 Open Access Article
Open Access ArticleRecent advances in photocatalytic self-cleaning performances of TiO2-based building materials
Yuanchen Wei†
a,
Que Wu†b,
Hong Mengb,
Yongqing Zhang*b and
Changlu Cao *a
*a
aLonghua District Bureau of Public Works of Shenzhen Municipality, Shenzhen 518028, China. E-mail: 896319369@qq.com
bSchool of Environment and Energy, Guangdong Provincial Key Laboratory of Atmospheric Environment and Pollution Control, South China University of Technology, Guangzhou 510006, China. E-mail: zhangyq@scut.edu.cn
First published on 11th July 2023
Abstract
TiO2-based photocatalytic building materials can keep the building surface clean, and have decontamination, antibacterial effects and so on, which greatly reduces the maintenance cost and the risk of cleaning work, and these materials have great application potential in pollution and carbon reduction in the future. However, due to the wide band gap of TiO2, the low utilization of solar energy and the instability of super hydrophilicity have always been the difficulties in the field of photocatalysis. Based on the relevant research of TiO2-based photocatalytic materials in recent years, this review summarizes the modification strategies that can effectively improve the photocatalytic activity of TiO2-based photocatalytic materials. At the same time, the influence of complex environmental factors and substrate properties on the self-cleaning behavior of TiO2-based building materials was analyzed. This paper aims to provide effective guidance for the future application of TiO2-based photocatalysts in the construction field, improve people's understanding of photocatalytic building materials (PBM) and photocatalytic self-cleaning characteristics, and provide more possibilities for the extensive application of photocatalysis technology in the construction field, as well as to promote the realization of global carbon neutrality and other strategic goals of pollution and carbon reduction.
Introduction
Global environmental problems such as global climate change are gradually worsening. In the face of the threat to the environment caused by the continuous large-scale emission of CO2, a series of economic, policy and technical means have been adopted globally to reduce CO2 emissions, trying to slow down global climate change. Recently, a global carbon neutrality initiative has been launched all over the world, and many countries/regions have made detailed descriptions and deployments of relevant technical priorities. There is no doubt that buildings are the main source of carbon emissions and the main end-user energy sector. According to relevant research, the total energy consumption of global buildings and their proportion in end-user energy consumption will also show an increasing trend, along with the growth of buildings and population as well as the improvement of people's living standards. Moreover, building materials will be in direct contact with the human body, and the indoor environment will also be strongly affected by the properties of them.1 So, it is crucial to promote the green and low-carbon development of buildings for achieving net zero carbon or near zero carbon emissions.It is a common phenomenon that the beauty and luster of ordinary architectural surfaces fade over time. Building surfaces can be contaminated by greasy and viscous sediments, resulting in the strong stickiness of environmental dust. Taking fatty acid molecules as an example, their carbide group (–COOH) enables them to adhere to the building surface through chemical bonding with Ca2+ ions in concrete; On the other hand, the long chain of fatty acids is connected with other hydrophobic molecules perpendicular to the surface, causing fat stains to capture many atmospheric particles and dust.2 Therefore, dirt on the surface layer will reduce the visual appearance. Restoring the aesthetic properties of a building is a difficult task without continuous and proper maintenance. However, traditional cleaning processes also bring high costs, large water consumption, labor demand and safety hazards. Building materials with self-cleaning function greatly reduce the cost of cleaning buildings, especially for high-rise buildings and skyscrapers, which are very difficult and expensive to clean and maintain. Self-cleaning building materials greatly reduce resource consumption and have great prospects for future building development. Aiming at the deficiencies of traditional building exterior wall cleaning technology and market demand, the application of self-cleaning building materials provides a solution. Since the discovery of wettability self-cleaning effects on biological surfaces in nature, materials for self-cleaning of architectural surfaces have been extensively developed. The research and development of building materials with self-cleaning property is of great significance in the future urban construction.
As a semiconductor material, titanium dioxide can generate electron–hole pairs through light excitation. Among various excellent semiconductor photocatalysts, titanium dioxide (TiO2) appears to be a promising and important prospect for use in air purification, sewage treatment, antibacterial disinfection, self-cleaning and solid waste treatment due to its excellent properties such as strong oxidizing property, chemical stability, low cost and non-toxicity.3,4 The application of TiO2 in building materials dates back to the early 1990s,5 and photocatalysis and surface wettability of TiO2-based materials endow buildings with ideal self-cleaning surfaces, making them strong candidates for applications in buildings.
Generally, the self-cleaning property of titanium dioxide relies on the synergistic effect of photocatalysis and photo-induced superhydrophilicity. TiO2-based materials usually have anti-bacterial qualities,6 and the introduction of TiO2 will also affect various properties of raw materials, such as mechanical properties, anti-fogging, wear resistance, etc. Since the TiO2 super-hydrophilic coating helps to maintain the initial high solar reflectivity of the building surface/facade, the water evaporates and releases heat when it contacts the building surface, the surface temperature decreases, and the power consumption of air conditioning and refrigeration can be reduced to a certain extent, thereby alleviating the Urban heat island effect. The application can also be extended to parking lots and roads to lower the temperature and cool the environment.1 There are endless photocatalytic self-cleaning products on the market, with various forms of building materials, such as anti-fog glass, ceramic tiles, as well as cement, paints, coatings, etc. Representative products have been used in public/in private construction, automotive glass and pavement engineering, such as self-cleaning ceramics produced by Japan TOTO based on Hydrotech™ technology, Activ™ self-cleaning anti-fog glass from Pilkington, TioCem® and TX Active® pavement materials,7–9 etc.
Despite TiO2 merits, some disadvantages limit its wide-spread utilization. Due to the wide band gap, TiO2 can only absorb in the UV (ultraviolet) region accounting for 4% of the entire solar spectrum, which greatly hinder its application potential. Because of the quick recombination of photo-induced e−/h+ pairs, the photocatalysis ratio of TiO2 is very low. Therefore, noticeable efforts have been made to improve visible-light-harvesting capability and efficient removal of pollutants of TiO2-based photocatalysts. In recent years, the research and discussion on the mechanism of photocatalytic degradation of pollutants and surface wettability based on TiO2 has mainly focused on solving the limitations of its narrow spectral sensitivity range and low quantum efficiency,10,11 in order to ultimately improve the photocatalytic activity of TiO2 in practical applications. Numerous modification methods including nanostructure preparation, noble metal deposition, ion doping, semiconductor recombination, dye sensitization, self-doping, polycrystalline and its mixtures, surface chelation, etc. have been studied and discussed.12–16
The self-cleaning properties and applications of TiO2-based building materials were extensively investigated. Herein, the reaction mechanism of photocatalytic degradation of organic pollutants, as well as the formation and transformation mechanism of photoinduced surface wettability were reviewed. Besides, the progress in modification techniques to effectively improve the photocatalytic efficiency of TiO2 were also summarized. The difficulties and key points of TiO2-based materials in realizing the self-cleaning function of buildings were also introduced. The aim was to provide an effective reference for the development of TiO2-based photocatalytic architecture in the future.
Basic principle of photocatalytic and self-cleaning property
A self-cleaning surface is a surface that keeps itself clean by natural phenomena and avoids manual work, usually achieved by surface hydrophobic or hydrophilic phenomena. Wang R et al. first reported that the self-cleaning properties of TiO2 were attributed to photoinduced super-hydrophilic surfaces.5 Since then, titanium dioxide, a promising semiconductor material for photoinduced wetting, has received much attention. The self-cleaning properties of TiO2 originate from the photoinduced properties under UV irradiation, and the self-cleaning function of these green building materials is mainly achieved by the following two factors (Fig. 1):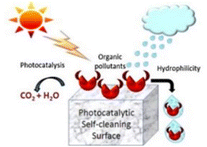 | ||
| Fig. 1 Principle of photocatalytic self-cleaning on TiO2 surface.3 | ||
(1) Adsorption and degradation of organic substrates. TiO2-based photocatalysts utilize sunlight/indoor light to decompose the dirt and other impurities. The pollutants on the building surface mainly come from the complex of atmospheric aerosol pollutants. The particulate matter and organic pollutants in the air are adhered to the surface of the building through organic binders.17 Electron–hole pairs are generated under excitation, and the pollutants adhering to the surface of the material undergo oxidation reactions with oxidizing electrons until the pollutants are decomposed into CO2 and H2O.
(2) Super hydrophilic. Superhydrophilicity is a phenomenon that occurs when TiO2 films exhibit a small water contact angle under UV light irradiation. The water contact angle (θ) is used to measure the surface wettability. The contact angle of the hydrophilic surface is generally less than 90°, while that of the super-hydrophilic surface can reach below 10°, or even almost 0°.18 This surface makes the water tend to spread flat on the surface, forming a uniform water film that can block dirt and organisms, and the surface contaminants can be washed away by the water flow. It is generally believed that the reciprocal of the contact angle is related to the density of the reconstructed hydroxyl groups on a UV-irradiated surface.19 Ultraviolet light irradiation can stimulate the hydrolysis/dissociation and adsorption of molecular water on the surface of TiO2 to achieve surface hydroxylation, thereby forming a super-hydrophilic region on the surface of TiO2. It is generally believed that the reciprocal of the contact angle is related to the density of the reconstructed hydroxyl groups on the UV irradiated surface.
TiO2 possesses both photocatalytic and photoinduced super-hydrophilicity. The application of super-hydrophilic technology is very wide. Take the preparation of antifogging surfaces through super-hydrophilic effect as an example. When humid air condenses, the surfaces of mirrors and glass will fog up, forming many small water droplets, which scatter light. On super-hydrophilic surfaces, no water droplets are formed, conversely, a uniform film of water can be scattered from the surface, which does not scatter light. Its superhydrophilicity also contributes to the self-cleaning process of TiO2, and the macroscopic effect of self-cleaning is actually the combined effect of superhydrophilicity and degradation of organic sediments. Both photocatalysis and superhydrophilicity are photo-activated, but the photochemical processes responsible for the two phenomena are not the same. Although they may act simultaneously on the surface. Therefore, it is difficult to distinguish which mechanism is more significant for self-cleaning process, and it is possible that the synergy of photocatalysis and superhydrophilicity will jointly promote self-cleaning. Furthermore, the adsorption of organic compounds on the membrane surface may lead to the transition of surface properties from hydrophilic to hydrophobic. Photocatalytic decomposition of these organic pollutants can restore the super-hydrophilicity of the surface. Consequently, the synergistic effect of photocatalysis and superhydrophilicity guarantees the self-cleaning properties of TiO2 films.
Photocatalytic degradation of organic pollutants
The adsorption and oxidative decomposition of pollutants attached to the substrate surface by TiO2 under irradiation is one of the important factors for its photocatalytic self-cleaning function. The pollutants attached to the building surface mainly come from the complex of atmospheric aerosol pollutants. The particulate matter and organic pollutants in the air are adhered to the building surface through organic binders.17The mechanism of TiO2 photocatalytic degradation of organic pollutants has been extensively reported in many papers. Generally, under irradiation with radiant light higher than its band gap energy (anatase TiO2 ≥ 3.20 eV), electrons in the valence band (VB) of TiO2 absorb the light energy to be excited and migrate to the conduction band (CB), resulting in an excited state electrons (e−), and leave positron holes (h+) in the valence band, the wavelength of this photon energy usually corresponds to λ < 400 nm, that is, only ultraviolet light of sunlight can be utilized. Furthermore, the formed excited electrons and holes are very unstable. Since photogenerated carriers will undergo multiple changes after being generated, recombination and capture are competing processes, and the excited electrons are easily recombined with the holes,20 Therefore, the trapping process of interface carriers must be rapid.
The unrecombined charge carriers can escape the charge annihilation reaction and migrate to the surface, where the reductive excited electrons (e−) reduce the oxygen adsorbed on the surface to superoxide radicals (˙O2−). And the holes in the valence band can oxidize the water or OH− adsorbed on the surface to generate ˙OH.3 After protonation, superoxide radicals can react with water to generate hydrogen oxide radicals (HO2˙), thereby becoming another source of hydroxyl radicals (Fig. 2). Moreover, surface oxygen can not only inhibit the recombination of photogenerated electrons and holes, but also oxidize intermediates that have been hydroxylated. These generated reactive oxygen species can oxidize the organic pollutants adsorbed on the surface of TiO2, and the final products obtained by complete oxidation are CO2 and H2O, therefrom the photocatalytic process achieves a clean surface (eqn. (1)–(6)).
| e− + O2 → ˙O−2 | (1) |
| h+ + OH− → ˙OH | (2) |
| h+ + H2O → ˙OH | (3) |
| ˙O−2 + H+ → HO2˙ | (4) |
| ˙O−2 + Pollutant → H2O + CO2 | (5) |
| ˙OH + Pollutant → H2O + CO2 | (6) |
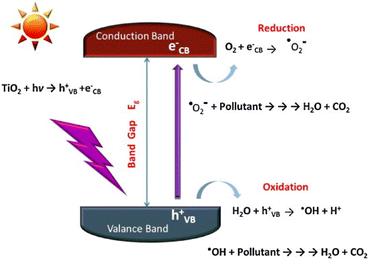 | ||
| Fig. 2 Diagram of reaction mechanism of TiO2 photocatalytic degradation of pollutants.3 | ||
Photoinduced superhydrophilicity
There are several explanations for the mechanism of photoinduced super-hydrophilic surface of TiO2 (ref. 3 and 5) The common mechanisms mainly include the formation of photoinduced surface oxygen vacancies and the photoinduced reconstruction of Ti–OH bonds on TiO2 surface, photocatalytic decomposition of organic adsorbents, and a combination of mechanisms. Generally, TiO2 superhydrophilicity can be regarded as two stages of surface wettability change: (1) the formation of surface oxygen vacancies induced by light; (2) Ti–O–Ti fractures to form Ti–OH bonds, resulting in the increase of surface hydroxyl groups.Owing to the binding energy between Ti and lattice oxygen atoms is weakened by the electrons and holes generated on the surface of TiO2 produced by UV irradiation, the photogenerated electrons are generated under UV light, Ti4+ in the TiO2 lattice is reduced to Ti3+. And holes on the valence band of TiO2 react with surface lattice oxygen ions to generate O2, leaving oxygen defects. Thus, the surface lattice oxygen is released and oxygen vacancies that can be filled by adsorbed water are produced. The vacancies break this bond by dissociating adsorbed water molecules, resulting in Ti–O–Ti being broken by water molecules and forming Ti–OH (Fig. 3), which in turn accelerate hydroxylation forms a super-hydrophilic region on the surface of TiO2.21,22 The hydrophilicity of –OH leads to the fact that when the surface of a building is washed with water, contaminants such as oil stains on the surface can be washed away.23,24
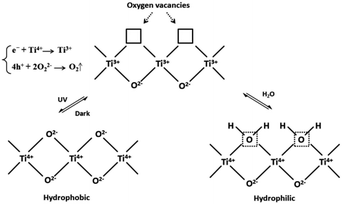 | ||
| Fig. 3 Photoinduced formation of Ti–OH bonds.23 | ||
Strategies of modifying TiO2 to improve the photocatalytic activity
Pure TiO2 photocatalysts suffer from some inherent and well-recognized drawbacks, mainly their low solar utilization and low quantum efficiency. The limitations of current TiO2 materials include high recombination of photogenerated electron–hole pairs and poor light utilization. Due to the relatively large band gap and narrow spectral sensitivity range, TiO2 can only be activated under a small amount of ultraviolet light in sunlight, that is, the utilization rate of solar energy is low. That may increase the additional use of energy-consuming ultraviolet lamps in practical applications, and result in higher purification costs. In addition, as the electron-cavity of sunlight excitation is easy to recombine, the recombination process of photogenerated charge carriers in TiO2 reduces the quantum efficiency of the entire photocatalytic reaction.10 Therefore, the development and application of TiO2 in engineering are largely limited by these defects. Researchers have remediated these limitations by modifying TiO2 to improve the efficiency of photocatalytic degradation.Noble metal deposition
Simply put, deposition of noble metals improves the visible light response of TiO2 through the surface plasmon resonance effect. The main mechanism is to capture photogenerated electrons through the noble metal coated on the surface of TiO2. The light captured by the noble metal forms a strong fluidity, which can be quickly transferred to the surface of TiO2, reducing the recombination rate of photogenerated carriers in TiO2,20 so the photocatalytic activity was improved. The most commonly used deposited precious metal is Pt, followed by Ag, Au, Pd, Cu, etc. When the surface of TiO2 is in contact with these precious metals, the carriers will be redistributed. Since the Fermi level of these precious metals is lower than that of TiO2, photogenerated electrons are efficient effectively transferred from the conduction band of the n-type semiconductor TiO2 with a higher Fermi level to metal particles with a lower Fermi level until their Fermi levels are aligned, while holes remain in TiO2 on the price band.Baba K et al.25 deposited Au and Pt on the TiO2 coating respectively, and obtained coatings with enhanced photocatalytic activity by noble metal deposition. Due to the enhanced electron capture in the noble metal layer, the ultraviolet photoactivity of the nanocomposite coating was enhanced. In comparison, it is found that this electron capture phenomenon of the Pt–TiO2 coating is particularly obvious, because of its larger work function, while Au–TiO2 exhibits a more pronounced visible-light response enhancement. The coating synthesized in their study exhibited higher photocatalytic activity than commercial self-cleaning glass from Pilkington (Activ), so it also shows great potential for water purification or self-cleaning surfaces.
