Open Access Article
Open Access ArticleA new desorption method of polyurethane foam for the determination of gold and a comparative study on four desorption methods based on meta-analysis
Yaru Houa,
Jilong Lu *a,
Mao Lib,
Qiaoqiao Weia,
Yuchao Fana and
Yongzhi Wanga
*a,
Mao Lib,
Qiaoqiao Weia,
Yuchao Fana and
Yongzhi Wanga
aCollege of Geo-exploration Science and Technology, Jilin University, Changchun, 130026, People's Republic of China. E-mail: lujl@jlu.edu.cn
bThe Second Hospital of Jilin University, Changchun, 130041, People's Republic of China
First published on 20th April 2023
Abstract
Accurate determination of gold in geological samples is an important prerequisite and guarantee for studying geological problems. There are many methods for digestion and enrichment of gold among which polyurethane foam (PUF) enrichment after aqua regia digestion is the most commonly used in the experiments. A new method to help the relief of gold from the PUF was put forward in this study, and it was applied to four certified reference materials (CRMs) together with three previously used methods, and the optimal extraction and enrichment conditions were determined through experiments. The four methods were compared by meta-analysis, and the thiourea liberation method was superior to the other three methods because of its simple operation and high accuracy. Out of consideration for the incomplete adsorption of gold in the solution by only one piece of PUF, repetitive adsorption of gold with a second and a third piece of PUF in the solution was proposed in this study. Results show that the gold content obtained by secondary and tertiary adsorption accounts for 11.03% of the total content, and the highest can reach 20.74%. When the third adsorption was carried out, the gold content in several samples was below the detection limit. Therefore, repeated adsorption of gold in the solution is necessary, and three times of adsorption is necessary.
1. Introduction
Gold is a noble metal with high ductility, corrosion resistance and chemical stability. It has important applications in geology, materials, chemistry, biology, medicine and other fields because of its special properties. Studies of the source, enrichment process, and state of gold are of great guiding significance in gold exploration. However, the distribution of gold in various geological samples is uneven and often exists in a low content which makes the separation, enrichment,1 and accurate determination of gold a difficult problem in geological analysis.The treatment methods before gold determination include dry ashing,2–4 and wet acid digestion. Enrichment, such as precipitation,5 ion exchange resin,6 solvent extraction,7–9 PUF adsorption,10,11 extraction chromatography,12 is followed. There are many testing methods for gold, including atomic absorption spectrometry,13 inductively coupled plasma mass spectrometry14,15 and so on. However, the uncertainty of gold determination is often high due to incomplete sample digestion, incomplete adsorption and desorption process of gold, high detection limit of instruments, and interference of instrument matrix.
PUF was widely used in various field16–18 and was first applied to adsorb gold in acidic medium in 1970 by Bowen.19 PUF is made of toluene diisocyanate and polyether polyol. The carbon dioxide generated in the synthesis process is left in the polymer to form spongy foam plastic with a certain degree of cross-chain. When the gold in the solution meets the foam, adsorption and exchange occur on the foam membrane potential. The coordination anions of gold would be bonded with the active groups of –CH2–O–CH2 and –O–C–NHR on the foam skeleton, so that the two would combine to form a stable ionic association.20–23 In recent years, PUF has been widely used in the determination of trace gold in geological samples because of its strong adsorption performance and low price. Desorption is required before determination. There are several methods of desorption of gold, so it is of great significance to choose the best way among them.24–27
Meta-analysis is widely used in medicine, psychology and other fields to conduct comprehensive quantitative analysis of many research results of the same subject with specific conditions, which is superior to the comprehensive analysis ability of conventional literature review.28 However, it is rare in the study of geological phenomena.29–32 Meta-analysis is applied to the correlation between a specific exposure factor and a specific outcome in medical analysis.33–35 It is feasible to transform the original geochemical data into valid data which can be identified by meta-analysis, and to obtain the mean differences of four different methods so as to compare different methods.
The desorption methods of PUF were discussed and a new method of desorption of PUF was proposed. Meta-analysis was applied to compare the four desorption methods of PUF. Considering the incomplete adsorption of one piece of PUF, the necessity of repeated adsorption was put forward and verified. This study aims to establish an accurate, simple, and rapid process for the determination of gold in geological samples through a comparative study of sample digestion method, the time of PUF adsorption, the way of PUF desorption.
