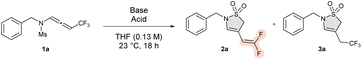Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
TBAF-promoted carbanion-mediated sulfonamide cyclization of CF3-substituted N-allenamides: an access to fluorinated γ-sultams†
Clément
Gommenginger
 a,
Yongxiang
Zheng
a,
Daniele
Maccarone
a,
Yongxiang
Zheng
a,
Daniele
Maccarone
 b,
Ilaria
Ciofini
b and
Laurence
Miesch
b,
Ilaria
Ciofini
b and
Laurence
Miesch
 *a
*a
aEquipe Synthèse Organique et Phytochimie, Institut de Chimie, CNRS-UdS, UMR 7177, 4 rue Blaise Pascal, CS 90032, 67081, Strasbourg, France. E-mail: lmiesch@unistra.fr
bChimie ParisTech, PSL University, CNRS, Institute of Chemistry for Life and Health Sciences, Chemical Theory and Modelling Group, F-75005 Paris, France
First published on 6th July 2023
Abstract
Upon treatment with TBAF, CF3-substituted N-allenamides were transformed into γ-sultams. Cyclic sulfonamides bearing an ene-gem-difluorinated tether could be obtained by addition of acetic acid to the ammonium salt whereas TBAF alone provided the corresponding trifluorinated ethyl sultams. A combined experimental and computationnal mechanistic study suggested that this transformation involves a 5-endo-dig cyclization on the ene-ynamide generated in situ.
Introduction
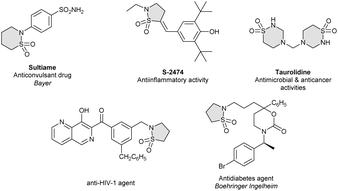
Since the discovery of sulfonamide antibacterials,1 sulfonamides have played a key role in medicinal chemistry. The cyclic counterparts of sulfonamide compounds (sultams) display enhanced biological activities compared to their acyclic congeners.2 Although not found in nature, these amide surrogates are considered privileged motifs that have found diverse applications in drug discovery such as in sultiame, an anticonlvulsant agent,3 S-2474, a non-steroidal anti-inflammatory drug,4 taurolidine, which exhibits antimicrobial and anticancer activities,5 a naphthyridine derivative that is an HIV-1 inhibitor,6 and an antidiabetes agent developed by Boehringer Ingelheim (Fig. 1).7In view of this important structural motif, numerous methods for their synthesis have been developed. The carbanion-mediated sulfonate (or sulfonamide) cyclization constitutes a well-known approach to these important scaffolds (Scheme 1A).8
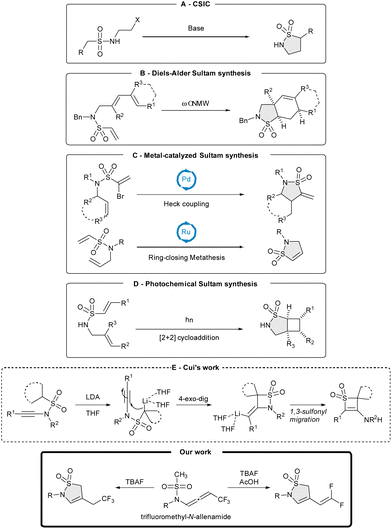
In this process, the carbanion resulting from deprotonation of the proton located at the α-position of the SO2 group reacts with a suitable electrophile to form various cyclic sulfonamides. Intramolecular Diels–Alder cycloadditions of vinylsulfonamides have proven to be essential to construct specific sultams (Scheme 1B).9 Although effective, these protocols require strong bases and harsh reaction conditions often detrimental to sensitive functional groups. Transition-metal catalyzed-processes,10 including intramolecular Heck reactions of α-bromovinylsulfonamides,11 rhodium-catalyzed intramolecular aziridination of unsaturated sulfonamides,12 as well as ring-closing metathesis,10,13 have emerged as powerful methods to prepare cyclic sulfonamides (Scheme 1C). Recently, Mykhailiuk reported a photochemical cyclization providing access to a new class of bicyclic sultams (Scheme 1D).14 Related to this present work, it is important to mention Cui's contribution, who observed an unusual reorganization divergence on N-sulfonyl ynamides upon basic treatment via a 4-exo-dig cyclization and subsequent 1,3 sulfonyl-migration (Scheme 1E).15 Because organofluorine compounds play a critical role in life science and agrochemistry, the judicious introduction of fluorine atoms into organic molecules has become an increasingly dominant research area.16 In particular, the gem-difluoro-ene group, a mimic of carbonyl compounds,17 and the CF3 moiety are attractive targets because of their impact on the development of marketed drugs.18
In view of our previous results on CF3-substituted N-allenamides, we anticipated that deprotonation at the α-position of the sulfonyl moiety of N-sulfonyl-allenamides might initiate an anionic 5-endo-dig cyclization to produce cyclic sulfonamides.
