Open Access Article
Open Access ArticleResearch on microbial community structure and treatment of dye wastewater with the enhancement of activated sludge by magnetic field at low temperature
Suo Liu ab,
He Li*a and
Yizhuo Wanga
ab,
He Li*a and
Yizhuo Wanga
aSchool of Civil Engineering, Southeast University, 2# Southeast University Road, Jiangning District, Nanjing, China. E-mail: liusuo2@outlook.com; ericlihe@seu.edu.cn; wyz1290@126.com
bKey Lab of Jiangsu Provincial Environmental Engineering, Jiangsu Provincial Academy of Environmental Science, #176 Jiangdong North Road, Gulou District, Nanjing, China
First published on 1st June 2023
Abstract
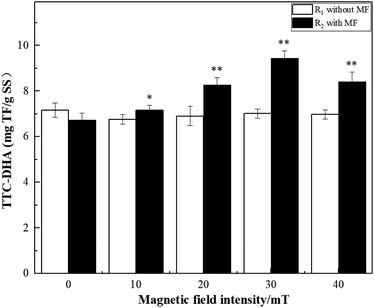
This study characterized the effect of different magnetic field (MF) intensities (10–40 mT) on the degradation of dye wastewater by activated sludge and the diversity of the microbial community at a low temperature (5 °C). The examined MF range promoted the degradation of dye wastewater by the microorganisms in the activated sludge at a low temperature. It was found that the optimal degradation performance was achieved at 30 mT. Additionally, the maximum degradation efficiency of COD and chromaticity (66.30% and 60.87%, respectively) were also achieved at 30 mT and the peak TTC-dehydrogenase activity (TTC-DHA) was 9.44 mg TF g−1 SS. Furthermore, it was revealed that MF enhancement increased the richness and diversity of activated sludge microorganisms, thus promoting the growth and reproduction of activated sludge microorganisms at low temperatures. Bacterial taxa known to effectively participate in the degradation of pollutants by activated sludge were enriched at 30 mT. The dominant bacteria under 30 mT were Flavobacterium, Hydrogenophaga, Gemmatimonadaceae, Zoogloea, Saprospiraceae, Pseudomonas, and Geothrix.
1. Introduction
It is reported that the highest amount of dyes used every year in the world is in the textile industry.1,2 However, some dyes are not effectively utilized, resulting in a large amount of dye wastewater, which is ultimately discharged into natural waters.3,4 Due to their complex composition, difficult biodegradability, and low recycling rate, the removal of dyes from wastewater is an ongoing challenge. In most countries, biochemical methods are commonly and extensively utilized to treat dye wastewater. Currently, the process of treating dye wastewater at room temperature is relatively mature, and extensive research has been conducted on the decolorization and degradation of printing and dyeing wastewater, and the performance of sewage treatment plants is generally considered to be excellent.1,2,5,6However, low temperatures are known to inhibit the microbial activity of activated sludge.7 In turn, low temperatures may also reduce the utilization of substrates by activated sludge microorganisms, as well as the adsorption and sedimentation capacity of activated sludge.8 Furthermore, microbial community structure and species richness are significantly affected by temperature.9,10 Specifically, low temperature can reduce the diversity and species richness of microbial communities, which may deteriorate activated sludge performance.11 As a result, a drop in wastewater temperature will unavoidably have an impact on microbial metabolism and the removal of pollutants.12 Current biochemical methods cannot effectively meet the requirements for the treatment of dyeing wastewater at low temperatures, making it difficult to comply with discharge standards. In turn, this has serious repercussions on water environment pollution. Nevertheless, previous studies have reported that activated sludge contains cold-adapting microorganisms at low temperatures, which can effectively degrade organic pollutants in sewage.8 Thus, magnetic field enhancement technology has been applied to the field of wastewater biological treatment to more effectively utilize these cold-adapted microorganisms.
Given the effects of low temperature on the biological treatment of wastewater, recent studies have focused on the enhancement of activated sludge by magnetic fields, which can improve wastewater treatment efficiency and maintain operational stability at low temperatures.13 Previous studies have demonstrated that static magnetic fields (SMF) with different intensity strengths (from 1 mT to 1 T) can affect many biological systems to some extent,14 and may directly affect the growth and metabolism of activated sludge microorganisms.15 Activated sludge biomass growth and dehydrogenase activity are positively correlated under low-intensity magnetic fields.16 Moreover, magnetic fields affect the enzyme activity and biofilm formation capacity of microorganisms, in addition to flocculation activity and microbial biomass.17 Therefore, magnetic fields can enhance the degradation of wastewater treatment performance by increasing dehydrogenase activity.18 Another study reported that exposing an activated sludge reactor to a weak magnetic field significantly increased its COD removal efficiency.19 Furthermore, previous studies have also demonstrated that exposing activated sludge to 5.0 and 20.0 mT magnetic fields increased the bacterial growth rate compared to the blank control group (0 mT), whereas the 200.0 and 500.0 mT bacterial growth lag period was prolonged and the growth rate was reduced, meaning that a low-intensity magnetic field promoted microorganism growth.20 Furthermore, the dehydrogenase activity of microbes can be stimulated by a magnetic field intensity of 20–40 mT to adapt to the cold environments, with cold-adapted Gram-negative bacteria being substantially enriched at 30 mT.21
This study focuses on the use of magnetic fields in conjunction with dye wastewater treatment technology, as well as the relationships between magnetic field intensity and treatment efficiency of dye wastewater, and dehydrogenase activity. Furthermore, high-throughput sequencing technology was used to study microbial community structure and diversity in activated sludge systems, as well as the effects of different magnetic field intensities on dye degradation performance. Thus this findings provided partial insights into the physiological mechanisms of microorganism-mediated dye degradation. Therefore, it provides a basis for future studies on the biological treatment of dye wastewater at low temperatures.