2. Materials and methods
2.1. Reagents and standards
Hydrochloric acid (HCl), nitric acid (HNO3) of analytical grade and deionized water were used in sample digestion. 250 g L−1 ferric chloride solution with 1% HCl was used during adsorption. 10 g L−1 thiourea solution, 200 g L−1 potassium chloride solution and 500 g L−1 potassium bromide solution, methyl isobutyl ketone (MIBK), and potassium chlorate of analytical grade were employed in the desorption process.National standard gold single element solution (GSB-1715-2004, 1000 mg L−1) was acquired from the National Center of Analysis and Testing for Nonferrous Metals and Electronic Materials. CRMs (GBW07248a, GBW07808b, GBW07809b) were obtained from the Institute of Geophysical and Geochemical Exploration, Chinese Academy of Geological Sciences (Langfang, China). CRM GBW07192 was obtained from the Central South Institute of Metallurgical Geology (Yichang, China).
2.2. Instrumentation
Samples were triturated by a planetary ball mill (QM-3SP4, Laibu, China) and dried in an air-dry oven (DHG-9423A, Shanghai Jinghong, China) at 105 °C. Cooled sample powder was weighed with an electronic balance (ATY124, Shimadzu, Japan) and ashed in a muffle furnace (SXL-1008, Shanghai Jinghong, China). The electric heating plate (SB-1.8-4, Shanghai Shiyan, China) was used in sample digestion. The cyclotron oscillator (HY-8A, Jintan Jingda, China) was used to ensure the gold was absorbed by PUF completely. The constant temperature water bath (HH-S26S, Jintan Instruments, China) was employed in the desorption of gold. Gold determination was performed on an inductively coupled plasma mass spectrometry (ICP-MS) (Nexion 350D, PerkinElmer, USA). The operating parameters of ICP-MS were listed in Table 1.| Parameter | Setting | Parameter | Setting |
|---|---|---|---|
| Radiofrequency power/W | 1150 | Sampling cone aperture/mm | 1.2 |
| Cooling gas flow/(L min−1) | 18 | Skimmer cone aperture/mm | 1 |
| Auxiliary gas flow/(L min−1) | 1.2 | Scanning times | 20 |
| Nebulizer gas flow/(L min−1) | 0.92 | Sampling time/s | 60 |
3. Experiments
3.1. Pretreatment of PUF
The internal residues left during the production of PUF such as carbamido, allophanate, biuret, and isocyanate will greatly reduce the adsorption performance of the PUF which leads to the degradation of the adsorption performance. Hence, it is necessary to pretreat the PUF before the adsorption experiment. First, the PUF was cut into small squares of about 0.2 g (3 × 2 × 1 cm3), socked into sodium hydroxide solution and boiled for 30 minutes, washed with deionized water until neutral. Then, it was transferred into 10% HCl and soaked for 2 h, washed again and dipped in deionized water for 2 h, squeeze the water out, dried naturally for later use.363.2. Sample digestion
Herein, the high-temperature ashing-acid digestion and PUF adsorption were performed to determine the content of gold in rock samples according to the geological and mineral industry standard DZ/T 0279.4-2016 of the People's Republic of China. 10.0000 g of sample was accurately weighed into a porcelain crucible and sent to a muffle furnace for 2 h at 700 °C, during which the furnace was opened twice for oxygen supplement to ensure that the sample powder was ashed completely. After cooling, the sample was transferred to a conical flask, several drops of water were added to moisten the powder, 30 mL 50% aqua regia was added to dissolve the sample, place the conical flask on the electric heating plate with the surface dish covered. Remove the surface dish one hour later and continue to evaporate until the solution is about 10 mL. 70 mL water and 3 mL FeCl3 were added with a PUF to the cooling solution. The conical flask was then placed on the cyclotron oscillator for 30 minutes.10,11 The PUF was taken out, the residue was washed and squeezed for later use.3.3. Desorption of gold
Four methods of desorption of gold from PUF were performed in the experiment, among which method III was a new method proposed in this study.4. Results and discussion
4.1. Validation of methods
Four CRMs with different content of gold were digested and absorbed under the same condition and extricated with four different methods. The logarithmic deviation (Δlg![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) ) between the measured mean value and the standard value of the CRMs and the relative standard deviation (RSD) between the measured value and the standard value of the CRMs were calculated to measure the accuracy and precision of the methods according to the geological and mineral industry standard DZ/T 0011-2015 of the People's Republic of China. Δlg
) between the measured mean value and the standard value of the CRMs and the relative standard deviation (RSD) between the measured value and the standard value of the CRMs were calculated to measure the accuracy and precision of the methods according to the geological and mineral industry standard DZ/T 0011-2015 of the People's Republic of China. Δlg ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) and RSD can be evaluated by the following equations:
and RSD can be evaluated by the following equations:
Δlg ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) = |lg = |lg ![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) i − lg Cs| i − lg Cs|
| (1) |
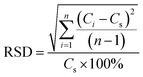 | (2) |
![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) is the mean of parallel measurements, Ci is the value of parallel measurements, Cs is the standard value of CRM, n = 5 is the number of parallel experiments. The Δlg
is the mean of parallel measurements, Ci is the value of parallel measurements, Cs is the standard value of CRM, n = 5 is the number of parallel experiments. The Δlg![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) and RSD acquired are all less than 0.11 and 10% respectively, indicating that the accuracy and precision of the methods are qualified.
