Open Access Article
Open Access ArticleDissimilar-at-boron N-BODIPYs: from light-harvesting multichromophoric arrays to CPL-bright chiral-at-boron BODIPYs†
César
Ray
a,
Edurne
Avellanal-Zaballa
b,
Mónica
Muñoz-Úbeda
cd,
Jessica
Colligan
e,
Florencio
Moreno
 a,
Gilles
Muller
a,
Gilles
Muller
 e,
Iván
López-Montero
e,
Iván
López-Montero
 cdf,
Jorge
Bañuelos
cdf,
Jorge
Bañuelos
 b,
Beatriz L.
Maroto
b,
Beatriz L.
Maroto
 *a and
Santiago
de la Moya
*a and
Santiago
de la Moya
 *a
*a
aDepto. de Química Orgánica, Facultad de CC. Químicas, Universidad Complutense de Madrid, Ciudad Universitaria s/n, 28040, Madrid, Spain. E-mail: santmoya@ucm.es; belora@ucm.es
bDepto. de Química Física, Universidad del Pais Vasco-EHU, Apartado 644, 48080, Bilbao, Spain
cInstituto de Investigación Biomédica Hospital Doce de Octubre (imas12), Avda. de Córdoba s/n, 28041, Madrid, Spain
dDepartamento de Química Física, Facultad de Ciencias Químicas, Universidad Complutense de Madrid, Ciudad Universitaria s/n, 28040, Madrid, Spain
eDepartment of Chemistry, San José State University, San José, CA 95192-0101, USA
fInstituto Pluridisciplinar, Universidad Complutense de Madrid, Paseo de Juan XXIII 1, 28040, Madrid, Spain
First published on 19th October 2023
Abstract
We report a workable and easy approach for the direct post-multifunctionalization of common BODIPYs (F-BODIPYs) with minimal interference to the starting photophysical behavior. It entails the easy transformation of an F-BODIPY into the corresponding N-BODIPY by using a dissimilarly-N,N′-disubstituted bis(sulfonamide), which is easily obtained from ethane-1,2-diamine. This approach is exemplified by the rapid synthesis of a selected battery of unprecedented dissimilar-at-boron N-BODIPYs, which are rationally designed to act as efficient multichromophoric arrays for light harvesting by excitation energy transfer, as specific bioprobes for fluorescent imaging, or as efficient chiroptical dyes exhibiting visible circular dichroism and circularly polarized luminescence. Noticeably, this approach has led to the synthesis of the first CPL-bright chiral-at-boron BODIPYs, a significant novelty in BODIPY chemistry and CPL emitters.
Introduction
The development of advanced organic dyes for sought-after photonic applications, from energy to health, has experienced exponential growth in the last few years. This is due to the inherent benefits of organic dyes and materials based on them. They exhibit a number of advantages derived from their organic nature and small molecular size, such as flexibility, lightness, membrane permeability or biocompatibility, and have the key advantages of easy synthesis and modulation by workable organic chemistry.1However, today's organic photonics increasingly demands sophisticated dyes that have a wide number of different properties (e.g., fluorescence, reactive oxygen species (ROS) photogeneration, biocompatibility and biospecificity for phototheragnosis1,2), including challenging ones such as bright circularly polarized luminescence (CPL)3,4 (e.g., to develop highly efficient CP-OLEDs vs. conventional OLEDs5). In fact, CPL organic materials are considered as next-generation materials.6
The post-functionalization versatility of the outstanding BODIPY (boron dipyrromethene) dyes is virtually ideal for achieving this goal,7,8 including the generation of bright CPL.9,10 Nevertheless, easily endowing photophysically-optimized BODIPYs with the additional properties required for a specific application is still challenging. Thus, in most cases, this post-multifunctionalization involves low-efficiency linear synthetic pathways, and the optimized starting photophysical behavior is usually affected during the functionalization process.11 Hence, the development of easy and direct (convergent) post-multifunctionalization strategies for BODIPYs, without causing significant photophysical interference, is pivotal to advance BODIPY chemistry and organic photonics.
Among the well-established BODIPY post-functionalization approaches,7a,11,12 those involving reactions at the boron center (mainly halogen substitutions) must be highlighted.10b,11,12b,13–15 The significance of post-functionalization at boron is the low influence on the dye photophysics, because the boron atom is not substantial part of the BODIPY chromophore (localized at the π-conjugated dipyrrin moiety).16 Interestingly, this approach has succeeded in easily endowing BODIPYs with valuable CPL, mainly through BINOL-based O-BODIPYs (involving a BO2 moiety)13a–c or more-sustainable terpene-based C-BODIPYs (involving a BC2 moiety).14c However, building dissimilar-at-boron BODIPYs (i.e., BODIPYs having two different moieties dangling from the boron atom) has been scarcely used for BODIPY multifuncionalization.17a This is probably due to the lack of efficient procedures for the substitution of only one of the groups attached to the boron.17 As a notable example, Harriman and Ziessel rationally developed dissimilar-at-boron C-BODIPYs as multichromophoric arrays for electronic energy transfer, where two different chromophores were attached to the boron atom.17a
Moreover, dissimilar-at-boron BODIPYs based on asymmetrically-substituted BODIPY cores present a chiral boron (chiral-at-boron BODIPYs). This would ideally lead to significant chiroptical behaviors, due to the closeness of the chiral boron to the BODIPY chromophore. With this idea in mind, Ziessel,18a and Işık and Tanyeli18b reported the few examples of chiral-at-boron BODIPYs based on C- and O-BODIPY, respectively. Unfortunately, these dyes showed low or even silent fluorescence, probably due to enhanced vibrational relaxation and intersystem crossing.18b
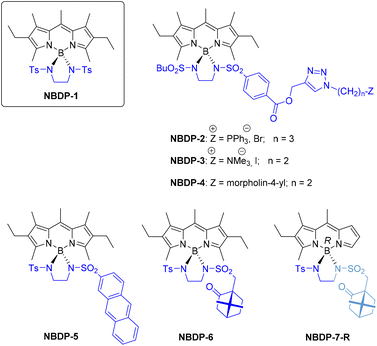
In the engaging and yet rather unexplored field of at-boron BODIPY functionalization,11,12b our group described in 2017 a simple methodology to synthesize the first stable N-BODIPYs (involving a BN2 moiety), from accessible bis(sulfonamide)s derived from ethane-1,2-diamine (e.g., see NBDP-1 in Fig. 1).19 These new BODIPYs are particularly important because of their demonstrated excellent photophysical signatures, which even surpass those of parent F-BODIPYs (e.g., laser emission),19 and also due to the possibility of BODIPY post-multifunctionalization, since up to two different moieties could be introduced at boron this way. This possibility has been preliminarily demonstrated by us through the successful preparation of N-BODIPYs NBDP-2 and NBDP-3 (Fig. 1), as potential photodynamic therapy (PDT) agents based on specific organelle accumulation, where one of the sulfonylamino moieties dangling from boron was rationally selected to gain the desired biospecificity, and the other one to address solubility issues.20
These preliminary results on the construction of dissimilar-at-boron N-BODIPYs as a versatile BODIPY post-multifunctionalization approach prompted us to investigate its scope by the rationally-guided design and efficient (convergent) synthesis of a selected set of dissimilar-at-boron N-BODIPYs (see Fig. 1). The dyes in this battery were designed to act as a highly fluorescent lysosome bioprobe (NBDP-4), as an efficient light-harvesting multichromophoric array based on workable Förster resonance energy transfer (FRET) (NBDP-5), as an efficient chiroptical BODIPY based on sustainable camphor (NBDP-6) and, which is more in the forefront, as the first highly fluorescent chiral-at-boron BODIPY (NBDP-7). Additionally, the selection of these dyes was made on the basis of using available starting materials to reach synthetically simple and low-cost dyes.
Results and discussion
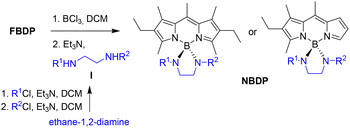
As expected, the functionally-designed dissimilar-at-boron N-BODIPY dyes NBDP-5, NBDP-6 and NBDP-7 were easily obtained from the corresponding F-BODIPY and bis(sulfonamide) (see the reaction scheme in Table 1), following the general procedure originally developed by us for the preparation of N-BODIPYs equally substituted at boron.19 This procedure uses a mixture of BCl3/Et3N to promote the nucleophilic substitution of the F-BODIPY fluorines under soft reaction conditions (see the scheme in Table 1 and the ESI† for experimental details). As the starting F-BODIPYs, we selected highly fluorescent and accessible ones, specifically symmetrically substituted 2,6-diethyl-1,3,5,7,8-pentamethyl-F-BODIPY (FBDP-1, a commercial laser dye known as PM567) for NBDP-5 and NBDP-6, and asymmetrically substituted 2-ethyl-1,3,8-trimethyl-F-BODIPY (FBDP-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21) for chiral-at-boron NBDP-7. Regarding the intermediate bis(sulfonamide)s, I-1 for the synthesis of NBDP-5 and I-2 for the synthesis of NBDP-6 and NBDP-7, they were easily prepared by reacting commercially available ethane-1,2-diamine with one equivalent of p-toluenesulfonyl chloride in a basic medium, followed by a second analogous reaction with anthracene-2-sulfonyl chloride for I-1 or (1S)-camphor-10-sulfonyl chloride for I-2 (65% and 78% yields, respectively; see Table 1 and the ESI† for experimental details).
21) for chiral-at-boron NBDP-7. Regarding the intermediate bis(sulfonamide)s, I-1 for the synthesis of NBDP-5 and I-2 for the synthesis of NBDP-6 and NBDP-7, they were easily prepared by reacting commercially available ethane-1,2-diamine with one equivalent of p-toluenesulfonyl chloride in a basic medium, followed by a second analogous reaction with anthracene-2-sulfonyl chloride for I-1 or (1S)-camphor-10-sulfonyl chloride for I-2 (65% and 78% yields, respectively; see Table 1 and the ESI† for experimental details).
| F-BODIPYa | R1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
R2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
I (yield)c | N-BODIPY (yield)d |
|---|---|---|---|---|
| DCM: dichloromethanea FBDP-1: 2,6-diethyl-1,3,5,7,8-pentamethyl-F-BODIPY; FBDP-2: 2-ethyl-1,3,8-trimethyl-F-BODIPY.b a: Tosyl; b: butane-1-sulfonyl; c: anthracene-2-sulfonyl; d: (1S)-camphor-10-sulfonyl; e: {4-[(propargyloxy)carbonyl]phenyl}sulfonyl.c Chemical yield in the preparation of I from ethane-1,2-diamine.d Chemical yield in the preparation of the N-BODIPY (NBDP) from the corresponding F-BODIPY (FBDP) and bis(sulfonamide) I.e Ref. 20. | ||||
| FBDP-1 | a | c | I-1 (65%) | NBDP-5 (35%) |
| FBDP-1 | a | d | I-2 (78%) | NBDP-6 (78%) |
| FBDP-1 | b | e | I-3 (87%)e | NBDP-8 (72%)e |
| FBDP-2 | a | d | I-2 (78%) | NBDP-7-R (37%) |
| NBDP-7-S (39%) | ||||
The chemical yields for the synthesis of N-BODIPYs from F-BODIPYs (Table 1) ranged from 35% for NBDP-5 to 78% for NBDP-7. Poor solubility of I-1 in the reaction media, caused by the anthryl moiety, should explain the low yield achieved in the preparation of NBDP-5. In the case of NBDP-7, the formed epimers NBDP-7-R (R configuration for boron; see Fig. 1) and NBDP-7-S (S for boron) were easily separated by simple elution chromatography in 37% and 39% yields, respectively (see experimental details in the ESI†), and their absolute configurations were determined by X-ray diffraction analysis (see Table S3 and Fig. S4 in the ESI†).
