Open Access Article
Open Access ArticleRegioselective intramolecular cyclization of o-alkynyl arylamines with the in situ formation of ArXCl to construct poly-functionalized 3-selenylindoles†
Minhui Yu‡
ab,
Tao Jin‡a,
Xiaohua Wang‡c,
Haohu Li‡a,
Decai Jia,
Jinzhong Yao *b,
Heyang Zenga,
Senlei Shia,
Kaimeng Xu*a and
Lianpeng Zhang
*b,
Heyang Zenga,
Senlei Shia,
Kaimeng Xu*a and
Lianpeng Zhang *a
*a
aYunnan Provincial Key Laboratory of Wood Adhesives and Glued Products, Southwest Forestry University, Kunming 650224, Yunnan, China. E-mail: xukm007@163.com; lpz@zju.edu.cn
bCollege of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, Zhejiang, China. E-mail: jzyao@zju.edu.cn
cTongji Zhejiang College, Jiaxing 314051, China
First published on 21st February 2023
Abstract
In this article, a practical and metal-free method for the synthesis of poly-functionalized 3-selenyl/sulfenyl/telluriumindoles from o-alkynyl arylamines has been achieved. In this protocol, the in situ formation of selenenyl chloride, sulfenyl chloride or tellurenyl chloride is considered as the key intermediate and the 3-selenyl/sulfenyl/telluriumindoles can be obtained in good to excellent yields. Furthermore, the product 2-phenyl-3-(phenylselanyl)-1-tosyl-1H-indole can be selectively oxidized to compounds 2-phenyl-3-(phenylseleninyl)-1-tosyl-1H-indole and 2-phenyl-3-(phenylselenonyl)-1-tosyl-1H-indole in good yields.
Introduction
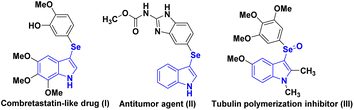
As an active nitrogen-containing heterocyclic skeleton, indole exists widely in biologically active natural products and synthetic bioactive products, and represents an important “privileged scaffold”.1 Among the numerous indoles, 3-arylselenoindoles are particularly attractive due to their potential use in therapeutics such as anti-oxidant, anti-tumor, and anti-cancer agents.2 For example, as a novel combretastatin-like drug, 3-selenylindole compound (I) exhibits highly potent inhibitory activity against cancer cells3 (Scheme 1). In vitro tubulin polymerization and immunostaining experiments showed that antineoplastic agent (II) significantly inhibits tubulin polymerization in vitro.4Acting as a tubulin polymerization inhibitor, the corresponding selenoxide of 3-(3,4,5-trimethoxyphenyl)seleno-1H-indole inhibits tumor cells by interfering with the tubulin system of tumor cells.4–6 Therefore in terms of the unique biological activity and the synthetic methodology, the building of selenium-containing compounds has become one of the most hotspot in the last decade.7
Direct selenylation based on the indole compounds is one of the most classical synthetic methods to construct 3-selenylindoles.8 Undeniably, 2-alkynylanilines are also powerful synthons for the synthesis of 3-selenylindoles9–13 (Scheme 2).
The metal-catalyzed (facilitated) selenoamination of 2-alkynylanilines with diorganyl diselenides had been achieved in the past decade9,10 (Scheme 2a). In 2009, Larock and co-workers reported a two-step process of synthesis 3-selenylindoles with arylselenyl chlorides in the presence of a stoichiometric amount of n-Bu4NI11 (Scheme 2b). Most recently, visible light catalysis for the construction of bioactive molecules has received increasing attention.14 In 2017, an efficient method for the preparation of 3-selenyl indoles through visible light-promoted tandem cyclization of 2-alkynylanilines with diaryl(alkyl)diselenides under transition metal-free and photocatalyst-free conditions was reported12 (Scheme 2c). Therefore, the development of a practical protocol of construct of 3-selenylindoles under metal-free conditions is still desirable.
Because of the unstable properties of the sulfenyl chlorides or selenenyl chlorides, Du and co-works have developed a metal-free protocol about the in situ formation of PhXCl from PhXXPh and PhICl2 to the synthesis of chalcogenides.15 In recent years, one of the research focuses of our group is the construction of heterocyclic compounds based on the controllable functionalization of alkynes.16 Inspired by the works above, herein, we report a simple metal-free method to afford the 3-selenyl/sulfenyl/telluriumindoles in the presence of the in situ generated ArSeCl, ArSCl, or ArTeCl from the reaction between the diselenides, disulfides, or ditellurides and PhICl2 (Scheme 2d).
Results and discussion
In order to verify the feasibility of this reaction, 4-methyl-N-(2-(phenylethynyl)phenyl)benzenesulfon-amide (1a) was initially employed to react with 1,2-diphenyldiselane (2a) under various conditions (Table 1). To our delight, after adding substrate 1a (0.20 mmol) to the solution of the reaction of substrate 2a (0.10 mmol) and PhICl2 (0.14 mmol) in MeCN (2.0 mL) at room temperature then stirring the mixtures for 12 hours in the absence of light delivered the desired product 2-phenyl-3-(phenylselanyl)-1-tosyl-1H-indole (3a) in a yield of 85% (Table 1, entry 1). Changing the hypervalent iodine(III) oxidants from PhICl2 to PIDA or PIFA gave the poor conversion under otherwise identical conditions (Table 1, entries 2 and 3). When using mCPBA as the oxidant, the expected product 3a was not detected (Table 1, entry 4). Hereafter, a series of solvents including DMF, DMAC, DCM, toluene, and EtOH were tested and the solvent screening revealed that DCM and EtOH provided superior results in yields of 88% and 97%, respectively (Table 1, entries 5–9). As we can see, increasing or decreasing the amount of the oxidant PhICl2 resulted in reduced conversions (Table 1, entries 10 and 11). Increasing the amount of PhSeSePh 2a to 0.15 mmol gave the similar result (Table 1, entry 12). Conducting the reaction under an ice-bath or 50 °C rather than at room temperature, lower yields were observed (Table 1, entries 13 and 14). Finally, the best conditions for the preparation of 3-selenylindoles were 0.5 equiv. of PhSeSePh and 0.7 equiv. of PhICl2 in EtOH at room temperature.| Entry | Additive (equiv.) | Solvent | T (°C) | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol) and 2a (0.10 mmol) in solvent (2.0 mL) were stirred for 12 hours in the absence of light.b Isolated yields.c 2a (0.15 mmol). | ||||
| 1 | PhICl2 (0.7) | MeCN | r.t. | 85 |
| 2 | PIDA (0.7) | MeCN | r.t. | 10 |
| 3 | PIFA (0.7) | MeCN | r.t. | 13 |
| 4 | mCPBA (0.7) | MeCN | r.t. | N.D. |
| 5 | PhICl2 (0.7) | DMF | r.t. | 42 |
| 6 | PhICl2 (0.7) | DMAC | r.t. | 40 |
| 7 | PhICl2 (0.7) | DCM | r.t. | 88 |
| 8 | PhICl2 (0.7) | Toluene | r.t. | 61 |
| 9 | PhICl2 (0.7) | EtOH | r.t. | 97 |
| 10 | PhICl2 (0.8) | EtOH | r.t. | 91 |
| 11 | PhICl2 (0.6) | EtOH | r.t. | 90 |
| 12c | PhICl2 (0.7) | EtOH | r.t. | 94 |
| 13 | PhICl2 (0.7) | EtOH | 0 | 72 |
| 14 | PhICl2 (0.7) | EtOH | 50 | 86 |
With the optimized reaction conditions in hand, a variety of o-alkynyl arylamines were prepared and we investigated the generality of these regioselective intramolecular cyclization under the in situ formation of ArXCl to construct poly-functionalized 3-selenyl/sulfenyl/telluriumindoles (Schemes 3 and 4). 2-Phenyl-3-(phenylselanyl)-1-tosyl-1H-indole (3a) was synthesized under the standard conditions which was identified by NMR, HRMS, IR, and the diffraction of X-ray (CCDC: 2001345†). Firstly, the reactions of various substituted N-(2-(argioethynyl)phenyl)-4-methylbenzenesulfonamides 1b–1e with 1,2-diphenyldiselane (2a) were examined, which afforded the corresponding products with the different functional groups on the aromatic rings 3b–3e in 93–99% yields. It is noteworthy that 3-(phenylselanyl)-2-(thiophen-3-yl)-1-tosyl-1H-indole 3e exhibited the high yield in 99%. Regarding the scope of sulfonyl functionality, fortunately, besides p-tolylsulfonyl substrates, naphthylsulfonyl 1f, 1-thiophen-2-sulfonyl 1g and ethylsulfonyl 1h could also successfully participated in the current selenium cyclization, leading to the desired products 3f, 3g and 3h in 81%, 99% and 99% yield, respectively. To our delight, the reaction underwent smoothly when using the substrate with no protecting group substituted, and gave the product 2-phenyl-3-(phenylselanyl)-1H-indole (3i) in 63% yield by extending the reaction time to 24 hours. 1,2-Diphenyldisulfane (2b) and 1,2-diphenylditellane (2c) were also used as the reaction substrate under the standard conditions, the expected products 3-(phenylthio)-2-(thiophen-3-yl)-1-tosyl-1H-indole (3j) and 3-(phenyltellanyl)-2-(thiophen-3-yl)-1-tosyl-1H-indole (3k) were synthesized in 74% and 67% yield, respectively.