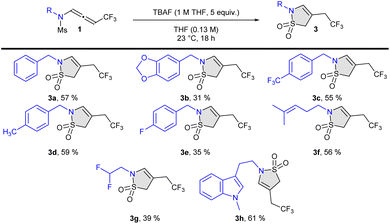
We report herein a tetra-n-butylammonium fluoride (TBAF)-promoted cyclization of CF3-substituted N-sulfonyl-allenamides for the preparation of trifluoethyl-substituted and difluoro-ene sultams (Scheme 1). Trifluoromethylated N-allenamides were obtained by treatment of terminal ynamides with trifluoromethylated diazomethane according to our previously developed strategy.19
Results and discussion
In consideration of the work of Cui et al.,15 who noticed that the deprotonation of the α-position if the sulfonyl moiety of N-sulfonyl ynamides initiated a 4-exo-dig cyclization, we performed our first experiment by treatment of trifluoromethylated N-allenamide 1a with lithium diisopropylamide (LDA) in THF. In this case, only the corresponding ene-ynamide 6 was observed along with some stating material (Table 1, entry 1).20 By employing weaker bases such as Cs2CO3, the starting material was totally recovered (Table 1, entry 2). Taking into consideration these results, we wondered whether tetraalkyl ammonium fluoride salts would provide an efficient solution to this problem. When TBAF was employed as a base, we noticed that a mixture of two cyclic unsaturated sulfonamides, a CF3-substituted and an ene-difluorinated sultam, 3a and 2a, respectively, were isolated along with some starting material 1a (Table 1, entry 3). By increasing the amount of TBAF, this transformation was exclusively directed toward the formation of CF3-substituated sultam 3a (Table 1, entry 4). To guide the reaction solely toward the difluorinated ene product 2a, our objective was to quench the released fluoride ion with an acid to avoid the re-introduction of the fluoride ion on the difluorinated ene moiety.20 First trials were carried out with a Lewis acid. Whether with BF3·Et2O, TMSOTf, or FeCl3 (Table 1, entries 5–7), the outcome of this transformation led to a mixture of compounds 2a and 3a, albeit the selectivity is poorer with the latter. We then turned our attention to the utilization of a Brønsted acid. Triflic acid (TfOH) and trifluoroacetic acid (TFA) led to a mixture of sultams 2a and 3a, whereas acetic acid provided the desired difluorinated ene-sultam 2a with 69% yield along with traces of the trifluorinated derivative 3a (Table 1, entries 8–10). Controlled experiments with Brønsted acids such as TFA and TfOH alone led to degradation of the starting material (Table 1, entries 11 and 12), whereas with acetic acid the starting material was totally recovered (Table 1, entry 13). When CF3-substituted N-allenamide 1a was subjected to hydrofluoric acid (HF), the corresponding mesylsulfonamide was exclusively isolated (Table 1, entry 14).| Entrya | Base ([equiv.]) | Acid ([equiv.]) | Yieldb [%] | ||
|---|---|---|---|---|---|
| 2a | 3a | 1a | |||
| a Reaction conditions: to a solution of 1a (0.25 mmol) in THF (2 mL), was added a solution of TBAF (1 M in THF, 1.25 mmol) at 23 °C, and the mixture was stirred for 18 h. b Isolated yields. c The mixture was stirred from −78 °C to 0 °C for 1 day. The corresponding ene-ynamide 6 was observed in mixture with 1a. d The mixture was stirred for 1 h instead 18 h. e Only degradation of starting material was observed. f 94% of mesylsulfonamide was isolated. | |||||
| 1c | LDA (1.2) | — | — | — | 30 |
| 2 | Cs2CO3 (1.2) | — | — | — | 100 |
| 3d | TBAF (1) | — | 14 | 41 | 27 |
| 4 | TBAF (5) | — | — | 57 | — |
| 5 | TBAF (5) | BF3·OEt2 (3) | 55 | 13 | — |
| 6 | TBAF (5) | TMSOTf (1.2) | 43 | 7 | 37 |
| 7 | TBAF (5) | FeCl3 (5) | 35 | 26 | — |
| 8 | TBAF (5) | TfOH (4.5) | 45 | 11 | — |
| 9 | TBAF (5) | TFA (2.5) | 36 | 26 | — |
| 10 | TBAF (5) | AcOH (5) | 69 | Traces | — |
| 11e | — | TFA (5) | — | — | — |
| 12e | — | TfOH (5) | — | — | — |
| 13 | — | AcOH (5) | — | — | >98 |
| 14f | — | HF (cat.) | — | — | — |
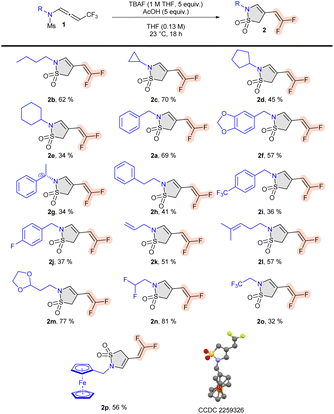