and RSD acquired are all less than 0.11 and 10% respectively, indicating that the accuracy and precision of the methods are qualified.
4.2. Comparison of methods
The gold in the sample was absorbed three times of oscillating and the desorption of PUF was performed with the above four different methods. Parallel experiments were carried out five times under the same conditions, and the experimental results were shown in Table 2.| CRM | Standard value (μg g−1) | Method | Content (μg g−1) | Recovery rate (%) | Relative error (%) |
|---|---|---|---|---|---|
| GBW07192 | 15.100 | I | 15.278 | 101.18 | 1.18 |
| II | 14.130 | 93.58 | 6.42 | ||
| III | 14.418 | 95.48 | 4.52 | ||
| IV | 14.298 | 94.69 | 5.31 | ||
| GBW07248a | 0.101 | I | 0.107 | 105.96 | 5.96 |
| II | 0.102 | 101.41 | 1.41 | ||
| III | 0.105 | 103.80 | 3.80 | ||
| IV | 0.105 | 103.96 | 3.96 | ||
| GBW07808b | 3.300 | I | 3.353 | 101.61 | 1.62 |
| II | 3.253 | 98.58 | 1.42 | ||
| III | 3.280 | 99.39 | 0.61 | ||
| IV | 3.245 | 98.32 | 1.68 | ||
| GBW07809a | 10.800 | I | 10.771 | 99.73 | 0.27 |
| II | 10.706 | 99.13 | 0.87 | ||
| III | 10.744 | 99.48 | 0.52 | ||
| IV | 10.443 | 96.69 | 3.31 |
All the above four methods can meet the test requirements, among which the method I is highly accurate and efficient which is suitable for laboratory testing except that it requires a large amount of acid and is dangerous to operate. Method II has been widely applied in geological samples determination especially during laboratory analysis and testing with high accuracy. This method requires the use of a glass bar to squeeze the PUF which leads to the fracture of the bottom of the colorimetric tube and the loss of the sample, so it is recommended to use a glass bar with a rubber plug at one end and squeeze the PUF for more than 200 times. Method III is relatively simple and convenient to operate with small consumption of reagent which is especially suitable for the extraction of large quantities of gold during industry work but has the disadvantages of cumbersome transfer extraction, long process, high energy consumption of muffle furnace. It is easy to cause the problem of unqualified accuracy and precision of method IV due to the unstable absorbance of the instrument of organic solvents and the use of organic solvents in the process is not environmentally friendly. In addition, it is unkind for ICP-MS to determine the content of solution with organic reagent as solvent.