When there is a methyl substituent on the benzene ring, the reaction proceeded smoothly and 3l was obtained in excellent yield. Moreover, using the reaction substrate containing alkyl alkyne, the product 3m was obtained in the yield of 83%. After the thorough exploration of the reaction scope of o-alkynylanilines, we came to investigate the reactivity of variously substituted 2-alkynyl-1-naphthylamines and the 3-alkynylpyridin-2-amine (Scheme 4). Changing the benzene ring to naphthalene ring did not have a great influence on the yields of the reaction. As expected, substrates bearing p-methoxyphenyl or 3-thienyl gave the corresponding products 3n and 3o in 85%, and 99% yield, respectively. Moreover, the reactivity of 1-(thiophen-3-ethynyl)naphthalen-2-amines which had various substituted groups on the nitrogen atom was also evaluated. It can be seen from the results that substrates bearing substituted benzenesulfonyl, 2-naphthalenesulfonyl and 2-thiophenesulfonyl were investigated, and the experimental results showed that these substrates also worked well. Especially when the starting material with a strong electron-withdrawing group of p-nitrobenzenesulfonyl (1p) was used as the substrate, the expected product 3p was still obtained in a yield of 63%. In addition, by changing the arylsulfonyl to ethylsulfonyl, the selenylated product 3t was isolated in 75% yield. The reaction worked well when using 4-methyl-N-(3-(phenylethynyl)pyridin-2-yl)benzenesulfonamide (1u) as the starting material, furnishing 2-phenyl-3-(phenylselanyl)-1-tosyl-1H-pyrrolo[2,3-b]pyridine (3u) in 86% yield. In the end, a 1.0 mmol scale experiment of 1o had been carried out and the desired product 3o was obtained in 95% yield.
In order to gain insights into the reaction mechanism, some control experiments were conducted. Firstly, 2.0 equiv. of TEMPO was added to the reaction of 4-methyl-N-(2-(phenylethynyl)phenyl)benzenesulfon-amide (1a) with 1,2-diphenyldiselane (2a) under the standard conditions. From the reaction results, it could be seen that the yield of the desired product 2-phenyl-3-(phenylselanyl)-1-tosyl-1H-indole (3a) was 91%, which excluded a free-radical pathway for this reaction (Scheme 5a). Next, considering the previous work about the in situ formation of RSCl/ArSeCl and their application to the synthesis of chalcogenides, we wondered if the ArXCl was the key intermediate. Subsequently, the reaction of 4-methyl-N-(2-(phenylethynyl)phenyl)benzenesulfon-amide (1a) with 1.2 equiv. PhSeCl in EtOH at room temperature for 12 hours afforded the corresponding selenylated product 3a in 93% yield (Scheme 5b).
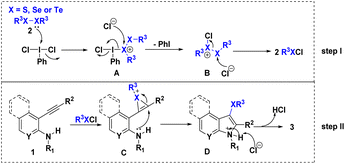
Based on the experimental results described above as well as previous literature reports,11,15 a plausible mechanism for this reaction is shown in Scheme 6 which involves R3XCl formation (step I) followed by a R3XCl-mediated electrophilic cyclization (step II). The intermediate A was formed through the attack of X (sulfur/selenium/tellurium) on the iodine center in PhICl2. Subsequently, the intermediate A is converted to intermediate B after elimination of PhI. Next, two molecules of PhXCl are produced through the chloride anion nucleophilically attacking the X atom of the intermediate B (step I). Subsequently, substrate 1 reacts with R3XCl to form the intermediate C. Then, the nitrogen atom acts as a nucleophile and attacks the X-onium center to afford the intermediate D. Finally, the removal of hydrogen proton from intermediate D assisted by the attack of chloride ion leads to the formation of the final product 3 (step II).
In order to expand the application of the synthesized 3-selenoindoles, we performed the selective oxidative reactions (Scheme 7). The product 2-phenyl-3-(phenylseleninyl)-1-tosyl-1H-indole (4a) was synthesized using 3a as the starting material and 1.1 equiv. mCPBA as the oxidant (Scheme 7a). When the oxidant of mCPBA was increased to 2.5 equiv., the product 2-phenyl-3-(phenylselenonyl)-1-tosyl-1H-indole (5a) was obtained in 78% yield (Scheme 7b).
Conclusions
In summary, we have developed a simple, practical, metal-free protocol for the synthesis of various functionalized 3-selenyl/sulfenyl/telluriumindoles from the reactions of in situ generated RXCl with o-alkynyl arylamines. Under the optimized reaction conditions, the reactions generated the corresponding products in good to excellent yields based on the high atom economy with excellent functional group tolerance. Through the experimental results and the related literature, a plausible reaction mechanism was proposed and ArXCl is proved to be the key intermediate. Furthermore, the product 2-phenyl-3-(phenylselanyl)-1-tosyl-1H-indole (3a) can be selectively oxidized to compounds 4a and 5a in good yields. Further studies on the synthetic application are currently under way.Experimental
General methods and materials
Unless stated otherwise, reactions were conducted in dried glassware. Commercially available reagents and solvents were used as received. 300–400 mesh silica gel was used for flash column chromatography. Visualization on TLC was achieved by the use of UV light (254 nm). 400 MHz and 100 MHz were used for the record of 1H NMR and 13C NMR spectra. Chemical shifts (δ ppm) were reported in parts per million referring to either the internal standard of TMS or the residue of the deuterated solvents. Splitting pattern was described as follows: s for singlet, d for doublet, t for triplet, q for quartet, and m for multiplet. Coupling constants were reported in Hz. The high-resolution mass spectrum (HRMS) was performed on Waters Xevo G2-S QTof mass spectrometer. All the substrates were synthesized with references to published literature.17 Deposition number 2001345 (for 3a) contain the ESI crystallographic data for this paper.†Typical procedure for the synthesis of 3a
Substrate 2a (0.10 mmol) and PhICl2 (0.14 mmol) were stirred in EtOH (2.0 mL) at room temperature for 15 minutes in the absence of light, then substrate 1a (0.20 mmol) was added. After the substrate 1a was completely consumed (monitored by TLC), the mixture was concentrated under reduced pressure. The residue was purified by column chromatography on silica gel (PE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the desired product 3a as white solid (97.6 mg, 97%).
1) to afford the desired product 3a as white solid (97.6 mg, 97%).
