Open Access Article
Open Access ArticlePreparation and characterization of doped hollow carbon spherical nanostructures with nickel and cobalt metals and their catalysis for the green synthesis of pyridopyrimidines†
Maryam Taheri ,
Hossein Naeimi
,
Hossein Naeimi * and
Amir Hossein Ghasemi
* and
Amir Hossein Ghasemi
Department of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, 87317-51167, Iran. E-mail: naeimi@kashanu.ac.ir; Fax: +983155912397; Tel: +983155912388
First published on 25th January 2023
Abstract
Fused heterocyclic systems containing the pyrimidine ring structure perform a significant role in numerous biological and pharmaceutical processes. Their properties include antibacterial, antifungal, anti-fever, anti-tumor, and antihistamine. As pyridopyrimidines are important in the essential fields of pharmaceutical chemistry, efficient methods for preparing these heterocycles are presented. In this study, a method for producing improved hollow carbon sphere nanostructures with cobalt and nickel (Co-Ni@HCSs) is presented. The nanocatalyst was prepared and identified by applying Fourier-transform infrared spectroscopy (FT-IR), X-ray powder diffraction (XRD), field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS), Brunauer–Emmett–Teller (BET), and elemental mapping techniques. The Co-Ni@HCSs nanocatalyst was proved to be highly efficient in synthesizing pyranopyrimidine derivatives. The sizeable active site, economic catalyst loading, easy workup, reusability, green reaction conditions, and excellent yields of all derivatives are some of the significant features of this process. Also, applying response surface methodology (RSM) and the Box–Behnken design (BBD) techniques allowed us to determine the influential factors of the laboratory variables and identify the optimum conditions for superior catalytic activity. Finally, synthesized organic compounds were identified by utilizing melting point, FT-IR, and hydrogen-1 nuclear magnetic resonance (1H NMR) analyses.
1. Introduction
Pyridopyrimidine is an organic heterocyclic compound consisting of a pyridine ring fused orthogonally at any location to a pyrimidine ring. Pyridopyrimidines and relevant fused heterocycles are of importance as potential bioactive molecules. They have been extensively applied in medicinal,1,2 and biological chemistry3–5 due to the range of their available products that includes vast chemical variety and their five feasible substitution localities.6Hollow nanostructures are a group of specific nanomaterials identified based on their morphologies. Hollow nanostructures can be classified into several groups. For example, some of the most common hollow nanostructures are hollow boxes, fibers, tubes, spheres, etc., reflecting different overall forms.7 Researchers have quickly discovered exciting properties related to fantastic hollow structures, such as high loading capacity, low density, and large surface area, and illustrated a vast range of applications,8 such as nano-drug delivery systems,9,10 catalysis,11,12 energy storage,13,14 sensors,15,16 biomedicine,17,18 and so forth.19 The importance of hollow sphere structures in catalysis is due to their shape. Three-dimensional (3D) hollow spheres have developed as potential structures due to their high catalytic activity, large surface area, specific high porosity, ultra-low density, and adequate adsorption positions for a synthesis reaction.20,21
Bimetallic catalysts are significant heterogeneous catalysts due to their superior catalytic activity compared to single metal catalysis components.22 Namely, metal particles combined with two metal ingredients present different catalytic activities to monometallic catalysts.23 Bimetallic catalysts show a combination of the properties associated with the presence of each of the two single metals and produce unique properties due to synergetic effects among the two metals present. The synergic effect is the main advantage of bimetallic catalysts. Sometimes, depending on the metals used and on the studied reaction, the catalytic activity of the bimetallic catalyst is more potent than the total activity of the respective single metallic catalysts.24,25 However, not all bimetallic catalysts exhibit a synergic effect.
Due to the significance of the bimetallic hollow nanostructure catalyst prepared from green materials that resulted in pyridopyrimidine, we hope to design and synthesize a nano hollow structure by a hydrothermal technique to form hollow carbon sphere nanostructures. Additionally, the influences of various experimental factors on the catalytic reaction of the synthesis of pyridopyrimidine derivatives were investigated and the reaction conditions were optimized by applying the Box–Behnken experimental design methodology to gain an excellent yield of product.
2. Experimental
2.1. Materials and apparatus
The solvents, chemical compounds, and reagents were purchased from Sigma-Aldrich, Merck, and Sinopharm Chemical Companies in high purities. The reagents were used without any further purification. The 1H NMR spectra were recorded in DMSO-d6 solvent using a Bruker Avance-400 MHz spectrometer in the presence of Si(CH3)4 as a chemical shift reference. The IR spectra were recorded on an FT-IR Magna 550 spectrometer using KBr plates in the range of 400–4000 cm−1. Melting points were measured by Yanagimoto micro melting point equipment. A BANDELIN ultrasonic HD 3200 with probe model KE76, with a diameter of 6 mm, was used for homogenizing the reaction mixture. A Philips X'Pert Pro apparatus was used to record XRD patterns with Cu Kα radiation (λ = 0.154056 Ångström wavelengths) from 10° to 80° (2θ). Nitrogen adsorption–desorption isotherms to estimate surface areas and pore size in the nanostructure at −196 °C were recorded by a BELSORP-mini II. Field emission scanning electron microscopy (FE-SEM) of the nanoparticles was performed on a Zeiss which operated at a 15 kV accelerating voltage. TEM images were obtained using a Philips EM208S that operated at an acceleration of 20–100 kV.2.2. General procedure for the preparation of the hollow carbon sphere nanocatalyst
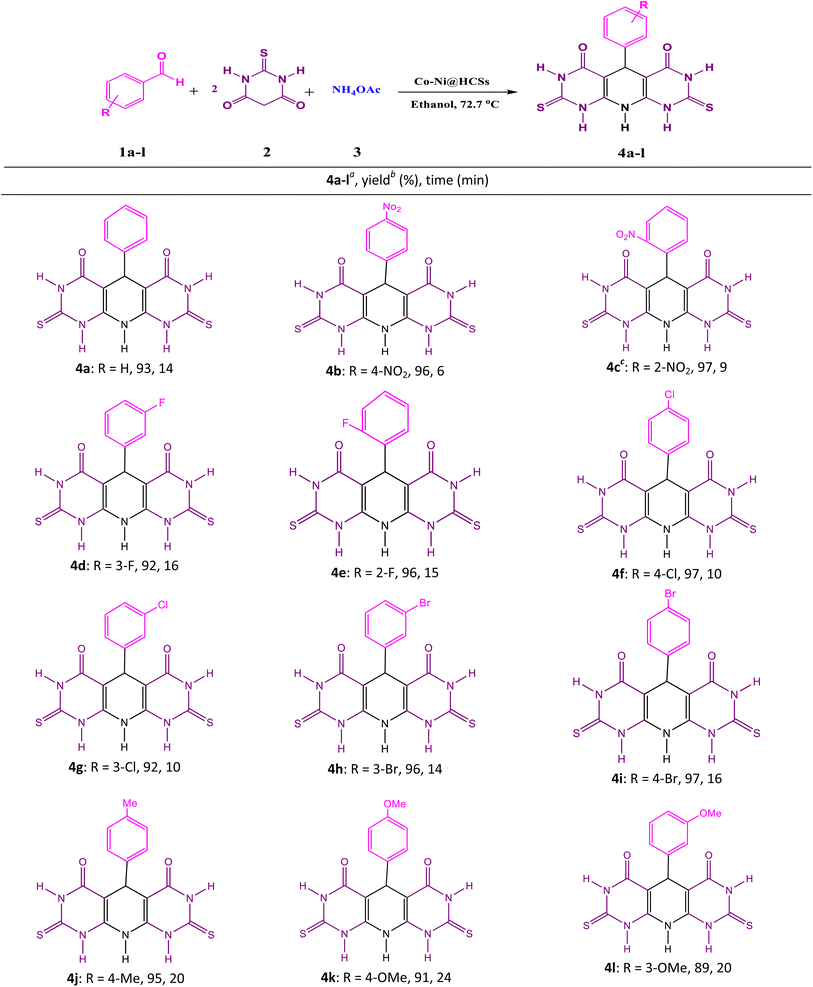
2.3. General procedure for the multicomponent synthesis of pyridopyrimidines
1 mmol of aryl aldehyde, 2 mmol of thiobarbituric acid, 1.4 mmol of ammonium acetate, 0.0125 g of Co-Ni@HCSs as a catalyst, and 15 mL of ethanol as a solvent were transferred into a round-bottomed flask. Next, the mixture was stirred by a magnetic stirrer at 72.7 °C. Throughout the reaction time, the progress was controlled by the thin-layer chromatography (TLC) technique. After the reaction was completed, the reaction mixture was cooled to room temperature, and the catalyst was isolated by filtration. The product was isolated by the recrystallization method from H2O and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 mL) to give the pure product. The pyridopyrimidine products were identified by melting points, Fourier-transform infrared spectroscopy (FT-IR), and hydrogen-1 nuclear magnetic resonance (1H NMR) analyses.
4 mL) to give the pure product. The pyridopyrimidine products were identified by melting points, Fourier-transform infrared spectroscopy (FT-IR), and hydrogen-1 nuclear magnetic resonance (1H NMR) analyses.
5-(4-Nitrophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4b); light brown solid; m.p.: >300 °C, decompose (lit. m.p. 330 °C);26 IR (KBr): v = 3077, 1598, 1550, 1509, 1429 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.65 (s, 2H, NH), 8.06 (d, J = 8.4 Hz, 2H, Ar), 7.25 (d, J = 8.4 Hz, 2H), 7.19 (s, 1H, NH), 7.06 (s, 1H, NH), 6.93 (s, 1H, NH), 6.05 (s, 1H, CH).
5-(2-Nitrophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4c); yellow solid; m.p.: 229–231 °C, decompose (lit. m.p. 230 °C);26 IR (KBr): v = 3487, 3195, 1614, 1542, 1426, 1358 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.58 (s, 2H, NH), 7.51 (d, J = 7.8 Hz, 1H), 7.46 (t, J = 7.6 Hz, 1H), 7.31 (t, J = 7.7 Hz, 1H), 7.21 (d, J = 8.0 Hz, 2H, NH, Ar), 7.07 (s, 1H, NH), 6.94 (s, 1H, NH), 6.10 (s, 1H, CH).
5-(3-Fluorophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4d); white solid; m.p.: 258–260 °C, decompose; IR (KBr): v = 3539, 3160, 1615, 1440, 1398 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.68 (s, 1H, NH), 11.54 (s, 1H, NH), 7.20 (s, 1H, Ar), 7.20 (s, 1H, NH), 7.06 (s, 1H, NH), 6.94 (s, 1H, NH), 6.88 (t, J = 8.0 Hz, 1H, Ar), 6.83 (d, J = 7.7 Hz, 1H, Ar), 6.70 (d, J = 11.1 Hz, 1H, Ar), 5.96 (s, 1H, CH3).
5-(2-Fluorophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4e); white solid; m.p.: 240–241 °C, decompose (lit. m.p. 240 °C);26 IR (KBr): v = 3127, 1609, 1550, 1480 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.66 (s, 1H, NH), 11.51 (s, 1H, NH), 7.11 (m, 4H), 6.99 (t, J = 8.0 Hz, 2H), 6.93 (d, J = 9.3 Hz, 1H), 6.01 (s, 1H, CH).
5-(4-Chlorophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4f); yellow solid; m.p.: 253–255 °C, decompose (lit. m.p. 257 °C);26 IR (KBr): v = 3117, 2885, 1625, 1562, 1520, 1442 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.61 (s, 1H, NH), 11.55 (s, 1H, NH), 7.20 (d, J = 8.7 Hz, 2H, Ar), 7.19 (s, 1H, NH), 7.06 (s, 1H, NH), 6.99 (d, J = 8.1 Hz, 2H, Ar), 6.93 (s, 1H, NH), 5.93 (s, 1H, CH).
5-(3-Chlorophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4g); white solid; m.p.: 253–255 °C, decompose; IR (KBr): v = 3106, 2885, 1666, 1620, 1520, 1546, 1435 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.65 (s, 1H, NH), 11.53 (s, 1H, NH), 7.19 (t, J = 3.9 Hz, 2H), 7.12 (d, J = 8.0 Hz, 1H), 7.06 (s, 1H), 6.95 (d, J = 12.3 Hz, 3H), 5.96 (s, 1H, CH).
5-(3-Bromophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4h); white solid; m.p.: 245–246 °C, decompose (lit. m.p. 246–248 °C);27 IR (KBr): v = 3111, 1619, 1547, 1434 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.70 (s, 1H, NH), 11.57 (s, 1H, NH), 7.26 (d, J = 8.1 Hz, 1H, Ar), 7.19 (s, 1H, Ar), 7.14 (t, J = 8.0 Hz, 1H, Ar), 7.07 (s, 2H, CH, Ar, NH), 6.99 (d, J = 8.1 Hz, 1H, Ar), 6.94 (s, 1H, NH), 5.96 (s, 1H, CH).
5-(4-Bromophenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4i); white solid; m.p.: 379–381 °C, decompose (lit. m.p. 380 °C);28 IR (KBr): v = 3325, 3081, 1594, 1533, 1486 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.65 (s, 1H, NH), 11.54 (s, 1H, NH), 7.33 (d, J = 8.2 Hz, 2H, Ar), 7.19 (s, 1H, NH), 7.06 (s, 1H, NH), 6.94 (d, J = 4.3 Hz, 2H, Ar), 6.93 (s, 1H, NH), 5.90 (s, 1H, CH).
2,8-Dithioxo-5-(p-tolyl)-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4j); white solid; m.p.: 199–201 °C, decompose (lit. m.p. 198–200 °C);29 IR (KBr): v = 3185, 3064, 2906, 1653, 1594, 1594, 1534, 1422 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.61 (s, 1H, NH), 11.48 (s, 1H, NH), 7.20 (s, 1H, NH), 7.07 (s, 1H, NH), 6.95 (d, J = 7.2 Hz, 3H, Ar, NH), 6.86 (d, J = 7.6 Hz, 2H, Ar), 5.90 (s, 1H, CH), 2.20 (s, 3H, Me).
5-(4-Methoxyphenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4k); orange solid; m.p.: 277–278 °C, decompose (lit. m.p. 277–279 °C);27 IR (KBr): v = 3068, 2899, 1650, 1595, 1521, 1431 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.60 (s, 1H, NH), 11.47 (s, 1H, NH), 7.08 (s, 3H, NH), 6.88 (d, J = 8.3 Hz, 2H, Ar), 6.72 (d, J = 8.2 Hz, 2H, Ar), 5.89 (s, 1H, CH), 3.66 (s, 3H, OMe).
5-(3-Methoxyphenyl)-2,8-dithioxo-2,3,5,8,9,10-hexahydropyrido[2,3-d:6,5-d′]dipyrimidine-4,6(1H,7H)-dione, (4l); orange solid; m.p.: 240–242 °C, decompose (lit. m.p. 242 °C);30 IR (KBr): v = 3155, 1605, 1537, 1437 cm−1; 1H NMR (400 MHz, DMSO-d6) δ (ppm): 11.35 (s, 2H, NH), 7.05 (m, 5H), 6.75 (m, 2H), 5.89 (s, 1H, CH), 3.59 (s, 3H, Me).
2.4. Design of the experiments and response surface methodology (RSM)
The response surface methodology was applied to optimize and improve the reaction conditions and product yield based on a Box–Behnken design (BBD) technique for the catalytic performance of the Co-Ni@HCSs nanocatalyst for the synthesis of pyridopyrimidine derivatives. The ranges and levels of the factors based on the Box–Behnken design technique are displayed in Table 1. Three factors of catalyst dose (A), temperature (B), and time of the reaction (C) at three levels (low level, central point, and high level) were chosen as the variables.| Variable | Factor | Range and level | ||
|---|---|---|---|---|
| Low level (−1) | Central point (0) | High level (+1) | ||
| Catalyst dose (mg) | A | 5 | 12.50 | 20 |
| Temperature (°C) | B | 25 | 52.50 | 80 |
| Time of reaction (min) | C | 5 | 15 | 25 |
The Design-Expert software (ver.13.0.5) was applied to design experiments and analyses of the experimental data. The number of experiments was determined by the Box–Behnken design method based on the following equation:31
| N = 2k(k − 1)+ C0 | (1) |
In eqn (1), k is the number of factors, and C0 is the replicate number of the central point. According to eqn (1), three factors and five central points are considered, so there was a total of 17 runs. The Box–Behnken experimental design model equation can be matched to a second-order polynomial model. RMS gives the second-order polynomial model that was applied to show the interactive effects between the experimental variables to optimize the reaction conditions and predict the yield of the product. The quadratic equation model can be expressed:32
| Y = β0 + β1A + β2B + β3C + β4AB + β5AC + β6BC + β7A2 + β8B2 + β9C2 + ε | (2) |
In eqn (2), Y is the predicted response (% product yield), β0 is the zero-order constant, which denotes the regression coefficients representing the offset term, β1−a is the main effect, β4–6 is the interaction effect, β7–9 is the quadratic effect, and the random error is ε.
3. Results and discussion
3.1. Preparation and characterization of the Co-Ni@HCS nanocatalyst
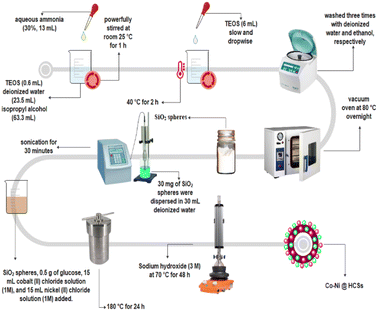
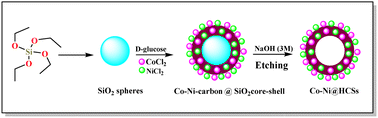
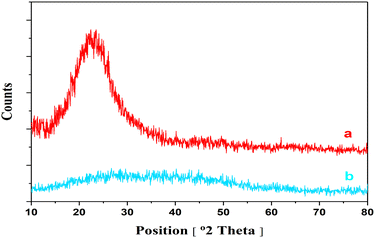
The Co-Ni@HCSs are prepared in three steps, as shown in Fig. 2. In the first step, SiO2 spheres were prepared from tetraethyl orthosilicate with the Stöber method.33 Then, the SiO2 spheres were coated with glucose, cobalt, and nickel through the hydrothermal method. Finally, the Co-Ni@HCSs were prepared by etching SiO2 spheres with concentrated sodium hydroxide.After the preparation of the Co-Ni@HCSs, the nanocatalyst was identified by different analyses such as XRD, FT-IR, FE-SEM, TEM, EDS, elemental mapping, and BET techniques. The X-ray diffraction patterns of the SiO2 spheres (Fig. 3a) and Co-Ni@HCSs (Fig. 3b) were studied. Fig. 3a illustrates a broad signal corresponding to the amorphous nature of the SiO2 spheres,34 and Fig. 3b is due to XRD patterns of the Co-Ni@HCSs. No specific signal of cobalt and nickel was observed in the XRD pattern of the Co-Ni@HCSs due to the prepared hollow spheres having amorphous morphology.
The FT-IR spectra of the SiO2 spheres (a), Co-Ni/SiO2@carbon core–shell spheres (b), and Co-Ni@HCSs are shown in Fig. 4. For the SiO2 spheres (Fig. 4a), the peak at about 953 cm−1 is due to the stretching vibration of the Si–OH bond. The peaks at 472 cm−1 and 800 cm−1 correspond to bending vibrations of the Si–O–Si bonds. The signal that appears at 1030 cm−1 corresponds to the stretching vibration of the Si–O–Si bonds. The signal at 1375 cm−1 is due to the stretching vibration of the aliphatic C–H bonds. Finally, the absorption peaks at 1633 and 3421 cm−1 are due to the O–H bond's bending and stretching vibrations.
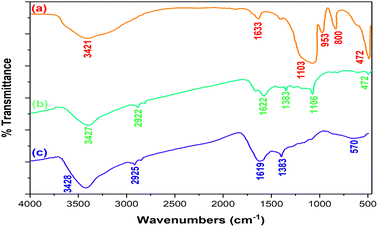 | ||
| Fig. 4 Comparison of the FT-IR spectra of SiO2 spheres (a), Co-Ni/SiO2@carbon core–shell spheres (b), and Co-Ni@HCSs (c). | ||
In the FT-IR spectrum of the Co-Ni/SiO2@carbon core–shell spheres (Fig. 4b), the peak at 1622 cm−1 was assigned to the vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O due to the glucose structure. The spectrum shows some peaks at 1000–1400 cm−1 corresponding to the OH and C–O bonds. Additionally, the signal at 3427 cm−1 is due to the O–H bond on the surface of the carbon spheres.
O due to the glucose structure. The spectrum shows some peaks at 1000–1400 cm−1 corresponding to the OH and C–O bonds. Additionally, the signal at 3427 cm−1 is due to the O–H bond on the surface of the carbon spheres.
In the spectrum of the Co-Ni@HCSs (Fig. 4c), the absorption peak at 1621 cm−1 is due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the broad peak at 3426 cm−1 is due to the O–H bond. No peaks corresponding to Si–O–Si or Si–OH were observed, which indicates the successful removal of the silicon and eventually the formation of hollow spheres.
O, and the broad peak at 3426 cm−1 is due to the O–H bond. No peaks corresponding to Si–O–Si or Si–OH were observed, which indicates the successful removal of the silicon and eventually the formation of hollow spheres.
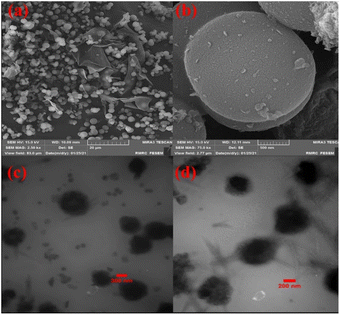
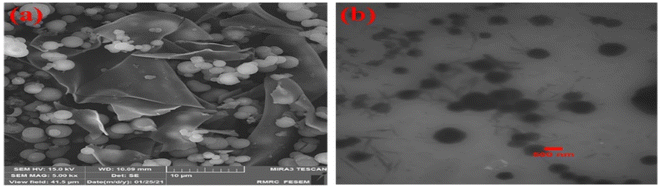
The scanning electron microscopy (FE-SEM) and transmission electron microscopy (TEM) images of the Co-Ni@HCSs are displayed in Fig. 5. The spherical morphology of the Co-Ni@HCSs nanocatalyst is easily identifiable in the SEM images (Fig. 5a and b). The average size of the hollow spheres is 400 nm, but the size of the cobalt and nickel nanoparticles stabilized on the surface of the hollow spheres is identified to be less than 30 nm.
Additionally, the TEM images (Fig. 5c and d) of the nanoparticles show the formation of the hollow spheres' nanostructure.
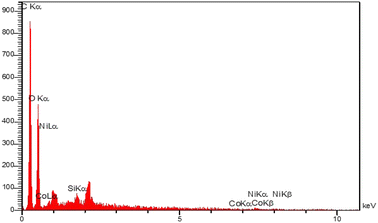
The elemental energy-dispersive X-ray (EDS) spectrum of the Co-Ni@HCSs nanocatalyst was investigated (Fig. 6). The EDS spectrum shows that the main elements in this fabricated nanocatalyst are carbon, cobalt, and nickel.
To determine the amount of Co and Ni in the catalyst structure, inductively coupled plasma atomic emission spectroscopy (ICP-OES) was used. The results showed 1.37 × 10−4 mol g−1 of Ni and 1.21 × 10−4 mol g−1 of Co were loaded on the Co-Ni@HCSs nanocatalyst.
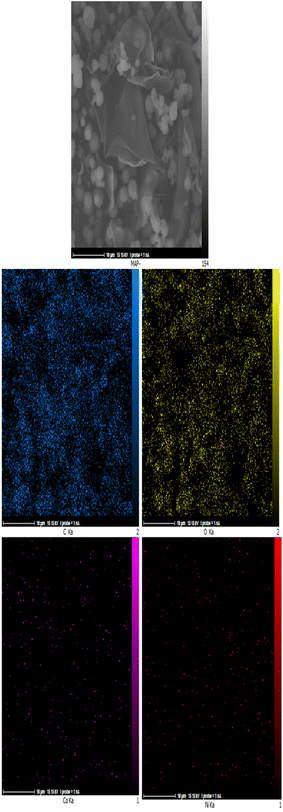
The energy-dispersive X-ray spectroscopy elemental mapping pictures of the Co-Ni@HCSs nanocatalyst are displayed in Fig. 7. As shown in Fig. 7, the cobalt and nickel nanoparticles are evenly distributed throughout the catalyst surface. The homogeneous distribution of cobalt and nickel nanoparticles increased the efficiency and increased the effective contact surface of the nanocatalyst. The homogeneous distribution of metal nanoparticles throughout the carbon spheres results in a similar catalyst effect throughout the catalyst surface. In addition, the homogeneous dispersion of the nanoparticles creates a synergetic effect between the metals and the raw reaction material, which leads to an increase in the catalyst efficiency.
The Brunauer–Emmett–Teller (BET) technique was used to estimate the surface area, pore volume, and pore size of Co-Ni@HCSs nanocatalyst (Fig. 8). The surface area is 240.99 m2 g−1, and the total pore volume (P/P0) is 1.0181 cm3 g−1 (Fig. 8a and b). The average pore diameter is 16.899 nm. Based on the Barrett–Joyner–Halenda (BJH) method, Fig. 8c shows the pore size and pore volume distribution. These results indicate a high contact surface of the hollow spheres, and as a result, increasing the catalyst contact surface improves the catalyst efficiency and reduces the reaction time.
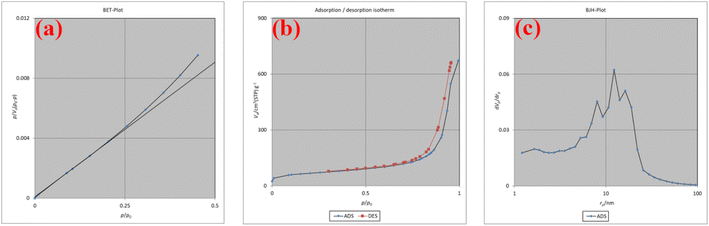 | ||
| Fig. 8 The BET plot (a), adsorption/desorption isotherm (b), and BJH plot (c) of the Co-Ni@HCSs nanocatalyst. | ||
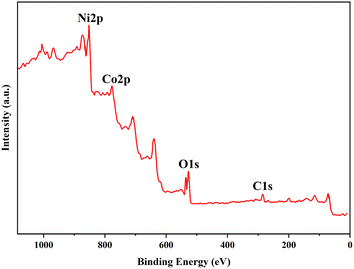
The X-ray photoelectron spectrum (XPS) of the Co-Ni@HCSs is shown in Fig. 9. The hollow carbon sphere nanostructures with cobalt and nickel were subjected to XPS measurements to obtain more detailed information about their elemental composition. As can be seen from the XPS spectrum, the catalyst structure contains elements such as carbon, oxygen, cobalt, and nickel. The C 1s region at 286.3 eV is generally associated with the sp2 carbon–carbon double bond or sp3 carbon–carbon single bond, as shown in Fig. 9. The O 1s XPS spectrum showed two peaks with binding energies at 527.3 and 532.6 eV, which are related to Ni–O and Ni–OH, respectively. A Co 2p XPS spectrum revealed two main peaks corresponding to the Co 2p3/2 and Co 2p1/2 energy levels, with binding energies of 783.0 and 797.9 eV. Based on the XPS spectrum of Ni 2p in this figure, peaks at binding energies of 858.6 eV and 854.2 eV were attributed to Ni 2p3/2, and 875.6 eV and 871.4 eV to Ni 2p1/2. The XPS spectrum clearly indicates the presence of Co2+ and Ni2+ in the catalyst structure.
3.2. Results of the statistical analysis of the process and product optimization
The response surface methodology and the Box–Behnken design method were utilized to determine the interactive effects of the experimental variables for finding the optimum conditions for the reactions. Primarily, the reaction of 2-nitrobenzaldehyde (1 mmol), thiobarbituric acid (2 mmol), and ammonium acetate (1.4 mmol) was chosen as a model reaction. To determine the optimum experimental conditions, the catalyst activity was investigated with a variety of factors, including temperature, time of the reaction, and catalyst dose in three solvents (EtOH, H2O, and EtOH/H2O). Based on laboratory data and calculations, the best reaction solvent was ethanol. To avoid increasing the computational data provided, calculations related to the ethanol solvent are included in the article, and the tables and graphs obtained from the other solvents (H2O and EtOH/H2O) are given in the ESI of the article (Fig. S28, S29, and Tables S1–S3, ESI†).Following the experimental ranges and three levels of the independent variables (Table 1), calculated outcomes from the Box–Behnken quadratic model are summarized in Table 2.
| Run | Factor A | Factor B | Factor C | The yield of product (%) in EtOH | ||
|---|---|---|---|---|---|---|
| A: catalyst dose (mg) | B: temperature (°C) | C: time (min) | Experimentala (%) | Predicted (%) | ||
| a Isolated yield. | ||||||
| 1 | 12.5 | 52.5 | 15 | 91 | 89.80 | |
| 2 | 20 | 80 | 15 | 97 | 97.88 | |
| 3 | 12.5 | 25 | 5 | 35 | 35.13 | |
| 4 | 5 | 25 | 15 | 13 | 12.13 | |
| 5 | 12.5 | 25 | 25 | 67 | 65.88 | |
| 6 | 12.5 | 80 | 5 | 93 | 94.13 | |
| 7 | 12.5 | 52.5 | 15 | 91 | 89.80 | |
| 8 | 12.5 | 52.5 | 15 | 89 | 89.80 | |
| 9 | 12.5 | 80 | 25 | 97 | 96.88 | |
| 10 | 5 | 80 | 15 | 64 | 62.13 | |
| 11 | 20 | 52.5 | 25 | 78 | 77.25 | |
| 12 | 12.5 | 52.5 | 15 | 88 | 89.80 | |
| 13 | 20 | 52.5 | 5 | 68 | 66.00 | |
| 14 | 5 | 52.5 | 25 | 40 | 42.00 | |
| 15 | 20 | 25 | 15 | 56 | 57.88 | |
| 16 | 12.5 | 52.5 | 15 | 90 | 89.80 | |
| 17 | 5 | 52.5 | 5 | 19 | 19.75 | |
The quadratic model to predict the percentage product yield of the model reaction with the Co-Ni@HCSs nanocatalyst in ethanol as a solvent is shown in the following equation:
| Y = 89.8 + 20.375A + 22.5B + 8.375C − 2.5AB − 2.75AC − 7BC − 27.025A2 − 5.275B2 − 11.525C2 | (3) |
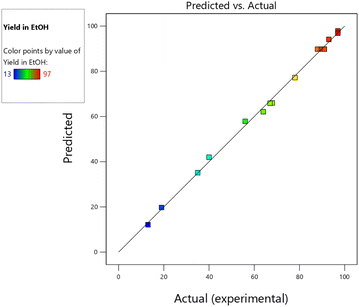 | ||
| Fig. 10 The predicted vs. actual (experimental) product yield of the model reaction with the Co-Ni@HCSs nanocatalyst in ethanol. | ||
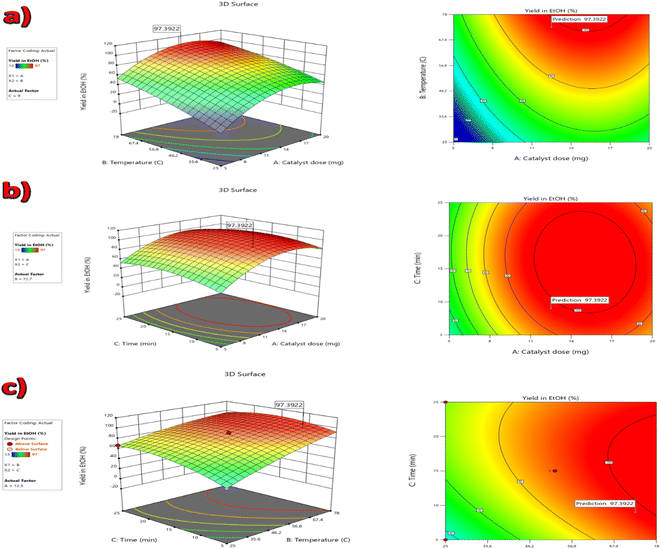
The standard analysis of variance (ANOVA) of the quadratic model is shown in Table 3, and the fitting quality was verified by ANOVA. In Table 3, the probability (P-value) is applied as a measure for evaluating the significance of the variables. A P-value smaller than 0.0500 shows that the model terms are significant. As a result, the A (catalyst dose), B (temperature), C (time), A2, B2, C2, and BC (temperature × time) factors, with P-values smaller than 0.0500, were the highly significant factors in the increase of catalytic activity and the reaction yield in ethanol. The AB (catalyst dose × temperature) and AC (catalyst dose × time) factors had lower significance than the previous factors; however, they were effective in the reaction yield due to the low P-value. The Box–Behnken design (BBD) technique and the predicted model show 3D response surface plots and corresponding contour plots in Fig. 11. The interaction influence among any pair of two experimental variables and their level (Table 1) is plotted in Fig. 11. All of the factors are significant in the yield of the product.
| Source | Sum of squares | DFa | Mean square | F-value | P-value | Significanceb |
|---|---|---|---|---|---|---|
| a Degree of freedom.b *Represents significant, **represents highly significant. | ||||||
| Model | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199.42 199.42 |
9 | 1355.49 | 350.77 | <0.0001 | ** |
| A – catalyst dose | 3321.13 | 1 | 3321.13 | 859.44 | <0.0001 | ** |
| B – temperature | 4050.00 | 1 | 4050.00 | 1048.06 | <0.0001 | ** |
| C – time | 561.13 | 1 | 561.13 | 145.21 | <0.0001 | ** |
| AB | 25.00 | 1 | 25.00 | 6.47 | 0.0385 | * |
| AC | 30.25 | 1 | 30.25 | 7.83 | 0.0266 | * |
| BC | 196.00 | 1 | 196.00 | 50.72 | 0.0002 | ** |
| A2 | 3075.16 | 1 | 3075.16 | 795.79 | <0.0001 | ** |
| B2 | 117.16 | 1 | 117.16 | 30.32 | 0.0009 | ** |
| C2 | 559.27 | 1 | 559.27 | 144.73 | <0.0001 | ** |
| Residual | 27.05 | 7 | 3.86 | |||
| Lack of fit | 20.25 | 3 | 6.75 | 3.97 | 0.1080 | |
| Pure error | 6.80 | 4 | 1.70 | |||
| Cor. total | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 226.47 226.47 |
16 | ||||
Based on the response surface methodology outcomes achieved from all 15 experimental runs, the optimal reaction conditions for the catalytic performance of the Co-Ni@HCSs nanocatalyst for the model reaction synthesis are as follows: A = 12.50 mg (catalyst dose), B = 72.7 °C (temperature), C = 9 min (time), and the predicted yield of the product is 97.39%. Four reactions were performed under the predicted conditions to ensure the validity of the predicted model and the optimal reaction conditions. An average product yield of 9.37% was obtained, in good accordance with the predicted model.
The synergistic effect of the Co-Ni@HCSs nanocatalyst was investigated, and the results are shown in Fig. 12. Four catalysts, hollow carbon spheres, Co@HCSs, Ni@HCSs, and Co-Ni@HCSs, were used in the model reaction, and the efficiency of each catalyst was obtained. As shown in Fig. 12, the hollow carbon spheres did not show any catalytic activity. The Co@HCSs nanocatalysts showed higher efficiency than the Ni@HCSs nanocatalysts. When two nanoparticles of cobalt and nickel participated together (Co-Ni@HCSs) in the reaction as a catalyst, we observed a synergistic effect, resulting in a significant increase in the percentage of products and a decrease in the reaction time.
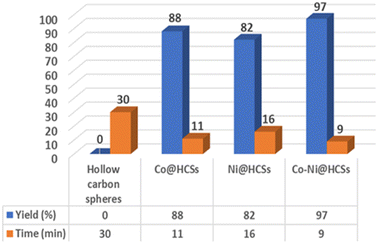 | ||
| Fig. 12 Comparison of the catalytic effects between hollow carbon spheres, Co@HCSs, Ni@HCSs, and Co-Ni@HCSs for the synthesis of 4c. | ||
After optimizing the reaction conditions with numerous solvents, catalyst dose, time of reaction, and temperature, the synthesis of pyridopyrimidine derivatives was performed using thiobarbituric acid, ammonium acetate, and the different aryl aldehydes. The results of the catalytic reaction are displayed in Table 4.
This study applied aryl aldehyde with electron-donating and electron-withdrawing substituents to synthesize pyridopyrimidine derivatives. The product yields were excellent, and the reaction times were short. Additionally, in the aryl aldehydes with electron-withdrawing substituents, the reaction times were shorter than in the aryl aldehydes with electron-donating ones.
To compare the catalytic performance of the Co-Ni@HCSs nanocatalyst with the previously published catalysts for the synthesis of pyridopyrimidine derivatives, the model reaction was investigated, and the outcomes are displayed in Table 5.
| Entry | Catalyst (conditions) | Time (min) | Yielda (%) | Ref. |
|---|---|---|---|---|
| a Isolated yield. | ||||
| 1 | Nano-[DMSPDE][Cl] (0.02 g, solvent-free, 110 °C) | 10 | 88 | 30 |
| 2 | Nano CuFe2O4 (10 mol%, H2O, US 40 W) | 12 | 95 | 35 |
| 3 | CuFe2O4 nanoparticles (10 mol%, H2O, microwave 100 W) | 2 | 95 | 36 |
| 4 | Co-Ni@HCSs (0.0125 g, ethanol, 72.7 °C) | 9 | 97 | This work |
As displayed in Table 5, the Co-Ni@HCSs nanocatalyst showed better catalytic activity than the reported catalysts. The synergistic effects due to the utilization of two metal nanoparticles and the high contact surface due to the hollow structure of the Co-Ni@HCSs nanocatalyst improved the catalytic activity of the catalyst (entry 4 vs. entries 1–3, Table 5).
3.3. Proposed reaction mechanism
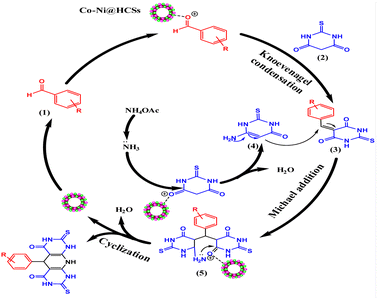
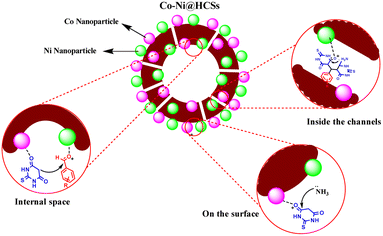
An acceptable mechanism for the formation of pyridopyrimidine derivatives is proposed35 in Scheme 1. The results show that the reaction occurs on the surface and inner surfaces of the hollow spheres. Initially, the CO-Ni@HCSs nanocatalyst acts as a Lewis acid due to the presence of an empty orbital and chelates with the carbonyl group of aldehyde 1. Due to the positive oxygen charge of the carbonyl group, favorable conditions are provided for a nucleophilic attack. The activated aldehyde 1 can be first condensed with 2-thiobarbituric acid 2 to afford heterodiene 3. This step was considered a quick Knoevenagel condensation reaction. Next, compound 3 is attacked by a Michael-type addition with extra 2-thiobarbituric acid that reacts with NH4OAc 4 and tautomerization occurs to synthesize intermediate 5. Then, the Co-Ni@HCSs nanocatalyst activates the carbonyl group of the intermediate 5. The attack of the amine group on the carbonyl group of intermediate 5 is enhanced by the Co-Ni@HCSs nanocatalyst, and finally, the cyclization of intermediate 5 occurs. After the cyclization and synthesis of the pyridopyrimidine derivatives, the Co-Ni@HCSs nanocatalyst starts a new catalytic cycle.30The active sites of the catalyst are shown in Fig. 13. According to the shape and hollow structure of the catalyst, the reaction can take place on the surface, inside the spheres, and inside the channels. The high contact surface of the catalyst increases the efficiency and reduces the reaction time. As a result, the amount of catalyst needed to carry out the reaction is also reduced.
The leaching of the Co-Ni@HCSs nanocatalyst was also studied with the hot filtration method. The reaction of 2-nitrobenzaldehyde, thiobarbituric acid, and ammonium acetate in the presence of the Co-Ni@HCSs nanocatalyst was stopped after 4.5 min, and then the nanocatalyst was separated by a paper filter from the reaction mixture. Next, the filtrate mixture was heated, and the progress of the reaction was monitored by the thin-layer chromatography technique. The monitored reaction did not progress after filtration, so the hot filtration analysis shows that Co-Ni@HCSs nanocatalyst leaching does not happen.
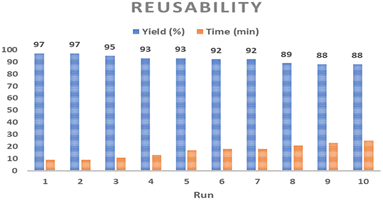
According to the FE-SEM image (Fig. 15a) and TEM image (Fig. 15b) of the Co-Ni@HCSs after 6 runs of recycling, no significant changes were observed in the structure and morphology of the catalyst. The morphological and structural stability of the catalyst after several recovery steps indicates its excellent stability and the maintenance of catalytic activity under the reaction conditions.
4. Conclusion
The basic purpose of this research was to design, prepare, and characterize economical, green, and high-performance nanocatalysts. In this study, hollow spherical structures that had a high contact surface were prepared using the hydrothermal method. The use of two metal nanoparticles caused a synergistic effect on the activity of the catalyst. The presence of the two factors of high contact surface and synergistic properties in the catalyst significantly increased the reaction efficiency and reduced the reaction time. Another essential feature of the synthesized nanocatalyst was the recovery and reuse of the catalyst with minimal reduction in performance. In this research, a catalyst was designed to synthesize pyridopyrimidine derivatives. The yield of the obtained products was excellent compared to the previous reports, and the reaction time was also short. The reaction conditions were optimized using the Box–Behnken design, which gave outcomes very close to the experimental results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the University of Kashan for supporting this work by Grant No. 159148/83.References
- A. Rifati-Nixha, M. Arslan, N. Gençer, K. Çıkrıkıçı, B. Gökçe and O. Arslan, J. Biochem. Mol. Toxicol., 2019, 33(6), 1–6 CrossRef PubMed.
- S. R. Dasari, S. Tondepu, L. R. Vadali and N. Seelam, Synth. Commun., 2020, 1–12 Search PubMed.
- R. B. Bakr and N. A. A. Elkanzi, J. Heterocycl. Chem., 2020, 57, 2977–2989 CrossRef CAS.
- P. P. Mohire, D. R. Chandam, A. A. Patravale, P. Choudhari, V. Karande, J. S. Ghosh and M. B. Deshmukh, Polycyclic Aromat. Compd., 2020, 1–19 Search PubMed.
- N. Kahriman, K. Peker, V. Serdaroğlu, A. Aydın, A. Usta, S. Fandaklı and N. Yaylı, Bioorg. Chem., 2020, 99, 103805 CrossRef CAS PubMed.
- F. Buron, J. Y. Mérour, M. Akssira, G. Guillaumet and S. Routier, Eur. J. Med. Chem., 2015, 95, 76–95 CrossRef CAS.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- N. Yan, Catal. Today, 2016, 278, 185–186 CrossRef CAS.
- M. Zhu, J. Tang, W. Wei and S. Li, Mater. Chem. Front., 2020, 4, 1105–1149 RSC.
- C. Zhang, D. Li, P. Pei, W. Wang, B. Chen, Z. Chu, Z. Zha, X. Yang, J. Wang and H. Qian, Biomaterials, 2020, 237, 119835 CrossRef CAS PubMed.
- J. Sun, Z. Dong, X. Sun, P. Li, F. Zhang, W. Hu, H. Yang, H. Wang and R. Li, J. Mol. Catal. A: Chem., 2013, 367, 46–51 CrossRef CAS.
- S. Mohammadi and H. Naeimi, Appl. Catal., A, 2020, 602, 117720 CrossRef CAS.
- X.-Y. Yu, L. Yu and X. W. D. Lou, Adv. Energy Mater., 2016, 6, 1501333 CrossRef.
- J. Wang, Y. Cui and D. Wang, Adv. Mater., 2019, 31, 1801993 CrossRef.
- J. E. Lee, C. K. Lim, H. J. Park, H. Song, S.-Y. Choi and D.-S. Lee, ACS Appl. Mater. Interfaces, 2020, 12, 35688–35697 CrossRef CAS.
- Z. Lou, Y. Wang, Y. Yang, Y. Wang, C. Qin, R. Liang, X. Chen, Z. Ye and L. Zhu, Nanomaterials, 2020, 10, 378 CrossRef CAS PubMed.
- H. Tang, Y. Zheng and Y. Chen, Adv. Mater., 2017, 29, 1604105 CrossRef.
- T. S. Atabaev and N. H. Hong, in Nano-Sized Multifunctional Materials, Elsevier, 2019, pp. 73–88 Search PubMed.
- J. W. Han, F. Hollmann, R. Luque, I. K. Song, G. Talarico, T. Tatsumi and N. Yan, Mol. Catal., 2022, 522, 112233 CrossRef CAS.
- D. V. Shinde, L. De Trizio, Z. Dang, M. Prato, R. Gaspari and L. Manna, Chem. Mater., 2017, 29, 7032–7041 CrossRef CAS.
- M. Rajabzadeh, R. Khalifeh, H. Eshghi and M. Bakavoli, J. Catal., 2018, 360, 261–269 CrossRef CAS.
- M. Sankar, N. Dimitratos, P. J. Miedziak, P. P. Wells, C. J. Kiely and G. J. Hutchings, Chem. Soc. Rev., 2012, 41, 8099 RSC.
- S. De, J. Zhang, R. Luque and N. Yan, Energy Environ. Sci., 2016, 9, 3314–3347 RSC.
- H. Naeimi and S. Mohammadi, ChemistrySelect, 2020, 5, 2627–2633 CrossRef CAS.
- A. H. Ghasemi and H. Naeimi, New J. Chem., 2020, 44, 5056–5063 RSC.
- H. Naeimi, A. Didar, Z. Rashid and Z. Zahraie, J. Antibiot., 2017, 70, 845 CrossRef CAS PubMed.
- A. Zare, A. Kohzadian, Z. Abshirini, S. S. Sajadikhah, J. Phipps, M. Benamara and M. H. Beyzavi, New J. Chem., 2019, 43, 2247–2257 RSC.
- M. S. Mohamed, S. M. Awad and A. I. Sayed, Molecules, 2010, 15, 1882–1890 CrossRef CAS PubMed.
- A. Zare, A. Kohzadian, H. Filian, M. S. G. Nezhad and A. Karami, Res. Chem. Intermed., 2022, 48, 1631–1644 CrossRef CAS.
- H. Naeimi and A. Didar, J. Mol. Struct., 2017, 1137, 626–633 CrossRef CAS.
- G. Zhang, H. Zhang, D. Yang, C. Li, Z. Peng and S. Zhang, Catal. Sci. Technol., 2016, 6, 6417–6430 RSC.
- X. Han, W. Yan, K. Chen, C.-T. Hung, L.-L. Liu, P.-H. Wu, S.-J. Huang and S.-B. Liu, Appl. Catal., A, 2014, 485, 149–156 CrossRef CAS.
- A. H. Ghasemi, A. Farazin, M. Mohammadimehr and H. Naeimi, Mater. Today Commun., 2022, 31, 103513 CrossRef CAS.
- A. Farazin, M. Mohammadimehr, A. H. Ghasemi and H. Naeimi, RSC Adv., 2021, 11, 32775–32791 RSC.
- H. Naeimi and A. Didar, Ultrason. Sonochem., 2017, 34, 889–895 CrossRef CAS PubMed.
- H. Naeimi, A. Didar and Z. Rashid, J. Iran. Chem. Soc., 2017, 14, 377–385 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra07152e |
| This journal is © The Royal Society of Chemistry 2023 |