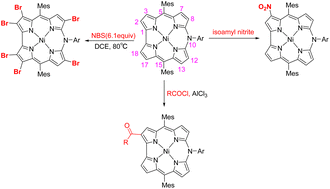
The peripheral functionalization of 10-azacorroles has been almost unexplored compared to the flourishing development of the functionalization of corroles. Here we explored the functionalization of 10-azacorroles through the study of nitration, bromination and acylation reactions affording novel 10-azacorrole derivatives with functional groups at peripheral positions. A one-pot reaction of 10-azacorroles with isoamyl nitrite under mild reaction conditions resulted in the consecutive formation of 3-nitro and 3,17-dinitroazacorroles in 70–85% yields. Treatment of 10-azacorroles with 6.1 equivalents of N-bromosuccinimide (NBS) in 1,2-dichloroethane at 80 °C resulted in hexabrominated azacorroles regioselectively. Acyl chlorides reacted with 10-azacorroles in dichloromethane in the presence of AlCl3 at room temperature, affording acylated products in moderate yields. The structures of all the products were characterized thoroughly by high-resolution mass spectrometry and NMR and UV-vis spectroscopy together with single-crystal X-ray diffraction analysis.