Characterization data
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 6.94 (d, J = 6.9 Hz, 2H), 7.02–7.10 (m, 5H), 7.26 (d, J = 4.0 Hz, 1H), 7.31 (d, J = 8.4 Hz, 4H), 7.39 (t, J = 7.6 Hz, 3H), 7.45 (d, J = 8.4 Hz, 2H), 8.37 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 110.5, 116.3, 121.2, 124.6, 125.7, 126.0, 126.9, 127.1, 129.0, 129.2, 129.3, 129.4, 130.9, 131.6, 131.8, 132.1, 135.0, 137.5, 144.9; IR (neat) 3329, 1655, 1574, 1451, 1239, 824, 730 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H22NO2SSe: 504.0536, found 504.0531.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 6.94 (d, J = 6.9 Hz, 2H), 7.02–7.10 (m, 5H), 7.26 (d, J = 4.0 Hz, 1H), 7.31 (d, J = 8.4 Hz, 4H), 7.39 (t, J = 7.6 Hz, 3H), 7.45 (d, J = 8.4 Hz, 2H), 8.37 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 110.5, 116.3, 121.2, 124.6, 125.7, 126.0, 126.9, 127.1, 129.0, 129.2, 129.3, 129.4, 130.9, 131.6, 131.8, 132.1, 135.0, 137.5, 144.9; IR (neat) 3329, 1655, 1574, 1451, 1239, 824, 730 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H22NO2SSe: 504.0536, found 504.0531.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 15
EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 3.60 (s, 3H), 6.90 (d, J = 8.3 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 7.06–7.13 (m, 8H), 7.23–7.25 (m, 1H), 7.37 (t, J = 7.8 Hz, 1H), 7.42–7.46 (m, 4H), 8.30 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 55.1, 109.8, 110.3, 115.3, 119.4, 120.3, 121.0, 124.0, 125.2, 125.9, 127.0, 128.8, 129.3, 129.6, 131.1, 131.6, 131.7, 132.9, 135.7, 137.1, 141.4, 144.6, 158.5; IR (neat) 3415, 3038, 1635, 1569, 1451, 1241, 823, 735 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H24NO3SSe: 534.0642, found 534.0634.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 3.60 (s, 3H), 6.90 (d, J = 8.3 Hz, 1H), 6.98 (t, J = 7.4 Hz, 1H), 7.06–7.13 (m, 8H), 7.23–7.25 (m, 1H), 7.37 (t, J = 7.8 Hz, 1H), 7.42–7.46 (m, 4H), 8.30 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 55.1, 109.8, 110.3, 115.3, 119.4, 120.3, 121.0, 124.0, 125.2, 125.9, 127.0, 128.8, 129.3, 129.6, 131.1, 131.6, 131.7, 132.9, 135.7, 137.1, 141.4, 144.6, 158.5; IR (neat) 3415, 3038, 1635, 1569, 1451, 1241, 823, 735 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H24NO3SSe: 534.0642, found 534.0634.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 15
EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 3.86 (s, 3H), 6.91–6.94 (m, 4H), 7.04 (t, J = 7.2 Hz, 2H), 7.09 (d, J = 8.1 Hz, 3H), 7.24–7.32 (m, 5H), 7.41 (t, J = 7.9 Hz, 1H), 7.45 (d, J = 7.8 Hz, 1H), 8.39 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 55.2, 110.1, 112.6, 116.4, 121.1, 123.0, 124.7, 125.6, 126.0, 126.9, 129.0, 129.4, 132.0, 132.3, 133.0, 134.9, 137.6, 144.9, 145.0, 160.3; IR (neat) 3238, 3101, 1638, 1571, 1455, 1231, 826, 738 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H24NO3SSe: 534.0642, found 534.0638.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 3.86 (s, 3H), 6.91–6.94 (m, 4H), 7.04 (t, J = 7.2 Hz, 2H), 7.09 (d, J = 8.1 Hz, 3H), 7.24–7.32 (m, 5H), 7.41 (t, J = 7.9 Hz, 1H), 7.45 (d, J = 7.8 Hz, 1H), 8.39 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 55.2, 110.1, 112.6, 116.4, 121.1, 123.0, 124.7, 125.6, 126.0, 126.9, 129.0, 129.4, 132.0, 132.3, 133.0, 134.9, 137.6, 144.9, 145.0, 160.3; IR (neat) 3238, 3101, 1638, 1571, 1455, 1231, 826, 738 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H24NO3SSe: 534.0642, found 534.0638.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.35 (s, 3H), 6.96 (d, J = 6.6 Hz, 2H), 7.05–7.11 (m, 5H), 7.28–7.47 (m, 10H), 7.63 (d, J = 7.8 Hz, 2H), 7.69 (d, J = 6.3 Hz, 2H), 8.40 (d, J = 8.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 110.8, 116.1, 116.4, 121.2, 124.7, 125.8, 126.1, 126.9, 127.2, 127.6, 127.7, 128.8, 128.9, 129.0, 129.2, 129.4, 129.5, 129.8, 131.8, 132.1, 132.2, 132.3, 134.9, 137.7, 140.4, 141.7, 144.7, 144.9; IR (neat) 3329, 3035, 1662, 1568, 1451, 1234, 821, 736 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H26NO2SSe: 580.0849, found 580.0853.
1). 1H NMR (400 MHz, CDCl3) δ 2.35 (s, 3H), 6.96 (d, J = 6.6 Hz, 2H), 7.05–7.11 (m, 5H), 7.28–7.47 (m, 10H), 7.63 (d, J = 7.8 Hz, 2H), 7.69 (d, J = 6.3 Hz, 2H), 8.40 (d, J = 8.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 110.8, 116.1, 116.4, 121.2, 124.7, 125.8, 126.1, 126.9, 127.2, 127.6, 127.7, 128.8, 128.9, 129.0, 129.2, 129.4, 129.5, 129.8, 131.8, 132.1, 132.2, 132.3, 134.9, 137.7, 140.4, 141.7, 144.7, 144.9; IR (neat) 3329, 3035, 1662, 1568, 1451, 1234, 821, 736 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H26NO2SSe: 580.0849, found 580.0853.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.32 (s, 3H), 6.92 (d, J = 7.4 Hz, 2H), 7.02 (t, J = 7.2 Hz, 2H), 7.07 (d, J = 8.2 Hz, 3H), 7.15 (d, J = 4.8 Hz, 1H), 7.18 (s, 1H), 7.23 (s, 1H), 7.28 (d, J = 8.5 Hz, 3H), 7.39 (t, J = 7.8 Hz, 1H), 7.45 (d, J = 7.7 Hz, 1H), 8.36 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 110.8, 116.2, 121.1, 123.7, 124.6, 125.7, 126.0, 126.8, 128.2, 129.0, 129.1, 129.4, 130.5, 130.9, 132.0, 132.1, 134.9, 137.5, 140.0, 144.9; IR (neat) 3332, 3041, 1648, 1572, 1451, 1235, 819, 728 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H20NO2S2Se: 510.0101, found 510.0105.
1). 1H NMR (400 MHz, CDCl3) δ 2.32 (s, 3H), 6.92 (d, J = 7.4 Hz, 2H), 7.02 (t, J = 7.2 Hz, 2H), 7.07 (d, J = 8.2 Hz, 3H), 7.15 (d, J = 4.8 Hz, 1H), 7.18 (s, 1H), 7.23 (s, 1H), 7.28 (d, J = 8.5 Hz, 3H), 7.39 (t, J = 7.8 Hz, 1H), 7.45 (d, J = 7.7 Hz, 1H), 8.36 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 110.8, 116.2, 121.1, 123.7, 124.6, 125.7, 126.0, 126.8, 128.2, 129.0, 129.1, 129.4, 130.5, 130.9, 132.0, 132.1, 134.9, 137.5, 140.0, 144.9; IR (neat) 3332, 3041, 1648, 1572, 1451, 1235, 819, 728 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H20NO2S2Se: 510.0101, found 510.0105.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 6.85–6.90 (m, 4H), 7.01 (t, J = 6.6 Hz, 1H), 7.28–7.31 (m, 3H), 7.36–7.42 (m, 3H), 7.44–7.49 (m, 3H), 7.57 (t, J = 7.2 Hz, 1H), 7.64 (t, J = 7.0 Hz, 1H), 7.75 (t, J = 7.4 Hz, 2H), 7.83 (d, J = 8.2 Hz, 1H), 7.94 (s, 1H), 8.49 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.6, 116.4, 121.3, 121.4, 124.8, 125.9, 126.0, 127.2, 127.7, 127.9, 128.97, 129.01, 129.1, 129.3, 129.4, 129.5, 130.8, 131.5, 131.7, 132.1, 134.7, 135.2, 137.7, 144.9; IR (neat) 3358, 3021, 1644, 1569, 1455, 1231, 829, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C30H22NO2SSe: 540.0536, found 540.0551.