2. Materials and methods
2.1 Source of activated sludge
The activated sludge was obtained from a wastewater treatment plant in Nanjing, China, and contained an activated sludge concentration of approximately 3320 mg L−1.2.2 Lab-scale reactor system and simulation of dye wastewater
The activated sludge reaction system consisted of a reactor with a 5 cm radius and a 30 cm height. Two plexiglass cylinder reactors (R1 and R2) were utilized, each with a working volume of 2 L. Each reactor was equipped with three drain valves, and the oxygen-filled aeration component consisted of an electromagnetic aeration pump, an aeration hose, and an aeration sand head. The amount of aeration in the reactor could be freely controlled using a knob. To generate a static magnetic field, two parallel heteropolar magnetic plates were attached to R2, as shown in Fig. 1, and the magnet size was 15 cm × 10 cm × 2.5 cm. A Tesla meter was used to measure the central magnetic field strength of the inner wall of the activated sludge reactor in this experiment, which was characterized by the intensity of the reactor's magnetic field. The magnetic field intensity was then controlled by adjusting the distance between the parallel magnets, and operation of entire process of the reactor was artificially controlled, and the period was operated for 24 hours. Each MF intensity was administered for 7 days, and both the R1 and R2 reactors were operated at 5 °C. The oxygen concentration in the two reactors was roughly 8 mg L−1 throughout the entire experimental period.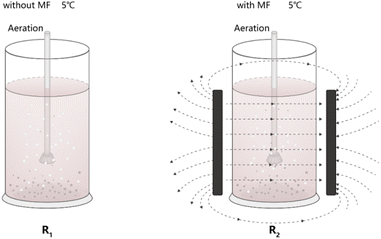 | ||
| Fig. 1 Schematic diagram of experimental setup (R1 refers to reactor #1 kept at 5 °C without magnetic field treatment; R2 refers to reactor #2 kept at 5 °C with different magnetic field intensities). | ||
The whole experimental design was divided into four stages. In the sludge acclimation stage, the inoculated sludge was cultured and acclimated to stabilize under low-temperature conditions. The second stage was the application of the magnetic field treatment, whereas R1 was the blank control without magnetic field, R2 was the experimental group with magnetic field, and then MF intensities (the magnetic field intensities were adjusted to 0, 10, 20, 30, and 40 mT, respectively). The third was the stable operation stage, activated sludge was adapted to different MF intensities and the effluent quality of the system remained stable. Finally, after identifying the most suitable magnetic field intensity for degrading dye wastewater, the reactor was operated under the selected magnetic field intensity for one month, after which high-throughput sequencing was conducted to characterize the microbial community structure in the activated sludge.
The dye wastewater used in this experiment was prepared in the laboratory. Acid red B (molecular formula, C20H14N2O7S2Na2) was the dye selected to conduct this experiments. The synthetic wastewater consisted of acid red B, NH4Cl, and KH2PO4, the COD concentration was 120 mg L−1 (Table 1), and the mass ratio of C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N
N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P was 100
P was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The composition of the synthetic dyeing wastewater is summarized in Table 1.