Under the optimized conditions, the reaction scope of this 5-endo-dig cyclization was examined. First, we investigated the chemical space of the unsaturated ene-difluorinated sultams. Linear alkyls (2b) and cyclic alkyls (2c–e) were tolerated for this transformation. Aryl substituents (2a–j) were accommodated as well. Pleasingly, functionalized sidechains suitable for late-stage transformations could be installed on the sultam ring through this transformation. In particular, unsaturated sidechains (2k–2l), protected aldehydes (2m), di- and tri-fluorinated sidechains (2n–o), and ferrocenyl derivatives (2p)21 were successfully adapted (Scheme 2). X-Ray analysis of 2p confirmed the structure of the difluoro-ene sultam (CCDC 2259326† contains the supplementary crystallographic data for the structure).22
We then focused on access of the unsaturated sultams to products bearing a CF3-tether. Aryl derivatives (3a–e), unsaturated sidechains (3f), di-fluorinated sidechains (3g), as well as tryptamine derivatives (3h) could be adapted, albeit with lower yields because of instability of the targeted compounds (Scheme 3).
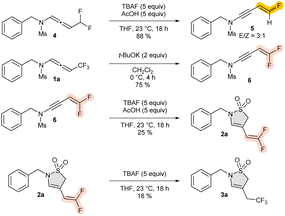
To gain greater insight into the reaction mechanism, a series of control experiments were performed. When the difluorinated substrate 1a was exposed to the same reaction conditions, i.e., TBAF/AcOH, the corresponding sultam was not observed; the monofluorinated ene-ynamide 5 was exclusively obtained in 88% yield as a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E- and Z-compounds. We have recently demonstrated that gem-difluorinated ene-ynamides 6 could be obtained directly through base-induced treatment of trifluoromethylated N-allenamides.20 Additionally, when the difluorinated ene-ynamide 6 was treated under the same conditions (i.e., a mixture of TBAF and AcOH) the corresponding ene-difluorinated sultam 2a was obtained, providing evidence that the ene-ynamide species 6 is an intermediate of the transformation. It is also possible to achieve the synthesis of CF3-substituted sultam 3a directly from the ene-difluorinated sultam 2a, which reinforces the theory that the fluoride ion must be quenched to access to sultams bearing an ene-difluorinated tether (Scheme 4).23
1 mixture of E- and Z-compounds. We have recently demonstrated that gem-difluorinated ene-ynamides 6 could be obtained directly through base-induced treatment of trifluoromethylated N-allenamides.20 Additionally, when the difluorinated ene-ynamide 6 was treated under the same conditions (i.e., a mixture of TBAF and AcOH) the corresponding ene-difluorinated sultam 2a was obtained, providing evidence that the ene-ynamide species 6 is an intermediate of the transformation. It is also possible to achieve the synthesis of CF3-substituted sultam 3a directly from the ene-difluorinated sultam 2a, which reinforces the theory that the fluoride ion must be quenched to access to sultams bearing an ene-difluorinated tether (Scheme 4).23
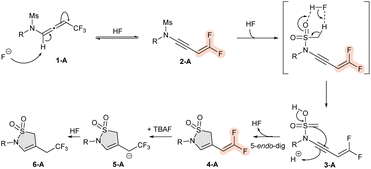
Based on the aforementioned experimental results, calculations based on Density Functional Theory (DFT) were performed to provide support for a plausible reaction mechanism. A simplified reaction scheme is depicted in Scheme 5, while the full computed reaction profile is reported in Scheme 6. Thanks to the presence of fluoride ions in the reaction medium, the N-allenamide 1-A is deprotonated α to the nitrogen, thus allowing a subsequent δ-elimination of a fluoride ion, providing difluorinated ene-ynamide 2-A. This is consistent with the formation of compound 5 (Scheme 4). Ylide 3-A is generated in situ through an HF-catalyzed process.24 5-endo-dig addition of ylide 3-A on the ynamide part of the molecule provides γ-sultam with a gem-difluorinated ene-tether 4-A. Further addition of the fluoride ion on compound 4-A led finally to the trifluorinated adduct 6-Avia compound 5-A (Scheme 5).
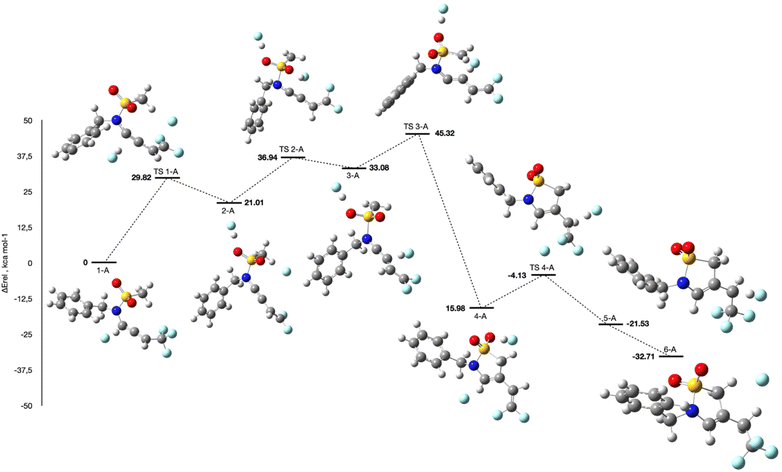
The full computed energy profile reported in Scheme 6 is more complex because of the formation of a larger number of reaction intermediates (refer to ESI† for Computational details). Nonetheless, it reveals that the energetically demanding step is the deprotonation, with an energetic barrier of about 29 kcal mol−1. Once the deprotonation has taken place, the reaction proceeds toward the thermodynamically more stable products with barriers all below 20 kcal mol−1. It is also important to stress the role played by the F-/HF pair in stabilizing the different reaction intermediates and transition states (TS). Analysis of the evolution of the carbon–carbon bonds (reported in the ESI†) along the molecular skeleton indicates that starting from the allene 1-A (showing two C–C double bonds of 1.306 Å for the allene and single bond of 1.488 Å), a triple- (1.208 Å) single- (1.409 Å) and double bond (1.330 Å) are formed in intermediates 2-A and 3-A before the formation of the cyclic product.
From intermediate 3-A, cyclization to yield a four-membered ring was also tested (see ESI†). Nonetheless, the associated TS is higher in energy with respect to the corresponding one leading to the formation of the experimentally observed sultam, and the four-membered ring product is less stable than compound 4-A by about 12 kcal mol−1. Calculations performed on the analogous difluorinated substrate (see ESI†) show that while the reaction barrier associated with the deprotonation of the proton α to the nitrogen is relatively similar, the transition state for the cyclization step is actually much higher (roughly 40 kcal mol−1), thus impeding the formation of the corresponding sultam as experimentally observed.