1). 1H NMR (400 MHz, CDCl3) δ 6.85–6.90 (m, 4H), 7.01 (t, J = 6.6 Hz, 1H), 7.28–7.31 (m, 3H), 7.36–7.42 (m, 3H), 7.44–7.49 (m, 3H), 7.57 (t, J = 7.2 Hz, 1H), 7.64 (t, J = 7.0 Hz, 1H), 7.75 (t, J = 7.4 Hz, 2H), 7.83 (d, J = 8.2 Hz, 1H), 7.94 (s, 1H), 8.49 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.6, 116.4, 121.3, 121.4, 124.8, 125.9, 126.0, 127.2, 127.7, 127.9, 128.97, 129.01, 129.1, 129.3, 129.4, 129.5, 130.8, 131.5, 131.7, 132.1, 134.7, 135.2, 137.7, 144.9; IR (neat) 3358, 3021, 1644, 1569, 1455, 1231, 829, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C30H22NO2SSe: 540.0536, found 540.0551.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 6.91 (t, J = 4.3 Hz, 1H), 6.98–7.00 (m, 2H), 7.06–7.09 (m, 3H), 7.28–7.32 (m, 2H), 7.41–7.49 (m, 8H), 8.33 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 111.5, 116.5, 121.4, 125.1, 125.9, 126.2, 127.1, 127.3, 129.1, 129.3, 129.4, 130.9, 131.4, 131.6, 132.5, 133.3, 133.4, 137.3, 137.5, 144.8; IR (neat) 3312, 3032, 1651, 1576, 1451, 1227, 821, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H18NO2S2Se: 495.9944, found 495.9939.
1). 1H NMR (400 MHz, CDCl3) δ 6.91 (t, J = 4.3 Hz, 1H), 6.98–7.00 (m, 2H), 7.06–7.09 (m, 3H), 7.28–7.32 (m, 2H), 7.41–7.49 (m, 8H), 8.33 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 111.5, 116.5, 121.4, 125.1, 125.9, 126.2, 127.1, 127.3, 129.1, 129.3, 129.4, 130.9, 131.4, 131.6, 132.5, 133.3, 133.4, 137.3, 137.5, 144.8; IR (neat) 3312, 3032, 1651, 1576, 1451, 1227, 821, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H18NO2S2Se: 495.9944, found 495.9939.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 1.09 (t, J = 7.4 Hz, 3H), 3.07–3.13 (m, 2H), 7.13 (s, 5H), 7.32 (t, J = 7.5 Hz, 1H), 7.41–7.42 (m, 6H), 7.60 (d, J = 7.7 Hz, 1H), 8.16 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 7.7, 49.0, 109.3, 115.0, 121.5, 124.5, 125.8, 126.3, 127.4, 127.8, 129.1, 129.3, 129.8, 130.8, 131.5, 131.6, 137.2, 144.8; IR (neat) 3419, 3033, 1649, 1578, 1445, 1241, 826, 732 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H20NO2SSe: 442.0380, found 442.0387.
1). 1H NMR (400 MHz, CDCl3) δ 1.09 (t, J = 7.4 Hz, 3H), 3.07–3.13 (m, 2H), 7.13 (s, 5H), 7.32 (t, J = 7.5 Hz, 1H), 7.41–7.42 (m, 6H), 7.60 (d, J = 7.7 Hz, 1H), 8.16 (d, J = 8.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 7.7, 49.0, 109.3, 115.0, 121.5, 124.5, 125.8, 126.3, 127.4, 127.8, 129.1, 129.3, 129.8, 130.8, 131.5, 131.6, 137.2, 144.8; IR (neat) 3419, 3033, 1649, 1578, 1445, 1241, 826, 732 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C22H20NO2SSe: 442.0380, found 442.0387.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 7.11–7.16 (m, 3H), 7.19–7.24 (m, 3H), 7.29 (t, J = 7.6 Hz, 1H), 7.40–7.46 (m, 4H), 7.68 (d, J = 7.9 Hz, 1H), 7.73 (d, J = 7.2 Hz, 2H), 8.56 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 95.7, 111.1, 120.9, 121.1, 123.3, 125.4, 128.2, 128.5, 128.7, 129.1, 132.0, 132.1, 134.1, 136.2, 142.1; IR (neat) 3409, 3042, 1647, 1572, 1452, 1237, 823, 732 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16NSe: 350.0448, found 350.0447.
1). 1H NMR (400 MHz, CDCl3) δ 7.11–7.16 (m, 3H), 7.19–7.24 (m, 3H), 7.29 (t, J = 7.6 Hz, 1H), 7.40–7.46 (m, 4H), 7.68 (d, J = 7.9 Hz, 1H), 7.73 (d, J = 7.2 Hz, 2H), 8.56 (s, 1H); 13C NMR (100 MHz, CDCl3) δ 95.7, 111.1, 120.9, 121.1, 123.3, 125.4, 128.2, 128.5, 128.7, 129.1, 132.0, 132.1, 134.1, 136.2, 142.1; IR (neat) 3409, 3042, 1647, 1572, 1452, 1237, 823, 732 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C20H16NSe: 350.0448, found 350.0447.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.36 (s, 3H), 6.78 (d, J = 8.6 Hz, 2H), 7.04 (d, J = 8.6 Hz, 2H), 7.12 (d, J = 8.4 Hz, 4H), 7.25–7.29 (m, 2H), 7.36–7.45 (m, 4H), 7.49–7.51 (m, 1H), 8.38 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 115.1, 116.5, 120.1, 124.8, 126.3, 126.5, 127.0, 128.0, 128.9, 129.3, 129.5, 130.5, 131.4, 132.4, 134.8, 135.1, 137.6, 137.8, 145.1; IR (neat) 3402, 3037, 1649, 1567, 1451, 1239, 822, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H20NO2S3: 462.0656, found 462.0661.
1). 1H NMR (400 MHz, CDCl3) δ 2.36 (s, 3H), 6.78 (d, J = 8.6 Hz, 2H), 7.04 (d, J = 8.6 Hz, 2H), 7.12 (d, J = 8.4 Hz, 4H), 7.25–7.29 (m, 2H), 7.36–7.45 (m, 4H), 7.49–7.51 (m, 1H), 8.38 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 115.1, 116.5, 120.1, 124.8, 126.3, 126.5, 127.0, 128.0, 128.9, 129.3, 129.5, 130.5, 131.4, 132.4, 134.8, 135.1, 137.6, 137.8, 145.1; IR (neat) 3402, 3037, 1649, 1567, 1451, 1239, 822, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H20NO2S3: 462.0656, found 462.0661.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 7.02–7.16 (m, 7H), 7.25–7.29 (m, 3H), 7.41–7.43 (m, 3H), 7.49–7.50 (m, 2H), 8.33 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 100.0, 114.8, 115.9, 123.5, 124.4, 126.0, 126.2, 126.9, 127.2, 128.5, 129.2, 129.5, 132.2, 132.3, 133.8, 135.3, 135.7, 138.0, 139.1, 144.9; IR (neat) 3410, 3101, 1644, 1569, 1451, 1235, 828, 728 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H20NO2S2Te: 559.9998, found 559.9992.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 7.02–7.16 (m, 7H), 7.25–7.29 (m, 3H), 7.41–7.43 (m, 3H), 7.49–7.50 (m, 2H), 8.33 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 100.0, 114.8, 115.9, 123.5, 124.4, 126.0, 126.2, 126.9, 127.2, 128.5, 129.2, 129.5, 132.2, 132.3, 133.8, 135.3, 135.7, 138.0, 139.1, 144.9; IR (neat) 3410, 3101, 1644, 1569, 1451, 1235, 828, 728 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H20NO2S2Te: 559.9998, found 559.9992.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (500 MHz, CDCl3) δ 2.38 (s, 3H), 2.41 (s, 3H), 6.95–6.89 (m, 2H), 7.07 (t, J = 7.5 Hz, 2H), 7.15–7.10 (m, 3H), 7.29–7.24 (m, 4H), 7.32 (d, J = 8.3 Hz, 2H), 7.41–7.35 (m, 2H), 8.26 (d, J = 8.4 Hz, 1H); 13C NMR (126 MHz, CDCl3) δ 21.4, 21.7, 110.9, 116.1, 121.1, 126.1, 126.8, 127.5, 128.9, 129.1, 129.4, 129.5, 131.9, 132.5, 132.8, 134.8, 134.8, 135.3, 135.8, 143.8, 145.0; IR (neat) 3319, 3023, 1658, 1571, 1448, 1233, 822, 732 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H23ClNO2SSe: 552.0303, found 552.0309.
1). 1H NMR (500 MHz, CDCl3) δ 2.38 (s, 3H), 2.41 (s, 3H), 6.95–6.89 (m, 2H), 7.07 (t, J = 7.5 Hz, 2H), 7.15–7.10 (m, 3H), 7.29–7.24 (m, 4H), 7.32 (d, J = 8.3 Hz, 2H), 7.41–7.35 (m, 2H), 8.26 (d, J = 8.4 Hz, 1H); 13C NMR (126 MHz, CDCl3) δ 21.4, 21.7, 110.9, 116.1, 121.1, 126.1, 126.8, 127.5, 128.9, 129.1, 129.4, 129.5, 131.9, 132.5, 132.8, 134.8, 134.8, 135.3, 135.8, 143.8, 145.0; IR (neat) 3319, 3023, 1658, 1571, 1448, 1233, 822, 732 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H23ClNO2SSe: 552.0303, found 552.0309.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 0.95 (t, J = 7.3 Hz, 3H), 1.44 (dd, J = 14.9, 7.4 Hz, 2H), 1.76–1.65 (m, 2H), 2.41 (s, 3H), 3.36–3.24 (m, 2H), 7.07 (dd, J = 8.0, 1.6 Hz, 2H), 7.19–7.10 (m, 3H), 7.30–7.20 (m, 3H), 7.40–7.33 (m, 1H), 7.48 (d, J = 7.7 Hz, 1H), 7.67 (d, J = 8.3 Hz, 2H), 8.27 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.8, 21.7, 22.8, 28.4, 33.3, 108.0, 115.3, 120.6, 124.2, 124.9, 126.0, 126.4, 128.8, 129.1, 129.9, 131.9, 132.0, 135.9, 137.1, 144.9, 147.2; IR (neat) 3329, 3044, 1651, 1571, 1455, 1237, 821, 727 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H26NO2SSe: 484.0849, found 484.0861.
1). 1H NMR (400 MHz, CDCl3) δ 0.95 (t, J = 7.3 Hz, 3H), 1.44 (dd, J = 14.9, 7.4 Hz, 2H), 1.76–1.65 (m, 2H), 2.41 (s, 3H), 3.36–3.24 (m, 2H), 7.07 (dd, J = 8.0, 1.6 Hz, 2H), 7.19–7.10 (m, 3H), 7.30–7.20 (m, 3H), 7.40–7.33 (m, 1H), 7.48 (d, J = 7.7 Hz, 1H), 7.67 (d, J = 8.3 Hz, 2H), 8.27 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 13.8, 21.7, 22.8, 28.4, 33.3, 108.0, 115.3, 120.6, 124.2, 124.9, 126.0, 126.4, 128.8, 129.1, 129.9, 131.9, 132.0, 135.9, 137.1, 144.9, 147.2; IR (neat) 3329, 3044, 1651, 1571, 1455, 1237, 821, 727 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C25H26NO2SSe: 484.0849, found 484.0861.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 3.89 (s, 3H), 6.94 (d, J = 8.4 Hz, 2H), 7.00–7.12 (m, 6H), 7.25–7.27 (m, 3H), 7.38 (d, J = 8.1 Hz, 2H), 7.46 (s, 2H), 7.88 (d, J = 9.1 Hz, 1H), 7.94 (d, J = 6.4 Hz, 1H), 8.65 (d, J = 9.2 Hz, 1H), 9.44 (d, J = 7.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 55.3, 108.6, 112.6, 115.6, 123.4, 123.6, 124.9, 125.2, 125.8, 126.2, 126.5, 126.8, 127.9, 128.2, 128.4, 129.2, 129.5, 131.4, 133.3, 133.6, 134.9, 135.3, 145.0, 145.2, 160.2; IR (neat) 3338, 3035, 1661, 1568, 1445, 1241, 822, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H26NO3SSe: 584.0799, found 584.0781.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 3.89 (s, 3H), 6.94 (d, J = 8.4 Hz, 2H), 7.00–7.12 (m, 6H), 7.25–7.27 (m, 3H), 7.38 (d, J = 8.1 Hz, 2H), 7.46 (s, 2H), 7.88 (d, J = 9.1 Hz, 1H), 7.94 (d, J = 6.4 Hz, 1H), 8.65 (d, J = 9.2 Hz, 1H), 9.44 (d, J = 7.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 55.3, 108.6, 112.6, 115.6, 123.4, 123.6, 124.9, 125.2, 125.8, 126.2, 126.5, 126.8, 127.9, 128.2, 128.4, 129.2, 129.5, 131.4, 133.3, 133.6, 134.9, 135.3, 145.0, 145.2, 160.2; IR (neat) 3338, 3035, 1661, 1568, 1445, 1241, 822, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H26NO3SSe: 584.0799, found 584.0781.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.33 (s, 3H), 6.96–7.10 (m, 8H), 7.16 (s, 1H), 7.32 (t, J = 3.9 Hz, 1H), 7.35 (d, J = 8.1 Hz, 2H), 7.43–7.48 (m, 2H), 7.86 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 6.8 Hz, 1H), 8.62 (d, J = 8.4 Hz, 1H), 9.45 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 109.2, 115.4, 123.6, 124.7, 125.2, 125.8, 126.3, 126.7, 127.9, 128.3, 128.4, 128.4, 129.2, 129.5, 130.7, 131.2, 131.3, 133.5, 135.0, 135.2, 140.2, 145.1; IR (neat) 3404, 3044, 1641, 1576, 1451, 1235, 824, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H22NO2S2Se: 560.0257, found 560.0259.
