Open Access Article
Open Access ArticleImpact of aza-substitutions on the preference of helix handedness for β-peptide oligomers: a DFT study†
Hae Sook Parka and
Young Kee Kang *b
*b
aDepartment of Nursing, Cheju Halla University, Cheju 63092, Republic of Korea
bDepartment of Chemistry, Chungbuk National University, Cheongju, Chungbuk 28644, Republic of Korea. E-mail: ykkang@chungbuk.ac.kr
First published on 19th January 2023
Abstract
We studied the helix preference of the heterochiral pentamers of cis-2-aminocyclohexanecarboxylic acid (c-ACHC) and cis-2-aminocyclopentanecarboxylic acid (c-ACPC) with alternating backbone configurations by replacing Cβ-aza- or Cα-aza-peptide residues using DFT methods in solution. The helix-handedness preferences of two pentamers were strongly affected by the replacement positions (i.e., chiralities) but not depending on the solvent polarity.
Azapeptides are peptide analogues in which the CHα or CHβ groups of the backbone of α- or β-amino acid residues were replaced by a nitrogen atom. There have been considerable attempts in syntheses and applications of azapeptides to improve biological potencies.1–5 Two kinds of β-peptide analogues can be generated by the aza-replacement in the CHβ or CHα groups of the backbone, which are Cβ-aza-peptide (3-aza-β2-peptide or α-Nα-hydrazino) or Cα-aza-peptide (2-aza- β3-peptide or ureidopeptide) residues, respectively (Fig. 1).6,7
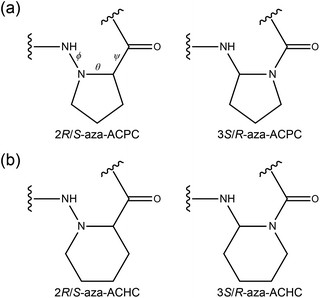 | ||
| Fig. 1 Chemical structures of aza-β-peptide residues: (a) Cβ-aza- (left) and Cα-aza-peptide (right) residues of cis-ACPC and (b) Cβ-aza- (left) and Cα-aza-peptide (right) residues of cis-ACHC. | ||
In particular, the isosteric substitution of the aza group in the backbone is known to affect the type and strength of H-bonds in helical foldamers of β-peptides.6–13 Homochiral oligomers of trans-2-aminocyclobutanecarboxylic acid (t-ACBC) adopted a left-handed (M)-12-helix conformation both in solution and in the solid state.8 However, the preferred conformation of oligomers of t-ACBC was switched into the (M)-8-helix structure, when one or two t-ACBC residues were replaced by the aza-substituted t-ACBC at the CHα group [i.e., N-aminoazetidine-2-carboxylic acid (AAzC) residue] not depending on the position.9–11
In the case of heterochiral oligomers of cis-2-aminocyclopentanecarboxylic acid (c-ACPC) with alternating backbone configurations, i.e., H-[(1S,2R)-ACPC-(1R,2S)-ACPC]3-NH2, the dominant conformation was a right-handed (P)-H10/12 helix in CD3OH, DMSO-d6, and water.12 However, the oligomer with same configurations was switched into a left-handed (M)-H12/10 helix by replacing the (1R,2S)-ACPC residue with the 2S-aza-ACPC residue (i.e., Cβ-aza-peptide) at every even positions in the same solvents.13
We studied the helix preference of the heterochiral pentamer of cis-2-aminocyclohexanecarboxylic acid (c-ACHC) with alternating backbone configurations, i.e., tBuO-(1S,2R)-ACHC-[(1R,2S)-ACHC-(1S,2R)-ACHC]2-NHMe, in solution.14 The dominant conformation was a left-handed (M)-H12/10 helix in solution but the population of the right-handed (P)-H10/12 helix somewhat increased as the increase of solvent polarity.
However, no report is available to study the helix preference of (i) heterochiral oligomers of c-ACHC by replacing Cβ-aza- or Cα-aza-peptide residues and (ii) heterochiral oligomers of c-ACPC by replacing Cα-aza-peptide residue. Here, we studied the helix preference of the heterochiral pentamers of c-ACHC and c-ACPC with alternating backbone configurations by replacing Cβ-aza- or Cα-aza-peptide residues (Fig. 1) depending on the position and solvent polarity using DFT methods in solution.