Finally, dissimilar-at-boron N-BODIPY NBDP-4 was obtained following the same procedure used preliminarily by us for the preparation of NBDP-2 and NBDP-3.20 This procedure involves first preparing the clickable ethynyl-based dissimilar-at-boron NBDP-8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 from FBDP-1 and bis(sulfonamide) I-3 (see Table 1) to subsequently conduct a standard click reaction (CuSO4/sodium ascorbate) with the corresponding functionalized azide carrying the morpholino moiety (see Fig. 2). In the case of NBDP-4, the click reaction of NBDP-8 with the commercial morpholine-based azide took place in 85% chemical yield (see experimental details in the ESI†). It must be highlighted here that using the butane-1-sulfonyl-derived bis(sulfonamide) I-3 (instead of p-toluenesulfonyl) facilitated the synthesis of NBDP-8, probably due to the observed enhanced solubility in the reaction medium (dichloromethane). This supports the feasibility of simply using one of the at-boron pending sulfonylamino groups to modulate dye solubility, which is an important property for in-solution applications and dye material processing.
20 from FBDP-1 and bis(sulfonamide) I-3 (see Table 1) to subsequently conduct a standard click reaction (CuSO4/sodium ascorbate) with the corresponding functionalized azide carrying the morpholino moiety (see Fig. 2). In the case of NBDP-4, the click reaction of NBDP-8 with the commercial morpholine-based azide took place in 85% chemical yield (see experimental details in the ESI†). It must be highlighted here that using the butane-1-sulfonyl-derived bis(sulfonamide) I-3 (instead of p-toluenesulfonyl) facilitated the synthesis of NBDP-8, probably due to the observed enhanced solubility in the reaction medium (dichloromethane). This supports the feasibility of simply using one of the at-boron pending sulfonylamino groups to modulate dye solubility, which is an important property for in-solution applications and dye material processing.
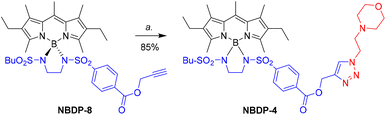 | ||
| Fig. 2 Synthesis of NBDP-4 from the previously-developed dissimilar-at-boron clickable N-BODIPY NBDP-820 by a click reaction. a. 4-(2-Azidoethyl)morpholine, CuSO4·5H2O, sodium ascorbate, t-butanol/H2O. | ||
All these synthetic results, including our preliminary ones,20 demonstrate the ease and versatility of constructing dissimilar-at-boron N-BODIPYs, as well as the utility of NBDP-8 (and related compounds) as a readily-accessible precursor of functionalized BODIPY dyes through workable click chemistry.
Regarding ground state photophysics, the recorded visible spectral signatures (see the ESI† for experimental details) of all the obtained PM567-derived dissimilar-at-boron N-BODIPYs, including preliminary NBDP-8, NBDP-2 and NBDP-3,20 closely resemble those reported for related both NBDP-119 (equally substituted at boron) and parent F-BODIPY FBDP-1 (see Table 2 and Fig. S1†). As expected, the unequal substitution of the starting F-BODIPY fluorines with different nitrogenated moieties does not affect the fluorescence capacity of the dissimilar-at-boron N-BODIPY dyes. They all exhibit fluorescence quantum yields higher than 80%, regardless of the solvent used for the measurement, with only slight bathochromic shifts (less than 10 nm) and some small changes in the absorption coefficient, together with a moderate increase in the lifetime (see Table 2, and Table S1 in the ESI†). The same behavior is found for chiral-at-boron N-BODIPYs NBDP-7-R and NBDP-7-S, which exhibit fluorescence signatures closely related to those of the corresponding parent F-BOPIPY FBDP-2, with the exception of some loss of absorption capability (see Table 2).
| Dye |
λ
ab![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (nm)
(nm) |
ε
max![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (104 × M−1cm−1)
(104 × M−1cm−1) |
λ
fl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (nm)
(nm) |
ϕ | τ (ns) |
|---|---|---|---|---|---|
| a Maximum absorption wavelength. b Maximum molar absorption coefficient. c Maximum fluorescence wavelength. d Fluorescence quantum yield. e Fluorescence lifetime. | |||||
| FBDP-1 | 516.0 | 7.9 | 532.0 | 0.91 | 6.10 |
| NBDP-1 | 525.5 | 6.2 | 542.0 | 0.82 | 8.37 |
| NBDP-2 | 524.5 | 5.1 | 544.0 | 0.80 | 7.77 |
| NBDP-3 | 524.5 | 5.2 | 541.5 | 0.73 | 7.76 |
| NBDP-4 | 524.5 | 5.5 | 543.5 | 0.88 | 7.82 |
| NBDP-5 | 522.5 | 6.2 | 538.0 | 0.80 | 7.93 |
| NBDP-6 | 524.0 | 5.6 | 541.5 | 0.82 | 8.31 |
| NBDP-8 | 524.5 | 6.0 | 542.5 | 0.80 | 8.16 |
| NBDP-9 | 523.0 | 5.5 | 542.0 | 0.93 | 8.28 |
| FBDP-2 | 495.5 | 4.3 | 510.5 | 0.80 | 5.80 |
| NBDP-7-R | 504.5 | 2.8 | 520.5 | 0.87 | 8.00 |
| NBDP-7-S | 502.5 | 2.6 | 519.5 | 0.82 | 7.56 |
Importantly, the strong fluorescence emission recorded for NBDP-7-R and NBDP-7-S, with fluorescence quantum yields up to ca. 90% in methanol (see Table 2), makes them the first highly fluorescent chiral-at-boron BODIPYs reported so far, a significant record in chiral-BODIPY chemistry and photonics.
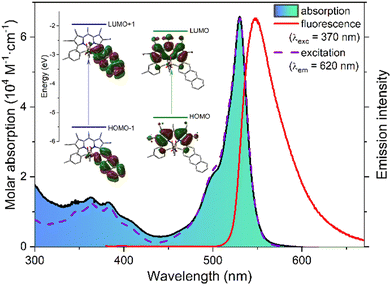
Regarding the FRET-based light-harvesting behavior designed for NBDP-5,13d,22 the UV-Vis absorption spectrum of this dye shows the two expected absorption bands: a sharp one that peaked at 530 nm, with higher intensity, ascribed to the BODIPY chromophore, and another one, broader and weaker, centered at 370 nm, featuring the typical resolved vibrational resolution of anthracene absorption22a (Fig. 3).
As designed, the fluorescence spectrum of NBDP-5 shows a single band regardless of the used irradiation wavelength, 530 nm for BODIPY chromophore excitation or 370 nm for anthracene chromophore excitation (Fig. 3). Moreover, the corresponding excitation spectrum, monitored at the long-wavelength tail of the fluorescence profile (620 nm), fully matches the absorption one, supporting electronic isolation for the involved anthracene and BODIPY chromophores. All these results demonstrate excitation-energy transfer (EET) from the anthracene moiety (energy donor) to the BODIPY one (energy acceptor), with ca. 100% EET efficiency, as calculated from the loss of anthracene-chromophore fluorescence (residual donor emission in NBDP-5 is almost negligible upon its direct excitation; see the ESI†). Possible competing electron-transfer between the acting chromophores was ruled out by electrochemical measurements, since the main oxidation and reduction bands in the cyclic voltammogram of NBDP-5 match those of isolated FBDP-1 and anthracene (see Fig. S2 in the ESI†). Besides, FRET23a must be the mechanism involved in the observed EET process, as supported by the spectral overlapping between the anthracene-chromophore emission and the BODIPY-chromophore absorption (see Fig. S3 in the ESI†), enabling the required dipole–dipole coupling. Besides, the at-boron linkage hampers any electronic exchange by the through-bond mechanism;23b however, it brings both acting chromophores close to each other (the B3LYP/6-311g* computed distance between their centers of masses is 8.3 Å; see Fig. S3 in the ESI†), allowing efficient through-space mediated EET.23c All these photophysical, electrochemical and computational results demonstrate the usefulness of constructing dissimilar-at-boron N-BODIPYs to develop valuable light-harvesting organic dyes.
In relation to the lysosomal probing behavior designed for NBDP-4, it must be noted that this dye has a basic morpholine moiety, which is known to promote specific accumulation in acidic lysosomes.24 Moreover, as in the case of preliminary NBDP-2 and NBDP-3,20 the involved molecular arrangement comprises a number of advantageous features for fluorescence bioprobing. On the one hand, the moiety responsible for the biospecificity is located far from the one responsible for the fluorescence signaling (the BODIPY core) and, therefore, no interference is expected from either photophysics or biospecificity viewpoints. On the other hand, their easy synthetic access from a common reactive intermediate (NBDP-8, see Fig. 2) facilitates not only the rapid generation of different bioprobes (labelling different biosystems), but also the rapid synthesis of related ones (labelling the same biosystem) for bioprobe optimization purposes.
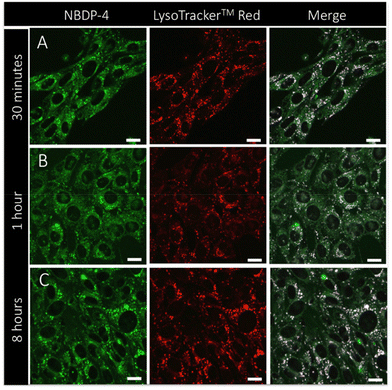
As expected, highly fluorescent morpholine-based NBDP-4 effectively functions as a specific lysosomal bioprobe. This was assessed in living mouse embryonic fibroblasts (MEFs), which were stained simultaneously with LysoTracker™ Red (4 nM) and NBDP-4 (500 nM) (see the ESI† for experimental details). Co-localization of both probes was imaged by fluorescence confocal scanning laser microscopy (CSLM; see the ESI† for experimental details). Although NBDP-4 was detected within the cellular cytoplasm as a dim fluorescent background, this N-BODIPY dye strongly co-localized with Lysotracker™ Red even with short incubation times. The calculated Pearson's coefficient (R) was above the threshold for high colocalization, with values equal to 0.93, 0.93 and 0.94 for incubation times of 30 min, 1 h and 2 h, respectively (Fig. 4). These biophotophysical results, together with the preliminary ones reported by us for NBDP-2 and NBDP-3,20 demonstrate the high capability of the involved dissimilar-at-boron N-BODIPY design for cellular uptake and specific fluorescence probing, as well as the workability of NBDP-8 to rapidly build subcellular fluorescent bioprobes.
Regarding the chiroptical behavior designed for NBDP-6 and epimers NBDP-7-R and NBDP-7-S, these dyes exhibit the expected visible electronic circular dichroism (ECD) in diluted chloroform solution (see the ESI† for experimental details), due to the chiral perturbation of the BODIPY chromophore caused by the involved chiral elements. However, the dichroic capability of NBDP-6 is significantly poorer than that exhibited by NBDP-7-R and NBDP-7-S, as measured using the corresponding calculated maximum |gabs‡| (0.1 × 10−3 for NBDP-6vs. 1.4 × 10−3 and 1.2 × 10−3 for NBDP-7-R and NBDP-7-S; see Table S2 in the ESI†). This suggests low chiral-perturbing capability from the hanging chiral camphor moiety, and higher from the chiral boron center (note the change of the gabs sign when changing the boron configuration in NBDP-7 epimers; Table S2 in the ESI†). The reason must be the high conformational mobility and long distance to the BODIPY chromophore of the camphor moiety, compared to the closer and conformationally-fixed chiral boron center. To prove the low perturbing capability of the camphor moiety in this structure, we decided to synthesize and chiroptically characterize C2-symmetric NBDP-9 (Fig. 5; see the ESI†), in order to compare it to known related CBDP,14c which has conformationally-restricted chiral terpene moieties located closer to the BODIPY chromophore (cf.CBDP and NBDP-9 in Fig. 5).
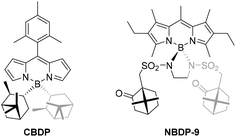 | ||
| Fig. 5 Related C2-symmetric BODIPYs functionalized at boron with hanging chiral terpene moieties: known isopinocampheyl-based C-BODIPY CBDP14c and camphor-based N-BODIPY NBDP-9. | ||
Satisfactorily, NBDP-9 exhibited the characteristic, excellent fluorescence signatures of the N-BODIPY family (see Table 2, and Table S1 in the ESI†). However, regarding chiroptics, while terpene-based C-BODIPY CBDP was reported to be ECD-active in the Vis region,14c related N-BODIPY NBDP-9 turned out to be ECD-silent under almost the same experimental conditions (see Table S2 in the ESI†). This result supports the hypothesis mentioned above on the low chiral-perturbing capability of the distant and moving terpene moieties of the developed camphor-based N-BODIPY dyes.