4.3. Meta-analysis
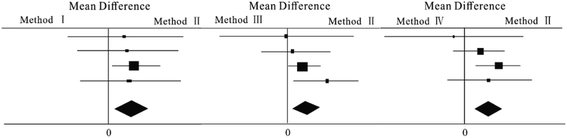
In the meta-analysis, when the probability of heterogeneity test is P > 0.05, multiple independent studies can be considered to be homogenous, and a fixed effect model can be selected to calculate their combined statistics. When the probability of the population effect test is P < 0.05, it is statistically significant. The diamond at the bottom of the forest plot represents the combined results of multiple randomized controlled trials (RCTs). The figure was divided into two halves, left and right, to judge whether the difference is statistically significant. The left side of the line is the experimental group, namely the treatment group, and the right side is the control group. When the diamond intersects the vertical line, there is no statistical significance between different methods in the RCT. The position and meaning of the diamond are as follows: for adverse outcomes such as disease events and death, when the diamond is completely on the left of the vertical line, the treatment group is more effective; when the diamond is completely on the right, the control group is more effective; For favorable outcomes such as remission and cure, the position and meaning of the diamond are opposite; The greater the distance of the rhombus from the vertical line, the more obvious the effectiveness difference between the two schemes.In this study, four methods were used to test the relative errors and relative standard deviations of four samples for meta-analysis. The forest map obtained is shown in Fig. 1. The analysis results are shown in Table 3. Results indicated that the four independent methods were homogeneous, and the probability of the overall effect test was less than 0.05, which had significant statistical significance. All the three mean differences were greater than 0, the mean difference of the method II and method III are equal in value and both less than method IV, which indicated that the effect of method I in the control group was more significant than that in the experimental groups. Method II and method III are more accurate and effective than method IV under the experimental conditions set up in this study.
| Treatment group | Control group | Heterogeneity test | Overall effect test | Significance |
|---|---|---|---|---|
| a I2 is the statistic; P is the probability of the statistic. | ||||
| Method I | Method II | P = 0.52; I2 = 0% | P = 0.006; MF = 0.02 | Significant |
| Method III | Method II | P = 0.99; I2 = 0% | P = 0.006; MF = 0.02 | Significant |
| Method IV | Method II | P = 0.57; I2 = 0% | P = 0.004; MF = 0.03 | Significant |
Of course, the experimental conditions employed in this study including the time and temperature of adsorption, desorption, and ashing burning, the amount of oxidant may introduce a certain degree of impact on the measurement results, which deserved to be explored and optimized in the subsequent study.
5. Repetitive adsorption
In order to avoid the incompleteness of single adsorption of PUF which led to the inaccurate determination of gold, the gold in the solution was adsorbed three times under the premise of ensuring the same PUF treatment mode, adsorption mode and foam treatment mode in this experiment, and finally, the sum of the three adsorption times was obtained as the total gold content.It can be seen from Table 4 that the gold content determined after the second and third adsorption accounted for more than 3.26% of the total gold content, with an average of 11.03% and a maximum of 20.74%. Among them, the gold content determined after the second adsorption accounted for more than 3.14% of the total gold content, with an average of 9.23% and the highest of 16.19%. After the third adsorption, the determined gold content decreased significantly, with an average value of 1.79%. When the third adsorption was carried out, the gold content of some samples was already below the detection limit.
| CRM | Method | Time of adsorption | Sum (μg g−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 (μg g−1) | ratio (%) | 2 (μg g−1) | ratio (%) | 3 (μg g−1) | ratio (%) | |||
| GBW07192 | I | 14.280 | 93.47 | 0.768 | 5.03 | 0.230 | 1.51 | 15.278 |
| II | 13.669 | 96.74 | 0.443 | 3.14 | 0.018 | 0.13 | 14.130 | |
| III | 13.560 | 94.05 | 0.630 | 4.37 | 0.228 | 1.58 | 14.418 | |
| IV | 12.961 | 90.65 | 0.947 | 6.62 | 0.390 | 2.73 | 14.298 | |
| GBW07248a | I | 0.086 | 80.37 | 0.015 | 14.02 | 0.006 | 5.61 | 0.107 |
| II | 0.086 | 84.31 | 0.014 | 13.73 | 0.002 | 1.96 | 0.102 | |
| III | 0.083 | 79.05 | 0.019 | 18.10 | 0.003 | 2.86 | 0.105 | |
| IV | 0.084 | 80.00 | 0.017 | 16.19 | 0.004 | 3.81 | 0.105 | |
| GBW07808b | I | 2.992 | 89.23 | 0.320 | 9.54 | 0.041 | 1.22 | 3.353 |
| II | 2.883 | 88.63 | 0.342 | 10.51 | 0.028 | 0.86 | 3.253 | |
| III | 2.840 | 86.59 | 0.359 | 10.95 | 0.081 | 2.47 | 3.280 | |
| IV | 2.831 | 87.24 | 0.392 | 12.08 | 0.022 | 0.68 | 3.245 | |
| GBW07809b | I | 10.102 | 93.79 | 0.554 | 5.14 | 0.115 | 1.07 | 10.771 |
| II | 10.281 | 96.03 | 0.339 | 3.17 | 0.086 | 0.80 | 10.706 | |
| III | 9.684 | 90.13 | 0.948 | 8.82 | 0.112 | 1.04 | 10.744 | |
| IV | 9.713 | 93.00 | 0.689 | 6.60 | 0.041 | 0.39 | 10.443 | |
6. Conclusions
The new method of the desorption of gold proposed in this study has the advantages of simple operation and time-saving. The accuracy and precision of all these four methods can meet the test requirements. The reagent dosage, proportion and detailed operation process of each method were proposed, which can provide a basis for the determination of gold in geological samples.The four PUF desorption methods were compared and the thiourea liberation method was determined as the best method to help gold relieve from the PUF by meta-analysis. The advantages of this method are high accuracy, high precision, and simple operation. Ashing burning method and HNO3–KClO3 decomposition method followed, while the organic solvent extraction method is not stable enough and the introduction of organic solvents was not environmentally friendly. The thiourea liberation method and HNO3–KClO3 decomposition method with high accuracy and efficiency were suitable for laboratory analysis and determination while the ashing burning method can be employed in industry work. Meta-analysis can quantitatively compare the results of different methods which has immeasurable application potential in the geochemical field.
In this study, repeated adsorption of gold in the solution was proposed. Repeated adsorption experiments were carried out for each desorption method. Experiment results showed that the gold content of secondary adsorption and tertiary adsorption accounted for 11.03% of the total content, and the highest was 20.74%. Moreover, the lower the gold content in the sample, the higher the ratio of determining gold content to total content after repeated adsorption. Therefore, it is necessary to carry out repeated adsorption of PUF under the adsorption and desorption conditions of setting according to this experiment, and three times of adsorption is enough.
Author contributions
Yaru Hou: Conceptualization, methodology, reparation, data processing, experimenting, validation, writing-reviewing. Jilong Lu: conceptualization, project administration, funding acquisition, reviewing. Mao Li: software, validation, investigation, writing-reviewing. Qiaoqiao Wei: methodology, reparation. Yuchao Fan: reparation, reviewing. Yongzhi Wang: data processing, writing-reviewing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the State Key Research and Development Program, grant number [2021YFC2901801, 2016YFC0600606].References
- L. Fischer, B. Moser and S. Hann, Molecules, 2021, 26 Search PubMed.
- C. I. Cerceau, C. F. Carvalho, A. C. S. Rabelo, C. G. Dos Santos, S. M. D. Goncalves and E. V. V. Varejao, J. Environ. Manage., 2016, 183, 771–776 CrossRef CAS PubMed.
- M.-R. Juvonen, A. Bartha, T. M. Lakomaa, L. A. Soikkeli, E. Bertalan, E. I. Kallio and M. Ballok, Geostand. Geoanal. Res., 2004, 28, 123–130 CrossRef CAS.
- K. A. Porter, C. Kirk, D. Fearey, L. J. Castrodale, D. Verbrugge and J. McLaughlin, Publ. Health Rep., 2015, 130, 440–446 CrossRef PubMed.
- J. G. S. Gupta, Talanta, 1989, 36, 651–656 CrossRef CAS PubMed.
- R. Al-Merey, Z. Hariri and J. Abu Hilal, Microchem. J., 2003, 75, 169–177 CrossRef CAS.
- S. Chen, M. Bas, S. Happel, P. Randhawa, S. McNeil, E. Kurakina, S. Zeisler, K. Maskell, C. Hoehr, C. F. Ramogida and V. Radchenko, J. Chromatogr. A, 2023, 1688, 463717 CrossRef CAS PubMed.
- I. De la Calle, F. Pena-Pereira, N. Cabaleiro, I. Lavilla and C. Bendicho, Talanta, 2011, 84, 109–115 CrossRef CAS PubMed.
- M. S. El-Shahawi, A. S. Bashammakh and S. O. Bahaffi, Talanta, 2007, 72, 1494–1499 CrossRef CAS PubMed.
- A. S. Bashammakh, S. O. Bahaffi, F. M. Al-Shareef and M. S. El-Shahawi, Anal. Sci., 2009, 25, 413–418 CrossRef CAS PubMed.
- X. Tang, H. Li, H. Liu, B. Li, Y. Zhao, J. Lu, J. Zhou and Q. Liu, J. Anal. Methods Chem., 2019, 2019, 1792792 Search PubMed.
- Y. H. Liu, B. Wan and D. S. Xue, Molecules, 2019, 24, 1778 CrossRef CAS PubMed.
- Y. Liu, Z. Wang, D. Xue, Y. Yang, W. Li, H. Cheng, C. Patten and B. Wan, At. Spectrosc., 2020, 41, 131–140 CrossRef CAS.
- H. Cheng, Z. Wang, K. Chen, K. Zong, Z. Zou, T. He, Z. Hu, M. Fischer-Gödde and Y. Liu, Geostand. Geoanal. Res., 2019, 43, 663–680 CrossRef CAS.
- D. Tao, W. Guo, W. Xie, L. Jin, Q. Guo and S. Hu, Microchem. J., 2017, 135, 221–225 CrossRef CAS.
- J. Chao, B. Lv, Y. Zhao, C. Gong, X. Hong, Y. Pan and L. Xu, Energy Technol., 2023, 202201502 Search PubMed.
- H. Wang, Q. Liu, H. Li, H. Zhang and S. Yan, Polymers, 2023, 15 CAS.
- Q. Xiong, J. Qu, R. Zhao, Y. Chen, Y. Li, W. Xu, B. Pan, P. Jin and Z. Zheng, Lett. Appl. Microbiol., 2023, 76 Search PubMed.
- H. J. M. Bowen, J. Chem. Soc. A, 1970, 1082–1085 RSC.
- L. Wang, Chem. Eng. News, 2006, 84, 48 Search PubMed.
- P. F. Woolrich, J. Occup. Med., 1970, 12, 529 CAS.
- C. Yang, Z. H. Zhuang and Z. G. Yang, J. Appl. Polym. Sci., 2014, 131 Search PubMed.
- B. Zide and R. Pardoe, Am. J. Surg., 1976, 132, 424–426 CrossRef CAS PubMed.
- A. B. Farag, M. H. Soliman, O. S. Abdel-Rasoul and M. S. El-Shahawi, Anal. Chim. Acta, 2007, 601, 218–229 CrossRef CAS PubMed.
- E. A. Moawed, N. Burham and M. F. El-Shahat, J. Liq. Chromatogr. Relat. Technol., 2007, 30, 1903–1914 CrossRef CAS.
- C. D. Pereira, J. L. Mantovano, C. C. Turci and E. D. M. Ferreira, Microchem. J., 2014, 115, 121–125 CrossRef CAS.
- D. S. Xue, H. Y. Wang, Y. H. Liu, P. Shen and J. F. Sun, Anal. Methods, 2016, 8, 29–39 RSC.
- C. A. Watt and J. E. Kennedy, Front. Psychol., 2016, 7, 2030 Search PubMed.
- M. Chasse, W. L. Griffin, O. Alard, S. Y. O'Reilly and G. Calas, Lithos, 2018, 310, 409–421 CrossRef.
- M. Delgado-Rodriguez, Journal of Epidemiology and Community Health, 2006, 60, 90–92 CrossRef PubMed.
- I. Kalantzi and I. Karakassis, Mar. Pollut. Bull., 2006, 52, 484–493 CrossRef CAS PubMed.
- M. Ul Islam, F. H. Jiang, Z. C. Guo and X. H. Peng, Soil Tillage Res., 2021, 209 Search PubMed.
- C. W. Osenberg, O. Sarnelle and D. E. Goldberg, Ecology, 1999, 80, 1103–1104 CrossRef.
- A. Shah, M. P. Jones and G. J. Holtmann, Indian J. Gastroenterol., 2020, 39, 503–513 CrossRef PubMed.
- I. R. White, Stata J., 2015, 15, 951–985 CrossRef.
- X. D. Tang, B. Li, J. L. Lu, H. Y. Liu and Y. Y. Zhao, J. Anal. Sci. Technol., 2020, 11 CAS.
- G. Xue, Analytical Chemistry of Gold, China Astronautic Publishing House, China, 1990, in Chinese Search PubMed.
- M. M. A. Nikje and F. H. A. Mohammadi, Polym.-Plast. Technol., 2010, 49, 818–821 CrossRef CAS.
- M. Turkowska, K. Karon, A. Milewski and A. Jakobik-Kolon, Materials, 2022, 15 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |