Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of pyrazolopyridine pharmaceuticals under mild conditions using an algin-functionalized silica-based magnetic nanocatalyst (Alg@SBA-15/Fe3O4)†
Fereshte Hassanzadeh-Afruzi ,
Zeinab Amiri-Khamakani,
Mahdi Saeidirad,
Mohammad Mehdi Salehi,
Reza Taheri-Ledari
,
Zeinab Amiri-Khamakani,
Mahdi Saeidirad,
Mohammad Mehdi Salehi,
Reza Taheri-Ledari and
Ali Maleki
and
Ali Maleki *
*
Catalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir; Fax: +98-21-73021584; Tel: +98-21-73228313
First published on 3rd April 2023
Abstract
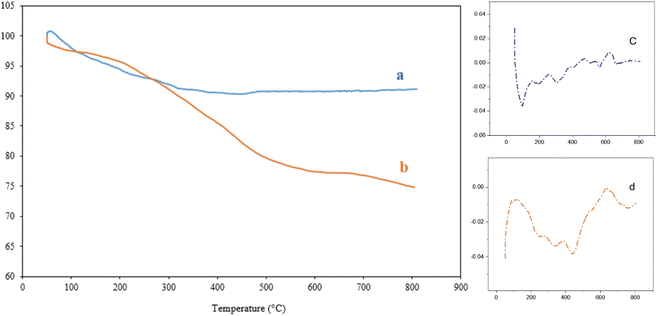
Pyrazolopyridines are common scaffolds in various bioactive compounds, which have several therapeutic effects and unique pharmacological properties. In this study, we fabricated a novel environmentally friendly silica-based nanocomposite as a multifunctional catalytic system for the synthesis of pyrazolopyridine derivatives. This novel heterogeneous nanocomposite named Alg@SBA-15/Fe3O4 (Alg stands for alginic acid), was prepared in several steps. In this regard, SBA-15 was synthesized by the hydrothermal method. Next, it was magnetized by Fe3O4 nanoparticles via an in situ co-precipitation process. Then, SBA-15/Fe3O4 particles were functionalized with 3-minopropyltriethoxysilane (APTES). Afterward, Alg@SBA-15/Fe3O4 was obtained by a nucleophilic substitution reaction between SBA-15/Fe3O4–NH2 and an as-synthesized methyl-esterified alginic. Different analyses such as Fourier-transform infrared (FTIR), energy-dispersive X-ray (EDX) spectroscopy, field-emission scanning-electron microscopy (FESEM), vibrating-sample magnetometer (VSM), X-ray diffraction (XRD), thermogravimetric analysis (TGA), and BET (Brunauer–Emmett–Teller) have been used to confirm the structure of the fabricated catalyst. The magnetic properties of the Alg@SBA-15/Fe3O4 catalytic system imparted by Fe3O4 MNPs enable it to be conveniently isolated from the reaction mixture by using an external magnet. According to the obtained results, the prepared nanocatalyst has high thermal stability and it lost approximately 26% of its weight up to 800 °C. Interestingly, a small amount of prepared nanocatalyst (0.02 g) has shown excellent catalytic performance in the synthesis of pyrazolopyridine derivatives (90–97%) in a short reaction time (20–30 min) at room temperature which can be attributed to its porous structure and large surface area, and the presence of many acidic and basic functional groups. In general, it can be argued that the Alg@SBA-15/Fe3O4 nanocomposite deserves more attention due to its non-toxicity, ease of preparation, good recyclability, and its high catalytic efficiency.
1. Introduction
It has been stated that nitrogen-containing heterocycles account for more than 90% of substances examined by pharmaceutical companies.1 Pyrazopyridine as a kind of nitrogen-rich heterocycle has attracted a lot of interest due to having valuable pharmacological and biological activities, including anti-virus2 and anti-leishmania,3 as well as anti-malarial4 properties. Tracazolate, cartazolate, and etazolate are some of pyrazolopyridine derivatives that are used as anxiolytic drugs. Other pyrazolopyridine-containing biologically-active compounds involve a GSK-3 inhibitor, and BAY 41-2272 can be utilized as cardiovascular therapeutic agents.5 Although various multi-step procedures for the construction of pyrazolopyridines have been developed in recent years, some of them