Ion doping
Ion doping is also an effective means to expand the spectral range and improve quantum efficiency, including metal ion doping, non-metal ion doping and multicomponent co-doping. The mechanism by which ion doping affects the photocatalytic efficiency of TiO2 is that it can introduce defect sites in the TiO2 lattice or change its crystalline state, and may even form capture centers, doping energy levels, etc., thereby affecting the recombination of electron–hole pairs; or acting as a traps of electrons or holes, prolong the life-span of the surface carriers; or act as recombination centers of photogenerated carrier, accelerate the recombination of electron–hole pairs, etc.13,26The charge, radius and concentration of metal ions are all important factors affecting the photocatalytic activity of metal-doped TiO2. For example, metal ions with a larger radius than Ti4+ are generally difficult to enter into the nano-TiO2 lattice or interstitial, so generally do not change its unit cell parameters, even if they enter, they will cause lattice expansion. Taking Ag+ as an example,26 due to the formation of Ti–O–Ag tetrahedra and oxygen vacancies, the particle arrangement/the arrangement of endoplasmic points in nano-TiO2 crystal is destroyed, the recombination of photogenerated electrons and holes is inhibited. The chemisorption of hydroxyl oxygen and oxygen vacancies are increased, and the photocatalytic activity of TiO2 is improved. Furthermore, when metal particle dopants have variable valence states, photogenerated electrons or holes can be captured by changing their valence states, so as to facilitate/promote the separation of electron–hole pairs.27 However, some transition metals have been proved to be toxic to a series of pathogenic microbial species, so the possible cytotoxicity of metals remains a problem.14 Some studies have analyzed the effects of the types and doses of doped metals on photocatalytic activity and human health,28,29 but the pity is that the study on the cytotoxicity of titanium dioxide modified with various metals is still not mature. In addition, since the photocatalytic activity depends on the concentration of metal ions, and as with noble metal deposition, metal-ion doped/doping materials bring relatively large manufacturing costs, so it is necessary to find more advantageous alternatives, and metal-doped TiO2 needs further research.
Non-metal doping can cause changes in the energy band structure of TiO2, and orbital hybridization increases the valence band of TiO2, making its band gap smaller, enabling effective visible light activation, and enhancing the adsorption of pollutants. For example, Surface fluorination of TiO2 is to utilize F− to induce the generation of oxygen vacancies, the increase of Ti3+ and surface acidity enhancement to increase the adsorption of water and other polar molecules, resulting in efficient photocatalytic conversion.30 Currently, there have been many studies on the improvement of hydrophilicity and electrical conductivity by nitrogen and carbon doping of TiO2, but the influence of non-metal ion doping on thermal stability and the controversial formation mechanism of doping energy level still needs further research, and the instability of photocatalytic activity and complicated procedures also limit its long-term efficiency in practical applications.
In addition, compared with the single-element doped catalyst system, the synergistic effect of multi-species co-doping enables TiO2 to exhibit better photocatalytic activity. The charge compensation between doping species can improve the mobility of photogenerated carriers and enhance the redox potential to increase the generation of defects. At present, co-doping between metal ions, non-metal elements, and between metal and non-metal has been widely studied and developed.31,32 A. Biswas et al.33 used N, F co-doped TiO2 nanoparticles to modify the surface of cotton, and obtained superhydrophobic cotton, which has the effective performance of light-induced cleaning under visible light illumination. The modified cotton can be used for self-cleaning and the application of visible light-based organic dye degradation and oil-water separation. This modification method can also avoid the use of expensive raw materials. However, the preparation processes of the modification technology of co-doped TiO2 are usually complicated.
Semiconductor materials composition
The construction of compound semiconductors can largely use the synergistic effect of different components to improve the photocatalytic performance of TiO2. In recent years, researches on the compounding of TiO2 and other materials to form binary or ternary compound semiconductors and other materials have been extensively carried out, which is essentially the modification of one particle to another. Based on the heterojunction of TiO2, the coupling of semiconductors with different band gaps27,34 (such as CdS, ZnO, V2O5, WO3, Bi2O3, etc.) and TiO2 can improve the photocatalytic efficiency. The band gap structure of heterojunction formed by TiO2 and low-bandgap semiconductor can lead to efficient spatial separation and transfer of charge carriers, prevent the recombination of photogenerated carriers and enhance the overall photoelectrochemical properties,35 enabling photocatalytic activation of TiO2 under visible light illumination.It has been found that the mixed phase TiO2 of anatase and rutile exhibits higher photocatalytic activity than the pure phase (anatase) TiO2.36 The commercially available TiO2 P25 is composed of 70% anatase and 30% rutile. The heterostructure composed of dual-phase TiO2 causes a mixed crystalline effect, which effectively improves the charge separation efficiency, and the smaller band gap of rutile extends the photoactivation spectrum to the visible light region. SiO2/TiO2 composite films have been widely used to realize the self-cleaning and antireflective functions of material surfaces. Although increasing the volume ratio of TiO2 in the composite film is conducive to enhancing the self-cleaning effect, while higher TiO2 content will reduce the anti-reflective performance. Based on this dilemma, the team of Li K et al.37 developed ultrasound assisted pickling method to effectively prepare well dispersed protonated titanate nanotube colloid. They obtained TiO2 nanotube film with excellent anti reflection and nearly perfect self-cleaning performance (Fig. 4), which proved the feasibility of manufacturing pure TiO2 antireflection coating for glass substrate. In addition, the effective charge separation at the interface of the heterostructure composed of TiO2/Si promotes the change of the surface charge state. This method is widely used to improve the efficiency of photoelectronic systems. The transforming trend of wettability surface can be dominated by controlling the ratio/proportion of photocarriers on TiO2,38 which also provides a reference for the transformation of controllable surface wettability of TiO2.
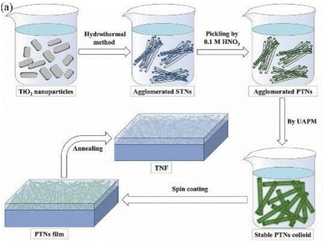 | ||
| Fig. 4 Preparation of self-cleaning nanotube films with high UV absorption and quantum efficiency in the form of stearic acid by ultrasonic assisted pickling.37 | ||
Combining semiconductors with carbon-based materials is also one of the most promising strategies to enhance the visible light response and suppress charge carrier recombination. Among the carbon-based materials composited with TiO2, graphene and its derivatives are inherently active photocatalysts with excellent properties such as high electrical conductivity and high specific surface area.14 The formation of C–Ti bond significantly improves the photocatalytic activity TiO2.39 Graphene is a suitable alternative to noble metals. The self-cleaning surface technology of graphene loaded titanium dioxide is expected to obtain cheap, highly conductive and highly photocatalytic transparent films. This modification method of graphene loading can effectively obtain self-cleaning and anti-fouling surfaces, which is well proved by the highly photocatalytic active GO (x wt%)/TiO2-CA hybrid membranes synthesized via a facile non-solvent induced phase inversion technique.40 Graphene oxide (GO) leads to a red shift of light absorption edge due to its influence on the band gap energy of TiO2, and at the same time generates new hydroxyl and oxygen vacancies, thereby affecting the transfer of TiO2 charge carriers.16 Moreover, the transparent properties of graphene are also recognized as being fully utilized in self-cleaning technologies. And reduced graphene oxide (rGO), which is thought to have the properties of defect-free graphene and GO, has also attracted the attention of researchers. He, H.Y. et al.41 reported a highly transparent rGO-TiO2: Nd nanohybrid film with enhanced self-cleaning properties. The Nd doping and rGO led to further obvious enhancement in hydrophilicity and photo-induced ultrahydrophilicity. Obvious synergistic effect of the rGO and Nd cation in enhancing the self-cleaning properties was observed and mainly ascribed to the decreased particle size and efficient interface-induced, which confirmed by the measurement of band energy level and optical conductivity. However, the detailed mechanism by which rGO achieves the visible light response of TiO2 does not seem to have reached a general consensus. Graphitic carbon nitride (g-C3N4) is also a research hotspot for photocatalytic composites due to its good photocatalytic properties and unique electronic structure.42
Compared with single materials, multifunctional ternary semiconductor composites have wider applications in practical fields. Semiconductors used for composites often have to satisfy the two necessary principles of lattice adaptation and energy band alignment.14 Benefiting from the synergistic coupling effect of the ternary interface realized by various semiconductors, the multifunctional ternary nanocomposites (such as CdS-Mn/MoS2/TiO2, Bi2WO6/TiO2/rGO, etc.) can exhibit excellent photocatalytic activity and good stability,43,44 as well as improved photoelectrochemical performance of photoelectrodes, which also provides a new perspective for exploring low-cost, high-efficiency photocatalytic composites. Of course, the TiO2 semiconductor composite technology still needs to be continuously improved to ensure effective interfacial charge transfer. Controlling the content of defects and impurities is the key, and the improvement of material stability is the basis for ensuring its reusability.14
It is worth mentioning that ALD (atomic layer deposition) is an excellent thin film deposition technology that can uniformly coat and precisely control the thickness of semiconductor thin films, and cover substrates of various shapes. It has huge practical implications for the production of photocatalytic self-cleaning materials.16 Kang et al.45 obtained TiO2 coating with unique fish scale structure on stainless steel mesh through ALD technology, which could decompose oil molecules under UV irradiation and restore the original wettability of the polluted membrane. This superoleophobic and underwater super oleophobic surface shows special self-cleaning performance and high mechanical strength.
