Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhanced photocatalytic activity in ZnO nanoparticles developed using novel Lepidagathis ananthapuramensis leaf extract†
Supin K Ka,
Parvathy Namboothiri P Ma and
M. Vasundhara *ab
*ab
aPolymers and Functional Materials Department, CSIR- Indian Institute of Chemical Technology, Hyderabad, 500007, India. E-mail: mvas@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India
First published on 6th January 2023
Abstract
The present study focuses on the green synthesis of zinc oxide nanoparticles (ZnO NPs) using a novel Lepidagathis ananthapuramensis (LA) leaf extract and a systematic study on the photocatalytic degradation of methylene blue (MB) dye. The structural, thermal, morphological, optical, and surface area analysis of prepared ZnO NPs were examined using X-ray diffraction (XRD), UV-visible spectroscopy, Raman spectroscopy, Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), Brunauer–Emmett–Teller (BET) analysis, thermogravimetric analysis (TGA), field emission-scanning electron microscopy (FE-SEM), energy dispersive X-ray analysis (EDAX) and high-resolution transmission electron microscopy (HR-TEM). The LA stabilised ZnO NPs produced NPs with diverse morphologies, low band gap and cost-effective high yield of production. A systematic study has been carried out to determine the crystallinity and crystallite size of ZnO NPs based on the concentration of Zn(NO3)2 precursor, concentration of LA leaf extract, calcination temperature and calcination time. The crystallinity and crystallite size of ZnO NPs were evaluated based on the XRD technique. The photocatalytic activity of ZnO NPs was thoroughly investigated for the degradation of MB dye based on various physicochemical parameters such as reaction time, concentration of catalyst, concentration of precursors, concentration of LA extract, concentration of MB, calcination temperature and calcination time. These systematic photocatalytic studies followed green protocols and provided an excellent photocatalytic efficiency result of 96–98.5% towards the decomposition of MB. Hence, this material can work as a potential candidate for waste water treatment by also degrading other toxic dyes.
1. Introduction
Over the last decade, various metal oxides such as ZnO,1–3 CuO,4 NiO,5 CoO,6 Fe2O3,7 MnO2,8 TiO2,9,10 SnO2,11 MgO,12 etc have been synthesised for numerous applications, among which photocatalytic applications and waste water treatment have been explored enormously. Among various metal oxides, ZnO and TiO2 NPs are studied the most due to their non-toxic nature, cost effectiveness, chemical stability and inertness, eco-friendliness, potential photocatalytic efficiency, etc.13,14. Even though both are effectively used, ZnO NPs are generally a better candidate for photocatalytic degradation of pollutants because of their high stability, non-toxicity,15 single oxidation state, natural abundance, suitable band gap16 and better biocompatibility.17 ZnO is a wide bandgap (3.37 eV)18 semiconductor which acts as an excellent photocatalyst for the decomposition of water pollutants.19 One of the top most priorities of the present-day research is for clean water in order to retain the ecosystem. Rapid growth of textile industries is one of the major causes for the water contamination. Dyes and its effluents from textile, food, and printing industries are the major pollutant released in water bodies. Generally, dyes are highly toxic and non-biodegradable because of its complex nature, causing serious threat to the ecosystem. methylene blue (MB) is a widely used dye in textile industries, which is extremely carcinogenic and toxic.20 Presently, more than 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes of dye are produced every year and its waste contents are released into water bodies by the industries.21 This is undoubtedly an alarming situation which needs to be addressed urgently. Various conventional methods such as coagulation, adsorption, ultrafiltration, ozonisation etc. are available, but they deal with the removal of dyes, effective in destroying some of the dyes only and need post treatment of secondary waste materials.22 So, semiconductor photocatalysis23 involving advanced oxidation process (AOP) has gained immense attention due to its effectiveness in degradation of the toxic dyes without generating any harmful products. Dye degradation using semiconductor photocatalyst is more environmentally friendly method, which can be a possible contender in waste water treatment.
000 tonnes of dye are produced every year and its waste contents are released into water bodies by the industries.21 This is undoubtedly an alarming situation which needs to be addressed urgently. Various conventional methods such as coagulation, adsorption, ultrafiltration, ozonisation etc. are available, but they deal with the removal of dyes, effective in destroying some of the dyes only and need post treatment of secondary waste materials.22 So, semiconductor photocatalysis23 involving advanced oxidation process (AOP) has gained immense attention due to its effectiveness in degradation of the toxic dyes without generating any harmful products. Dye degradation using semiconductor photocatalyst is more environmentally friendly method, which can be a possible contender in waste water treatment.
The major worldwide challenge is to synthesise these metal oxide NPs in a cost effective, eco-friendly and sustainable manner. There are various conventional methods available such as physical methods (ball milling, solid state, physical vapor deposition etc.), chemical methods (sol–gel, coprecipitation, solution combustion, hydrothermal etc.) etc for synthesising metal oxide NPs.1–12 But these methods fail to address the problems related to toxicity, cost, waste production and environmental contamination. In order to overcome them, today's researchers have been immensely focusing on the green chemistry route for synthesizing metal oxides. Green approach towards the synthesis of metal oxide NPs is more eco-friendly,24 economically viable, socially accepted, safer, sustainable to environment etc. and account to increase in the yields of production.25 Synthesis of metal oxide NPs through greener route involves various biological components such as bacteria, fungi, algae, leaves, root etc. Among the available greener methods, the use of leaf extracts is the best way as it involves simplest, less investment, shorter time period, highly efficient, non-pathogenic26 and feasible process to prepare metal oxide at larger scale relative to bacteria, fungi and algae mediated synthesis. Various leaf extract such as Cayratia pedata27 Raphanus sativus var. Longipinnatus28, Tectona grandis (L.)29, Cassia fstula, Melia azedarach30 etc. were used as the stabilising agent for the synthesis of ZnO NPs. Therefore, in this regard, we have focused on synthesis of ZnO NPs from the Lepidagathis ananthapuramensis (LA) leaf extract, which is a highly effective green approach.
LA is a new species recently discovered in the Ananthapuram region of Kasaragod district, Kerala.31 It belongs to the Acanthaceae family, whose advanced studies related to medicinal or any other applications need to be done. The use of this LA leaf is a green mediated route for the synthesis of ZnO NPs. The leaf extract plays a crucial role in the synthesis process as it contains phytochemicals. These phytochemicals act as effective capping, stabilizing and reducing agents in the synthesis process. The phytochemicals present in the LA leaf extract are shown in the supplementary file,† which has been evaluated based on the method described in.32,33 The phytochemicals present in the LA leaf extract like alkaloids, flavonoids, terpenoids, glycosides, saponins, tannins, cardiac glycosides, reducing sugars, quinones, phenols etc. facilitate the production of ZnO NPs to give a high yield. In this one pot synthesis of ZnO NPs, the functional groups present in these phytochemicals play a binary role i.e., they act as effective reducing and capping agents. The use of LA extract for the synthesis of ZnO NPs is a novel approach which can provide diverse nature of particles with the effectiveness in controlling its size and shape. Moreover, this leaf extract can provide better results related to other properties of ZnO NPs which has been clearly discussed in the entire work. Also, particles and crystallites size, shape, surface area and band gap are highly affected by the type of precursor, concentration of precursor, calcinations temperature, doping etc.34,35
Focusing on the eco-friendly approach, the present study emphasised on the novel LA leaf mediated synthesis of ZnO NPs and its capability of degrading the MB dye using solar driven photocatalytic process. The great band gap tuning, varied morphologies, outstanding stability, etc. of ZnO NPs enable them to achieve good photocatalytic outcomes. A systematic study of evaluating the photocatalytic efficiency of ZnO NPs was done based on various physicochemical parameters like importance of sunlight and ZnO catalyst, catalyst loading, concentrations of dye, zinc nitrate hexahydrate Zn(NO3)2·6H2O and LA extract, calcination temperatures and calcination hours. This systematic study can help to decide the best material for the excellent decomposition of water pollutant.
2. Experimental set-up
2.1 Materials
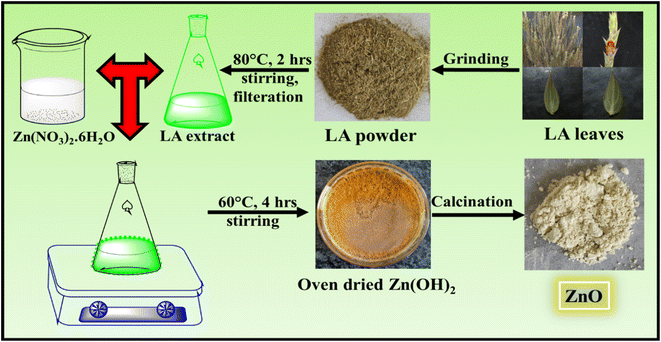
LA leaves were procured from Ananthapuram region (Anandashram), Kasaragod district, Kerala. Zn(NO3)2·6H2O (99% purity) was purchased from Sisco Research Laboratory (SRL) Pvt. Ltd. Methylene blue was purchased from Sigma-Aldrich. Distilled water was utilised for the synthesis.2.2 Synthesis of LA leaf extract
LA leaves were collected, washed with distilled water, dried under shade for 1 week and hot air oven dried for couple of days. Then the leaves were grinded to fine powder using mixing grinder. This fine powder was used as a stock powder for future prospects.10 g of fine powder of LA leaves were added to 250 mL distilled water taken in a 500 mL conical flask. The LA solution was stirred continuously for 2 hours at 80 °C. The solution was then cooled down to room temperature and filtered using a cotton cloth. The filtrate was used as the LA extract for the synthesis of ZnO NPs and stored in the refrigerator for future uses.
2.3 Synthesis of ZnO NPs using LA extract
Firstly, the synthesis of ZnO was done using 25 mL 0.1 M Zn(NO3)2·6H2O and 5 mL LA extract. Both the solutions were mixed well and stirred continuously at 60 °C for 4 hours in a 100 mL conical flask using a hot magnetic stirrer. As the reaction proceeds, the colour changes from green to dark green. The final product obtained was oven dried and calcined at 500 °C for 2 hours. The product calcined at 500 °C was grinded well using a mortar and pestle. Then this calcined sample was used for the characterisation to confirm the formation of ZnO NPs.Further property study of ZnO NPs was done with respect to concentration of Zn(NO3)2·6H2O, concentrations of LA extract, calcination temperatures and calcination hours following the same procedure mentioned earlier. (Table 1) The following table shows the different parameter used for the property studies and their corresponding sample name which is mentioned in their characterisation plots (Fig. 1).
| Variable parameters | No. of samples synthesised | Sample name | Concentration of Zn(NO3)2 | Leaf extract | Calcination temperature | Calcination hours |
|---|---|---|---|---|---|---|
Sample names (ZnO-0.10, ZnO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, ZnO-500 and ZnO-2) are the same material. These sample names are given as common code ZnO-LA, reflected in the characterisation plots. 5, ZnO-500 and ZnO-2) are the same material. These sample names are given as common code ZnO-LA, reflected in the characterisation plots. |
||||||
| Concentration of Zn(NO3)2 | 1 | ZnO-0.025 | 25 mL 0.025M | 5 mL | 500 °C | 2 |
| 2 | ZnO-0.05 | 25 mL 0.05 M | ||||
| 3 | ZnO-0.10 | 25 mL 0.10 M | ||||
| 4 | ZnO-0.15 | 25 mL 0.15 M | ||||
| 5 | ZnO-0.20 | 25 mL 0.20 M | ||||
| 6 | ZnO-0.30 | 25 mL 0.30 M | ||||
| 7 | ZnO-0.50 | 25 mL 0.50 M | ||||
| 8 | ZnO-0.70 | 25 mL 0.70 M | ||||
| 9 | ZnO-1.00 | 25 mL 1.00 M | ||||
| Concentration of LA extract | 10 | ZnO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
25 mL 0.10 M | 5 mL | 500 °C | 2 |
| 11 | ZnO-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
10 mL | ||||
| 12 | ZnO-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
15 mL | ||||
| 13 | ZnO-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
20 mL | ||||
| 14 | ZnO-5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
25 mL | ||||
| Calcination temperature | 15 | ZnO-500 | 25 mL 0.10 M | 5 mL | 500 °C | 2 |
| 16 | ZnO-600 | 600 °C | ||||
| 17 | ZnO-700 | 700 °C | ||||
| 18 | ZnO-800 | 800 °C | ||||
| 19 | ZnO-900 | 900 °C | ||||
| 20 | ZnO-1000 | 1000 °C | ||||
| Calcination hours | 21 | ZnO-2 | 25 mL 0.10 M | 5 mL | 500 °C | 2 |
| 22 | ZnO-4 | 4 | ||||
| 23 | ZnO-6 | 6 | ||||
The crystallite size and crystallinity percentage are calculated using the following equations:
Crysatallite size, D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
2.4 Photocatalytic set-up
The photocatalytic studies were done using ZnO NPs as a semiconductor photocatalyst and MB dye as the model dye. The photocatalytic activity of ZnO NPs was analysed based on the observation of degradation (decolouration) of MB dye. The photocatalytic efficiency is calculated using the following equations; [A0 − A/A0] × 100 or [C0 − C/C0] × 100 where A0 and A is the initial (0 minute) and final (90 minute) absorbance values of the MB solution without and with ZnO semiconductor photocatalyst and C0 and C are the 0 minute and 90 minute MB dye concentrations without and with ZnO semiconductor photocatalyst at 0 minute and 90 minutes respectively.The photocatalytic experimental set-up is developed by exposing reaction mixture (ZnO-MB dye solution) in sunlight under constant stirring of 500 rpm in a magnetic stirrer. The solar light intensity was measured with the help of lux meter. Initially for trial basis, 5 mg of ZnO-1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was used and added to the 50 mL of 10−5 M MB dye solution. The solution then stirred continuously under sunlight. At a regular interval of 15 minutes, 5 mL of reaction mixture is taken out using a micropipette by switching of the stirring process. Up to 90 minutes, 6 such aliquots were taken and each mixture was centrifuged at 10
5 was used and added to the 50 mL of 10−5 M MB dye solution. The solution then stirred continuously under sunlight. At a regular interval of 15 minutes, 5 mL of reaction mixture is taken out using a micropipette by switching of the stirring process. Up to 90 minutes, 6 such aliquots were taken and each mixture was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes. Each mixture was then analysed using the UV-visible spectroscopy over the wavelength range of 200–800 nm, in order to check the absorbance values. The change in absorbance values is the guidance towards the MB degradation studies.
000 rpm for 10 minutes. Each mixture was then analysed using the UV-visible spectroscopy over the wavelength range of 200–800 nm, in order to check the absorbance values. The change in absorbance values is the guidance towards the MB degradation studies.
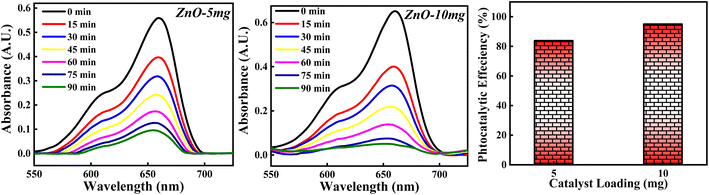
The similar procedure was followed for the systematic study of photocatalysis based on various parameters i.e., influence of sunlight and no sunlight (dark reactions), catalyst loading, effect of concentrations of Zn(NO3)2·6H2O precursor, LA extract and MB dye, effect of calcinations temperatures and effect of calcination hours. Most of the photocatalytic activity of ZnO NPs based on various parameters has been compared with ZnO-LA and 5 mg ZnO photocatalyst is used for the photocatalytic studies.
2.5 Characterisation techniques
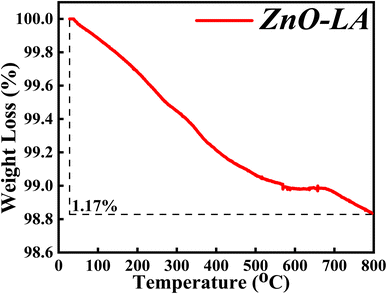
The structural characterisation was done based on XRD analysis with the help of PAN analytical X'Pert Pro diffractometer using the wavelength of Cu-Kα (λ = 1.54060 Å) radiation. The materials were slow scanned in the range of 20–80° with the step size of 0.02. The optical properties were studied with the help of UV-1800 Shimadzu spectrophotometer. The characteristic structural information of ZnO was analysed using LabRam HR evolution Raman spectrometer having a 633 nm laser (Horiba scientific, France). FT-IR spectroscopy was performed using PerkinElmer 100 spectrometer (USA) in the scan range of 4000–400 cm−1. The electronic states were analysed using Thermo Scientific K-Alpha XPS instrument having monochromatic Al Kα radiation, Ephoton = 1486.6 eV. TGA was employed to check the chemical and thermal stability using TA Q500 Instrument (USA) in the range of 0–800 °C at 5° min−1 heating rate under nitrogen atmosphere. The BET was employed to check the surface area using Microtrac BEL instrument (BELPREP vacII, JAPAN). The surface and elemental composition were studied using FE-SEM/EDAX analysis with the help of Hitachi-S520 (Oxford link ISISSEM Model), Japan.3. Results and discussions
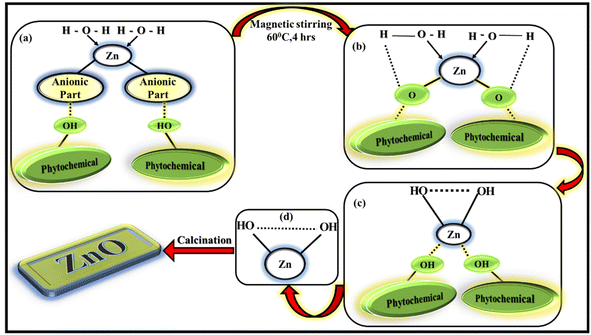
3.1 Possible mechanism of synthesising ZnO NPs from LA leaf template
The importance of leaf extract for synthesising ZnO NPs is because of the availability of phytochemicals present in leaf extract. The phytochemicals contain different types of functional groups like ketone, aldehyde, hydroxyl, carboxylic acid etc. which helps in the formation of ZnO NPs involving chemical reduction and oxidation process. The present LA extract contain alkaloids, flavonoids, phenols, quinones etc, as mentioned in ESI† file. These phytochemicals will act as effective capping agents as well as reducing-oxidising agents in the synthesis process. The exact mechanism of forming ZnO NPs from leaf extract is not yet understood completely, several research works are being performed to study the exact mechanism. (Fig. 2) The possible mechanism of forming ZnO NPs via greener way involves two steps as mentioned in:351. Formation of Zn(OH)2 from the corresponding Zn(NO3)2·6H2O salt.
2. Calcination of the subsequent reaction product into ZnO NPs.
Zn2+ ions present in the zinc electrolytic solution [Zn(NO3)2·6H2O solution]is reduced to the stabilised compound by chelating to the phytochemicals such as flavonoids and polyphenols, present in the leaf extract during the reaction. The anionic part of zinc salt will be eliminated as corresponding acid by taking proton from the phytochemicals. The water molecules will try to donate its proton to the corresponding phytochemicals. As a result, the hydroxyl group present in the reaction mixture (biomolecule or in solution) will try to make bond with stabilised metal species leading to the generation of zinc hydroxide (Zn(OH)2). All these processes occur simultaneously. The obtained solution is oven-dried overnight and a yellow–orange pre-product was obtained which was calcined to eliminate the hydroxides and the impurities to finally obtain ZnO NPs.
3.2 Analysis of synthesized ZnO NPs
Crysatallite size, D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
| Sample | ZnO |
|---|---|
| Cell parameter | |
| Crystal structure | Hexagonal |
| Space group | P63mc |
| Point group | 6 mm or C6v |
| a (Å) | 3.25 |
| b (Å) | 3.25 |
| c (Å) | 5.21 |
| α | 90 |
| β | 90 |
| ϒ | 120 |
| Volume (Å3) | 47.61 |
| Density (g cm−3) | 5.68 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Positional parameter | |
| Zn x | 0.3333 |
| Zn y | 0.6667 |
| Zn z | 0.0024 |
| O x | 0.3333 |
| O y | 0.6667 |
| O z | 0.3814 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Site occupation | |
| Zn | 1.0 |
| O | 1.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| Agreement factors | |
| RWP | 15.8 |
| RP | 11.7 |
| REXP | 12.0 |
| χ2 | 1.74 |
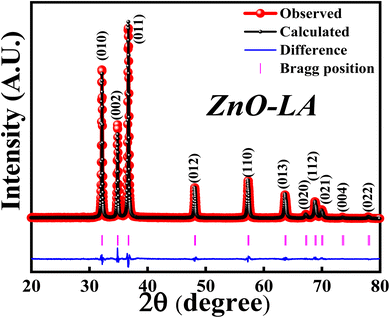
It was observed that the synthesized ZnO NPs have the 81.40% crystallinity with the average crystallite size of 28.12 nm. This indicates that the sample is highly crystalline possessing nano-crystallites. Further study of XRD analysis with respect to effect of concentrations of precursor and LA extract, effect of calcinations temperatures and calcinations hours are discussed later.