suffer from one or more challenges for instance long reaction time, low yields, laborious work-up procedure, as well as generation of by-products. It seems that the development of efficient methods for the synthesis of pyrazolopyridines is still an important topic in chemistry because of their extensive biological activities. Multicomponent reactions (MCRs) is a practical approach to addressing the challenges of the pyrazolopyridine synthesis. MCRs are one-pot processes in which three or more reactants are reacted to form a particular product containing all of the reactants' atoms.6,7 These reactions have been broadly utilized for the synthesis of complicated bio-active organic compounds, particularly heterocyclic materials for instance the pyrazolopyridine, owing to their outstanding advantages such as atom economy, experimental simplicity, and the formation of several bonds in a single operation.8 The use of magnetic nanocatalysts in MCRs is an attractive idea for the development of sustainable methods in green synthetic chemistry.9–11Porous materials with outstanding properties such as, uniform pore size, adjustable porous structure, and high surface area have piqued the curiosity of scientists.12 According to the IUPAC classification, porous materials can be categorized as microporous materials, whose pore width is less than 2 nm, mesoporous materials, whose pore width is 2–50 nm, and macroporous materials, whose pore width is more than 50 nm.13 Mesoporous materials were first discovered by Mobil Research and Development Corporation in 1992, and their synthesis and application have received great attention since then.14 Zhao and coworkers at the University of California, Santa Barbara reported the SBA-15 mesoporous silica for the first time in 1998.13,15–17 Well-defined pore size between 4 to 30 nm, thick pore walls, pore architecture,11,18 wide surface area (up to 1000 m2 g−1), high thermal and hydrothermal stability, and excellent capability to be modified/functionalized are among the features that make materials based on SBA-15 have different applications, including adsorption, drug delivery, imaging, sensor, and catalysis.13,19–22 One-pot synthesis or co-condensation, and grafting technique or post-synthesis are the two main methods used to modify/functionalize silica mesoporous materials.13 Incorporation of Fe3O4 magnetic nanoparticles as a super-paramagnetic substance into silica-based mesoporous supports is a very practical step towards green chemistry, as it can lead to the easy separation of these materials from the reaction medium.23–27 Chemical modification of SBA-15 can lead to the formation of versatile highly-efficient porous catalysts by additional active sites for interaction with the reactants.28 A catalytic application on SBA-15-based materials have been extensively studied in the literature.29,30 Safaei Ghomi et al. reported several of supported SBA-15 catalysts. Generally, in these reported works, the SBA-15 mesoporous silica was employed as a support metal species, and a highly dispersed metal catalyst was utilized in different multicomponent reactions,31–33 for example, they prepared functionalized-SBA-15 using a tetradentate ligand to construct a new catalyst. They investigated the efficiency of this catalyst in the synthesis of 2-azapyrrolizidine alkaloids, which have different biological properties.34 In 2019, by grafting the nickel Schiff base complex on the SBA-15 surface, Noroozi et al. prepared a nano-catalyst called Ni(II)-Schiff base/SBA-15. It was an eco-friendly, and effective catalyst for the synthesis of 2-amino-4-aryl-5-oxo-4H,5H-pyrano[3,2-c]chromene-3-carbonitriles derivatives through a tandem reaction.35 In another example, in 2021, research on the synthesis of dihydropyranopyrazole compounds by using a guanidine functionalized SBA-15/Fe3O4 mesoporous nanocomposite was performed. In this research, synthesis efficiency of dihydropyranopyrazole derivatives by employing a guanidine functionalized SBA-15/Fe3O4, SBA-15/Fe3O4, and Fe3O4 reported 95%, 71%, and negligible, respectively, which is a good indication of the ability of SBA-15 in catalytic applications.13
Alginic acid, also called algin, is generally extracted from cell-wall of brown seaweeds such as macroalgae, ascophyllum, and kelp has attracted so much attention due to its natural availability and non-toxicity.36,37 Since this natural linear polysaccharide is obtained from natural resources, it can be utilized for construction of green catalysis of organic reactions.38 Brilliant properties such as chemical versatility, renewability, hydrophilicity, cost-effectiveness, and biodegradability make alginic acid as an environmentally friendly organocatalyst.39,40 However, the use of this natural polymer as a catalyst may be associated with weaknesses such as low chemical and physical stability, relatively low chemical variability, and undesirable catalytic efficiency that limit its catalytic application. Modification of alginic acid and adding it to heterogeneous systems seems to be a suitable solution to overcome these limitations and obtain a heterogeneous catalyst with high catalytic capacity.38
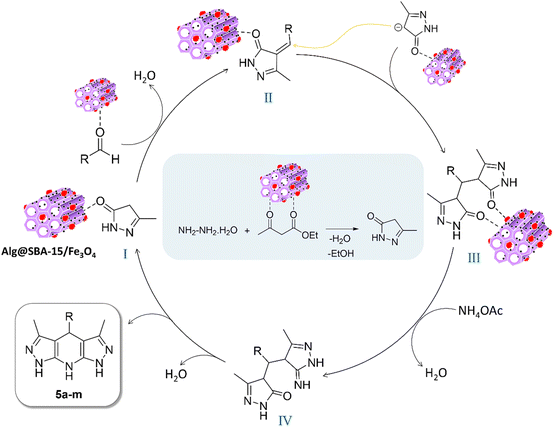
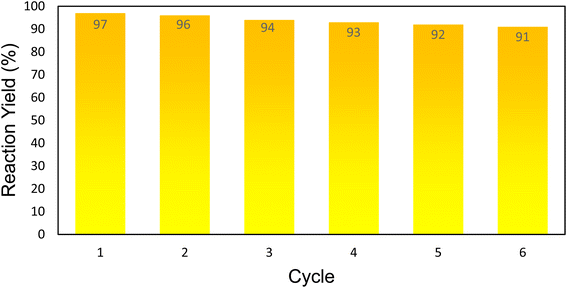
Herein, we introduce a new multifunctional catalytic system for the synthesis of pyrazolopyridine derivatives which mainly composed of SBA-15 as a mesoporous component, iron oxide MNPs as magnetic part, and modified alginic acid as natural polymer. In the fabrication of this novel mesoporous nanocomposite, formulated as “Alg@SBA-15/Fe3O4”, first SBA-15 was magnetized with Fe3O4 MNPs, then magnetized SBA-15 was modified with APTES to obtain SBA-15/Fe3O4–NH2. An important step in the preparation of the final composite is the conversion of alginic acid to methyl-esterified alginic acid via chemical esterification reaction. After the preparation of methyl-esterified alginic acid, in the final stage, the SBA-15/Fe3O4–NH2 formed a covalent bond with methyl-esterified alginic acid through nucleophilic substitution reaction. This inorganic–organic mesoporous nanocomposite has exhibited unique physicochemical characteristics which originate from the combination of its components. The outstanding characteristic properties of the fabricated heterogeneous catalytic system, for instance its porosity, average size, and thermal stability were carefully examined utilizing various analyses. Afterward, the ability of this catalyst in the organic synthesis was carefully investigated. In summary, it was found that using the Alg@SBA-15/Fe3O4 catalytic system at room temperature can lead to the synthesis of various high-yield pyrazolopyridine derivatives in short times. Also, the catalytic reusability of Alg@SBA-15/Fe3O4 was investigated for five successive runs, which showed that less than 10% of its catalytic capacity decreases after consecutive times compared to the first time. The easy separation of Alg@SBA-15/Fe3O4 catalytic system from the reaction medium stemmed from its magnetic property, is an effective step toward green chemistry. Overall, because of its cost-effectiveness and efficiency, the presented nanocatalyst is suggested for large-scale synthesis.