Regarding visible CPL, all the synthesized camphor-based BODIPYs are CPL active, including ECD-silent NBDP-9 (e.g., see the CPL spectra of NBDP-9, and NBDP-7 epimers in chloroform solution in Fig. 6; see the ESI† for experimental details), with the maximum |glum§| values ranging from ca. 0.5 × 10−3 for NBDP-7-R to ca. 1 × 10−3 for NBDP-9 and NBDP-7-S (see Table S2 in the ESI†).
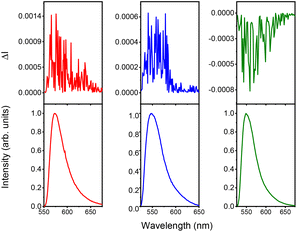 | ||
| Fig. 6 CPL (upper curves) and total luminescence (lower curves) spectra of NBDP-9 (red), NBDP-7-R (blue) and NBDP-7-S (green) in degassed chloroform (1 mM). | ||
The clear CPL activity of ECD-silent NBDP-9 could be explained by the participation of a more electronically-forbidden transition for the emission band, when compared with that for the absorption.25 Noteworthily, for the ECD-active N-BODIPYs NBDP-6, NBDP-7-R and NBDP-7-S, the signs of the visible maximum gabs and the corresponding visible maximum glum are the same (see Table S2 in the ESI†). This behavior is different to those reported for related at-boron-substituted BINOL-based O-BODIPYs13a and isopinocampheyl-based C-BODIPYs,14c and can be explained on the basis of the low probability of the investigated N-BODIPYs for enabling intramolecular charge transfer (ICT) emission upon light absorption,26 as supported by their high fluorescence capabilities, regardless of the solvent polarity (see Table S1 in the ESI†).
Finally, it must be noted that despite the precautions that must be taken when handling glum data of small organic molecules (note the common very small values and the inherent experimental errors), significant visible glum values are obtained from the developed highly fluorescent chiral-at-boron N-BODIPYs NBDP-7, i.e., having a stereogenic boron. It is true that these BODIPYs include a chiral moiety (from natural camphor) in their molecular structure, but as demonstrated before, its role is to facilitate epimer separation upon synthesis, rather than influencing the dye chiroptical activity. All these characteristics of the reported chiral-at-boron N-BODIPYs (high fluorescence, chirality coming from the sustainable, natural chiral pool, and CPL activity within the range of the best CPL-enabling simple organic molecules, CPL-SOMs3b,9) support them as the first examples of a valuable new family of chiral BODIPYs for advancing CPL BODIPYs and CPL-SOM-based materials and applications.
Conclusions
Construction of dissimilar-at-boron N-BODIPYs from the corresponding F-BODIPYs and accessible ethane-1,2-diamine-based bis(sulfonamide)s is demonstrated to be a workable new approach for easy and direct BODIPY post-multifunctionalization, with minimal perturbation of the dye ground state photophysics. Such post-multifunctionalization has great value, since it allows the rapid implementation of more than one property in a selected, photophysically-optimized BODIPY dye and should accelerate the achievement of improved BODIPYs for advancing organic photonics. This is supported by the reported rapid design and synthesis of an efficient light-harvesting multichromophoric array based on FRET, a clickable dye reagent, a lysosomal fluorescent bioprobe, and a set of chiroptical SOMs, including CPL-SOMs. Regarding chiroptics, the new approach has allowed the rapid achievement of the first highly-fluorescent chiral-at-boron BODIPYs. Their high fluorescence and their efficient CPL-activity and sustainable chirality coming from the natural chiral pool make this new family of BODIPY dyes highly interesting to advance CPL-SOMs and sought-after photonic materials and applications based of them (e.g., full-organic probes for CPL bioimaging6 or more sustainable CP-OLEDs5).Author contributions
Conceptualization: B.L.M., S.d.l.M.; funding acquisition: S.d.l.M., J.B., G.M., I.L.-M.; investigation: C.R., E.A.-Z., M.M.-Ú., J.C.; methodology: B.L.M., F.M., J.B., G.M., I.L.-M.; supervision: B.L.M., F.M., J.B., G.M., I.L.-M.; writing – original draft: B.L.M., S.d.l.M., with input from all the authors; writing – review & editing: B.L.M., S.d.l.M., J.B., G.M., I.L.-M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research received financial support from the Spanish Ministerio de Ciencia e Innovación (MICIN)/Agencia Estatal de Investigación PID2020-114755GB-C32 and C-33 AEI/10.13039/501100011033 and from the Basque Government (Grant IT1639-22). This work was also supported by the TECNOLOGÍAS 2018 program, funded by the Regional Government of Madrid, Spain (Grant S2018/BAA-4403 SINOXPHOS-CM, to I.L-M.). M.M.-Ú. was recipient of a Sara Borrell fellowship (CD15/00190) financed by the Spanish Ministry of Health. G.M. is thankful for the financial support by the California State University Program for Education and Research in Biotechnology (CSUPERB) Research Development Grant. C.R. thanks Comunidad de Madrid-UCM for a research contract (Programa Operativo de Empleo Juvenil, YEI).References
- For example, see: (a) B. N. G. Giepmans, S. R. Adams, M. H. Ellisman and R. Y. Tsien, The fluorescent toolbox for assessing protein location and function, Science, 2006, 312, 217–224 CrossRef CAS PubMed; (b) A. Bessette and G. S. Hanan, Design, synthesis and photophysical studies of dipyrromethene-based materials: insights into their applications in organic photovoltaic devices, Chem. Soc. Rev., 2014, 43, 3342–3405 RSC; (c) R. Strack, Organic dyes for live imaging, Nat. Methods, 2021, 18, 30 CrossRef CAS PubMed; (d) X. Zhang, J. Gao, Y. Tang, J. Yu, S. S. Liew, C. Qiao, Y. Cao, G. Liu, H. Fan, Y. Xia, J. Tian, K. Pu and Z. Wang, Bioorthogonally activatable cyanine dye with torsion-induced disaggregation for in vivo tumor imaging, Nat. Commun., 2022, 13, 3513 CrossRef CAS PubMed; (e) L. Sun, Y. Chen, M. Sun and Y. Zheng, Organic Solar Cells: Physical Principle and Recent Advances, Chem. - Asian J., 2023, e202300006 CrossRef CAS PubMed.