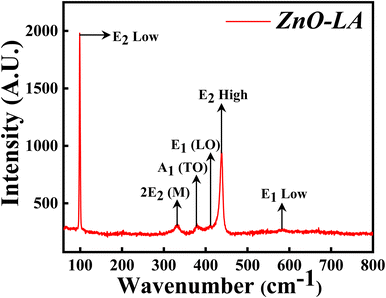
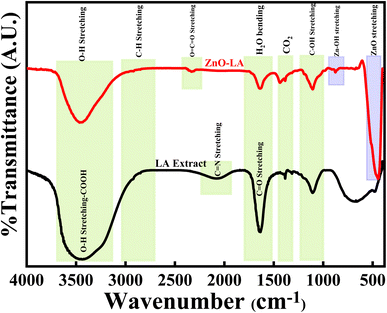
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency, C
N stretching frequency, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching frequency and C–OH stretching frequency respectively. These peaks arised due to various phytochemicals content such as quinones, alkaloids etc. present in the leaf extract. However, some of the peaks were absent in the case of calcined ZnO NPs. ZnO NPs showed characteristic strong intense peak around 445 cm−1, small intense shoulder peak around 525 cm−1 and small intense at 874 cm−1 which belong to Zn–O and Zn–OH stretching vibrations. All these vibrations are due to the Zn–O bond present in the crystal lattice. Also, ZnO NPs showed peaks at 3640–3140 cm−1, 1632 cm−1 and 1420 cm−1 that are attributed to the O–H stretching, H2O bending vibrations of adsorbed moisture and atmospheric CO2 interference during analysis. All the peaks obtained are well matched with earlier results47–51
C stretching frequency and C–OH stretching frequency respectively. These peaks arised due to various phytochemicals content such as quinones, alkaloids etc. present in the leaf extract. However, some of the peaks were absent in the case of calcined ZnO NPs. ZnO NPs showed characteristic strong intense peak around 445 cm−1, small intense shoulder peak around 525 cm−1 and small intense at 874 cm−1 which belong to Zn–O and Zn–OH stretching vibrations. All these vibrations are due to the Zn–O bond present in the crystal lattice. Also, ZnO NPs showed peaks at 3640–3140 cm−1, 1632 cm−1 and 1420 cm−1 that are attributed to the O–H stretching, H2O bending vibrations of adsorbed moisture and atmospheric CO2 interference during analysis. All the peaks obtained are well matched with earlier results47–51
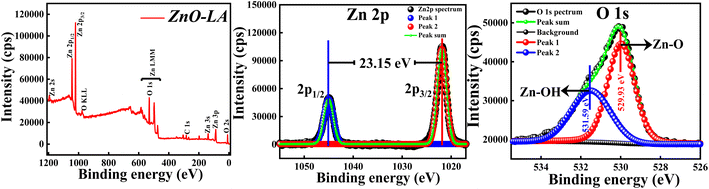
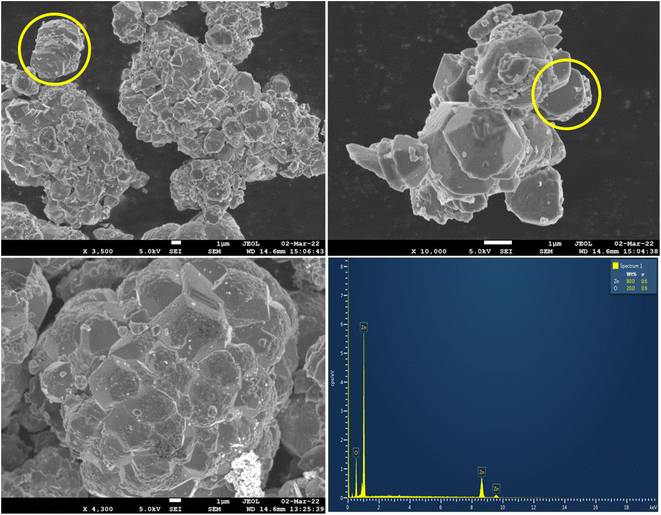
The elemental analysis of ZnO NPs showed four absorption peaks pertaining to Zn and O with the weight percentage of 80 and 20. There are no additional peaks observed indicating that the synthesised ZnO NPs is of high purity.
3.3 Crystallinity and crystallize size studies based on XRD analysis
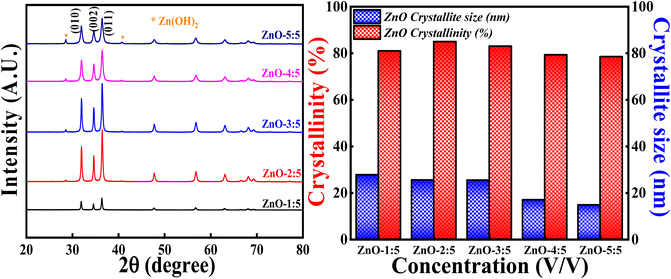 | ||
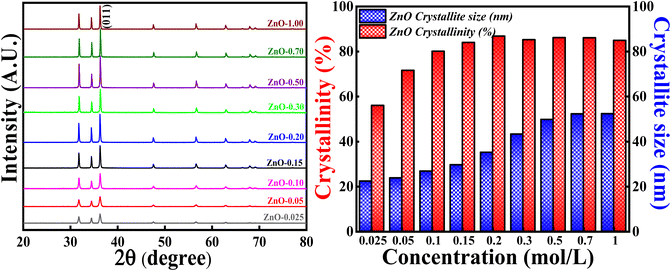
| Fig. 13 (Left) XRD patterns and crystallinity, (Right) crystallite size studies based on volume ratio LA: Zn(NO3)2·6H2O. | ||
The broadness of the highly intense (011) peak can be seen as the concentration of LA is increased, as represented in Fig. 13 and S5.† The broadness of this peak signifies the formation of the small crystallites due to the large FWHM value (Scherrer's equation). This is clearly reflected in the crystallite size also, as the concentration of LA extract is increased, crystallite size simultaneously decreased. The lowest crystallite size is obtained for ZnO-5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 i.e., 14.72 nm. The decrement in the crystallite size may be due to the increase in the concentration of stabilising agent (effective capping), which will prevent the particles (crystallites) to agglomerate.55 So, the smaller crystallite size can be obtained for ZnO NPs involving more LA content in the synthesis process. But increase in the concentration of LA extract generates other impurity phases also. It is also observed that the degree of crystallinity is first increasing slightly and then decreasing as the concentration of LA content is increased. This can be due to the formation of Zn(OH)2 impurity phase. The obtained results are in well agreement with earlier report.56 Hence, the degree of crystallinity and size of the crystallites are significantly influenced with the amount of LA extract used in the synthesis process.
5 i.e., 14.72 nm. The decrement in the crystallite size may be due to the increase in the concentration of stabilising agent (effective capping), which will prevent the particles (crystallites) to agglomerate.55 So, the smaller crystallite size can be obtained for ZnO NPs involving more LA content in the synthesis process. But increase in the concentration of LA extract generates other impurity phases also. It is also observed that the degree of crystallinity is first increasing slightly and then decreasing as the concentration of LA content is increased. This can be due to the formation of Zn(OH)2 impurity phase. The obtained results are in well agreement with earlier report.56 Hence, the degree of crystallinity and size of the crystallites are significantly influenced with the amount of LA extract used in the synthesis process.