1. The composition of the synthetic dyeing wastewater is summarized in Table 1.
| Dye | COD (mg L−1) | Composition | Concentration (mg L−1) |
|---|---|---|---|
| Acid red B | 120 | NH4Cl | 18.3 |
| KH2PO4 | 4.5 |
2.3 TTC dehydrogenase activity (TTC-DHA)
Triphenyl tetrazolium chloride-dehydrogenase activity-dehydrogenase activity (TTC-DHA) is an important indicator of microbial metabolism and organic degradation capacity. Dehydrogenase is an extracellular enzyme produced by microbial cells, which can effectively participate in the degradation of organic matter. It is an essential enzyme for microbial biodegradation of organic pollutants in wastewater. Therefore, dehydrogenase is not only an indicator of microbial activity,22 but also reflects the ability of microbial cells to degrade substrates.23 The TTC-DHA analysis was conducted as described previously with minor modifications.23,24 Centrifuge tube containing the activated sludge (15 mL) and a stopper were centrifuged at 4000 rpm for 5 minutes, after which the supernatant was discarded. Next, 15 mL of distilled water was added, the mixture was stirred, and the supernatant was discarded once again after a 5 minute centrifugation. The samples were then washed three times and diluted with distilled water to the original volume for use. Next, 2 mL of activated sludge solution was transferred to a stoppered test tube, followed by 1.5 mL of Tris–HCl buffer solution, 0.5 mL of 0.1 mol L−1 glucose solution, 0.5 mL of 4% TTC solution, and 0.5 mL of 0.36% Na2SO3 solution. The samples were then immediately placed in a 5 °C incubator for 2 h. The reaction was terminated by adding 0.5 mL of formaldehyde, and 5 mL of toluene was rapidly added after every termination reaction. The sample was extracted by shaking at room temperature for 10 min, centrifuged at 4000 rpm for 5 min, and the absorbance of the supernatant was measured at 485 nm. Under the aforementioned conditions, an enzyme activity unit was defined as the production of 1 mg of triphenyl formazan (TF) in 1 h.2.4 Microbial community structure
Based on the results of the above-described experiments, this study selected the magnetic field intensity that was most suitable for strengthening the treatment of dye wastewater by activated sludge. Both the R1 (without MF) and R2 (with MF) reactors were placed in a biochemical incubator at 5 °C and only R2 was exposed to the selected magnetic field intensity. The reactors were then stably operated for 30 days and sludge samples were recovered at 15 and 30 days. Next, the 1–1.5 mL activated sludge samples from R1 and R2 were centrifuged, after which genomic DNA was extracted using the OMEGA Soil DNA kit. The isolated DNA was then examined using 1% agarose gel electrophoresis. The extracted DNA was PCR amplified by using 16S rRNA gene V3–V4 region universal primers with 515F (5′-GTGCCAGCMGCCGCGG-3′) and 907R (5′-CCGTCAATTCMTTTRAGTTT-3′). The acquired PCR products were sequenced by Biozeron Co., Ltd using a Miseq Illuminasequencer (Shanghai, China). Based on a 97% identity, the resultant sequences were grouped into operational taxonomic units (OTUs). The OTU representative sequences were compared to the matching database's template sequence, the taxonomic information for each OTU was acquired, and the four diversity indexes of Chao1, Coverage, Shannon, and Simpson diversity indices were calculated. Based on the OTU classification and classification status identification results, the composition of the microbial community of the activated sludge and the number of microorganisms were analyzed.2.5 Statistical analysis
Statistical analysis was performed using SPSS version 22.0 for Windows. One-way analysis of variance was used to examine the differences between the control and experimental groups. Values of p < 0.05 were considered significant, and values of p < 0.01 considered highly significant. OTUs with 97% similarity were selected and analyzed by high throughput sequencing using the Illumina PE250 platform. Normalized data were used to calculate the alpha diversity index of different samples using Mothur (e.g., Chao, Shannon, and Simpson indices).25 Based on the taxonomic analysis, the community structure composition at different classification levels (e.g., phylum, genus) was obtained. Beta diversity analysis was carried out using R language and STAMP for statistical analysis and mapping.3. Results and discussion
3.1 Bioreactor efficiency
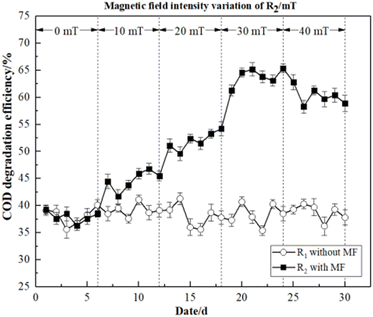
In this study, the COD degradation efficiency of R1 and R2 and the performance of the activated sludge were close to synchronization when the magnetic field intensity was 0 mT. Additionally, both the low temperature and the intensity of the magnetic field had an impact on the initial performance of R2 at 10 mT. Compared with R1, microbial activity was temporarily inhibited in a more complex environment. Therefore, the degradation efficiency of dye wastewater by activated sludge was not obvious. After adjusting MF intensity of R2, the COD degradation efficiency increased with higher magnetic field intensity, and reached its maximum at 30 mT (66.30%). However, as the MF intensity increased further, the COD degradation efficiency dropped until it reached a stable state. These findings suggested that magnetic fields can increase the permeability and cold adaptation of the microbial membranes enhanced.27 Therefore, magnetic fields can improve the COD degradation efficiency of activated sludge in dye wastewater.