In this work, the helix preference was investigated for two heterochiral pentamers of c-ACHC and c-ACPC with alternating backbone configurations, i.e., Ac-(1S,2R)-ACHC-[(1R,2S)-ACHC-(1S,2R)-ACHC]2-NHMe (1) and Ac-(1S,2R)-ACPC-[(1R,2S)-ACPC-(1S,2R)-ACPC]2-NHMe (2).
All DFT calculations were performed using the M06-2X,15 functional implemented in the Gaussian 09 program.16 The geometry optimizations were carried out at the M06-2X/6-31+G(d) level of theory and followed by single-point energy (Esp) calculations at the M06-2X/def2-TZVP level of theory. The M06-2X/6-31+G(d) level of theory exhibited good performance against the observed rotational constants of the most stable conformer of Ac-Ala-NHMe with RMSD = 0.9 MHz.17 The M06-2X/def2-TZVP//M06-2X/6-31+G(d) level of theory well reproduced the relative CCSD(T)/CBS-limit energies of local minima of Ac-Ala-NHMe and Ac-Pro-NHMe (ref. 17) with RMSD = 0.10 and 0.08 kcal mol−1, respectively. The solvation free energies (ΔGs) were calculated at the M06-2X/6-311G(d,p) level of theory using the implicit PCM18 solvation method in chloroform, acetonitrile, DMSO, and water. The population of each helix was estimated using the relative energy (ΔEs) obtained by the sum of ΔEsp and ΔΔGs at 25 °C in solution. Recently, the M06-2X/def2-TZVP//M06-2X/6-31+G(d) level of theory with the PCM method appeared to be appropriate in predicting the conformational preferences and the cis–trans isomerization of the longer peptides containing Pro or Pro derivatives in chloroform.19
The structures of (M)- and (P)-helices of cis-ACHC pentamer 1 optimized at the M06-2X/6-31G(d) level of theory14 were used to generate the initial structures of cis-ACPC pentamer 2 and the corresponding both pentamer analogues containing aza-residues. Aza-containing analogues of pentamers 1 and 2 considered here were constructed by aza-substitutions only at even or odd positions to investigate the positional dependence. This is because the residues at even or odd positions have the same chiralities. GaussView20 was used in constructing the initial structures for optimization. All helices of pentamer 1 and 2 and their aza-analogues were reoptimized at the M06-2X/6-31+G(d) level of theory.
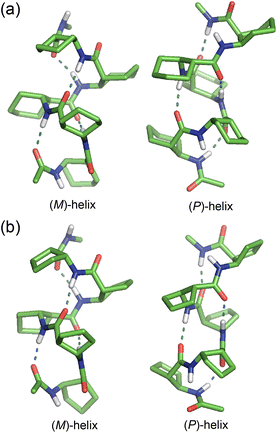
The optimized structures of c-ACHC pentamer 1 and c-ACPC pentamer 2 are shown in Fig. 2. In the (M)-H12/10 helix of pentamer 1, there were two C12 H-bonds between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (i − 1) and H–N (i + 2) with the distances 2.94 and 2.90 Å; and two C10 H-bonds between H–N (i) and O
O (i − 1) and H–N (i + 2) with the distances 2.94 and 2.90 Å; and two C10 H-bonds between H–N (i) and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (i +1) with the distances 2.97 and 2.98 Å. The (P)-H10/12 helix of pentamer 1 also had two C10 H-bonds with the distances 2.90 and 2.93 Å; and two C12 H-bonds with the distances 2.93 and 2.90 Å. The C10 H-bonds were somewhat shorter in the (P)-helix than those in the (M)-helix. In both (M)- and (P)-helices of pentamer 2, the types of H-bonds were quite similar to those of pentamer 1. However, both C12 and C10 H-bonds became about 0.05 Å shorter in pentamer 2 than those in pentamer 1. Because the types and distances of H-bonds were similar in both (M)- and (P)-helices of aza-substituted pentamers 1 and 2, no further comparison was made in detail. The optimized structures of (M)- and (P)-helices of both pentamer analogues containing aza-residues are shown in Fig. S1–S4 of ESI.† The corresponding relative conformational energies, and relative solvation free energies, and absolute electronic energies are shown in Tables S1 and S2 of ESI.† The Cartesian coordinates of all optimized structures are also listed in ESI.†
C (i +1) with the distances 2.97 and 2.98 Å. The (P)-H10/12 helix of pentamer 1 also had two C10 H-bonds with the distances 2.90 and 2.93 Å; and two C12 H-bonds with the distances 2.93 and 2.90 Å. The C10 H-bonds were somewhat shorter in the (P)-helix than those in the (M)-helix. In both (M)- and (P)-helices of pentamer 2, the types of H-bonds were quite similar to those of pentamer 1. However, both C12 and C10 H-bonds became about 0.05 Å shorter in pentamer 2 than those in pentamer 1. Because the types and distances of H-bonds were similar in both (M)- and (P)-helices of aza-substituted pentamers 1 and 2, no further comparison was made in detail. The optimized structures of (M)- and (P)-helices of both pentamer analogues containing aza-residues are shown in Fig. S1–S4 of ESI.† The corresponding relative conformational energies, and relative solvation free energies, and absolute electronic energies are shown in Tables S1 and S2 of ESI.† The Cartesian coordinates of all optimized structures are also listed in ESI.†
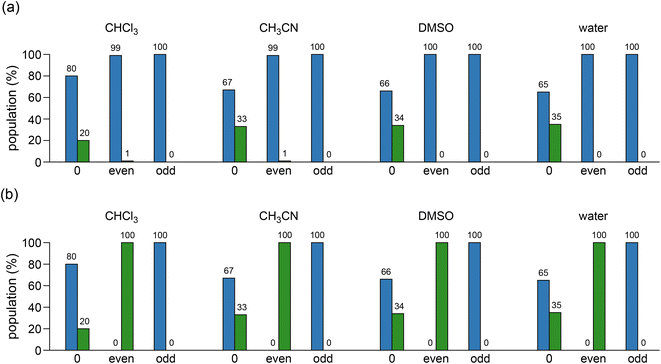
The relative populations of (M)- and (P)-helices for c-ACHC pentamer 1 and their aza-substituted analogues were shown in Fig. 3. The relative populations of (M)- and (P)-helices for pentamers 1 (shown in the first column of each set of histograms in Fig. 3a and b) were calculated as 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 67
20 and 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 in chloroform and acetonitrile, respectively, which are consistent with the observed values of 80
33 in chloroform and acetonitrile, respectively, which are consistent with the observed values of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and 63
20 and 63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37, respectively.14 There was somewhat the increase of the population of the right-handed (P)-helix as the increase of solvent polarity. However, there were dramatic shifts from (P)-helix to (M)-helix for pentamer 1 when either even or odd residues with (1R,2S) or (1S,2R) configurations, respectively, were replaced by Cβ-aza residues in all four solvents. However, the replacements by Cα-aza residues at even or odd positions resulted in only (P)- or (M)-helix, respectively in all solvents.
37, respectively.14 There was somewhat the increase of the population of the right-handed (P)-helix as the increase of solvent polarity. However, there were dramatic shifts from (P)-helix to (M)-helix for pentamer 1 when either even or odd residues with (1R,2S) or (1S,2R) configurations, respectively, were replaced by Cβ-aza residues in all four solvents. However, the replacements by Cα-aza residues at even or odd positions resulted in only (P)- or (M)-helix, respectively in all solvents.
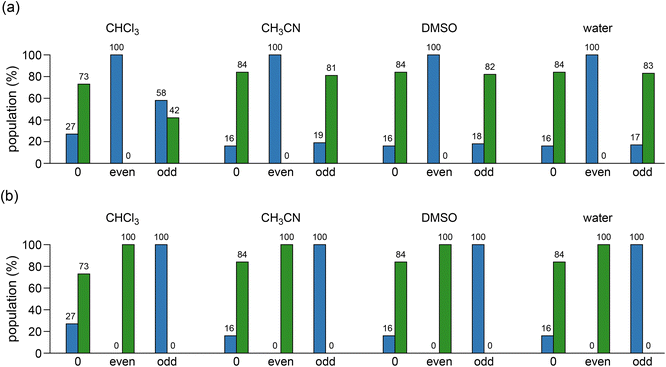
Fig. 4 depicted the relative populations of (M)- and (P)-helices for c-ACPC pentamer 2 and their aza-substituted analogues. The (P)-H10/12 helix of pentamer 2 was preferred by 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 (shown in the first column of each set of histograms in Fig. 4a and b), which is consistent with the experimental results in CD3OH, DMSO-d6, and water,12 its population was somewhat increased in acetonitrile, DMSO, and water (Fig. 4). The dramatic shifts of (P)-helix to (M)-helix were found for pentamer 2 when even residues were replaced by Cβ-aza residues in all four solvents, whereas the replacements at odd residues retained the preference of the (P)-helix in acetonitrile, DMSO, and water but some decrease in its population in chloroform. However, the replacements by Cα-aza residues at even or odd positions for pentamer 2 resulted in only (P)- or (M)-helix, respectively in all solvents, as the same as for pentamer 1.
27 (shown in the first column of each set of histograms in Fig. 4a and b), which is consistent with the experimental results in CD3OH, DMSO-d6, and water,12 its population was somewhat increased in acetonitrile, DMSO, and water (Fig. 4). The dramatic shifts of (P)-helix to (M)-helix were found for pentamer 2 when even residues were replaced by Cβ-aza residues in all four solvents, whereas the replacements at odd residues retained the preference of the (P)-helix in acetonitrile, DMSO, and water but some decrease in its population in chloroform. However, the replacements by Cα-aza residues at even or odd positions for pentamer 2 resulted in only (P)- or (M)-helix, respectively in all solvents, as the same as for pentamer 1.
In summary, the replacement of Cβ-aza residues brought c-ACHC pentamer 1 to only the (M)-helix not depending on the position (i.e., chirality) and the solvent polarity, whereas the helix-handedness preference of c-ACPC pentamer 2 was affected by the replacements at even or odd positions independent of the solvent polarity. However, the replacements by Cα-aza residues at even positions or odd positions resulted in only (P)- or (M)-helix, respectively, for both pentamers 1 and 2 not depending on the solvent polarity. Hence, the results obtained in this work would provide useful structural information for the design of bioactive helical β-peptides with either left- or right-handedness.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2020R1I1A3053400).References
- T. A. Martinek and F. Fülöp, Chem. Soc. Rev., 2012, 41, 687 RSC.
- I. Avan, C. D. Hall and A. R. Katritzky, Chem. Soc. Rev., 2014, 43, 3575 RSC.
- R. Chingle, C. Proulx and W. D. Lubell, Acc. Chem. Res., 2017, 50, 1541 CrossRef CAS PubMed.
- A. Begum, D. Sujatha, K. V. S. R. G. Prasad and K. Bharathi, Asian J. Chem., 2017, 29, 1879 CrossRef CAS.
- K. Tarchoun, M. Yousef and Z. Bánóczi, Future Pharm., 2022, 2, 293 CrossRef.
- L. Fischer, C. Didierjean, F. Jolibois, V. Semetey, J. M. Lozano, J.-P. Briand, M. Marraud, R. Poteau and G. Guichard, Org. Biomol. Chem., 2008, 6, 2596 RSC.
- R.-O. Moussodia, E. Romero, E. Wenger, B. Jamart-Grégoire and S. Acherar, J. Org. Chem., 2017, 82, 9937 CrossRef CAS PubMed.
- C. Fernandes, S. Faure, E. Pereira, V. Théry, V. Declerck, R. Guillot and D. J. Aitken, Org. Lett., 2010, 12, 3606 CrossRef CAS PubMed.
- A. Altmayer-Henzien, V. Declerck, J. Farjon, D. Merlet, R. Guillot and D. J. Aitken, Angew. Chem., Int. Ed., 2015, 54, 10807 CrossRef CAS PubMed.
- V. Declerck and D. J. Aitken, J. Org. Chem., 2018, 83, 8793 CrossRef CAS PubMed.
- Z. Imani, R. Guillot, V. Declerck and D. J. Aitken, J. Org. Chem., 2020, 85, 6165 CrossRef CAS PubMed.
- T. A. Martinek, I. M. Mándity, L. Fülöp, G. K. Tóth, E. Vass, M. Hollósi, E. Forroó and F. Fülöp, J. Am. Chem. Soc., 2006, 128, 13539 CrossRef CAS PubMed.
- A. Hetényi, G. K. Tóth, C. Somlai, E. Vass, T. A. Martinek and F. Fülöp, Chem.–Eur. J., 2009, 15, 10736 CrossRef PubMed.
- S. Shin, M. Lee, I. A. Guzei, Y. K. Kang and S. H. Choi, J. Am. Chem. Soc., 2016, 138, 13390 CrossRef CAS PubMed.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09 (Revision D.01), Gaussian, Inc., Wallingford, CT, 2013 Search PubMed.
- H. S. Park and Y. K. Kang, Chem. Phys. Lett., 2014, 600, 112 CrossRef.
- B. Mennucci, R. Cammi and J. Tomasi, J. Chem. Phys., 1999, 110, 6858 CrossRef CAS.
- H. S. Park and Y. K. Kang, New J. Chem., 2019, 43, 17159 RSC.
- R. D. Dennington II, T. A. Keith and J. Millam, GaussView (Version 6.0), Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07575j |
| This journal is © The Royal Society of Chemistry 2023 |