2. Results and discussion
2.1. Preparation of Alg@SBA-15/Fe3O4 nanocomposites
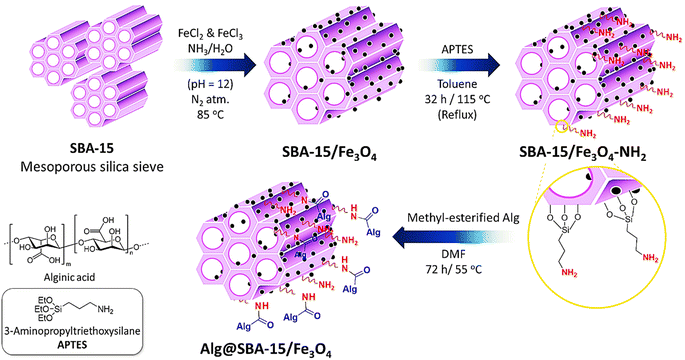
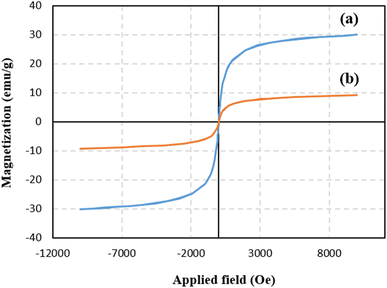
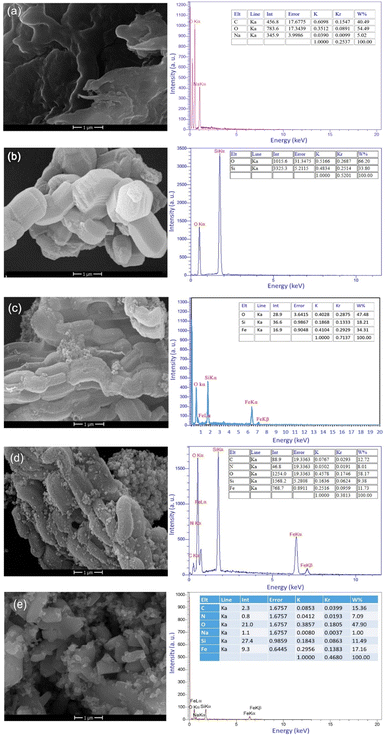
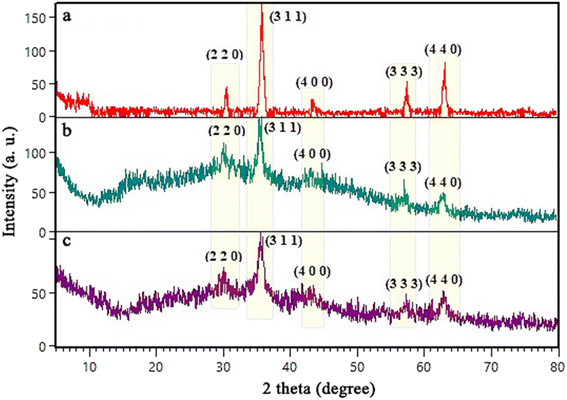
To prepare Alg@SBA-15/Fe3O4 nanocomposite, several steps were performed which are briefly described in this section. After preparing SBA-15, the in situ co-precipitation method was used to magnetize the SBA-15, resulting in SBA-15/Fe3O4. Then, the modification of the magnetic SBA-15 surface was performed with APTES. The reason for using APTES in this study was that the surface of SBA-15 obtained the appropriate functional groups to bind to methyl-esterified alginic acid. Eventually, to prepare the Alg@SBA-15/Fe3O4 nanocomposites, the methyl-esterified alginic acid was attached to SBA-15/Fe3O4–NH2 through an amide bond (Fig. 1).2.2. Characterization of Alg@SBA-15/Fe3O4 nanocomposites
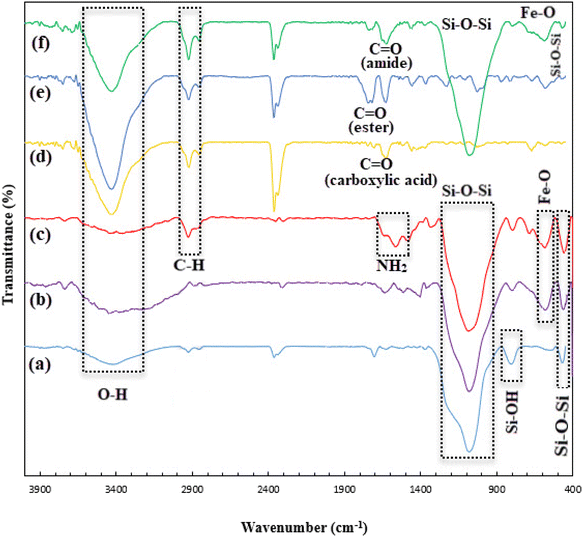
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at about 1624 cm−1, which probably belongs to the amide functional group. This peak is important in that it can be a reason for the creation of amide bonds between SBA-15/Fe3O4–NH2 and methyl-esterified alginic acid.
O peak at about 1624 cm−1, which probably belongs to the amide functional group. This peak is important in that it can be a reason for the creation of amide bonds between SBA-15/Fe3O4–NH2 and methyl-esterified alginic acid.
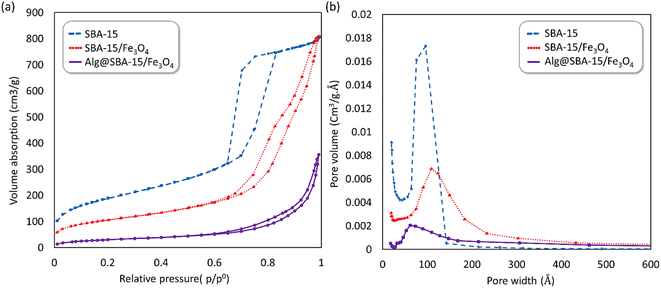 | ||
| Fig. 7 (a) N2 adsorption–desorption isotherms and (b) the pore size distribution curve of SBA-15 (blue), SBA-15/Fe3O4 (red) and Alg@SBA-15/Fe3O4 (purple). | ||
2.3. Application of Alg@SBA-15/Fe3O4 composite in the synthesis of pyrazolopyridine derivatives
| Entry | Catalyst | Catalyst loading (g) | Solvent | Temp. (°C) | Yielda (%) |
|---|---|---|---|---|---|
| a Multicomponent reaction conditions: ethyl acetoacetate (2 mmol), hydrazine hydrate (2 mmol), 3-nitrobezaldehyde (1 mmol) and ammonium acetate (1 mmol), catalyst (10–25 mg). The yields relate to the isolated product. | |||||
| 1 | — | — | — | r.t. | Trace |
| 2 | — | — | — | 80 | Trace |
| 3 | Alg@SBA-15/Fe3O4 | 0.02 | — | r.t. | <65 |
| 4 | Alg@SBA-15/Fe3O4 | 0.02 | H2O | r.t. | 78 |
| 5 | Alg@SBA-15/Fe3O4 | 0.02 | EtOH | r.t. | 97 |
| 6 | Alg@SBA-15/Fe3O4 | 0.02 | EtOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t. | 89 |
| 7 | Alg@SBA-15/Fe3O4 | 0.015 | EtOH | r.t. | 94 |
| 8 | Alg@SBA-15/Fe3O4 | 0.010 | EtOH | r.t. | 92 |
| 9 | Alg@SBA-15/Fe3O4 | 0.025 | EtOH | r.t. | 97 |
| 10 | Alg | 0.020 | EtOH | r.t. | 57 |
| 11 | SBA-15/Fe3O4 | 0.020 | EtOH | r.t. | 58 |
| 12 | Methyl-esterified Alg | 0.020 | EtOH | r.t. | 48 |
| 13 | Mixture of Alg and SBA-15/Fe3O4 | 0.020 | EtOH | r.t. | 91 |
| 14 | Mixture of methyl-esterified Alg and SBA-15/Fe3O4 | 0.020 | EtOH | r.t. | 90 |
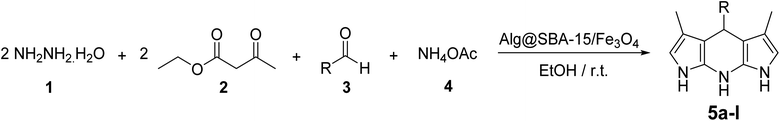 | ||
| Scheme 1 Synthesis of pyrazolopyridine derivatives (5a–l) in the presence of Alg@SBA-15/Fe3O4 nanocatalyst. | ||
| Entry | Aldehyde | Product | Time (min) | Yielda (%) | Mp (°C) | |
|---|---|---|---|---|---|---|
| Found | Reported | |||||
| a The yields relate to the isolated product. | ||||||
| 1 | Benzaldehyde | 5a | 25 | 95 | 240–243 | 240–241 (ref. 8) |
| 2 | 3-NO2 benzaldehyde | 5b | 20 | 97 | 287–290 | 285–287 (ref. 8) |
| 3 | 2,4-Cl2 benzaldehyde | 5c | 20 | 97 | 307–310 | 308 (ref. 8) |
| 4 | 4-Cl benzaldehyde | 5d | 20 | 93 | 257–259 | 255–257 (ref. 8) |
| 5 | 4-Me benzaldehyde | 5e | 25 | 97 | 246–248 | 244–246 (ref. 8) |
| 6 | 4-OMe benzaldehyde | 5f | 30 | 92 | 184–186 | 185–186 (ref. 50) |
| 7 | 3-Me benzaldehyde | 5g | 30 | 90 | 279–280 | 280–282 (ref. 51) |
| 8 | 2-Cl benzaldehyde | 5h | 30 | 93 | 220–223 | 219–221 (ref. 52) |
| 9 | 2-Me benzaldehyde | 5i | 25 | 91 | 290–293 | 290–292 (ref. 53) |
| 10 | 4-F benzaldehyde | 5j | 20 | 92 | 258–260 | 259–261 (ref. 8) |
| 11 | 2-OH, 3-OMe benzaldehyde | 5k | 30 | 91 | 202–204 | 200–202 (ref. 54) |
| 12 | 3,4,5-(OMe)3 benzaldehyde | 5l | 25 | 93 | 249–251 | 248–250 (ref. 51) |
| 13 | 4-CH(Me)2 benzaldehyde | 5m | 30 | 91 | 281–284 | 280–282 (ref. 51) |
3. Experimental section
3.1. Materials and equipment
All used chemicals in present work, were bought from Merck and Sigma-Aldrich companies. In order to show the correct construction of the Alg@SBA-15/Fe3O4 catalyst and its characteristics, several analyses were used. A Shimadzu IR-470 spectrometer was used to get Fourier-transform infrared (FT-IR) spectra of the samples. Energy dispersive X-ray analysis (EDX) of the neat materials and prepared samples was performed employing a Numerix DXP-X10P and TESCAN, MIRA II. The morphology and surface property of neat materials and prepared compounds were examined with the help of images obtained from FESEM (model KYKY-EM8000). By employing a Lakeshore 7407, the magnetic behavior of the samples was studied at ambient temperature. By utilizing an STA504 device, thermal stability of compounds was assessed by TGA. The produced samples' X-ray diffraction (XRD) pattern was achieved utilizing an X-ray diffractometer (JEOL JDX-8030 (20 mA, 30 kV)). The N2 adsorption–desorption isotherm of this study was also obtained using Micromeritics ASAP 2010. Also, a, KQ-250 DE (40 kHz) device was utilized as an ultrasound cleaning bath.3.2. Preparation method
3.3. Procedure for the synthesis of pyrazolopyridine derivatives with the Alg@SBA-15/Fe3O4 catalytic system
Four reactants including; ethyl acetoacetate (2.0 mmol), hydrazine hydrate (2.0 mmol), aromatic aldehydes (1.0 mmol) and ammonium acetate (1.0 mmol) were mixed and reacted in the presence of 0.02 g of the Alg@SBA-15/Fe3O4 catalytic system in the presence of 2.0 mL EtOH at room temperature. TLC (Thin layer chromatography) technique was employed to investigate the reaction completion process. After the reaction was finished, the magnetic nanocatalyst was effortlessly removed from the reaction medium by an external magnet. In the final step, by adding water to the reaction mixture, pure products were got, filtered and washed with further water. Most of the time, additional purification was not required.3.3.1.1 3,5-Dimethyl-4-phenyl-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4′,3′-e] pyridine (5a). FT-IR (KBr) νmax: 3540, 3301, 2925, 1614, 1515, 1375 cm−1; 1H-NMR (300 MHz, DMSO-d6): δH (ppm) = 2.06 (6H, 2-CH3, singlet), 4.81 (1H, –CH, singlet), 7.14–7.20 (5H, H-aromatic, multi), 11.24–11.32 (3H, –NH, broad singlet); 13C NMR (75 MHz, DMSO-d6): δ (ppm) = 10.8, 33.1, 104.6, 125.8, 127.9, 128.1, 140.2, 143.7, 161.5. CHN Anal. Calcd for C15H15N5: C: 67.90, H: 5.70, N: 26.40 found C: 67.86, H: 5.67, N: 26.37, mass for C15H15N5: [M–H] 265.133 found 265.122.
3.3.1.2 4-(4-Chlorophenyl)-3,5-dimethyl-1, 4,7,8-tetrahydrodipyrazolo[3,4-b:4′,3′-e] pyridine (5d). FT-IR (KBr) νmax: 3100, 2854, 1612, 1526, 1368 cm−1;1H-NMR (300 MHz, DMSO-d6): δH (ppm) = 2.06 (6H, 2-CH3, singlet), 4.8 (1H, –CH, singlet), 7.12 (5H, H-aromatic, multi),7.24 (2H, H-aromatic, broad singlet), 11.35 (3H, –NH, broad singlet). 13C NMR (75.47 MHz, DMSO-d6): δ(ppm) = 10.7, 32.6, 104.3, 128.0, 129.8, 130.4, 140.1, 142.7, 161.4. CHN Anal. Calcd for C15H14ClN5: C: 60.10, H: 4.71, N: 23.36 found C: 60.08, H: 4.69, N: 23.35.
3.3.1.3 4-(4-Fluorophenyl)-3,5-dimethyl-1,4,7,8-tetrahydrodipyrazolo[3,4-b:4′,3′-e]pyridine (5j). FT-IR (KBr) νmax: 3165, 3067, 1602, 1511; 1HNMR (300.13 MHz, DMSO-d6): δH (ppm) = 2.07 (6H, 2-CH3, singlet), 4.81 (1H, –CH, singlet), 7.01–7.05 (2H, H-aromatic, multi), 7.08–7.13 (m, 2H, H-Aromatic, multi), 11.34 (3H, –NH, broad singlet); 13C NMR (75.47 MHz, DMSO-d6): δ = 10.8, 32.5, 104.6, 114.8, 129.6, 129.7, 139.8, 161.4,162.4; Anal. Calcd for C15H14FN5: C: 63.59, H: 4.98, N: 24.72 found C: 63.57, H: 5.01, N: 24.69; mass for C15H14FN5 [M–H] 283.12 found 283.11.
4. Conclusion
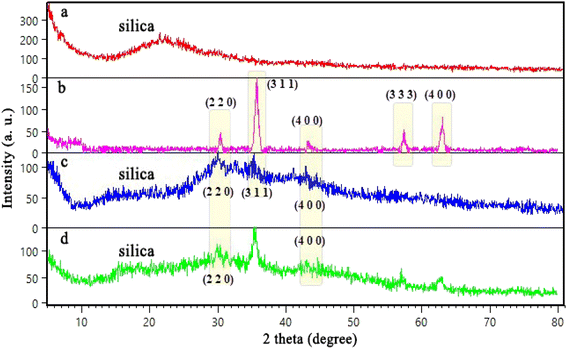
In this study, a novel mesoporous nanocomposite based on SBA-15 was fabricated in which SBA-15 was modified with Fe3O4 nanoparticles, APTES and methyl-esterified alginic acid. For the synthesis of pyrazolopyridine derivatives, the prepared nanocomposite demonstrated excellent catalytic performance. The corresponding products were synthesized in high yields without using a complicated work-up process. TGA result revealed that this nanocomposite is thermally stable, losing only around 25% of its weight up to 800 °C. The XRD analysis also confirmed that Alg@SBA-15/Fe3O4 has properly been prepared. Also, the results of other analyzes, including VSM, FESEM, BET and EDX, were consistent with the confirmation of the correct synthesis of Alg@SBA-15/Fe3O4. The catalytic system synthesized in this study can be considered efficient catalyst for the synthesis of pyrazolopyridine derivatives. One of the reasons for this is its superparamagnetic property, which was also confirmed by VSM analysis, which allows this catalyst to be easily separated from the medium and reused for several times. In the model reaction of this study for the synthesis of various derivatives of pyrazolopyridine, while a small amount of Alg@SBA-15/Fe3O4 (0.02 g) was used, high yield products were obtained in a short time.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are grateful of Iran University of Science & Technology (IUST) for the partial support.References
- A. P. Marjani, J. Khalafy and S. Akbarzadeh, Green Process. Synth., 2019, 8, 533–541 CAS.
- K. S. Gudmundsson, S. D. Boggs, P. R. Sebahar, L. D. A. Richardson, A. Spaltenstein, P. Golden, P. B. Sethna, K. W. Brown, K. Moniri, R. Harvey and K. R. Romines, Bioorg. Med. Chem. Lett., 2009, 19, 4110–4114 CrossRef CAS PubMed.
- H. De Mello, A. Echevarria, A. M. Bernardino, M. Canto-Cavalheiro and L. L. Leon, J. Med. Chem., 2004, 47, 5427–5432 CrossRef CAS PubMed.
- R. G. Stein, J. H. Biel and T. Singh, J. Med. Chem., 1970, 13, 153–155 CrossRef CAS PubMed.
- R. Navari, S. Balalaie, S. Mehrparvar, F. Darvish, F. Rominger, F. Hamdan and S. Mirzaie, Beilstein J. Org. Chem., 2019, 15(85), 874–880 CrossRef CAS PubMed.
- R. Taheri-Ledari, S. M. Hashemi and A. Maleki, RSC Adv., 2019, 9, 40348–40356 RSC.
- F. Hassanzadeh-Afruzi, M. M. Salehi, G. Heidari, A. Maleki and E. N. Zare, J. Mol. Struct., 2023, 1274, 134490 CrossRef CAS.
- R. Taheri-Ledari, J. Rahimi and A. Maleki, Ultrason. Sonochem., 2019, 59, 104737 CrossRef PubMed.
- R. Taheri-Ledari and A. Maleki, J. Pept. Sci., 2020, 26, e3277 CrossRef CAS PubMed.
- F. Hassanzadeh-Afruzi, Z. Amiri-Khamakani, S. Bahrami, M. R. Ahghari and A. Maleki, Sci. Rep., 2022, 12(1), 1–16 CrossRef PubMed.
- F. Hassanzadeh-Afruzi, A. Maleki and E. N. Zare, J. Nanostruct. Chem., 2022, 2022, 1–12 Search PubMed.
- R. Taheri-Ledari, W. Zhang, M. Radmanesh, N. Cathcart, A. Maleki and V. Kitaev, J. Nanobiotechnol., 2021, 19, 1–21 CrossRef PubMed.
- F. Hassanzadeh-Afruzi, S. Asgharnasl, S. Mehraeen, Z. Amiri-Khamakani and A. Maleki, Sci. Rep., 2021, 11(1), 1–15 CrossRef PubMed.
- J. Safaei-Ghomi and R. Teymuri, Appl. Organomet. Chem., 2019, 33, e5150 Search PubMed.
- P. Verma, Y. Kuwahara, K. Mori, R. Raja and H. Yamashita, Nanoscale, 2020, 12, 11333–11363 RSC.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- I. M. El-Nahhal, J. K. Salem, N. S. Tabasi, R. Hempelmann and F. S. Kodeh, Chem. Phys. Lett., 2018, 691, 211–218 CrossRef CAS.
- S. Singh, R. Kumar, H. D. Setiabudi, S. Nanda and D. V. N. Vo, Appl. Catal., A, 2018, 559, 57–74 CrossRef CAS.
- B. N. Mahato and T. Krithiga, Can. J. Chem., 2022, 100, 9–17 CrossRef CAS.
- J. Wang, H. Ge and W. Bao, Mater. Lett., 2015, 145, 312–315 CrossRef CAS.
- A. Ghorbani-Choghamarani, M. Mohammadi, T. Tamoradi and M. Ghadermazi, Polyhedron, 2019, 158, 25–35 CrossRef CAS.
- F. Hassanzadeh-Afruzi, F. Esmailzadeh, S. Asgharnasl, F. Ganjali, R. Taheri-Ledari and A. Maleki, Sep. Purif. Technol., 2022, 291, 120956 CrossRef CAS.
- K. Valadi, S. Gharibi, R. Taheri-Ledari and A. Maleki, Solid State Sci., 2020, 101, 106141 CrossRef CAS.
- F. Ganjali, A. Kashtiaray, S. Zarei-Shokat, R. Taheri-Ledari and A. Maleki, Nanoscale Adv., 2022, 4, 1263–1307 RSC.
- A. Maleki, R. Taheri-Ledari and M. Soroushnejad, ChemistrySelect, 2018, 3, 13057–13062 CrossRef CAS.
- Z. Hajizadeh, F. Hassanzadeh-Afruzi, D. F. Jelodar, M. R. Ahghari and A. Maleki, RSC Adv., 2020, 10, 26467–26478 RSC.
- F. Hassanzadeh-Afruzi, H. Dogari, F. Esmailzadeh and A. Maleki, Appl. Organomet. Chem., 2021, 35, e6363 CrossRef CAS.
- A. Hakiki, B. Boukoussa, H. Habib Zahmani, R. Hamacha, N. H. Hadj Abdelkader, F. Bekkar, F. Bettahar, A. P. Nunes-Beltrao, S. Hacini, A. Bengueddach and A. Azzouz, Mater. Chem. Phys., 2018, 212, 415–425 CrossRef CAS.
- S. Yuan, M. Wang, J. Liu and B. Guo, J. Environ. Manage., 2020, 254, 109787 CrossRef CAS PubMed.
- M. Mureddu, F. Ferrara and A. Pettinau, Appl. Catal., B, 2019, 258, 117941 CrossRef CAS.
- J. Safaei Ghomi, Z. Akbarzadeh and A. Bakhtiari, J. Coord. Chem., 2019, 72, 826–840 CrossRef CAS.
- J. S. Ghomi and A. Bakhtiari, ChemistrySelect, 2018, 3, 12704–12711 CrossRef CAS.
- J. Safaei-Ghomi, R. Teymuri and A. Bakhtiari, BMC Chem., 2019, 13, 1–12 CrossRef PubMed.
- J. Safaei-Ghomi and A. Bakhtiari, RSC Adv., 2019, 9, 19662–19674 RSC.
- N. Noroozi Pesyan, G. Rezanejade Bardajee, E. Kashani, M. Mohammadi and H. Batmani, Res. Chem. Intermed., 2019, 46(1), 347–367 CrossRef.
- A. Srivastava, A. Yadav and S. Samanta, Tetrahedron Lett., 2015, 56, 6003–6007 CrossRef CAS.
- I. P. S. Fernando, D. Kim, J. W. Nah and Y. J. Jeon, Chem. Eng. J., 2019, 355, 33–48 CrossRef CAS.
- A. Maleki, Z. Varzi and F. Hassanzadeh-Afruzi, Polyhedron, 2019, 171, 193–202 CrossRef CAS.
- Z. Durmus, H. Sözeri, B. Unal, A. Baykal, R. Topkaya, S. Kazan and M. S. Toprak, Polyhedron, 2011, 30, 322–328 CrossRef CAS.
- X. Guo, Y. Wang, Y. Qin, P. Shen and Q. Peng, Int. J. Biol. Macromol., 2020, 162, 618–628 CrossRef CAS PubMed.
- R. Taheri-Ledari, W. Zhang and M. Radmanesh, et al., Small, 2020, 16, 2002733 CrossRef CAS PubMed.
- A. Maleki, R. Taheri-Ledari and J. Rahimi, et al., ACS Omega, 2019, 4, 10629–10639 CrossRef CAS PubMed.
- R. Taheri-Ledari, A. Maleki, E. Zolfaghari, M. Radmanesh, H. Rabbani, A. Salimi and R. Fazel, Ultrason. Sonochem., 2020, 61, 104824 CrossRef CAS PubMed.
- R. Taheri-Ledari, K. Valadi, S. Gharibi and A. Maleki, Mater. Res. Bull., 2020, 130, 110946 CrossRef CAS.
- M. Munir, M. Ahmad, M. Mubashir, S. Asif, A. Waseem, A. Mukhtar, S. Saqib, H. Siti Halimatul Munawaroh, M. K. Lam, K. Shiong Khoo, A. Bokhari and P. Loke Show, Bioresour. Technol., 2021, 328, 124859 CrossRef CAS PubMed.
- A. Maleki, R. Taheri-Ledari, R. Ghalavand and R. Firouzi-Haji, J. Phys. Chem. Solids, 2020, 136, 109200 CrossRef CAS.
- Z. P. Zanele, F. M. Mtunzi, S. M. Nelana, A. N. Ebelegi, N. Ayawei, E. D. Dikio, D. Wankasi and P. N. Diagboya, Langmuir, 2021, 37, 9764–9773 CrossRef CAS PubMed.
- E. F. Aboelfetoh, M. E. Zain Elabedien and E. Z. M. Ebeid, J. Environ. Chem. Eng., 2021, 9, 104817 CrossRef CAS.
- K. Dashtian, R. Zare-Dorabei, R. Jafarinia and M. Saghanejhad Tehrani, J. Environ. Chem. Eng., 2017, 5, 5233–5240 CrossRef CAS.
- Kavita, P. Verma, D. K. Verma, B. Kumar, A. K. Singh, N. Shukla, V. Srivastava and R. B. Rastogi, RSC Adv., 2020, 10, 10188–10196 RSC.
- R. R. Chinthaparthi, V. L. Chittiboena, S. Jorepalli and C. S. R. Gangireddy, J. Heterocycl. Chem., 2021, 58, 1104–1116 CrossRef CAS.
- F. Tamaddon and D. Arab, RSC Adv., 2019, 9, 41893–41902 RSC.
- M. Dashteh, M. Yarie, M. A. Zolfigol, A. Khazaei and S. Makhdoomi, Appl. Organomet. Chem., 2021, 35, e6222 CrossRef CAS.
- J. Safaei-Ghomi, R. Sadeghzadeh and H. Shahbazi-Alavi, RSC Adv., 2016, 6, 33676–33685 RSC.
- H. Zhao, M. Cheng, J. Zhang and M. Cai, Green Chem., 2014, 16, 2515–2522 RSC.
- Z. Abbasi, S. Rezayati, M. Bagheri and R. Hajinasiri, Chin. Chem. Lett., 2017, 28, 75–82 CrossRef CAS.
- S. Sadjadi, J. Mol. Liq., 2021, 323, 114994 CrossRef CAS.
- T. Taubner, M. Marounek and A. Synytsya, Int. J. Biol. Macromol., 2017, 103, 202–207 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07228a |
| This journal is © The Royal Society of Chemistry 2023 |