Conclusions
In conclusion, we have developed a mild and efficient access to trifluorinated- and ene-gem-difluorinated γ-sultams from CF3-substituted N-allenamides. Addition of TBAF allowed the formation of CF3-substituted sultams, whereas addition of acetic acid to the ammonium salt led to the formation of cyclic sulfonamides bearing a difluorinated ene moiety. The developed strategy shows broad functional group tolerance and a good substrate scope. Furthermore, DFT calculations corroborated a transformation proceeding by a 5-endo-dig cyclization on the ene-ynamide formed in situ.Conflicts of interest
There are no conflicts to declare.Author contributions
L. M., C. G., and Y. Z. conceived and designed the experiments. L. M. directed the project. C. G. performed the experiments. I. C. and D. M. performed the DFT calculations. L. M. and I. C. wrote the paper. L. M., I. C., Y. Z., C. G., and D. M. discussed the results and commented on the manuscript.Acknowledgements
Support for this work was provided by CNRS and Université de Strasbourg. C. G. thanks M.R.T. for a research fellowship, and Y. Z. thanks C.S.C. for a research fellowship.References
- J. E. Lesch, The First Miracle Drugs: How the Sulfa Drugs Transformed Medicine, Oxford University Press, Oxford, 2007 Search PubMed.
- S. Debnath and S. Mondal, Sultams: Recent Syntheses and Applications, Eur. J. Org. Chem., 2018, 933–956 CrossRef CAS.
- K. M. Gorman and A. Shahwan, Sultiame revisited: treatmentof refractory absence seizures, Epileptic Disord., 2016, 18, 329–330 Search PubMed.
- M. Inagaki, T. Tsuri, H. Jyoyama, T. Ono, K. Yamada, M. Kobayashi, Y. Hori, A. Arimura, K. Yasui, K. Ohno, S. Kakudo, K. Koizumi, R. Suzuki, M. Kato, S. Kawai and S. Matsumoto, Novel Antiarthritic Agents with 1,2-Isothiazolidine-1,1-dioxide (γ-Sultam) Skeleton: Cytokine Suppressive Dual Inhibitors of Cyclooxygenase-2 and 5-Lipoxygenase, J. Med. Chem., 2000, 43, 2040–2048 CrossRef CAS PubMed.
- (a) P. M. Neary, P. Hallihan, J. H. Wang, R. W. Pfirrmann, D. J. Bouchier-Hayes and H. P. Redmond, The Evolving Role of Taurolidine in Cancer Therapy, Ann. Surg. Oncol., 2010, 17, 1135–1143 CrossRef PubMed; (b) Y. Liu, A.-Q. Zhang, L. Cao, H.-T. Xia and J.-J. Ma, Taurolidine Lock Solutions for the Prevention of Catheter-Related Bloodstream Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, PLoS One, 2013, 8, e79417 CrossRef PubMed.
- L. Zhuang, J. S. Wai, M. W. Embrey, T. E. Fisher, M. S. Egbertson, L. S. Payne, J. P. Guare, J. P. Vacca, D. J. Hazuda, P. J. Felock, A. L. Wolfe, K. A. Stillmock, M. V. Witmer, G. Moyer, W. A. Schleif, L. J. Gabryelski, Y. M. Leonard, J. J. Lynch, S. R. Michelson and S. D. Young, Design and Synthesis of 8-Hydroxy-[1,6]Naphthyridines as Novel Inhibitors of HIV-1 Integrase in Vitro and in Infected Cells, J. Med. Chem., 2003, 46, 453–456 CrossRef CAS PubMed.
- D. A. Claremon, L. Zhuang, K. Leftheris, C. M. Tice, Y. Ye, S. B. Singh and F. Himmelsbach, Cyclic inhibitors of 11BETA-hydroxysteroid dehydrogenase 1, US 8592410B2, 2013.
- (a) D. Postel, A. N. Van Nhien and J. L. Marco, Chemistry of Sulfonate- and Sulfonamide-Stabilized Carbanions – The CSIC Reactions, Eur. J. Org. Chem., 2003, 3713–3726 CrossRef CAS; (b) A. V. Dobrydnev and J. Marco-Contelles, Updating the CSIC Reaction (2003–2020), Eur. J. Org. Chem., 2021, 1229–1248 CrossRef CAS.
- (a) P. Metz, D. Seng, R. Fröhlich and B. Wibbeling, Intramolecular Diels-Alder Reactions of Vinylsulfonamides, Synlett, 1996, 741–742 CrossRef CAS; (b) A. D. Brosius, L. E. Overman and L. Schwink, Total Synthesis of (+)-Aloperine. Use of a Nitrogen-Bound Silicon Tether in an Intramolecular Diels–Alder Reaction, J. Am. Chem. Soc., 1999, 121, 700–709 CrossRef CAS.
