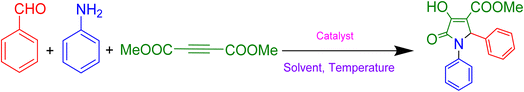Open Access Article
Open Access Articleβ-Cyclodextrin: a green supramolecular catalyst assisted eco-friendly one-pot three-component synthesis of biologically active substituted pyrrolidine-2-one†
Subhankar Paula,
Sharmistha Dasa,
Bijeta Mitraa,
Gyan Chandra Pariyarb and
Pranab Ghosh *a
*a
aDepartment of Chemistry, University of North Bengal, District-Darjeeling, West Bengal, India. E-mail: pizy12@yahoo.com; Fax: +91 0353 2699001; Tel: +91 0353 2776381
bDepartment of Food Technology, University of North Bengal, District-Darjeeling, West Bengal, India
First published on 13th February 2023
Abstract
A green, novel and eco-efficient synthetic route towards the synthesis of highly substituted bio-active pyrrolidine-2-one derivatives was demonstrated using β-cyclodextrin, a water-soluble supramolecular solid as a green and eco-benign catalyst at room temperature under water–ethanol solvent medium. The exploration of the green catalyst β-cyclodextrin for the metal-free one-pot three-component synthesis of a wide range of highly functionalized bio-active heterocyclic pyrrolidine-2-one moieties from easily available aldehydes and amines explains the superiority and uniqueness of this protocol.
1. Introduction
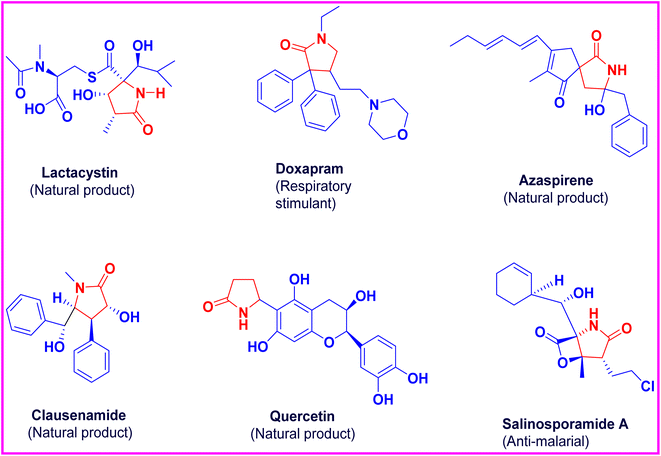
A significant amount of research work is dedicated towards the development of novel nitrogen-based heterocyclic molecules. Not only are they present in abundance in nature, but their unique structural skeleton also demonstrates their importance in the biological system and medicinal chemistry. Among them, the five-membered ring systems with a pyrrolidine moiety have gained increased attention lately due to their diverse medicinal properties, which include antibacterial, antibiotics, antitumor and cytotoxic effects.The pyrrolidine ring system that includes nicotine,1 tryptamine and vinblastine possesses considerable biochemical, pharmaceutical, agricultural importance. These natural compounds may have hydroxyproline, 2-pyrrolidone, streptopyrrolidine or diphenylprolinol rings as a component in their well-defined conformations. The pyrrolidinone nucleus is rated as one of the most important heterocyclic compounds, featuring notable pharmaceutical effects and acting as a versatile precursor in the design of powerful bio-active agents and intricate medicinally important compounds. Polysubstituted 3-hydroxy-2-pyrrolidinones are an important part of the framework in many biologically active compounds and have found wide applications in pharmaceutical and agricultural sectors.2 Some of the biologically active compounds and natural products containing the pyrrolidinone moiety are aniracetam, doxapram,3 cotinine, clausenamide, lactacystin,4 ethosuximide, codonopsinine, pyrrolidinone quercetin,5 azaspirene6 and salinosporamide A7 (Fig. 1). This fascinating group of compounds has diverse pharmacological activities, such as antidepressant, anti-malarial, antibacterial, antifungal, anti-cancer and anticonvulsant. Substituted 3-pyrrolin-2-ones with a 2-pyrrolidinone8 moiety are also used in medicinal chemistry, as many derivatives show significant pharmacological and biological activities, such as anti-cancer agents,9 anti-tumour,10 HIV-1 integrase inhibitors,11 anti-microbial,12 antibacterial13 and anti-inflammatory.14
Nowadays, there is a rising interest towards using supramolecular chemistry and homogeneous catalysts, as they play an important role in synthesizing organic heterocyclic compounds15 and pharmaceutically important compounds. β-Cyclodextrin, a major class of cyclodextrins, is a family of cyclic oligosaccharides consisting of a macrocyclic ring of 7 glucose subunits joined by α-1,4 glycosidic bonds and is an important enzyme model.16 As with other cyclodextrins, β-cyclodextrin also uses its internal hydrophobic cavity to encapsulate biologically active molecules from aqueous solution and to selectively bind the organic substrates. β-Cyclodextrin catalyzes the organic transformations with high selectivity17 by non-covalent supramolecular bonding with the reversible formation of host–guest complexes as seen in enzymes, which gradually increases the bioavailability and stability of drug molecules. β-Cyclodextrin activates a variety of organic compounds like aldehydes, ketones, anhydride, oximes, amines, nitriles and increases the rate of reactions.18 These properties of β-CD recognises it as an important asset for pharmaceutical industries, as well as for synthetic chemistry. Moreover, β-CD is easily available, recyclable, inexpensive, and water-soluble due to the presence of a hydrophilic external part, and is a mild and non-toxic supramolecule.19 This account summarises our efforts in designing an important organic transformation using β-cyclodextrin, as well as a greener synthetic route to a variety of biologically relevant organic molecules.
In the last few decades, many research works for the synthesis of biologically active heterocyclic compounds have been empowered by one-pot multi-component reactions (MCRs).20 These reactions have considerable importance in organic synthesis, as well as in the medicinal and pharmacological sectors. In comparison with multi-step reactions, a multi-component synthesis involves three or more starting materials that react together in the same reaction vessel to exclusively produce the desired product molecule without the need of separating the intermediates. This makes the reaction time shorter, reduces the wastage of solvents, and completes the reaction with low consumption of energy.
In organic synthesis using the MCR strategy, the catalyst and solvents play a very pivotal role. Use of highly volatile solvents or transition metal and lanthanide metal catalysts may have detrimental environmental effects. Furthermore, the catalysts might be associated with drawbacks such as high cost, low compatibility, difficulty in handling, and being easily ruined by impurities. These limitations of metal catalysts and organic solvents can be overcome by using any green organo-catalyst and green eco-friendly solvent, which will be beneficial to our environment. Water and ethanol are versatile solvents, and the water–ethanol system is now broadly used from the view of a greener approach. The aqueous–ethanol mixture as a reaction medium has been greatly beneficial in many instances.21 This not only offers a homogenous medium for many reactions, but also provides rate accelerations in several others. The water–ethanol reaction medium has been demonstrated to be an efficient system for the multi-component synthesis of biologically potent heterocyclic derivatives.22–24
Numerous examples of multi-component synthesis of pyrrolidinone derivatives using a variety of catalysts, such as the ionic liquids [BBSI][HSO4] and [BBSI][Cl],25 magnetic solid acid catalysts [Fe3O4@SiO2@NMPs], [Fe3O4@SiO2@Cl NMPs] and [Fe3O4@SiO2@propyl-ANDSA],26 metal catalyst [Cu(OAc)2],27 metal–organic framework [UiO-66-SO3H],28 TiO2-nanoparticle in aqueous CTAB solution,29 tetragonal-ZrO2 nanocatalyst,30 ethanolic citric acid under US condition,31 ionic liquid [bmim]BF4 in PEG-400,32 photocatalyst Rose Bengal in CH3CN–H2O under visible light,33 acid catalyst PTSA,34 and carbocatalyst graphene oxide,35 have been reported in the literature. These working routes for synthesising pyrrolidinones include the condensation of aryl anilines, aromatic aldehydes and dialkylacetylenedicarboxylates using various metal catalysts, lanthanide catalysts, ionic liquids, nanoparticles, and acids. Although these synthetic routes of pyrrolidinone derivatives may have advantages, they still suffer from limitations such as the use of hazardous solvents, harsh reaction conditions, high temperature, long reaction time, complex synthetic pathways, low yield, limited structural diversity, functional group tolerability and metal contamination at the end of the reaction. Synthesising and characterising the metal catalysts, magnetic nanoparticles, lanthanide metal catalysts, and ionic liquids make these processes expensive, complex, time-consuming, and difficult to carry out under mild conditions or separate the products in a simple way. Consequently, better quality approaches for synthesising pyrrolidinone derivatives with a precise, straightforward, green, eco-efficient one-pot multi-component reaction in the absence of transition metal or lanthanide metal catalyst and harmful organic solvents are still unknown, and will be well accepted in synthetic organic chemistry.
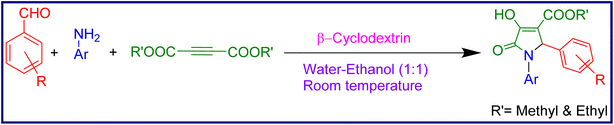
Taking into consideration all the above mentioned factors, we have succeeded in employing a combination of water–ethanol and β-cyclodextrin at room temperature for a greener synthesis of substituted pyrrolidinone derivatives (Scheme 1).
2. Experimental section
2.1 General procedure for the synthesis of methyl/ethyl 2-aryl-4-hydroxy-5-oxo-1-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate and methyl/ethyl 1-aryl-4-hydroxy-5-oxo-2-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate
β-Cyclodextrin (10 mg) and 2 ml of water–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) were added to the mixture of aldehydes (1 mmol), amines (1 mmol) and dimethylacetylenedicarboxylate (1.2 mmol) or diethylacetylenedicarboxylate (1.2 mmol) contained in a reaction vessel. The resulting reaction mixture was then stirred in a magnetic stirrer at room temperature for 8 h in open air. The progress of the reaction was monitored by TLC using petroleum ether and ethyl acetate as the eluent solvent in a silica bed on an aluminium sheet. After completion of the reaction as indicated by TLC, a large amount of water and ethyl acetate were added to the reaction mixture, and the product was extracted thrice by ethyl acetate with the help of a separating funnel. The extracted ethyl acetate solution was then passed through the bed of anhydrous sodium sulphate (Na2SO4) to make it a completely water-free organic solution, and evaporated to achieve a significant volume reduction. The crude product or crystal product obtained was then purified by filtering and washing with ethyl acetate and petroleum ether mixture. The products obtained were known compounds, and identified through 1H-NMR, 13C-NMR and 19F-NMR spectroscopies.