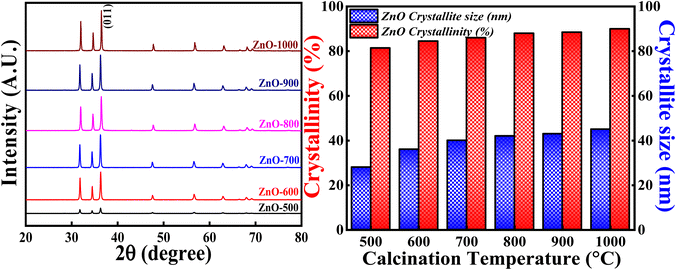 | ||
| Fig. 14 (Left) XRD patterns, (Right) crystallinity and crystallite size studies based on calcinations temperature. | ||
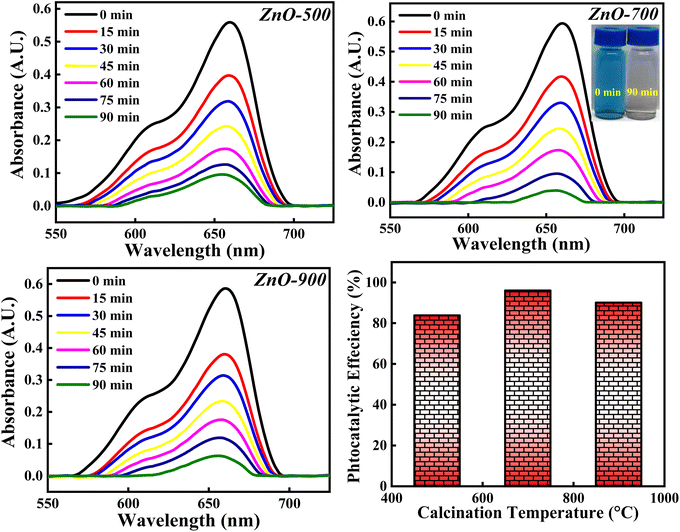
Similarly, the crystallite size showed increasing trend as the calcinations temperature is increased. But the crystallite size increased more from 500 °C to 700 °C and then slight increment from 700 °C to 1000 °C. The more increment in the crystallite size from 500 °C to 700 °C can be due to the coalescence process.47,48 This process can cause major crystal and grain growth enhancing surface roughness and porosity. The later slight increment in the crystallite size from 700 °C to 1000 °C can be due to the higher mobility of atoms leading them to agglomerate. Sometimes, random motion of the atoms follows dispersion and diffusion as well. As a result, all the factors compete with each other in order to determine the crystallite size. Generally, the increase in the crystallite size can be related to the merging of particles as the consequence of thermal calcination. Hence, the calcinations temperature plays a vital role in tuning the crystallite size and degree of crystallinity.
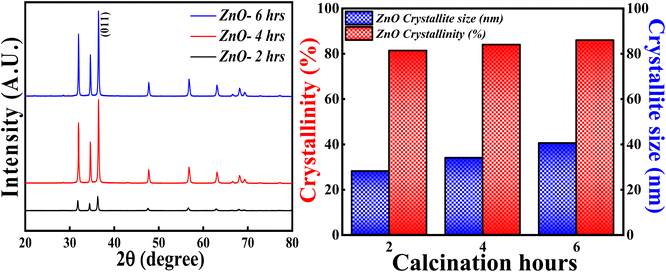 | ||
| Fig. 15 (Left) XRD patterns, (Right) crystallinity and crystallite size studies based on calcinations hours. | ||
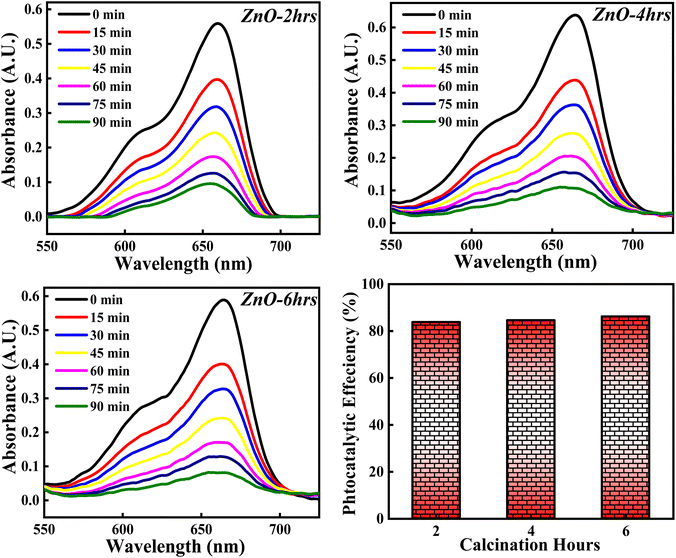
The main observation from the XRD results is that the degree of crystallinity and crystallite size can be tuned based on the concentration of precursors, leaf extract, calcinations temperatures and calcinations hours. The appropriate sample can be used for the effective decomposition of MB dye based on their properties.
3.4 Photocatalytic studies
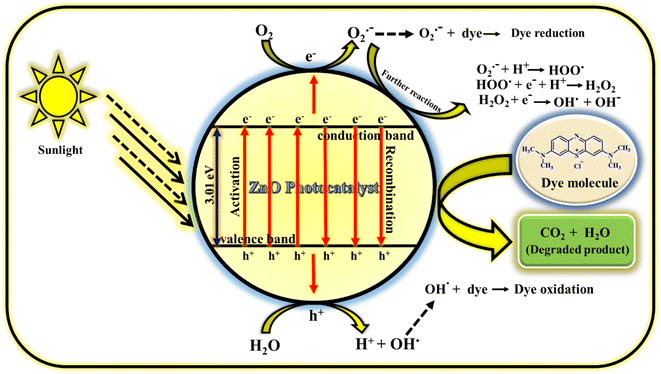 | ||
| Fig. 16 Mechanism of photocatalytic performance of ZnO NPs using MB dye.60 | ||
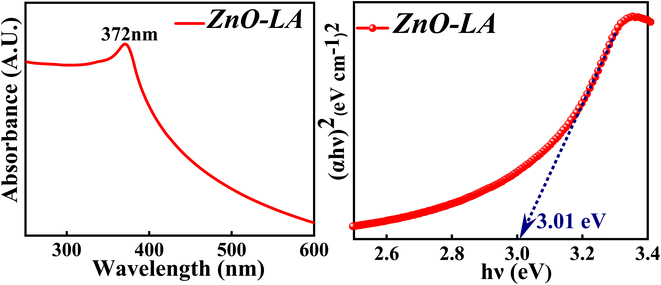
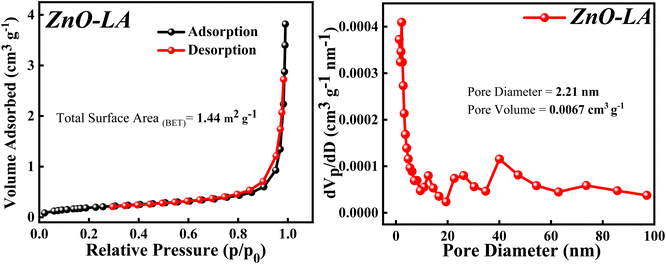
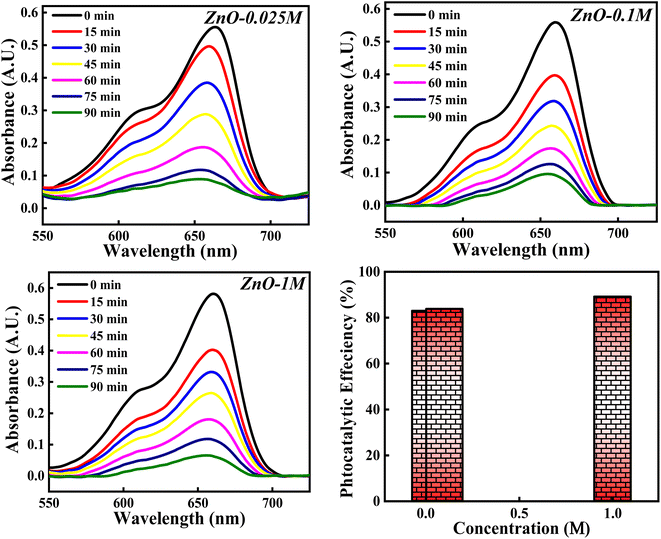
There is a slight variation of in band gap values (3.00–3.01 eV) for 0.025 M, 0.05 M and 0.1 M concentration was observed, as depicted in Fig. S6.† Above the 0.1 M concentrations, the band gap was not able to calculate which can be due to the poor dispersion of the particles in solution as a result of agglomeration for the higher concentration. Similarly, total surface area calculated from BET didn't show much variation, which can reflect in the photocatalytic results (Fig. S7†). The 5.02% increment in the photocatalytic efficiency for 1 M from 0.1 M concentrations can be due to the defect generation (Ov, Zni) which can introduce defect level in between the conduction and valence bands. This impurity level can act as trappers for charge carriers which can enhance the recombination process65 and thus improving the degradation process. Moreover, as the particle size increases, band gap decreases (quantum size effect). Hence, the photocatalytic activity of ZnO NPs catalyst for the degradation of MB dye depends on the precursor concentrations as well.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, ZnO-3
5, ZnO-3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and ZnO-5
5 and ZnO-5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
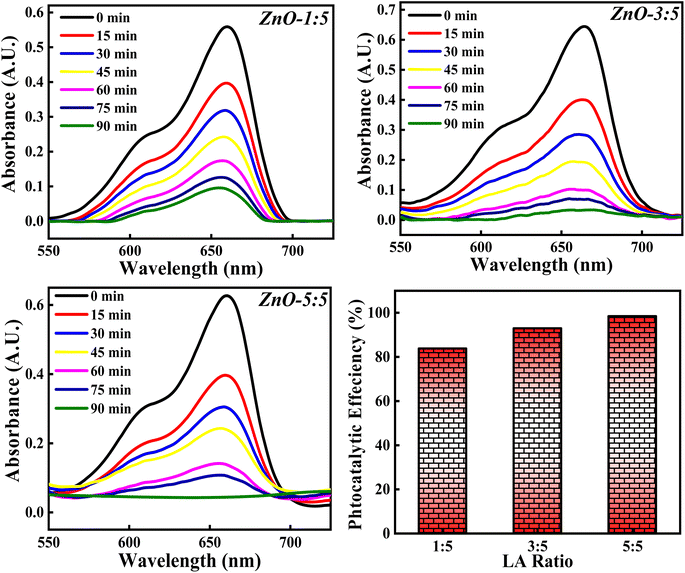
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 is 84.10%, 92.36% and 98.50%. It is clearly noticed that the photocatalytic potency is increasing significantly with the increase in the concentration of LA extract. This significant increase in the photocatalytic degradation of MB can be due to various reasons such as Zn(OH)2 impurity phase generation, small size crystallites, higher surface area, enhanced recombination process etc. From the XRD results, it is clearly observed that the crystallite size decreases and the concentration of Zn(OH)2 increases as the LA extract concentrations are increased. Firstly, the presence of Zn(OH)2 can introduce an impurity level below the conduction band, which can act as scavengers of charge carriers enhancing the recombination time,66 thus reducing the band gap. Moreover, the presence of surface hydroxyl group, facilitate the formation of non-covalent bond with the dye molecules. Thus, enhancing the adsorption can improve the photocatalytic process. Secondly, as the concentration of LA is increased, the crystallite size is decreased confirming the presence of small size particles providing higher surface area for adsorption of dye molecules. Thirdly, the presence of hydroxyl group on the catalyst surface can produce OH˙, enhancing the photocatalytic decomposition of MB dye.12 So, the factors such as presence of OH group, small size crystallites, and high surface area enhance the photocatalytic process. So, the concentration of LA extract should be optimised in order to get small size ZnO particles for 100% MB degradation, without generating any impurity phases such as Zn(OH)2. Hence, the concentration of LA extract is greatly influencing the dye degradation process.
5 is 84.10%, 92.36% and 98.50%. It is clearly noticed that the photocatalytic potency is increasing significantly with the increase in the concentration of LA extract. This significant increase in the photocatalytic degradation of MB can be due to various reasons such as Zn(OH)2 impurity phase generation, small size crystallites, higher surface area, enhanced recombination process etc. From the XRD results, it is clearly observed that the crystallite size decreases and the concentration of Zn(OH)2 increases as the LA extract concentrations are increased. Firstly, the presence of Zn(OH)2 can introduce an impurity level below the conduction band, which can act as scavengers of charge carriers enhancing the recombination time,66 thus reducing the band gap. Moreover, the presence of surface hydroxyl group, facilitate the formation of non-covalent bond with the dye molecules. Thus, enhancing the adsorption can improve the photocatalytic process. Secondly, as the concentration of LA is increased, the crystallite size is decreased confirming the presence of small size particles providing higher surface area for adsorption of dye molecules. Thirdly, the presence of hydroxyl group on the catalyst surface can produce OH˙, enhancing the photocatalytic decomposition of MB dye.12 So, the factors such as presence of OH group, small size crystallites, and high surface area enhance the photocatalytic process. So, the concentration of LA extract should be optimised in order to get small size ZnO particles for 100% MB degradation, without generating any impurity phases such as Zn(OH)2. Hence, the concentration of LA extract is greatly influencing the dye degradation process.
A comparative study of photocatalytic efficiency of ZnO NPs of current work with the previous results is represented in Table 3.
| Sample name | Synthesis method | Light source | % Efficiency (time) | Photocatalyst/MB concentration | Reference |
|---|---|---|---|---|---|
| MxZn1−xO (M = Al3+, Fe3+, Cr3+) | Chemical method | Visible light | 91% (120 min) | — | 68 |
| PQ/ZnO | Chemical method | Sunlight | 80% (90 min) | 50 mg/10 ppm | 69 |
| ZnO | Chemical method (autoclave) | Mercury vapor lamp | 92% (60 min) | 50 mg/10 ppm | 70 |
| ZnO NPs | Green method-Sambucus ebulus extract | UV light | 80% (200 min) | 20 mg/50 ppm | 71 |
| ZnO NPs | Green method-LA leaf extract | Sunlight | 98.5% (120 min) | 5 mg/10 ppm | Present work |
4. Conclusion
The green approach towards the synthesis of ZnO NPs using a novel LA leaf extract offered a simple, stable, economically viable, less invested, sustainable and eco-friendly method. The systematic study of ZnO NPs showed diverse morphologies, low band gap (2.81–3.01 eV) with increase in calcinations temperatures, and generation of Zn(OH)2 with the increase in LA concentration. The crystallinity percentage and crystallite size were significantly affected with the variation in concentration of precursor, concentration of LA leaf extract, calcinations temperatures and calcinations hours which played a crucial role in the activity of ZnO NPs as a photocatalyst. The mentioned synthesis process can be employed in industrial scale so that waste minimisation can be increased as well as enhancing simple high yield production. To be employed in industrial scale, all the synthesis parameters should be optimised in such a way that ZnO NPs can be accumulated in a larger amount. The photocatalytic application of ZnO NPs showed an excellent result by degrading the MB dye completely. The dye degradation study was done systematically based on various physicochemical parameters which guided towards the efficient photocatalytic result, 96–98.5% (5 mg degrading 10 ppm MB dye completely). Hence, ZnO NPs photocatalyst synthesised through systematic green chemistry protocol can be used effectively for degrading other toxic dyes and can be employed in large-scale for the effective treatment of waste water.Author contributions
Supin K. K: writing – original draft, methodology. Formal analysis, investigation, conceptualization, validation and visualization, Parvathy Namboothiri P M: partial analysis and visualization, M. Vasundhara: conceptualization, writing – review & editing, visualization, supervision, project administration and funding acquisition.Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this article.Acknowledgements
The authors gratefully acknowledge the financial support from the Science and Engineering Research Board (SERB), Government of India, by a grant no.: CRG/2019/001574. M. Vasundhara would like to acknowledge the support offered by Department of K&IM of Indian Institute of Chemical Technology (IICT/Pubs./2022/324).References
- V. Jawale, G. Gugale, M. Chaskar, S. Pandit, R. Pawar, S. Suryawanshi, V. Pandit, G. Umarji and S. Arbuj, J. Mater. Res., 2021, 36, 1573–1583 CrossRef CAS.
- Q. Li, P. Wang, S. Liu, Y. Fei and Y. Deng, Green Chem., 2016, 18, 6091–6098 RSC.
- X. Jiang, W. Wang, H. Wang, Z. H. He, Y. Yang, K. Wang, Z. T. Liu and B. Han, Green Chem., 2022, 24, 7652–7660 RSC.
- A. D. Kute, R. P. Gaikwad, I. R. Warkad and M. B. Gawande, Green Chem., 2022, 24, 3502–3573 RSC.
- L. Cai, B. Yan, Q. Yue, J. L. Li, P. Liu, X. Qi and G. Yang, J. Mater. Chem. A, 2022, 10, 18939–18949 RSC.
- L. Yan, D. Chu, X. Q. Chu, D. Ge and X. Chen, New J. Chem., 2022, 46, 15071–15079 RSC.
- S. Yu, V. M. H. Ng, F. Wang, Z. Xiao, C. Li, L. B. Kong, W. Que and K. Zhou, J. Mater. Chem. A, 2018, 6, 9332–9367 RSC.
- J. Dai, A. F. Patti, G. N. Styles, S. Nanayakkara, L. Spiccia, F. Arena, C. Italiano and K. Saito, Green Chem., 2019, 21, 2005–2014 RSC.
- Y. Cao, D. Chen, Y. Meng, S. Saravanamurugan and H. Li, Green Chem., 2021, 23, 10039–10049 RSC.
- V. R. Akshay, B. Arun, M. Mukesh, A. Chanda and M. Vasundhara, Vacuum, 2021, 184, 109955 CrossRef CAS.
- M. Periyasamy and A. Kar, J. Mater. Chem. C, 2020, 8, 4604–4635 RSC.
- K. K. Supin, A. Saji, A. Chanda and M. Vasundhara, Opt. Mater., 2022, 132, 112777 CrossRef.
- C. B. Anucha, I. Altin, E. Bacaksiz and V. N. Stathopoulos, Chem. Eng. J. Adv., 2022, 100262 CrossRef CAS.
- Z. Warren, T. T. Guaraldo, J. Wenk and D. Mattia, J. Mater. Chem. C, 2022, 10, 11542–11552 CAS.
- C. Manjunatha, V. Chirag, B. W. Shivaraj, N. Srinivasa and S. Ashoka, J. Nanostruct., 2020, 10, 531–539 CAS.
- C. Vidya, C. Manjunatha, M. N. Chandraprabha, M. Rajshekar and A. R. MAL, J. Environ. Chem. Eng., 2017, 5, 3172–3180 CrossRef CAS.
- H. Liu, H. Li, K. Du and H. Xu, New J. Chem., 2022, 46, 14867–14878 RSC.
- S. A. Ansari, C. Manjunatha, N. Parveen, B. W. Shivaraj and R. H. Krishna, Dalton Trans., 2021, 50, 14891–14907 RSC.
- H. Widiyantari, N. A. K. Umiati and R. D. Herdianti, J. Phys.: Conf. Ser., 2018, 1025, 012004 CrossRef.
- Z. Z. Vasiljevic, M. P. Dojcinovic, J. D. Vujancevic, I. Jankovic-Castvan, M. Ognjanovic, N. B. Tadic, S. Stojadinovic, G. O. Brankovicand and M. V. Nikolic, R. Soc. Open Sci., 2020, 7, 200708 CrossRef CAS PubMed.
- H. B. Slama, A. ChenariBouket, Z. Pourhassan, F. N. Alenezi, A. Silini, H. Cherif-Silini, T. Oszako, L. Luptakova, P. Golinska and L. Belbahri, Appl. Sci., 2021, 11, 6255 CrossRef CAS.
- R. Aliand and B. S. Ooi, J. Teknol., 2006, 45, 31–42 Search PubMed.
- C. Belver, J. Bedia, A. Gómez-Avilés, M. Peñas-Garzón and J. J. Rodriguez, Nanoscale Mater. Water Purif., 2016, 581–651 Search PubMed.
- C. Vidya, C. Manjunatha, M. Sudeep, S. Ashoka and L. A. Raj, SN Appl. Sci., 2020, 2, 1–15 Search PubMed.
- C. P. Devatha and A. K. Thalla, Synth. Inorg. Nanomater., 2018, 169–184 CAS.
- D. Zhang, X. L. Ma, Y. Gu, H. Huang and G. W. Zhang, Front. Chem., 2020, 8, 799 CrossRef CAS PubMed.
- A. Jayachandran, T. R. Aswathy and A. S. Nair, Biochem. Biophys. Rep., 2021, 26, 100995 CAS.
- A. Umamaheswari, S. L. Prabu, S. A. John and A. Puratchikody, Biotechnol. Rep., 2021, 29, e00595 CrossRef CAS PubMed.
- N. Senthilkumar, E. Nandhakumar, P. Priya, D. Soni, M. Vimalan and I. V. Potheher, New J. Chem., 2017, 41, 10347–10356 RSC.
- M. Naseer, U. Aslam, B. Khalid and B. Chen, Sci. Rep., 2020, 10, 1–10 CrossRef PubMed.
- A. VS, S. Arya, P. Biju, E. J. Josekutty and J. Augustine, Phytotaxa, 2020, 460, 269–276 CrossRef.
- K. S. Banu and L. Cathrine, Int. j. adv. res. chem. sci., 2015, 2, 25–32 Search PubMed.
- I. A. Ajayi, O. Ajibade and R. A. Oderinde, Res. J. Chem. Sci., 2011, 1, 58–62 CAS.
- M. Jena, C. Manjunatha, B. W. Shivaraj, G. Nagaraju, S. Ashoka and M. S. Aan, Mater. Today Chem., 2019, 12, 187–199 CrossRef CAS.
- J. Osuntokun, D. C. Onwudiwe and E. E. Ebenso, Green Chem. Lett. Rev., 2019, 12, 444–457 CrossRef CAS.
- U. Wijesinghe, G. Thiripuranathar, F. Menaa, H. Iqbal, A. Razzaq and H. Almukhlifi, Catalysts, 2021, 11, 831 CrossRef CAS.
- J. Estrada-Urbina, A. Cruz-Alonso, M. Santander-González, A. Méndez-Albores and A. Vázquez-Durán, J. Nanomater., 2018, 8, 247 CrossRef CAS PubMed.
- K. Davis, R. Yarbrough, M. Froeschle, J. White and H. Rathnayake, RSC Adv., 2019, 9, 14638–14648 RSC.
- V. Srikant and D. R. Clarke, J. Appl. Phys., 1998, 83, 5447–5451 CrossRef CAS.
- J. D. O. Primo, C. Bittencourt, S. Acosta, A. Sierra-Castillo, J. F. Colomer, S. Jaerger, V. C. Teixeira and F. J. Anaissi, Front. Chem., 2020, 8, 571790 CrossRef PubMed.
- S. Faisal, H. Jan, S. A. Shah, S. Shah, A. Khan, M. T. Akbar, M. Rizwan, F. Jan, Wajidullah, N. Akhtar, A. Ghattak and S. Syed, ACS Omega, 2021, 6, 9709–9722 CrossRef CAS PubMed.
- D. N. Montenegro, V. Hortelano, O. Martínez, M. C. Martínez-Tomas, V. Sallet, V. Muñoz-Sanjosé and J. Jiménez, J. Phys. D, 2013, 46, 235302 CrossRef.
- R. Cusco, E. Alarcon-Llado, J. Ibanez and L. Artus, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 165202 CrossRef.
- F. Friedrich and N. H. Nickel, Appl. Phys. Lett., 2007, 91, 111903 CrossRef.
- J. Sann, J. Stehr, A. Hofstaetter, D. M. Hofmann, A. Neumann, M. Lerch, U. Haboeck, A. Hoffman and C. Thomsen, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 195203 CrossRef.
- R. Al-Gaashani, S. Radiman, A. R. Daud, N. Tabet and Y. Al-Douri, Ceram. Int., 2013, 39, 2283–2292 CrossRef CAS.
- M. B. Nayan, K. Jagadish, M. R. Abhilash, K. Namratha and S. Srikantaswamy, J Water Resource Prot., 2019, 11, 357 CrossRef CAS.
- V. Hoseinpour, M. Souri, N. Ghaemi and A. Shakeri, Heath biotechnol. biopharma, 2017, 1, 39–49 Search PubMed.
- C. Vivek, B. Balraj and S. Thangavel, J. Mater. Sci.: Mater. Electron., 2019, 30, 11220–11230 CrossRef CAS.
- A. Sangeetha, S. Jaya Seeli, K. P. Bhuvana, M. A. Kader and S. K. Nayak, J. Sol-Gel Sci. Technol., 2019, 91, 261–272 CrossRef CAS.
- D. Saravanakkumar, S. Sivaranjani, K. Kaviyarasu, A. Ayeshamariam, B. Ravikumar, S. Pandiarajan, C. Veeralakshmi, M. Jayachandran and M. Maaza, J. Semicond., 2018, 39, 033001 CrossRef.
- M. Pudukudy and Z. Yaakob, J. Cluster Sci., 2015, 26, 1187–1201 CrossRef CAS.
- S. V. Nistor, D. Ghica, M. Stefan, I. Vlaicu, J. N. Barascu and C. Bartha, J. Alloys Compd., 2013, 548, 222–227 CrossRef CAS.
- M. Wang, L. Jiang, E. J. Kim and S. H. Hahn, RSC Adv., 2015, 5, 87496–87503 RSC.
- M. G. Demissie, F. K. Sabir, G. D. Edossa and B. A. Gonfa, J. Chem., 2020, 1–9 CrossRef.
- C. A. Soto-Robles, P. A. Luque, C. M. Gómez-Gutiérrez, O. Nava, A. R. Vilchis-Nestor, E. Lugo-Medina, R. Ranjithkumar and A. Castro-Beltrán, Results Phys., 2019, 15, 102807 CrossRef.
- Z. N. Kayani, F. Saleemi and I. Batool, Appl. Phys. A, 2015, 119, 713–720 CrossRef CAS.
- Z. B. Fang, Z. J. Yan, Y. S. Tan, X. Q. Liu and Y. Y. Wang, Appl. Surf. Sci., 2005, 241, 303–308 CrossRef CAS.
- Q. P. Zhang, X. N. Xu, Y. T. Liu, M. Xu, S. H. Deng, Y. Chen, H. Yuan, F. Yu, Y. Huang, K. Zhao, S. Xu and G. Xiong, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- S. Narath, S. K. Koroth, S. S. Shankar, B. George, V. Mutta, S. Wacławek, M. Cernik, V. V. T. Padil and R. S. Varma, Nanomater, 2021, 11, 1558 CrossRef CAS PubMed.
- A. Elaziouti and B. Ahmed, J. Chem. Eng. Process Technol., 2011, 2, 1–9 Search PubMed.
- K. H. Rahman and A. K. Kar, Bull. Mater. Sci., 2022, 45, 1–10 CrossRef.
- S. K. Lee and A. Mills, Chem. Commun, 2003, 18, 2366–2367 RSC.
- A. Akyol, H. C. Yatmaz and M. Bayramoglu, Appl. Catal., 2004, 54, 19–24 CrossRef CAS.
- F. H. Mustapha, A. A. Jalil, M. Mohamed, S. Triwahyono, N. S. Hassan, N. F. Khusnun, C. N. C. Hitam, A. F. A. Rahman, L. Firmanshah and A. S. Zolkifli, J. Cleaner Prod., 2017, 168, 1150–1162 CrossRef CAS.
- M. Faheem, H. M. Siddiqi, A. Habib, M. Shahid and A. Afzal, Front. environ. sci., 2022, 1159 Search PubMed.
- M. H. Razali, E. C. E. Ali, M. Kasah and A. H. Omar, ICASTOR J. Eng., 2012, 5, 95 Search PubMed.
- C. Manjunatha, B. Abhishek, B. W. Shivaraj, S. Ashoka, M. Shashank and G. Nagaraju, Chem. Pap., 2020, 74, 2719–2731 CrossRef CAS.
- V. Jawale, A. Al-fahdawi, S. Salve, S. Pandit, G. Dawange, G. Gugale, M. Chaskar, D. Hammiche, S. Arbuj and V. Pandit, Mater. Today: Proc., 2022, 52, 17–20 CAS.
- D. G. Kumbhar, V. U. Pandit, S. D. Deshmukh, J. D. Ambekar, S. S. Arbuj and S. B. Rane, J. Nanoeng. Nanomanuf., 2015, 5, 227–231 CrossRef CAS.
- S. Alamdari, M. Sasani Ghamsari, C. Lee, W. Han, H. H. Park, M. J. Tafreshi, H. Afarideh and M. H. M. Ara, Appl. Sci., 2020, 10, 3620 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ra06967a |
| This journal is © The Royal Society of Chemistry 2023 |