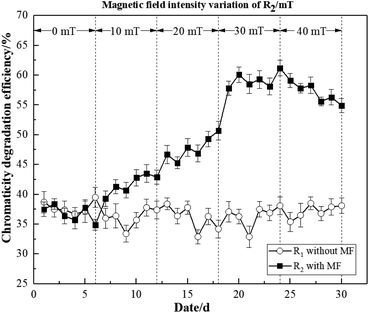
As illustrated in Fig. 3, the average degradation efficiencies of R2 under four different magnetic field intensities (10, 20, 30, 40 mT) were 41.56%, 47.60%, 58.90%, and 56.76%, respectively. In contrast, R1 without magnetic field exhibited values of 35.97%, 35.81%, 36.53 and 39.67%, respectively. As the effect of the MF on microorganisms was cumulative, some time was required for it to exert a reinforcing effect.23 The degradation efficiency of activated sludge began to increase at 20 mT. The degradation efficiency of R2 exhibited an interesting trend. Particularly, we observed a positive correlation between degradation efficiency and MF intensity, and the peak value was 60.87% at 30 mT, which decreased slightly with a higher MF intensity. These findings indicated that the activity of the activated sludge microorganisms was inhibited by 40 mT to some extent. However, activated sludge microorganisms may still be strengthened by the 40 mT magnetic field to degrade dye wastewater. Therefore, it demonstrated that the magnetic field was beneficial to the activated sludge, thus improving the chromaticity degradation efficiency of dye wastewater.
Based on these findings, it was concluded that the reactor with MF improvement was able to adapt to the low-temperature environment more quickly, and achieved a greater COD and chromaticity degradation efficiency.
These findings thus demonstrated that the magnetic field intensities of 10–40 mT could improve degradation efficiency. However, the most significant magnetic field strengthening effects were observed at 30 mT.
3.2 Microbial community structure
Several studies have demonstrated that temperature is among the environmental factors with the strongest influence on the composition of microbial communities in activated sludge.30,31 In turn, the compositions and dynamics of bacteria have a major impact on the treatment performance of activated sludge.32 Therefore, we next analyzed the effects of magnetic field treatment on the microbial community structure of the activated sludge samples.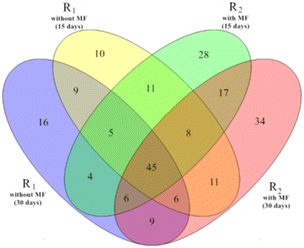 | ||
| Fig. 5 Venn diagram of four samples at the genus level (R1 refers to reactor #1, without magnetic field; R2 refers to reactor #2, kept at 5 °C at a magnetic field intensity of 30 mT). | ||
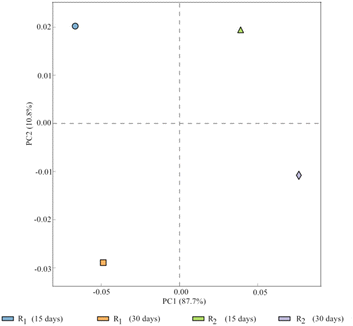
As illustrated in Fig. 6, PCA was conducted to observe the overall variation in bacterial communities among the four activated sludge samples. PC1 and PC2 represent 87.7% and 10.8% of the variations between the samples, respectively. PCA revealed the four samples had significant variance, with the variance between R1 and R2 (30 days) being larger than that between R1 and R2 (15 days). Furthermore, the variance between R1 (15 days) and R1 (30 days) and that between R2 (15 days) and R2 (30 days) was explained by both on PC1 and PC2, whereas the variance between R1 and R2 (15 days) and that of R1 and R2 (30 days) both were only explained by PC2. This indicated that the similarity of the microbial community structure between two given samples was significant, when the distance between two sample points was closer. The magnetic field was one of the primary factors that influenced microbial growth and contributed to the variation in microbial composition. Moreover, the variances of R1 and R2 were represented in PC1. Similarly the factor of time contributed substantially to PC2.
Therefore, magnetic field treatment was identified among the primary environmental elements that directly affect microbial diversity, which may influence the cold adaptability and physiological function of microorganisms in activated sludge. Thus, the microbial community structure of activated sludge changed significantly at a MF intensity of 30 mT.
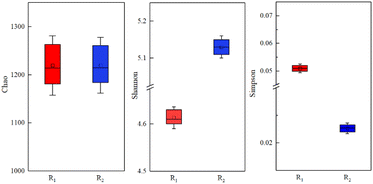
The obtained sequence number, coverage, and alpha indices are summarized in Table 2 and Fig. 7. The coverage of the activated sludge samples in R1 and R2 was above 99%, which indicates that the probability of the sequence being detected in the two sludge samples was high. Therefore, the sequencing results were sufficient to reflect the true status of the microorganisms in the sample, meaning that the samples were representative.34 The Shannon and Simpson indices were employed to assess the diversity of the microbial community, whereas the Chao index was utilized to assess the microbial richness.35 In this study, the total number of sequences detected in the R2 sample exposed to a MF intensity of 30 mT was as high as 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 801, whereas that of R1 without magnetic field treatment was 38
801, whereas that of R1 without magnetic field treatment was 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615. Although there was no significant difference in the Chao indices of the two reactors, the microbial diversity in R2 was significantly higher than in R1. As summarized in Table 1, the Shannon and Simpson indices of R2 were 5.13 and 0.022, respectively.