1 ratio) were added to the mixture of aldehydes (1 mmol), amines (1 mmol) and dimethylacetylenedicarboxylate (1.2 mmol) or diethylacetylenedicarboxylate (1.2 mmol) contained in a reaction vessel. The resulting reaction mixture was then stirred in a magnetic stirrer at room temperature for 8 h in open air. The progress of the reaction was monitored by TLC using petroleum ether and ethyl acetate as the eluent solvent in a silica bed on an aluminium sheet. After completion of the reaction as indicated by TLC, a large amount of water and ethyl acetate were added to the reaction mixture, and the product was extracted thrice by ethyl acetate with the help of a separating funnel. The extracted ethyl acetate solution was then passed through the bed of anhydrous sodium sulphate (Na2SO4) to make it a completely water-free organic solution, and evaporated to achieve a significant volume reduction. The crude product or crystal product obtained was then purified by filtering and washing with ethyl acetate and petroleum ether mixture. The products obtained were known compounds, and identified through 1H-NMR, 13C-NMR and 19F-NMR spectroscopies.
2.2 Spectroscopic data of the synthesized compounds
3. Result and discussion
3.1 Optimisation of reaction conditions
For the optimisation of the reaction conditions, reaction parameters such as the solvent, temperature, time and amount of catalyst used were observed to have a wide influence on the reaction, and also on the yield of the desired product. To begin our study, we have taken benzaldehyde, aniline and dimethylacetylenedicarboxylate in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 ratios as a model reaction. We have performed the reaction in various solvents, such as acetonitrile, DMF, methanol, ethanol, water, water–ethanol mixture, as well as in solvent-free condition, and all the experimental results are summarised in Table 1.
1.2 ratios as a model reaction. We have performed the reaction in various solvents, such as acetonitrile, DMF, methanol, ethanol, water, water–ethanol mixture, as well as in solvent-free condition, and all the experimental results are summarised in Table 1.
| Entry | Solvent | Catalyst loading (mg) | Temperature (°C) | Time (h) | Yieldc (%) |
|---|---|---|---|---|---|
| a The bold numbers represent the optimised condition.b Reaction of benzaldehyde (1 mmol), aniline (1 mmol), and dimethylacetylenedicarboxylate (1.2 mmol).c Isolated yield after purification. | |||||
| 1 | Neat | 30 | 100 | 24 | 45 |
| 2 | Acetonitrile | 30 | Reflux | 24 | Trace |
| 3 | DMF | 30 | 100 | 24 | Trace |
| 4 | Methanol | 30 | Reflux | 12 | 60 |
| 5 | Ethanol | 30 | Reflux | 12 | 65 |
| 6 | Water | 30 | Reflux | 12 | 70 |
| 7 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
30 | Reflux | 12 | 75 |
| 8 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
20 | Reflux | 12 | 75 |
| 9 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10 | Reflux | 12 | 75 |
| 10 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5 | Reflux | 12 | 40 |
| 11 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
— | Reflux | 12 | — |
| 12 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10 | 70 | 12 | 77 |
| 13 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10 | 50 | 12 | 79 |
| 14 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
10 | r.t. | 12 | 80 |
| 15 | Water + ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1) |
10 | r.t. | 8 | 82 |
At first, the reaction was carried out in solvent-free condition with 30 mg of the β-CD at 100 °C for 24 h. It was observed that only a small amount of the desired product (Table 1, entry 1) was formed. Then, the same reaction was performed in various aprotic solvents, such as acetonitrile and DMF, with 30 mg β-CD under reflux condition and 100 °C, respectively. Under such reaction conditions, only a trace amount of the desired product was formed even after 24 h (Table 1, entries 2 and 3). After that, various polar protic solvents such as methanol and ethanol were screened with 30 mg β-CD under reflux condition for 12 h, and we were happy to observe a good amount of the desired product formation. With ethanol, the yield was quite high (Table 1, entries 4 and 5). Then, we carried out the same reaction in aqueous medium under reflux condition for 12 h, where an excellent amount of the expected product was formed (Table 1, entry 6). From this observation, it was concluded that water was necessary to perform the reaction perfectly with better conversion since β-CD is highly water-soluble. Then, we tried the same reaction with water and ethanol in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio under reflux condition for 12 h with 30 mg catalyst, and the expected product was formed in high yield (Table 1, entry 7). From this, it was clear that water–ethanol in a 1
1 ratio under reflux condition for 12 h with 30 mg catalyst, and the expected product was formed in high yield (Table 1, entry 7). From this, it was clear that water–ethanol in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is the ideal solvent for this organic transformation.
1 ratio is the ideal solvent for this organic transformation.
We then reduced the amount of the catalyst to 20 mg and 10 mg, where almost the same amount of the desired product was obtained (Table 1, entries 8 and 9). Since 30 mg, 20 mg and 10 mg of the catalyst gave nearly the same amount of the desired product under the same reaction condition, it can be concluded that 10 mg of the catalyst was sufficient for the reaction. After that, on further reducing the amount of catalyst to 5 mg, a low amount of the product was obtained (Table 1, entry 10). No desired product was obtained without any catalyst (Table 1, entry 11). Now, from these observations, it can be concluded that for our reaction, 10 mg β-CD was adequate enough to get good yield of the products.
After that, we started optimizing the temperature and time. The reaction was carried out in a solvent mixture containing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethanol–water ratio for 12 h with 10 mg of β-CD at different temperatures, such as 70 °C, 50 °C and room temperature (Table 1, entries 12–14). It was observed that the best result was obtained at room temperature (Table 1, entry 14). Furthermore, the expected product separation became easier for the reaction carried out at room temperature since the side product formation was minimized. Now, when the time was reduced to 8 h, it was found that the yield of the desired product was almost the same (Table 1, entry 15). Hence, from these above-mentioned observations during the screening of the reaction conditions, it can be concluded that the reaction with 10 mg of β-CD and 1
1 ethanol–water ratio for 12 h with 10 mg of β-CD at different temperatures, such as 70 °C, 50 °C and room temperature (Table 1, entries 12–14). It was observed that the best result was obtained at room temperature (Table 1, entry 14). Furthermore, the expected product separation became easier for the reaction carried out at room temperature since the side product formation was minimized. Now, when the time was reduced to 8 h, it was found that the yield of the desired product was almost the same (Table 1, entry 15). Hence, from these above-mentioned observations during the screening of the reaction conditions, it can be concluded that the reaction with 10 mg of β-CD and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of water–ethanol solvent at room temperature for 8 h was the best condition for our work.
1 ratio of water–ethanol solvent at room temperature for 8 h was the best condition for our work.
3.2 Investigating products
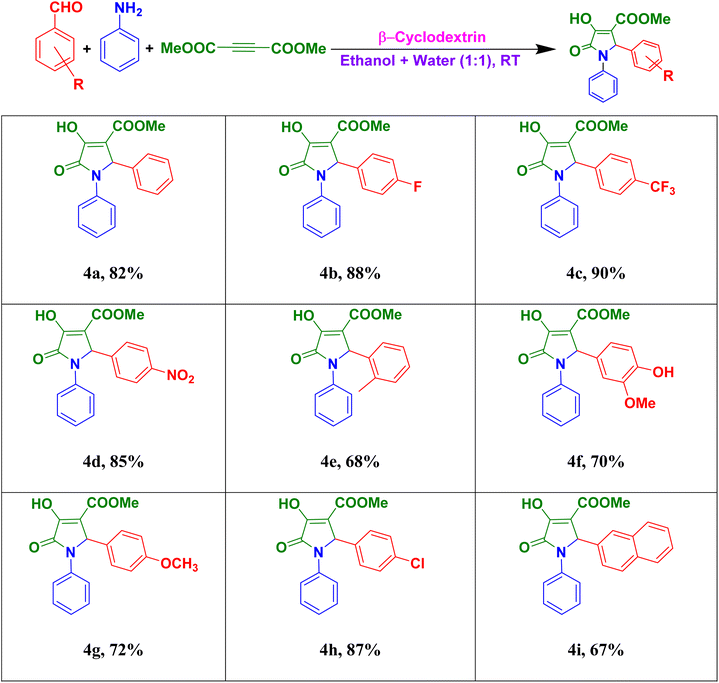
For the summary of this research scheme, numerous aldehydes and amines were treated with dialkylacetylenedicarboxylate (alkyl = methyl & ethyl) under the optimised reaction condition to project a versatile range of alkyl-2-aryl-4-hydroxy-5-oxo-1-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate and alkyl-1-aryl-4-hydroxy-5-oxo-2-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate (alkyl = methyl ðyl) derivatives (Tables 2–4). Here, all the pure solid products were isolated without column chromatography.a Reaction with aldehydes (1 mmol), aniline (1 mmol), dimethylacetylenedicarboxylate (1.2 mmol) with 10 mg of β-CD in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of water–ethanol at room temperature for 8 h. 1 ratio of water–ethanol at room temperature for 8 h. |
|---|
 |
a Reaction with benzaldehyde (1 mmol), aryl amines (1 mmol), dimethylacetylenedicarboxylate (1.2 mmol) with 10 mg of β-CD in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of water–ethanol at room temperature for 8 h. 1 ratio of water–ethanol at room temperature for 8 h. |
|---|
 |
a Reaction with aldehydes (1 mmol), aniline (1 mmol), diethylacetylenedicarboxylate (1.2 mmol) with 10 mg of β-CD in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of water–ethanol at room temperature for 10 h.b Reaction with benzaldehyde (1 mmol), aryl amines (1 mmol), diethylacetylenedicarboxylate (1.2 mmol) with 10 mg of β-CD in 1 1 ratio of water–ethanol at room temperature for 10 h.b Reaction with benzaldehyde (1 mmol), aryl amines (1 mmol), diethylacetylenedicarboxylate (1.2 mmol) with 10 mg of β-CD in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of water–ethanol at room temperature for 10 hours. 1 ratio of water–ethanol at room temperature for 10 hours. |
|---|
 |
In Table 2, from the result of investigation, it was detected that aldehydes with electron-withdrawing groups on the benzene ring, e.g., 4-fluorobenzaldehyde (Table 2, 4b), 4-trifluoromethylbenzaldehyde (Table 2, 4c), 4-nitrobenzaldehyde (Table 2, 4d) and 4-chlorobenzaldehyde (Table 2, 4h) gave good yield of the desired product, whereas the aldehydes with electron-donating groups on the benzene ring, e.g., o-tolubenzaldehyde (Table 2, 4e), vanillin (Table 2, 4f), 4-methoxybenzaldehyde (Table 2, 4g) and 2-naphthaldehyde (Table 2, 4i) offered quite low yields of the desired product. It was also found that benzaldehyde itself (Table 2, 4a) gave high yield of the desired product.
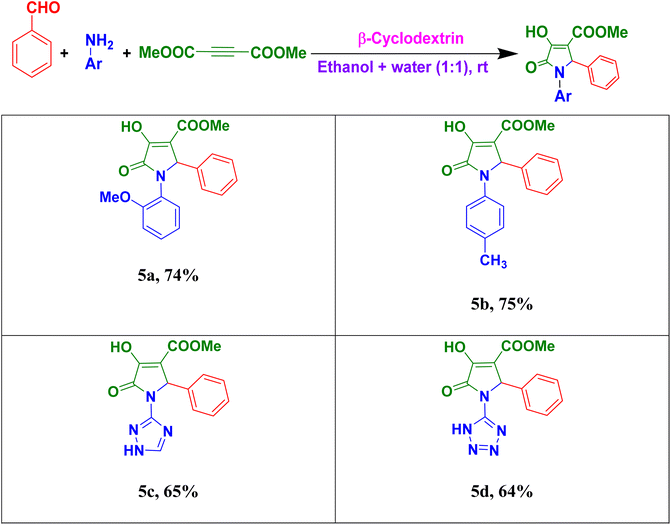
We further extended our protocol for the synthesis of methyl-1-aryl-4-hydroxy-5-oxo-2-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate derivatives using various aryl amines with benzaldehyde and dimethylacetylenedicarboxylate (Table 3). Here, aromatic amines having electron-donating groups on the benzene ring (like before) also gave good yield of the product. With heterocyclic amines, e.g., triazole (Table 3, 5c) and tetrazole (Table 3, 5d) amines, a low yield of the desired products was observed.
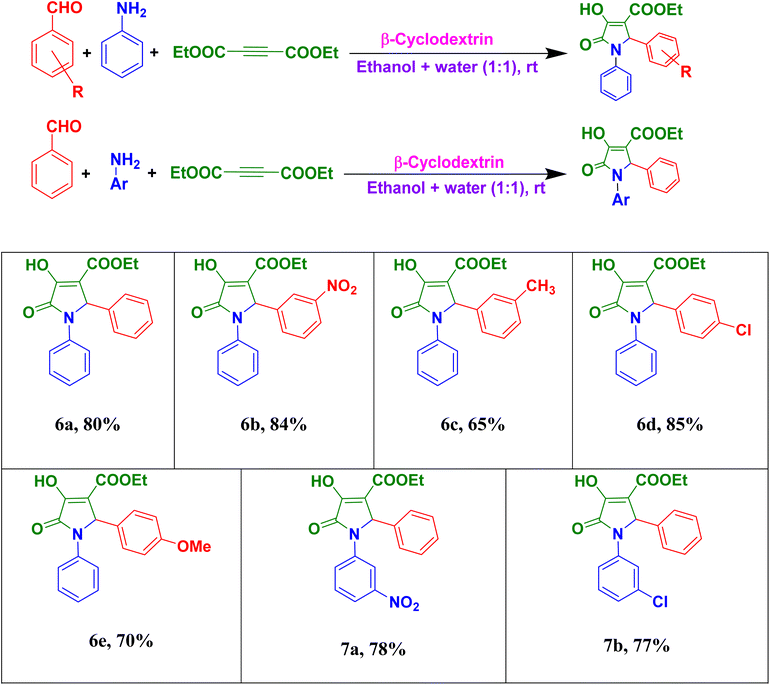
We further used the protocol for the synthesis of ethyl-2-aryl-4-hydroxy-5-oxo-1-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate and ethyl-1-aryl-4-hydroxy-5-oxo-2-phenyl-2,5-dihydro-1H-pyrrole-3-carboxylate derivatives using various aromatic aldehydes, aniline and diethylacetylenedicarboxylate and various aryl amines, benzaldehyde and diethylacetylenedicarboxylate, respectively (Table 4). These procedures needed 10 h to complete under the optimized conditions.
3.3 Mechanism
A plausible mechanism for the synthesis of various 1,2,3-functionalised 4-hydroxy pyrrolidine-5-ones is shown in Scheme 2. The supramolecular catalyst β-CD synergistically behaves as an effective host molecule for various types of organic compounds due to the presence of its seven free primary –OH groups. Our catalyst β-CD possibly activates both aryl aldehyde (1) and di(methyl/ethyl) acetylene dicarboxylate (3, 4) derivatives simultaneously as the active electrophilic species, and hence facilitates the further reaction. The condensation reaction of aryl amines (2) with these activated electrophilic aldehydes with the loss of water, along with the reaction of water with activated electrophilic di(methyl/ethyl) acetylene dicarboxylate derivatives, may then generate the corresponding intermediates (5) and (6), respectively. After that, the reaction between these two intermediates (5) and (6) will yield the corresponding intermediate (7). Finally, a series of tautomerization, cyclization and aromatisation reactions generate the intermediates (8), (9), and finally the product molecule (10).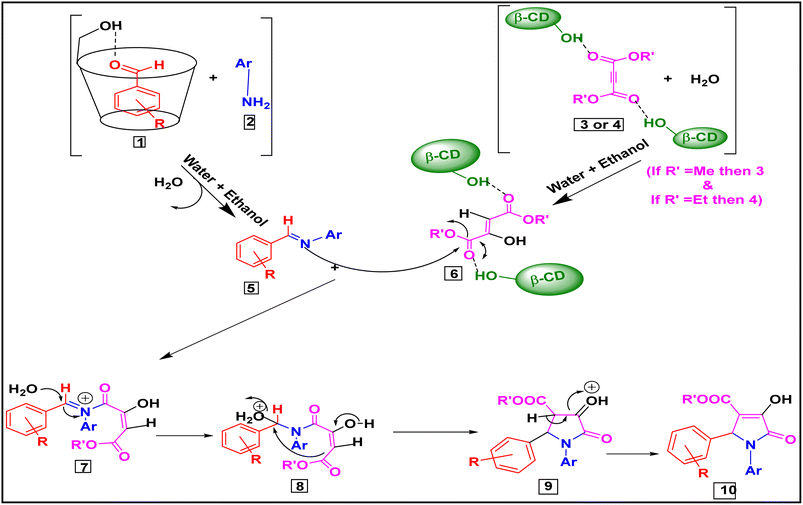 | ||
| Scheme 2 Plausible mechanism for β-cyclodextrin-mediated synthesis of functionalised pyrrolidine-2-ones. | ||
4. Conclusion
In conclusion, we present a straightforward, vigorous, simplistic, eco-friendly and green approach for the synthesis of 1,2,3-functionalised 4-hydroxy pyrrolidine-5-one derivatives using a bio-degradable, eco-benign, green, supramolecular catalyst β-cyclodextrin, in water–ethanol medium. Furthermore, the reaction was carried out without using any additional metal catalyst or metal salt, volatile organic solvent under mild reaction conditions which boosts the benefits of the procedure. The replacement of toxic and costly metal catalysts with an eco-friendly, bio-degradable, green and inexpensive organo-catalyst is the unique benefit of this synthetic procedure. This environmentally-benign approach is likely to reach widespread applications in the pharmaceutical industry, organic synthetic chemistry and natural product synthesis.Author contributions
S. Paul and S. Das have completed the whole experimental work, and contributed equally to these methodologies. Dr B. Mitra and Dr G. C. Pariyar helped them in the selection of work and preparation of manuscript under the supervision of Prof. P. Ghosh.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
G. C. P. is grateful to the University of North Bengal for financial support. S. Das is grateful to West Bengal for financial support through the state JRF fellowship.References
- L. P. Dwoskin, L. Teng, S. T. Buxton and P. A. Crooks, J. Pharmacol. Exp. Ther., 1999, 288, 905–911 CAS.
- S. Tu and C. Zhang, Org. Process Res. Dev., 2015, 19, 2045–2049 CrossRef CAS.
- P. Singh, V. Dimitriou, R. P. Mahajan and A. W. A. Crossley, Br. J. Anaesth., 1993, 71, 685–688 CrossRef CAS PubMed.
- S. Omura, T. Fujimoto, K. Moriguchi, H. Tanaka, Y. Sasaki, K. Moriguchi, H. Tanaka and Y. Sasaki, J. Antibiot., 1991, 44, 113–116 CrossRef CAS PubMed.
- V. Ilkei, L. S. Hazai, S. Antus and H. Bolcskei, Stud. Nat. Prod. Chem., 2018, 56, 247–283 CAS.
- Y. Asami, H. Kakeya, R. Onose, A. Yoshida, H. Matsuzaki and H. Osada, Org. Lett., 2002, 4, 2845–2848 CrossRef CAS PubMed.
- R. H. Feling, G. O. Buchanan, T. J. Mincer, C. A. Kauffman, P. R. Jensen and W. Fenical, Angew. Chem., Int. Ed., 2003, 42, 355–357 CrossRef CAS PubMed.
- H. Ahankar, A. Ramazani, K. Slepokura, T. Lis and S. W. Joo, Green Chem., 2016, 18, 3582–3593 RSC.
- (a) T. Michael, A. Michael, T. Andreas, H. Ulrich, B. Mirko and N. A. Johannes, WO 2008055945(A1), 2008; (b) V. O. Koz’minykh, N. M. Igidov, S. S. Zykova, V. E. Kolla, N. S. Shuklina and T. Odegova, Pharm. Chem. J., 2002, 36, 188–191 CrossRef.
- Y. Geng, X. Wang, L. Yang, H. Sun, Y. Wang, Y. Zhao, R. She, M. X. Wang, D. X. Wang and J. Tang, PLoS One, 2015, 10, 1–15 Search PubMed.
- (a) K. Ma, P. Wang, W. Fu, X. Wan, L. Zhou, Y. Chu and D. Ye, Bioorg. Med. Chem. Lett., 2011, 21, 6724–6727 CrossRef CAS PubMed; (b) A. Pendri, T. L. Troyer, M. J. Sofia, M. A. Walker, B. N. Naidu, J. Banville, N. A. Meanwell, I. Dicker, Z. Lin, M. Krystal and S. W. Gerritz, J. Comb. Chem., 2010, 12, 84–90 CrossRef CAS PubMed.
- V. L. Gein, M. N. Armisheva, N. A. Rassudikhina, M. I. Vakhrin and E. V. Voronina, Pharm. Chem. J., 2011, 45, 162–164 CrossRef CAS.
- V. L. Gein, V. A. Mihalev, N. N. Kasimova, E. V. Voronina, M. I. Vakhrin and E. B. Babushkina, Pharm. Chem. J., 2007, 41, 208–210 CrossRef CAS.
- V. L. Gein, V. V. Yushkov, N. N. Kasimova, N. S. Shuklina, Y. M. Vasil'eva and M. V. Gubanova, Pharm. Chem. J., 2005, 39, 484–487 CrossRef CAS.
- (a) S. S. Reddy, M. V. K. Reddy and P. V. G. Reddy, ChemistrySelect, 2018, 3, 4283–4288 CrossRef CAS; (b) S. N. Murthy and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 4481–4484 CrossRef; (c) M. Abbasi, J. Chin. Chem. Soc., 2017, 64, 896–917 CrossRef CAS; (d) S. V. Akolkar, N. D. Kharat and A. A. Nagargoje, Catal. Lett., 2020, 150, 450–460 CrossRef CAS; (e) A. Ghorad, S. Mahalle and L. D. Khillare, Catal. Lett., 2017, 147, 640–648 CrossRef CAS; (f) G. Dhananjaya, A. V. D. Rao, K. A. Hossain, V. R. Anna and M. Pal, Tetrahedron Lett., 2020, 61, 151972 CrossRef CAS; (g) Y. A. Tayade, S. A. Padvi, Y. B. Wagh and D. S. Dalal, Tetrahedron Lett., 2020, 56, 2441–2447 CrossRef; (h) B. Mitra, G. C. Pariyar and P. Ghosh, RSC Adv., 2021, 11, 1271–1281 RSC.
- (a) L. R. Reddy, N. Bhanumathi and K. R. Rao, Chem. Commun., 2000, 2321–2322 RSC; (b) M. A. Reddy, N. Bhanumathi and K. R. Rao, Chem. Commun., 2001, 1974–1975 RSC; (c) K. Surendra, N. S. Krishnaveni, R. Sridhar and K. R. Rao, Tetrahedron Lett., 2006, 47, 2125–2127 CrossRef CAS; (d) O. Z. Tee, C. Mazza, R. L. Hemmer and J. B. Giorgi, J. Org. Chem., 1994, 59, 7602–7608 CrossRef CAS.
- (a) L. Marchetti and M. Levine, ACS Catal., 2011, 1, 1090–1118 CrossRef CAS; (b) R. Breslow and U. Maitra, Tetrahedron Lett., 1983, 24, 1901–1904 CrossRef CAS; (c) A. Gonzalez and S. Holt, J. Org. Chem., 1982, 47, 3186–3188 CrossRef CAS; (d) H. J. Schneider and N. K. Sangwan, J. Chem. Soc., Chem. Commun., 1986, 24, 1787–1789 RSC; (e) D. D. Sternbach and D. M. Rossana, J. Am. Chem. Soc., 1982, 104, 5853–5854 CrossRef CAS.
- D. K. Dalal, D. R. Patil and Y. A. Tayade, Chem. Rec., 2018, 18, 1560–1582 CrossRef CAS PubMed.
- (a) A. L. Laza-Knoerr, R. Gref and P. J. Couvreur, J. Drug Targeting, 2010, 18, 645–656 CrossRef CAS PubMed; (b) J. Heng-Bing, S. DongPo, S. Ming, L. Zhong, W. Le-Fu, H. B. Ji, D. P. Shi, M. Shao, Z. Li and L. F. Wang, Tetrahedron Lett., 2005, 46, 2517–2520 CrossRef.
- (a) K. Datta, B. Mitra, B. S. Sharma and P. Ghosh, ChemistrySelect, 2022, 7, e202103602 CAS; (b) A. R. Nesaragi, R. R. Kamble, S. R. Hoolageri, A. Mavazzan, S. F. Madar, A. Anand and S. D. Joshi, Appl. Organomet. Chem., 2022, 36, e6469 CrossRef CAS PubMed; (c) M. Esmati and B. Zeynizadeh, Appl. Organomet. Chem., 2022, 36, e6496 CrossRef CAS; (d) S. Zeinali, L. Z. Fekri and L. H. Zadeh, Appl. Organomet. Chem., 2022, 36, e6560 CrossRef CAS; (e) P. Basak, S. Dey and P. Ghosh, RSC Adv., 2021, 11, 32106–32118 RSC; (f) B. Mitra, G. C. Pariyar and P. Ghosh, RSC Adv., 2021, 11, 1271–1281 RSC; (g) M. Kalhor and S. Banibairami, RSC Adv., 2020, 10, 41410–41423 RSC; (h) Z. Elyasi, J. S. Ghomi, G. R. Najafia, M. Reza and Z. Monfareda, RSC Adv., 2020, 10, 44159–44170 RSC; (i) S. Dey, P. Basak and P. Ghosh, ChemistrySelect, 2020, 5, 15209–15217 CrossRef CAS; (j) P. Basak, S. Dey and P. Ghosh, ChemistrySelect, 2020, 5, 626–636 CrossRef CAS; (k) P. Choudhury, P. Ghosh and B. Basu, Mol. Diversity, 2020, 24, 283–294 CrossRef CAS PubMed; (l) B. Mitra, G. C. Pariyar and P. Ghosh, ChemistrySelect, 2019, 4, 5476–5483 CrossRef CAS; (m) B. Mitra, S. Mukherjee, G. C. Pariyar and P. Ghosh, Tetrahedron Lett., 2018, 59, 1385–1389 CrossRef CAS; (n) A. Maleki, E. Akhlaghi and R. Paydar, Appl. Organomet. Chem., 2016, 30, 382–386 CrossRef CAS; (o) R. G. Vaghei, N. Sarmast and J. Mahmoodi, Appl. Organomet. Chem., 2017, 31, e3681 CrossRef; (p) S. Moradi, M. A. Zolfigol, M. Zarei, D. A. Alonso, A. Khoshnood and A. Tajally, Appl. Organomet. Chem., 2018, 32, e4043 CrossRef; (q) R. G. Vaghei and V. Izadkhah, Appl. Organomet. Chem., 2018, 32, e4025 Search PubMed; (r) F. Panahi, E. Niknam, S. Sarikhani, F. Haghighi and A. K. Nezhad, New J. Chem., 2017, 41, 12293–12302 RSC; (s) M. Afradi, S. A. Pour, M. Dolat and A. Y. E. Abadi, Appl. Organomet. Chem., 2018, 32, e4103 CrossRef; (t) M. Zahedifar, P. Mohammadi and H. Sheibani, Lett. Org. Chem., 2017, 14, 315–323 CrossRef CAS; (u) F. Nemati, A. Elhampour, H. Farrokhi and M. B. Natanzi, Catal. Commun., 2015, 66, 15–20 CrossRef CAS; (v) F. Keshavarzipour and H. Tavakol, Appl. Organomet. Chem., 2017, 31, e3682 CrossRef; (w) S. Sadjadi, M. M. Heravi and M. Malmir, Appl. Organomet. Chem., 2018, 32, e4029 Search PubMed; (x) S. Shojaei, Z. Ghasemi and A. Shahrisa, Tetrahedron Lett., 2017, 58, 3957–3965 CrossRef CAS; (y) J. Lu, E. Q. Ma, Y. H. Liu, Y. M. Li, L. P. Mo and Z. H. Zhang, RSC Adv., 2015, 5, 59167–59185 RSC; (z) M. Zhang, J. Lu, J. N. Zhang and Z. H. Zhang, Catal. Commun., 2016, 78, 26–32 CrossRef CAS.
- (a) D. Reinhardt, F. Ilgen, D. Kralisch, B. König and G. Kreisel, Green Chem., 2008, 10, 1170–1181 RSC; (b) P. Alaimo, Org. Lett., 2008, 10, 5111–5114 CrossRef CAS PubMed; (c) A. Laitinen, Y. Takebayashi, I. Kylänlahti, J. Yli-Kauhaluoma, T. Sugeta and K. Otake, Green Chem., 2004, 6, 49–52 RSC; (d) C. K. Pai and M. B. Smith, J. Org. Chem., 1995, 60, 3731–3735 CrossRef CAS; (e) C. J. Li and L. Chen, Chem. Soc. Rev., 2006, 35, 68–82 RSC; (f) S. Kobayashi, T. Hamada, S. Nagayama and K. Manabe, Org. Lett., 2001, 3, 165–167 CrossRef CAS PubMed; (g) S. Sadjadi, Appl. Organomet. Chem., 2018, 32, e4211 CrossRef.
- M. N. K. Reddy, S. K. Reddy, M. Balaji and I. Kim, ChemistrySelect, 2019, 4, 644–649 CrossRef.
- S. Payra, A. Saha and S. Banerjee, ChemistrySelect, 2018, 3, 7535–7540 CrossRef CAS.
- C. B. Li, L. S. Huang, R. S. Wu and D. Z. Xu, ChemistrySelect, 2019, 4, 1635–1639 CrossRef CAS.
- N. G. Khaligh, T. Mihankhah and M. R. Johan, Synth. Commun., 2019, 49, 1334–1342 CrossRef CAS.
- R. G. Vaghei, N. Sarmast and J. Mahmoodi, Appl. Organomet. Chem., 2016, 31, e3681 CrossRef.
- L. Lv, S. Zheng, X. Cai, Z. Chen, Q. Zhu and S. Liu, ACS Comb. Sci., 2013, 15, 183–192 CrossRef CAS PubMed.
- R. G. Vaghei, D. Azarifar, S. Dalirana and A. R. Oveisi, RSC Adv., 2016, 6, 29182–29189 RSC.
- R. Sarkar and C. Mukhopadhyay, Tetrahedron Lett., 2013, 54, 3706–3711 CrossRef CAS.
- A. Saha, S. Payra and S. Banerjee, RSC Adv., 2016, 6, 101953–101959 RSC.
- H. Ahankar, A. Ramazani, K. Slepokura, T. Lis and S. W. Joo, Green Chem., 2016, 18, 3582–3593 RSC.
- S. Pervaram, D. Ashok, C. V. R. Reddy, M. Sarasija and A. Ganesh, Chem. Data Collect., 2020, 29, 100508 CrossRef CAS.
- A. Dutta, M. A. Rohman, R. Nongrum, A. Thongani, S. Mitra and R. Nongkhlaw, New J. Chem., 2021, 45, 8136–8148 RSC.
- J. Sun, Q. Wu, E. Y. Xia and C. G. Yun, Eur. J. Org. Chem., 2011, 2011, 2981–2986 CrossRef.
- M. Saha and A. R. Das, ChemistrySelect, 2017, 2, 10249–10260 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: All of the experimental details and the spectroscopic details, i.e., spectroscopic data and all the scanned copies of 1H, 13C &19F NMR spectra of the synthesised derivatives. See DOI: https://doi.org/10.1039/d2ra08054k |
| This journal is © The Royal Society of Chemistry 2023 |