- A. Zhou, D. Rayabarapu and P. R. Hanson, “Click, Click, Cyclize”: A DOS Approach to Sultams Utilizing Vinyl Sulfonamide Linchpins, Org. Lett., 2009, 11, 531–534 CrossRef CAS PubMed.
- S. Merten, R. Fröhlich, O. Kataeva and P. Metz, Synthesis of Sultams by Intramolecular Heck Reaction, Adv. Synth. Catal., 2005, 347, 754–758 CrossRef CAS.
- J.-L. Liang, S.-X. Yuan, P. W. H. Chan and C.-M. Che, Rhodium(II,II) Dimer as an Efficient Catalyst for Aziridination of Sulfonamides and Amidation of Steroids, Org. Lett., 2002, 4, 4507–4510 CrossRef CAS PubMed.
- (a) S. Hanessian, H. Sailes and E. Therrien, Synthesis of functionally diverse bicyclic sulfonamides as constrained proline analogues and application to the design of potential thrombin inhibitors, Tetrahedron, 2003, 59, 7047–7056 CrossRef CAS; (b) S. Mondal and S. Debnath, Synthesis of Sultams by Ring-Closing Metathesis, Synthesis, 2014, 368–374 Search PubMed.
- D. Dibchak, V. Shcherbacova, A. V. Denisenko and P. K. Mykhailiuk, Convenient Access to Conformationally Rigid Sultams, Org. Lett., 2019, 21, 8909–8914 CrossRef CAS PubMed.
- L. Zeng, Y. Lin, J. Li, H. Sajiki, H. Xie and S. Cui, Skeletal reorganization divergence of N-sulfonyl ynamides, Nat. Commun., 2020, 11, 5639–5649 CrossRef CAS PubMed.
- (a) J.-P. Bégué and D. Bonnet-Delpon, Bioorganic and Medicinal Chemistry of Fluorine, Wiley, Hoboken, NJ, 2008 CrossRef; (b) W. K. Hagmann, The Many Roles for Fluorine in Medicinal Chemistry, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed; (c) P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, Germany, 2nd edn, 2013 CrossRef.
- N. A. Meanwell, Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design, J. Med. Chem., 2011, 54, 2529–2591 CrossRef CAS.
- (a) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011), Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS; (b) H. Mei, J. Han, S. Fustero, M. Medio-Simon, D. M. Sedgwick, C. Santi, R. Ruzziconi and V. A. Soloshonok, Fluorine-Containing Drugs Approved by the FDA in 2018, Chem. – Eur. J., 2019, 25, 11797–11819 CrossRef CAS.
- Y. Zheng, B. Moegle, S. Ghosh, A. Perfetto, D. Luise, I. Ciofini and L. Miesch, Copper-Catalyzed Synthesis of Terminal vs. Fluorine-Substituted N-Allenamides via Addition of Diazo Compounds to Terminal Ynamides, Chem. – Eur. J., 2022, 28, e202103598 CAS.
- M. Hourtoule and L. Miesch, Regio- and Stereoselective Addition to gem-Difluorinated Ene–Ynamides: Access to Stereodefined Fluorinated Dienes, Org. Lett., 2022, 24, 3896–3900 CrossRef CAS PubMed.
- C. Mahe, O. Blacque, G. Gasser, V. Gandon and K. Cariou, N-Metallocenyl Ynamides: Preparation, Reactivity, and Synthesis of ansa[3]-Ferrocenylamides, Org. Lett., 2023, 25, 624–629 CrossRef CAS PubMed.
- CCDC 2259326 (2p) contains the supplementary crystallographic data for this paper.†.
- Y. Qiao, T. Si, M.-H. Yang and R. A. Altman, Metal-Free Trifluoromethylation of Aromatic and Heteroaromatic Aldehydes and Ketones, J. Org. Chem., 2014, 79, 7122–7131 CrossRef CAS PubMed.
- A. J. Mota, A. Klein, F. Wendling, A. Dedieu and M. Miesch, Controlled Synthesis of α-Allenic Ester and Spiro Ketone Derivatives from Tailored α-Substituted Cycloalkanones Through Cascade Reactions: Exploring the Possible Reaction Pathways by Means of Semiempirical MO Calculations, Eur. J. Org. Chem., 2005, 4346–4358 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: All experimental data and detailed procedures, including computational details. CCDC 2259326. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo00781b |
| This journal is © the Partner Organisations 2023 |