Dye photosensitization
Dye photosensitization can make photoactive compounds (organic and inorganic sensitizers) adsorb on the surface of TiO2, which can extend the excitation wavelength and broaden the absorption spectrum of TiO2. This surface modification has been successfully applied to dye-sensitized solar cells (DSSCs) and dye-sensitized photocatalysts (DSPs). Dye sensitization of TiO2 generally involves three basic processes: dye adsorption, the excitation of the adsorbed dye, and the injection of electrons into the conduction band of TiO2 by the excited dye molecules. Dye molecules excite triplet states through visible light absorption, and transfer electrons to electron acceptors, so dyes are oxidized and reactive oxygen species are produced. TiO2 works as an essential electron transfer media in this process.15 Organic sensitizers can effectively change the surface structure of TiO2, but they have always had the disadvantage of low stability and durability. During the photocatalytic reaction, the dye molecules attached to the surface of TiO2 may be desorbed and decomposed, resulting in significant loss of DSP activity. There may also be competitive adsorption between organic pollutants and sensitizers, leading to a very complicated degradation process.46 These are directions for further improvement and innovation in dye sensitization methods.In recent years, the hybridization of organic/inorganic component with specific functions, which can combine some advantages of optimizing organic and inorganic materials, has been recognized as an effective method to obtain ideal new materials. Based on the fact that the sensitizers containing pyridine groups shows better stability than common carboxylates and phosphates on the surface of TiO2, it was found that the four pyridine groups of porous organic-inorganic hybrid material C4BTP-TiO2 can interact with Ti (OC4H9)4 through Ti–N bond to form a cross-linking structure, which greatly improves the stability of doped dyes in the hybrid system.47 This new photocatalyst has more stable structure and better durability, not only with improved separation and transport efficiency of photogenerated carriers, but also with the adsorption capacity and activation degree of the reactants.
Self-doping
Ti3+ self-doping is also considered as an effective method to heighten the solar energy utilization of TiO2. On the one hand, the introduction of Ti3+ can form the impurity energy level in the band gap of TiO2, narrow the band gap and prolong the transport lifetime of photogenerated carriers.48 On the other hand, Ti3+ also forms a site to capture photogenerated electrons by adsorbing molecular oxygen, so as to achieve strong photocatalytic activity under visible light irradiation. Like the generation of oxygen vacancies in the lattice, this modified TiO2 photocatalysts prepared by self-doping has visible light-driven photocatalytic activity, and also avoid the introduction of other impurities. Self-doping can also be carried out in concert with other element-modified modification methods.49Others
Augmenting surface acidity, surface derivatization, and chelation techniques50 can enhance the photocatalytic activity of TiO2. By increasing the number of active sites of TiO2, adsorption of organic pollutants onto the material is intensified, thereby improving its photocatalytic activity. Furthermore, controlling the size and size distribution of nano-sized crystals can improve the dispersion and stability of TiO2 materials, leading to enhanced self-cleaning performance. In brief, various modification techniques can expand the photo-response of TiO2 to visible light, thereby enhancing its photocatalytic activity. Any factor that effectively inhibits the recombination of photogenerated carriers and increases the lifetime of electron–hole pairs can improve the quantum efficiency, thereby significantly enhancing the photocatalytic performance of TiO2. Photocatalysts can be developed at the macroscopic level, and TiO2 can also be modified at the microscopic level through morphology and crystal phase control.Moreover, the latest trend is to develop composite self-cleaning materials through the integration and modification of various eco-friendly technologies. One such example is the incorporation of multiple mechanisms, including the use of TiO2 self-cleaning coating combined with graphene, silver, and Fe additives,51 and the simultaneous deposition of doped TiO2 hybrid materials with Ag,52 etc. Wang et al.53 developed A-SiO2/N-TiO2 composites by incorporating A-SiO2 microspheres and N-TiO2 particles, which were further functionalized with HDTMS and deposited onto a substrate to form a superhydrophobic coating. The coating exhibited self-healing properties and sustained its self-cleaning efficacy even after 3 months of outdoor exposure. Upon UV irradiation, the coating decomposed methyl orange solution and recovered its superhydrophobicity following 8 hours of dark storage. As the self-cleaning efficiency of TiO2 photocatalysis is dependent on its surface wettability, it is essential to comprehensively analyze the distinct impact of each component on the surface properties (including wettability, surface energy, and adhesion) of self-cleaning materials, when multiple factors are involved in the modification of photocatalysis self-cleaning materials. Table 1 presents a partial compilation of studies investigating the correlation between modified catalysts and the relationship between contact angle and performance. The conversion of surface properties can be measured by water contact angle.51
| Photocatalyst | Wettability | Contact angle | Performance | Publication year/literature |
|---|---|---|---|---|
| A-SiO2/N-TiO2 | Superhydrophobic | Water contact angle (WCA) of 157.2° and contact angle hysteresis (CAH) of 2.7° | Methyl orange solution was degraded under UV irradiation, and the coating maintained its self-cleaning performance after being placed outdoors for 3 months | 2021 (ref. 54) |
| Nanocellulose-titanium dioxide-(3-aminopropyl) trimethoxysilane (NCC-TiO2-APTMS) | Superhydrophilic | WCA ≈ 6° | Improved cleaning efficiency of coating, excellent self-cleaning properties after exposure to sunlight | 2022 (ref. 55) |
| SiO2–TiO2 | Superhydrophilic | WCA = 0° | The degradation rate of methyl orange (10 ppm) was more than 98% under UV irradiation for 40 min, and RhB and MB can be degraded efficiently under sunlight irradiation | 2022 (ref. 56) |
| Coating deposited by titanium dioxide and chemically modified by OTS | Superhydrophobic | Static water contact angle of 158° ± 2° and sliding angle of 4° ± 1° | Maintain good performance after UV irradiation, chemical immersion, and physical abrasion; excellent self-cleaning ability after rinsed with water droplets | 2020 (ref. 57) |
| Fluorosilicone/SiO2–TiO2 (FSi/SiO2–TiO2) coating | Superhydrophobic | WCA = 165° ± 8° | The TiO2/SiO2 coatings were optically transparent and achieved a superhydrophilic state after UV irradiation for almost 2 h. Superhydrophilicity is a building block to self-cleaning ability and crucial for reliability | 2021 (ref. 58) |
| Wood modified by titanium dioxide nanoparticles and PFOTS | Superhydrophobic | Static contact angle of more than 150° and tilt angle of less than 10° | Excellent self-cleaning ability, stain resistance property, durability and chemical stability | 2020 (ref. 59) |
| TiO2 nanoparticles and Pluronic F-127 coated glass | Superhydrophilic | WCA = 4.9° ± 0.5° | Great self-cleaning effect against concentrated syrup and methylene blue and great antifog performance that maintains high transparency of around 89% when the coated glass is placed above hot-fog vapor for 10 min | 2022 (ref. 60) |
| Sponge-like TiO2–SiO2 nanoparticles coated glass | Hydrophilic | WCA ≤ 12° | Photocatalytic degradation rate relative to OG up to 7.9%, anti-fogging | 2020 (ref. 61) |
| TiO2-APTES-cotton fabrics | Superhydrophobic | Contact angle of 155° ± 1°, and tilting angle of 6° ± 1° | Good mechanical, chemical, and thermal stability; impart self-cleaning, antifouling, antibacterial, anti-stain, and UV-blocking properties | 2023 (ref. 62) |
| Stearic acid (STA)-TiO2/zinc composite (TZC) coating | Superhydrophobic | The water contact angle of 160 ± 1.4° and sliding angle of 5.4 ± 0.2° | Excellent self-cleaning property, corrosion resistance, and superhydrophobic stability | 2021 (ref. 63) |
| Titanium substrate in NH4F/H3PO4 electrolyte by anodization | Superhydrophobic | Best superhydrophobicity surface with a maximum CA of similar to 170.3° and a SA of approximately 0° | Ultralow adhesion, a long-term stability, excellent self-cleaning effect and good anti-icing property | 2021 (ref. 64) |
| Novel g-C3N4/TiO2/PAA/PTFE ultrafiltration membrane | Hydrophilic | WCA = 62.3° | Highly resistant to fouling in the prolonged filtration of 1000 mg L−1 bovine serum albumin (BSA) solution | 2019 (ref. 65) |
| A-SiO2/N-TiO2@PDMS coating | Superhydrophobic | WCA = 17.5° | Methyl orange solution could be degraded; good adaptation to different substrates and outdoor environments; exhibited the same liquid repellency towards different droplets | 2021 (ref. 66) |
| Titanium dioxide-composited cotton fabrics | Superhydrophobic | WCA = 176.3 ± 1° | Good self-cleaning ability, excellent performance of oil-water separation, photocatalytic, sustainability and stability | 2020 (ref. 67) |
Another effective approach to enhance the photocatalytic activity of TiO2-based photocatalysts is to develop round-the-clock photocatalysts (RCPs) with surface modification, such as template and defect engineering. The RCPs act as photo-induced charge traps, enabling the enhancement of light absorption and the increase in active sites of photocatalysis, while also increasing the storage capacity of electricity to achieve higher photocatalytic activity. Mokhtarifar et al.68 successfully improved the performance of TiO2/WO3 photocatalyst by using soft templates and hydrogen treatment methods, as well as incorporating Pt plasma nanoparticles as a cocatalyst. The composite catalyst demonstrated efficient self-cleaning performance under both sunlight and low-light conditions, by expanding the visible light absorption through the synergy of the composite system (TiO2, WO3 and Pt). After seven days of simulated artificial acid rain experiments, it was observed that TiO2 and TiO2/WO3 films were unable to protect the surface from discoloration and had obvious cracks. This was attributed to the growth of an oxide film as a result of titanium oxidation, which caused further damage to the coatings. On the other hand, the H2:TiO2/WO3@Pt film was able to protect the surface from discoloration and maintain its structural integrity after degradation. This self-cleaning coating has been successfully applied to protect both cultural heritage and modern cultural relics.
Application status and future prospects of TiO2 self-cleaning building materials
Practical application and long-term field test
Table 2 lists some of the research reports on self-cleaning building materials. TiO2 can be effectively integrated with various building materials such as cement board, cement mortar, dolomite, cement sand slurry, calcareous stone, clay brick, glass, and concrete, through some methods, such as mixing, brushing, and spraying, to functionalize the building materials. Visual comparison of the self-cleaning activities of various photocatalysts is challenging due to differences in radiation properties, intensity of light used, radiation time, and other factors. Nonetheless, current research results have confirmed that the application of photocatalysis technology in the construction field can effectively achieve the self-cleaning function of building materials.| Photocatalyst | Building material | Preparation method | Light source | Self-cleaning effect | Publication year/literature |
|---|---|---|---|---|---|
| FeO-P25 | Cement mortar | Mixed use of cement mortar (5 wt% P25, 2–4% iron oxide) | Ultraviolet | RhB is removed by 75% after 18 h | 2013 (ref. 69) |
| P25-SiO2 | Dolomite | Spray P25 silica sol on the stone surface | Ultraviolet | Discoloration of methylene blue (70% removed after 72 h) | 2012 (ref. 70) |
| P25-SiO2 | Mixed cement slurry (7 wt% P25, 15 wt% silica) | Mixed cement slurry (7 wt% P25, 15 wt% silica) | Ultraviolet | Pyronin Y is removed by 90% after 3.5 h | 2014 (ref. 71) |
| Original commercial P25 | Cement sand [ash] slurry | Mix with cement mortar (5 wt%) | Ultraviolet | RhB is removed by 65% after 26 h | 2015 (ref. 72) |
| Ag-SiO2-P25 | Stone | Spraying silver-modified P25-SiO2 sol on stone surface | Ultraviolet | Remove 50% of MB after 72 h | 2015 (ref. 73) |
| Original P25 | Calcareous calculus | Spray P25 sol on the stone surface | Sunlight | RhB is removed by 90% after 7.5 h | 2016 (ref. 74) |
| P25-Ag | Clay brick | Spray photocatalyst sol on brick surface | Ultraviolet | Methylene blue is removed by 45% after 26 h | 2016 (ref. 75) |
| Au-TiO2/SiO2 | Kapuli limestone | Spray catalyst on the material surface | Xenon arc lamp 300–800 nm | The removal rate of MB reaches 100% in 90 minutes | 2019 (ref. 76) |
| WO@TiO2 | P25 (80% anatase and 20% rutile) | The prepared coating precursor is directly sprayed on the substrate and cured at room temperature for 48 h to obtain MWT coating | Ultravioletand visible light | Decreased by 11.74% and 12.05% respectively under ultraviolet and visible light | 2021 (ref. 77) |
| N-doped TiO2/SWCNT | Glass | Brush the modified photocatalytic material on the surface of glass material | Visible light | The highest degradation efficiency of methylene blue is 72.43% | 2019 (ref. 78) |
| TiO2 | Concrete | Using internal doping method (IDM) and spraying method (SPM) to combine photocatalysis mixed crystal nano-TiO2 particles with concrete | Ultraviolet | Methyl orange has the maximum photocatalytic degradation efficiency of 73.82%. The photocatalytic degradation efficiency of unpolished nano-TiO2 concrete is much higher than that of polished nano-TiO2 concrete | 2020 (ref. 79) |
| Cu-TiO2/SiO2 | Fiberglass reinforced concrete (GFRC) board | Cu(I)–TiO2 NPs mixed slurry is prepared by impregnation method, and added to silica oligomer, and the synthetic sol is sprayed on the white concrete surface | The photocatalyst containing 5% copper has the highest efficiency, and the maximum damage to methylene blue and soot within 60 min and 168 h of irradiation is 95% and 50% respectively. Higher copper load leads to lower performance | 2021 (ref. 80) |
With the expansion of research in this area, the scope of photocatalysis self-cleaning characteristics in architecture is broadening, including building exteriors, road construction projects, and the protection of architectural cultural heritage. Commercial products of photocatalytic self-cleaning, such as self-cleaning glass, ceramic tiles, fabrics, cement, and paint, have emerged in the construction market. Numerous demonstration projects have confirmed the feasibility of applying photocatalytic self-cleaning materials to the construction industry, such as the self-cleaning window glass of Marunouchi building in Tokyo,81 the self-cleaning dome glass of the National Opera House of China82 (Fig. 5), etc., More information regarding the application cases of photocatalytic self-cleaning building materials can be found in Table 3.
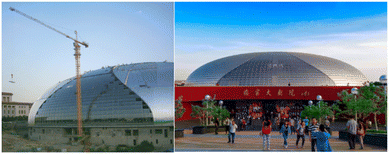 | ||
| Fig. 5 Self-cleaning dome glass of China National Opera House under construction and appearance drawing.82 | ||
| Type of building materials | Catalyst used | Application/product case |
|---|---|---|
| Self-cleaning glass | TiO2 photocatalytic coating | Type B public buildings, such as self-cleaning window glass of Marunouchi building in Tokyo,81 self-cleaning dome glass of the National Opera House of China,82 etc. Activ™ self-cleaning anti-fog glass from Pilkington, UK,83 etc. |
| Self-cleaning concrete and cement | TiO2 NPs | The exterior wall of the building, such as Misericordia Church in Roma,84 the Italian Pavilion of the World Expo in Milan, Italy;81 TioCem® and TX active® pavement materials,85 etc. |
| Self-cleaning pavement materials (cement, concrete blocks, etc.) | TiO2 NPs | Large-scale application in road field test, such as Hengelo Castorweg Street,81 Leopold II tunnel in Brussels,86 Asphalt pavement of Wenxing Tunnel in Xiamen, China87 |
| Self-cleaning hanging plate | TiO2 photocatalytic coating | KMEW photocatalytic photoceramic Cladding, Japan |
| Self-cleaning paint and paint | TiO2 NPs | Self-cleaning binder and paint produced by Japan TOTO based on Hydrotech™ technology.88 |
H. Oladipoa et al.7 tested the Pilkington activity of glass with automatic cleaning function C (PAC) and Pilkington activity. Photocatalytic performance of B (PAB), and the energy saving potential of different glass configurations of PAB and PAC is simulated by building energy modeling. The results show that the self-cleaning glass has a large specific surface area, high TiO2 content, and high reactivity under ultraviolet light. The simulation results show that the PAB of double-layer glass is the most energy-saving, has the comprehensive ability to promote building energy conservation, and its ability to reduce VOC.