1). 1H NMR (400 MHz, CDCl3) δ 2.33 (s, 3H), 6.96–7.10 (m, 8H), 7.16 (s, 1H), 7.32 (t, J = 3.9 Hz, 1H), 7.35 (d, J = 8.1 Hz, 2H), 7.43–7.48 (m, 2H), 7.86 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 6.8 Hz, 1H), 8.62 (d, J = 8.4 Hz, 1H), 9.45 (d, J = 8.4 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 109.2, 115.4, 123.6, 124.7, 125.2, 125.8, 126.3, 126.7, 127.9, 128.3, 128.4, 128.4, 129.2, 129.5, 130.7, 131.2, 131.3, 133.5, 135.0, 135.2, 140.2, 145.1; IR (neat) 3404, 3044, 1641, 1576, 1451, 1235, 824, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C29H22NO2S2Se: 560.0257, found 560.0259.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 6.96–6.98 (m, 2H), 7.01–7.09 (m, 4H), 7.20 (s, 1H), 7.35 (s, 1H), 7.49–7.50 (m, 2H), 7.60 (d, J = 8.3 Hz, 2H), 7.91 (d, J = 9.2 Hz, 1H), 7.95 (d, J = 7.5 Hz, 1H), 8.11 (d, J = 8.6 Hz, 2H), 8.57 (d, J = 9.2 Hz, 1H), 9.47 (d, J = 7.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.9, 115.0, 123.6, 124.1, 124.2, 125.1, 125.7, 126.3, 126.7, 127.4, 127.8, 128.0, 128.5, 128.9, 129.3, 130.2, 131.1, 131.6, 133.1, 134.8, 139.7, 143.0, 150.5; IR (neat) 3403, 3041, 1646, 1572, 1453, 1243, 821, 737 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H19N2O4S2Se: 590.9951, found 590.9956.
1). 1H NMR (400 MHz, CDCl3) δ 6.96–6.98 (m, 2H), 7.01–7.09 (m, 4H), 7.20 (s, 1H), 7.35 (s, 1H), 7.49–7.50 (m, 2H), 7.60 (d, J = 8.3 Hz, 2H), 7.91 (d, J = 9.2 Hz, 1H), 7.95 (d, J = 7.5 Hz, 1H), 8.11 (d, J = 8.6 Hz, 2H), 8.57 (d, J = 9.2 Hz, 1H), 9.47 (d, J = 7.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.9, 115.0, 123.6, 124.1, 124.2, 125.1, 125.7, 126.3, 126.7, 127.4, 127.8, 128.0, 128.5, 128.9, 129.3, 130.2, 131.1, 131.6, 133.1, 134.8, 139.7, 143.0, 150.5; IR (neat) 3403, 3041, 1646, 1572, 1453, 1243, 821, 737 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H19N2O4S2Se: 590.9951, found 590.9956.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 6.94 (d, J = 7.4 Hz, 2H), 7.05–7.10 (m, 4H), 7.17 (s, 1H), 7.25 (d, J = 5.7 Hz, 2H), 7.31–7.36 (m, 3H), 7.46–7.48 (m, 2H), 7.88 (d, J = 9.2 Hz, 1H), 7.92–7.94 (m, 1H), 8.59 (d, J = 9.2 Hz, 1H), 9.46 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.0, 115.3, 123.6, 123.8, 125.0, 125.5, 126.0, 126.5, 127.0, 128.1, 128.3, 128.5, 128.6, 129.2, 129.3, 130.5, 131.1, 131.4, 133.4, 135.0, 136.3, 140.0, 140.6; IR (neat) 3389, 3045, 1651, 1577, 1455, 1231, 821, 733 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H19ClNO2S2Se: 579.9711, found 579.9706.
1). 1H NMR (400 MHz, CDCl3) δ 6.94 (d, J = 7.4 Hz, 2H), 7.05–7.10 (m, 4H), 7.17 (s, 1H), 7.25 (d, J = 5.7 Hz, 2H), 7.31–7.36 (m, 3H), 7.46–7.48 (m, 2H), 7.88 (d, J = 9.2 Hz, 1H), 7.92–7.94 (m, 1H), 8.59 (d, J = 9.2 Hz, 1H), 9.46 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.0, 115.3, 123.6, 123.8, 125.0, 125.5, 126.0, 126.5, 127.0, 128.1, 128.3, 128.5, 128.6, 129.2, 129.3, 130.5, 131.1, 131.4, 133.4, 135.0, 136.3, 140.0, 140.6; IR (neat) 3389, 3045, 1651, 1577, 1455, 1231, 821, 733 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C28H19ClNO2S2Se: 579.9711, found 579.9706.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 6.83–6.89 (m, 4H), 6.95–6.96 (m, 1H), 7.04–7.05 (m, 1H), 7.11 (s, 1H), 7.28 (s, 1H), 7.38–7.47 (m, 3H), 7.53–7.57 (m, 1H), 7.62 (t, J = 7.2 Hz, 1H), 7.70 (d, J = 8.8 Hz, 1H), 780 (t, J = 10.0 Hz, 2H), 7.88–7.93 (m, 2H), 7.98 (s, 1H), 8.71 (d, J = 9.2 Hz, 1H), 9.42 (d, J = 7.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 109.2, 115.4, 121.1, 123.6, 124.6, 125.2, 125.8, 126.3, 126.8, 127.8, 127.9, 128.1, 128.4, 128.6, 128.9, 129.2, 129.3, 129.5, 129.6, 130.6, 131.2, 131.3, 131.5, 133.4, 134.9, 135.2, 135.2, 140.1; IR (neat) 3411, 3028, 1651, 1568, 1451, 1233, 825, 729 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H22NO2S2Se: 596.0257, found 596.0261.
1). 1H NMR (400 MHz, CDCl3) δ 6.83–6.89 (m, 4H), 6.95–6.96 (m, 1H), 7.04–7.05 (m, 1H), 7.11 (s, 1H), 7.28 (s, 1H), 7.38–7.47 (m, 3H), 7.53–7.57 (m, 1H), 7.62 (t, J = 7.2 Hz, 1H), 7.70 (d, J = 8.8 Hz, 1H), 780 (t, J = 10.0 Hz, 2H), 7.88–7.93 (m, 2H), 7.98 (s, 1H), 8.71 (d, J = 9.2 Hz, 1H), 9.42 (d, J = 7.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 109.2, 115.4, 121.1, 123.6, 124.6, 125.2, 125.8, 126.3, 126.8, 127.8, 127.9, 128.1, 128.4, 128.6, 128.9, 129.2, 129.3, 129.5, 129.6, 130.6, 131.2, 131.3, 131.5, 133.4, 134.9, 135.2, 135.2, 140.1; IR (neat) 3411, 3028, 1651, 1568, 1451, 1233, 825, 729 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H22NO2S2Se: 596.0257, found 596.0261.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 6.88 (t, J = 4.3 Hz, 1H) 6.99–7.04 (m, 5H), 7.14 (d, J = 4.92 Hz, 1H), 7.24 (s, 1H), 7.31–7.32 (m, 2H), 7.45–7.46 (m, 3H), 7.87 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 7.8 Hz, 1H), 8.53 (d, J = 9.2 Hz, 1H), 9.45 (d, J = 7.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.0, 115.4, 123.6, 123.8, 125.1, 125.4, 125.9, 126.4, 126.9, 127.1, 127.9, 128.2, 128.3, 128.4, 129.3, 130.7, 130.9, 131.5, 133.4, 133.7, 134.6, 137.9, 140.2; IR (neat) 3401, 3043, 1642, 1563, 1451, 1241, 829, 726 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H18NO2S3Se: 551.9665, found 551.9667.
1). 1H NMR (400 MHz, CDCl3) δ 6.88 (t, J = 4.3 Hz, 1H) 6.99–7.04 (m, 5H), 7.14 (d, J = 4.92 Hz, 1H), 7.24 (s, 1H), 7.31–7.32 (m, 2H), 7.45–7.46 (m, 3H), 7.87 (d, J = 9.2 Hz, 1H), 7.92 (d, J = 7.8 Hz, 1H), 8.53 (d, J = 9.2 Hz, 1H), 9.45 (d, J = 7.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 110.0, 115.4, 123.6, 123.8, 125.1, 125.4, 125.9, 126.4, 126.9, 127.1, 127.9, 128.2, 128.3, 128.4, 129.3, 130.7, 130.9, 131.5, 133.4, 133.7, 134.6, 137.9, 140.2; IR (neat) 3401, 3043, 1642, 1563, 1451, 1241, 829, 726 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H18NO2S3Se: 551.9665, found 551.9667.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 20
EA = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 1.10 (t, J = 7.4 Hz, 3H), 3.18 (q, J = 7.4 Hz, 2H), 7.09–7.17 (m, 6H), 7.34–7.35 (m, 2H), 7.49–7.51 (m, 2H), 7.84 (d, J = 9.2 Hz, 1H), 7.92–7.94 (m, 1H), 8.38 (d, J = 9.2 Hz, 1H), 9.53 (d, J = 9.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 7.7, 49.7, 108.3, 114.3, 123.6, 123.8, 124.1, 125.3, 126.1, 126.5, 126.9, 127.9, 128.3, 128.5, 128.7, 129.3, 130.6, 130.9, 131.1, 133.4, 134.7, 139.8; IR (neat) 3398, 3029, 1639, 1551, 1450, 1238, 831, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H20NO2S2Se: 498.0101, found 498.0111.