- For example, see: (a) S. Atilgan, T. Ozdemir and E. U. Akkaya, A Sensitive and Selective Ratiometric Near IR Fluorescent Probe for Zinc Ions Based on the Distyryl−Bodipy Fluorophore, Org. Lett., 2008, 10, 4065–4067 CrossRef CAS PubMed; (b) G. Duran-Sampedro, A. R. Agarrabeitia, I. Garcia-Moreno, L. Gartzia-Rivero, S. de la Moya, J. Banuelos, I. Lopez-Arbeloa and M. J. Ortiz, An asymmetric BODIPY triad with panchromatic absorption for high-performance red-edge laser emission, Chem. Commun., 2015, 51, 11382–11385 RSC; (c) V.-N. Nguyen, Y. Yim, S. Kim, B. Ryu, K. M. K. Swamy, G. Kim, N. Kwon, C.-Y. Kim, S. Park and J. Yoon, Molecular Design of Highly Efficient Heavy-Atom-Free Triplet BODIPY Derivatives for Photodynamic Therapy and Bioimaging, Angew. Chem., Int. Ed., 2020, 59, 8957–8962 CrossRef CAS PubMed.
- (a) Y. Zhang, S. Yu, B. Han, Y. Zhou, X. Zhang, X. Gao and Z. Tang, Circularly polarized luminescence in chiral materials, Matter, 2022, 5, 837–875 CrossRef CAS; (b) E. M. Sánchez-Carnerero, A. R. Agarrabeitia, F. Moreno, B. L. Maroto, G. Muller, M. J. Ortiz and S. de la Moya, Circularly Polarized Luminescence from Simple Organic Molecules, Chem. – Eur. J., 2015, 21, 13488–13500 CrossRef PubMed; (c) J. Kumar, T. Nakashima and T. Kawai, Circularly Polarized Luminescence in Chiral Molecules and Supramolecular Assemblies, J. Phys. Chem. Lett., 2015, 6, 3445–3452 CrossRef CAS PubMed.
- For example, see: (a) K. Ma, W. Chen, T. Jiao, X. Jin, Y. Sang, D. Yang, J. Zhou, M. Liu and P. Duan, Boosting the circularly polarized luminescence of small organic molecules via multi-dimensional morphology control, Chem. Sci., 2019, 10, 6821–6827 RSC; (b) C. Maeda, K. Nagahata, T. Shirakawa and T. Ema, Azahelicene-Fused BODIPY Analogues Showing Circularly Polarized Luminescence, Angew. Chem., Int. Ed., 2020, 59, 7813–7817 CrossRef CAS PubMed; (c) J.-F. Chen, Q.-X. Gao, L. Liu, P. Chen and T.-B. Wei, A pillar[5]arene-based planar chiral charge-transfer dye with enhanced circularly polarized luminescence and multiple responsive chiroptical changes, Chem. Sci., 2023, 14, 987–993 RSC.
- (a) S. Feuillastre, M. Pauton, L. Gao, A. Desmarchelier, A. J. Riives, D. Prim, D. Tondelier, B. Geffroy, G. Muller, G. Clavier and G. Pieters, Design and Synthesis of New Circularly Polarized Thermally Activated Delayed Fluorescence Emitters, J. Am. Chem. Soc., 2016, 138, 3900–3993 CrossRef PubMed; (b) K. Dhbaibi, L. Abella, S. Meunier-Della-Gatta, T. Roisnel, N. Vanthuyne, B. Jamoussi, G. Pieters, B. Racine, E. Quesnel, J. Autschbach, J. Crassous and L. Favereau, Achieving high circularly polarized luminescence with push–pull helicenic systems: from rationalized design to top-emission CP-OLED applications, Chem. Sci., 2021, 12, 5522–5533 RSC.
- (a) H. Koike, K. Nozaki and M. Iwamura, Microscopic Imaging of Chiral Amino Acids in Agar Gel through Circularly Polarized Luminescence of EuIII Complex, Chem. - Asian J., 2020, 15, 85–90 CrossRef CAS PubMed; (b) P. Stachelek, L. MacKenzie, D. Parker and R. Pal, Circularly polarised luminescence laser scanning confocal microscopy to study live cell chiral molecular interactions, Nat. Commun., 2022, 13, 553 CrossRef CAS PubMed.
- (a) J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, The triplet excited state of Bodipy: formation, modulation and application, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC; (b) H. Lu, J. Mack, T. Nyokong, N. Kobayashi and Z. Shen, Optically active BODIPYs, Coord. Chem. Rev., 2016, 318, 1–15 CrossRef CAS; (c) J. Bañuelos, BODIPY Dye, the Most Versatile Fluorophore Ever?, Chem. Rec., 2016, 16, 335–348 CrossRef PubMed; (d) S. Kolemen and E. U. Akkaya, Reaction-based BODIPY probes for selective bio-imaging, Coord. Chem. Rev., 2018, 354, 121–134 CrossRef CAS; (e) E. Bassan, A. Gualandi, P. G. Cozzi and P. Ceroni, Design of BODIPY dyes as triplet photosensitizers, Chem. Sci., 2021, 12, 6607–6628 RSC.
- For example, see: (a) M. Chapran, E. Angioni, N. J. Findlay, B. Breig, V. Cherpak, P. Stakhira, T. Tuttle, D. Volyniuk, J. V. Grazulevicius, Y. A. Nastishin, O. D. Lavrentovich and P. J. Skabara, An Ambipolar BODIPY Derivative for a White Exciplex OLED and Cholesteric Liquid Crystal Laser toward Multifunctional Devices, ACS Appl. Mater. Interfaces, 2017, 9, 4750–4757 CrossRef CAS PubMed; (b) J. Zou, P. Wang, Y. Wang, G. Liu, Y. Zhang, Q. Zhang, J. Shao, W. Si, W. Huang and X. Dong, Penetration depth tunable BODIPY derivatives for pH triggered enhanced photothermal/photodynamic synergistic therapy, Chem. Sci., 2019, 10, 268–276 RSC; (c) A. Cortés-Villena, D. A. Caminos, R. E. Galian and J. Pérez-Prieto, Singlet Sensitization of a BODIPY Rotor Triggered by Marriage with Perovskite Nanocrystals, Adv. Opt. Mater., 2023, 2300138 CrossRef.
- M. J. Hall and S. de la Moya, BODIPY based emitters of circularly polarized luminescence, in Circularly polarized luminescence of isolated small organic molecules, ed. T. Mori, Springer, Singapore, 2020, pp. 117–149 Search PubMed.
- For example, see: (a) Y. Gobo, M. Yamamura, T. Nakamura and T. Nabeshima, Synthesis and Chiroptical Properties of a Ring-Fused BODIPY with a Skewed Chiral π Skeleton, Org. Lett., 2016, 18, 2719–2721 CrossRef CAS PubMed; (b) N. Algoazy, R. G. Clarke, T. J. Penfold, P. G. Waddell, M. R. Probert, R. Aerts, W. Herrebout, P. Stachelek, R. Pal, M. J. Hall and J. G. Knight, Near-Infrared Circularly Polarised Luminescence from Helically Extended Chiral N,N,O,O-Boron Chelated Dipyrromethenes, ChemPhotoChem, 2022, 6, e202200090 CrossRef CAS; (c) C. Ray, C. Díaz-Norambuena, M. Johnson, F. Moreno, B. L. Maroto, J. Bañuelos, G. Muller and S. de la Moya, Tuning CPL by helical pitch modulation in helically flexible small organic multichromophores, J. Mater. Chem. C, 2023, 11, 456–461 RSC; (d) L. Cui, K. Deyama, T. Ichiki, Y. Konishi, A. Horioka, T. Harada, K. Ishibashi, Y. Hisaeda and T. Ono, Color-tuning and boosting circularly polarized luminescence performance of axially chiral tetra-BF2 complexes by post-modifications, J. Mater. Chem. C, 2023, 11, 2574–2581 RSC.
- E. Bodio and C. Goze, Investigation of B-F substitution on BODIPY and aza-BODIPY dyes: Development of B-O and B-C BODIPYs, Dyes Pigm., 2019, 160, 700–710 CrossRef CAS.
- (a) N. Boens, B. Verbelen, M. J. Ortiz, L. Jiao and W. Dehaen, Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core, Coord. Chem. Rev., 2019, 399, 213024 CrossRef CAS; (b) R. L. Gapare and A. Thompson, Substitution at boron in BODIPYs, Chem. Commun., 2022, 58, 7351–7359 RSC.
- For examples of BODIPYs with O-substitution at boron (O-BODIPYs), see: (a) E. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, M. J. Ortiz, B. G. Vo, G. Muller and S. de la Moya, Circularly Polarized Luminescence by Visible-Light Absorption in a Chiral O-BODIPY Dye: Unprecedented Design of CPL Organic Molecules from Achiral Chromophores, J. Am. Chem. Soc., 2014, 136, 3346–3349 CrossRef PubMed; (b) S. Zhang, Y. Wang, F. Meng, C. Dai, Y. Cheng and C. Zhu, Circularly polarized luminescence of AIE-active chiral O-BODIPYs induced via intramolecular energy transfer, Chem. Commun., 2015, 51, 9014–9017 RSC; (c) J. Jiménez, C. Díaz-Norambuena, S. Serrano, S. C. Ma, F. Moreno, B. L. Maroto, J. Bañuelos, G. Muller and S. de la Moya, BINOLated aminostyryl BODIPYs: a workable organic molecular platform for NIR circularly polarized luminescence, Chem. Commun., 2021, 57, 5750–5753 RSC; (d) C. O. Obondi, G. N. Lim, P. Martinez, V. Swamy and F. D'Souza, Controlling electron and energy transfer paths by selective excitation in a zinc porphyrin–BODIPY–C60 multi-modular triad, Nanoscale, 2017, 9, 18054 RSC.
- For examples of BODIPYs with C-substitution at boron (C-BODIPYs), see: (a) C. Uriel, A. M. Gómez, E. García Martínez de la Hidalga, J. Bañuelos, I. Garcia-Moreno and J. C. López, Access to 2,6-Dipropargylated BODIPYs as “Clickable” Congeners of Pyrromethene-567 Dye: Photostability and Synthetic Versatility, Org. Lett., 2021, 23, 6801–6806 CrossRef CAS PubMed; (b) Y. Zhang, S. Yuan, P. Liu, L. Jing, H. Pan, X.-K. Ren and Z. Chen, J-aggregation induced emission enhancement of BODIPY dyes via H-bonding directed supramolecular polymerization: the importance of substituents at boron, Org. Chem. Front., 2021, 8, 4078–4085 RSC; (c) J. Jiménez, F. Moreno, T. Arbeloa, T. A. Cabreros, G. Muller, J. Bañuelos, I. García-Moreno, B. L. Maroto and S. de la Moya, Isopinocampheyl-based C-BODIPYs: a model strategy to construct cost-effective boron-chelate emitters of circularly polarized light, Org. Chem. Front., 2021, 8, 4752–4757 RSC; (d) Y. Zhou, Y. Gao, L. Pang, W. Kang, K. Man and W. Wang, A green light-enhanced cytosolic protein delivery platform based on BODIPY-protein interactions, Nano Res., 2023, 16, 1042–1051 CrossRef CAS.
- For examples of BODIPYs with COO-substitution at boron (COO-BODIPYs), see: (a) C. Ray, C. Schad, F. Moreno, B. L. Maroto, J. Bañuelos, T. Arbeloa, I. García-Moreno, C. Villafuerte, G. Muller and S. de la Moya, BCl3-Activated Synthesis of COO-BODIPY Laser Dyes: General Scope and High Yields under Mild Conditions, J. Org. Chem., 2020, 85, 4594–4601 CrossRef CAS PubMed. For examples of BODIPYs with other substitutions at boron, see: (b) A. Blazquez-Moraleja, I. Saenz-de-Santa Maria, M. D. Chiara, D. Alvarez-Fernandez, I. Garcia-Moreno, R. Prieto-Montero, V. Martinez-Martinez, I. López Arbeloa and J. L. Chiara, Shedding light on the mitochondrial matrix through a functional membrane transporter, Chem. Sci., 2020, 11, 1052–1065 RSC; (c) M. Wang, G. Zhang, P. Bobadova-Parvanova, K. M. Smith and M. G. H. Vicente, Syntheses and Investigations of Conformationally Restricted, Linker-Free α-Amino Acid–BODIPYs via Boron Functionalization, J. Org. Chem., 2021, 86, 18030–18041 CrossRef PubMed; (d) C. Schad, E. Avellanal-Zaballa, E. Rebollar, C. Ray, E. Duque-Redondo, F. Moreno, B. L. Maroto, J. Bañuelos, I. García-Moreno and S. de la Moya, Triplet–triplet sensitizing within pyrene-based COO-BODIPY: a breaking molecular platform for annihilating photon upconversion, Phys. Chem. Chem. Phys., 2022, 24, 27441–27448 RSC.
- F. López Arbeloa, J. Bañuelos, V. Martínez, T. Arbeloa and I. López Arbeloa, Structural, photophysical and lasing properties of pyrromethene dyes, Int. Rev. Phys. Chem., 2005, 24, 339–374 Search PubMed.
- (a) A. Harriman, L. Mallon, S. Goeb, G. Ulrich and R. Ziessel, Electronic Energy Transfer to the S2 Level of the Acceptor in Functionalised Boron Dipyrromethene Dyes, Chem. – Eur. J., 2009, 15, 4553–4564 CrossRef CAS PubMed; (b) A. B. More, S. Mula, S. Thakare, N. Sekar, A. K. Ray and S. Chattopadhyay, J. Org. Chem., 2014, 79, 10981–10987 CrossRef CAS PubMed.
- (a) A. Haefele, C. Zedde, P. Retainlleau, G. Ulrich and R. Ziessel, Boron Asymmetry in a BODIPY Derivative, Org. Lett., 2010, 12, 1672–1675 CrossRef CAS PubMed; (b) M. Işık, E. Dündar, E. Şahin and C. Tanyeli, A boron dipyrromethene chiral at boron and carbon with a bent geometry: synthesis, resolution and chiroptical properties, Chem. Commun., 2022, 58, 7188–7191 RSC.
- C. Ray, L. Díaz-Casado, E. Avellanal-Zaballa, J. Bañuelos, L. Cerdán, I. García-Moreno, F. Moreno, B. L. Maroto, Í. López-Arbeloa and S. de la Moya, N-BODIPYs Come into Play: Smart Dyes for Photonic Materials, Chem. – Eur. J., 2017, 23, 9383–9390 CrossRef CAS PubMed.
- C. Ray, A. García-Sampedro, C. Schad, E. Avellanal-Zaballa, F. Moreno, M. J. Ortiz, J. Bañuelos, A. Villanueva, P. Acedo, B. L. Maroto and S. de la Moya, Alkynyl N-BODIPYs as Reactive Intermediates for the Development of Dyes for Biophotonics, Chem. Proc., 2021, 3, 15 Search PubMed.
- J. Bañuelos, I. Lopez-Arbeloa, I. García-Moreno, A. Costela, L. Infante, E. Perez-Ojeda, M. Palacios-Cuesta and M. J. Ortiz, Controlling Optical Properties and Function of BODIPY by Using Asymmetric Substitution Effects, Chem. – Eur. J., 2010, 16, 14094–14105 CrossRef PubMed.
- For example, see: (a) C.-W. Wan, A. Burghart, J. Chen, F. Bergström, L. B.-Å. Johansson, M. F. Wolford, T. G. Kim, M. R. Topp, R. M. Hochstrasser and K. Burgess, Anthracene–BODIPY Cassettes: Syntheses and Energy Transfer, Chem. – Eur. J., 2003, 9, 4430–4441 CrossRef CAS PubMed; (b) C. Ray, C. Schad, E. Avellanal-Zaballa, F. Moreno, B. L. Maroto, J. Bañuelos, I. García-Moreno and S. de la Moya, Multichromophoric COO-BODIPYs: an advantageous design for the development of energy transfer and electron transfer systems, Chem. Commun., 2020, 56, 13025–13028 RSC.
- (a) T. Förster, 10th Spier memorial lecture. Transfer mechanisms of electronic excitation, Discuss. Faraday Soc., 1959, 27, 7–17 RSC; (b) G. D. Scholes, K. P. Ghiggino, A. M. Oliver and M. N. Paddon-Row, Through-Space and Through-Bond Effects on Exciton Interactions in Rigidly Linked Dinaphthyl Molecules, J. Am. Chem. Soc., 1993, 115, 4345–4349 CrossRef CAS; (c) S. Speiser, Photophysics and Mechanisms of Intramolecular Electronic Energy Transfer in Bichromophoric Molecular Systems: Solution and Supersonic Jet Studies, Chem. Rev., 1996, 96, 1953–1976 CrossRef CAS PubMed.
- For example, see: X. Kong, L. Di, Y. Fan, Z. Zhou, X. Feng, L. Gai, J. Tian and H. Lu, Lysosome-targeting turn-on red/NIR BODIPY probes for imaging hypoxic cells, Chem. Commun., 2019, 55, 11567–11570 RSC.
- H. Tanaka, Y. Inoue and T. Mori, Circularly Polarized Luminescence and Circular Dichroisms in Small Organic Molecules: Correlation between Excitation and Emission Dissymmetry Factors, ChemPhotoChem, 2018, 2, 386 CrossRef CAS.
- J. Jiménez, F. Moreno, B. L. Maroto, T. A. Cabreros, A. S. Huy, G. Muller, J. Bañuelos and S. de la Moya, Modulating ICT emission: a new strategy to manipulate the CPL sign in chiral emitters, Chem. Commun., 2019, 55, 1631–1634 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, dye characterization and supplementary tables and figures. CCDC 2269820. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01561k |
| ‡ The degree of ECD is given by the Kunh's dissymmetry ratio, gabs(λ) = 2(εL − εR)/(εL + εR), where εL and εR are the molar absorption coefficients of left and right circularly polarized absorption, respectively. |
| § The degree of CPL is given by the luminescence dissymmetry factor, glum(λ) = 2(IL − IR)/(IL + IR), where IL and IR are the intensities of left and right circularly polarized emission, respectively. |
| This journal is © the Partner Organisations 2023 |