Open Access Article
Open Access ArticleCarboxymethyl cellulose-based materials as an alternative source for sustainable electrochemical devices: a review
Maida Akhlaq†
,
Umair Mushtaq†
,
Sadia Naz
 and
Maliha Uroos
and
Maliha Uroos
 *
*
Centre for Research in Ionic Liquids, School of Chemistry, University of the Punjab, Lahore-54590, Pakistan. E-mail: malihauroos.chem@pu.edu.pk
First published on 15th February 2023
Abstract
In electrochemistry, bio-based materials are preferred over the traditional costly and synthetic polymers due to their abundance, versatility, sustainability and low cost. One of the bio-based polymers is carboxymethyl cellulose (CMC) which has become an overarching material in electrochemical devices pertaining to its amphiphilic nature with multi-carbon functional groups. Owing to its flexible framework with fascinating groups on its surface like hydroxide (–OH) and carboxylate (–COO−), CMC is able to be modified into conducting materials by blending it with other biopolymers, synthetic polymers, salts, acids and others. This blending has improved the profile of CMC by exploiting the ability of hydrogen bonding, swelling, adhesiveness and dispersion of charges and ions. These properties of CMC have made it possible to utilize this bio-sourced polymer in several applications as a conducting electrolyte, binder in electrodes, detector, sensor and active material in fuel cells, actuators and triboelectric nanogenerators (TENG). Thus, CMC based materials are cheap, environment friendly, hydrophilic, biodegradable, non-toxic and biocompatible which render it a desirable material in energy storage devices.
1. Introduction
Polymers, giant molecules containing multiple repeating units, are equally important for their technological applications as well as from a fundamental scientific point of view. Usually, polymer materials are considered as insulators, however, they could be upgraded into conducting materials.1,2 Modified polymer conducting materials are envisioned as host materials in energy storage media in the near future. These have been widely used in sensors, batteries, fuel cells, capacitors, photoelectrochemical solar cells and electrochromic displays.3 They have stable structure and low volatility, therefore, can be used in modern technology devices.4,5Ion conductivity behavior of polymers was first uncovered by Wright in 1975 (ref. 6) who discovered the ionic conductive behavior of polyethylene oxide–alkaline metal ion complex. There were a lot of struggles at that time to improve the ionic conductivity of polymer electrolytes and making facile the applications of polymers in power applications. Much attention was paid on synthetic polymer such as polyethylene oxide (PEO) and polyvinyl alcohol (PVA).7,8 But these synthetic polymers are costly and depleting petroleum resources. Comparatively, natural polymers like cellulose and cellulose derivatives are ecofriendly, abundantly available and low-priced.9,10 One of such exciting polymers is carboxymethyl cellulose (CMC).11
CMC is a polysaccharide comprising of fibrous tissues of plants. It is water soluble, low-cost polymer containing hydroxyl and sodium carboxymethyl groups (–CH2COONa).12,13 It has emerged as a valuable chemical due to its anticipated properties such as biodegradability, nontoxicity, high hydrophilicity, biocompatibility and excellent film forming ability.14 It exhibits variety of applications in textile, drugs, flocculation, food processing, detergents and many more.15,16
Role of CMC in electrochemistry is based on its parent molecule “cellulose” which is a biodegradable natural biopolymer with linear polysaccharide chain. The acidification of cellulose yields its linear bulky derivative ‘CMC’.17,18 It is an abundant carbon resource due to its multi-carbon functional groups which could be modified to obtain dense carbon conducting framework.19,20 However, as a single polymer, CMC has the limited stability and miscibility in electrochemical devices.21 To enhance the efficiency, CMC has been mixed with other biodegradable polymers like PVA, PEO, cellulose, hemicellulose, chitosan, kappa carrageenan (KC) and so on. The mixing of these polymers with CMC tend to increase its physicochemical properties such as miscibility, tensile strength, ionic conductivity and reduce the crystallinity.22
Herein, we will initially present CMC applications in energy storage devices as an electrolyte or binder in detail. After that, applications of CMC in energy conversion and generation devices such as fuel cells and TENG will be discussed. Lastly, we will discuss it's applications in electrical devices like biosensors and actuators. Moreover, we are well aware of recent reviews on role of biopolymers in electrochemistry.23,24 But to the best of our knowledge, there is no published review on applications of CMC based materials in electrochemistry, specifically.
2. CMC based materials in energy storage devices as electrolyte/binder
In energy storage devices, energy is stored in different forms such as electromagnetic, electrochemical, chemical, potential, kinetic and thermal.25 In view of, power density, energy density, technical maturity and cyclability rechargeable batteries have been popularized.26 Among them, CMC has found wide range of applications as an electrolyte, binder for electrode in batteries and cells that have been discussed here in detail.2.1. General advantages of solid polymer electrolytes (SPEs) over liquid electrolytes
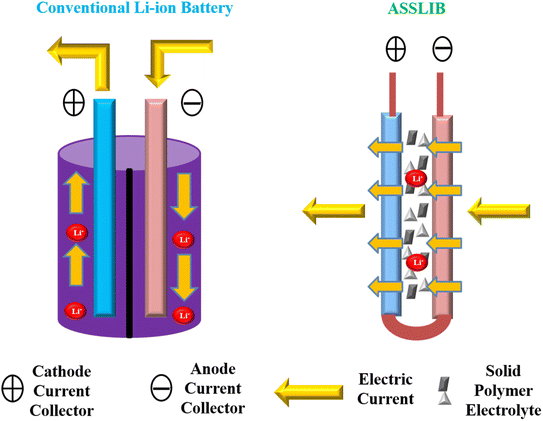
Traditionally, liquid electrolytes have been used in electrochemical applications because they are conductive even at low temperature. But they have certain drawbacks which make them unsuitable for electrochemical devices such as leakage, deterioration, instability and so on.27 Moreover, they could react with electrode, lead to corrosion and decrease the life span of the device.28 Additionally, boiling point of solvent restricts the temperature of the device, showing lower electrochemical firmness.29 With the discovery of solid polymer electrolytes (SPEs) in 1975,11 these were predicted as suitable substitute for liquid electrolytes due to their better conductivity as well as improved mechanical and thermal stability (Fig. 1).29,30 From there on, SPEs are preferred due to their high elasticity, low self-discharge, no leakage, easy processibility and good compatibility with electrodes.31 The only serious disadvantage of solid electrolyte is their crystallinity but it can be solved by using organic or inorganic acids/salts.32 Nowadays, SPEs have gained considerable attention due to their potent applications in energy density batteries, electrochemical cells, electrochromic devices and fuel cells.33 But natural polymer electrolytes are preferred over earlier synthetic polymers due to low cost, biocompatibility and availability.28,34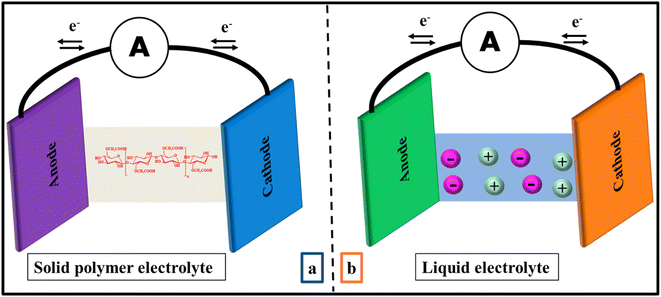 | ||
| Fig. 1 Schematic illustration of electrolytes based on (a) solid polymer electrolyte (SPE) (b) conventional liquid polymer electrolyte in electrochemical storage devices. | ||
2.2. CMC based blends as solid-state electrolyte
The use of cellulose and its derivatives as solid polymer electrolyte (SPE) is noteworthy because these are predicting green future unlike toxic and harmful materials. One of the derivatives of cellulose is CMC which has been extensively used as solid electrolyte in batteries and cells. CMC, when combined with other biopolymers or salts, has been showing excellent conductivity with cost-effectiveness which makes it desirable as electrolyte in energy storage devices. CMC based blends reported as solid-state electrolytes are briefly summarized in next sections.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The ionic conductivity and physiochemical properties were developed by using 1–5 wt% of lithium perchlorate (LiClO4) as doped salt. The highest tensile strength was obtained with 3 wt%, and ionic conductivity with 5 wt% of LiClO4. The 5 wt% loading of LiClO4 in films was considered outstanding for ionic conductance.44
3. The ionic conductivity and physiochemical properties were developed by using 1–5 wt% of lithium perchlorate (LiClO4) as doped salt. The highest tensile strength was obtained with 3 wt%, and ionic conductivity with 5 wt% of LiClO4. The 5 wt% loading of LiClO4 in films was considered outstanding for ionic conductance.44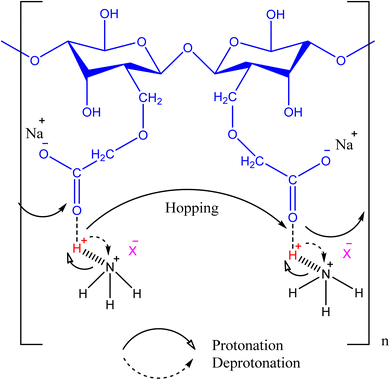 | ||
| Fig. 2 A schematic representation of hopping mechanism involving protonation and deprotonation by the coordination of CMC with ammonium salts. | ||
In another study by the same research group, NH4Br-doped CMC served as the polymer host in the biopolymer electrolyte (BE) system. Analysis of the BE system's ionic conduction has been carried out using EIS and the most conducting sample was found to contain 25% NH4Br. When different concentrations of plasticizer were introduced to the BE system, the ionic conductivity increased to 3.31 × 10−3 S cm−1. Proton batteries may be made using CMC based BE systems with high conductivities, according to linear sweep voltammetry (LSV) measurements. In comparison to previous studies that used polymer electrolytes in their solid-state batteries, these solid-state proton batteries produced higher open-circuit voltages (OCVs) of 1.36 and 1.48 V at room temperature and outperformed previous studies that used polymer electrolytes in their solid-state batteries.41 On the same ground, Yang et al., developed proton conducting BE by combining different NH4Br compositions (wt%) with biopolymer materials CMC. It has been revealed that the conducting material is mostly available proton (H+) in this study, as verified by FTIR and transference number measurement (TNM) analyses. The Zn + ZnSO4·7H2O/BE/MnO2 design of a rechargeable proton conducting BE battery generated an all-out open circuit potential (OCP) of 1.36 V at room temperature and demonstrated excellent rechargeability.42
Samsudin et al., also investigated CMC based BE systems containing NH4Br. FTIR spectroscopy revealed a putative coordination relationship between CMC and NH4Br, which was validated by the Grotthus mechanism. The BE sample containing 25% NH4Br had the maximum ionic conductivity at room temperature (1.12 × 104 S cm−1) and the least weight loss as evidenced by TGA. Ionic transference number of mobile ions determined by direct current polarization technique was 0.98, indicating that conducting species are ions (H+). The BE was electrochemically stable up to 1.42 ± 0.01 V, making it appropriate for proton batteries. Zn + ZnSO4·7H2O/BE sample/MnO2 rechargeable proton-conducting BE batteries were made. The proton battery had a starting cell potential of 1.52 V and a constant OCP of 1.36 V. It was recharged 10 times without losing cell potential.43
