M06-2X/6-311++G(d,p)//M06-2X/6-31+G(d)) level of theory, whereas the kinetic results were investigated by using the M06-2X/6-31+G(d). This level was sufficient for geometry optimization with acceptable accuracy, confirmed by previous studies.22,34,46
3. Results and discussion
3.1. The HOO˙ radical scavenging of feruloylmonotropeins in the gas phase
| Positions | BDE | ΔGo | PA | ΔGo | IE | ΔGo |
|---|---|---|---|---|---|---|
| 6FMT–O4–H | 116.0 | 30.0 | 321.0 | 202.9 | 187.7 | 163.0 |
| 6FMT–C5–H | 82.6 | –2.9 | ||||
| 6FMT–O24–H | 97.6 | 11.0 | 333.6 | 215.6 | ||
| 10FMT–O4–H | 113.8 | 24.9 | 329.4 | 211.3 | 174.3 | 150.8 |
| 10FMT–C5–H | 78.8 | –9.4 | ||||
| 10FMT–O24–H | 87.0 | –1.3 | 325.9 | 207.0 |
The above results suggest that the lowest BDE value of O–H bonds was at C5 and O24 for both studied compounds. The BDE values of these positions of 6-FMT are 82.6 kcal mol−1 and 97.6 kcal mol−1, and that of 10-FMT are 78.8 kcal mol−1 and 87.0 kcal mol−1, for the bonds at C5–H and O24–H, respectively. These O24-BDE values are higher than that of typical antioxidants such as viniferifuran (82.7 kcal mol−1),48 resveratrol (83.9 kcal mol−1),48 and vanillic acid (85.2 kcal mol−1);49 thus the radical scavenging activity of these positions is expected to be weaker than the reference antioxidants.
The Gibbs free energy changes of the first step of the FMT + HOO˙ reaction following each pathway i.e. FHT, SET (single electron transfer-SET, the first step for SETPT mechanism), and proton loss (PL, the first step of SPLET mechanism) were also calculated to confirm the favored pathway of FMT antiradical activity. The results show that the reaction at 6-FMT–C5–H, 10-FMT–C5–H, and 10-FMT–O24–H are spontaneous due to the ΔGo < 0, except for 6-FMT–O24–H position with ΔGo = 11.0. Whereas the ΔGo of the reaction following the SP and SET mechanism are much higher than that of FHT mechanism. The above data show that the radical scavenging activity of FMT in the gas phase will not follow SETPT nor SPLET mechanism. Thus, the kinetic study of FHT pathway of C5–H and O24–H positions should be investigated in the next section.
3.1.1.1 Kinetic study. From the data above, the preferred mechanism is FHT at C5–H and O24–H position. Accordingly, the kinetic parameters were calculated for the lowest BDE values of each type of bond. The results are presented in Table 2 and Fig. 2.
| Comp. | Mechanisms | Positions | ΔG‡ | κ | kEck | Γ |
|---|---|---|---|---|---|---|
| 6-FMT | FHT | C5–H | 21.0 | 457.6 | 1.20 | 0.0 |
| O24–H | 17.2 | 3322.0 | 5.30 × 103 | 100.0 | ||
| koverall | 5.30 × 103 | |||||
| 10-FMT | FHT | C5–H | 16.2 | 24.1 | 1.99 × 102 | 76.7 |
| O24–H | 17.6 | 72.7 | 6.02 × 101 | 23.3 | ||
| koverall | 2.59 × 102 | |||||
 | ||
| Fig. 2 Optimized geometries of TSs between the studied compounds and HOO˙ radical in the gas phase following the FHT pathway. | ||
According to the above data, both 6-FMT and 10-FMT are weak antioxidants, with koverall = 5.30 × 103 and 2.59 × 102 M−1 s−1, respectively. The O24–H contribute 100% of the antioxidant activity of 6-FMT, while that of 10-FMT is only 23.3%. The results show that the substitution of ferulic acid at the 17 position of pyranosyl moiety (6-FMT) causes the higher HOO˙ radical scavenging activity of FMT than at the 10 position of iridoid skeleton moiety (10-FMT). Besides, those low reaction rates correspond to the high BDE values in the prior section.
3.2. The HOO˙ radical scavenging of feruloylmonotropeins in the physiological environments
| Positions | BDE | ΔGo | PA | ΔGo | IE | ΔGo |
|---|---|---|---|---|---|---|
| 6-FMT–O4–H | 298.6 | 98.2 | 153.3 | 46.1 | ||
| 6-FMT–C5–H | 79.2 | –16.8 | ||||
| 6-FMT–O24–H | 93.9 | –3.1 | 308.8 | 108.4 | ||
| 10-FMT–O4–H | 298.1 | 97.6 | 141.7 | 35.7 | ||
| 10-FMT–C5–H | 75.7 | –24.0 | ||||
| 10-FMT–O24–H | 84.7 | –15.1 | 299.5 | 98.2 |
| Positions | BDE | ΔGo | PA | ΔGo | IE | ΔGo |
|---|---|---|---|---|---|---|
| 6-FMT–O4–H | 40.5 | 35.9 | 116.5 | 34.4 | ||
| 6-FMT–C5–H | 76.6 | –20.5 | ||||
| 6-FMT–O24–H | 90.9 | –7.2 | 50.0 | 45.5 | ||
| 10-FMT–O4–H | 35.7 | 31.2 | 107.1 | 26.3 | ||
| 10-FMT–C5–H | 75.5 | –21.9 | ||||
| 10-FMT–O24–H | 81.7 | –15.9 | 38.7 | 33.4 |
As per Table 3, the BDE values of the C5–H and O24–H position of both studied substances in pentyl ethanoate are lower than in the gas phase, corresponding to negative free Gibbs energies. The lowest PA values of 6-FMT and 10-FMT range from 298.6 kcal mol−1 to 308.8 kcal mol−1, and from 298.1 kcal mol−1 to 299.5 kcal mol−1, respectively. The IE value of 6-FMT is 153.3 kcal mol−1 and that of 10-FMT is 141.7 kcal mol−1. The computed data suggested that the HOO˙ radical scavenging of 6-FMT and 10-FMT in apolar environments do not follow either the SPLET or SETPT mechanism, and those pathways can be omitted in the kinetic calculation.
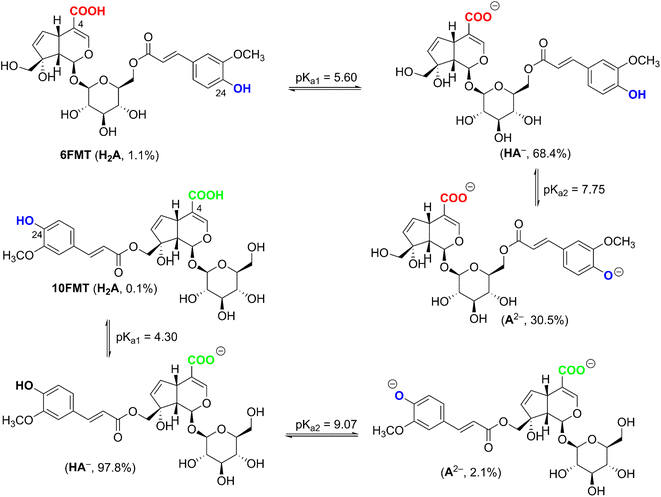
From the data of Table 4, the BDE values of C5–H and O24–H position of 6-FMT and 10-FMT in water are even over than that in a–polar solvent, consistent with the bond-weakening effect of the high dielectric constant of the medium. The lower BDE correspond to the lower negative ΔGo values which range from −7.2 kcal mol−1 to −21.9 kcal mol−1. The lowest PA values of OH bonds of 6-FMT and 10-FMT were predicted for O4–H at 40.5 kcal mol−1 and 35.7 kcal mol−1, and that of O24–H at 50.0 kcal mol−1 and 38.7 kcal mol−1, respectively. Because of the lower PA value at O4–H position, both 6-FMT and 10-FMT would most likely be dissociated at O4–H follow by O24–H position. The pKa was calculated following the literature,50,51 and the results are presented in Fig. 3.
The pKa1 and pKa2 of 6-FMT are 5.60 and 7.75, at pH 7.4 yielding state populations of 1.1% of neutral (HA), 68.4% of anion (HA−), and 30.5% of dianion (A2−). The pKa1 and pKa2 of 10-FMT are 4.30 and 9.07, corresponding to 0.1% of neutral, 97,8% of anion, and 2.1% of dianion populations. Thus, these populations were used for the kinetic calculation.
| Mechanism | Position | ΔG‡ | κ | kEck | kapp | Γ |
|---|---|---|---|---|---|---|
| FHT | 6-FMT–C5–H | 19.8 | 116.6 | 24.0 | 24.0 | 99.6 |
| 6-FMT–O24–H | 18.9 | 0.1 | 9.4 × 10−3 | 9.4 × 10−3 | 0.4 | |
| koverall | 24.0 | |||||
| FHT | 10-FMT-C5-H | 19.1 | 43.2 | 2.50 | 2.50 | 9.7 |
| 10-FMT-O24–H | 19.3 | 488.0 | 23.0 | 23.0 | 90.3 | |
| koverall | 25.5 | |||||
| Comp. | States | ΔG‡ | κ | kD | kapp | f | kf | Γ |
|---|---|---|---|---|---|---|---|---|
| 6-FMT | HA− | 29.8 | 17.0 | 8.80 × 109 | 9.50 × 10−10 | 0.684 | 6.50 × 10−10 | 0.0 |
| A2– | 6.4 | 12.9 | 8.80 × 109 | 1.20 × 108 | 0.305 | 3.66 × 107 | 100.0 | |
| 3.66 × 107 | Chem | |||||||
| 10-FMT | HA− | 33.5 | 15.9 | 8.70 × 109 | 1.70 × 10−12 | 0.978 | 1.66 × 10−12 | 0.0 |
| A2− | 5.6 | 16.0 | 8.70 × 109 | 4.50 × 108 | 0.021 | 9.45 × 106 | 100.0 | |
| 9.45 × 106 | Chem | |||||||
In the pentyl ethanoate solvent:
| koverall = ∑kapp(FHT–neutral) | (2) |
In water:
| koverall = ∑kapp(SET–anion) + ∑kapp(SET–dianion) | (3) |
As per calculated data, the koverall of HOO˙ radical scavenging reaction of 6-FMT and 10-FMT in pentyl ethanoate are 24.0 M−1 s−1 and 25.5 M−1 s−1, respectively. These rate constants suggest that 6-FMT and 10-FMT are weak antioxidants in non-polar environments. In contrast, both 6-FMT and 10-FMT have good radical scavenging activity in water with the koverall at 3.66 × 107 M−1 s−1 for 6-FMT and 9.45 × 106 M−1 s−1 for 10-FMT. The radical scavenging activity of FMT+HOO· reactions are dominated by the SET mechanism of A2− in both cases, with kf(A2−) = 3.66 × 107 M−1 s−1, Γ = 100% for 6-FMT and kf (A2−) = 9.45 × 106 M−1 s−1 for 10-FMT, while the HA− state do not make any contribution (Γ = 0.0%). Those calculated rate of activity of FMTs are nearly equivalent to their moieties, such as ferulic acid (koverall = 5.80 × 107 M−1 s−1),53 guaiacol (koverall = 2.38 × 106 M−1 s−1).54 Based on calculated data, we can conclude that 6-FMT and 10-FMT are better HOO˙ radical scavengers in the aqueous solution than the reference antioxidant trolox, with the rate of activity approximately 105–408 times faster than that of trolox (k = 8.96 × 104 M−1 s−1).55 The rate constants are similar to ascorbic acid (k = 9.97 × 107 M−1 s−1)34 and resveratrol ((k = 5.62 × 107 M−1 s−1),56 suggesting that 6-FMT and 10-FMT are good antioxidants in the polar environment.
4. Conclusion
In this study, the HOO˙ radical scavenging activity of 6-FMT and 10-FMT were calculated by M06-2X functional. The results show that both of the studied substances are weak antioxidant in apolar environment, but they show good antioxidant activity in the polar environment, with koverall = 3.66 × 107 M−1 s−1 for 6-FMT and 9.45 × 106 M−1 s−1 for 10-FMT, via SET pathway at O4–H and O24–H position. The activity is exerted by the dianion A2− with Γ = 100% while the anion HA− does not make any contribution. These rate constants of radical scavenging activity are substantially better than reference antioxidant trolox and similar to ascorbic acid and resveratrol, confirming that 6-FMT and 10-FMT are good antioxidants in water at physiological pH.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Nguyen Quang Trung was funded by Vingroup JSC and supported by the Master, PhD Scholarship Programme of Vingroup Innovation Foundation (VINIF), Institute of Big Data, code VINIF2021.TS.114.References
- Y. L. Kim, Y.-W. Chin, J. Kim and J. H. Park, Chem. Pharm. Bull., 2004, 52, 1356–1357 CrossRef CAS PubMed.
- G. J. Kapadia, S. C. Sharma, H. Tokuda, H. Nishino and S. Ueda, Cancer Lett., 1996, 102, 223–226 CrossRef CAS PubMed.
- Y.-F. Chen, N. Li, Y.-L. Jiao, P. Wei, Q.-Y. Zhang, K. Rahman, H.-C. Zheng and L.-P. Qin, Phytomedicine, 2008, 15, 427–436 CrossRef CAS PubMed.
- Y. Ma, L.-L. Zhou, H.-Y. Yan and M. Liu, Am. J. Chin. Med., 2009, 37, 669–683 CrossRef CAS PubMed.
- H. Yan, Y. Ma, M. Liu and L. Zhou, Planta Med., 2008, 74, 1345–1350 CrossRef CAS PubMed.
- H. Otsuka, Nat. Med., 2002, 56, 59–62 CAS.
- J. S. Chen, X. L. Wang and K. Y. Ding, Chin. Chem. Lett., 2010, 21, 437–439 CrossRef.
- M. Yangmin, M. Yuan and F. Jianxi, Acta Bot. Boreali-Occident. Sin., 2000, 20, 145–148 Search PubMed.
- Z. Fang, S. Guo, H. Lin, X. Lin, L. Hao-Long, Y. Liu, J. Yan and Y. Yang, Hubei Nong Ye Ke, 2014, 53, 912–914 Search PubMed.
- K.-j. HE, B.-m. LIU, X.-m. DONG, L. CHEN, M.-s. CHEN and J.-g. MO, Guangxi Sci., 2010, 17, 138–140 CAS.
- N. Ishikura, Z.-q. Yang, K. Yoshitama and K. Kurosawa, Z. Naturforsch. C, 1990, 45, 1081–1084 CrossRef CAS.
- X. Zou, J. Liang, L.-S. Ding and S.-L. Peng, Zhongguo Zhongyao Zazhi, 2006, 31, 1436–1441 CAS.
- X. Zou, S. Peng, X. Liu, B. Bai and L. Ding, Fitoterapia, 2006, 77, 374–377 CrossRef CAS PubMed.
- C. Zhuang, X. Wang, L. Miao, H. Zhou and T. Wu, Chem. Nat. Compd., 2013, 49, 379–380 CrossRef CAS.
- Y.-W. Chin, K.-D. Yoon, M.-J. Ahn and J.-W. Kim, Bull. Korean Chem. Soc., 2010, 31, 1070–1072 CrossRef CAS.
- Đ. N. Quang, Vietnam J. Chem., 2009, 47, 95 Search PubMed.
- Y. Li, C. Zheng and L. Qin, Drugs, 2012, 43, 658–660 CAS.
- W. Peng, X.-Q. Qiu, Z.-H. Shu, Q.-C. Liu, M.-B. Hu, T. Han, K. Rahman, L.-P. Qin and C.-J. Zheng, J. Ethnopharmacol., 2015, 174, 317–321 CrossRef CAS PubMed.
- X. Yin, M. H. Ji, T. Y. WU FX, Z. Xia, Z. Wan and M. Shan, Int. J. Clin. Exp. Med., 2012, 48, 1466–1469 CAS.
- E. Alvareda, P. A. Denis, F. Iribarne and M. Paulino, Comput. Theor. Chem., 2016, 1091, 18–23 CrossRef CAS.
- P. A. Denis, Theor. Chem. Acc., 2011, 129, 219–227 Search PubMed.
- N. Q. Trung, A. Mechler, N. T. Hoa and Q. V. Vo, R. Soc. Open Sci., 2022, 9, 220177 Search PubMed.
- A. Galano and J. Raúl Alvarez-Idaboy, Int. J. Quantum Chem., 2019, 119, e25665 CrossRef.
- A. Galano, J. Mex. Chem. Soc., 2015, 59, 231–262 Search PubMed.
- D. T. N. Hang, N. T. Hoa, H. N. Bich, A. Mechler and Q. V. Vo, Phytochemistry, 2022, 201, 113281 CrossRef CAS PubMed.
- M. V. Bay, P. C. Nam, D. T. Quang, A. Mechler, N. K. Hien, N. T. Hoa and Q. V. Vo, ACS Omega, 2020, 5, 7895–7902 CrossRef CAS PubMed.
- H. Boulebd, A. Mechler, N. T. Hoa and Q. V. Vo, New J. Chem., 2020, 4–8 Search PubMed.
- M. Carreon-Gonzalez, A. Vivier-Bunge and J. R. Alvarez-Idaboy, J. Comput. Chem., 2019, 2–3 Search PubMed.
- E. Dzib, J. L. Cabellos, F. Ortíz-Chi, S. Pan, A. Galano and G. Merino, Int. J. Quantum Chem., 2019, 119, e25686 CrossRef.
- Y. Zhao, N. E. Schultz and D. G. Truhlar, J. Chem. Theory Comput., 2006, 2, 364–382 CrossRef PubMed.
- A. Galano and J. R. Alvarez-Idaboy, J. Comput. Chem., 2014, 35, 2019–2026 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, J. Phys. Chem. A, 2008, 112, 1095–1099 CrossRef CAS PubMed.
- J. R. l. Alvarez-Idaboy and A. Galano, J. Phys. Chem. B, 2012, 116, 9316–9325 CrossRef CAS PubMed.
- A. Galano and J. R. Alvarez-Idaboy, J. Comput. Chem., 2013, 34, 2430–2445 CrossRef CAS PubMed.
- Q. V. Vo, M. V. Bay, P. C. Nam and A. Mechler, J. Phys. Chem. B, 2019, 123, 7777–7784 CrossRef CAS PubMed.
- E. Dzib, J. L. Cabellos, F. Ortiz-Chi, S. Pan, A. Galano and G. Merino, Eyringpy 1.0.2, Cinvestav, Mérida, Yucatán, 2018 Search PubMed.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1935, 31, 875–894 RSC.
- H. Eyring, J. Chem. Phys., 1935, 3, 107–115 CrossRef CAS.
- D. G. Truhlar, W. L. Hase and J. T. Hynes, J. Phys. Chem., 1983, 87, 2664–2682 CrossRef CAS.
- T. Furuncuoglu, I. Ugur, I. Degirmenci and V. Aviyente, Macromolecules, 2010, 43, 1823–1835 CrossRef CAS.
- E. Vélez, J. Quijano, R. Notario, E. Pabón, J. Murillo, J. Leal, E. Zapata and G. Alarcón, J. Phys. Org. Chem., 2009, 22, 971–977 CrossRef.
- E. Pollak and P. Pechukas, J. Am. Chem. Soc., 1978, 100, 2984–2991 CrossRef CAS.
- A. Fernández-Ramos, B. A. Ellingson, R. Meana-Pañeda, J. M. Marques and D. G. Truhlar, Theor. Chem. Acc., 2007, 118, 813–826 Search PubMed.
- C. Eckart, Phy. Rev., 1930, 35, 1303 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. aramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 16, Revision A.01, Gaussian, Inc, Wallingford, CT, 2016 Search PubMed.
- M. Nowicki, M. Zaranek, P. Pawluć and M. Hoffmann, Catal. Sci. Technol., 2020, 10, 1066–1072 RSC.
- Y. J. Sung, J. Y. H. Kim, H. I. Choi, H. S. Kwak and S. J. Sim, Sci. Rep., 2017, 7, 1–11 CAS.
- Y. Shang, H. Zhou, X. Li, J. Zhou and K. Chen, New J. Chem., 2019, 43, 15736–15742 RSC.
- C.-H. Hsu, Y. M. Hung, K.-A. Chu, C.-F. Chen, C.-H. Yin and C.-C. Lee, Sci. Rep., 2020, 10, 1–10 CrossRef PubMed.
- Q. V. Vo, M. V. Bay, P. C. Nam, D. T. Quang, M. Flavel, N. T. Hoa and A. Mechler, J. Org. Chem., 2020, 85, 15514–15520 CrossRef CAS PubMed.
- A. Galano, A. Pérez-González, R. Castañeda-Arriaga, L. Muñoz-Rugeles, G. Mendoza-Sarmiento, A. Romero-Silva, A. Ibarra-Escutia, A. M. Rebollar-Zepeda, J. R. León-Carmona and M. A. Hernández-Olivares, J. Chem. Inf. Model., 2016, 56, 1714–1724 CrossRef CAS PubMed.
- H. Boulebd, D. M. Pereira, I. A. Khodja, N. T. Hoa, A. Mechler and Q. V. Vo, J. Mol. Liq., 2022, 346, 118277 CrossRef CAS.
- J. R. León-Carmona, J. R. Alvarez-Idaboy and A. Galano, Phys. Chem. Chem. Phys., 2012, 14, 12534–12543 RSC.
- A. Galano, J. R. León-Carmona and J. R. l. Alvarez-Idaboy, J. Phys. Chem. B, 2012, 116, 7129–7137 CrossRef CAS PubMed.
- M. E. Alberto, N. Russo, A. Grand and A. Galano, Phys. Chem. Chem. Phys., 2013, 15, 4642–4650 RSC.
- C. Iuga, J. R. l. Alvarez-Idaboy and N. Russo, J. Org. Chem., 2012, 77, 3868–3877 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00458a |
| This journal is © The Royal Society of Chemistry 2023 |