615. Although there was no significant difference in the Chao indices of the two reactors, the microbial diversity in R2 was significantly higher than in R1. As summarized in Table 1, the Shannon and Simpson indices of R2 were 5.13 and 0.022, respectively.
| Sample | Quality sequence number | Chao | Coverage | Shannon | Simpson |
|---|---|---|---|---|---|
| R1 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 615 615 |
1214 | 0.995 | 4.61 | 0.051 |
| R2 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 801 801 |
1215 | 0.997 | 5.13 | 0.022 |
These findings suggest that magnetic field could increase the richness and diversity of activated sludge microorganisms, and thus promote the growth and reproduction of activated sludge microorganisms at low temperatures.
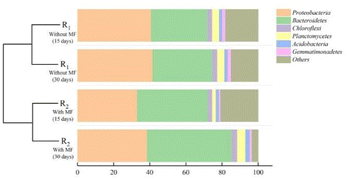
After 30 days of cultivation of the activated sludge, the samples exhibited unique patterns of microbial diversity. Proteobacteria and Bacteroidetes were the bacterial phyla with the highest abundance in the activated sludge of the two reactors. Studies have shown that Proteobacteria was widely distributed in activated sludge treatment systems, which was the main type population of activated sludge systems in urban sewage plants. These systems contain a variety of bacteria with uniquemetabolic types, mainly for the degradation of organic pollutants, as well as for nitrogen and phosphorus removal.36–38 The relative abundance of Proteobacteria in R1 and R2 was 41.61%, and 38.57%, respectively. It suggested that the bacteria were widely distributed and highly diverse, including many bacteria that survived at low temperatures.37 Bacteroidetes have a strong metabolic capacity to metabolize complex organic matter, proteins, and lipids. The members of this phylum can decompose complex macromolecular substances into simple compounds, and play an important role in the ecosystem.39 Bacteroidetes accounted for 33.09% and 46.97% of the bacterial diversity of the activated sludge samples from R1 and R2, respectively. Interestingly, the abundance of Bacteroidetes was higher in R2 exposed to the magnetic field compared to R1, indicating that the magnetic field enhances the survival of Bacteroides. Compared with R1, the relative abundance of Chloroflexi in R2 exposed to the magnetic field was relatively high (2.96%). A previous study reported that Chloroflexi was mostly composed of filamentous bacteria, and existed in the form of a floc skeleton within the sludge microbial floc, which promoted the flocculation of sludge and was capable of degrading macromolecular organic matter and removing biological phosphorus.40 Planctomycetes are small aquatic bacteria or aerobic bacteria, which play an important role in sewage treatment.41,42 The relative abundance of Planctomycetes in R1 and R2 was 3.97% and 4.56%, respectively, suggesting that the bacteria were more likely to survive in an activated sludge system treated with a magnetic field. Moreover, the relative abundance of Acidobacteria in R1 and R2 was 1.82% and 2.36%, showing that the magnetic field was beneficial to maintain the reproductive activity of Acidobacteria in activated sludge. Acidobacteria is an important class of microorganisms in activated sludge, which could degrade macromolecular organic matter,43 and significantly contribute to the stabilization of the ecological environment.44 Gemmatimonadetes is a type of Gram-negative bacterium that greatly contributes to biological phosphorus removal and contaminant degradation,45 and its relative abundance in R1 and R2 was 1.88% and 1.14%, respectively.
It demonstrated that the structure of the activated sludge's microbial community changed noticeably under a 30 mT magnetic field intensity, and the continuous culture time of activated sludge exposed to magnetic field was also a factor affecting the changes in the microbial communities. As the activated sludge culture time increased, the relative abundance of the six dominant phyla in all samples with an magnetic field intensity of 30 mT, Proteobacteria, Bacteroidetes, Chloroflexi, Planctomycetes, Acidobacteria and Gemmatimonadetes were increased by 46.97, 2.96, 4.56, 2.36, and 1.14, respectively.