Abutalib et al., reported the good optical, dielectric and electrical properties of CMC films grafted with polyvinyl alcohol (PVA) and graphene nanotubes. The optical energy band gap confirmed the optical characteristics of prepared polymer blends and dynamic behavior of ions of all samples were investigated by frequency dependence AC conductance. The nanocomposite films ionic conductivity increased by addition of graphene nanotubes concentration. The highest conductivity was observed 4.53 × 10−4 at 30 °C.49
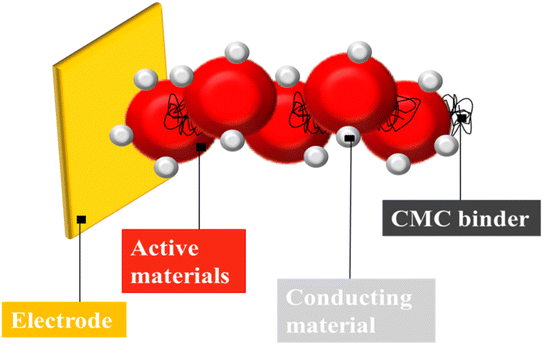
In ASSLIB, there are different ways to upsurge the energy density of the batteries two of them are: (1) by improving the specific capacity of the cell, development of the high-energy density electrodes55 and (2) by improving the volumetric capacity of the cell, and designing a thick electrode with compact cell configuration.56 But, the use of thicker electrode causes the enhanced diffusion of Li-ion which causes resistance to the charge transport with degradation of electrochemical performance.57 Binders as major battery components, provide integrity with enhanced physiochemical properties to the electrodes (Fig. 4).58 Conventional CMC based aqueous binders are reliable, low cost, environment friendly and provides high energy density to the anodes of Li-ion batteries (Table 1).59,60 Yang et al., reported CMC with humic as a potential binder for lithium batteries for the enhancement of electrochemical performance. Both water soluble polymers acted as combined binder for charge–discharge and cathode preparation process. The binder forms stable interface layer of cathode with significant increase in electrical conductivity of electrode. After 100 cycles, the reversible capacity was observed which was higher than cathode with traditional binder. The cathode specific capacity was 105 mA h g−1 at 8 °C with discharge platform of 3.30 V.61 Jeong et al., reported that lithium incorporated hierarchically porous carbon monoliths (PCMs), a potential anode material in Li-ion batteries, were prepared with lithium salt of water-soluble CMC. The porous structure of carbon and homogeneity of lithium throughout the PCMs have enhanced the electrochemical properties of composites. PCMs have low initial irreversible capacity, stable life cycle at high current density and increase in resistance at the end of the discharge. Moreover, the galvanostatic intermittent titration technique indicated the high diffusion coefficient of prepared material at the end of the discharge.62 Karkar et al., compared polyacrylic acid (PAA) and CMC/citric acid as binders for silicon-based negative Li-ion battery electrodes and found later one as more effective. Concentration of binder affects the electrode slurry rheology, dry electrode shape, and physical characteristics. Electrochemical behavior of electrodes is a function of active mass loading (1 to 4.5 mg cm−2). Increasing the binder concentration from 4 to 12 wt% enhances 1st cycle efficiency due to its role as a pre-formed artificial solid electrolyte interphase layer. Increasing the binder concentration improves the cyclability of electrodes with active mass loading over 1 mg cm−2. CMC/citric acid is more effective at low concentration and PAA at high content, perhaps due to their molecular structures. For both binders, post-processing, called maturation improves electrochemical performance.63
| CMC source | Blends/composite material | Specific capacitance (mA h g−1) | Current density (mA g−1) | Charge/discharge cycles | Applications in electrochemistry | Ref |
|---|---|---|---|---|---|---|
| Commercial; Sigma-Aldrich | Na-CMC/SBR as binder | 450 | 0.2 | 30 | • Carbon coated silicon/boron/graphite anode in Li-ion batteries | 72 |
| Commercial | Na-CMC/SBR as binder | 2221 | 30 | • Modified elastomeric binder for silicon anode in Li-ion batteries | 73 | |
| Commercial | CMC as binder | 149.2 | 200 | • CMC as cheap and green binder for LiNi1/3Mn1/3Co1/3O2 (NMC) as cathode material for Li-ion batteries | 74 | |
| Synthetic CMC based on cotton cellulose | CMC-Li binder | 180 | 200 | • CMC-Li is cheaper, eco-friendly, biodegradable and safer binder for Li-ion batteries' electrodes | 65 | |
| Commercial; Sigma-Aldrich | Na-CMC/SBR/acrylic acid hydrogels as binder | 157/162 | 0.5 | 50 | • Physiochemical effect of CMC based binder for graphite anode in Li-ion batteries | 71 |
| Commercial; Sigma-Aldrich | CMC derived Li incorporated hierarchically porous carbon monoliths | 312 | 30 | 50 | • Pre-lithiated CMC derived porous carbon monoliths can act as an efficient material for high power anode | 62 |
Cotton is also used to make the water-based CMC binder with lithium (CMC-Li). CMC-Li can enhance the lithium content, boosting the diffusion efficiency and specific capacity. The CMC-Li battery preserved 98% of its original reversible capacity after 200 cycles at 176 mA h g−1, exceeding lithium iron phosphate (LiFePO4, LFP) predicted specific capacity. The batteries are electrochemically sound, pollution-free, and stable.64 Due to small loss and consistent performance, CMC-Li with high degree of substitution is preferred.65 Qiu et al., studied electrochemical characteristics of lithium iron phosphate and water-soluble binder cathodes, CMC-Na and CMC-Li. After 200 cycles, the CMC with lithium as binder enhanced LFP cathode cyclic ability by 96.7% at 175 mA h g−1 compared to poly(vinylidene difluoride) (PVdF). The LFP electrode with CMC-Li as the binder has the best capacity rate as compared to other binders. EIS studies demonstrate that CMC-Li has lower charge transfer resistance than CMC and PVdF alone.66 CMC-Li extracted from cotton after treatment with lithium ethoxide was reported to be used as lithium-replenishing binders in the negative electrode of batteries with water as a dispersant to assemble a battery. The batteries showed better results due to reduced resistance, improved impedance, cycle period with 2500 circles and increase in life span by 20% which could be attributed to lithium supplementation. By increasing the lithium content, batteries showed improved ion conduction rate and ion conductivity.67
On one side of a pristine polyethylene battery separator, a ceramic layer composed of aluminum oxide (Al2O3) powder, CMC, and styrene-butadiene rubber (SBR) mixed binder was effectively formed to create a coating separator made of ceramics for lithium batteries. In the preparation of separator, water as solvent was used with very little quantity of SBR–CMC combination as binder to improve the thermostability. The studies have shown that the ceramic-coated separator with SBR–CMC binder had excellent thermal stability, high fluid electrolyte absorption, and superior hydrophilicity. Tests of pouch cells with a ceramic-coated separator also demonstrate high cycle stability. The ceramic coating separator (CCS) membrane significantly improved thermal contraction, liquid electrolyte hydrophilicity, and electrolyte absorption. As the ceramic-coating layer increased on the CCS membrane, the absorption of electrolytes increased, and the thermal contraction was decreased. The punch cells constructed with ceramic-coated separators have superior cycling performance compared to those with polyethylene separators. Because just a minimal quantity of binder is required and the solvent is water, the CCS membrane is appropriate for use in secondary lithium batteries.68 Yeo et al., investigated the combination of rechargeable lithium-ion batteries with lumichrome cathodes and aqueous SBR-CMC binders. A 2S6P battery module was successfully made to power blue LEDs. Positive electrode lumichrome revealed no structural alterations compared to pristine powder. Large pouch cells had cathodic and anodic peaks with 2.58 and 2.26 V average potentials. Ethylene carbonate-dimethyl carbonate (EC-DMC) and triethylene glycol-dimethoxyethane (TEGDME) both have initial discharge capabilities of 142 mA h g−1. EC-DMC-injected pouch cells performed better than TEGDME-injected ones. This may be due to the decreased lithium-ion migration resistance at the electrode/electrolyte interphase and increased EC-DMC ionic conductivity. EC-DMC-injected pouch cells cycled more than TEGDME-injected ones. After 40 cycles, EC-DMC and TEGDME-injected cells retained 56.4% and 22.3% of their capacity. TEGMDE electrolyte decomposes and swells lithium metal negative electrodes. To enhance lumichrome active materials' electrochemical characteristics, novel electrolyte systems and negative electrode materials are being studied.69 Li et al., reported that CMC Oxa-Michael addition with sulfobetaine methacrylate (SBMA) resulted in zwitterionic modification because zwitterion segment can simultaneously provide quaternary ammonium and sulfonic group for electron and lithium-ion receptors. The limiting molar conductivity increased (61 S cm2 mol−1) and activation energy decreased (0.058 eV). This composite CMC/SBMA used as binder in graphite anode of lithium-ion batteries with discharge voltage plateau at 3.35 V and specific capacitance 140 mA h g−1. This binder had also been used in activated carbon electrode for supercapacitor with low diffusion parameter of 0.03, low internal resistance and high specific capacitance of 277.9 F g−1.70 Shin et al., increased the thickness of graphite anode which created a perfect cell of increased mass loading and density of the Li-ion battery. Increasing the duration of ionic migration route may reduce Li+ diffusivity. Using SBR as a binder, cross-linked poly acrylic acid–carboxymethyl cellulose hydrogels were generated to improve adhesion strength with diffusivity. Adhesion strength of electrode with electrolyte permeability were increased by 2.0 and 2.5 times, respectively, as compared to a standard CMC–SBR combination, leading to good electrochemical characteristics owing to improve interpolymeric and electrolyte properties. Retention capacity increased from 80 to 90% at 1 °C.71
| CMC source | Blends/composite material | Capacity (mA h g−1) | Current density (mA g−1) | Capacity retention (%) | Cycles | Application | Ref |
|---|---|---|---|---|---|---|---|
| Commercial; Daicel, Ltd | CMC/poly (vinylidene difluoride) binder | 250 | 25 | 97 | 100 | • CMC as binder for hard carbon negative electrode in sodium ion batteries | 85 |
| Commercial; Sigma-Aldrich Co. Ltd | CMC as binder | 400 | 25 | 20 | • Comparison of CMC binder with other kind of binders in sodium ion batteries | 76 | |
| Commercial | CMC as binder | 627.2 | 50 | 73.4 | 100 | • CuO nanosheets as anode with CMC binder showed satisfactory electrochemical performance | 87 |
| Commercial | CMC/SBR as binder | 97 | 200 | 200 | • Spinel oxide-FeV2O4 with CMC/SBR binder used as conversion- based anode material in sodium ion batteries | 86 | |
| Commercial; TCI | Na-CMC as binder | 635 | 73 | 60 | • BiFeO3-CMC electrode in sodium ion batteries | 88 |
Transition metal oxides were also used as electrodes for sodium-ion batteries. FeV2O5 with eco-friendly and cost-effective binders of sodium carboxymethyl cellulose/styrene butadiene rubber has proved potential anode material for batteries. The transition metal oxide worked on the principle of pseudo-capacitive process and binders showed highly stable capacity of 97 mA h g−1 after 200 cycles. The higher stability might be due to the strong hydrogen bonding between carboxyl and hydroxyl groups which leads to superior binding with active material and current collector. By comparing the transition metal oxide capacitance with PVdF capacity, the later showed detachment of the electrode material from copper foil after 200 runs.86 Hydrothermally produced copper oxide (CuO) nanosheets were also evaluated as anodes of sodium-ion batteries. CuO nanosheets performed well as anodes due to their unique nanosheet structure. CuO nanosheets electrode with CMC binder demonstrated much better electrochemical performance than PVdF electrode. CuO nanosheet electrodes with CMC binder exhibited 627.2 mA h g−1 at 50 mA g−1. CuO nanosheet preserved 50.3% of its original capacity after 300 cycles at a current density of 2000 mA g−1. CuO nanosheets are attractive electrode materials because of their high reversible capacity, steady cycling performance, and excellent rate capability.87
| Blends/composite material | Specific capacitance (mA h g−1) | Current density (mA g−1) | Charge/discharge cycles | Ionic conductance (mS cm−2) | Applications in electrochemistry | Ref |
|---|---|---|---|---|---|---|
| CMC/PVA as polyelectrolyte | — | 10 | 300 | 119.8 | • CMC/PVA based polyelectrolyte separator in zinc iodine batteries | 95 |
| CMC/cellulose acetate | 315 | 200 | 500 | — | • CMC based green binders in zinc ion batteries | 90 |
| CMC and KOH as additive | — | 100 | — | — | • CMC as additive in zinc paste electrodes | 91 |
| Na-CMC as electrolyte | — | 268.2 | 2800 | 10.08 | • Na-CMC based moderately concentrated electrolyte | 92 |
| CMC/poly(N-isopropylacrylamide) based electrolyte | — | 0.25–5 | 150 | 1.68 × 10−4 | • Physiochemical effect of CMC based binder for graphite anode in zinc ion batteries | 94 |
| CMC source | Blends/composite material | Specific capacitance (F g−1) | Current density (A g−1) | Cycles | Voltage (V) | Applications in electrochemistry | Ref |
|---|---|---|---|---|---|---|---|
| Commercial; Tianjing Yuanli Chemical Co., China | Sodium carboxymethyl cellulose (Na-CMC)/polyaniline nanorods | 425.25 | 1 | 1000 | −0.2 to 0.8 | • CMC/polyaniline nanorods are used in high performance redox supercapacitors | 116 |
| Commercial; Anli (Henan, China) | Na-CMC aerogels | 152.6 | 0.5 | −0.1 to 0 in 6 M KOH solution | • Na-CMC aerogel enhanced electrochemical performance supercapacitors | 110 | |
| Commercial; Zhiyuan Chemical Reagent Co., Ltd | CMC/bacterial cellulose/citric acid based hierarchical composite porous carbon | 350 | 0.5 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
• Renewable biomass-derived carbon material and its application in supercapacitors | 103 | |
| Commercial | CMC based polypyrrole–vanadium oxide composite on graphite electrode in acetonitrile | 800 | 5.3 | 1000 | 1 mA cm−2 | • CMC based composite applications in asymmetric supercapacitors | 39 |
| Commercial; Anli LLC (Henan, China) | Na-CMC derived nitrogen doped carbon aerogels | 185.3 | 0.5 | 5000 | −1 to 0 | • Na-CMC derived aerogels can be used as electrodes in supercapacitors | 101 |
| Commercial; Sigma-Aldrich | Porous carbon pearls of CMC | 217 | 1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 at 30 A g−1 000 at 30 A g−1 |
0 to 1 | • Porous CMC derived pearls as active material for double-layer capacitors | 108 |
CMC based carbon aerogels with nitrogen doping also exhibit the potential to be utilized as electrode in supercapacitors. These were prepared via multistep advanced approach comprising sol–gel, freeze drying, carbonization and base stimulation process with ferric trichloride (FeCl3), collagen as cross-linker and nitrogen source. These carbon aerogels showed porous, three dimensional and higher surface area with magnetic properties. As an electrode material, the aerogel displayed current density 0.5 A g−1 and specific capacitance 185.3 F g−1 in a 6 M KOH electrolyte. The retention of specific capacitance was 90.2% after charge and discharge cycle showing cycling stability.101
CMC intercalated reduced graphene oxide films are also gaining considerable attention in charge storage devices due to their flexible, wearable and bendable nature. Reduced graphene oxide usage is challenging due to its density stacked structure with poor wettability to the electrolyte. Addition of CMC in reduced graphene oxide has acted as activating agent. The composite films were reduced to improve the electrochemical properties due to the increase in assessable area for electrolytic ions. The polymer composite films showed drastic increase in capacitance after charging/discharging cycles owing to electro activation and pseudo capacitance as compared to reduced graphene oxide film alone.102 Shu et al., have employed hierarchical porous carbon materials prepared from composite material of CMC/bacterial cellulose/citric acid as an electrode in supercapacitors. The prepared material has excellent rate capability of 254 F g−1 at current density 15 A g−1, 96% capacitance retention of cyclic stability after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles tests with enhanced rate capability and high specific capacitance of 350 F g−1 with current density at 0.5 A g−1. In aqueous KOH electrolyte, the symmetric porous carbon electrode showed higher specific capacitance and electron density of 28 W h kg−1.103
000 cycles tests with enhanced rate capability and high specific capacitance of 350 F g−1 with current density at 0.5 A g−1. In aqueous KOH electrolyte, the symmetric porous carbon electrode showed higher specific capacitance and electron density of 28 W h kg−1.103
Ali et al., have employed bidirectional approach by using CMC samples from two different sources, bamboo (BCMC) and oil palm empty fruit bunch (OCMC), prepared by simple incipient wetness impregnation method proceeded by calcination process and incorporation of manganese oxide (Mn2O3). The carbonization step has converted CMC into porous CMC and surface area in case of BCMC was more than OCMC. After incorporation of metal oxide, the crystalline size of both CMC has reduced. Comparatively, BCMC/Mn2O3 composite film comprised of higher electrochemical performance (31.98 mF cm−2) then OCMC/Mn2O3 (24.15 mF cm−2). However, both CMC showed fairly high cyclic stability after 1000 charge/discharge cycles.104
An antifouling nanohybrid membrane comprising of CMC, graphene oxide nanosheet and magnesium oxide (MgO) nanoparticles was synthesized for water treatment and as an electrode in supercapacitor applications. TEIS Nyquist plots revealed the supercapacitor and energy applications of hybrid material.105 After that, another hybrid nanomaterial containing yttrium oxide (Y2O3) with modified graphene oxide and CMC has been prepared to utilize it as electrode in supercapacitor. The electrochemical properties were inspected by CV, Tafel plot and EIS which showed nanomaterial as promising candidate for energy storage and supercapacitors applications.106
CMC based macrostructure in combination with graphene oxide showed the potential to be used as antifouling membrane for pollutant detection and as an electrode in supercapacitor applications. Impedance analysis of fabricated nanocomposite approved the movement of charges between electrolyte and electrode for the accomplishment of specific capacitance.107
BiFeO3-CMC electrode has excellent electrochemical performance in combination with fluoroethylene carbonate (FEC) as electrolytes. These electrodes delivered initial capacity values of 635 and 453 mA h g−1 in charge/discharge process in the electrolyte composition of 1 M NaPF6 in EC/DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with 2% FEC additive. The capacity retention of 73% with capacity value stabilized around 10th cycle after 60 cycles.88 Chang et al., have developed porous carbon pearls (PCPs) from concentrated solution of CMC followed by ice-templating and carbonization. The PCPs with pearly luster had well developed bi-model pore structure with total pore volume of 0.81 cm3 g−1, specific surface area 1338.6 m2 g−1. The PCPs electrode showed high super-capacitive performance in three-electrode system with maximum specific capacitance of 217 F g−1 at 1 A g−1 in aqueous KOH electrolyte, excellent cyclic stability (100%) after 10
1, v/v) with 2% FEC additive. The capacity retention of 73% with capacity value stabilized around 10th cycle after 60 cycles.88 Chang et al., have developed porous carbon pearls (PCPs) from concentrated solution of CMC followed by ice-templating and carbonization. The PCPs with pearly luster had well developed bi-model pore structure with total pore volume of 0.81 cm3 g−1, specific surface area 1338.6 m2 g−1. The PCPs electrode showed high super-capacitive performance in three-electrode system with maximum specific capacitance of 217 F g−1 at 1 A g−1 in aqueous KOH electrolyte, excellent cyclic stability (100%) after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 30 A g−1. To infer concrete super-capacitive potential, a symmetric capacitor device was equipped by utilizing a coin cell which showed specific capacitance of 37 F g−1, power density of 5.0 kW kg−1, energy density of 2.88 W h kg−1 and current density of 1 A g−1. Moreover, this device had superior capacitance retention 98.5% and cycling stability of 10
000 cycles at 30 A g−1. To infer concrete super-capacitive potential, a symmetric capacitor device was equipped by utilizing a coin cell which showed specific capacitance of 37 F g−1, power density of 5.0 kW kg−1, energy density of 2.88 W h kg−1 and current density of 1 A g−1. Moreover, this device had superior capacitance retention 98.5% and cycling stability of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at current density of 10 A g−1.108
000 cycles at current density of 10 A g−1.108
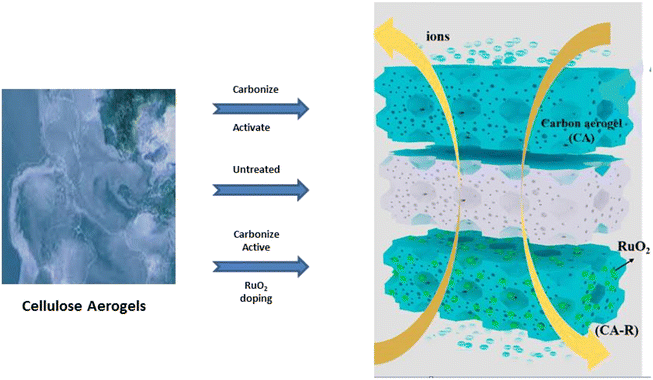 | ||
| Fig. 6 A simple representation of activating relation between cellulose and carbon aerogels in the presence of metal oxide. | ||
Sodium salt CMC as a carbon source and nickel sulfate as a nickel precursor, were treated by a sol–gel procedure, freeze-drying, and pyrolysis.113 The prepared carbon aerogel (CA)/NiO composites have a three-dimensional network structure, broad surface area, and outstanding magnetic characteristics. NiSO4 affects composites' electrochemical characteristics.114 The CA-1.0 composites demonstrate better specific capacity (81.67 mA h g−1 at 0.5 A g−1) in KOH electrolyte solution (6 M), based on the coupling of CA double-layer capacitance and NiO redox reaction. After 5000 charge–discharge cycles, the CA-1.0 electrode retains 94.5% of its capability. A simple and affordable approach for making carbon/NiO composites has excellent possibilities for enhanced energy storage systems.115
3. Applications of CMC in energy conversion and generation devices
The efficiency of energy conversion is a central parameter for optimization and development of power generation devices.117 Recently, CMC has gained importance as an active material in energy conversion devices such as fuel cells and triboelectric nanogenerator (TENG) which are listed below.3.1. CMC in fuel cells
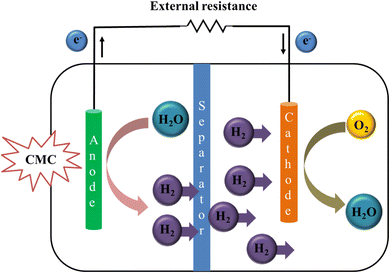
Microbial fuel cell is the device that can convert chemical energy stored in organic matter into the electrical energy by utilizing microorganisms as anode catalyst.118 These cells are considered environment friendly, highly efficient, superiorly performing and new for the growth in green energy.119,120 But their practical applications still face problems like lack of energy storage and low power generation.121,122 Anodes are crucial for these cells as they are main thing in current output and affecting power.123,124 Anode material must be promising for electricity producing bacteria and conductivity.125,126 Conducting polymers due to their high conductivity and redox behavior have received great attention in this field. CMC based conducting materials are already used in electrochemistry and biosensors (Fig. 7).126 As CMC forms a transparent adhesive liquid after swelling in water, so CMC is highly effective as water-based binder.85 Small quantity of unreacted CMC in composites is effective for the binding of substrate with composite material which is also good for electrode stability.127 Wang et al., studied microbial fuel cells which utilize wastewater stored chemical energy and modified them by alteration of anode with ternary composite hydrogel of CMC/polypyrrole/titanium nitride/carbon brush hydrogel. This bioanode has enhanced the energy output of microbial fuel cell. SEM analysis confirmed the three-dimensional macroporous structure with enlarged area on surface for the growth of microorganisms. The energy density (14.11 W m−3) and current density of ternary hybrid anode were 4.72 and 10.12 times larger than blank carbon brush anode. The synergistic effect of polymers has improved the potential of electrochemical anode.128 A single medium sediment microbial fuel cells (SMFC) differs from traditional SMFC in that the cathode is immersed in an anoxic sediment environment to prevent oxygen from competing for electrons and to improve the likelihood of electron acceptors copper ions reduction. The sediment containing higher copper ions restricts microbial growth, necessitating the development of a viable solution to this issue. The copper tolerant bacteria immobilized on carboxymethyl cellulose (CMC) beads serve variety of purposes, such as the adsorption of copper ions, defense against inhibition by large levels of copper ions, and enhancement of copper tolerant bacteria activities as co-substrate. The results demonstrate increased organic removal (85%) by copper tolerant Ochrobactrum oryzae at high copper ion concentrations, which was 1.48–1.65 times and 5 times more efficient than those comprising other strains and blended bacteria. Ochrobactrum oryzae’s inhibition by copper in the sediment was lessened as a result of the immobilization of the organism with CMC, which also assured the SMFC's ability to generate electricity steadily. The highest output voltage (173.9 mV) and copper ion removal rate (>70%) attained with the addition of cell-immobilized beads to the SMFC anode zone were 2.89 and 1.15 times greater than those values without the beads, accordingly. On 70th day, electrochemically active species of bacteria that could break down organic molecules dominated the anode zone.1293.2. CMC in triboelectric nanogenerator (TENG)
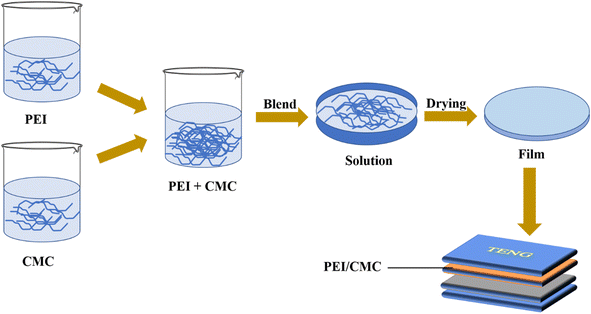
TENG is a green energy conversion device which can convert low quality and low frequency mechanical energy into low current and high voltage electric energy.130 It works on the principle of contact electrification and electrostatic induction coupling.131 TENG is composed of three components; negative layer, positive layer and conductive electrodes.132 Recently, CMC based materials have been used in negative and positive layers of TENG and provide a new idea for environment friendly TENG. Jaiban et al., developed cellulose extracted from soybean and its CMC based biofilm as negative part of triboelectric generator (TENG) with maximum voltage of 28 V and current of 80 nA. This designed system had the capacity to lit up three commercial LEDs.133 Similarly, Qu et al., used CMC, epichlorohydrin and polyethyleneimine (PEI) based aerogel as positive layer material of TENG. The corresponding aerogel and TENG produced voltage of 203 V and current density of 8.2 μA cm−2. The tribopositive performance of CMC was increased by physical mixing with PEI (Fig. 8). This developed open circuit voltage and current can reach 385 V and 51 μA, respectively.134Recently, a very interesting application of TENG has been reported by Kang et al. The babies who like to bite, TENG has come to their rescue. The prepared TENG based on Na-CMC and carboxymethyl chitosan dissolves quickly in aqueous environment without any side effects. It has excellent electric performance with output power of 120 mW m−2.135 Likewise, Ya et al., studied the MXene/CMC based TENG for mechanical energy harvest. This TENG exhibits the power density of 402.94 mW m−2 with external resistance 18 MΩ. Moreover, this TENG has bright potential to harvest energy and can protect the humans from electromagnetic radiations.136
4. Applications of CMC in electrical devices
In flexible electronics, CMC has been used as carbonized CMC and acts as smart fabric for manufacturing of devices.137 In electrical actuators and biosensors, CMC based hybrids can sense biogenic molecules and external stimuli (Table 5). Moreover, CMC has range of applications in biogel ion electrical actuators as listed below.| Material | pH | Electrochemical at method | Linear range | Limit of detection | Chemical detection | Ref |
|---|---|---|---|---|---|---|
| Multiwalled carbon nanotube/hydroxypropyl-β-cyclodextrin functionalized CMC | 5.7 | DPV, CV, EIS | 500–9000 μM | 1.9 μM | Tryptophan | 145 |
| Polyaniline/multiwalled carbon nano tubes/CMC | 7 | CV | 0.05–5 mM | 0.01 mM | Ascorbic acid | 144 |
| Carbon nanotube/CMC | CV | Catecholamines | 149 | |||
| Reduced graphene oxide/CMC | 7.4 | EIS | 0.5–10.0 μg mL−1 | 61 ng mL−1 | Adiponectin | 150 |
| Oxidized-CMC-sulfate/sulfated polyaniline composite | 5.7 | DPV, CV, EIS | 0.05 μM | Nitro-phenol | 148 | |
| Hybrid PANI@dialdehyde CMC/ZnO nanocomposite | 5.7 | DPV | 0.50 to 5 μM | 0.45 M | H2O2 | 151 |
4.1. CMC based blends as biosensor
CMC or CMC-based hybrid materials have been effectively used to sense biogenic compounds in the humans and other living organisms. In 2004, Wu et al. designed a glucose biosensor to entrap an enzyme, glucose oxidase, into xerogel matrix of hydroxyethyl CMC polymer and tetraethyl-orthosilicate. This fabricated sensor exhibited excellent analytical ability to detect glucose in urine samples.138 After that, CMC based materials have been in fashion as sensors for detecting biomolecules (amino acids, neurotransmitters, proteins), pollutants, microorganisms and body fluids. Applications of CMC as biosensors for H2O2 and some biomolecules are summarized in succeeding sections.Ji et al., have performed an interesting work by examining electrochemical chiral recognition of tryptophan (trp) enantiomer based on biosensor developed by grafting CMC with hydroxypropyl-β-cyclodextrin, muti-walled carbon nanotubes and copper ions on the external surface of glassy carbon electrodes. The DPV showed oxidation peak current ratio reached at 2.2 under optimal experimental conditions. The highest electrochemical signal was observed with L-trp due to its stronger affinity than D-trp. The as-prepared biosensor also showed significant utilization for the detection of D-trp in the racemic mixture.145 CMC based carbon nanotube electrode has been used in the detection of neurotransmitter catecholamines which exhibits electron transfer chemical reaction. The catecholamines electrochemical affinity based on kinetic property of intramolecular cyclization rate of chemical reaction. The apparent ECE profile in CV of composite electrode was justified by DigiElch simulation program. The cyclic voltammetry (CV) results verified the electrochemical performance of catecholamines due to cyclization which involves hydrogen ions with pH effect.146 A new electrochemical immunosensor for the detection of the adiponectin cytokine was developed using a reduced graphene oxide–carboxymethyl cellulose hybrid (CMC–rGO). Covalent bonding of oxidized CMC to GO layers was followed by chemical reduction using sodium borohydride (NaBH4) to produce the hybrid material. Anti-APN capture antibody was immobilized in a sandwich-type immunoassay using the commercial metal-complex-based polymer Mix&Go™. HRP-Strept and Biotin-APN were employed in the assay design. Comparing findings from ELISA kits with the developed immunosensor showed that the 61 ng mL−1 detection limit and excellent storage stability were adequate for the measurement of the target analyte in human serum. It's worth noticing that the immunosensor has a major advantage over the ELISA kit in terms of the overall assay time. The ELISA kit may be used in as little as 2 hour after the antibody has been immobilized, however the total duration is close to 5 hours.147 The role of deposition of oxidized-sulfated-CMC and sulfated polyamide on glassy carbon electrode were investigated by Alamry et al., in non-enzymatic, mediator free electrochemical sensing of ortho-nitrophenol in orange juice samples, milk and water. Electrochemical characterizations were confirmed through CV, EIS and DPV. The functional groups on modified CMC like –OH, –SO3 and –COOH were excellent for polymerization and adsorption of 2-NP. Under optimal concentration 2-NP showed linear correlation and low detection limit of 0.05 μM was observed.148
4.2. CMC in electrical actuators
In this era, biogel based actuators152 are considered darling of scholar due to their smartness. They are driving force for robots and intelligent mechanical system,153 can sense the external stimuli154 like temperature, magnetic field, voltage with generation of expansion, reversible contraction and rotation.155,156 Recently, they have become research focus in the field of bionics due to their properties which are as same as biological muscle157 such as low weight, low energy consumption, flexible movement, no noise, small driving voltage and large deflection displacement.156,158 Lv et al., investigated a biogel ion electrical actuator that was prepared by using raw materials, sodium alginate (SCIA sample) and CMC. The influence of sodium alginate and CMC ratios on deflection displacement and output force was investigated which proved that optimal mass 0.25 g of CMC was helpful in improving the response speed of 0.176 mm s−1, deflection displacement of 17.137 mm and output force of 2.675 mN. The maximum output force density 15.970 mN g−1 and specific capacitance 96.95 mF g−1 were improved as compared to carboxymethyl chitosan and sodium alginate cross linking reaction. When ratios of both polymers increased, the output force displacement and deflection displacement declined due to excessive cross linking which caused blockage of three-dimensional network structure and ion channel decreased, resulting in degradation. Infrared indicated that combination of both polymers has resulted in macromolecular chain structure which enhanced the electrostatic effect due to the attraction of internal charge of the molecule. Addition of glycerin had improved the flexibility, expansion of ion channels and increase in deflection amplitude. The water preservation in the SCIA sample was helpful in the increase of working life of biogel ion actuator.159 Again, this group studied biogel based actuators as new smart materials. Chitosan properties enhanced by crosslinking with CMC and tannic acid through freezing technology were employed to design novel actuators. The freezing treatment enhanced the elastic modulus, specific capacitance and output force by 0.014 MPa, 1.590 F g−1 and 5.943 mN than the unfrozen treated samples. FTIR showed the interaction of sample after freezing treatment, SEM indicated a channel type macroporous structure due to fine distribution of water molecules.160 Wei et al., proposed to fabricate the actuating material that can meet the balance between mechanical integrity (specifically tensile strength) and humidity response for generators, soft robotics, artificial muscles and biomimetic devices. They employed a simple evaporation-induced self-assembly strategy to create a durable yet extremely sensitive merged film-based humidity actuator by utilizing biopolymer CMC, aluminum ions (Al3+) and MXene nanosheets. The closely packed hierarchical microstructure, in combination with hydrogen and ionic bonding with the complementary reinforcement effects of MXene nanosheets and Al3+, results in a remarkable tensile strength of 154.2 MPa at >95% humidity, as well as strong mechanical properties of 273.6 MPa and preferable toughness of 7.95 MJ m−3 (Fig. 9). Interestingly, when exposed to humidity gradients, the composite film exhibits substantial shape deformation, sensitive actuation (less than 2.3 s), and outstanding cycling stability (above 1500 cycles) attributed to the exceptional lamination structure and water induced swelling action of biopolymer. Owing to the aforementioned advantages, the composite film actuator can be effectively built to fabricate smart materials like artificial muscle, human finger and moist electrical generator.161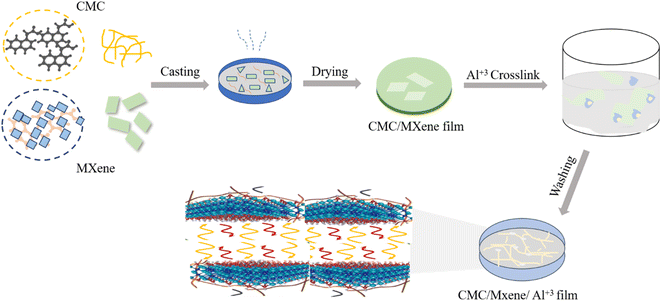 | ||
| Fig. 9 A graphic representation of CMC and MXene based film for the development of sensitive actuator. | ||
5. Conclusion and future outlook
In rapidly growing field of electrochemistry, the aqueous processing of solid electrolytes, binders and electrodes is still much unexplored field. One of the water-soluble polymers is CMC, which has certain desirable attributes that perfectly fit it for application in electrochemical devices. Its characteristics to form hydrogen bonds with other polymers and swelling in water to form thick paste are giving it an edge over other synthetic polymers. Thus, CMC can be turned into a conducting material via modifying it with certain biopolymers and active functional groups that make it suitable for energy storage appliances. From past many years, potential of modified CMC has been explored as a conducting material in electrolytes, binder in electrodes of storage cells, active material in fuel cells, TENG and electrical actuators as discussed in this review. This developing work on electrochemical potential of CMC has made us to anticipate for its full exploitation in storage cells and electricity devices. Future researchers must focus to explore the following aspects:(1) Bio-sourced polymers must be preferred over synthetic polymers to lower the cost and enhance the lifetime of batteries.
(2) CMC composites as activating materials in fuel cells, TENG and actuators is less investigated, there must be substantial progress in these fields.
(3) In order to fully access the applications of CMC based materials, the process must be validated on large scale.
Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
The Higher Education Commission of Pakistan is acknowledged for providing funding through TDF03-294 and HEC/NRPU-8639. The School of Chemistry is acknowledged for its support of this project.References
- K. Maurya, et al., Proton conducting polymer electrolyte: II poly ethylene oxide + NH4l system, J. Mater. Sci., 1992, 27(23), 6357–6364 CrossRef CAS.
- A. Ali, N. Mohamed and A. Arof, Polyethylene oxide (PEO)–ammonium sulfate ((NH4)2SO4) complexes and electrochemical cell performance, J. Power Sources, 1998, 74(1), 135–141 CrossRef CAS.
- A. Samsudin, E. Kuan and M. Isa, Investigation of the potential of proton-conducting biopolymer electrolytes based methyl cellulose–glycolic acid, Int. J. Polym. Anal. Charact., 2011, 16(7), 477–485 CrossRef CAS.
- N. Srivastava, A. Chandra and S. Chandra, Dense branched growth of (SCN)x and ion transport in the poly(ethyleneoxide) NH4SCN polymer electrolyte, Phys. Rev. B, 1995, 52(1), 225 CrossRef CAS PubMed.
- A. Samsudin and M. Isa, Structural and electrical properties of carboxy methylcellulose-dodecyltrimethyl ammonium bromide-based biopolymer electrolytes system, Int. J. Polym. Mater., 2012, 61(1), 30–40 CrossRef CAS.
- P. V. Wright, Electrical conductivity in ionic complexes of poly(ethylene oxide), Br. Polym. J., 1975, 7(5), 319–327 CrossRef CAS.
- S. Hashmi, et al., Proton-conducting polymer electrolyte. I. The polyethylene oxide + NH4ClO4 system, J. Phys. D: Appl. Phys., 1990, 23(10), 1307 CrossRef CAS.
- M. Hema, et al., Structural, vibrational and electrical characterization of PVA–NH4Br polymer electrolyte system, Phys. B, 2008, 403(17), 2740–2747 CrossRef CAS.
- M. Buraidah, et al., Ionic conductivity by correlated barrier hopping in NH4I doped chitosan solid electrolyte, Phys. B, 2009, 404(8–11), 1373–1379 CrossRef CAS.
- N. Nik Aziz, N. Idris and M. Isa, Solid polymer electrolytes based on methylcellulose: FT-IR and ionic conductivity studies, Int. J. Polym. Anal. Charact., 2010, 15(5), 319–327 CrossRef CAS.
- J. Li, et al., Boosting the performance of poly(ethylene oxide)-based solid polymer electrolytes by blending with poly(vinylidene fluoride-co-hexafluoropropylene) for solid-state lithium-ion batteries, Int. J. Energy Res., 2020, 44(9), 7831–7840 CrossRef CAS.
- A. P. Rokhade, et al., Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine, Carbohydr. Polym., 2006, 65(3), 243–252 CrossRef CAS.
- D. Zhang, et al., Transparent, conductive, and flexible carbon nanotube films and their application in organic light-emitting diodes, Nano Lett., 2006, 6(9), 1880–1886 CrossRef CAS PubMed.
- D. A. Rees, Structure, conformation, and mechanism in the formation of polysaccharide gels and networks, Adv. Carbohydr. Chem. Biochem., 1969, 24, 267–332 CrossRef CAS PubMed.
- F. He and D. Zhao, Manipulating the size and dispersibility of zerovalent iron nanoparticles by use of carboxymethyl cellulose stabilizers, Environ. Sci. Technol., 2007, 41(17), 6216–6221 CrossRef CAS PubMed.
- F. He, et al., Stabilization of Fe–Pd nanoparticles with sodium carboxymethyl cellulose for enhanced transport and dechlorination of trichloroethylene in soil and groundwater, Ind. Eng. Chem. Res., 2007, 46(1), 29–34 CrossRef CAS.
- J. Figueiredo, et al., Cellulose and derivatives from wood and fibers as renewable sources of raw-materials, Top. Curr. Chem., 2010, 117–128 CrossRef CAS PubMed.
- Y. Hu and J. M. Catchmark, Integration of cellulases into bacterial cellulose: toward bioabsorbable cellulose composites, J. Biomed. Mater. Res., Part B, 2011, 97(1), 114–123 CrossRef PubMed.
- A.-M. Oprea, et al., Synthesis and characterization of some cellulose/chondroitin sulphate hydrogels and their evaluation as carriers for drug delivery, Carbohydr. Polym., 2012, 87(1), 721–729 CrossRef CAS PubMed.
- L. Xie, et al., Superhydrophobicity of CMCAB fibrous mats produced by electrospinning, Integr. Ferroelectr., 2012, 135(1), 55–61 CrossRef CAS.
- H. Huang, et al., Electrochemical and electrocatalytic properties of myoglobin and hemoglobin incorporated in carboxymethyl cellulose films, Bioelectrochemistry, 2003, 61(1–2), 29–38 CrossRef CAS PubMed.
- A. Siddhanta, et al., Profiling of cellulose content in Indian seaweed species, Bioresour. Technol., 2009, 100(24), 6669–6673 CrossRef CAS PubMed.
- Z. Wang, et al., Why cellulose-based electrochemical energy storage devices?, Adv. Mater., 2021, 33(28), 2000892 CrossRef CAS PubMed.
- D. Bresser, et al., Alternative binders for sustainable electrochemical energy storage–the transition to aqueous electrode processing and bio-derived polymers, Energy Environ. Sci., 2018, 11(11), 3096–3127 RSC.
- X. Wang, et al., Flexible energy-storage devices: design consideration and recent progress, Adv. Mater., 2014, 26(28), 4763–4782 CrossRef CAS PubMed.
- L. Kong, et al., Advanced energy materials for flexible batteries in energy storage: a review, SmartMat, 2020, 1(1), 1–35 CrossRef.
- M. Chai and M. Isa, Electrical characterization and ionic transport properties of carboxyl methylcellulose-oleic acid solid polymer electrolytes, Int. J. Polym. Anal. Charact., 2013, 18(4), 280–286 CrossRef CAS.
- K. R. Reddy, K. P. Lee and A. I. Gopalan, Novel electrically conductive and ferromagnetic composites of poly(aniline-co-aminonaphthalenesulfonic acid) with iron oxide nanoparticles: synthesis and characterization, J. Appl. Polym. Sci., 2007, 106(2), 1181–1191 CrossRef CAS.
- M. Hassan, et al., Hierarchical assembly of graphene/polyaniline nanostructures to synthesize free-standing supercapacitor electrode, Compos. Sci. Technol., 2014, 98, 1–8 CrossRef CAS.
- A. Samsudin, W. M. Khairul and M. Isa, Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes, J. Non-Cryst. Solids, 2012, 358(8), 1104–1112 CrossRef CAS.
- L. Zhu, et al., Toward high performance solid-state lithium-ion battery with a promising PEO/PPC blend solid polymer electrolyte, Int. J. Energy Res., 2020, 44(13), 10168–10178 CrossRef CAS.
- S. B. Aziz, et al., A conceptual review on polymer electrolytes and ion transport models, J. Sci.: Adv. Mater. Devices, 2018, 3(1), 1–17 Search PubMed.
- A. Samsudin, M. Aziz and M. Isa, Natural polymer electrolyte system based on sago: structural and transport behavior characteristics, Int. J. Polym. Anal. Charact., 2012, 17(8), 600–607 CrossRef CAS.
- F. Z. Benabid and F. Zouai, Natural polymers: cellulose, chitin, chitosan, gelatin, starch, carrageenan, xylan and dextran, Algerian Journal of Natural Products, 2016, 4(3), 348–357 Search PubMed.
- Y. Xu and Y. Zhang, Synthesis of polypyrrole/sodium carboxymethyl cellulose nanospheres with enhanced supercapacitor performance, Mater. Lett., 2015, 139, 145–148 CrossRef CAS.
- Y. Yan, et al., Growth of polyaniline nanowhiskers on mesoporous carbon for supercapacitor application, J. Power Sources, 2011, 196(18), 7835–7840 CrossRef CAS.
- L. Yang, et al., Synthesis and characterization of polypyrrole nanotubes/multi-walled carbon nanotubes composites with superior electrochemical performance, J. Mater. Sci.: Mater. Electron., 2014, 25(2), 1047–1052 CrossRef CAS.
- A. Rajeh, M. Morsi and I. Elashmawi, Enhancement of spectroscopic, thermal, electrical and morphological properties of polyethylene oxide/carboxymethyl cellulose blends: combined FT-IR/DFT, Vacuum, 2019, 159, 430–440 CrossRef CAS.
- E. Karaca, K. Pekmez and N. Ö. Pekmez, Electrosynthesis of polypyrrole–vanadium oxide composites on graphite electrode in acetonitrile in the presence of carboxymethyl cellulose for electrochemical supercapacitors, Electrochim. Acta, 2018, 273, 379–391 CrossRef CAS.
- N. Zainuddin, et al., Characterization on conduction properties of carboxymethyl cellulose/kappa carrageenan blend-based polymer electrolyte system, Int. J. Polym. Anal. Charact., 2018, 23(4), 321–330 CrossRef CAS.
- M. Pumera, et al., Graphene for electrochemical sensing and biosensing, TrAC, Trends Anal. Chem., 2010, 29(9), 954–965 CrossRef CAS.
- I. Ojeda, et al., Electrochemical immunosensor for rapid and sensitive determination of estradiol, Anal. Chim. Acta, 2012, 743, 117–124 CrossRef CAS PubMed.
- S. Rudhziah, et al., Biopolymer electrolytes based on blend of kappa-carrageenan and cellulose derivatives for potential application in dye sensitized solar cell, Electrochim. Acta, 2015, 175, 162–168 CrossRef CAS.
- P. Weerasooriya, et al., Isolation and characterization of hemicellulose blended carboxymethylcellulose films incorporated with lithium perchlorate as a potential ion conductive biopolymer, Mater. Lett., 2021, 299, 130085 CrossRef CAS.
- M. M. Pérez-Madrigal, et al., Pastes and hydrogels from carboxymethyl cellulose sodium salt as supporting electrolyte of solid electrochemical supercapacitors, Carbohydr. Polym., 2018, 200, 456–467 CrossRef PubMed.
- R. Badry, et al., Spectroscopic and thermal analyses for the effect of acetic acid on the plasticized sodium carboxymethyl cellulose, J. Mol. Struct., 2021, 1224, 129013 CrossRef CAS.
- M. S. A. Rani, A. Ahmad and N. S. Mohamed, Influence of nano-sized fumed silica on physicochemical and electrochemical properties of cellulose derivatives-ionic liquid biopolymer electrolytes, Ionics, 2018, 24(3), 807–814 CrossRef CAS.
- G. Asnag, A. Oraby and A. Abdelghany, Green synthesis of gold nanoparticles and its effect on the optical, thermal and electrical properties of carboxymethyl cellulose, Composites, Part B, 2019, 172, 436–446 CrossRef CAS.
- M. M. Abutalib and A. Rajeh, Boosting optical and electrical characteristics of polyvinyl alcohol/carboxymethyl cellulose nanocomposites by GNPs/MWCNTs fillers as an application in energy storage devices, Int. J. Energy Res., 2022, 46(5), 6216–6224 CrossRef CAS.
- M. Singh, J. Kaiser and H. Hahn, Thick electrodes for high energy lithium ion batteries, J. Electrochem. Soc., 2015, 162(7), A1196 CrossRef CAS.
- J. B. Goodenough and Y. Kim, Challenges for rechargeable Li batteries, Chem. Mater., 2010, 22(3), 587–603 CrossRef CAS.
- J.-M. Tarascon, Key challenges in future Li-battery research, Philos. Trans. R. Soc., A, 2010, 368(1923), 3227–3241 CrossRef PubMed.
- N. Kamaya, et al., A lithium superionic conductor, Nat. Mater., 2011, 10(9), 682–686 CrossRef CAS PubMed.
- J. Li, R. Lewis and J. Dahn, Sodium carboxymethyl cellulose: a potential binder for Si negative electrodes for Li-ion batteries, Electrochem. Solid-State Lett., 2006, 10(2), A17 CrossRef.
- J. S. Wang, et al., Formulation and characterization of ultra-thick electrodes for high energy lithium-ion batteries employing tailored metal foams, J. Power Sources, 2011, 196(20), 8714–8718 CrossRef CAS.
- L. Hu, et al., Lithium-ion textile batteries with large areal mass loading, Adv. Energy Mater., 2011, 1(6), 1012–1017 CrossRef CAS.
- H. Zheng, et al., A comprehensive understanding of electrode thickness effects on the electrochemical performances of Li-ion battery cathodes, Electrochim. Acta, 2012, 71, 258–265 CrossRef CAS.
- J.-H. Lee, et al., Aqueous processing of natural graphite particulates for lithium-ion battery anodes and their electrochemical performance, J. Power Sources, 2005, 147(1–2), 249–255 CrossRef CAS.
- B. Bitsch, et al., A novel slurry concept for the fabrication of lithium-ion battery electrodes with beneficial properties, J. Power Sources, 2014, 265, 81–90 CrossRef CAS.
- H. Buqa, et al., Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries, J. Power Sources, 2006, 161(1), 617–622 CrossRef CAS.
- S. Yang, et al., Hybrid humics/sodium carboxymethyl cellulose water-soluble binder for enhancing the electrochemical performance of a Li-ion battery cathode, Powder Technol., 2019, 351, 203–211 CrossRef CAS.
- C.-U. Jeong, et al., Li-incorporated porous carbon monoliths derived from carboxymethyl cellulose as anode material for high power lithium-ion batteries, J. Power Sources, 2021, 506, 230050 CrossRef CAS.
- Z. Karkar, et al., A comparative study of polyacrylic acid (PAA) and carboxymethyl cellulose (CMC) binders for Si-based electrodes, Electrochim. Acta, 2017, 258, 453–466 CrossRef CAS.
- A. Chiappone, et al., Nanoscale microfibrillated cellulose reinforced truly-solid polymer electrolytes for flexible, safe and sustainable lithium-based batteries, Cellulose, 2013, 20(5), 2439–2449 CrossRef CAS.
- L. Qiu, et al., Carboxymethyl cellulose lithium (CMC-Li) as a novel binder and its electrochemical performance in lithium-ion batteries, Cellulose, 2014, 21(4), 2789–2796 CrossRef CAS.
- L. Qiu, et al., Novel polymer Li-ion binder carboxymethyl cellulose derivative enhanced electrochemical performance for Li-ion batteries, Carbohydr. Polym., 2014, 112, 532–538 CrossRef CAS PubMed.
- L. Qiu, et al., High Performance Study of Lithium Carboxymethylcellulose as Water-Soluble Binder for Lithium Supplementation in Lithium Batteries, Starch-Stärke, 2022, 2200049 CrossRef CAS.
- C. Shi, et al., Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries, J. Power Sources, 2014, 270, 547–553 CrossRef CAS.
- J.-S. Yeo, et al., Electrochemical properties of large-sized pouch-type lithium ion batteries with bio-inspired organic cathode materials, J. Power Sources, 2016, 313, 91–95 CrossRef CAS.
- W.-C. Li, et al., Superior performances of supercapacitors and lithium-ion batteries with carboxymethyl cellulose bearing zwitterions as binders, J. Taiwan Inst. Chem. Eng., 2022, 133, 104263 CrossRef CAS.
- D. Shin, H. Park and U. Paik, Cross-linked poly (acrylic acid)-carboxymethyl cellulose and styrene-butadiene rubber as an efficient binder system and its physicochemical effects on a high energy density graphite anode for Li-ion batteries, Electrochem. Commun., 2017, 77, 103–106 CrossRef CAS.
- H. S. Kim, K. Y. Chung and B. W. Cho, Electrochemical properties of carbon-coated Si/B composite anode for lithium ion batteries, J. Power Sources, 2009, 189(1), 108–113 CrossRef CAS.
- T. Li, J.-y. Yang and S.-g. Lu, Effect of modified elastomeric binders on the electrochemical properties of silicon anodes for lithium-ion batteries, Int. J. Miner., Metall. Mater., 2012, 19(8), 752–756 CrossRef CAS.
- J. Xu, et al., The effect of different binders on electrochemical properties of LiNi1/3Mn1/3Co1/3O2 cathode material in lithium ion batteries, J. Power Sources, 2013, 225, 172–178 CrossRef CAS.
- S. Kim, et al., Adv. Energy Mater., 2012, 2, 710 CrossRef CAS; M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater, 2013, 23, 947 CrossRef.
- Y. Yui, et al., Electrochemical properties of Sn–Co electrode with various kinds of binder materials for sodium ion batteries, Solid State Ionics, 2016, 288, 219–223 CrossRef CAS.
- Y. Zhao and A. Manthiram, High-capacity, high-rate Bi–Sb alloy anodes for lithium-ion and sodium-ion batteries, Chem. Mater., 2015, 27(8), 3096–3101 CrossRef CAS.
- P. R. Abel, et al., Nanocolumnar germanium thin films as a high-rate sodium-ion battery anode material, J. Phys. Chem. C, 2013, 117(37), 18885–18890 CrossRef CAS.
- M. K. Datta, et al., Tin and graphite based nanocomposites: potential anode for sodium ion batteries, J. Power Sources, 2013, 225, 316–322 CrossRef CAS.
- A. M. Sukeshini, et al., Physicochemical characterization of CuFeO2 and lithium intercalation, Solid State Ionics, 2000, 128(1–4), 33–41 CrossRef CAS.
- A. Magasinski, et al., Toward efficient binders for Li-ion battery Si-based anodes: polyacrylic acid, ACS Appl. Mater. Interfaces, 2010, 2(11), 3004–3010 CrossRef CAS PubMed.
- S. Komaba, et al., Comparative study of sodium polyacrylate and poly(vinylidene fluoride) as binders for high capacity Si–graphite composite negative electrodes in Li-ion batteries, J. Phys. Chem. C, 2012, 116(1), 1380–1389 CrossRef CAS.
- B. Son, et al., Measurement and analysis of adhesion property of lithium-ion battery electrodes with SAICAS, ACS Appl. Mater. Interfaces, 2014, 6(1), 526–531 CrossRef CAS PubMed.
- S. Komaba, et al., Study on polymer binders for high-capacity SiO negative electrode of Li-ion batteries, J. Phys. Chem. C, 2011, 115(27), 13487–13495 CrossRef CAS.
- M. Dahbi, et al., Sodium carboxymethyl cellulose as a potential binder for hard-carbon negative electrodes in sodium-ion batteries, Electrochem. Commun., 2014, 44, 66–69 CrossRef CAS.
- I. V. B. Maggay, et al., Electrochemical properties of novel FeV2O4 as an anode for Na-ion batteries, Sci. Rep., 2018, 8(1), 1–11 CAS.
- M. Fan, H. Yu and Y. Chen, High-capacity sodium ion battery anodes based on CuO nanosheets and carboxymethyl cellulose binder, Mater. Technol., 2017, 32(10), 598–605 CrossRef CAS.
- A. Surendran, et al., Unveiling the Electrochemical Mechanism of High-Capacity Negative Electrode Model-System BiFeO3 in Sodium-Ion Batteries: An In Operando XAS Investigation, ACS Appl. Mater. Interfaces, 2022, 14(6), 7856–7868 CrossRef CAS PubMed.
- C. Li, et al., Cathode materials for rechargeable zinc-ion batteries: from synthesis to mechanism and applications, J. Power Sources, 2020, 449, 227596 CrossRef CAS.
- N. Jaikrajang, et al., Impact of Binder Functional Groups on Controlling Chemical Reactions to Improve Stability of Rechargeable Zinc-Ion Batteries, ACS Appl. Energy Mater., 2021, 4(7), 7138–7147 CrossRef CAS.
- C. Yang, et al., Effects of carboxymethyl cellulose on the electrochemical characteristics and dendrite growth of zinc in alkaline solution, J. Electrochem. Soc., 2016, 163(9), A1836 CrossRef CAS.
- L. Li, et al., Electrolyte concentration regulation boosting zinc storage stability of high-capacity K0. 486V2O5 cathode for bendable quasi-solid-state zinc ion batteries, Nano-Micro Lett., 2021, 13(1), 1–14 CrossRef CAS PubMed.
- Y. Mao, et al., Modifying hydrogel electrolyte to induce zinc deposition for dendrite-free zinc metal anode, Electrochim. Acta, 2021, 393, 139094 CrossRef CAS.
- I. Dueramae, et al., Properties enhancement of carboxymethyl cellulose with thermo-responsive polymer as solid polymer electrolyte for zinc ion battery, Sci. Rep., 2020, 10(1), 12587 CrossRef PubMed.
- P. Tangthuam, et al., Carboxymethyl cellulose-based polyelectrolyte as cationic exchange membrane for zinc-iodine batteries, Heliyon, 2020, 6, e05391 CrossRef CAS PubMed.
- M. Armand and J. Tarascon, Researchers must find a sustainable way of providing the power our modern lifestyles demand, Nature, 2008, 451(652), e657 Search PubMed.
- J.-H. Choi, et al., High capacitance and energy density supercapacitor based on biomass-derived activated carbons with reduced graphene oxide binder, Carbon, 2018, 132, 16–24 CrossRef CAS.
- W. Tian, et al., Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material, J. Mater. Chem. A, 2015, 3(10), 5656–5664 RSC.
- C. Clasen and W.-M. Kulicke, Determination of viscoelastic and rheo-optical material functions of water-soluble cellulose derivatives, Prog. Polym. Sci., 2001, 26(9), 1839–1919 CrossRef CAS.
- Y. Hu, et al., Biomass-based porous N-self-doped carbon framework/polyaniline composite with outstanding supercapacitance, ACS Sustainable Chem. Eng., 2017, 5(10), 8663–8674 CrossRef CAS.
- M. Yu, et al., Magnetic N-doped carbon aerogel from sodium carboxymethyl cellulose/collagen composite aerogel for dye adsorption and electrochemical supercapacitor, Int. J. Biol. Macromol., 2018, 115, 185–193 CrossRef CAS PubMed.
- Y.-R. Son and S.-J. Park, Influence of carboxymethyl cellulose content on structures and electrochemical behaviors of reduced graphene oxide films, Electrochim. Acta, 2020, 330, 135219 CrossRef CAS.
- Y. Shu, et al., Hierarchical porous carbons from polysaccharides carboxymethyl cellulose, bacterial cellulose, and citric acid for supercapacitor, Carbohydr. Polym., 2020, 227, 115346 CrossRef CAS PubMed.
- M. Ali, et al., Porous carboxymethyl cellulose carbon of lignocellulosic based materials incorporated manganese oxide for supercapacitor application, Int. J. Biol. Macromol., 2021, 180, 654–666 CrossRef CAS PubMed.
- N. M. El-Shafai, et al., Enhancement of the photocurrent and electrochemical properties of the modified nanohybrid composite membrane of cellulose/graphene oxide with magnesium oxide nanoparticle (GO@CMC. MgO) for photocatalytic antifouling and supercapacitors applications, Electrochim. Acta, 2021, 392, 138989 CrossRef CAS.
- N. M. El-Shafai, et al., Graphene oxide/cellulose derivative nanohybrid membrane with Yttrium oxide: upgrading the optical and electrochemical properties for removing organic pollutants and supercapacitors implementations, J. Energy Storage, 2021, 44, 103344 CrossRef.
- N. M. El-Shafai, et al., Synthesis, characterization, and cytotoxicity of self-assembly of hybrid nanocomposite modified membrane of carboxymethyl cellulose/graphene oxide for photocatalytic antifouling, energy storage, and supercapacitors application, Colloids Surf., A, 2021, 626, 127035 CrossRef CAS.
- H. S. Chang, et al., Preparation and electrochemical characterization of porous carbon pearls from carboxymethyl cellulose for electrical double-layer capacitors, Korean J. Chem. Eng., 2022, 39(5), 1232–1239 CrossRef CAS.
- Q. Zhang, et al., Nanocellulose-enabled, all-nanofiber, high-performance supercapacitor, ACS Appl. Mater. Interfaces, 2019, 11(6), 5919–5927 CrossRef CAS PubMed.
- M. Yu, J. Li and L. Wang, KOH-activated carbon aerogels derived from sodium carboxymethyl cellulose for high-performance supercapacitors and dye adsorption, Chem. Eng. J., 2017, 310, 300–306 CrossRef CAS.
- T. Cai, et al., Cellulose as an adhesive for the synthesis of carbon aerogel with a 3D hierarchical network structure for capacitive energy storage, ChemElectroChem, 2019, 6(9), 2586–2594 CrossRef CAS.
- T. Méndez-Morales, et al., Performance of microporous carbon electrodes for supercapacitors: comparing graphene with disordered materials, Energy Storage Mater., 2019, 17, 88–92 CrossRef.
- W. Gan, et al., High-performance supercapacitor device with ultrathick electrodes fabricated from all-cellulose-based carbon aerogel, Energy Fuels, 2021, 35(9), 8295–8302 CrossRef.
- J. Park, et al., Rational design of a redox-active nonaqueous electrolyte for a high-energy-density supercapacitor based on carbon nanotubes, ACS Sustainable Chem. Eng., 2019, 7(8), 7728–7735 CrossRef CAS.
- M. Yu, et al., One-step synthesis of sodium carboxymethyl cellulose-derived carbon aerogel/nickel oxide composites for energy storage, Chem. Eng. J., 2017, 324, 287–295 CrossRef CAS.
- H. Peng, et al., In situ synthesis of polyaniline/sodium carboxymethyl cellulose nanorods for high-performance redox supercapacitors, J. Power Sources, 2012, 211, 40–45 CrossRef CAS.
- C. D. Richards, et al., Efficiency of energy conversion for devices containing a piezoelectric component, J. Micromech. Microeng., 2004, 14(5), 717 CrossRef CAS.
- D. Zhong, et al., Enhanced electricity generation performance and dye wastewater degradation of microbial fuel cell by using a petaline NiO@ polyaniline-carbon felt anode, Bioresour. Technol., 2018, 258, 125–134 CrossRef CAS PubMed.
- L. Xu, et al., The integrated processes for wastewater treatment based on the principle of microbial fuel cells: a review, Crit. Rev. Environ. Sci. Technol., 2016, 46(1), 60–91 CrossRef.
- S. J. Dunaj, et al., Relationships between soil organic matter, nutrients, bacterial community structure, and the performance of microbial fuel cells, Environ. Sci. Technol. Libr., 2012, 46(3), 1914–1922 CrossRef CAS PubMed.
- N. Zhao, et al., Polyaniline/reduced graphene oxide-modified carbon fiber brush anode for high-performance microbial fuel cells, Int. J. Hydrogen Energy, 2018, 43(37), 17867–17872 CrossRef CAS.
- A. Deeke, et al., Influence of the thickness of the capacitive layer on the performance of bioanodes in microbial fuel cells, J. Power Sources, 2013, 243, 611–616 CrossRef CAS.
- S. Zhao, et al., Three-dimensional graphene/Pt nanoparticle composites as freestanding anode for enhancing performance of microbial fuel cells, Sci. Adv., 2015, 1(10), e1500372 CrossRef PubMed.
- H. Xu, et al., A 3D porous NCNT sponge anode modified with chitosan and Polyaniline for high-performance microbial fuel cell, Bioelectrochemistry, 2019, 129, 144–153 CrossRef CAS PubMed.
- Q. Du, et al., Polydopamine as a new modification material to accelerate startup and promote anode performance in microbial fuel cells, J. Power Sources, 2017, 343, 477–482 CrossRef CAS.
- D. Hidalgo, et al., Surface modification of commercial carbon felt used as anode for microbial fuel cells, Energy, 2016, 99, 193–201 CrossRef CAS.
- S. Xu, et al., Nanocellulose-assisted dispersion of graphene to fabricate poly (vinyl alcohol)/graphene nanocomposite for humidity sensing, Compos. Sci. Technol., 2016, 131, 67–76 CrossRef CAS.
- Y. Wang, et al., Conductive polypyrrole–carboxymethyl cellulose–titanium nitride/carbon brush hydrogels as bioanodes for enhanced energy output in microbial fuel cells, Energy, 2020, 204, 117942 CrossRef CAS.
- S.-H. Liu, et al., Enhanced copper removal and bioelectricity generation in sediment microbial fuel cells through biostimulation and bioaugmentation, J. Cleaner Prod., 2022, 350, 131458 CrossRef CAS.
- S. Niu, et al., Theoretical study of contact-mode triboelectric nanogenerators as an effective power source, Energy Environ. Sci., 2013, 6(12), 3576–3583 RSC.
- Z. L. Wang, On Maxwell's displacement current for energy and sensors: the origin of nanogenerators, Mater. Today, 2017, 20(2), 74–82 CrossRef.
- K. Dong, X. Peng and Z. L. Wang, Fiber/fabric-based piezoelectric and triboelectric nanogenerators for flexible/stretchable and wearable electronics and artificial intelligence, Adv. Mater., 2020, 32(5), 1902549 CrossRef CAS PubMed.
- P. Jaiban, et al., The biofilm from soybean meal for application in triboelectric generator, Mater. Lett., 2022, 325, 132862 CrossRef CAS.
- J. He, et al., High-Performance Flexible Triboelectric Nanogenerator Based on Environmentally Friendly, Low-Cost Sodium Carboxymethylcellulose for Energy Harvesting and Self-Powered Sensing, ACS Appl. Electron. Mater., 2022, 291–301 Search PubMed.
- K. Yan, et al., A non-toxic triboelectric nanogenerator for baby care applications, J. Mater. Chem. A, 2020, 8(43), 22745–22753 RSC.
- Y. Cheng, et al., Lightweight and flexible MXene/carboxymethyl cellulose aerogel for electromagnetic shielding, energy harvest and self-powered sensing, Nano Energy, 2022, 98, 107229 CrossRef CAS.
- M. S. Rahman, et al., Recent developments of carboxymethyl cellulose, Polymers, 2021, 13(8), 1345 CrossRef CAS PubMed.
- X. J. Wu and M. M. Choi, An optical glucose biosensor based on entrapped-glucose oxidase in silicate xerogel hybridised with hydroxyethyl carboxymethyl cellulose, Anal. Chim. Acta, 2004, 514(2), 219–226 CrossRef CAS.
- X. WANG, et al., The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival, Biochem. J., 1998, 333(2), 291–300 CrossRef CAS PubMed.
- J.-i. Abe and B. C. Berk, Fyn and JAK2 Mediate Ras Activation by Reactive Oxygen Species*, J. Biol. Chem., 1999, 274(30), 21003–21010 CrossRef CAS PubMed.
- M. Mittal, M. R. Siddiqui, K. Tran, S. P. Reddy and A. B. Malik, Reactive Oxygen Species in Inflammation and Tissue Injury, Antioxid. Redox Signaling, 2014, 20(7), 1126–1167 CrossRef CAS PubMed.
- Z. Medříková, et al., Carboxymethylcellulose-based magnetic Au or Ag nanosystems: eminent candidates in catalysis, sensing applications based on SERS, and electrochemistry, Appl. Mater. Today, 2019, 14, 143–150 CrossRef.
- R. H. Althomali, et al., Hybrid PANI@dialdehyde carboxymethyl cellulose/ZnO nanocomposite modified glassy carbon electrode as a highly sensitive electrochemical sensor, Diamond Relat. Mater., 2022, 122, 108803 CrossRef CAS.
- V. Gautam, K. P. Singh and V. L. Yadav, Preparation and characterization of green-nano-composite material based on polyaniline, multiwalled carbon nano tubes and carboxymethyl cellulose: for electrochemical sensor applications, Carbohydr. Polym., 2018, 189, 218–228 CrossRef CAS PubMed.
- J. Ji, et al., A facile electrochemical chiral sensor for tryptophan enantiomers based on multiwalled carbon nanotube/hydroxypropyl-β-cyclodextrin functionalized carboxymethyl cellulose, Microchem. J., 2022, 175, 107133 CrossRef CAS.
- M. Seki, R. Wada and H. Muguruma, Electrochemical behavior of intramolecular cyclization reaction of catecholamines at carbon nanotube/carboxymethylcellulose electrode, J. Electroanal. Chem., 2022, 116486 CrossRef CAS.
- C. Arenas, et al., An electrochemical immunosensor for adiponectin using reduced graphene oxide–carboxymethylcellulose hybrid as electrode scaffold, Sens. Actuators, B, 2016, 223, 89–94 CrossRef CAS.
- K. A. Alamry, et al., Sensitive electrochemical detection of toxic nitro-phenol in real environmental samples using enzymeless oxidized-carboxymethyl cellulose-sulfate/sulfated polyaniline composite based electrode, Microchem. J., 2022, 172, 106902 CrossRef CAS.
- M. Seki, R. Wada and H. Muguruma, Electrochemical behavior of intramolecular cyclization reaction of catecholamines at carbon nanotube/carboxymethylcellulose electrode, J. Electroanal. Chem., 2022, 918, 116486 CrossRef CAS.
- C. B. Arenas, et al., An electrochemical immunosensor for adiponectin using reduced graphene oxide–carboxymethylcellulose hybrid as electrode scaffold, Sens. Actuators, B, 2016, 223, 89–94 CrossRef CAS.
- R. H. Althomali, et al., Hybrid PANI@dialdehyde carboxymethyl cellulose/ZnO nanocomposite modified glassy carbon electrode as a highly sensitive electrochemical sensor, Diamond Relat. Mater., 2022, 122, 108803 CrossRef CAS.
- N. Terasawa, et al., A multi-walled carbon nanotube/polymer actuator that surpasses the performance of a single-walled carbon nanotube/polymer actuator, Carbon, 2012, 50(1), 311–320 CrossRef CAS.
- Q. Peng, et al., Shape-memory polymer nanocomposites with a 3D conductive network for bidirectional actuation and locomotion application, Nanoscale, 2016, 8(42), 18042–18049 RSC.
- D. Y. Lee, et al., Ionic polymer–metal composite bending actuator loaded with multi-walled carbon nanotubes, Sens. Actuators, A, 2007, 133(1), 117–127 CrossRef CAS.
- B. Strachotová, et al., Super porous organic–inorganic poly(N-isopropylacrylamide)-based hydrogel with a very fast temperature response, Polymer, 2007, 48(6), 1471–1482 CrossRef.
- J. T. Zhang, S. W. Huang and R. X. Zhuo, Preparation and characterization of novel temperature sensitive poly(N-isopropylacrylamide-co-acryloyl beta-cyclodextrin) hydrogels with fast shrinking kinetics, Macromol. Chem. Phys., 2004, 205(1), 107–113 CrossRef CAS.
- C.-A. Dai, et al., A membrane actuator based on an ionic polymer network and carbon nanotubes: the synergy of ionic transport and mechanical properties, Smart Mater. Struct., 2009, 18(8), 085016 CrossRef.
- Y. Gu, et al., Research strategies to develop environmentally friendly marine antifouling coatings, Mar. Drugs, 2020, 18(7), 371 CrossRef CAS PubMed.
- Y. Lv, et al., Effect of carboxymethyl cellulose on the output force and electrochemical performance of sodium alginate ion electric actuator, Sens. Actuators, A, 2022, 339, 113269 CrossRef CAS.
- Y. Lv, et al., Study on the Response Properties of Chitosan-Based Biogel Actuators Enhanced by Carboxymethyl Cellulose and Tannic Acid Using Freezing Technology, SSRN Electronic Journal, DOI:10.2139/ssrn.4111261.
- J. Wei, et al., Nacre-inspired composite film with mechanical robustness for highly efficient actuator powered by humidity gradients, Chem. Eng. J., 2023, 451, 138565 CrossRef CAS.
Footnote |
| † Joint first authors. |
| This journal is © The Royal Society of Chemistry 2023 |