1). 1H NMR (400 MHz, CDCl3) δ 1.10 (t, J = 7.4 Hz, 3H), 3.18 (q, J = 7.4 Hz, 2H), 7.09–7.17 (m, 6H), 7.34–7.35 (m, 2H), 7.49–7.51 (m, 2H), 7.84 (d, J = 9.2 Hz, 1H), 7.92–7.94 (m, 1H), 8.38 (d, J = 9.2 Hz, 1H), 9.53 (d, J = 9.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 7.7, 49.7, 108.3, 114.3, 123.6, 123.8, 124.1, 125.3, 126.1, 126.5, 126.9, 127.9, 128.3, 128.5, 128.7, 129.3, 130.6, 130.9, 131.1, 133.4, 134.7, 139.8; IR (neat) 3398, 3029, 1639, 1551, 1450, 1238, 831, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C24H20NO2S2Se: 498.0101, found 498.0111.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 15
EA = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.36 (s, 3H), 7.07–7.11 (m, 5H), 7.16–7.22 (m, 3H), 7.38–7.40 (m, 2H), 7.43–7.49 (m, 3H), 7.70 (d, J = 7.8 Hz, 1H), 7.84 (d, J = 8.3 Hz, 2H), 8.52 (d, J = 3.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 106.4, 119.9, 124.3, 126.5, 127.4, 128.0, 129.1, 129.3, 129.4, 129.4, 129.9, 130.9, 131.1, 131.2, 135.7, 144.7, 145.1, 145.5, 149.2; IR (neat) 3411, 3040, 1637, 1566, 1451, 1239, 822, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H21N2O2SSe: 505.0489, found 505.0488.
1). 1H NMR (400 MHz, CDCl3) δ 2.36 (s, 3H), 7.07–7.11 (m, 5H), 7.16–7.22 (m, 3H), 7.38–7.40 (m, 2H), 7.43–7.49 (m, 3H), 7.70 (d, J = 7.8 Hz, 1H), 7.84 (d, J = 8.3 Hz, 2H), 8.52 (d, J = 3.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.7, 106.4, 119.9, 124.3, 126.5, 127.4, 128.0, 129.1, 129.3, 129.4, 129.4, 129.9, 130.9, 131.1, 131.2, 135.7, 144.7, 145.1, 145.5, 149.2; IR (neat) 3411, 3040, 1637, 1566, 1451, 1239, 822, 731 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C26H21N2O2SSe: 505.0489, found 505.0488.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 10
EA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 7.11–7.17 (m, 3H), 7.32–7.56 (s, 13H), 7.71 (d, J = 7.8 Hz, 1H), 8.30 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 115.6, 120.7, 124.7, 126.0, 126.3, 126.7, 126.9, 127.9, 128.2, 128.9, 129.5, 129.7, 130.3, 130.9, 131.0, 132.3, 135.0, 137.2, 145.6; IR (neat) 3329, 1641, 1568, 1442, 1251, 856, 741 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H22NO3SSe: 520.0486, found 520.0482.
1). 1H NMR (400 MHz, CDCl3) δ 2.34 (s, 3H), 7.11–7.17 (m, 3H), 7.32–7.56 (s, 13H), 7.71 (d, J = 7.8 Hz, 1H), 8.30 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 115.6, 120.7, 124.7, 126.0, 126.3, 126.7, 126.9, 127.9, 128.2, 128.9, 129.5, 129.7, 130.3, 130.9, 131.0, 132.3, 135.0, 137.2, 145.6; IR (neat) 3329, 1641, 1568, 1442, 1251, 856, 741 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H22NO3SSe: 520.0486, found 520.0482.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 10
EA = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1H NMR (400 MHz, CDCl3) δ 2.36 (s, 3H), 7.11–7.18 (m, 3H), 7.32–7.57 (m, 13H), 7.71 (d, J = 7.9 Hz, 1H), 8.30 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 105.0, 115.6, 120.7, 124.7, 126.0, 126.3, 126.9, 127.9, 128.1, 128.9, 129.5, 129.7, 130.3, 130.9, 132.3, 135.0, 137.2, 140.3, 143.3, 145.6; IR (neat) 3256, 1652, 1588, 1465, 1241, 851, 733 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H22NO4SSe: 536.0435, found 536.0441.
1). 1H NMR (400 MHz, CDCl3) δ 2.36 (s, 3H), 7.11–7.18 (m, 3H), 7.32–7.57 (m, 13H), 7.71 (d, J = 7.9 Hz, 1H), 8.30 (d, J = 8.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 21.6, 105.0, 115.6, 120.7, 124.7, 126.0, 126.3, 126.9, 127.9, 128.1, 128.9, 129.5, 129.7, 130.3, 130.9, 132.3, 135.0, 137.2, 140.3, 143.3, 145.6; IR (neat) 3256, 1652, 1588, 1465, 1241, 851, 733 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C27H22NO4SSe: 536.0435, found 536.0441.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Natural Science Foundation of China (22161043 and 21801096), Natural Science Foundation of Zhejiang Province (LY21B020009), Yunnan Fundamental Research Project (202201AU070071), “High-level Talent Introduction Program” Project of Yunnan Province (YNQR-QNRC-2019-065), Yunnan Provincial Department of Education Project (2022Y554), Department of Education of Zhejiang Province (Y202147909), The Science and Technology Project of Jiaxing (2022AY10018), and The Start Up Funding of Southwest Forestry University (112126), also supported by the 111 Project (D21027).Notes and references
- For selected examples: (a) M. Suleman, P. Lu and Y. Wang, Org. Chem. Front., 2021, 8, 2059 RSC; (b) X.-Y. Liu and Y. Qin, Acc. Chem. Res., 2019, 52, 1877 CrossRef CAS PubMed; (c) J. Bariwal, L. G. Voskressensky and E. V. V. Eycken, Chem. Soc. Rev., 2018, 47, 3831 RSC; (d) P. V. Thanikachalam, R. K. Maurya, V. Garg and V. Monga, Eur. J. Med. Chem., 2019, 180, 562 CrossRef CAS PubMed; (e) M. Chauhan, A. Saxena and B. Saha, Eur. J. Med. Chem., 2021, 218, 113400 CrossRef CAS PubMed; (f) Y. Zhu, J. Zhao, L. Luo, Y. Gao, H. Bao, P. Li and H. Zhang, Eur. J. Med. Chem., 2021, 223, 113665 CrossRef CAS PubMed; (g) B. Qin, S. Huang, J.-Q. Chen, W. Xiao and J. Wu, Org. Chem. Front., 2022, 9, 3521 RSC.
- For selected examples: (a) C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255 CrossRef CAS PubMed; (b) Q.-B. Zhang, Y.-L. Ban, P.-F. Yuan, S.-J. Peng, J.-G. Fang, L.-Z. Wu and Q. Liu, Green Chem., 2017, 19, 5559 RSC; (c) X. Zhang, C. Wang, H. Jiang and L. Sun, Chem. Commun., 2018, 54, 8781 RSC; (d) S. K. Bhunia, P. Dasa and R. Jana, Org. Biomol. Chem., 2018, 16, 9243 RSC.
- Z. Wen, X. Li, D. Zuo, B. Lang, Y. Wu, M. Jiang, H. Ma, K. Bao, Y. Wu and W. Zhang, Sci. Rep., 2016, 6, 23986 CrossRef PubMed.
- Q. Guan, C. Han, D. Zuo, M. Zhai, Z. Li, Q. Zhang, Y. Zhai, X. Jiang, K. Bao, Y. Wu and W. Zhang, Eur. J. Med. Chem., 2014, 87, 306 CrossRef CAS PubMed.
- G. L. Regina, R. Bai, W. M. Rensen, E. D. Cesare, A. Coluccia, F. Piscitelli, V. Famiglini, A. Reggio, M. Nalli, S. Pelliccia, E. D. Pozzo, B. Costa, I. Granata, A. Porta, B. Maresca, A. Soriani, M. L. Iannitto, A. Santoni, J. Li, M. M. Cona, F. Chen, Y. Ni, A. Brancale, G. Dondio, S. Vultaggio, M. Varasi, C. Mercurio, C. Martini, E. Hamel, P. Lavia, E. Novellino and R. Silvestri, J. Med. Chem., 2013, 56, 123 CrossRef PubMed.
- Z. Wen, J. Xu, Z. Wang, H. Qi, Q. Xu, Z. Bai, Q. Zhang, K. Bao, Y. Wu and W. Zhang, Eur. J. Med. Chem., 2015, 90, 184 CrossRef CAS PubMed.
- For selected examples: (a) I. P. Beletskaya and V. P. Ananikov, Chem. Rev., 2011, 111, 1596 CrossRef CAS PubMed; (b) D. Luo, G. Wu, H. Yang, M. Liu, W. Gao, X. Huang, J. Chen and H. Wu, J. Org. Chem., 2016, 81, 4485 CrossRef CAS PubMed; (c) M. Iwasaki, N. Miki, Y. Tsuchiya, K. Nakajima and Y. Nishihara, Org. Lett., 2017, 19, 1092 CrossRef CAS PubMed.
- For selected examples: (a) Y. Cao, J. Liu, F. Liu, L. Jiang and W. Yi, Org. Chem. Front., 2019, 6, 825 RSC; (b) Q.-Y. Han, C.-L. Zhao, T. Dong, J. Shi, K.-L. Tana and C.-P. Zhang, Org. Chem. Front., 2019, 6, 2732 RSC; (c) V. Rathore and S. Kumar, Green Chem., 2019, 21, 2670 RSC; (d) S. K. Bhunia, P. Dasa and R. Jana, Org. Biomol. Chem., 2018, 16, 9243 RSC.
- (a) H.-A. Du, R.-Y. Tang, C.-L. Deng, Y. Liu, J.-H. Li and X.-G. Zhang, Adv. Synth. Catal., 2011, 353, 2739 CrossRef CAS; (b) A. Sperança, B. Godoi, P. H. Menezes and G. Zeni, Synlett, 2013, 24, 1125 CrossRef.
- For selected examples: (a) S. Lapcinska and P. Arsenyan, Eur. J. Org. Chem., 2020, 784 CrossRef CAS; (b) R. Gou, Y. Zhang, S.-W. Wu and F. Liu, Synlett, 2019, 30, 207 CrossRef CAS; (c) Z. Li, L. Hong, R. Liu, J. Shen and X. Zhou, Tetrahedron Lett., 2011, 52, 1343 CrossRef CAS.
- (a) Y. Chen, C.-H. Cho and R. C. Larock, Org. Lett., 2009, 11, 173 CrossRef CAS PubMed; (b) Y. Chen, C.-H. Cho, F. Shi and R. C. Larock, J. Org. Chem., 2009, 74, 6802 CrossRef CAS PubMed; (c) B. Z. Lu, W. Zhao, H.-X. Wei, M. Dufour, V. Farina and C. H. Senanayake, Org. Lett., 2006, 8, 3271 CrossRef CAS PubMed; (d) D. Yue and R. C. Larock, Org. Lett., 2004, 6, 1037 CrossRef CAS PubMed.
- Q. Shi, P. Li, Y. Zhang and L. Wang, Org. Chem. Front., 2017, 4, 1322 RSC.
- (a) M. C. D. F. Xavier, E. M. A. Sandagorda, J. S. S. Neto, R. F. Schumacher and M. S. Silva, RSC Adv., 2020, 10, 13975 RSC; (b) Y.-L. Ban, L. You, K.-W. Feng, F.-C. Ma, X.-L. Jin and Q. Liu, J. Org. Chem., 2021, 86, 5274 CrossRef CAS PubMed.
- For selected examples, see: (a) Z.-G. Yuan, Q. Wang, A. Zheng, K. Zhang, L.-Q. Lu, Z. Tang and W.-J. Xiao, Chem. Commun., 2016, 52, 5128 RSC; (b) D. Staveness, I. Bosque and C. R. J. Stephenson, Acc. Chem. Res., 2016, 49, 2295 CrossRef CAS PubMed; (c) K. P. S. Cheung, S. Sarkar and V. Gevorgyan, Chem. Rev., 2022, 122, 1543 CrossRef PubMed; (d) M. Zora, D. Demirci, A. Kivrak and Y. Kelgokmen, Tetrahedron Lett., 2016, 57, 993 CrossRef CAS; (e) L. C. Wilkins, B. A. R. Günther, M. Walther, J. R. Lawson, T. Wirth and R. L. Melen, Angew. Chem., Int. Ed., 2016, 55, 11292 CrossRef CAS PubMed.
- For selective examples: (a) L. Xing, Y. Zhang, B. Li and Y. Du, Org. Lett., 2019, 21, 3620 CrossRef CAS PubMed; (b) B. Zhang, X. Li, X. Li, Z. Yu, B. Zhao, X. Wang and Y. Du, J. Org. Chem., 2021, 86, 17274 CrossRef CAS PubMed; (c) Z. Shang, Q. Chen, L. Xing, Y. Zhang, L. Wait and Y. Du, Adv. Synth. Catal., 2019, 361, 4926 CrossRef; (d) Z. Yu, D. Zhang, X. Li, B. Zhang, Z. Yang, Y. Qian and Y. Du, Asian J. Org. Chem., 2021, 10, 3015 CrossRef CAS.
- For selective examples: (a) Q. Wang, L. Zhang, J. Yao, G. Qiu, X. Li and H. Zhou, J. Org. Chem., 2018, 83, 4092 CrossRef CAS PubMed; (b) Y. Zhang, W. Lai, L. Zhang, X. Gao, G. Qiu and H. Zhou, Org. Biomol. Chem., 2021, 19, 1827 RSC; (c) X. Chen, L. Zhang, Y. Wang, G. Qiu, Q. Liang and H. Zhou, J. Org. Chem., 2020, 85, 12526 CrossRef CAS PubMed; (d) C. Bi, L. Zhang, G. Qiu, X. Li, J. Yao and H. Zhou, Org. Biomol. Chem., 2018, 16, 3006 RSC; (e) Y. Hua, Z.-Y. Chen, H. Diao, L. Zhang, G. Qiu, X. Gao and H. Zhou, J. Org. Chem., 2020, 85, 9614 CrossRef CAS PubMed; (f) Y. Zhang, T. Liu, L. Liu, H. Guo, H. Zeng, W. Bi, G. Qiu, W. Gao, X. Ran, L. Yang, G. Du and L. Zhang, J. Org. Chem., 2022, 87, 8515 CrossRef CAS PubMed.
- For selective examples: (a) X. Li, B. Zhang, J. Zhang, X. Wang, D. Zhang, Y. Du and K. Zhao, Chin. J. Chem., 2021, 39, 1211 CrossRef CAS; (b) S. S. K. Boominathan, G. C. Senadi, J. K. Vandavasi, J. Y.-F. Chen and J.-J. Wang, Chem.–Eur. J., 2015, 21, 3193 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2001345. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra00030c |
| ‡ Equal contributions to this manuscript. |
| This journal is © The Royal Society of Chemistry 2023 |