In addition, jubilee church Dives in Misericordiade built with photocatalysis concrete in 2003 has been paid attention to since its service period began. After six years of continuous monitoring, only the external and internal values of the luminosity are slightly different, and the color change of the surface plate caused by inorganic substances can be completely eliminated by water cleaning.89
A. Chabas et al.90 evaluated the aging degree and self-cleaning performance of self-cleaning glass materials exposed in urban environment for eight years in combination with previous work experience. They found that the affinity and super hydrophilicity of TiO2 coated glass to water vapor were not significantly reduced in a short time, and the transparency and wettability of the glass were not affected by the exposure conditions (no shielding). However, after a long time of exposure (from 12 months to 100 months of experimental period), without manual cleaning, the cleaning efficiency of the photocatalysis glass is reduced due to the negative feedback of sediment aging and super-waterness on the photocatalysis efficiency, and the transparency of the final glass is also reduced after a long time of exposure.
Therefore, although there are cases of photocatalytic coatings that can maintain super hydrophilicity and self-cleaning activity after long-term exposure, demonstrating the stability of photocatalytic self-cleaning, it is doubtful whether self-cleaning materials can maintain lasting activity in complex environments and completely replace manual cleaning.
Future perspective and related problem discussions
In the ever-changing external environment, the durability of performance is a crucial factor in the practical application of photocatalytic self-cleaning materials in the construction industry. Currently, most self-cleaning products focus on short-term benefits and the instantaneous self-cleaning effect achieved through light induction for buildings, while ignoring long-term stability. In reality, the self-cleaning performance of photocatalysis falls short of expectations when exposed to complex and harsh environments. In order to achieve large-scale industrial application, it is essential to prioritize the review of photocatalyst materials that possess both high efficiency and long life. Consequently, the following efforts must be made.Decreasing the cost of photocatalytic functional building materials
Currently, glass and ceramic are the most commonly used substrates for TiO2 due to their resistance to high calcination temperatures and strong adhesion properties. After high temperature treatment, the photocatalytic coating on ceramic tiles and glass is typically stable and permanent. However, organic building materials are unable to withstand the high calcination temperature required to anchor the photocatalytic layer. Furthermore, organic building materials are often decomposed by the photocatalytic reaction, resulting in a reduction in photocatalytic activity and destruction of the structure and strength. As a result, an intermediate layer must be placed between the organic material and the photocatalytic material, significantly increasing the manufacturing difficulty and economic cost. Moreover, for large-scale construction using photocatalytic building materials, the effects of acid rain and heavy rainfall must be considered. Over time, the TiO2 coating may become thinner, making it essential for designers to comprehensively consider the balance between performance, cost, and risk.Developing the TiO2 based building materials with high catalytic activity and stability
The complex surface characteristics of porous building materials is one of the key reasons why TiO2 coatings cannot adhere to the substrate throughout its entire service life. Thus, the quality of the TiO2 coating is critical for achieving long-term stability of its photocatalytic self-cleaning properties. Long-term field exposure tests have shown that changes in outdoor environment and human destruction inevitably lead to the loss of TiO2 photocatalyst. The aging of the substrate (when TiO2 is used as a coating) or the substrate itself (when TiO2 is used for mixing) is a major factor affecting the long-term stability of TiO2 photocatalyst self-cleaning characteristics. Effective conversion of surface wettability can be achieved through the control of hydrophobic and hydrophilic components, irradiation and other means, as well as the control and maintenance of surface wettability. However, achieving the reversibility of this wettability transformation over a longer period of time is the key challenge. Furthermore, maintaining a smooth and even surface during the reapplication process remains difficult. Additionally, due to the transparency of the coating, it is not possible to check its efficiency, especially on high-rise buildings. These are important considerations that designers must take into account.Utilizing artificial intelligence, machine learning, and big data to assist in developing novel photocatalytic building materials
In the future, the development and application of TiO2 based photocatalysts will continue to focus on improving their photocatalytic performance. The combination of machine learning, big data, and artificial intelligence may accelerate the discovery and optimization of new photocatalytic materials. Theoretical calculations can help predict the properties of new materials before synthesis, saving time and resources. The integration of machine learning, big data, and artificial intelligence may also provide a new approach for high-throughput screening of photocatalytic materials. However, the use of machine learning, artificial intelligence, big data and density functional theory auxiliary development of titanium dioxide-based building materials and its performance prediction research rarely reported, therefore, the future for using machine learning, artificial intelligence, big data and density functional theory auxiliary development stable and excellent performance of photocatalytic building materials also need more efforts and research.Reasonable installation and design
The development of self-cleaning materials involves the design of TiO2 coatings in various application scenarios. In particular, for sunshades or photovoltaic solar panels, the location and equipment pose unique challenges that must be considered. The shape of buildings can also impact the performance of self-cleaning materials, as irregular rain flow can negatively affect photocatalytic activity. To mitigate these issues, designers must carefully consider the location and shape of the building in their design. For instance, the surface wettability of the self-cleaning material must be adjusted according to the specific location and shape of the building to achieve optimal performance. Additionally, factors such as the intensity and direction of sunlight and rainfall must be taken into account to ensure effective self-cleaning. Furthermore, it is important to select appropriate additives and modification techniques to enhance the stability of the TiO2 coating under different environmental conditions. Through the careful consideration of these factors in the design process, self-cleaning materials can be effectively utilized in various applications, providing sustainable and eco-friendly solutions to modern architectural challenges.Improve relevant laws and regulations
The general public still lacks a full understanding of the effectiveness and durability of TiO2 coating performance. Many users may not be aware of the limitations of TiO2 coating, such as its ability to only perform the self-cleaning function on organic dirt but not on large particles. Moreover, many studies merely evaluate the self-cleaning performance by the removal effect of common dyes, like methylene blue, and the change in surface color, which fails to reflect the actual performance of materials. In reality, pollution in the environment is often more complex. S. Khannyra et al.91 tend to use soot to evaluate the self-cleaning effect of their TiO2/SiO2 samples, because it is the real pollutant of building exterior walls in urban areas. To formulate and implement legal standards, it is necessary to issue updated and comprehensive evaluation standards for the self-cleaning characteristics of such materials. Strict requirements should be set for the performance of coating technology or processing products to further ensure the effectiveness of TiO2 self-cleaning in practical applications. Incorporating factors like simulated climate change and man-made destruction into material performance testing and evaluation, integrating the concept of maintainability into design, and determining the design criteria for maintainability of TiO2 coatings will ensure the quality of these green technologies in design, installation, and maintenance practices.Conclusions
The self-cleaning performance of TiO2 is generally considered to be a synergism effect of photocatalytic degradation and photo-induced super-hydrophilicity. The mechanism of photocatalytic degradation of pollutants has been reported in many papers, and the acquisition and transformation of surface super-hydrophilicity and hydrophobicity have also been discussed. However, the relevant mechanisms have not yet formed a unified consensus, and there is a lack of complete evaluation systems to accurately measure the surface wettability in practical applications. Self-cleaning surfaces resulting from the strong photocatalytic activity of titanium dioxide and light-induced superhydrophilicity seem to have attracted wider attention. In contrast, there are not many articles that deal with superhydrophobic self-cleaning activity of TiO2.In order to obtain high photocatalytic activity, in recent years, activities have focused on the research of TiO2 modification methods including noble metal deposition, ion-doping modification, semiconductor coupling, dye sensitization, self-doping and nano-sized crystals, etc. The focus is mainly on solving the limitations of low visible light utilization and low quantum efficiency of TiO2. However, other aspects such as adsorption and surface superhydrophilicity have not been fully considered. In addition, reversible photo-controlled surfaces with reversible hydrophilicity and hydrophobicity are also one of the research fields of TiO2 photocatalytic self-cleaning applications.
Although there are existing outdoor monitoring schemes to evaluate the self-cleaning property of building materials, a large number of environmental factors and the complexity of substrate properties involved in the process bring challenges for the monitoring on real building surface. Therefore, further research on the proportion, process and other conditions of TiO2 photocatalytic self-cleaning materials in practical applications, as well as the evaluation and improvement of the self-cleaning effect of different substrates, the long-term stability and durability of coatings, are the issues that need to be further tackled in the large-scale application of photocatalytic self-cleaning materials.
Author contributions
All authors verify their contribution to the current review article as follows: design, study conception, and supervision of the whole article is done by Yuanchen Wei and Yongqing Zhang; data collection is done by Xiaoyu Bai, Que Wu, Hong Meng, and Yuqi Liu; analysis and interpretation of results by Xiaoyu Bai, Que Wu and Hong Meng; manuscript preparation and proofreading by Xiaoyu Bai, Que Wu, Yuanchen Wei and Yongqing Zhang.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2016YFC0400708), Guangdong Basic and Applied Basic Research Foundation, China (2019A1515011761) and the Foundation of Longhua District Bureau of Public Works of Shenzhen Municipality, Shenzhen, China.Notes and references
- A. I. Gopalan, J. C. Lee, G. Saianand, K. P. Lee, P. Sonar, R. Dharmarajan, Y. L. Hou, K. Y. Ann, V. Kannan and W. J. Kim, Nanomaterials, 2020, 10 Search PubMed.
- L. Peruchon, E. Puzenat, A. Girard-Egrot, L. Blum, J. M. Herrmann and C. Guillard, J. Photochem. Photobiol., A, 2008, 197, 170–176 CrossRef CAS.
- S. Banerjee, D. D. Dionysiou and S. C. Pillai, Appl. Catal., B, 2015, 176, 396–428 CrossRef.
- N. Rahimi, R. A. Pax and E. M. Gray, Prog. Solid State Chem., 2016, 44, 86–105 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Nature, 1997, 388, 431–432 CrossRef CAS.
- F. Hamidi and F. Aslani, Nanomaterials, 2019, 9, 1444 CrossRef CAS PubMed.
- H. Oladipo, C. Garlisi, K. Al-Ali, E. Azar and G. Palmisano, J. Environ. Chem. Eng., 2019, 7, 102980 CrossRef CAS.
- A. Mills, A. Lepre, N. Elliott, S. Bhopal, I. P. Parkin and S. A. O'Neill, J. Photochem. Photobiol., A, 2003, 160, 213–224 CrossRef CAS.
- A. A. Bawono, Z. H. Tan, A. H. Hamdany, N. NguyenDinh, S. Z. Qian, B. Lechner and E. H. Yang, Cem. Concr. Compos., 2020, 114 Search PubMed.
- V. Etacheri, C. Di Valentin, J. Schneider, D. Bahnemann and S. C. Pillai, J. Photochem. Photobiol., C, 2015, 25, 1–29 CrossRef CAS.
- H. R. Dong, G. M. Zeng, L. Tang, C. Z. Fan, C. Zhang, X. X. He and Y. He, Water Res., 2015, 79, 128–146 CrossRef CAS PubMed.
- A.-I. Gopalan, J.-C. Lee, G. Saianand, K.-P. Lee, P. Sonar, R. Dharmarajan, Y.-l. Hou, K.-Y. Ann, V. Kannan and W.-J. Kim, Nanomaterials, 2020, 10, 1854 CrossRef CAS PubMed.
- N. U. Saqib, R. Adnan and I. Shah, Environ. Sci. Pollut. Res., 2016, 23, 15941–15951 CrossRef CAS PubMed.
- X. Du, J. P. Luo, Q. S. Qin, J. H. Zhang and D. Fu, Catal. Surv. Asia, 2022, 26, 16–34 CrossRef CAS.
- E. T. Yun, H. Y. Yoo, W. Kim, H. E. Kim, G. Kang, H. Lee, S. Lee, T. Park, C. Lee, J. H. Kim and J. Lee, Appl. Catal., B, 2017, 203, 475–484 CrossRef CAS.
- N. Justh, T. Firkala, K. Laszlo, J. Labar and I. M. Szilagyi, Appl. Surf. Sci., 2017, 419, 497–502 CrossRef CAS.
- L. Peruchon, E. Puzenat, A. Girard-Egrot, L. Blum, J. M. Herrmann and C. Guillard, J. Photochem. Photobiol., A, 2008, 197, 170–176 CrossRef CAS.
- X. F. Luo, Z. P. Zhu, Y. Tian, J. You and L. Jiang, Catalysts, 2021, 11 CAS.
- K. Hashimoto, et al., J. Phys. Chem. B, 2003, 107, 1028–1035 CrossRef.
- L. Q. Jing, Y. C. Qu, B. Q. Wang, S. D. Li, B. J. Jiang, L. B. Yang, W. Fu, H. G. Fu and J. Z. Sun, Sol. Energy Mater. Sol. Cells, 2006, 90, 1773–1787 CrossRef CAS.
- C. Garlisi, G. Scandura, G. Palmisano, M. Chiesa and C. Y. Lai, Langmuir, 2016, 32, 11813–11818 CrossRef CAS PubMed.
- Y. Takata, S. Hidaka, J. M. Cao, T. Nakamura, H. Yamamoto, M. Masuda and T. Ito, Energy, 2005, 30, 209–220 CrossRef CAS.
- T.P. Nisha and H. John, J.Environ.Chem.Eng., 2020, 8(5), 1041211 Search PubMed.
- R. W. Wang, K. H. Hashimoto, A. F. Fujishima, M. C. Chikuni, E. K. Kojima, A. K. Kitamura, M. S. Shimohigoshi and T. W. Watanabe, Nature, 1997, 388, 31 Search PubMed.
- K. Baba, S. Bulou, M. Quesada-Gonzalez, S. Bonot, D. Collard, N. D. Boscher and P. Choquet, ACS Appl. Mater. Interfaces, 2017, 9, 41200–41209 CrossRef CAS PubMed.
- Y. W. Ren, S. Xing, J. Wang, Y. Liang, D. Y. Zhao, H. L. Wang, N. Wang, W. W. Jiang, S. M. Wu, S. M. Liu, C. Q. Liu, W. Y. Ding, Z. H. Zhang, J. F. Pang and C. Dong, Opt. Mater., 2022, 124 Search PubMed.
- H. Khan, Z. R. Jiang and D. Berk, Sol. Energy, 2018, 162, 420–430 CrossRef CAS.
- A. Malankowska, A. Mikolajczyk, J. Medrzycka, I. Wysocka, G. Nowaczyk, M. Jarek, T. Puzyn and E. Mulkiewicz, Environ. Sci.: Nano, 2020, 7, 3557–3574 RSC.
- A. Nel, T. Xia, L. Madler and N. Li, Science, 2006, 311, 622–627 CrossRef CAS PubMed.
- N. S. Leyland, J. Podporska-Carroll, J. Browne, S. J. Hinder, B. Quilty and S. C. Pillai, Sci. Rep., 2016, 6, 24770 CrossRef CAS PubMed.
- Y. Q. Bu, L. Zhang, D. W. Ma and F. Zhuge, Catal. Lett., 2021, 151, 2396–2407 CrossRef CAS.
- T. D. Pham and B. K. Lee, Appl. Catal., A, 2017, 529, 40–48 CrossRef CAS.
- A. Biswas and N. R. Jana, ACS Appl. Nano Mater., 2021, 4, 877–885 CrossRef CAS.
- M. Endo-Kimura, M. Janczarek, Z. Bielan, Z. Dong, W. Kunlei, A. Markowska-Szczupak and E. Kowalska, ChemEngineering, 2019, 3(18), 13–18 Search PubMed.
- M. Tavakolian, K. Keshavarz and M. Hosseini-Sarvari, Mol. Catal., 2021, 514 Search PubMed.
- V. Etacheri, M. K. Seery, S. J. Hinder and S. C. Pillai, Inorg. Chem., 2012, 51, 7164–7173 CrossRef CAS PubMed.
- K. Li, M. Li, C. Xu, Z. Du, J. Chen, F. Zou, C. Zou, S. Xu and G. Li, J. Mater. Sci. Technol., 2021, 88, 11–20 CrossRef CAS.
- A. V. Rudakova, A. V. Emeline, A. I. Romanychev and D. W. Bahnemann, J. Alloys Compd., 2021, 872 Search PubMed.
- Z. J. Shi, M. G. Ma and J. F. Zhu, Catalysts, 2019, 9, 20 CrossRef.
- R. Khurram, A. Javed, R. H. Ke, C. Lena and Z. Wang, Nanomaterials, 2021, 11 Search PubMed.
- H. Y. He, Part. Sci. Technol., 2019, 605–611 CAS.
- Y. H. Li, Y. J. Sun, F. Dong and W. K. Ho, J. Colloid Interface Sci., 2014, 436, 29–36 CrossRef CAS PubMed.
- L. W. Ji, B. J. Liu, Y. J. Qian, Q. Yang and P. Gao, Adv. Powder Technol., 2020, 31, 128–138 CrossRef CAS.
- X. Li, Y. Li, H. J. Wang, H. Y. Miao, H. Y. Zhu, X. F. Liu, H. B. Lin and G. Shi, ACS Appl. Energy Mater., 2021, 4, 730–736 CrossRef CAS.
- H. J. Kang, Y. Sun, Y. Li, W. Qin and X. H. Wu, Ind. Eng. Chem. Res., 2020, 59, 21088–21096 CrossRef CAS.
- E. Can, B. Uralcan and R. Yildirim, ACS Appl. Energy Mater., 2021, 4, 10931–10939 CrossRef CAS.
- Y. H. Zhong, Y. Lei, J. F. Huang, L. M. Xiao, X. L. Chen, T. Luo, S. Qin, J. Guo and J. M. Liu, J. Mater. Chem. A, 2020, 8, 8883–8891 RSC.
- D. N. Zhang, X. Y. Ma, H. W. Zhang, Y. L. Liao and Q. J. Xiang, Mater. Today Energy, 2018, 10, 132–140 CrossRef.
- J. T. Zhang, M. M. Yuan, X. Liu, X. Y. Wang, S. R. Liu, B. Han, B. K. Liu and H. Z. Shi, Chem. Eng. J., 2020, 387 CAS.
- L. G. C. Rego, R. da Silva, J. A. Freire, R. C. Snoeberger and V. S. Batista, J. Phys. Chem. C, 2010, 114, 1317–1325 CrossRef CAS.
- R. A. Prawira and D. Ariyanti, Mater. Today: Proc., 2022, 63, S214–S221 CAS.
- L. Yang, Q. Sang, J. Du, M. Yang, X. Li, Y. Shen, X. Han, X. Jiang and B. Zhao, Phys. Chem. Chem. Phys., 2018, 20, 15149–15157 RSC.
- X. Wang, H. Ding, C. Wang, R. Zhou, Y. Li, W. Li and W. Ao, Appl. Surf. Sci., 2021, 567, 150808 CrossRef CAS.
- X. Wang, H. Ding, C. H. Wang, R. Zhou, Y. Z. Li, W. Li and W. H. Ao, Appl. Surf. Sci., 2021, 567 CrossRef CAS.
- P. Y. Borse, S. U. Mestry and S. T. Mhaske, Polym. Bull., 2022, 79, 9371–9395 CrossRef CAS.
- X. Wang, H. Ding, G. C. Lv, R. Zhou, R. X. Ma, X. F. Hou, J. M. Zhang and W. Li, Ceram. Int., 2022, 48, 20033–20040 CrossRef CAS.
- N. Pratiwi, Z. Zulhadjri, S. Arief, A. Admi and D. V. Wellia, J. Sol-Gel Sci. Technol., 2020, 96, 669–678 CrossRef CAS.
- L. J. Zong, Y. P. Wu, X. G. Li and B. Jiang, J. Coat. Technol. Res., 2021, 18, 1245–1259 CrossRef CAS.
- S. K. Pandit, B. K. Tudu, I. M. Mishra and A. Kumar, Prog. Org. Coat., 2020, 139 Search PubMed.
- A. Syafiq, V. Balakrishnan and N. A. Abd Rahim, Pigm. Resin Technol., 2022, 8 DOI:10.1108/prt-06-2022-0080.
- M. Boutamart, S. Briche, K. Nouneh, S. Rafqah and Y. Agzenai, Chemistryselect, 2020, 5, 8522–8531 CrossRef CAS.
- N. Ahmad, S. Rasheed, K. Ahmed, S. G. Musharraf, M. Najam-ul-Haq and D. Hussain, Sep. Purif. Technol., 2023, 306 Search PubMed.
- C. A. B. Hu, X. Y. Xie and K. N. Ren, J. Alloys Compd., 2021, 882 Search PubMed.
- G. X. Xiang, S. Y. Li and B. H. Ma, Surf. Coat. Technol., 2021, 423 Search PubMed.
- L. N. Chi, Y. J. Qian, J. Q. Guo, X. Z. Wang, H. Arandiyan and Z. Jiang, Catal. Today, 2019, 335, 527–537 CrossRef CAS.
- X. Wang, W. H. Ao, S. J. Sun, H. Zhang, R. Zhou, Y. Z. Li, J. Wang and H. Ding, Nanomaterials, 2021, 11 Search PubMed.
- T. T. He, H. Y. Zhao, Y. H. Liu, C. K. Zhao, L. Y. Wang, H. Y. Wang, Y. Zhao and H. Wang, Colloids Surf., A, 2020, 585 Search PubMed.
- M. Mokhtarifar, D. T. Nguyen, M. Sakar, A. Lucotti, M. Asa, R. Kaveh, M. V. Diamanti, M. Pedeferri and T.-O. Do, J. Environ. Chem. Eng., 2022, 10, 106891 CrossRef CAS.
- M. V. Diamanti, B. Del Curto, M. Ormellese and M. P. Pedeferri, Constr. Build. Mater., 2013, 46, 167–174 CrossRef.
- L. Pinho, F. Elhaddad, D. S. Facio and M. J. Mosquera, Appl. Surf. Sci., 2013, 275, 389–396 CrossRef CAS.
- A. A. Essawy and S. Abd El Aleem, Constr. Build. Mater., 2014, 52, 1–8 CrossRef.
- M.-Z. Guo, A. Maury-Ramirez and C. S. Poon, Build. Environ., 2015, 94, 395–402 CrossRef.
- L. Pinho, M. Rojas and M. J. Mosquera, Appl. Catal., B, 2015, 178, 144–154 CrossRef CAS.
- A. Calia, M. Lettieri and M. Masieri, Build. Environ., 2016, 110, 1–10 CrossRef.
- L. Graziani, E. Quagliarini and M. D'Orazio, Constr. Build. Mater., 2016, 129, 116–124 CrossRef CAS.
- M. Luna, M. J. Mosquera, H. Vidal and J. M. Gatica, Build. Environ., 2019, 164 Search PubMed.
- G. Liu, H. Xia, Y. Niu, X. Zhao, G. Zhang, L. Song and H. Chen, Chem. Eng. J., 2021, 409 Search PubMed.
- J. S. Appasamy and J. C. Kurnia, IOP Conf. Ser.: Earth Environ. Sci., 2019, 268, 012049 CrossRef.
- Z. Guo, C. Huang and Y. Chen, Nanotechnol. Rev., 2020, 9, 219–229 CrossRef CAS.
- S. Khannyra, M. J. Mosquera, M. Addou and M. L. A. Gil, Constr. Build. Mater., 2021, 313, 125419 CrossRef CAS.
- R. Paolini, D. Borroni, M. Pedeferri and M. V. Diamanti, Constr. Build. Mater., 2018, 182, 126–133 CrossRef CAS.
- C. L. Bai, Science, 2005, 309, 61–63 CrossRef CAS PubMed.
- A. Mills, A. Lepre, N. Elliott, S. Bhopal, I. P. Parkin and S. A. O'Neill, J. Photochem. Photobiol., A, 2003, 160, 213–224 CrossRef CAS.
- L. Cassar, MRS Bull., 2004, 29, 328–331 CrossRef CAS.
- A. A. Bawono, Z. H. Tan, A. H. Hamdany, N. NguyenDinh, S. Qian, B. Lechner and E.-H. Yang, Cem. Concr. Compos., 2020, 114, 103788 CrossRef CAS.
- E. Boonen, V. Akylas, F. Barmpas, L. Bottalico, A. Boreave, M. Cazaunau, H. Chen, V. Daele, T. De Marco, J. F. Doussin, C. Gaimoz, M. Gallus, C. George, N. Grand, B. Grosselin, G. L. Guerrini, H. Herrmann, S. Ifang, J. Kleffmann, R. Kurtenbach, M. Maille, G. Manganelli, A. Mellouki, K. Miet, F. Mothes, N. Moussiopoulos, L. Poulain, R. Rabe, P. Zapf and A. Beeldens, J. Environ. Manage., 2015, 155, 136–144 CrossRef CAS PubMed.
- W. Liu, S. Y. Wang, J. Zhang and J. F. Fan, Constr. Build. Mater., 2015, 81, 224–232 CrossRef.
- H. Oladipo, C. Garlisi, K. Al-Ali, E. Azar and G. Palmisano, J. Environ. Chem. Eng., 2019, 7, 102980 CrossRef CAS.
- J. Chen and C.-s. Poon, Build. Environ., 2009, 44, 1899–1906 CrossRef.
- A. Chabas, S. Alfaro, T. Lombardo, A. Verney-Carron, E. Da Silva, S. Triquet, H. Cachier and E. Leroy, Build. Environ., 2014, 79, 57–65 CrossRef.
- S. Khannyra, M. Luna, M. L. A. Gil, M. Addou and M. J. Mosquera, Build. Environ., 2022, 211, 108743 CrossRef.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |