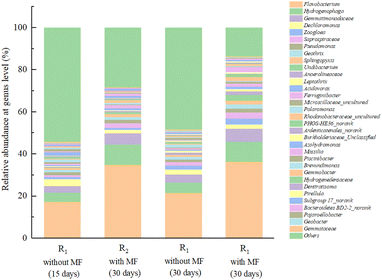
As illustrated in Fig. 9, the bacterial community had undergone significant changes, as the magnetic field continued to contribute to the acclimation of the activated sludge in the reactors. Flavobacterium was the most abundant genus in the activated sludge in R1 and R2 accounting for 21.47% and 36.33% of the overall abundance, respectively. Moreover, the biomass produced by proliferation of R2 under the magnetic field was significantly higher than that of R1. Previous studies have reported that Flavobacterium is strictly aerobic, and it cannot produce acid or gas in the reaction solutions containing carbohydrates. Additionally, the members of this genus were the main contributors to micelle formation, which not only has the function of degrading macromolecular organic matter such as high molecular substances, proteins, lipids, and cellulose, but also participates in nitrification,46 and could be involved in nitrogen removal from sewage.47 Therefore, Flavobacterium became a dominant genus in the R2 with magnetic field enhancement. Hydrogenophaga was the second most abundant genus in R2, accounting for 9.59% of the relative abundance. Which was reported to be facultative bacterium that was able to survive in a carbon source environment and was capable of denitrification.48 Gemmatimonadaceae, accounting for 3.85% and 6.17% of the bacterial abundance in R1 and R2 respectively, were assigned to Gemmatimonadetes, which is an important genus in the process of biological phosphorus removal, and consumed substantial amounts of organic matter during this period.47 Dechloromonas accounted for 2.51%, 1.92% in R1 and R2, and played an important role in the biological phosphorus removal process. However, these bacteria are autotrophic bacteria,49,50 and do not use external organic carbon sources, meaning that there is no significant effect on the degradation of organic matter. Moreover, Zoogloea was also to be found in the samples, accounting for up to 1.98%, 2.95% of the total bacterial abundance in R1 and R2, was capable of degrading refractory organics, including phenols and NHCs,51 and some of the fungi in Zoogloea can promote the flocculation of activated sludge. Combined with the experimental observations, a suitable magnetic field intensity could effectively promote the flocculation and sedimentation performance of activated sludge. Saprospiraceae was reported to be frequently detected in activated sludge flocs, which may degrade proteins by producing extracellular enzymes. In this study, these bacteria were more abundant in R2 (2.81%) higher than R1 (1.33%). Furthermore, Pseudomonas and Geothrix were also detected in all samples. Pseudomonas is a common genus in the natural environment, which are an aerobic heterotrophic microorganisms that can metabolize various organic materials, including some complex organic compounds that are not easily degraded by other microorganisms. These microorganisms can also decompose proteins and fats,52 and some of them can participate in the process of biological nitrogen removal.53 Furthermore, Geothrix belongs to the phylum Acidobacteria and the members of this genus accounted for 1.36% and 2.01% in R1 and R2, respectively, which may be benefit the degradation of organic pollutants.
It revealed that an appropriate magnetic field intensity of 30 mT encouraged the growth of a wide variety of microbial taxa in the activated sludge community, including Flavobacterium, Hydrogenophaga, Gemmatimonadaceae, Dechloromonas, Zoogloea, Saprospiraceae_uncultured, Pseudomonas and Geothrix.
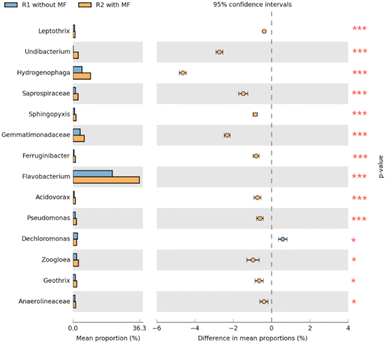 | ||
| Fig. 10 Differences in the abundance of bacterial genera between samples of R1 and R2 based on Fisher's exact test. | ||
The genus abundances in the R1 and R2 samples differed significantly (P < 0.05), meaning that the microbial communities were highly susceptible to magnetic field treatment. The abundance of Dechloromonas, Zoogloea, Geothrix, and Anaerolineaceae under magnetic field varied only slightly (0.01 < P value ≤ 0.05). In contrast, the abundance of Flavobacterium, Hydrogenophaga, Gemmatimonadaceae, Undibacterium, Saprospiraceae, Leptothrix, Acidovorax, Ferruginibacter, Pseudomonas, and Sphingopyxis showed highly significant differences (P value ≤ 0.001). Studies have shown that these bacteria were widely present in activated sludge, and may effectively degrade pollutants in wastewater.45,46,48,49,51,52 Moreover, Zoogloea can promote the flocculation of activated sludge.51
Collectively, it demonstrated that magnetic field treatment increased the abundances of the aforementioned genera, which enabled the reactor to adapt more easily to low-temperature environments and maintain a high pollutant degradation performance in the activated sludge. Moreover, these results were consistent with the results of the bioreactor efficiency.
4. Conclusions
In this study, it demonstrated that magnetic field intensities of 10–40 mT could promote microorganism growth in activated sludge, thus promoting the degradation of dye wastewater at low temperatures. Particularly, a 30 mT magnetic field intensity at a low temperature enhanced the microbial activity in the activated sludge, thus improving the efficiency of activated sludge degradation for dye wastewater. More importantly, our findings revealed that magnetic field treatment effectively enriched bacterial communities, thus enabling the reactor to more easily adapt to low-temperature environments and maintain the pollutant removal performance of the activated sludge reactor. The dominant microbial taxa in the activated sludge reactor treated with a 30 mT magnetic field, which effectively participate in the degradation of pollutants, included Flavobacterium, Hydrogenophaga, Gemmatimonadaceae, Zoogloea, Saprospiraceae, Pseudomonas, and Geothrix.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (51508241). The authors gratefully acknowledge for the experimental equipment support provided by Key Lab of Jiangsu Provincial Environmental Engineering.References
- V. Katheresan, J. Kansedo and S. Y. Lau, J. Environ. Chem. Eng., 2018, 6, 4676–4697 CrossRef CAS.
- K. A. Adegoke and O. S. Bello, Water Resour. Ind., 2015, 12, 8–24 CrossRef.
- T. A. Nguyen and R.-S. Juang, Chem. Eng. J., 2013, 219, 109–117 CrossRef CAS.
- M. A. Rauf and S. Salman Ashraf, Chem. Eng. J., 2012, 209, 520–530 CrossRef CAS.
- R. L. Singh, P. K. Singh and R. P. Singh, Int. Biodeterior. Biodegrad., 2015, 104, 21–31 CrossRef CAS.
- N. Manavi, A. S. Kazemi and B. Bonakdarpour, Chem. Eng. J., 2017, 312, 375–384 CrossRef CAS.
- J. Xu, J. He, M. Wang and L. Li, Chemosphere, 2018, 211, 1219–1227 CrossRef CAS PubMed.
- J. Guo, J. Wang, D. Cui, L. Wang, F. Ma, C. C. Chang and J. Yang, Bioresour. Technol., 2010, 101, 6622–6629 CrossRef CAS PubMed.
- A. Karkman, K. Mattila, M. Tamminen and M. Virta, Biotechnol. Bioeng., 2011, 108, 2876–2883 CrossRef CAS PubMed.
- Z. Ma, X. Wen, F. Zhao, Y. Xia, X. Huang, D. Waite and J. Guan, Bioresour. Technol., 2013, 133, 462–468 CrossRef CAS PubMed.
- Y. Chen, S. Lan, L. Wang, S. Dong, H. Zhou, Z. Tan and X. Li, Chemosphere, 2017, 174, 173–182 CrossRef CAS PubMed.
- H. Zhou, X. Li, G. Xu and H. Yu, Sci. Total Environ., 2018, 643, 225–237 CrossRef CAS PubMed.
- A. Tomska and L. Wolny, Desalination, 2008, 222, 368–373 CrossRef CAS.
- F. Javani Jouni, P. Abdolmaleki and M. Movahedin, In Vitro Cell. Dev. Biol.: Anim., 2013, 49, 212–219 CrossRef PubMed.
- A. Yadollahpour and S. Rashidi, Res. J. Pharm. Technol., 2017, 6, 641–644 CrossRef.
- M. Lebkowska, A. Rutkowska-Narozniak, E. Pajor and Z. Pochanke, Bioresour. Technol., 2011, 102, 8777–8782 CrossRef CAS PubMed.
- S. Ghodbane, A. Lahbib, M. Sakly and H. Abdelmelek, BioMed Res. Int., 2013, 2013, 602987 Search PubMed.
- N. S. Zaidi, J. Sohaili, K. Muda and M. Sillanpää, Sep. Purif. Rev., 2013, 43, 206–240 CrossRef.
- H.-x. Lan, R. Chen, P. Ma, H. Zhang, S.-h. Lan and Y.-d. Wang, Desalin. Water Treat., 2013, 53, 27–35 CrossRef.
- Y. Ji, Y. Wang, J. Sun, T. Yan, J. Li, T. Zhao, X. Yin and C. Sun, Bioresour. Technol., 2010, 101, 8535–8540 CrossRef CAS PubMed.
- C. Niu, W. Liang, H. Ren, J. Geng, L. Ding and K. Xu, Bioresour. Technol., 2014, 159, 48–54 CrossRef CAS PubMed.
- Y. F. Cheng, Q. Zhang, G. F. Li, Y. Xue, X. P. Zheng, S. Cai, Z. Z. Zhang and R. C. Jin, Bioresour. Technol., 2019, 289, 121707 CrossRef CAS PubMed.
- C. Niu, J. Geng, H. Ren, L. Ding, K. Xu and W. Liang, Bioresour. Technol., 2013, 150, 156–162 CrossRef CAS PubMed.
- T. Burdock, M. Brooks, A. Ghaly and D. Dave, Adv. Biosci. Biotechnol., 2011, 02, 214–225 CrossRef CAS.
- P. D. Schloss, D. Gevers and S. L. Westcott, PLoS One, 2011, 6, e27310 CrossRef CAS PubMed.
- C. Niu, J. Geng, H. Ren, L. Ding and K. Xu, Bioresour. Technol., 2012, 123, 66–71 CrossRef CAS PubMed.
- B. Kayranli and A. Ugurlu, Desalination, 2011, 278, 77–83 CrossRef CAS.
- H. Yavuza and S. S. Celebi, Enzyme Microb. Technol., 2000, 26, 22–27 CrossRef.
- M. Iwasaka, M. Ikehata, J. Miyakoshi and S. Ueno, Bioelectrochemistry, 2005, 65, 59–68 CrossRef PubMed.
- X. Lu, X. X. Zhang, Z. Wang, K. Huang, Y. Wang, W. Liang, Y. Tan, B. Liu and J. Tang, PLoS One, 2015, 10, e0125549 CrossRef PubMed.
- K. Meerbergen, M. Van Geel, M. Waud, K. A. Willems, R. Dewil, J. Van Impe, L. Appels and B. Lievens, MicrobiologyOpen, 2017, 6, 1–13 CrossRef PubMed.
- L. Zhang, Z. Shen, W. Fang and G. Gao, Sci. Total Environ., 2019, 689, 1181–1191 CrossRef CAS PubMed.
- D. E. Fouts, S. Szpakowski, J. Purushe, M. Torralba, R. C. Waterman, M. D. MacNeil, L. J. Alexander and K. E. Nelson, PLoS One, 2012, 7, e48289 CrossRef CAS PubMed.
- J. Qu, X. Chen, J. Zhou, H. Li and W. Mai, Bioresour. Technol., 2019, 289, 121714 CrossRef CAS PubMed.
- Q. Yang, J. Wang, H. Wang, X. Chen, S. Ren, X. Li, Y. Xu, H. Zhang and X. Li, Bioresour. Technol., 2012, 117, 155–163 CrossRef CAS PubMed.
- S. L. McLellan, S. M. Huse, S. R. Mueller-Spitz, E. N. Andreishcheva and M. L. Sogin, Environ. Microbiol., 2010, 12, 1376 CrossRef CAS.
- T. Zhang, M.-F. Shao and L. Ye, ISME J., 2011, 6, 1137–1147 CrossRef PubMed.
- X. X. Zhang and T. Zhang, Environ. Sci. Technol., 2011, 45, 2598–2604 CrossRef CAS PubMed.
- V. R. Hill, A. M. Kahler, N. Jothikumar, T. B. Johnson, D. Hahn and T. L. Cromeans, Appl. Environ. Microbiol., 2007, 73, 6327 CrossRef CAS.
- C. Kragelund, C. Levantesi, A. Borger, K. Thelen, D. Eikelboom, V. Tandoi, Y. Kong, J. van der Waarde, J. Krooneman, S. Rossetti, T. R. Thomsen and P. H. Nielsen, FEMS Microbiol. Ecol., 2007, 59, 671–682 CrossRef CAS PubMed.
- Y. Song, G. Mao, G. Gao, M. Bartlam and Y. Wang, Microb. Ecol., 2019, 78, 428–445 CrossRef CAS PubMed.
- T. Xiang and D. Gao, Bioresour. Technol., 2019, 289, 121710 CrossRef CAS PubMed.
- J. Huang, C. Yan, J. Liu, W. Guan, R. P. Singh, C. Cao and J. Xiao, J. Environ. Manage., 2019, 245, 28–36 CrossRef CAS PubMed.
- C. Ren, W. Liu, F. Zhao, Z. Zhong, J. Deng, X. Han, G. Yang, Y. Feng and G. Ren, Catena, 2019, 181, 104071 CrossRef CAS.
- H. Zhang, Y. Sekiguchi, S. Hanada, P. Hugenholtz, H. Kim, Y. Kamagata and K. Nakamura, Int. J. Syst. Evol. Microbiol., 2003, 53, 1155–1163 CrossRef CAS PubMed.
- S. M. Blunt, J. D. Sackett, M. R. Rosen, M. J. Benotti, R. A. Trenholm, B. J. Vanderford, B. P. Hedlund and D. P. Moser, Sci. Total Environ., 2018, 622–623, 1640–1648 CrossRef CAS PubMed.
- X. Dong and G. B. Reddy, Bioresour. Technol., 2010, 101, 1175–1182 CrossRef CAS PubMed.
- Y. Liu, D. Wei, W. Xu, R. Feng, B. Du and Q. Wei, Bioresour. Technol., 2019, 288, 121504 CrossRef CAS PubMed.
- M. P. Ginige, P. Hugenholtz, H. Daims, M. Wagner, J. Keller and L. L. Blackall, Appl. Environ. Microbiol., 2004, 70, 588–596 CrossRef CAS PubMed.
- Y. Liu, T. Zhang and H. H. Fang, Bioresour. Technol., 2005, 96, 1205–1214 CrossRef CAS PubMed.
- Z. Zhang, Y. Han, C. Xu, H. Han, D. Zhong, M. Zheng and W. Ma, Bioresour. Technol., 2019, 287, 121465 CrossRef CAS PubMed.
- Y. Huang, X. Hou, S. Liu and J. Ni, Chem. Eng. J., 2016, 304, 864–872 CrossRef CAS.
- H. Zhang, Z. Zhao, S. Li, S. Chen, T. Huang, N. Li, S. Yang, Y. Wang, L. Kou and X. Zhang, Chem. Eng. J., 2019, 372, 26–36 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |