Open Access Article
Open Access ArticleDevelopment of NaY9Si6O26:Yb3+ phosphors with high thermal stability for NIR anti-counterfeiting: study of its crystal structure and luminescent properties
Tae Wook Kang†
a,
Yeon Bin Choi†a,
Chae Ha Kanga,
Young Ji Parka,
Jin Ho Kim a,
Byungseo Bae
a,
Byungseo Bae *b and
Sun Woog Kim
*b and
Sun Woog Kim *a
*a
aAdvanced Materials Convergence R&D Division, Display Materials Center, Korea Institute of Ceramic Engineering and Technology, Jinju 52851, Korea. E-mail: skim80@kicet.re.kr
bAdvanced Resources Team, Yeongwol Industrial Promotion Agency, 21-28 Palgoe 1 Nonggongdanji, Yeongwolgun, 26240, Korea
First published on 8th March 2023
Abstract
Near-infrared (NIR) radiation has generated considerable industrial and research interest. However, NIR phosphors for this are limited by low quantum efficiency and broad spectra. Rare-earth-containing compounds doped with activators as host systems for NIR phosphors may resolve these limitations. Yb3+-doped NaY9Si6O26 phosphors were synthesized using a conventional solid-state reaction method. The main phase of the synthesized phosphor samples exhibited a hexagonal structure NaY9Si6O26 phase, and had an angular-shape with an average grain size of 1–3 μm. The NaY9Si6O26:Yb3+ phosphors showed a near-infrared emission from 950 to 1100 nm, which was attributed to the 2F5/2 → 2F7/2 transition of Yb3+ ions under 270 and 920 nm excitation. The excitation spectra, recorded by monitoring the emission at 985 nm, showed two bands in the ultraviolet and infrared regions, which correspond to the charge transfer transition and the 2F7/2 → 2F5/2 transition of Yb3+ ions. At 300 °C, the emission intensity of the NaY9Si6O26:Yb3+ phosphor remained constant at 82%. Furthermore, the thermal degradation was negligible after cooling, suggesting the possibility of application in advanced anti-counterfeiting applications.
Introduction
Since its discovery, near-infrared (NIR) radiation has attracted considerable industrial and research interest. This type of radiation has promising potential for varied applications, mostly related to the characterization of chemicals, security, pharmaceutical, medical, cosmetic, food, and agricultural industries, which contribute to the continued advancement of modern technology.1–5 Radiation sources such as tungsten halogen lamps, NIR light-emitting diodes (LEDs), and phosphor-converted LEDs (pc-LEDs) can emit in the infrared range.6 However, the selection of these radiation sources for varied applications is based on the wavelength, full width at half maximum (FWHM), lifetime, efficiency, and thermal stability. For commercial applications, considering the manufacturing cost and size of radiation sources is essential. Conventional NIR tungsten halogen lamps have low efficiency, a large size, high thermal effect, and short lifetimes.7 NIR LEDs also do not meet the commercial requirements owing to their narrow FWHM.8,9 NIR pc-LEDs, conversely, are considered the most suitable light sources because they provide suitable emission, high efficiency, a long lifetime, and excellent durability.10 Therefore, pc-LEDs are highly preferred alternative radiation sources for NIR applications in devices such as automotive sensors, security applications, remote controls, and spectrometers.11–13 In recent years, the limitations associated with NIR phosphors, including low quantum efficiency and broad spectra, have been extensively studied.14–17Yb3+ has been widely investigated as a simple electronic structure with two multiplets: the 2F5/2 level in the excited state and 2F7/2 level in the ground state. In recent years, numerous studies have been conducted on the development of Yb3+-doped inorganic materials, and their potential use in optical materials such as lasers,18 solar cells,19,20 upconversion phosphors,21–23 and biological applications.24 Rare-earth-containing compounds have been studied as host systems for NIR phosphors because of their large stokes shifts and emission with doped activators. Among them, compounds with an apatite structure (space group P63/m) have been extensively investigated as effective hosts for luminous materials owing to their excellent chemical stability and high efficiency for activated ions.25–27 NaY9Si6O26 is a type of oxyapatite compound, M10(AO4)6B2 (M = Ca, Ba, La, Y, …; A = P, Si, …; B = F, Cl, OH, …), which has two types of Y3+ lattice sites (Wyckoff 4f and 6h).28
In the present study, a new NIR phosphor Yb3+-doped NaY9Si6O26 was synthesized using a solid-state reaction, and their crystal structures were identified using Rietveld refinement. The addition of an excess amount of Na2CO3 as a raw material results in a drastic increase in the ratio of the NaY9Si6O26 phase. As a result, a compound close to a single phase was obtained. The synthesized NaY9Si6O26:Yb3+ phosphor had an angular shape with an average grain size of 1–3 μm. The emission and excitation properties of NaY9Si6O26:Yb3+ phosphor were investigated to obtain a better understanding of its thermal stability.
Experimental
Yb3+-doped NaY9Si6O26 phosphors were synthesized using a conventional solid-state reaction. In the powdered form, Na2CO3 (Junsei Chemical Co., Ltd, 99%), Y2O3 (Daejung Chemical & Metal Co., Ltd, 99%), SiO2 (Junsei Chemical Co., Ltd, 99%), and Yb2O3 (Wako, 99.9%) were used as starting materials to synthesize NaY9Si6O26:Yb3+ phosphor. The amount of Yb3+ was adjusted between 1 and 15 mol% in the Yb3+-doped NaY9Si6O26 phosphor. Using an agate mortar, these raw materials were mixed in a nonstoichiometric ratio of 30 mol% with an excess of Na2CO3 as the chemical parameter for the single phase. For 8 h, the mixture was sintered at 1400 °C. After sintering, the samples were ground using an agate mortar.The crystal structures of the synthesized powder samples were identified using X-ray powder diffraction (XRD, Bruker D8 Advance). Rietveld analysis using the RIETAN-FP package was carried out to obtain detailed crystallographic data. The morphology of the powder was characterized by scanning electron microscopy (SEM, JEOL, JSM6700F). Photoluminescence emission (PL) and excitation (PLE) spectra were measured at room temperature using a fluorescence spectrophotometer (PSI, DARSA PRO 3400). The PL spectra of NaY9Si6O26:Yb3+ phosphor were obtained under 270 and 920 nm excitation, and the PLE spectra were recorded under 985 nm emission. Temperature-dependent PL spectra were measured in the temperature range 25–300 °C as appropriate.
Results and discussion
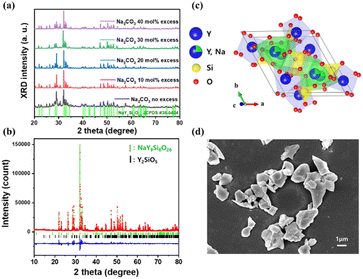
Fig. 1(a) shows the XRD patterns of the synthesized NaY9Si6O26:Yb3+ phosphors with an excess amount of Na2CO3 as raw material. NaY9Si6O26:Yb3+ was synthesized with a large amount of yttrium silicate impurities in a sample synthesized by stoichiometric mixing without the addition of Na2CO3. Yttrium silicate impurities are formed by the remaining Y and Si due to the volatilization behaviour of Na2CO3 raw material during sintering. The amount of impurities decreased with an increase in the amount of excess of Na2CO3. The sample with the least amount of impurities was synthesized at the addition of 30 mol% Na2CO3, and the impurities increased when more than 40 mol% Na2CO3 was added. The Rietveld refinement results for the XRD data of Na(Y0.9Yb0.1)9Si6O26 phosphor are shown in Fig. 1(b). The refined crystallographic data and parameters of the XRD patterns of Na(Y0.9Yb0.1)9Si6O26 phosphor are summarized in Table 1. The data from the Joint Committee on Powder Diffraction Standards (JCPDS No. 35-0404) corresponding to the NaY9Si6O26 phase with hexagonal structure obtained were used as the starting model. As shown in Table 1, the final R-factor values, Rwp, Rp, and S, converged to 5.84, 4.08, and 3.20%, respectively, which verifies the phase purity of the as-prepared sample. The XRD pattern of Na(Y0.9Yb0.1)9Si6O26 indexed mostly with the reported data, and also detected the presence of a small amount of Y2SiO5 impurity phase, which accounted for 6%. The final refined Na(Y0.9Yb0.1)9Si6O26 phase was hexagonal in the space group P63/m (No. 176) with refined lattice parameters a = b = 0.93386(0) nm, c = 0.67562(0) nm, and V = 0.58920 nm3. Fig. 1(c) shows a schematic of the NaY9Si6O26 crystal structure produced using VESTA,29 which corresponds to the apatite structure with two other Y3+ sites: Wyckoff 4f and 6h.28 The Y3+ situated at the 6h site (Y1) was surrounded by seven oxygen atoms, whereas the Na+ and Y3+ situated at the 4f sites (Y2) were coordinated by nine oxygen atoms. Simultaneously, the Si4+ ion was 4-fold coordinated by four oxygen atoms to form a tetrahedron. The ionic radii of 7-coordinated Y3+ and Yb3+ were calculated to be 0.0960 and 0.0925 nm, respectively, whereas those of 9-coordinated Y3+ and Yb3+ were calculated to be 0.1075 and 0.1042 nm, respectively. Therefore, based on their similar ionic radii and identical valences, the Yb3+ ions were expected to substitute the Y3+ lattices in the NaY9Si6O26 crystal structure. The shapes of NaY9Si6O26:Yb3+ phosphors measured by SEM are shown in Fig. 1(d). The phosphor powder particles were composed of angular-shaped fine grains with an average size of 1–3 μm.| Atom | Site | Occ. | x | y | z | Beq. |
|---|---|---|---|---|---|---|
| Y1 | 6h | 0.85 | 0.23336 | 0.23788 | 0.25 | 0.5 |
| Yb1 | 6h | 0.15 | 0.23336 | 0.23788 | 0.25 | 0.5 |
| Y2 | 4f | 0.75 | 0.33333 | 0.66667 | 0.49888 | 0.5 |
| Na1 | 4f | 0.25 | 0.33333 | 0.66667 | 0.49888 | 0.5 |
| Si1 | 6h | 1 | 0.39760 | 0.02662 | 0.25 | 0.5 |
| O1 | 2a | 1 | 0.0 | 0.0 | 0.25 | 0.5 |
| O2 | 6h | 1 | 0.59474 | 0.12790 | 0.25 | 0.5 |
| O3 | 6h | 1 | 0.33109 | −0.15883 | 0.25 | 0.5 |
| O4 | 12i | 1 | 0.33882 | 0.08590 | 0.06434 | 0.5 |
Fig. 2 shows the XRD patterns of NaY9Si6O26:Yb3+ phosphors with varying concentrations. The XRD peaks of all the samples were almost identical to those of the NaY9Si6O26 phase (JCPDS No. 35-0404), which had hexagonal structure with space group P63/m. As the Yb3+ ion concentration in the NaY9Si6O26:Yb3+ phosphors increased, a peak shift to a higher diffraction angle was observed because Y3+ ions (ionic radius: 0.0960 nm for 7 coordination) in the host material were partially substituted with smaller Yb3+ ions (ionic radius: 0.0925 nm for 7 coordination) to form a solid solution.
 | ||
| Fig. 2 XRD patterns of NaY9Si6O26:Yb3+ phosphor doped with varying concentrations and NaY9Si6O26 as reference (JCPDS #35-0404). | ||
The excitation and emission spectra of NaY9Si6O26:Yb3+ phosphors with varying concentrations of Yb3+ ions are shown in Fig. 3. The PLE spectra recorded by monitoring the emission at 985 nm exhibited a excitation band from 220 to 300 nm. A peak was observed at 270 nm in the ultraviolet region owing to the charge transfer transition, which involves transfer of electrons from the ligand anion O2− to the central cation Yb3+ ion.30 The PL spectra of the NaY9Si6O26:Yb3+ phosphors recorded under 270 nm excitation showed a strong NIR emission band in the range 950–1100 nm, which corresponds to the 2F5/2 → 2F7/2 spin-allowed transitions of Yb3+ ions. The simple 4f13 electron configuration of Yb3+ has a unique spectral term with two multiplets: ground state multiplets 2F7/2 and excited state multiplets 2F5/2. Variation in the splitting of the Stark level of Yb3+ ions was observed because of the differences in the local crystal fields. The ground state, 2F7/2 splits into four Stark levels, and the excited state, 2F5/2 splits into three levels.31 The emission peak intensity of NaY9Si6O26:Yb3+ phosphors increased with increasing Yb3+ concentration up to 10 mol% and then decreased, probably because of the concentration quenching effect.
 | ||
| Fig. 3 (a) Excitation (λem = 985 nm), and (b) emission (λex = 270 nm) spectra of NaY9Si6O26:Yb3+ phosphors with varying concentrations of Yb3+ ions. | ||
In recent years, counterfeiting technology of security materials has advanced, making anti-counterfeiting technology an important tool for protecting the legitimate rights of consumers and enterprises.32,33 It is therefore imperative to develop new materials for advanced anti-counterfeiting security. For this application, it is necessary to develop an NIR-emitting material that is sensitive in the NIR region beyond the existing method of checking security through ultraviolet excitation. In terms of security level, it can be applied to level 3 (forensic), which can be read only with dedicated equipment, beyond level 1, which can be visually identified, and level 2, which can be verified with general equipment. In order to explore the possibilities of these applications, the excitation and emission spectra of NaY9Si6O26:Yb3+ phosphors in the NIR region and are shown in Fig. 4. The PLE spectra recorded by monitoring the emission at 985 nm exhibited a excitation band from 870 to 930 nm, as shown in Fig. 4(a). This can be attributed to the transition from the lowest ground state Stark level to the excited state Stark level in Yb3+ ions. In Fig. 4(b), the PL spectra of NaY9Si6O26:Yb3+ phosphors excited at 920 nm show NIR emission from 960 to 1100 nm, which was attributed to the transition from the lowest Stark level of 2F5/2 to four Stark levels of the ground state 2F7/2 in Yb3+ ions.
 | ||
| Fig. 4 (a) Excitation (λem = 985 nm), and (b) emission (λex = 920 nm) spectra of NaY9Si6O26:Yb3+ phosphors with varying concentrations of Yb3+ ions in NIR region. | ||
The thermal degradation behavior of NIR phosphors is an imperative factor for advanced anti-counterfeiting security because excellent thermal conductivity is necessary to employ the NIR emitting material in fiber application products (clothes, banknotes, passports, etc.). The temperature dependence of NaY9Si6O26:Yb3+ phosphor emission was measured to investigate its thermal stability. Fig. 5 shows the temperature-dependent PL intensity under 270 nm excitation in the temperature range 25–300 °C. The emission intensity of NaY9Si6O26:Yb3+ phosphor decreased with increasing temperature. The relative PL intensity of NaY9Si6O26:Yb3+ phosphor was 82% of the initial PL intensity at 300 °C, indicating its excellent thermal stability. Moreover, its thermal quenching behavior can be attributed to the non-radiative relaxation of thermally activated electron–phonon coupling.34 Finally, after heating the NaY9Si6O26:Yb3+ phosphor to 300 °C and then cooling to room temperature, the NIR emission intensity recovered nearly 100% of its initial intensity, indicating that thermal degradation was minimal. This suggests applicability to advanced anti-counterfeiting applications.
 | ||
| Fig. 5 Temperature dependence of the PL emission intensity of NaY9Si6O26:Yb3+ phosphor. The inset shows the emission spectra. | ||
Conclusions
We developed the new NIR-emitting Yb3+-doped NaY9Si6O26 phosphors using conventional solid-state reaction. The synthesized phosphors showed a hexagonal crystal structure corresponding to the apatite compound with a small amount of yttrium silicate impurities. It showed angular-shaped fine grains with an average grain size of 1–3 μm. The PLE spectra of the NaY9Si6O26:Yb3+ phosphors, monitored at an emission wavelength of 985 nm, showed two excitation bands, 220–300 nm and 870–970 nm, respectively. The former was attributed to the charge transfer transition of Yb3+ ions, and the latter was attributed to the transition from the lowest Stark level of the ground 2F7/2 to three Stark levels of the excited 2F5/2 in Yb3+ ions. The emission spectra under 270 and 920 nm excitation presented a NIR emission band from 950 to 1100 nm, which corresponds to the transition of Yb3+ ions from the lowest Stark level of the excited 2F5/2 to four Stark levels of the ground 2F7/2. These luminescent properties indicate that it can be used as a material in the security field that is sensitive to the NIR as well as the ultraviolet region. The excellent thermal stability of NaY9Si6O26:Yb3+ phosphor was evident from the fact that the relative PL intensity of NaY9Si6O26:Yb3+ phosphor was 82% of the initial PL intensity at 300 °C. The emission intensity that showed thermal quenching up to 300 °C recovered to its initial emission intensity when cooled to 25 °C. Thus, it was confirmed that a minimal amount of thermal degradation occurred. The results of our study suggest that NaY9Si6O26:Yb3+ phosphors are promising next-generation candidates for advanced anti-counterfeiting applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Technology Innovation Program (20021925, Development of Environment-Friendly Security Fiber and Application Products Using Near-Infrared Light Pigments Over 1000 nm) funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).Notes and references
- H. W. Siesler, Y. Ozaki, S. Kawata and H. M. Heise, Near-Infrared Spectroscopy: Principles, Instruments, and Applications, John Wiley & Sons, Germany, 2008 Search PubMed.
- Z. Y. Wang, Near-Infrared Organic Materials and Emerging Applications, CRC Press, Boca Raton, 2013 Search PubMed.
- K. Svoboda and S. M. Block, Biological Applications of Optical Forces, Annu. Rev. Biophys. Biomol. Struct., 1994, 23, 247–285 CrossRef CAS PubMed.
- M. Wolf, M. Ferrari and V. Quaresima, Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications, J. Biomed. Opt., 2007, 12, 062104 CrossRef PubMed.
- V. Ntziachristos, C. Bremer and R. Weissleder, Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging, Eur. Radiol., 2003, 13, 195 CrossRef PubMed.
- V. Rajendran, H. Chang and R. S. Liu, Recent progress on broadband near-infrared phosphors-converted light-emitting diodes for future miniature spectrometers, Opt. Mater.: X, 2019, 1, 100011 CAS.
- V. Rajendran, M.-H. Fang, G. N. D. Guzman, T. Lesniewski, S. Mahlik, M. Grinberg, G. Leniec, S. M. Kaczmarek, Y.-S. Lin, K.-M. Lus, C.-M. Lin, H. Chang, S.-F. Hu and R.-S. Liu, Super broadband near-infrared phosphors with high radiant flux as future light sources for spectroscopy applications, ACS Energy Lett., 2018, 3, 2679 CrossRef CAS.
- D. Kim, Y. Jung, K.-A. Toh, B. Son and J. Kim, An empirical study on iris recognition in a mobile phone, Expert Syst. Appl., 2016, 54, 328 CrossRef.
- R. Filippo, E. Taralli and M. Rajteri, LEDs: sources and intrinsically bandwidth-limited detectors, Sensors, 2017, 17, 1673 CrossRef PubMed.
- S. Fuchi and Y. Takeda, Wideband near-infrared phosphor by stacking Sm3+ doped glass underneath Yb3+, Nd3+ co-doped glass, Phys. Status Solidi C, 2011, 8, 2653 CrossRef CAS.
- Q. Shao, H. Ding, L. Yao, J. Xu, C. Liang and J. Jiang, Photoluminescence properties of a ScBO3:Cr3+ phosphor and its applications for broadband near-infrared LEDs, RSC Adv., 2018, 8, 12035 RSC.
- Q. Shao, H. Ding, L. Yao, J. Xu, C. Liang, Z. Li, Y. Dong and J. Jiang, Broadband near infrared light source derived from Cr3+-doped phosphors and a blue LED chip, Opt. Lett., 2018, 43, 5251 CrossRef CAS PubMed.
- Z. Pan, Y.-Y. Lu and F. Liu, Sunlight-activated long-persistent luminescence in the near infrared from Cr3+-doped zinc gallo germanates, Nat. Mater., 2011, 11, 58 CrossRef PubMed.
- H. Zeng, T. Zhou, L. Wang and R. J. Xie, Two-site occupation for exploring ultrabroadband near-infrared phosphor—double-perovskite La2MgZrO6:Cr3+, Chem. Mater., 2019, 31, 5245 CrossRef CAS.
- T. Gao, W. Zhuang, R. Liu, Y. Liu, C. Yan, J. Tian, G. Chen, X. Chen, Y. Zheng and L. Wang, Site occupancy and enhanced luminescence of broadband NIR gallo germanate phosphors by energy transfer, J. Am. Ceram. Soc., 2019, 103, 202 CrossRef.
- Y. Zhong, S. Gai, M. Xia, S. Gu, Y. Zhang, X. Wu, J. Wang, N. Zhou and Z. Zhou, Enhancing quantum efficiency and tuning photoluminescence properties in far-redemitting phosphor Ca14Ga10Zn6O35:Mn4+ based on chemical unit engineering, Chem. Eng. J., 2019, 374, 381 CrossRef.
- L. Zhang, D. Wang, Z. Hao, X. Zhang, G. h. Pan, H. Wu and J. Zhang, Cr3+-doped broadband NIR garnet phosphor with enhanced luminescence and its application in NIR spectroscopy, Adv. Opt. Mater., 2019, 7, 1900185 CrossRef.
- L. Sánchez-García, M. O. Ramírez, R. M. Solé, J. J. Carvajal, F. Díaz and L. E. Bausá, Plasmon-induced dual-wavelength operation in a Yb3+ laser, Light Sci. Appl., 2019, 8, 1 CrossRef PubMed.
- B. T. Huy, D. H. Kwon, S. S. Lee, V. D. Dao, H. B. Truong and Y. I. Lee, Optical properties of Sr2YF7 material doped with Yb3+, Er3+, and Eu3+ ions for solar cell application, J. Alloys Compd., 2022, 897, 163189 CrossRef CAS.
- Y. Xu, L. Zhang, L. Dong, S. Yin, X. Wu and H. You, Novel SrGd2Al2O7:Mn4+, Nd3+, and Yb3+ phosphors for c-Si solar cells, Dalton Trans., 2021, 50, 7017 RSC.
- J. Miao, M. Chen, Z. Chen, L. Zhang, S. Wei and X. Yang, Effect of Yb3+ concentration on upconversion luminescence and optical thermometry sensitivity of La2MoO6: Yb3+, Er3+ phosphors, Appl. Opt., 2021, 60, 1508 CrossRef PubMed.
- S. Liu, H. Ming, J. Cui, S. Liu, W. You, X. Ye, Y. Yang, H. Nie and R. Wang, Color-tunable upconversion luminescence and multiple temperature sensing and optical heating properties of Ba3Y4O9:Er3+/Yb3+ phosphors, J. Phys. Chem. C, 2018, 122, 16289 CrossRef CAS.
- K. Pavani, J. Suresh Kumar, K. Srikanth, M. J. Soares, E. Pereira, A. J. Neves and M. P. E. Graca, Highly efficient upconversion of Er3+ in Yb3+ codoped non-cytotoxic strontium lanthanum aluminate phosphor for low temperature sensors, Sci. Rep., 2017, 7, 17646 CrossRef CAS PubMed.
- D. K. Zharkov, A. G. Shmelev, A. V. Leontyev, V. G. Nikiforov, V. S. Lobkov, M. H. Alkahtani, P. R. Hemmer and V. V. Samartsev, Light converting Yb3+/Er3+ doped YVO4 nanoparticles for biological applications, Laser Phys. Lett., 2020, 17, 075901 CrossRef CAS.
- M. Shang, D. Geng, D. Yang, X. Kang, Y. Zhang and J. Lin, Luminescence and Energy Transfer Properties of Ca2Ba3(PO4)3Cl and Ca2Ba3(PO4)3Cl:A (A = Eu2+/Ce3+/Dy3+/Tb3+) under UV and Low-Voltage Electron Beam Excitation, Inorg. Chem., 2013, 52, 3102 CrossRef CAS PubMed.
- L. Wang, B. K. Moon, B. C. Choi, J. H. Kim, J. Shi and J. H. Jeong, Photoluminescent properties and site occupation preference in Bi3+, Eu3+ doped CaY4(SiO4)3O phosphor, Ceram. Int., 2016, 42, 12971 CrossRef CAS.
- W. Zhou, F. Pan, L. Zhou, D. Hou, Y. Huang, Y. Tao and H. Liang, Site Occupancies, Luminescence, and Thermometric Properties of LiY9(SiO4)6O2:Ce3+ Phosphors, Inorg. Chem., 2016, 55, 10415 CrossRef CAS PubMed.
- G. R. Redhammer and G. Roth, Lithium and Sodium Yttrium Orthosilicate Oxyapatite, LiY9(SiO4)6O2 and NaY9(SiO4)6O2, at Both 100 K and Near Room Temperature, Acta Crystallogr., 2003, 59, i120 Search PubMed.
- K. Momma and F. Izumi, VESTA: a three-dimensional visualization system for electronic and structural analysis, J. Appl. Crystallogr., 2008, 41, 653 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer, Heidelberg, 1994 Search PubMed.
- L. Sánchez-García, M. O. Ramírez, C. Tserkezis, R. Sole, J. J. Carvajal, M. Aguiló, F. Díaz and L. E. Bausá, Anisotropic Enhancement of Yb3+ Luminescence by Disordered Plasmonic Networks Self assembled on RbTiOPO4 Ferroelectric Crystals, Nanoscale, 2017, 9, 16166 RSC.
- G. Cai, T. Delgado, C. Richard and B. Viana, ZGSO Spinel Nanoparticles with Dual Emission of NIR Persistent Luminescence for Anti-Counterfeiting Applications, Materials, 2023, 16, 1132 CrossRef CAS PubMed.
- Y. Xiao, P. Xiong, S. Zhang, K. Chen, S. Tian, Y. Sun, P. Shao, K. Qin, M. G. Brik, S. Ye, D. Chen and Z. Yang, Deep-red to NIR mechanoluminescence in centrosymmetric perovskite MgGeO3: Mn2+ for potential dynamic signature anti-counterfeiting, Chem. Eng. J., 2023, 453, 139671 CrossRef CAS.
- M. Kaczkan, M. Malinowski, A. Suchocki, D. A. Pawlak and S. Turczyński, Temperature and concentration dependent luminescence of Yb3+ centers in YAM, J. Alloys Compd., 2020, 842, 155893 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |