Open Access Article
Open Access ArticleCytotoxicity profiling of choline chloride-based natural deep eutectic solvents†
Boris M. Popović *a,
Nevenka Gligorijevićb,
Sandra Aranđelovićb,
Ana Catarina Macedocd,
Tatjana Jurić
*a,
Nevenka Gligorijevićb,
Sandra Aranđelovićb,
Ana Catarina Macedocd,
Tatjana Jurić a,
Denis Uka
a,
Denis Uka a,
Karolina Mocko-Blažek
a,
Karolina Mocko-Blažek a and
Ana Teresa Serracd
a and
Ana Teresa Serracd
aChemistry and Biochemistry Laboratory, Department of Field and Vegetable Crops, Faculty of Agriculture, University of Novi Sad, Trg Dositeja Obradovića 8, 21000 Novi Sad, Serbia. E-mail: boris.popovic@polj.uns.ac.rs; Fax: +381 21 450 857; Tel: +381 21 485 3424
bDepartment of Experimental Oncology, Institute for Oncology and Radiology of Serbia, Pasterova 14, 11000 Belgrade, Serbia
ciBET, Instituto de Biologia Experimental e Tecnológica, Avenida da República, Quinta do Marquês, 2780-157 Oeiras, Portugal
dInstituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa (ITQB NOVA), Avenida da República, Quinta do Marquês, 2780-157 Oeiras, Portugal
First published on 25th January 2023
Abstract
This study aims to examine in detail for the first time the cytotoxic profile of twelve choline chloride-based deep eutectic solvents (NADES) against HT-29, Caco-2, MCF-7, and MRC-5 cell lines. All NADES systems were synthesized by microwave synthesis using choline chloride as a hydrogen bond acceptor (HBA) and selected sugars, alcohols, organic acids, and urea as hydrogen bond donors (HBD) with the addition of 20% water (w/w) to all systems. It was observed that the cytotoxic effect predominantly depended on the structure of HBD. Acidic systems, where HBDs were organic acids showed the highest cytotoxic effects in all investigated cell lines. The cytotoxicity depended mostly on the concentration of the NADES system in the cell medium as well as on the chemical constitution of the investigated systems. The highest cytotoxic effects showed acidic systems, especially to the HT-29 cell line. The EC50 value for the citric acid-based system was 3.91 mg mL−1 for the HT-29 cell line which was the most vulnerable to acidic NADES systems.
Introduction
In recent years, eco-friendly practices in applied chemistry and technology have engaged the attention of the scientific community. The growing global trend in modern research toward decreasing the use of hazardous and toxic solvents and processes is should lead to the design of an alternative that will follow the principles of green chemistry. In the last couple of decades, a generation of innovative, ecologically benign, recyclable, and economically viable liquids named natural deep eutectic solvents (NADES) has been developed.1Considering the physicochemical properties of NADES (decreased melting point, low volatility, non-flammable nature, adjustable viscosity), the origin of their constituents (they are often a complex of two or more cellular metabolites), and their versatile properties and application (a selective extraction media for both polar and non-polar compounds, solubilizing agents, electrochemical reagents), these mixtures are regarded as a greener strategy to avoid organic solvents that are traditionally applied for such purposes.2
NADES systems meet the major principles of green chemistry including natural character and low toxicity of the ingredients, and no waste is generated upon their production.3 In comparison with other designer solvents such as ionic liquids, NADES are much more cost-effective, since the raw materials have a lower cost, and their synthesis is fast and simple without any by-products obtained in the preparation.3,4
NADES exhibit a wide spectrum of bioactivities, including antioxidant, anticancer, and antimicrobial activities.5 They also have been regarded as promising mixtures for pharmaceutical industries, particularly because of their potential to enhance the solubility, penetration as well as absorption of drugs even better than water.6 Moreover, the beneficial features of NADES to act as a drug delivery vehicle have been introduced in a recent study.7 The improved dissolution and enhanced bioavailability of poorly-soluble compounds nominate NADES as an advantageous strategy to overcome the weakness of the current pharmaceutical and food industries. An essential function of natural DES as a liquid phase in biological systems has recently been demonstrated, and it involves the solubilization of metabolites and macromolecules that are poorly soluble in water.8 Deep eutectic solvents show specific interactions with biological macromolecules mainly phospholipids, proteins, nucleic acids, and polysaccharides. However, water must be added to eutectic mixes with care, as it tends to disrupt the DES' network and hence alter its characteristics.8
Even though NADES are composed of naturally occurring substances, i.e. primary metabolites that are assumed to be non-toxic, the safety of eutectic mixtures for the application in the mentioned areas should not be taken a priori. To date, scarce studies performed to exhibit the toxicological profile of eutectics, so the majority of NADES systems are not sufficiently explored.9 Due to the unlimited molar combination of components forming NADES10 and the increased number of research related to the application of NADES in drug formulation, the evaluation of their toxicological nature is urgently needed. There are just a few publications related to the cytotoxicity of selected NADES systems against HT-29, MCF-7, HCT-16, HeLa, and HaCaT cell lines.11–14 Nevertheless, it was found that the cytotoxicity of NADES was much higher than their components.15
This investigation aimed to explore the cytotoxic profile of twelve choline chloride-based NADES systems with different hydrogen bond donors including organic acids, sugars, alcohols, and urea to evaluate the effect of HBD structure on the cytotoxic activity. In the investigation, three human cancer cell lines HT-29, MCF-7, and Caco-2, and one normal MRC-5 were used.
Experimental
Chemicals
The following materials were obtained from Alfa Aesar: choline chloride (ChCl; >98% purity), L-malic acid (MalA; 99%), 1,2-propanediol (1,2PD; >98%), 1,3-propanediol (1,3PD; 99%), 1,3-butanediol (1,3BD; 99%), and 1,4-butanediol (1,4BD; 99%). Centrohem supplied citric acid monohydrate (CitA; >99.5%). (Stara Pazova, Serbia). D-Fructose (Fru; 99%) and L-(+)-lactic acid (80% solution in water) were obtained from Lach-Ner (Neratovice, Czech Republic). Merck provided urea (Ur; 99.5%) and L-ascorbic acid (AscA; >99.5%) (Darmstadt, Germany). Sigma Aldrich (St. Louis, MO, USA) provided sorbitol (Sor; 98%), whereas Beta Hem provided glycerol (Gly; >99.5%) (Beograd, Serbia).Cell lines
The human tumor breast adenocarcinoma cells MCF-7 and normal fibroblast cells, MRC-5 were maintained in the Roswell Park Memorial Institute (RPMI) 1640 nutrient medium (Sigma Chemicals Co., USA) supplemented with penicillin (100 IU mL−1), streptomycin (200 μg mL−1), 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES) (25 mM), L-glutamine (3 mM), and 10% of heat-inactivated fetal bovine serum (FBS). The human colorectal adenocarcinoma cells HT-29 (ATCC, USA) and Caco-2 (DSMZ, Germany) were cultured, respectively, in RPMI 1640 medium (Gibco, UK) supplemented with 10% FBS (Biowest, USA) and Dulbecco's Modified Eagle Medium (DMEM; Gibco, USA) supplemented with 1% non-essential amino acids (NEAA; Biowest, USA), 10% FBS and 1% (v/v) penicillin–streptomycin solution (Gibco, USA).The cells were routinely maintained as monolayers in 75 cm2 culture flasks and incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Preparation of NADES
The microwave irradiation approach was used to produce twelve eutectic systems.16,17 Except for ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AscA, all NADES systems were generated by combining ChCl in a 1
AscA, all NADES systems were generated by combining ChCl in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with various HBDs. The molar ratio of choline chloride to ascorbic acid (ChCl
1 molar ratio with various HBDs. The molar ratio of choline chloride to ascorbic acid (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AscA) that produced a stable eutectic system that was transparent at room temperature was 2
AscA) that produced a stable eutectic system that was transparent at room temperature was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Each combination of HBA
1. Each combination of HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD was diluted with 20% water (w/w), and the glass bottles were put in a household microwave oven (Bosch, model AM817ALS) at 180 W for 40 seconds, or until a clear and transparent liquid was formed. To minimize overheating the combination and probable NADES breakdown, a brief gap in the irradiation process was inserted to cool the mixture. For NADES preparation purified water by reverse osmosis system (AquaSana, Novi Sad, Serbia) was used, with a conductivity of fewer than 2 μS cm−1 was used.
HBD was diluted with 20% water (w/w), and the glass bottles were put in a household microwave oven (Bosch, model AM817ALS) at 180 W for 40 seconds, or until a clear and transparent liquid was formed. To minimize overheating the combination and probable NADES breakdown, a brief gap in the irradiation process was inserted to cool the mixture. For NADES preparation purified water by reverse osmosis system (AquaSana, Novi Sad, Serbia) was used, with a conductivity of fewer than 2 μS cm−1 was used.
Cytotoxicity assay
Cytotoxicity assays were performed using confluent and non-differentiated colon adenocarcinoma cells Caco-2 and HT-29, breast adenocarcinoma cells MCF-7 and normal human fibroblast cells MRC-5. Caco-2 cells were seeded at a density of 2 × 104 cells per well in 96-well plates and allowed to grow for 7 days with medium renewal at day 3 after seeding. At day 7, (complete confluence), Caco-2 cells were incubated with different concentrations of the solvents diluted in a culture medium (DMEM with 0.5% FBS and 1% NEAA). Human tumor cells HT-29, MCF-7, and normal fibroblast MRC-5 cells were cultured in 96-well microplates at a density of 1 × 104 cells per well. After 24 h of incubation at 37 °C in 5% CO2, cells were exposed to different concentrations of the solvents diluted in a culture medium (RPMI with 0.5% FBS).The tested concentrations of NADES systems or DMSO ranged between 0.063 and 15% (v/v), according to the solvents assessed.
After 48 h of incubation at 37 °C with 5% CO2 in a humidified atmosphere, the cell viability was assessed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, USA) containing the MTS reagent according to the manufacturer's instructions. Absorbance was measured at 490 nm using a microplate spectrophotometer. Results were expressed in terms of the percentage (%) of cell viability relative to control (cells grown in culture medium only). The EC50 values represent concentration (volume ration) of the mixture causing 50% growth inhibition, and were determined from dose–response curves.
Morphological analysis
The morphology of cells was monitored using an inverted microscope (Carl Zeiss, Jena, Germany, magnification 103.75-fold) equipped with a digital camera (Olimpus, USA). For that purpose, human tumor cells Caco-2, HT-29, MCF-7, and non-tumor cells MRC-5, were seeded into 6-well plates (Thermo Scientific Nunc™), and following 24 h of growth, exposed to the tested NADES systems. Following 48 h of treatment, cells were visualized.Statistical analysis
All experiments were made in triplicate and the results were expressed with the mean ± standard deviation.Results and discussion
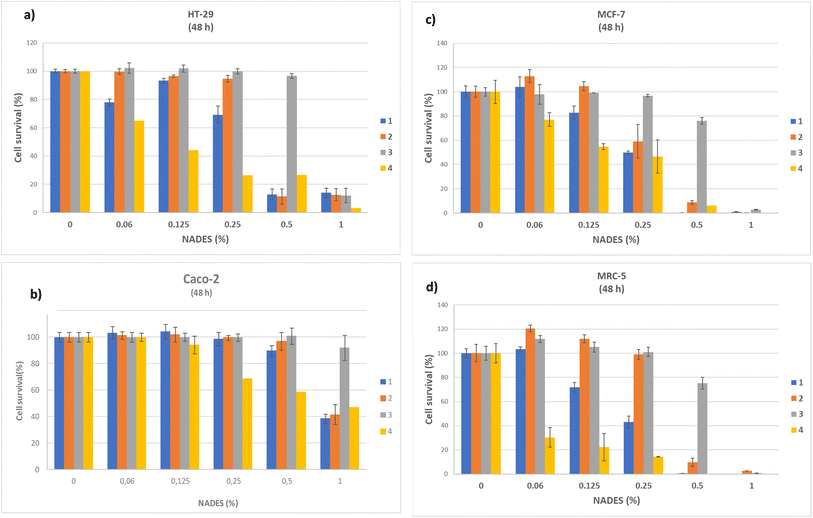
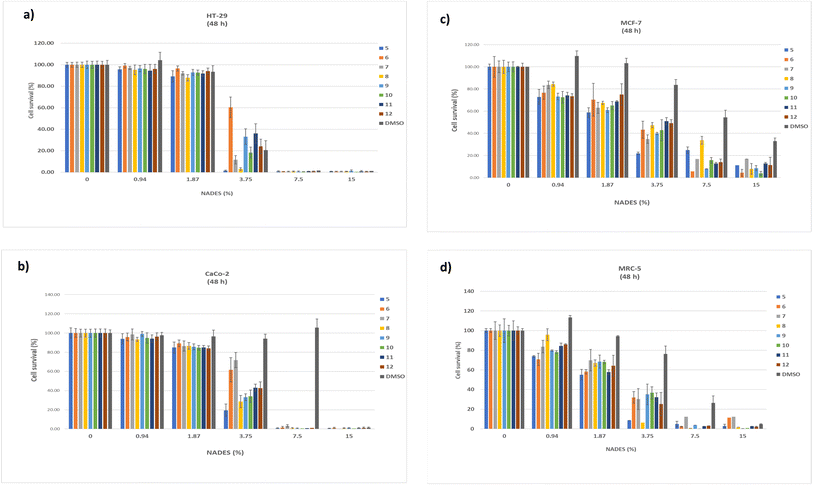
The results related to the cytotoxic effects of twelve NADES systems and comparison to DMSO as control are presented in Fig. 1 and 2, dose–response curves are presented in Fig. S2,† and calculated EC50 values are presented in Table 1. All prepared NADES systems were previously analyzed by thermogravimetric analysis (TGA) to determine water content after MW synthesis and experimental values of water content and molar ratios of prepared systems were presented in the publication Jurić et al. (2021).17| No. | NADES system | EC50 (%) ± S.D. | |||
|---|---|---|---|---|---|
| Caco-2 | HT-29 | MCF-7 | MRC-5 | ||
| a Results expressed in mg mL−1 are given in the ESI.b EC50 values for ascorbic acid-based system were not reproducible, and therefore EC50 was not presented in the table. | |||||
| 1 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA(1:1) CitA(1:1) |
0.88 ± 0.03 | 0.31 ± 0.02 | 0.44 ± 0.10 | 0.67 ± 0.03 |
| 2 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MalA(1:1) MalA(1:1) |
0.94 ± 0.03 | 0.37 ± 0.03 | 0.48 ± 0.09 | 0.44 ± 0.17 |
| 3 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LacA(1:1) LacA(1:1) |
2.90 ± 0.99 | 0.77 ± 0.04 | 1.13 ± 0.20 | 0.86 ± 0.03 |
| 4b | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AscA(2:1) AscA(2:1) |
n.d. | n.d. | n.d. | n.d. |
| 5 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ur(1:1) Ur(1:1) |
2.74 ± 0.10 | 2.37 ± 0.09 | 1.94 ± 0.12 | 1.93 ± 0.10 |
| 6 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly(1:1) Gly(1:1) |
4.09 ± 0.17 | 4.00 ± 0.10 | 2.76 ± 0.12 | 2.10 ± 0.20 |
| 7 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fru(1:1) Fru(1:1) |
4.42 ± 0.28 | 2.74 ± 0.05 | 2.45 ± 0.15 | 2.61 ± 0.40 |
| 8 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sor(1:1) Sor(1:1) |
2.97 ± 0.09 | 2.42 ± 0.09 | 3.42 ± 0.02 | 2.13 ± 0.16 |
| 9 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3PD(1:1) 1,3PD(1:1) |
3.08 ± 0.06 | 3.23 ± 0.09 | 2.34 ± 0.09 | 2.73 ± 0.03 |
| 10 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2PD(1:1) 1,2PD(1:1) |
3.07 ± 0.11 | 2.90 ± 0.07 | 2.96 ± 0.31 | 2.67 ± 0.10 |
| 11 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3BD(1:1) 1,3BD(1:1) |
3.33 ± 0.12 | 3.28 ± 0.12 | 2.66 ± 0.10 | 2.44 ± 0.11 |
| 12 | ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4BD(1:1) 1,4BD(1:1) |
3.31 ± 0.12 | 3.06 ± 0.09 | 3.05 ± 0.33 | 2.36 ± 0.22 |
| 13 | DMSO | ∼13.26 | 2.98 ± 0.13 | 2.34 ± 0.30 | 2.73 ± 0.28 |
MTS assay showed that tested NADES systems exhibited certain antiproliferative effects which varied depending on the tumor cell line and at the same time exerted mostly reduced toxicity towards non-tumor MRC-5 cells, compared to the control (DMSO). Human fibroblast MRC-5 cells were used as a model system for in vitro toxicity evaluation.
It can be generally stated that the first four systems, which contain acids, showed a significantly higher cytotoxic effect on all examined cell lines. For this reason, a concentration range of 0–1% (v/v) was chosen for these systems. Sample 3 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LacA(1:1)) didn't reach EC50 value with concentrations lower than 1%, so the concentration range was widened up to 7.5% and the results are presented in Fig. S1.† Other NADES systems, in which HBD is from the class of sugar, alcohol, or urea, showed significantly lower cytotoxicity, so a concentration range of up to 15% was chosen.
LacA(1:1)) didn't reach EC50 value with concentrations lower than 1%, so the concentration range was widened up to 7.5% and the results are presented in Fig. S1.† Other NADES systems, in which HBD is from the class of sugar, alcohol, or urea, showed significantly lower cytotoxicity, so a concentration range of up to 15% was chosen.
For all cell lines EC50 value was calculated and expressed in % v/v (Table 1) and recalculated in mg mL−1 (Table S1†). For Caco-2, EC50 ranged from 11.09 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA) – 55.69 mg mL−1 (ChCl
CitA) – 55.69 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fru), for HT-29: from 3.91 mg mL−1 (ChCl
Fru), for HT-29: from 3.91 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA) – 45.60 mg mL−1 (ChCl
CitA) – 45.60 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly), for MCF-7 from 5.54 mg mL−1 (ChCl
Gly), for MCF-7 from 5.54 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA) – 42.07 mg mL−1 (ChCl
CitA) – 42.07 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sor) and MRC-5 cell line, from 5.23 mg mL−1 (ChCl
Sor) and MRC-5 cell line, from 5.23 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MalA) – 30.17 mg mL−1 (ChCl
MalA) – 30.17 mg mL−1 (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,3PD). DMSO, a widely used solvent, was used for the comparison and it was significantly less toxic to Caco-2, MCF-7, and MRC-5, compared with NADES solvents. However, the toxicity of DMSO to HT-29 cells was similar to the toxicity expressed by ChCl
1,3PD). DMSO, a widely used solvent, was used for the comparison and it was significantly less toxic to Caco-2, MCF-7, and MRC-5, compared with NADES solvents. However, the toxicity of DMSO to HT-29 cells was similar to the toxicity expressed by ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4BD system (Table 1).
1,4BD system (Table 1).
Many previously published manuscripts reported that deep eutectic solvents are benign and non-toxic solvents.18–21 Meanwhile, several papers examined the cytotoxicity of eutectic mixtures to selected cell lines (Hayyan et al. 2013, 2015, 2016; Paiva et al. 2014; Radošević et al. 2015, 2018; Macário et al. 2019).10,11,15,22–24
Numerous researchers indicated that certain organic acids including malic acid could express diverse biological effects like antioxidant, anti-inflammatory, antiplatelet, antiapoptotic, and slight cytotoxic activity against HeLa and MCF-7 cell lines.25–28 Radošević et al. (2018)10 have also found that the choline chloride–oxalic acid NADES system showed cytotoxic activity to MCF-7 cell line with EC50 = 0.054 mg mL−1 which is about ten-fold higher activity compared with ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA and ChCl
CitA and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MalA in our investigation. Enhanced cytotoxicity of the oxalic acid-based NADES system was explained by the formation of calcium oxalate crystals inside the cells.22 Light microscopy photos of HT-29, Caco-2, MCF-7, and MRC-5 cells without (control) and after 48 h of treatment with 1% ChCl
MalA in our investigation. Enhanced cytotoxicity of the oxalic acid-based NADES system was explained by the formation of calcium oxalate crystals inside the cells.22 Light microscopy photos of HT-29, Caco-2, MCF-7, and MRC-5 cells without (control) and after 48 h of treatment with 1% ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA(1:1) are presented in Fig. 3.
CitA(1:1) are presented in Fig. 3.
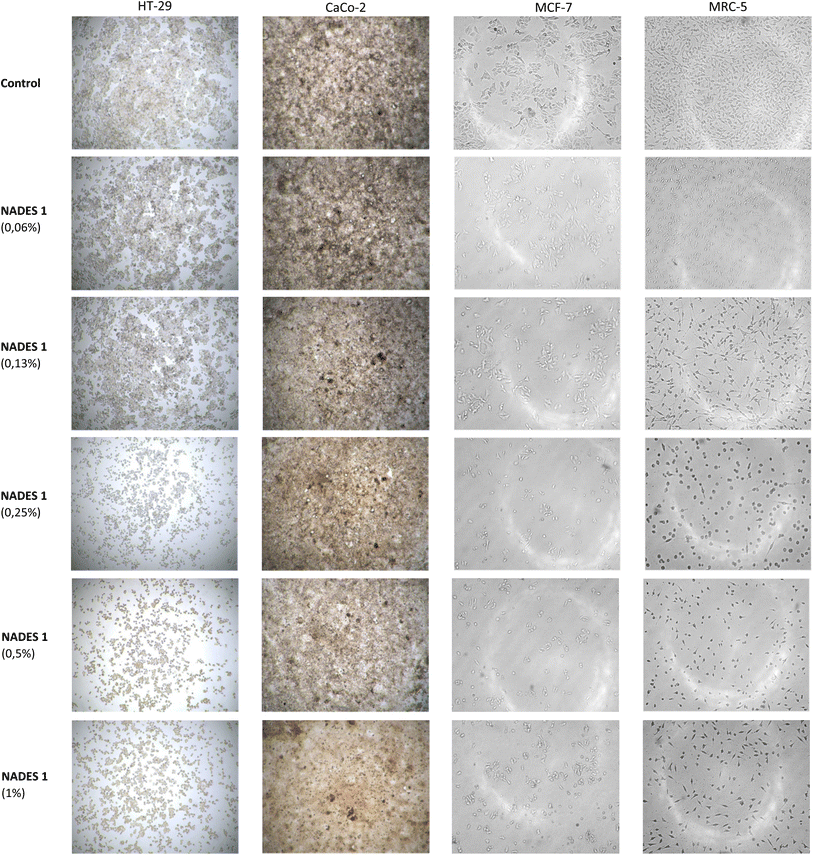 | ||
Fig. 3 Light microscopy of HT-29, CaCo-2, MCF-7 and MRC-5 cells without (control) and after 48 h of treatment with 1% acidic NADES system (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA(1:1)). CitA(1:1)). | ||
The morphological analysis of treated HT-29, Caco-2, MCF-7, and MRC-5 cells showed a dose-dependent decrease in cell number (only representative micrographs following action of acidic NADES system ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA(1:1) are shown in Fig. 3). Non-treated control cells have grown in monolayers, attached to the well bottom. Following treatment tumor cells HT-29, Caco-2, MCF-7 gradually lost their normal morphology, became shrunk, more rounded, and reduced cell-to-cell contacts. Morphological characteristics in non-tumor MRC-5 cells however were less affected by NADES treatment and cells mostly maintained typical spindle shape.
CitA(1:1) are shown in Fig. 3). Non-treated control cells have grown in monolayers, attached to the well bottom. Following treatment tumor cells HT-29, Caco-2, MCF-7 gradually lost their normal morphology, became shrunk, more rounded, and reduced cell-to-cell contacts. Morphological characteristics in non-tumor MRC-5 cells however were less affected by NADES treatment and cells mostly maintained typical spindle shape.
It was observed that among acidic systems, the lactic acid based system (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LacA) showed the lowest cytotoxicity to all four cell lines (Fig. 1), with EC50 values significantly higher than EC50 values for malic and citric acid-based systems. Lactic acid is also an essential component of the bioresorbable and biocompatible polymers (polyesters) and nanocarriers made to be more compatible with human cells.5,29 Still, as a component of the tested ChCl
LacA) showed the lowest cytotoxicity to all four cell lines (Fig. 1), with EC50 values significantly higher than EC50 values for malic and citric acid-based systems. Lactic acid is also an essential component of the bioresorbable and biocompatible polymers (polyesters) and nanocarriers made to be more compatible with human cells.5,29 Still, as a component of the tested ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LacA system at higher concentrations perhaps may contribute to the increased LacA concentration in the tumor cell surrounding and cause the acidic shifts in the extracellular space.30 This might also be the factor responsible for the similar cytotoxic effect of tested acid-based NADES systems.
LacA system at higher concentrations perhaps may contribute to the increased LacA concentration in the tumor cell surrounding and cause the acidic shifts in the extracellular space.30 This might also be the factor responsible for the similar cytotoxic effect of tested acid-based NADES systems.
The most acidic system among the four acidic systems in this research was the citric acid-based system with pH 1.5.17 The buffer system of the used culture medium could not buffer it and, for example, the pH of the DMEM after addition of 5% of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CitA was 2.83 which is still much lower than the optimal pH for cell culture cultivation.12
CitA was 2.83 which is still much lower than the optimal pH for cell culture cultivation.12
The ascorbic acid-based system showed a concentration dependent increase of cytotoxic effect, however, results were not reproducible, and standard deviations were high for all investigated cell lines, so EC50 was not calculated. An effect of ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AscA system on cell viability can be explained by a drop of pH below the optimal values for mammalian cell growth (i.e., pH 7–7.4).31 According to previous reports, cytotoxicity of higher ascorbate doses to tumor cells (millimolar concentrations) may be mediated also through the generation of reactive oxygen species (ROS) such as H2O2 in the preferably acidic and ferritin reach tumor cell microenvironment, and further ROS dependent damages to the cell membrane or other cell structures may occur.32 Studies of the ascorbic acid (vitamin C) effect in tumor cells up to the date indicated that both “protective” or “destructive” features, affecting cellular homeostasis, may be obtained by modulation of the pharmacological doses33–35 High-dose intravenously administered ascorbic acid is however widely used in cancer treatment as complementary and alternative medicine practice, due to its potential effectiveness as an anti-cancer agent which enhances chemosensitivity of cancer cells and reduces chemotherapy-related toxicities. Data obtained in the present study suggested that the additional tuning of the proportion of the components in the acidic NADES systems is required and needs to be precisely addressed.
AscA system on cell viability can be explained by a drop of pH below the optimal values for mammalian cell growth (i.e., pH 7–7.4).31 According to previous reports, cytotoxicity of higher ascorbate doses to tumor cells (millimolar concentrations) may be mediated also through the generation of reactive oxygen species (ROS) such as H2O2 in the preferably acidic and ferritin reach tumor cell microenvironment, and further ROS dependent damages to the cell membrane or other cell structures may occur.32 Studies of the ascorbic acid (vitamin C) effect in tumor cells up to the date indicated that both “protective” or “destructive” features, affecting cellular homeostasis, may be obtained by modulation of the pharmacological doses33–35 High-dose intravenously administered ascorbic acid is however widely used in cancer treatment as complementary and alternative medicine practice, due to its potential effectiveness as an anti-cancer agent which enhances chemosensitivity of cancer cells and reduces chemotherapy-related toxicities. Data obtained in the present study suggested that the additional tuning of the proportion of the components in the acidic NADES systems is required and needs to be precisely addressed.
Comparing all the results, no selectivity was detected comparing investigated cell lines. ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ur system expressed higher cytotoxicity to all investigated cell lines, especially to MCF-7 and MRC-5, compared with other non-acidic systems. The toxic effect of the urea-based system in MCF-7 cells could be attributed to urea basicity10,36 since the optimal pH for cell cultivation is neutral. On the other hand, HT-29 cell line was less affected pointing out that cytotoxicity depended on cell type.
Ur system expressed higher cytotoxicity to all investigated cell lines, especially to MCF-7 and MRC-5, compared with other non-acidic systems. The toxic effect of the urea-based system in MCF-7 cells could be attributed to urea basicity10,36 since the optimal pH for cell cultivation is neutral. On the other hand, HT-29 cell line was less affected pointing out that cytotoxicity depended on cell type.
Hayyan et al. (2015)11 investigated the cytotoxic effects of choline chloride-based NADES solvents comprising glycerol, ethylene glycol, and urea on OKF6, MCF-7, PC3, A375, HepG2, HT29, and H413 cell lines and compared them with their components. They came out to a similar conclusion that cell toxicity is dependent on NADES composition and concentration. Furthermore, they discovered that the toxicity of synthesized NADES systems was not equal to the simple sum of individual components' contribution, revealing that synergism or antagonism between components could exist.11 It has been implied that one mechanism by which DESs induces cancer cell death is through disruption of the cell membrane. Cell membrane disruption was observed in three different cancer cell lines (MCF-7, a human gastric cancer cell line [AGS], and a human cervical cancer cell line [HelaS3]) when the permeability dye stained cells were treated with ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fructose (2
fructose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and ChCl
1) and ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose (2
glucose (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).5
1).5
In the experiment, NADES systems were compared with dimethyl sulfoxide (DMSO) as a widely used solvent. DMSO is one of the most commonly used solvents for dissolving both polar and non-polar compounds, because of its polar aprotic character and amphiphilic nature.37 Although research from 1960 to 1990 pointed to a lot of concerns about the biological safety of DMSO, later investigations categorized DMSO in the same class as ethanol (class 3 solvent),.38 It is generally accepted that DMSO is nontoxic below 10% (v/v),37 but in the investigations dealing with cell lines in vitro, concentrations up to 1% DMSO are permissible to most cell lines. For example, OECD genetic toxicity test guidelines recommend no more than 1% final concentration of organic solvents including DMSO.39
However, it was documented in laboratory-based studies that dimethyl sulfoxide [DMSO, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S(CH3)2] may interfere with ligand displacement of metal-based pro-drugs and anticancer drugs such as cisplatin, that need substitution reaction under physiological (intracellular) conditions for their activation. Concerns that DMSO might not be quite suitable either for storage or continuous drug exposure, rises certain constraints for its wide usage, besides its toxicity.40,41 Rising awareness of the significant issue in medicinal chemistry considering appropriate drug solvation, which may provide higher solubility and availability while ensuring environmental and health safety is why the development of deep eutectic solvents (DESs) is gaining deep interest as alternative vehicles for the preparation of pharmaceutical formulations.
S(CH3)2] may interfere with ligand displacement of metal-based pro-drugs and anticancer drugs such as cisplatin, that need substitution reaction under physiological (intracellular) conditions for their activation. Concerns that DMSO might not be quite suitable either for storage or continuous drug exposure, rises certain constraints for its wide usage, besides its toxicity.40,41 Rising awareness of the significant issue in medicinal chemistry considering appropriate drug solvation, which may provide higher solubility and availability while ensuring environmental and health safety is why the development of deep eutectic solvents (DESs) is gaining deep interest as alternative vehicles for the preparation of pharmaceutical formulations.
Comparing the cytotoxicity of DMSO on investigated cell lines, differential susceptibility was found between them. The most tolerant was the Caco-2 cell line with EC50 ≈ 13.26% followed by MCF-7 and MRC-5 cell lines with EC50 = 4.25% and 4.36% respectively. The most vulnerable was HT-29 cell line with EC50 = 2.98%. The increased sensitivity of this cell line could be explained by the influence of DMSO on cell membranes. It was documented that DMSO easily penetrates most tissue membranes.42 It also enhances the permeability of other molecules dissolved in DMSO allowing them to penetrate deep into the tissue.43 Therefore, DMSO is used in preparations and formulations for topical application.37 All the mentioned features are also present in NADES solvents, with the exceptional possibility of solubilization of small molecules, an increase of permeability, and bioavailability.6,31,44 Radošević et al. (2018)10 pointed out that the mechanism of NADES action is determined by the composition of the system and is most likely associated with its interactions with cell membranes. Our research confirmed that the toxicity of NADES, which is variable and primarily depends on the biological characteristics of the cell line itself, and also on the concentration of the system, must be taken into account.
Conclusion
The use of green products of plant origin from renewable resources and generally biological materials such as NADES solvents represents the technology of the future. The fact that NADES systems are the solvents of the 21st century is supported by dramatically increased scientific interest in the use of NADES in the last few years. Herein we presented the cytotoxic profile of twelve choline chloride-based NADES systems determined in human tumor cells HT-29, Caco-2, MCF-7, and non-tumor MRC-5 cell lines, assuming that there is still a lack of data related to the cytotoxicity of frequently used NADES solvents. Our results supported the fact that NADES solvents showed relatively low cytotoxicity, except for those in which at least one component is acidic. The most acidic system, based on citric acid, also showed the highest cytotoxicity, especially to HT-29 and MCF-7 cell lines. This fact must be taken into account when talking about the applications of NADES systems as food additives, bio-based cosmetics, and mediums for making drug formulations.Data availability
All data generated or analyzed during this study are included in this published article.Author contributions
Boris M. Popović: conceptualization, methodology, validation, resources, writing – original draft, writing – review & editing, supervision. Nevenka Gligorijević: formal analysis, investigation, data curation. Sandra Aranđelović: writing – review & editing, supervision. Ana Catarina Macedo: investigation, validation. Tatjana Jurić: resources, investigation, software, validation, visualization, writing-review & editing. Denis Uka: investigation, software, validation. Karolina Mocko-Blažek: formal analysis, investigation. Teresa Serra: resources, validation, writing – review & editing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This research was financially supported by the Science Fund of the Republic of Serbia, #grant no. 7731993, Active Pharmaceutical Ingredient Deep Eutectic Solvents as Novel Therapeutic Agents and Food Supplements – APIDES, and the Ministry of Education, Science and Technological Development of the Republic of Serbia, grant no. 451-03-68/2022-14/200117 and grant no. 451-03-68/2022-14/200043.References
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063–1071 CrossRef CAS.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- V. I. B. Castro, R. Craveiro, J. M. Silva, R. L. Reis, A. Paiva and A. R. Ana, Cryobiology, 2018, 83, 15–26 CrossRef CAS PubMed.
- R. Craveiro, I. Aroso, V. Flammia, T. Carvalho, M. T. Viciosa, M. Dionísio, S. Barreiros, R. L. Reis, A. R. C. Duarte and A. Paiva, J. Mol. Liq., 2016, 215, 534–540 CrossRef CAS.
- M. H. Zainal-Abidin, M. Hayyan, G. C. Ngoh, W. F. Wong and C. Y. Looi, J. Controlled Release, 2019, 316 Search PubMed.
- S. Trombino, C. Siciliano, D. Procopio, F. Curcio, A. S. Laganà, M. L. Di Gioia and R. Cassano, Pharmaceutics, 2022, 14, 333 CrossRef CAS PubMed.
- M. Faggian, S. Sut, B. Perissutti, V. Baldan, I. Grabnar and S. Dall'Acqua, Molecules, 2016, 21, 1531 CrossRef PubMed.
- T. El Achkar, S. Fourmentin and H. Greige-Gerges, J. Mol. Liq., 2019, 288 Search PubMed.
- A. Mišan, J. Nađpal, A. Stupar, M. Pojić, A. Mandić, R. Verpoorte and Y. H. Choi, Crit. Rev. Food Sci. Nutr., 2020, 60 Search PubMed.
- K. Radošević, I. Čanak, M. Panić, K. Markov, M. C. Bubalo, J. Frece, V. G. Srček and I. R. Redovniković, Environ. Sci. Pollut. Res., 2018, 25, 14188–14196 CrossRef PubMed.
- M. Hayyan, C. Y. Looi, A. Hayyan, W. F. Wong and M. A. Hashim, PLoS One, 2015, 10(2), e0117934 CrossRef PubMed.
- M. Panić, M. Radić Stojković, K. Kraljić, D. Škevin, I. Radojčić Redovniković, V. Gaurina Srček and K. Radošević, Food Chem., 2019, 283, 628–636 CrossRef PubMed.
- P. Manuela, S. Drakula, G. Cravotto, R. Verpoorte, M. Hruškar, I. Radojčić Redovniković and K. Radošević, Innovative Food Sci. Emerging Technol., 2020, 66, 102514 CrossRef CAS.
- E. Shawky, S. S. Takla, H. M. Hammoda and F. A. Darwish, J. Ethnopharmacol., 2018, 227, 139–149 CrossRef PubMed.
- M. Hayyan, M. A. Hashim, A. Hayyan, M. A. Al-Saadi, I. M. AlNashef, M. E. S. Mirghani and O. K. Saheed, Chemosphere, 2013, 90(7), 2193–2195 CrossRef CAS PubMed.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Food Chem., 2015, 187, 14–19 CrossRef CAS PubMed.
- T. Jurić, D. Uka, B. B. Holló, B. Jović, B. Kordić and B. M. Popović, J. Mol. Liq., 2021, 343, 116968 CrossRef.
- H. R. Jhong, D. S. H. Wong, C. C. Wan, Y. Y. Wang and T. C. Wei, Electrochem. Commun., 2009, 11(1), 209–211 CrossRef CAS.
- A. Hayyan, F. S. Mjalli, I. M. Alnashef, T. Al-Wahaibi, Y. M. Al-Wahaibi and M. A. Hashim, Thermochim. Acta, 2012, 541, 70–75 CrossRef CAS.
- B. S. Singh, H. R. Lobo and G. S. Shankarling, Catal. Commun., 2012, 24, 70–74 CrossRef CAS.
- S. H. Wu, A. R. Caparanga, R. B. Leron and M. H. Li, Thermochim. Acta, 2012, 544, 1–5 CrossRef CAS.
- K. Radošević, M. Cvjetko Bubalo, V. Gaurina Srček, D. Grgas, T. Landeka Dragičević and R. I. Redovniković, Ecotoxicol. Environ. Saf., 2015, 112, 46–53 CrossRef PubMed.
- M. Hayyan, Y. P. Mbous, C. Y. Looi, W. F. Wong, A. Hayyan, Z. Salleh and O. Mohd-Ali, Springerplus, 2016, 5, 913 CrossRef PubMed.
- I. P. E. Macário, H. Oliveira, A. C. Menezes, S. P. M. Ventura, J. L. Pereira, A. M. M. Gonçalves, J. A. P. Coutinho and F. J. M. Gonçalves, Sci. Rep., 2019, 9, 3932 CrossRef PubMed.
- K. Radošević, N. Ćurko, V. Gaurina Srček, M. Cvjetko Bubalo, M. Tomašević, K. Kovačević Ganić and I. Radojčić Redovniković, LWT--Food Sci. Technol., 2016, 73, 45–51 CrossRef.
- K. K. Dharmappa, R. V. Kumar, A. Nataraju, R. Mohamed, H. V. Shivaprasad and B. S. Vishwanath, Planta Med., 2020, 89, 103470 Search PubMed.
- R. Varì, M. D’Archivio, C. Filesi, S. Carotenuto, B. Scazzocchio, C. Santangelo, C. Giovannini and R. Masella, J. Nutr. Biochem., 2011, 22(5), 409–417 CrossRef PubMed.
- K. Kim, O. N. Bae, K. M. Lim, J. Y. Noh, S. Kang, K. Y. Chung and J. H. Chung, J. Pharmacol. Exp. Ther., 2012, 343(3), 704–711 CrossRef CAS PubMed.
- X. Guo, Q. Mei, Y. Xing, L. Ye, H. Zhang, X. Shi and Z. Zhang, Drug Delivery, 2012, 143–148 CrossRef PubMed.
- W. Walz and S. Mukerji, Neurosci. Lett., 1988, 86(3), 296–300 CrossRef CAS PubMed.
- J. M. Silva, R. L. Reis, A. Paiva and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2018, 6(8), 10355–10363 CrossRef CAS.
- B. Deubzer, F. Mayer, Z. Kuçi, M. Niewisch, G. Merkel, R. Handgretinger and G. Bruchelt, Cell. Physiol. Biochem., 2010, 25, 767–774 CrossRef CAS PubMed.
- R. Bakalova, Z. Zhelev, T. Miller, I. Aoki and T. Higashi, Oxid. Med. Cell. Longevity, 2020, 2020 Search PubMed.
- A. S. Pires, C. R. Marques, J. C. Encarnação, A. M. Abrantes, A. C. Mamede, M. Laranjo, A. C. Gonçalves, A. B. Sarmento-Ribeiro and M. F. Botelho, Eur. J. Cell Biol., 2016, 95(6–7), 208–218 CrossRef CAS PubMed.
- A. Zasowska-Nowak, P. J. Nowak and A. Ciałkowska-Rysz, Nutrients, 2021, 13 Search PubMed.
- M. Cvjetko Bubalo, M. Mazur, K. Radošević and I. Radojčić Redovniković, Process Biochem., 2015, 50(11), 1788–1792 CrossRef CAS.
- M. Verheijen, M. Lienhard, Y. Schrooders, O. Clayton, R. Nudischer, S. Boerno, B. Timmermann, N. Selevsek, R. Schlapbach, H. Gmuender, S. Gotta, J. Geraedts, R. Herwig, J. Kleinjans and F. Caiment, Sci. Rep., 2019, 9, 4641 CrossRef CAS PubMed.
- Q3C — Tables and List Guidance for Industry, Center for Drug Evaluation and Research, Food Drug Adm., 2012, 9765, 301–827 Search PubMed.
- OCDE, Genetic toxicology Guidance Document, 2015, 1–58 Search PubMed.
- M. D. Hall, K. A. Telma, K. E. Chang, T. D. Lee, J. P. Madigan, J. R. Lloyd, I. S. Goldlust, J. D. Hoeschele and M. M. Gottesman, Cancer Res., 2014, 74(14), 3913–3922 CrossRef CAS PubMed.
- M. Capula, C. Corno, B. E. L. Hassouni, G. L. I. Petri and S. Aranđelović, Anticancer Res., 2019, 39 Search PubMed.
- S. W. Jacob and R. Herschler, Cryobiology, 1986, 23(1), 14–27 CrossRef CAS PubMed.
- Z. W. Yu and P. J. Quinn, Biosci. Rep., 1994, 14 Search PubMed.
- A. Shamseddin, C. Crauste, E. Durand, P. Villeneuve, G. Dubois, T. Durand, J. Vercauteren and F. Veas, Eur. J. Lipid Sci. Technol., 2017, 119, 1700171 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07488e |
| This journal is © The Royal Society of Chemistry 2023 |